ABSTRACT
Cartilage injuries are common problems that increase with the population aging. Cartilage is an avascular tissue with a relatively low level of cellular mitotic activity, which makes it impossible to heal spontaneously. To compensate for this problem, three-dimensional bio-printing has attracted a great deal of attention in cartilage tissue engineering. This emerging technology aims to create three-dimensional functional scaffolds by accurately depositing layer-by-layer bio-inks composed of biomaterial and cells. As a novel bio-ink, a decellularized extracellular matrix can serve as an appropriate substrate that contains all the necessary biological cues for cellular interactions. Here, this review is intended to provide an overview of decellularized extracellular matrix-based bio-inks and their properties, sources, and preparation process. Following this, decellularized extracellular matrix-based bio-inks for cartilage tissue engineering are discussed, emphasizing cell behavior and in-vivo applications. Afterward, the current challenges and future outlook will be discussed to determine the conclusing remarks.
Keywords: 3D bio-printing, cartilage, decellularization, dECM, tissue engineering
Introduction
The aging population in developed societies has exacerbated the problems associated with cartilage defects, especially osteoarthritis, affecting millions of people across the globe each year.1 Patients suffering from these injuries often experience severe pain or disability that can adversely affect their quality of life.2 It has been observed that men and women in their fifth to seventh decades of life often suffer from osteoarthritis and lose the function of their joints, such as the knees, hips, and shoulders as a result of degeneration of hyaline cartilage.3
On the other hand, cartilage is an avascular connective tissue that is supplied by repairing cells and exhibits a very limited self-healing capacity. As a traditional form of cartilage repair, allografts, autografts, and bone marrow stimulation are employed. All of these techniques are subject to limitations, such as secondary surgery, scarcity of donors, and rejection.4, 5 As a result, researchers are attempting to develop a method for cartilage regeneration; accordingly, the concept of tissue engineering is presented as a potentially promising method to repair cartilage defects.6
The natural cartilage structure has a complex architecture with four distinct zones: surface zone, middle zone, deep zone, and calcified zone. Each zone consists of a unique combination of cell phenotypes with diverse biochemical compositions, microstructures, and the physiological environment.7 Therefore, a suitable method needs to be developed for building cartilaginous structures that can support cells and ultimately lead to the regeneration of complex cartilage tissue. Scaffolding methods conventionally used for the fabrication of synthetic matrices cannot meet the requirements for cartilage healing.8 The emergence of the three-dimensional (3D) bio-printing technology was a revolution in cartilage tissue engineering and offers a number of advantages over conventional techniques, such as the ability to generate complex tissues containing multiple cell types and biomaterials, patient specificity, creation of predefined structures, and reproducibility.9 Bandyopadhyay et al.10 proposed a photo-cross-linkable bio-ink containing different concentrations of silk methacrylate, polyethylene glycol diacrylate and chondrocytes. This study demonstrated that the bio-printing technique has capability of maintaining cell viability while showing high mechanical properties, considering cartilage tissue requirements.
Today, the high capability of 3D printing to produce accurate structures based on a predetermined design is well-known. Still, creating an ideal bio-ink that can mimic the body’s natural microenvironment is still a major challenge. The cartilage is composed of water, proteoglycans, and type I, II, and X collagen. Glycosaminoglycans (GAGs) are an important component of cartilage, and in order for cartilage differentiation, the right amount of GAGs must be present.11 Therefore, the designed bio-inks should provide both mechanical and chemical properties for the target tissue as well as demonstrate biocompatibility for cell-laden printing.12 In this regard, extracellular matrix (ECM), a natural substance, can provide biochemical factors and a microenvironment for cells. The ECM components and their native structure serve as a guide for cell activities and, as a consequence, cell differentiation. Numerous studies attemp to mimic natural ECM to improve the regeneration capacity of the constructs.13-15 In this respect, decellularized ECM (dECM)-based bio-inks have gained considerable attention in the field of tissue regeneration, specifically in cartilage tissue engineering.16 The decellularization process is widely recognized as a prerequisite for the preparation of any ECM-derived material, referring to treatment process of removing cellular and nuclear components efficiently while protecting the composition and integrity of native ECM.17
Taken together, this review will discuss decellularization procedures and dECM properties with the aim of facilitating cartilage healing. This review will provide an overview of the dECM bio-inks used in cartilage tissue engineering and identify in-vitro and in-vivo challenges as well as present suggestions for future studies and clinical applications.
Decellularization Procedure
Decellularization is aimed at eliminating all cells from the tissue while maintaining its native composition and structure.18-20 The cartilage-derived dECM promotes chondrocytes adhesion, proliferation, and growth, which ultimately leads to cartilage regeneration in-vivo; furthermore, it enhances chondrogenic differentiation of stem cells in-vitro.21 A proper decellularization procedure, including chemical, enzymatic, and physical methods, should demonstrate the following features: < 50 ng double stranded DNA per mg of ECM dry weight, < 200 bp of DNA fragment length, and have no visible nuclear elements in the 4′,6-diamidino-2-phenylindole or hematoxylin and eosin-stained tissue.22
Chemical decellularization
Decellularization materials are classified as either alkaline or acidic. Among the alkaline and acidic agents, acidic is a highly effective way for decellularization. However, it can influence the mechanical properties.23 Peracetic acid is among the minimal invasive acids used for decellularization. The main benefit of alkaline and acid decellularization is the simultaneous disinfection process. Kheir et al.24 used 0.1% (v/v) peracetic acid for disinfection of porcine cartilage bone matrix after incubating the tissues in hypotonic tris buffer and 0.1% (w/v) sodium dodecyl sulfate (SDS) in hypotonic buffer with protease inhibitors. A histological examination confirmed the removal of cells. Decellularization may also be accomplished with hydrogen chloric acid,25 sulfuric acid,26 and ammonium hydroxide.27 The bases may remove all the growth factors and reduce the matrix’s mechanical properties.28
Chemical decellularization can also be achieved by using ionic detergents. SDS,29 sodium deoxycholate,30 zwitterionic detergents such as 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate31 are some of the famous example of ionic detergents. Most of these agents cause ECM disruption and protein removal from ECM, but, zwitterionic detergents have net-zero electrical charges, which can preserve the proteins during the decellularization procedure.32 One of the most common detergents is SDS. In a study, Bordbar et al.33 used three cycles of freeze-thawing for two minutes in liquid nitrogen, as well as two different chemical treatments that used 10% (v/v) ethylenediaminetetraacetic acid disodium salt and 5% and 10% (w/v) SDS. The findings demonstrated that SDS 5% (v/v) resulted in better decellularization capability after freezing cycles as compared to SDS 10% (v/v) and ethylenediaminetetraacetic acid. Therefore, it appears that the lower concentration of SDS had better infusion into dense cartilage.
Among non-ionic chemical detergents, Triton X-100 is the most widely used. Although Ghassemi et al.34 demonstrated complete decellularization with 3% Triton X-100, in some cases may lead to complete loss of GAGs.35
Physical decellularization
Application of osmotic pressure,26 freeze-thaw cycles,36 bath ultrasonication, and using a direct sonicator37 are examples of typical physical decellularization methods. The physical treatment is usually employed in combination with the other techniques to achieve high throughput decellularization; because these agents lyse cells and the cellular residues will remain when used alone.23 for example, osmotic pressure only loosens the ECM components, whereas the use of chemical agents improves the decellularization.38 Guimaraes et al.39 used a physical-chemical protocol for tracheal decellularization. The pig tracheal was cut into small pieces followed by freezing-thawing, and cycles of agitation (10 times). Then sodium deoxycholate was added and washed. The results demonstrated that DNA content was reduced to less than 2% (w/w) of untreated samples. In addition, the skin and mixed glands were removed properly. In another study, Al-Qurayshi et al.40 used a biological-physical-chemical decellularization technique for human larynges. The decellularization process took long 12 days. The decellularization procedure contained several agitations, freeze-thawing, enzymatic, chemical agent soaking, and washing steps. The results demonstrated high reduction in DNA content with high ECM structure preservation and adequate mechanical properties.
