Abstract
Purpose
To determine the levels of aromatase in atypical ductal hyperplasia (ADH) lesions, tissue surrounding the ADH, and in dense and non-dense normal breast tissue. We postulated that excess aromatase in breast tissue might, through production of increased estrogen, drive the carcinogenic process. Estrogens and their metabolites are thought to contribute to the development of breast cancer through estrogen receptor-mediated mechanisms and genotoxic effects of estrogen metabolites. ADH is a benign lesion of the breast which is associated with substantially increased risk for subsequent development of breast cancer. After 25 years, approximately 30% of women with ADH develop breast cancer. In women with three or more separate ADH lesions at the same time, 47% will develop breast cancer over that time period. Another important risk factor for breast cancer is the presence of mammographically dense breast tissue.
Methods
We utilized quantitative immunochemical analysis of aromatase in biopsy tissue to test this possibility. Previously published results comparing dense with non-dense breast tissue in normal women (Vachon et al. Breast Cancer Res Treat 125:243–252, 2011) were used for comparisons with ADH. A well-characterized histochemical H-score was employed for quantitative assessment of aromatase in the various tissue studied.
Results
The H-score of aromatase staining was statistically significantly higher (p = 0.003) in the ADH epithelium than surrounding epithelial tissue. In order of H-score from highest to lowest were ADH, issue surrounding ADH, dense normal and non-dense normal breast tissues. The levels of aromatase in a subset of women with ADH who went on to develop breast cancer were not higher than in women who did not.
Conclusions
We suggest from these studies that overexpression of aromatase in breast tissue and its resultant increase in estradiol levels may contribute to the later development of breast cancer in women with ADH.
Keywords: Aromatase, Atypical ductal hyperplasia, Breast cancer, Risk factors
Introduction
Breast cancer was diagnosed in 244,000 women in 2015 in the United States and 44,000 women died of this disease [1]. Treatment with endocrine-, biologic-, and chemotherapy as well as early diagnosis has reduced the death rate by 38% from 1989 to 2014 [1]. Breast cancer prevention with hormonal agents such as tamoxifen or aromatase inhibitors has been successful but has not been well accepted by women because of perceived side effects and risk/benefit ratio [2–7]. In prior studies of the effects of tamoxifen or aromatase inhibitors as cancer prevention agents with populations of unselected women, the number needed to treat (NNT) to prevent one breast cancer was approximately 50 [4]. These factors reduced the enthusiasm of physicians and their patients to initiate prevention therapy [4]. An effective means to decrease the NNT for prevention and enhance benefit/risk ratio is to focus hormone cancer prevention efforts on women who have both a high risk of breast cancer as well as a likely response rate to hormonal prevention. Atypical hyperplasia (AH), a benign breast lesion, is associated with a 24–47% incidence of breast cancer at 25 years, depending upon the number of independent lesions detected [8–11]. Eighty to ninety percent of these lesions are estrogen receptor positive [12–16]. In the first ten years after diagnosis, the majority of lesions are in the same breast (ipsilateral) but thereafter are equally contralateral and ipsilateral [9]. These findings suggest that AH is likely both a precursor lesion for breast cancer and a marker for later development of cancer or a “field defect” or “mutator phenotype” [17, 18].
Although no head-to-head comparisons have been conducted, treatment of AH patients with tamoxifen or aromatase inhibitors appears to reduce the incidence of breast cancer development by 64–75% versus 38% in women selected based on the Gail or similar models [8] (Table 1). Accordingly, the NNT in AH patients approximates 5, a number based on a 70% reduction of cancers from 30 to 9 in 100 women which equals 21/100 prevented or an NNT of 5 [8]. The enhanced risk of breast cancer with ADH taken together with the improved ability to prevent this neoplasm suggest that AH patients are excellent candidates for prevention with hormonal therapy.
Table 1.
Chemo-prevention trials of breast cancer in ADH patients in comparison with overall study findings
| Study | Name of study | Agent used | Results |
|---|---|---|---|
| Four studies in ADH patients | NSABP P-1 (2005) | Tamoxifen | HR 0.25 (0.10–0.52) |
| IBIS-! (2011) | Tamoxifen | HR 0.38 (0.07–2.21) | |
| MAP-3 (2011) | Exemestane MAP-3 | HR 0.36 (0.11–1.12) | |
| IBIS-II (2014) | Anastrozole IBIS-11 | HR 0.37 (0.12–1.11) | |
| Meta-analysis of overall studies based on Gail or other models | Meta-analysis | Tamoxifen | HR 0.62 (95% CI 0.56–0.69) |
Taken from Hartmann et al. NEJM [8]
A key unanswered question is the mechanism whereby AH lesions develop into cancer or herald an increase in contralateral cancer [8, 9]. We hypothesized that increased aromatase in these lesions might result in higher levels of estradiol in AH lesions themselves or in surrounding tissue. Increased estrogen is known to drive BC development both through estrogen receptor-mediated mechanisms and through estrogen metabolites acting as genotoxins independently of receptor function [19–24]. These effects result in accumulated mutations that drive BC development [25, 26] AH lesions are associated with loss of heterozygosity which suggests accumulation of genetic alterations [27].
In this study, we utilized immunohistochemistry (IHC) to assess the levels of aromatase in AH tissue and in the surrounding epithelial cells. To achieve relative tissue uniformity, we studied only the subset of women with atypical ductal hyperplasia (ADH) [8]. The large Mayo Clinic database of patients with benign breast disease [9] was interrogated to select the appropriate subjects for study. Our findings revealed higher histologic (“H”) scores for aromatase in ADH lesions than in the surrounding glandular tissue. As dense breast tissue is also a marker of breast cancer risk [28–30], we sought to compare our prior data on dense and non-dense breast tissue aromatase [31] with the ADH lesions. These data demonstrated a rank order of aromatase “H scores” from the highest to lowest with ADH > glandular tissue surrounding ADH, > dense breast tissue > non-dense breast tissue. These results have substantial implications for the use of hormone prevention therapies in women with ADH.
Materials and methods
Patient selection
In order to reduce possible heterogeneity, we selected post-menopausal women with ADH rather than all women with AH (which includes atypical lobular hyperplasia). The strict criteria of Page and Dupont were used to diagnose ADH and to differentiate ADH from ductal carcinoma in situ DCIS [32]. A cohort of 17 women with ADH who later developed BC over a period of 20 years (cases) was selected and matched on age and date of biopsy with 17 control women who were diagnosed with ADH but who did not develop BC. Unstained tissue slides prepared from 10% formalin fixed, paraffin-embedded specimens were utilized to detect aromatase with immunohistochemical (IHC) staining as described in a previously published manuscript [33]. The well-characterized #677 monoclonal anti-aromatase antibody was used for aromatase detection [34, 35]. The analyses included determination of H-score by IHC in the ADH lesions and in the surrounding epithelial tissue. As the surrounding epithelial tissue contained lobules with different numbers and sizes, the H-score was determined on the entire sections not containing ADH.
We had previously compared the aromatase H- scores in dense and non-dense breast biopsies from normal women using identical methods [31] and utilized these data as controls for the ADH patients. The methods for examining dense and non-dense tissue were published in detail before [31] and briefly here. On mammograms, the study radiologist identified areas of high and low density in the right breast. The areas identified as dense and non-dense were then localized by ultrasound. Using a 14-gauge needle, an ultrasound-guided core-needle biopsy was performed in the identified dense and non-dense regions.
To address the possibility that BMI and age might skew interpretation of the aromatase H- scores, we performed multivariate analyses on the patients in the dense and non–dense cohort and found no differences in our results (data not shown).
Modified H-score
A modified H-score (histological scoring system) previously described by Santen et al. and modified by Vachon et al. [31, 36] was used to semi-quantitate the amount of aromatase in ADH lesions and the surrounding epithelial tissue. In brief, the H-score represents the product of the degree of staining intensity, the proportion of cells positive for aromatase and the fraction of epithelial cells among all cell types in the section. The median of categories for fraction of cell type and extent of staining was used to calculate this score (i.e., 0.5% for <1%; 12.5% for category 1–24%; 37.5% for category 25–50%, 75% for >50%). The pathologists were blinded at the follow-up as to breast cancer development vs no breast cancer. This methodology was identical to that utilized when comparing normal dense versus non-dense breast tissue in our previous study [31]. Examples of each immune-staining category are illustrated in Fig. 1a, b, and c.
Fig. 1.