Enzymatic decellularization
Enzymatic decellularization offers the advantage of removing cellular components such as DNA. In order to hydrolyze ribonucleotide or deoxyribonucleotide chains or to cleave peptide chains; different enzymes must be employed; however, these may have some structural or functional effects.41 Nucleases (DNase and RNase),42 trypsin,43 and dipase44 are some of the most common enzymes for enzymatic decellularization. Each of these approaches has shown some drawbacks in decellularization that should be considered. In using Nuclease method, induction of severe distortion of ECM structure, incompleted cell removal, prevention of recellularization and transplantation were illustrated. Difficulty in sufficient decellularization and increasing incubation time were found in use of trypsin method, and in dipase method, damage to basement membranes and ECM should be considered.45 On the other hand, all these enzymatic approaches are used for decellularization, due to their specificities to removal of DNA content while proteins retain, and their ability in decellularization without inducing cytotoxic effects.46, 47 Decellularization of tracheal cartilage requires strong decellularization agents, such as using ionic methods, which can lead to disruption of the tissue’s native structure. Therefore, Zang et al.48 used a detergent-enzymatic treatment in order to decellularize the tracheal matrix. Following washing, the harvested rat trachea pieces were modified with 4% sodium deoxycholate and 1 mM sodium chloride containing 50 kU/mL of deoxyribonuclease I. Using five repeating cycles of detergent-enzymatic treatment, the matrix is decellularized with acceptable percentage and with sufficient compressive strength. Moreover, chelating agents such as ethylenediaminetetraacetic acid49 and ethylene glycol tetraacetic acid50 can also be used to decellularize cartilage. The binding between the chelating agents and metal ions, such as Ca2+ and Mg2+, results in cell separation.51
Combination techniques
Several chemical, physical, and enzymatic procedures are carried out simultaneously in order to complete decellularization. Schneider et al.26 examined 24 different decellularization protocols for preparing human articular cartilage material for use in tissue engineering procedures. Among the protocols included were several stages of freezing and thawing, the addition of a decellularization agent, an enzymatic step, and decontamination with various steps of decellularization. In different protocols, each of these steps may be modified, combined, or omitted entirely. The decellularization agents used included SDS, Triton X-100, hydrogen peroxide, sodium deoxycholate, 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate, sodium hydroxide, and hydrogen chloride (HCl). According to the results, although SDS shows good efficiency for reducing DNA content, it reduces cell cytocompatibility. As a result of this study, the combination of two steps, HCl treatment, and pepsin digestion, in addition to freeze and thaw cycles, as well as osmotic shock steps, has been proposed as the optimal method, which preserves the collagen structure and has superior mechanical properties to commercial cartilage scaffolds that can maintain one-third of native compressive modulus. In a study by Visscher et al.52 decellularization was performed by the subsequent steps of freeze-thawing, adding Tritron X-100 and protease inhibitor, washing, and soaking in Hanks buffered salt solution supplemented with DNase, washing, and freezing Yorkshire pigs’ ear cartilage. The double stranded DNA content measurement indicated a concentration of 9.4 ± 0.8 ng/mg. Additionally, according to second-harmonic generation and two-photon excited autofluorescence imaging and Masson’s trichrome staining, the collagen bundles and GAGs were preserved after the decellularization process, while hematoxylin and eosin staining confirmed that cellular component had been removed entirely. Also, Pati et al.53 performed hyaline cartilage decellularization with Tris-HCl buffer solution and repeated cycles of freezing and thawing followed by trypsin addition, washing, and 1% Tritron X-100 treatment. Hematoxylin and eosin staining demonstrated the removal of all cells and cell debris. Shen et al.54 prepared the dECM by incubating it in sodium hydroxide, washing, homogenizing, and freeze-drying it. In another study, Tian et al.55 used porcine cartilage ECM for cartilage tissue engineering. Therefore, washing of the cartilage pieces was performed with phosphate-buffered saline containing 3.5% (w/v) phenylmethylsulfonyl fluoride and 0.1% (w/v) ethylenediaminetetraacetic acid. Afterward, another chemical treatment was carried out, followed by the addition of acetic acid, deionized water, and nucleases were added followed by centrifugation and repeated washing.
Decellularized Extracellular Matrix Properties and Sources
dECM can be classified into three different types, including autogenous, allogeneic, and xenogeneic dECM, based on how it was derived. Since autogenous dECM scaffolds have limitations regarding tissue and surgical complications, allogeneic and xenogeneic dECM scaffolds are more promising. However, it should be considered that the allogeneic and xenogeneic dECM may show immunogenicity problems.19, 56
In another classification, dECM are classified based on the source of ECM with two main classes, including organ/tissue- and cell-derived dECM structures.19, 45 dECM scaffolds driven from tissues or organs exhibit the natural 3D microstructure of the specific organ/tissue, without the immunogenic cellular components. In cell-derived dECM scaffolds, cells cultured in-vitro are decellularized to form a substrate for producing large numbers of cells.45
There are some tissues and organs that are used as dECM sources, these include liver, heart, adipose tissue, cartilage, and skin from different sources, including humans, pigs, rats, goats, and cows. Among these dECM bio-ink-derived organ/tissue sources, pigs are the major source,57-59 due to the availability of porcine organs in large quantities.60
In comparison between animal and human origin, the potential for infectious disease transmission poses a challenge to the use of animals as a source of dECM. A controlled breeding animal could minimize this disease, and the disease could be almost eliminated by using xenogeneic ECM sources. Another disadvantage of using animal sources is the possibility of immunological reactions due to the presence of some specific animal antigens. In order to overcome this problem, gene editing and cloning techniques have been utilized.61 In addition, a number of factors, including animal age, may impact the composition, degradation rate, and mechanical properties of final the dECM.62 Whereas, animal tissue has shown greater stability and induction of stem cell differentiation compared to human tissue. Although organ/tissue ECM is derived from decellularized tissues, it retains its position as the most successful biomaterial due to its architectural and mechanical similarity to native ECM, as well as its ease of preparation at a large scale. However, several challenges must be overcome before the material can be used clinically. When animal tissue is used, incomplete decellularization carries the risk of disease transmission. Moreover, some specific issues, such as stem cell niches, are difficult to isolate. The large batch-to-batch differences make it impossible to use for specific applications.19, 63 To prevent disease transmission, human tissue could be substituted as a source of dECM bio-inks resources.64 Among the human tissue sources, adipose tissue is one of the human sources used to produce dECM bio-ink. In fact, this compound can induce proper cell viability as well as several adipogenic proteins expression without needing to supplement with adipogenic factors.65 However, a limited supply of human cadaveric tissue is one of the challenges of the human source.
Alternatively, cell-derived ECM may be able to offset some of the disadvantages of organ/tissue sources. This kind of dECM can be produced by the complete decellularization of human cell cultures, which removes all immunogenic components, maintaining the bioactivity. The preparation of dECM in vitro allows for the creation of ECM with specific properties. This could be accomplished by selecting a modification of appropriate cell types.66, 67 For example, a biomimetic hydrogel has been developed from mesenchymal stem cell (MSC) dECM (mdECM), which promotes cartilage regeneration with a complex mixture of macromolecules and signaling factors, enabling the engineering of cartilage tissue. Both 3% and 6% (w/v) mdECM hydrogels poses homogenous micropores. However, the size of the porous inside of the 3% (w/v) mdECM (2.72 ± 1.54 µm) was larger than that of the 6% (w/v) (1.35 ± 0.77 µm). Viability of MSCs was greater than 90% on day 1. The 6% (w/v) mdECM hydrogel maintained high cell viability over time, however, this was not the case for 3% (w/v), although this value (83.3%) was indicative of viability on day28. As a result, the highest concentration of cells exhibited a more similar shape to chondrocytes embedded.68
In addition to all of the above, plant tissues have been decellularized recently to create scaffolds that can be used to engineer tissues. Following decellularization, it can be seeded by specific cells for different biomedical applications. In addition to drug delivery systems, it can also be used to for cartilage and vascular regeneration. These tissues can be obtained from different parts of a plant, such as leaves, stems or fruits, and even vegetables. This source is known for its low cost, accessibility, sustainability, large surface area, interconnected pores, different hydrophilicity, and various mechanical properties.18, 20, 69-71 For example, nano fibrils cellulose with alginate was used as a bio-ink for cartilage tissue engineering. As a result of alginate’s low viscosity, the printing resolution is decreased. However, nano fibrils cellulose possesses a high viscosity, which could be printed without gelation. Therefore, the combination of these two materials served as a successful bio-ink. Printed bio-inks were examined with and without cross-linking in order to evaluate their printability and stability. Compressive stiffness measurements for all combinations of nano fibrils cellulose/alginate (Ink9010, Ink8020, Ink7030, Ink6040) at 30% strain showed that Ink9010 displayed lower compressive stiffness than Ink7030, which was about 60 kPa and 250 kPa, respectively. Moreover, Ink6040 showed reduced compressive stiffness in comparison with Ink7030. This indicated that a high alginate concentration compromised the mechanical properties of bio-ink. In the end, it should be noted that each application may need different mechanical properties for bio-ink.72 All discussed categories are illustrated in Figure 1 and Table 1 presents all the positive and negative properties of each category.