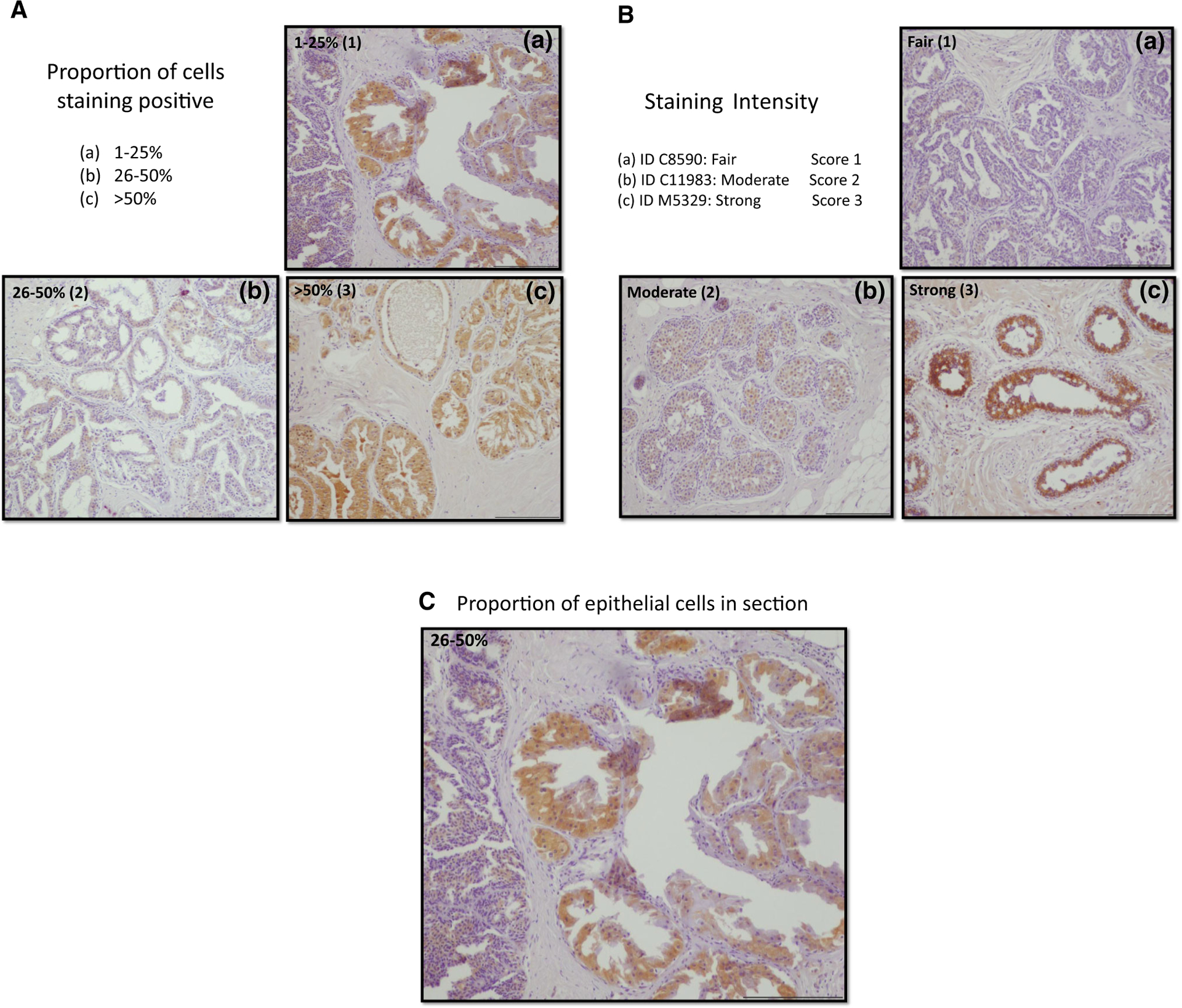
a Example of proportion of cells staining positive for aromatase. b Example of staining intensity of armatase IHC. c Example of fraction of epithelial calls in the section
Statistical analysis
For comparing the aromatase H-score in the ADH lesions versus the surrounding tissue, paired T-tests were used. We also compared the ADH and surrounding tissue H-scores with the epithelial cell H-scores reported previously in dense versus non-dense tissue using unpaired T tests [31]. Analyses were also performed utilizing multi-variable linear regression methods to look at differences between groups after adjusting for age and BMI differences.
Results
Aromatase H-scores
We observed no differences in the aromatase H-scores in ADH lesions between the group of women who later developed breast cancer (cases) and the group who did not (controls): 0.40 ± 0.09 (mean ± SEM) and 0.66 ± 0.09, respectively, P = NS. In the tissue surrounding the ADH, the differences just reached statistical significance (cases 0.17 ± 0.05, controls 0.34 ± 0.06 p = 0.04). Accordingly, we pooled the 34 patients into one group and compared the H-score in ADH lesions with the surrounding glandular tissue. We detected statistically significantly higher H-scores (p = 0.003) in the ADH lesions when compared to the non-ADH surrounding epithelial tissue (Fig. 2a). To determine whether this reflected intensity and proportion positive, we determined that intensity and proportion positive were statistically significantly different in ADH vs non-ADH tissue (Fig. 2b, c). The same sections were utilized for these comparisons, and therefore the fraction of glandular cells was identical in the ADH vs non-ADH analyses. These data indicate that aromatase protein levels are higher in the ADH lesions than in the surrounding epithelial cells.
Fig. 2.
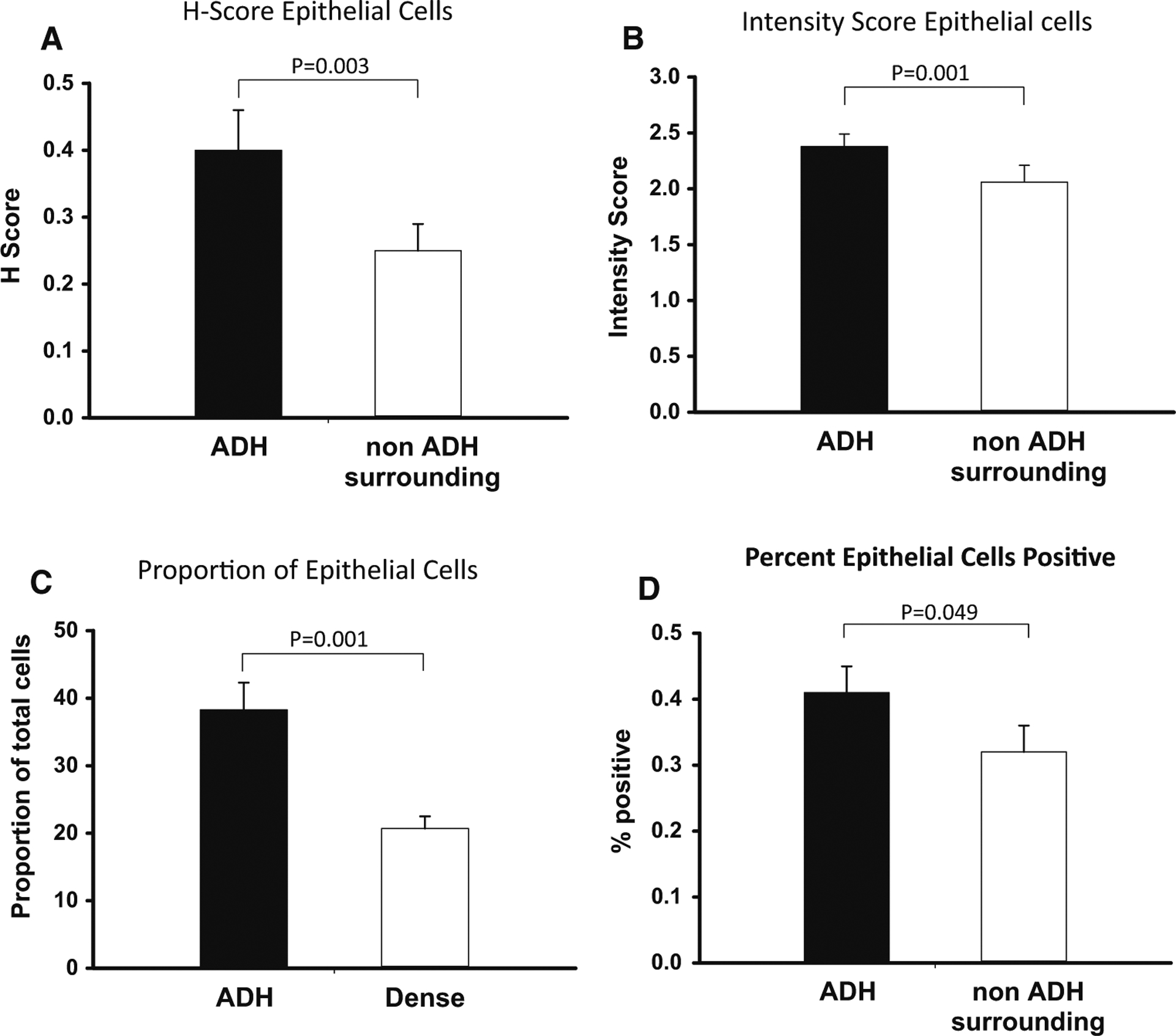
a H-score of aromatase in epithelial cells. b Intensity of aromatase staining in epithelial cells. c Percent of epithelial calls that stained for aromatase. d percent epithelial cells positive
As we had previously determined H-scores in dense versus non-dense breast tissue [31], we compared these values with our current data. As shown in Fig. 3, we found a statistically significant, graded level of H-score with the ADH lesions highest, followed by tissue surrounding the ADH lesions, then dense normal breast tissue and finally non-dense normal breast tissue as the lowest. Similar results were seen when adjusting for age and BMI differences in the comparison between tissue surrounding ADH lesion and dense normal breast tissue.
Fig. 3.