Figure 1. The schematic of different decellularized extracellular matrix sources and decellularization methods.
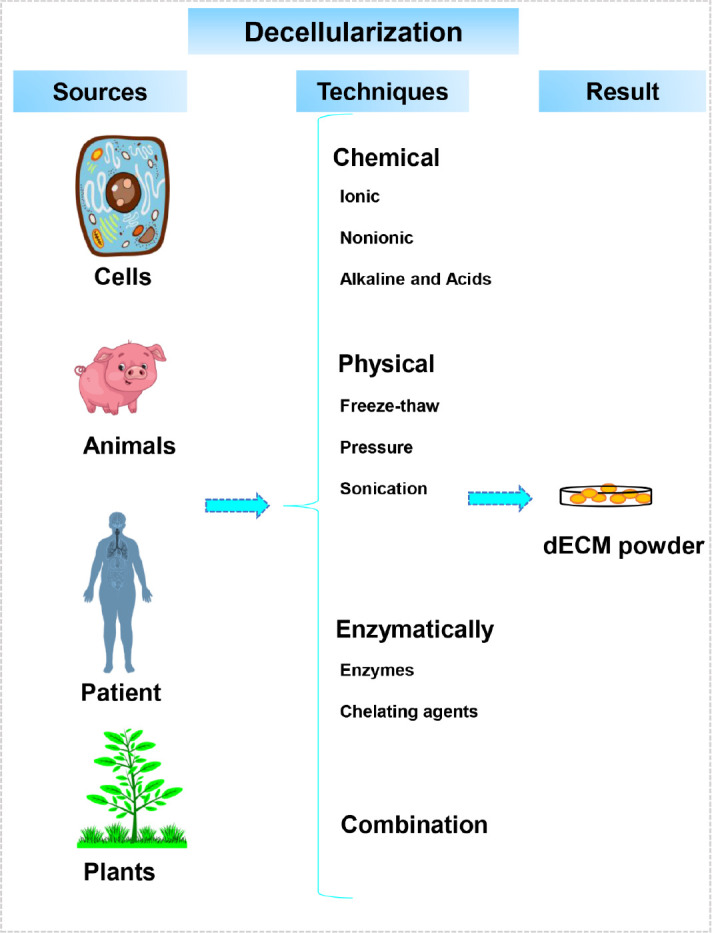
Table 1. Differences between organ/tissue dECM and cell-derived dECM.
| Advantages | Disadvantages | |
|---|---|---|
| Organ/tissue-derived dECM | Similarity to native ECM (architectural/mechanical) | Availability or lack thereof |
| Easy preparation at large scale | Present stem cell niche | |
| - | Large batch-to-batch differences | |
| Cell-derived dECM | Possibility of preparation in limited regions | Low similarity to native ECM |
| Present stem cells, cells | Difficult to preparation at large scale | |
| Small batch-to-batch differences | - |
Cartilage Bio-Printing Using the Decellularized Extracellular Matrix-Based Bio-Inks
A novel application of 3D bio-printing is the creation of cartilage scaffolds that contain living cells for the purpose of providing an appropriate microenvironment for cartilage regeneration.73 Different cell types can be used for cartilage tissue engineering, including chondrocytes,74 MSCs,75 (induced) pluripotent stem cells76 and even fibroblasts.77 It is imperative to support the adhesion, proliferation, and growth of cartilage cells in order to fabricate successful tissue engineering structures. There should be interconnected porous structures with pore sizes of at least > 200 μm for providing oxygen and nutrition supplements.78 A healthy human articular cartilage requires 5-25 MPa, 15-35 MPa, 0.24-0.85 MPa, and 0.2-2.0 MPa Tensile Young modulus, ultimate tensile stress, compression Young’s modulus, and complex shear modulus, respectively.79 Therefore, the mechanical properties of scaffolds should be taken into account in their design. Various studies have previously examined dECM bio-inks for cartilage regeneration. In this review, in order to facilitate the reading, the studies have been divided into two categories of in-vitro and in-vivo studies.
In-vivo investigations
The dECM based bio-ink is a novel topic in tissue engineering especially in cartilage regeneration. The number of studies conducted on this topic is limited. However, in this study, an attempt has been made to discuss the studies performed separately. The in-vitro studies section begins with cell laden dECM bio-ink studies and is followed by studies of the cell-free dECM bio-inks.
In-vitro investigations of cell-laden decellularized extracellular matrix bio-inks
A combination of biological signals in the printed structures can induce cell fate and provide an optimal microenvironment for cartilage healing.80 Zhang et al.6 proposed a novel silk fibroin/dECM/bone marrow MSCs constructs for cartilage tissue regeneration. Silk fibroin was incorporated into dECM to improve its mechanical stability. Printable bio-ink was prepared by mixing a solution of 0-15% (w/v) silk fibroin and 0-6% (w/v) dECM were mixed with phosphate-buffered saline and an equal volume of 80% polyethylene glycol to enhance the gelation of silk fibroin and finally mixing it with bone marrow MSCs. The printing was performed with a speed of 4-7 mm/s, a pressure of 0.20-0.30 MPa, a stage temperature of 37°C, and a room temperature of 15°C. The results demonstrated that the higher concentration of dECM (3% (w/v)) created a highly viscous solution requiring high printing pressure that inhibits the viability of the cells; while the concentration below 2% (w/v) was unable to provide adequate structural stability. Another study synthesized a chondrocytes-laden photo-cross-linkable bio-ink by methacrylating cartilage-derived ECM. Results of the viability assay demonstrated that a higher concentration of cartilage-derived ECM resulted in higher levels of viability and 40 mg/mL cartilage-derived ECM produced the highest level of cell proliferation after 4 weeks the chondrocytes had a triangular and ovoid shape, which is typical of chondrocytes.52
In a study by Pati et al.53 cell-laden bio-printing with ECM bio-ink was evaluated with the aim of regenerating adipose, cartilage, and heart tissue. The cartilage tissue constructs were printed using a polycaprolactone (PCL) having a 200 μm framework. The disadvantage of this technique is that the cells near the border of the PCL framework may be influenced by the PCL environment instead of the cartilage dECM. However, the majority of the cells were encapsulated in the dECM bio-ink. The gene expression studies demonstrated the high capability of the construct for cartilage differentiation. The expression of SRY-box transcription factor 9, as a marker of chondrogenic transcription, increased and the adhesion-related genes and integrin β1 were highly expressed after 3 days, suggesting the potential for chondrogenesis. In addition, the type II collagen, and F-actin staining demonstrated that the printed structures were capable of forming tissue.
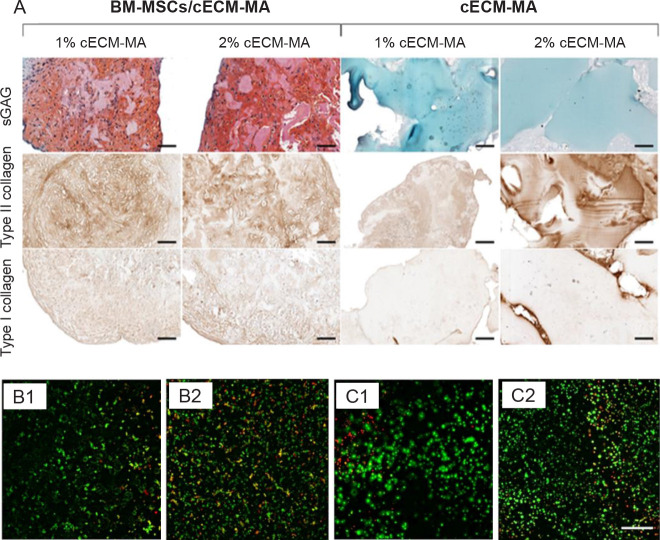
The photo-cross-linking created by methacrylated type II collagen can improve the stability of printed structures.81 In a recent study, Behan et al.82 proposed methacrylate cartilage dECM bio-ink to overcome the slow gelation of type II collagen as the base material for a collagen ECM. The designed bio-ink was loaded with bone marrow MSCs and the cartilage structure was formed by micro-extrusion and then photo-cross-linked with ultraviolet light. According to the rheological results, increasing the dECM concentration from 1% to 6% (w/v) led to better printability, and storage modulus (G’) increased 40-fold to 6% (w/v) dECM. Since elastic gels can be formed at high concentrations, methacrylation was carried out. The methacrylated dECM bio-ink demonstrated both higher storage and loss moduli. The cell encapsulation had a negligible effect on the rheological properties. The histological and biochemical assays confirmed the high presence of type II collagen and low level of type I collagen, indicating the formation of hyaline-like cartilage (Figure 2A). Sun et al.83 tried another dECM bioink for cartilage regeneration. The porcine skin was decellularized and after dellularization assurance the printing was performed with the combining chondrocytes and dECM in different concentrations and using printing speeds. The best concentration and printing speed were obtained 9 g/L and 8 mm/s, respectively. In addition, Terpstra et al.84 believed cartilage dECM could be used to alleviate vascularization. Here, bio-inks containing endothelial cells are pro-and anti-angiogenic supplemented with bioactive matrix-derived microfibres created by cartilage dECM and type I collagen. There was evidence of neovascularization after 2 days. The meniscus-like bio-printed structure was fabricated with two different zone formulations. The outer zone consists of human umbilical endothelial cells, mural cells, and type I collagen matrix-derived microfibre-laden pro-angiogenic bio-ink, while the inner zone consists of progenitor cells and cartilage dECM matrix-derived microfibre-laden anti-angiogenic bio-ink (Figure 2B, and C). The observation revealed the emergence of vascular network in the outer layer after 14 days. In Setayeshmehr et al.’s study,85 the solubilized dECM was mixed with poly(vinyl alcohol) to maintain printability and shape. The formulations were based on two different approaches; first, the poly(vinyl alcohol) was functionalized with amine groups, while the second formulation used cis-5-norbornene-endo-2,3-dicarboxylic anhydride to modify the poly(vinyl alcohol). The amine-modified bio-inks were cross-linked with genipin, they demonstrated good printability but low shape maintenance due to the slow cross-linking. The second modification resulted in a better cross-linking speed by using light curing. The dECM containing bio-inks exhibited high cell viability, bio-printability, and a regulated swelling ratio for cartilage tissue regeneration. In a more recent study, Govindharaj et al.86 used a Phallusia nigra tunicate dECM for cartilage regeneration. The dECM was applied in two different methods, once seeded with human mesenchymal stem cells and secondly was processed for bioprinting. The results showed the structure preserved its special honeycomb-shaped microstructure and its important functional groups. The results proved the efficiency of both methods with high biocompatibility and mechanical stability for cartilage tissue engineering.
Figure 2. (A) The histological staining for type I and II collagens and sGAGs of cECM-MA bio-inks contained/free BM-MSCs. Cell contained bio-inks as expected showed higher type I and II collagens and sGAGs. Reprinted from Behan et al.82 (B, C) The cell viability of cis-5-norbornene-endo-2,3-dicarboxylic anhydride-modified PVA samples (B) and cis-5-norbornene-endo-2,3-dicarboxylic anhydride-modified PVA contained solubilized dECM (C) after 1 and 7 days. Reprinted from Setayeshmehr et al.85 Scale bars: 100 µm. BM-MSCs: Bone marrow-derived mesenchymal stem cells; cECM-MA: methacrylated cartilage ECM-based hydrogel/bio-ink; dECM: decellularized extracellular matrix; PVA: poly (vinyl alcohol); sGAGs: sulphated glycosaminoglycans.
In-vitro cellular investigations of decellularized extracellular matrix bioinks
In another effort, Wiggenhauser et al.87 added dECM of porcine nasal cartilage to PCL printed structures to enhance cartilage healing. The cartilage differentiation was higher in dECM-contained scaffolds in comparison with pure PCL scaffolds. It was suggested that the sufficient micro-milieu provided by dECM can enhance cartilage regeneration. Zare et al.88 printed PCL scaffolds and alginate-sulfate added to improve the biomimetic environment. dECM was used to enhance the chondrogenesis. The in-vitro cellular investigation demonstrated that the 1% dECM had high, printability, cell viability, and proliferation. The mechanical tests showed the designed scaffolds had similar mechanical properties to natural nasal cartilage. In another study, Jung et al.89 combined dECM with silk fibroin for cartilage tissue engineering. The silk fibroin was used as the support material of dECM due to its high controllable cross-linking and viscosity properties. According to the bioprintability test, 18% and 20% (w/v) bio-inks demonstrated the fine resolution. The cell survival results showed over 80% cell viability and Safranin-O staining proved the high cartilaginous synthesized GAGs in the dECM-silk fibroin scaffolds.
In-vivo investigations
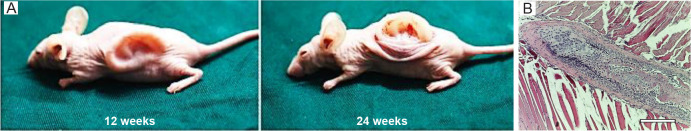
Although few in-vivo dECM bio-ink investigation have been performed so far, this section tries to collect and discuss the studies that have worked on this subject. Here, the means of in-vivo structures is the implanted 3D bio-printed structures using dECM bio-inks in in-vivo models. In an in-vivo trial, Jia et al.90 proposed a photo-cross-linkable bio-ink containing methacrylate-modified acellular cartilage matrix, gelatin methacrylate, poly(ethylene oxide), and PCL. The dECM provides native cues for cartilage regeneration, while the polymers are responsible for maintaining printability and mechanical properties for precise printing. After 24 weeks, the human auricle shape was successfully maintained (Figure 3A), and histological investigations demonstrated a high level of GAG and type II collagen. In another in-vivo investigation, 4% (w/v) collagen, and 2.5% (w/v) decellularized ECM granules bio-ink was formulated and bio-printed with the aim of promoting cartilage growth. The bio-inks were applied in three groups, including dECM, MSC, and both dECM and MSC. dECM granules with a diameter up to 280 µm increase the surface area for cell interactions and act as chondrogenic microenvironments. The cartilaginous tissue was formed on both the scaffold site and within the muscle fibres, indicating vascularization. Bone formation was observed in the treated groups, which is undesirable and can be attributed to the cell sources (Figure 3B). MSC derived from adipose tissue are equally capable of differentiating into cells such as osteoblasts, chondroblasts, and adipocytes.91 In addition, Chen et al.92 introduced a method combining electrospinning and 3D printing for cartilage tissue engineering. A major objective of this combination was to improve the mechanical properties of the dECM and create a customized shape structure with the proper pore size. To accomplish this, the electrospun gelatin/ poly(lactic-co-glycolic acid) fibres were cut and mixed with different concentrations of dECM and hyaluronic acid ink. The printed structures were analyzed by in-vitro tests, and articular cartilage regeneration capability was assessed in-vivo in healthy male New Zealand white rabbits. Following 12 weeks of surgery, the histological examination demonstrated complete defects filled with dECM/hyaluronic acid containing 50% (w/w) electrospun gelatin/poly(lactic-co-glycolic acid) fibres. Staining the tissue revealed the emergence of new chondrocytes and the uniform formation of cartilage tissue.
Figure 3. (A) The in-vivo auricular tissue regeneration on the back of nude mice after 12 and 24 weeks. Reprinted from Jia et al.90 (B) Microscopic observation of in-vivo investigation using decellularized extracellular matrix and mesenchymal stem cell bio-ink after 1 week. There was indirect osteogenesis in the muscle tissue near the scaffold. Scale bar: 800 μm. Reprinted from Isaeva et al.91.