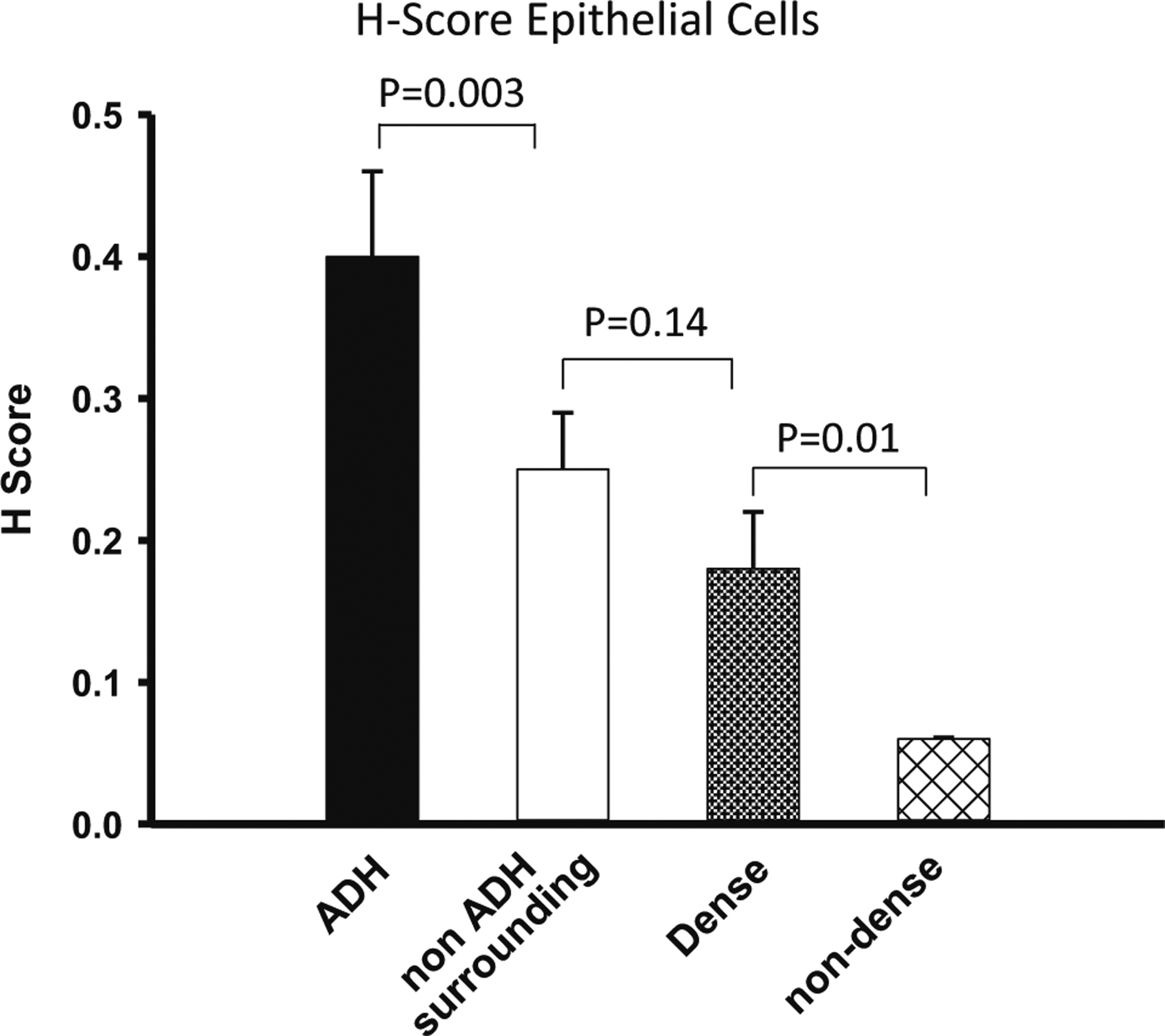
H-score comparing ADH, its surrounding tissue, dense breast tissue and non-dense breast tissue
Discussion
We observed higher levels of aromatase in ADH lesions than in the surrounding breast epithelial tissue. Both these levels were higher than in dense and non-dense breast tissue [31]. These data suggest that local production of estrogen may be higher in ADH lesions and in the tissue surrounding ADH than in dense or non-dense normal tissue [34, 37, 38]. In support of the concept that aromatase levels reflect estrogen concentrations, one of the co-authors (H.S.) [34, 38] previously reported that DCIS lesions with high aromatase immunoreactivity also have high tissue estrogen levels when measured by highly sensitive and specific estrogen assays [38, 39]. As ADH lesions have both high aromatase and estrogen receptor levels [12, 13], it is not surprising that women with these lesions appear to respond at a disproportionately higher rate to tamoxifen or aromatase inhibitors for breast cancer prevention than women selected by the Gail or other models [8] (Table 1).