Current Challenges and Future Outlook for Clinical Applications and Concluding Remarks
Although dECM-based bio-ink demonstrated unique properties for cartilage tissue engineering, there are some challenges that should be considered for future clinical applications. Due to the loss of native cartilage structure during the preparation process, pure cartilage dECM bio-inks cannot provide sufficient mechanical properties.6 Therefore it is usually combined with other materials or cross-linked to increase the mechanical performance, or a framework can be designed to enhance dECM properties, while using a polymeric framework for dECM printing may lead to decrease cell viability along with the polymer borders.53
It is proposed to study other creative techniques to enhance the mechanical properties of dECM bio-ink in the future. Powder-based plotting was found to overcome the low viscosity of the concentrated dECM bio-ink derived from cartilage.16 In addition, another study proposed to enhance thermal gelation of type II collagen through the use photocross-linking of methacrylate dECM.82 Protein heterogeneity, caused by different donors, tissues, and cells makes the comparison process difficult, especially in large measurements.52 Therefore, it is preferable to define a standard for comparing future studies.
Despite the fact that decellularization techniques can eliminate most cells, the DNA and chondrocytes may persist even after 25 cycles of decellularization. In addition, the reduction of the GAG content can lead to a decrease in mechanical properties. Therefore, decellularization techniques should be optimized to maintain structural integrity and remove cell debris for future clinical applications.93 It has been recently proposed that using the bioreactors enhances the decellularization procedure. Bioreactors are capable of reducing the number of decellularization cycles with an increased output and a reduced loss of matrix integrity.94
The above-mentioned current challenges should be minimized in order to create functional cell-laden dECM-based bio-ink for cartilage tissue regeneration. The development of the dECM bio-inks with high throughput will therefore require the application of new compositions with other natural and synthetic materials as well as new techniques. In addition, the new investigation techniques should be applied to the new composition in order to fully understand the designed bio-ink properties and replicate the natural microenvironment of cartilage tissue. In-vivo assays need to be carried out with greater focus in order to optimize the bio-inks properties in order to be used in clinical stages. As a result, according to the previous studies and mentioned properties, it appears that dECM-based bio-inks are suitable for cartilage tissue repair and are recommended for future studies aimed at addressing current challenges and improving properties. Since dECM bio-printing is a new field, the number of reviewed works is limited, and many aspects of this field need further consideration in the future.
Funding Statement
The work was supperted by the Alexander von Humboldt foundation (to FG).
Footnotes
Acknowledgement: None.
Conflicts of interest statement: The authors whose names are listed certify that they have no affiliations with or involvement in any organization or entity with any financial interest, or non-financial interest in the subject matter or materials discussed in this manuscript.
References
- 1.Lee S., Choi J., Youn J., Lee Y., Kim W., Choe S., Song J., Reis R. L., Khang G. Development and evaluation of gellan gum/silk fibroin/ chondroitin sulfate ternary injectable hydrogel for cartilage tissue engineering. Biomolecules. 2021;11:1184. doi: 10.3390/biom11081184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Y., Liu X., Zeng L., Zhang J., Zuo J., Zou J., Ding J., Chen X. Polymer fiber scaffolds for bone and cartilage tissue engineering. Adv Funct Mater. 2019;29:1903279. [Google Scholar]
- 3.Kreller T., Distler T., Heid S., Gerth S., Detsch R., Boccaccini A. R. Physico-chemical modification of gelatine for the improvement of 3D printability of oxidized alginate-gelatine hydrogels towards cartilage tissue engineering. Mater Des. 2021;208:109877. [Google Scholar]
- 4.Gan D., Xu T., Xing W., Wang M., Fang J., Wang K., Ge X., Chan C. W., Ren F., Tan H., Lu X. Mussel-inspired dopamine oligomer intercalated tough and resilient gelatin methacryloyl (GelMA) hydrogels for cartilage regeneration. J Mater Chem B. 2019;7:1716–1725. doi: 10.1039/c8tb01664j. [DOI] [PubMed] [Google Scholar]
- 5.Ghorbani F., Zamanian A., Kermanian F., Shamoosi A. A bioinspired 3D shape olibanum-collagen-gelatin scaffolds with tunable porous microstructure for efficient neural tissue regeneration. Biotechnol Prog. 2020;36:e2918. doi: 10.1002/btpr.2918. [DOI] [PubMed] [Google Scholar]
- 6.Zhang X., Liu Y., Luo C., Zhai C., Li Z., Zhang Y., Yuan T., Dong S., Zhang J., Fan W. Crosslinker-free silk/decellularized extracellular matrix porous bioink for 3D bioprinting-based cartilage tissue engineering. Mater Sci Eng C Mater Biol Appl. 2021;118:111388. doi: 10.1016/j.msec.2020.111388. [DOI] [PubMed] [Google Scholar]
- 7.Fu L., Li P., Li H., Gao C., Yang Z., Zhao T., Chen W., Liao Z., Peng Y., Cao F., Sui X., Liu S., Guo Q. The application of bioreactors for cartilage tissue engineering: advances, limitations, and future perspectives. Stem Cells Int. 2021;2021:6621806. doi: 10.1155/2021/6621806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sahranavard M., Zamanian A., Ghorbani F., Shahrezaee M. H. A critical review on three dimensional-printed chitosan hydrogels for development of tissue engineering. Bioprinting. 2020;17:e00063. [Google Scholar]
- 9.Lee H., Han W., Kim H., Ha D. H., Jang J., Kim B. S., Cho D. W. Development of liver decellularized extracellular matrix bioink for three-dimensional cell printing-based liver tissue engineering. Biomacromolecules. 2017;18:1229–1237. doi: 10.1021/acs.biomac.6b01908. [DOI] [PubMed] [Google Scholar]
- 10.Bandyopadhyay A., Mandal B. B., Bhardwaj N. 3D bioprinting of photo-crosslinkable silk methacrylate (SilMA)-polyethylene glycol diacrylate (PEGDA) bioink for cartilage tissue engineering. J Biomed Mater Res A. 2022;110:884–898. doi: 10.1002/jbm.a.37336. [DOI] [PubMed] [Google Scholar]
- 11.Choi J. H., Park A., Lee W., Youn J., Rim M. A., Kim W., Kim N., Song J. E., Khang G. Preparation and characterization of an injectable dexamethasone-cyclodextrin complexes-loaded gellan gum hydrogel for cartilage tissue engineering. J Control Release. 2020;327:747–765. doi: 10.1016/j.jconrel.2020.08.049. [DOI] [PubMed] [Google Scholar]
- 12.Ni T., Liu M., Zhang Y., Cao Y., Pei R. 3D bioprinting of bone marrow mesenchymal stem cell-laden silk fibroin double network scaffolds for cartilage tissue repair. Bioconjug Chem. 2020;31:1938–1947. doi: 10.1021/acs.bioconjchem.0c00298. [DOI] [PubMed] [Google Scholar]
- 13.Tsai W. B., Chen W. T., Chien H. W., Kuo W. H., Wang M. J. Poly(dopamine) coating of scaffolds for articular cartilage tissue engineering. Acta Biomater. 2011;7:4187–4194. doi: 10.1016/j.actbio.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 14.Chen Z., Xiao H., Zhang H., Xin Q., Zhang H., Liu H., Wu M., Zuo L., Luo J., Guo Q., Ding C., Tan H., Li J. Heterogenous hydrogel mimicking the osteochondral ECM applied to tissue regeneration. J Mater Chem B. 2021;9:8646–8658. doi: 10.1039/d1tb00518a. [DOI] [PubMed] [Google Scholar]