As the tissue surrounding the ADH lesions also has higher aromatase levels than dense and non-dense breast tissue, expression of aromatase might possibly act as the “field defect or “mutator phenotype” [18]. The increased local estrogen production and subsequent estrogen receptor (ER) and genotoxic metabolite actions could enhance the process of BC development. This could explain why ADH lesions are associated with both ipsilateral (likely daughter lesions) as well as contralateral BC development. Although we expected the women who developed invasive BC on subsequent follow-up to have higher levels of aromatase than those who did not, this was not the case. As the development of breast cancer is a multifactorial process with influences in the intrauterine state and puberty [40], our findings must be interpreted in this context.
While this study is the first to demonstrate high aromatase levels in ADH tissue, further studies are required to provide insight into the functional consequences of these findings. Measurement of estradiol and estrone levels in ADH lesions and surrounding tissue is needed to confirm the enzymatic effects of the increased aromatase expression. Randomized, head-to-head clinical trials should be performed to clearly demonstrate the efficacy of breast cancer prevention with aromatase inhibitors and tamoxifen in women with ADH as compared to women selected by Gail or other model parameters.
This study compared ADH and surrounding epithelial cells with the same cells in dense and non-dense benign breast tissue. In the prior study of Vachon et al., the aromatase IHC staining was higher in stromal cells and adipocytes than in the epithelial cells [31]. If the autocrine production of estrogen in epithelial cells is the principal driver of BC development, our data interpretation is likely correct. Specifically, under these circumstances, the estrogen synthesized in the glandular tissue itself via aromatase would act through ER mediated and ER independent mechanisms [41] to induce mutations. If, on the other hand, aromatase expressed in the stroma and adipocytes is a substantial driver of BC development through paracrine actions on epithelial cells, then a more sophisticated interpretation of our model would be needed. In the later instance, the total tissue aromatase and not epithelial would be the most important parameter (Table 2).
Table 2.
Demographic parameters in women with ADH in study
| Parameter | Mean ± standard deviation |
|---|---|
| Post-menopausal | 33 |
| Unknown menopausal status | 1 |
| Age at biopsy | 53.7 ± 9.5 |
| BMI | 26.6 ± 5.2 |
| Parous | 29 |
| Average # children | 2.37 ± 1.45 |
| Age first live birth | 23.9 ± 4.3 |
| No menopausal hormone therapy | 9 |
| Unknown menopausal hormone therapy | 3 |
An important question from these data is why the onset of breast cancer in women with ADH takes as long as 25 years. Our prior tumor kinetic models suggest that it takes an average of 16 years for a new breast cancer cell to undergo the 30 doubling times needed for it to exceed the imaging threshold for diagnosis [42], and it may take even longer to progress from an ADH lesion to cancer. Accordingly, it would appear that our data fit with overall clinical observations about occult breast cancer and the time needed for clinical diagnosis, namely up to 25 years.
Taking into account all of the aspects of ADH [8, 10, 43–45], we suggest that the management of patients with this lesion should be considered separately from women with a high risk of breast cancer assessed by other methods. The higher rate of ER positivity, aromatase expression, and of breast cancer prevention suggests that patients with ADH are more suitable candidates for prevention with aromatase inhibitors or anti-estrogens than are women selected from the Gail or IBIS-I-risk models [8].
In summary, ADH lesions have a higher level of aromatase than the epithelial cells surrounding ADH and normal dense and non-dense breast tissue have lower levels still. These data suggest high levels of local estrogen production in ADH lesions, an issue that will require further studies to validate.
Acknowledgements
This study has been supported by two NIH Grants, NCI CA187112 to Dr. Amy Degnim and R21 CA 186734 to Dr. Celine Vachon.