- 15.Xu Y., Shi G., Tang J., Cheng R., Shen X., Gu Y., Wu L., Xi K., Zhao Y., Cui W., Chen L. ECM-inspired micro/nanofibers for modulating cell function and tissue generation. Sci Adv. 2020;6:eabc2036. doi: 10.1126/sciadv.abc2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nam S. Y., Park S. H. ECM based bioink for tissue mimetic 3D bioprinting. Adv Exp Med Biol. 2018;1064:335–353. doi: 10.1007/978-981-13-0445-3_20. [DOI] [PubMed] [Google Scholar]
- 17.Kim B. S., Das S., Jang J., Cho D. W. Decellularized extracellular matrix-based bioinks for engineering tissue- and organ-specific microenvironments. Chem Rev. 2020;120:10608–10661. doi: 10.1021/acs.chemrev.9b00808. [DOI] [PubMed] [Google Scholar]
- 18.Adamski M., Fontana G., Gershlak J. R., Gaudette G. R., Le H. D., Murphy W. L. Two methods for decellularization of plant tissues for tissue engineering applications. J Vis Exp. 2018:57586. doi: 10.3791/57586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porzionato A., Stocco E., Barbon S., Grandi F., Macchi V., De Caro R. Tissue-engineered grafts from human decellularized extracellular matrices: a systematic review and future perspectives. Int J Mol Sci. 2018;19:4117. doi: 10.3390/ijms19124117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris A. F., Lacombe J., Zenhausern F. The emerging role of decellularized plant-based scaffolds as a new biomaterial. Int J Mol Sci. 2021;22:12347. doi: 10.3390/ijms222212347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim H. S., Mandakhbayar N., Kim H. W., Leong K. W., Yoo H. S. Protein-reactive nanofibrils decorated with cartilage-derived decellularized extracellular matrix for osteochondral defects. Biomaterials. 2021;269:120214. doi: 10.1016/j.biomaterials.2020.120214. [DOI] [PubMed] [Google Scholar]
- 22.Crapo P. M., Gilbert T. W., Badylak S. F. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233–3243. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kabirian F., Mozafari M. Decellularized ECM-derived bioinks: prospects for the future. Methods. 2020;171:108–118. doi: 10.1016/j.ymeth.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 24.Kheir E., Stapleton T., Shaw D., Jin Z., Fisher J., Ingham E. Development and characterization of an acellular porcine cartilage bone matrix for use in tissue engineering. J Biomed Mater Res A. 2011;99:283–294. doi: 10.1002/jbm.a.33171. [DOI] [PubMed] [Google Scholar]
- 25.Galliger Z., Panoskaltsis-Mortari A. Tracheal cartilage isolation and decellularization. Methods Mol Biol. 2018;1577:155–160. doi: 10.1007/7651_2017_52. [DOI] [PubMed] [Google Scholar]
- 26.Schneider C., Lehmann J., van Osch G. J., Hildner F., Teuschl A., Monforte X., Miosga D., Heimel P., Priglinger E., Redl H., Wolbank S., Nürnberger S. Systematic comparison of protocols for the preparation of human articular cartilage for use as scaffold material in cartilage tissue engineering. Tissue Eng Part C Methods. 2016;22:1095–1107. doi: 10.1089/ten.TEC.2016.0380. [DOI] [PubMed] [Google Scholar]
- 27.Ravichandran A., Murekatete B., Moedder D., Meinert C., Bray L. J. Photocrosslinkable liver extracellular matrix hydrogels for the generation of 3D liver microenvironment models. Sci Rep. 2021;11:15566. doi: 10.1038/s41598-021-94990-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reing J. E., Brown B. N., Daly K. A., Freund J. M., Gilbert T. W., Hsiong S. X., Huber A., Kullas K. E., Tottey S., Wolf M. T., Badylak S. F. The effects of processing methods upon mechanical and biologic properties of porcine dermal extracellular matrix scaffolds. Biomaterials. 2010;31:8626–8633. doi: 10.1016/j.biomaterials.2010.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z., Li Z., Li Z., Wu B., Liu Y., Wu W. Cartilaginous extracellular matrix derived from decellularized chondrocyte sheets for the reconstruction of osteochondral defects in rabbits. Acta Biomater. 2018;81:129–145. doi: 10.1016/j.actbio.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Rahman S., Griffin M., Naik A., Szarko M., Butler P. E. M. Optimising the decellularization of human elastic cartilage with trypsin for future use in ear reconstruction. Sci Rep. 2018;8:3097. doi: 10.1038/s41598-018-20592-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Neill J. D., Anfang R., Anandappa A., Costa J., Javidfar J., Wobma H. M., Singh G., Freytes D. O., Bacchetta M. D., Sonett J. R., Vunjak-Novakovic G. Decellularization of human and porcine lung tissues for pulmonary tissue engineering. Ann Thorac Surg. 2013;96:1046–1055. doi: 10.1016/j.athoracsur.2013.04.022. discussion 1055-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keane T. J., Swinehart I. T., Badylak S. F. Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance. Methods. 2015;84:25–34. doi: 10.1016/j.ymeth.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Bordbar S., Lotfi Bakhshaiesh N., Khanmohammadi M., Sayahpour F. A., Alini M., Baghaban Eslaminejad M. Production and evaluation of decellularized extracellular matrix hydrogel for cartilage regeneration derived from knee cartilage. J Biomed Mater Res A. 2020;108:938–946. doi: 10.1002/jbm.a.36871. [DOI] [PubMed] [Google Scholar]
- 34.Ghassemi T., Saghatoleslami N., Mahdavi-Shahri N., Matin M. M., Gheshlaghi R., Moradi A. A comparison study of different decellularization treatments on bovine articular cartilage. J Tissue Eng Regen Med. 2019;13:1861–1871. doi: 10.1002/term.2936. [DOI] [PubMed] [Google Scholar]
- 35.Zhou J., Fritze O., Schleicher M., Wendel H. P., Schenke-Layland K., Harasztosi C., Hu S., Stock U. A. Impact of heart valve decellularization on 3-D ultrastructure, immunogenicity and thrombogenicity. Biomaterials. 2010;31:2549–2554. doi: 10.1016/j.biomaterials.2009.11.088. [DOI] [PubMed] [Google Scholar]
- 36.Tavassoli A., Matin M. M., Niaki M. A., Mahdavi-Shahri N., Shahabipour F. Mesenchymal stem cells can survive on the extracellular matrix-derived decellularized bovine articular cartilage scaffold. Iran J Basic Med Sci. 2015;18:1221–1227. [PMC free article] [PubMed] [Google Scholar]
- 37.Azhim A., Ono T., Fukui Y., Morimoto Y., Furukawa K., Ushida T. Preparation of decellularized meniscal scaffolds using sonication treatment for tissue engineering. Annu Int Conf IEEE Eng Med Biol Soc. 2013;2013:6953–6956. doi: 10.1109/EMBC.2013.6611157. [DOI] [PubMed] [Google Scholar]
- 38.Mendibil U., Ruiz-Hernandez R., Retegi-Carrion S., Garcia-Urquia N., Olalde-Graells B., Abarrategi A. Tissue-specific decellularization methods: rationale and strategies to achieve regenerative compounds. Int J Mol Sci. 2020;21:5447. doi: 10.3390/ijms21155447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guimaraes A. B., Correia A. T., Alves B. P., Da Silva R. S., Martins J. K., Pêgo-Fernandes P. M., Xavier N. S., Dolhnikoff M., Cardoso P. F. G. Evaluation of a physical-chemical protocol for porcine tracheal decellularization. Transplant Proc. 2019;51:1611–1613. doi: 10.1016/j.transproceed.2019.01.042. [DOI] [PubMed] [Google Scholar]
- 40.Al-Qurayshi Z., Wafa E. I., Hoffman H., Chang K., Salem A. K. Tissue-engineering the larynx: Effect of decellularization on human laryngeal framework and the cricoarytenoid joint. J Biomed Mater Res B Appl Biomater. 2021;109:2030–2040. doi: 10.1002/jbm.b.34851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh S., Afara I. O., Tehrani A. H., Oloyede A. Effect of decellularization on the load-bearing characteristics of articular cartilage matrix. Tissue Eng Regen Med. 2015;12:294–305. [Google Scholar]
- 42.Khajavi M., Hajimoradloo A., Zandi M., Pezeshki-Modaress M., Bonakdar S., Zamani A. Fish cartilage: a promising source of biomaterial for biological scaffold fabrication in cartilage tissue engineering. J Biomed Mater Res A. 2021;109:1737–1750. doi: 10.1002/jbm.a.37169. [DOI] [PubMed] [Google Scholar]
- 43.Giraldo-Gomez D. M., Leon-Mancilla B., Del Prado-Audelo M. L., Sotres-Vega A., Villalba-Caloca J., Garciadiego-Cazares D., Piãa-Barba M. C. Trypsin as enhancement in cyclical tracheal decellularization: Morphological and biophysical characterization. Mater Sci Eng C Mater Biol Appl. 2016;59:930–937. doi: 10.1016/j.msec.2015.10.094. [DOI] [PubMed] [Google Scholar]
- 44.Keane T. J., Saldin L. T., Badylak S. F. 4 - Decellularization of mammalian tissues: Preparing extracellular matrix bioscaffolds. In: Tomlins P., editor. Characterisation and design of tissue scaffolds. Woodhead Publishing; 2016. pp. 75–103. [Google Scholar]
- 45.Zhang X., Chen X., Hong H., Hu R., Liu J., Liu C. Decellularized extracellular matrix scaffolds: Recent trends and emerging strategies in tissue engineering. Bioact Mater. 2022;10:15–31. doi: 10.1016/j.bioactmat.2021.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li J., Cai Z., Cheng J., Wang C., Fang Z., Xiao Y., Feng Z. G., Gu Y. Characterization of a heparinized decellularized scaffold and its effects on mechanical and structural properties. J Biomater Sci Polym Ed. 2020;31:999–1023. doi: 10.1080/09205063.2020.1736741. [DOI] [PubMed] [Google Scholar]
- 47.Phan N. V., Wright T., Rahman M. M., Xu J., Coburn J. M. In vitro biocompatibility of decellularized cultured plant cell-derived matrices. ACS Biomater Sci Eng. 2020;6:822–832. doi: 10.1021/acsbiomaterials.9b00870. [DOI] [PubMed] [Google Scholar]
- 48.Zang M., Zhang Q., Chang E. I., Mathur A. B., Yu P. Decellularized tracheal matrix scaffold for tissue engineering. Plast Reconstr Surg. 2012;130:532–540. doi: 10.1097/PRS.0b013e31825dc084. [DOI] [PubMed] [Google Scholar]
- 49.Kang H., Peng J., Lu S., Liu S., Zhang L., Huang J., Sui X., Zhao B., Wang A., Xu W., Luo Z., Guo Q. In vivo cartilage repair using adipose-derived stem cell-loaded decellularized cartilage ECM scaffolds. J Tissue Eng Regen Med. 2014;8:442–453. doi: 10.1002/term.1538. [DOI] [PubMed] [Google Scholar]
- 50.Hayrapetyan L., Arestakesyan H., Margaryan A., Oganesyan A., Grigoryan V., Karapetyan A. Comparison of articular and auricular cartilages: decellularization, cell proliferation rate, and infiltration in scaffolds. Res Biomed Eng. 2021;37:193–200. [Google Scholar]
- 51.Amirazad H., Dadashpour M., Zarghami N. Application of decellularized bone matrix as a bioscaffold in bone tissue engineering. J Biol Eng. 2022;16:1. doi: 10.1186/s13036-021-00282-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Visscher D. O., Lee H., van Zuijlen P. P. M., Helder M. N., Atala A., Yoo J. J., Lee S. J. A photo-crosslinkable cartilage-derived extracellular matrix bioink for auricular cartilage tissue engineering. Acta Biomater. 2021;121:193–203. doi: 10.1016/j.actbio.2020.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pati F., Jang J., Ha D. H., Won Kim S., Rhie J. W., Shim J. H., Kim D. H., Cho D. W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun. 2014;5:3935. doi: 10.1038/ncomms4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shen Y., Xu Y., Yi B., Wang X., Tang H., Chen C., Zhang Y. Engineering a highly biomimetic chitosan-based cartilage scaffold by using short fibers and a cartilage-decellularized matrix. Biomacromolecules. 2021;22:2284–2297. doi: 10.1021/acs.biomac.1c00366. [DOI] [PubMed] [Google Scholar]
- 55.Tian G., Jiang S., Li J., Wei F., Li X., Ding Y., Yang Z., Sun Z., Zha K., Wang F., Huang B., Peng L., Wang Q., Tian Z., Yang X., Wang Z., Guo Q., Guo W., Liu S. Cell-free decellularized cartilage extracellular matrix scaffolds combined with interleukin 4 promote osteochondral repair through immunomodulatory macrophages: In vitro and in vivo preclinical study. Acta Biomater. 2021;127:131–145. doi: 10.1016/j.actbio.2021.03.054. [DOI] [PubMed] [Google Scholar]
- 56.Solarte David V. A., Güiza-Argüello V. R., Arango-Rodríguez M. L., Sossa C. L., Becerra-Bayona S. M. Decellularized tissues for wound healing: towards closing the gap between scaffold design and effective extracellular matrix remodeling. Front Bioeng Biotechnol. 2022;10:821852. doi: 10.3389/fbioe.2022.821852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garreta E., Oria R., Tarantino C., Pla-Roca M., Prado P., Fernández-Avilés F., Campistol J. M., Samitier J., Montserrat N. Tissue engineering by decellularization and 3D bioprinting. Mater Today. 2017;20:166–178. [Google Scholar]
- 58.Jang J., Park H. J., Kim S. W., Kim H., Park J. Y., Na S. J., Kim H. J., Park M. N., Choi S. H., Park S. H., Kim S. W., Kwon S. M., Kim P. J., Cho D. W. 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair. Biomaterials. 2017;112:264–274. doi: 10.1016/j.biomaterials.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 59.An J., Teoh J. E. M., Suntornnond R., Chua C. K. Design and 3D printing of scaffolds and tissues. Engineering. 2015;1:261–268. [Google Scholar]
- 60.Goodale H. D. The progeny test as a means of evaluating the breeding potentialities of farm animals. Am Nat. 1933;67:481–499. [Google Scholar]
- 61.Dzobo K., Motaung K., Adesida A. Recent trends in decellularized extracellular matrix bioinks for 3D printing: an updated review. Int J Mol Sci. 2019;20:4628. doi: 10.3390/ijms20184628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tottey S., Johnson S. A., Crapo P. M., Reing J. E., Zhang L., Jiang H., Medberry C. J., Reines B., Badylak S. F. The effect of source animal age upon extracellular matrix scaffold properties. Biomaterials. 2011;32:128–136. doi: 10.1016/j.biomaterials.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aamodt J. M., Grainger D. W. Extracellular matrix-based biomaterial scaffolds and the host response. Biomaterials. 2016;86:68–82. doi: 10.1016/j.biomaterials.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johnson T. D., Dequach J. A., Gaetani R., Ungerleider J., Elhag D., Nigam V., Behfar A., Christman K. L. Human versus porcine tissue sourcing for an injectable myocardial matrix hydrogel. Biomater Sci. 2014;2014:60283D. doi: 10.1039/C3BM60283D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pati F., Ha D. H., Jang J., Han H. H., Rhie J. W., Cho D. W. Biomimetic 3D tissue printing for soft tissue regeneration. Biomaterials. 2015;62:164–175. doi: 10.1016/j.biomaterials.2015.05.043. [DOI] [PubMed] [Google Scholar]
- 66.Assunção M., Dehghan-Baniani D., Yiu C. H. K., Später T., Beyer S., Blocki A. Cell-derived extracellular matrix for tissue engineering and regenerative medicine. Front Bioeng Biotechnol. 2020;8:602009. doi: 10.3389/fbioe.2020.602009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hoshiba T. Cultured cell-derived decellularized extracellular matrix (cultured cell-derived dECM): Future applications and problems — a mini review. Curr Opin Biomed Eng. 2021;17:100256. [Google Scholar]
- 68.Antich C., Jiménez G., de Vicente J., López-Ruiz E., Chocarro-Wrona C., Griãán-Lisón C., Carrillo E., Montaãez E., Marchal J. A. Development of a biomimetic hydrogel based on predifferentiated mesenchymal stem-cell-derived ecm for cartilage tissue engineering. Adv Healthc Mater. 2021;10:e2001847. doi: 10.1002/adhm.202001847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee S. E., Park Y. S. The role of bacterial cellulose in artificial blood vessels. Mol Cell Toxicol. 2017;13:257–261. [Google Scholar]