Footnotes
Conflict of interest None of the authors has reported conflicts of interest with respect to the information covered in this manuscript
References
- 1.Siegel RL, Miller KD, Jemal A (2017) Cancer statistics, 2017. CA Cancer J Clin 67(1):7–30 [DOI] [PubMed] [Google Scholar]
- 2.Ozanne EM, Wittenberg E, Garber JE, Weeks JC (2010) Breast cancer prevention: patient decision making and risk communication in the high risk setting. Breast J 16(1):38–47 [DOI] [PubMed] [Google Scholar]
- 3.Ganz PA, Land SR (2008) Risks, benefits, and effects on quality of life of selective estrogen-receptor modulator therapy in post-menopausal women at increased risk of breast cancer. [Review] [34 refs]. Menopause 15(4:Suppl):Suppl-803 [DOI] [PubMed] [Google Scholar]
- 4.Fisher B, Costantino JP, Wickerham DL et al. (1998) Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 90(18):1371–1388 [DOI] [PubMed] [Google Scholar]
- 5.Fisher MD, O’Shaughnessy J (2002) Anastrozole may be superior to tamoxifen as adjuvant treatment for postmenopausal patients with breast cancer. Clin Breast Cancer 4:269–271 [Google Scholar]
- 6.Tchou J, Hou N, Rademaker A, Jordan VC, Morrow M (2004) Acceptance of tamoxifen chemoprevention by physicians and women at risk. Cancer 100(9):1800–1806 [DOI] [PubMed] [Google Scholar]
- 7.Visvanathan K, Hurley P, Bantug E et al. (2013) Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline. [Review][Erratum appears in J Clin Oncol. 2013 Dec 1;31(34):4383]. J Clin Oncol 31(23):2942–2962 [DOI] [PubMed] [Google Scholar]
- 8.Hartmann LC, Degnim AC, Santen RJ, Dupont WD, Ghosh K (2015) Atypical hyperplasia of the breast–risk assessment and management options. N Engl J Med 372(1):78–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartmann LC, Radisky DC, Frost MH et al. (2014) Understanding the premalignant potential of atypical hyperplasia through its natural history: a longitudinal cohort study. Cancer Prev Res 7(2):211–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Page DL, Dupont WD, Rogers LW, Rados MS (1985) Atypical hyperplastic lesions of the female breast. A long-term follow-up study. Cancer 55(11):2698–2708 [DOI] [PubMed] [Google Scholar]
- 11.Degnim AC, Dupont WD, Radisky DC et al. (2016) Extent of atypical hyperplasia stratifies breast cancer risk in 2 independent cohorts of women. Cancer 122(19):2971–2978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allred DC, Mohsin SK, Fuqua SA (2001) Histological and biological evolution of human premalignant breast disease. Endocr-Relat Cancer 8(1):47–61 [DOI] [PubMed] [Google Scholar]
- 13.Barr FE, Degnim AC, Hartmann LC et al. (2011) Estrogen receptor expression in atypical hyperplasia: lack of association with breast cancer. Cancer Prev Res 4(3):435–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shoker BS, Jarvis C, Clarke RB et al. (1999) Estrogen receptor-positive proliferating cells in the normal and precancerous breast. Am J Pathol 155(6):1811–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shoker BS, Jarvis C, Clarke RB et al. (2000) Abnormal regulation of the oestrogen receptor in benign breast lesions. J Clin Pathol 53(10):778–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shoker BS, Jarvis C, Sibson DR, Walker C, Sloane JP (1999) Oestrogen receptor expression in the normal and pre-cancerous breast. J Pathol 188(3):237–244 [DOI] [PubMed] [Google Scholar]
- 17.Santen R, Pinkerton J (2002) Benign breast disorders. In: DeG-root LJ, Jameson JL (eds) Endocrinology. W.B. Saunders Company, Philadelphia, pp 2189–2198 [Google Scholar]
- 18.Santen RJ, Mansel R (2005) Benign breast disorders. [Review] [89 refs]. N Engl J Med 353(3):275–285 [DOI] [PubMed] [Google Scholar]
- 19.Yue W, Wang JP, Li Y et al. (2010) Effects of estrogen on breast cancer development: role of estrogen receptor independent mechanisms. Int J Cancer 127(8):1748–1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Preston-Martin S, Pike MC, Ross RK, Jones PA, Henderson BE (1990) Increased cell division as a cause of human cancer. Cancer Res 50(23):7415–7421 [PubMed] [Google Scholar]
- 21.Jefcoate CR, Liehr JG, Santen RJ et al. (2000) Tissue-specific synthesis and oxidative metabolism of estrogens. J Natl Cancer Inst Monogr 27:95–112 [DOI] [PubMed] [Google Scholar]
- 22.Lavigne JA, Helzlsouer KJ, Huang HY et al. (1997) An association between the allele coding for a low activity variant of catechol-O-methyltransferase and the risk for breast cancer. Cancer Res 57(24):5493–5497 [PubMed] [Google Scholar]
- 23.Yager JD, Liehr JG (1996) Molecular mechanisms of estrogen carcinogenesis. Annu Rev Pharmacol Toxicol 36:203–232 [DOI] [PubMed] [Google Scholar]