- 70.Sun B., Zhang M., Shen J., He Z., Fatehi P., Ni Y. Applications of cellulose-based materials in sustained drug delivery systems. Curr Med Chem. 2019;26:2485–2501. doi: 10.2174/0929867324666170705143308. [DOI] [PubMed] [Google Scholar]
- 71.Müller F. A., Müller L., Hofmann I., Greil P., Wenzel M. M., Staudenmaier R. Cellulose-based scaffold materials for cartilage tissue engineering. Biomaterials. 2006;27:3955–3963. doi: 10.1016/j.biomaterials.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 72.Markstedt K., Mantas A., Tournier I., Martínez Ávila H., Hägg D., Gatenholm P. 3D bioprinting human chondrocytes with nanocellulose-alginate bioink for cartilage tissue engineering applications. Biomacromolecules. 2015;16:1489–1496. doi: 10.1021/acs.biomac.5b00188. [DOI] [PubMed] [Google Scholar]
- 73.Hong H., Seo Y. B., Kim D. Y., Lee J. S., Lee Y. J., Lee H., Ajiteru O., Sultan M. T., Lee O. J., Kim S. H., Park C. H. Digital light processing 3D printed silk fibroin hydrogel for cartilage tissue engineering. Biomaterials. 2020;232:119679. doi: 10.1016/j.biomaterials.2019.119679. [DOI] [PubMed] [Google Scholar]
- 74.Nuernberger S., Cyran N., Albrecht C., Redl H., Vécsei V., Marlovits S. The influence of scaffold architecture on chondrocyte distribution and behavior in matrix-associated chondrocyte transplantation grafts. Biomaterials. 2011;32:1032–1040. doi: 10.1016/j.biomaterials.2010.08.100. [DOI] [PubMed] [Google Scholar]
- 75.Cao Y., Cheng P., Sang S., Xiang C., An Y., Wei X., Yan Y., Li P. 3D printed PCL/GelMA biphasic scaffold boosts cartilage regeneration using co-culture of mesenchymal stem cells and chondrocytes: in vivo study. Mater Des. 2021;210:110065. [Google Scholar]
- 76.Uto S., Hikita A., Sakamoto T., Mori D., Yano F., Ohba S., Saito T., Takato T., Hoshi K. Ear cartilage reconstruction combining induced pluripotent stem cell-derived cartilage and three-dimensional shape-memory scaffold. Tissue Eng Part A. 2021;27:604–617. doi: 10.1089/ten.TEA.2020.0106. [DOI] [PubMed] [Google Scholar]
- 77.Sommar P., Pettersson S., Ness C., Johnson H., Kratz G., Junker J. P. Engineering three-dimensional cartilage- and bone-like tissues using human dermal fibroblasts and macroporous gelatine microcarriers. J Plast Reconstr Aesthet Surg. 2010;63:1036–1046. doi: 10.1016/j.bjps.2009.02.072. [DOI] [PubMed] [Google Scholar]
- 78.Dhandayuthapani B., Yoshida Y., Maekawa T., Kumar D. S. Polymeric scaffolds in tissue engineering application: a review. Int J Polym Sci. 2011;2011:290602. [Google Scholar]
- 79.Izadifar Z., Chen X., Kulyk W. Strategic design and fabrication of engineered scaffolds for articular cartilage repair. J Funct Biomater. 2012;3:799–838. doi: 10.3390/jfb3040799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Keeney M., Lai J. H., Yang F. Recent progress in cartilage tissue engineering. Curr Opin Biotechnol. 2011;22:734–740. doi: 10.1016/j.copbio.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 81.Yang K., Sun J., Wei D., Yuan L., Yang J., Guo L., Fan H., Zhang X. Photo-crosslinked mono-component type II collagen hydrogel as a matrix to induce chondrogenic differentiation of bone marrow mesenchymal stem cells. J Mater Chem B. 2017;5:8707–8718. doi: 10.1039/c7tb02348k. [DOI] [PubMed] [Google Scholar]
- 82.Behan K., Dufour A., Garcia O., Kelly D. Methacrylated cartilage ECM-based hydrogels as injectables and bioinks for cartilage tissue engineering. Biomolecules. 2022;12:216. doi: 10.3390/biom12020216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sun B., Han Y., Jiang W., Dai K. 3D printing bioink preparation and application in cartilage tissue reconstruction in vitro. J Shanghai Jiaotong Univ (Sci) 2021;26:267–271. [Google Scholar]
- 84.Terpstra M. L., Li J., Mensinga A., de Ruijter M., van Rijen M. H. P., Androulidakis C., Galiotis C., Papantoniou I., Matsusaki M., Malda J., Levato R. Bioink with cartilage-derived extracellular matrix microfibers enables spatial control of vascular capillary formation in bioprinted constructs. Biofabrication. 2022;14:034104. doi: 10.1088/1758-5090/ac6282. [DOI] [PubMed] [Google Scholar]
- 85.Setayeshmehr M., Hafeez S., van Blitterswijk C., Moroni L., Mota C., Baker M. B. Bioprinting via a dual-gel bioink based on poly(vinyl alcohol) and solubilized extracellular matrix towards cartilage engineering. Int J Mol Sci. 2021;22:3901. doi: 10.3390/ijms22083901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Govindharaj M., Hashimi N. A., Soman S. S., Kanwar S., Vijayavenkataraman S. 3D bioprinting of human mesenchymal stem cells in a novel tunic decellularized ECM bioink for cartilage tissue engineering. Materialia. 2022;23:101457. [Google Scholar]
- 87.Wiggenhauser P. S., Schwarz S., Koerber L., Hoffmann T. K., Rotter N. Addition of decellularized extracellular matrix of porcine nasal cartilage improves cartilage regenerative capacities of PCL-based scaffolds in vitro. J Mater Sci Mater Med. 2019;30:121. doi: 10.1007/s10856-019-6323-x. [DOI] [PubMed] [Google Scholar]
- 88.Zare P., Pezeshki-Modaress M., Davachi S. M., Chahsetareh H., Simorgh S., Asgari N., Haramshahi M. A., Alizadeh R., Bagher Z., Farhadi M. An additive manufacturing-based 3D printed poly ε-caprolactone/alginate sulfate/extracellular matrix construct for nasal cartilage regeneration. J Biomed Mater Res A. 2022;110:1199–1209. doi: 10.1002/jbm.a.37363. [DOI] [PubMed] [Google Scholar]
- 89.Jung C. S., Kim B. K., Lee J., Min B. H., Park S. H. Development of printable natural cartilage matrix bioink for 3D printing of irregular tissue shape. Tissue Eng Regen Med. 2018;15:155–162. doi: 10.1007/s13770-017-0104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jia L., Hua Y., Zeng J., Liu W., Wang D., Zhou G., Liu X., Jiang H. Bioprinting and regeneration of auricular cartilage using a bioactive bioink based on microporous photocrosslinkable acellular cartilage matrix. Bioact Mater. 2022;16:66–81. doi: 10.1016/j.bioactmat.2022.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Isaeva E. V., Beketov E. E., Demyashkin G. A., Yakovleva N. D., Arguchinskaya N. V., Kisel A. A., Lagoda T. S., Malakhov E. P., Smirnova A. N., Petriev V. M., Eremin P. S., Osidak E. O., Domogatsky S. P., Ivanov S. A., Shegay P. V., Kaprin A. D. Cartilage formation in vivo using high concentration collagen-based bioink with MSC and decellularized ECM granules. Int J Mol Sci. 2022;23:2703. doi: 10.3390/ijms23052703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen W., Xu Y., Li Y., Jia L., Mo X., Jiang G., Zhou G. 3D printing electrospinning fiber-reinforced decellularized extracellular matrix for cartilage regeneration. Chem Eng J. 2020;382:122986. [Google Scholar]
- 93.Partington L., Mordan N. J., Mason C., Knowles J. C., Kim H. W., Lowdell M. W., Birchall M. A., Wall I. B. Biochemical changes caused by decellularization may compromise mechanical integrity of tracheal scaffolds. Acta Biomater. 2013;9:5251–5261. doi: 10.1016/j.actbio.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 94.Tchoukalova Y. D., Hintze J. M., Hayden R. E., Lott D. G. Tracheal decellularization using a combination of chemical, physical and bioreactor methods. Int J Artif Organs. 2017 doi: 10.5301/ijao.5000648. [DOI] [PubMed] [Google Scholar]