- 24.Yager JD (2000) Endogenous estrogens as carcinogens through metabolic activation. J Natl Cancer Inst Monogr 27:67–73 [DOI] [PubMed] [Google Scholar]
- 25.Wood LD, Parsons DW, Jones S et al. (2007) The genomic landscapes of human breast and colorectal cancers.[see comment]. Science 318(5853):1108–1113 [DOI] [PubMed] [Google Scholar]
- 26.Leary RJ, Lin JC, Cummins J et al. (2008) Integrated analysis of homozygous deletions, focal amplifications, and sequence alterations in breast and colorectal cancers. Proc Natl Acad Sci USA 105(42):16224–16229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Connell P, Pekkel V, Fuqua SA, Osborne CK, Clark GM, Allred DC (1998) Analysis of loss of heterozygosity in 399 premalignant breast lesions at 15 genetic loci. J Natl Cancer Inst 90(9):697–703 [DOI] [PubMed] [Google Scholar]
- 28.Boyd NF, Lockwood GA, Byng JW, Tritchler DL, Yaffe MJ (1998) Mammographic densities and breast cancer risk. Cancer Epidemiol Biomark Prev 7(12):1133–1144 [PubMed] [Google Scholar]
- 29.Boyd NF, Jensen HM, Cooke G, Han HL, Lockwood GA, Miller AB (2000) Mammographic densities and the prevalence and incidence of histological types of benign breast disease. Reference Pathologists of the Canadian National Breast Screening Study. Eur J Cancer Prev 9(1):15–24 [DOI] [PubMed] [Google Scholar]
- 30.Santen RJ, Boyd NF, Chlebowski RT et al. (2007) Critical assessment of new risk factors for breast cancer: considerations for development of an improved risk prediction model. [Review] [95 refs]. Endocr Relat Cancer 14(2):169–187 [DOI] [PubMed] [Google Scholar]
- 31.Vachon CM, Sasano H, Ghosh K et al. (2011) Aromatase immunoreactivity is increased in mammographically dense regions of the breast. Breast Cancer Res Treat 125(1):243–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Page DL, Rogers LW (1992) Combined histologic and cytologic criteria for the diagnosis of mammary atypical ductal hyperplasia. Hum Pathol 23(10):1095–1097 [DOI] [PubMed] [Google Scholar]
- 33.Berg JC, Visscher DW, Vierkant RA et al. (2008) Breast cancer risk in women with radial scars in benign breast biopsies. Breast Cancer Res Treat 108(2):167–174 [DOI] [PubMed] [Google Scholar]
- 34.Sasano H, Miki Y, Shibuya R, Suzuki T (2010) Aromatase and in situ estrogen production in DCIS (ductal carcinoma in situ) of human breast. J Steroid Biochem Mol Biol 118(4–5):242–245 [DOI] [PubMed] [Google Scholar]
- 35.Sasano H, Anderson TJ, Silverberg SG et al. (2005) The validation of new aromatase monoclonal antibodies for immunohisto-chemistry—a correlation with biochemical activities in 46 cases of breast cancer. J Steroid Biochem Mol Biol 95(1–5):35–39 [DOI] [PubMed] [Google Scholar]
- 36.Santen RJ, Martel J, Hoagland M et al. (1994) Stromal spindle cells contain aromatase in human breast tumors. J Clin Endocrinol Metab 79(2):627–632 [DOI] [PubMed] [Google Scholar]
- 37.Sasano H, Suzuki T, Nakata T, Moriya T (2006) New development in intracrinology of breast carcinoma. [Review] [54 refs]. Breast Cancer 13(2):129–136 [DOI] [PubMed] [Google Scholar]
- 38.Takagi K, Ishida T, Miki Y et al. (2013) Intratumoral concentration of estrogens and clinicopathological changes in ductal carcinoma in situ following aromatase inhibitor letrozole treatment. Br J Cancer 109(1):100–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shibuya R, Suzuki T, Miki Y et al. (2008) Intratumoral concentration of sex steroids and expression of sex steroid-producing enzymes in ductal carcinoma in situ of human breast. Endocr Relat Cancer 15(1):113–124 [DOI] [PubMed] [Google Scholar]
- 40.Ritte R, Lukanova A, Tjonneland A et al. (2013) Height, age at menarche and risk of hormone receptor-positive and -negative breast cancer: a cohort study. Int J Cancer 132(11):2619–2629 [DOI] [PubMed] [Google Scholar]
- 41.Yue W, Wang JP, Li Y et al. (2010) Effects of estrogen on breast cancer development: role of estrogen receptor independent mechanisms. Int J Cancer 127(8):1748–1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Santen RJ, Yue W, Heitjan DF (2012) Modeling of the growth kinetics of occult breast tumors: role in interpretation of studies of prevention and menopausal hormone therapy. Cancer Epidemiol Biomark Prev 21(7):1038–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dupont WD, Parl FF, Hartmann WH et al. (1993) Breast cancer risk associated with proliferative breast disease and atypical hyperplasia. Cancer 71(4):1258–1265 [DOI] [PubMed] [Google Scholar]
- 44.Dupont WD, Page DL, Parl FF et al. (1999) Estrogen replacement therapy in women with a history of proliferative breast disease. Cancer 85(6):1277–1283 [DOI] [PubMed] [Google Scholar]
- 45.Page DL, Dupont WD (1998) Benign breast diseases and premalignant breast disease. Arch Pathol Lab Med 122(12):1048–1050 [PubMed] [Google Scholar]


