Abstract
It was recently discovered that the aarF gene in Providencia stuartii is required for coenzyme Q (CoQ) biosynthesis. Here we report that yigR, the Escherichia coli homologue of aarF, is ubiB, a gene required for the first monooxygenase step in CoQ biosynthesis. Both the P. stuartii aarF and E. coli ubiB (yigR) disruption mutant strains lack CoQ and accumulate octaprenylphenol. Octaprenylphenol is the CoQ biosynthetic intermediate found to accumulate in the E. coli strain AN59, which contains the ubiB409 mutant allele. Analysis of the mutation in the E. coli strain AN59 reveals no mutations within the ubiB gene, but instead shows the presence of an IS1 element at position +516 of the ubiE gene. The ubiE gene encodes a C-methyltransferase required for the synthesis of both CoQ and menaquinone, and it is the 5′ gene in an operon containing ubiE, yigP, and ubiB. The data indicate that octaprenylphenol accumulates in AN59 as a result of a polar effect of the ubiE::IS1 mutation on the downstream ubiB gene. AN59 is complemented by a DNA segment containing the contiguous ubiE, yigP, and ubiB genes. Although transformation of AN59 with a DNA segment containing the ubiB coding region fails to restore CoQ biosynthesis, transformation with the ubiE coding region results in a low-frequency but significant rescue attributed to homologous recombination. In addition, the fre gene, previously considered to correspond to ubiB, was found not to be involved in CoQ biosynthesis. The ubiB gene is a member of a predicted protein kinase family of which the Saccharomyces cerevisiae ABC1 gene is the prototypic member. The possible protein kinase function of UbiB and Abc1 and the role these polypeptides may play in CoQ biosynthesis are discussed.
Ubiquinone (coenzyme Q, or CoQ) is a prenylated benzoquinone that functions in the respiratory electron transport chain of the inner mitochondrial membranes of eukaryotes and in the plasma membrane of prokaryotes (7). In addition to respiratory electron and proton transport, the redox properties of CoQ allow the reduced form (CoQH2) to scavenge lipid peroxyl radicals either directly or indirectly as mediated through α-tocopherol (6, 16, 23). This antioxidant function of CoQH2 serves to protect cells from the oxidative, damaging effect of polyunsaturated fatty acids (15). Although CoQ is found primarily in the mitochondria of eukaryotes, it is also found in other organelles and in the plasma membrane, where it participates in a plasma membrane electron transport system (reviewed in reference 41). In the plasma membrane of prokaryotes, CoQ also functions in disulfide bond formation of periplasmic proteins (3, 27).
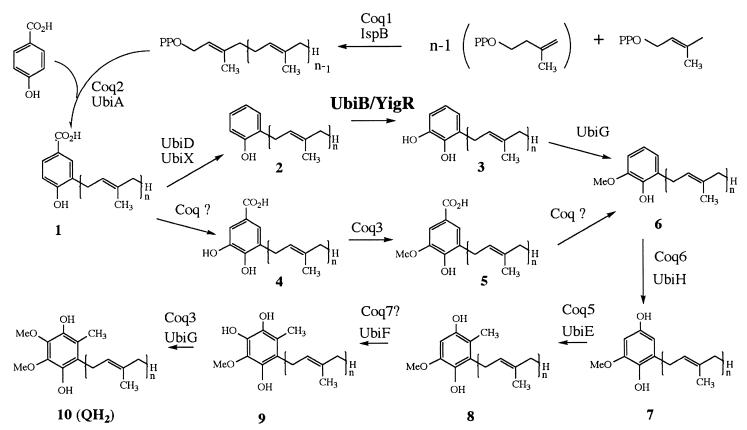
Generally, cells rely on de novo synthesis for their source of CoQ. Numerous studies carried out with bacteria and yeast have enabled the pathway to be elucidated (for reviews, see references 21, 32, and 38). Briefly, CoQ biosynthesis begins with the formation of compound 1 (Fig. 1) from the precursors polyprenyldiphosphate and 4-hydroxybenzoic acid, followed by a series of ring modifications. In Escherichia coli, prenylation is followed by decarboxylation, hydroxylation, and methylation, whereas in Saccharomyces cerevisiae, the order is hydroxylation, methylation, and then decarboxylation. The pathways then converge at compound 6 in both eukaryotes and prokaryotes. In E. coli, the tail of CoQ has eight isoprene groups and hence is designated CoQ8.
FIG. 1.
Biosynthesis of CoQ in prokaryotes and eukaryotes. After formation of 3-polyprenyl-4-hydroxybenzoic acid (compound 1), the proposed biosynthetic pathways for CoQ in eukaryotes and in prokaryotes are thought to diverge. The intermediates in the pathway are 2-polyprenylphenol (compound 2); 2-polyprenyl-6-hydroxy-phenol (compound 3); 3,4-dihydroxy-5-polyprenylbenzoic acid (compound 4); 3-methoxy-4-hydroxy-5-polyprenylbenzoic acid (compound 5); 2-polyprenyl-6-methoxy-phenol (compound 6); 2-polyprenyl-6-methoxy-1,4-benzoquinol (compound 7); 2-polyprenyl-3-methyl-6-methoxy-1,4-benzoquinol or 5-demethoxyubiquinol (compound 8); 2-polyprenyl-3-methyl-5-hydroxy-6-methoxy-1,4-benzoquinol or demethyl-QH2 (compound 9); and CoQnH2 (compound 10). In E. coli, n = 8, and compound 2 is referred to as octaprenylphenol. E. coli gene products are identified as Ubi; S. cerevisiae gene products are identified as Coq.
A number of studies have recently focused on the methylation steps in CoQ biosynthesis. The ubiE gene, encoding the E. coli C-methyltransferase enzyme, was identified and found to be necessary for both CoQ8 and menaquinone-8 (MK8) biosynthesis (28). The corresponding C-methyltransferase in yeast was identified as COQ5 (4, 14). One O-methyltransferase, identified as UbiG in E. coli and Coq3 in yeast, catalyzes both O-methylation steps in CoQ biosynthesis (36). However, the gene-enzyme relationships involving the monooxygenase steps in CoQ biosynthesis are not well understood. Early studies of bacteria identified mutants blocked in each of the three monooxygenase steps of CoQ8 synthesis, and the affected genes were designated ubiB (12) ubiF (43), and ubiH (44). Only the ubiH gene has been isolated (33). Studies of yeast have identified a mitochondrial protein, Coq7p, that is necessary for the last monooxygenase step (20, 31). Homologues of Coq7 in rat, human, and the nematode Caenorhabditis elegans each function to restore biosynthesis of CoQ in the yeast coq7Δ mutant (17, 19, 40). However, the COQ7 gene does not contain any homology to ubiH or any other known monooxygenase or hydroxylase. There is no known homologue for this gene in E. coli, but it is present in Rickettsia (2). Interestingly, the C. elegans COQ7 homologue, clk-1, has been implicated in a life extension phenotype (17). Thus, the exact function of Coq7 in CoQ biosynthesis and its numerous pleiotropic effects remain largely unknown. The genes responsible for the other monooxygenase steps in eukaryotes have yet to be identified.
The analysis of the E. coli genome by Daniels et al. (13) suggested that the fre gene, coding for NAD(P)H flavin oxidoreductase, corresponds to ubiB based on its chromosome location and its sequence similarity to flavin-dependent monooxygenases. E. coli ubiB mutants lack CoQ8 and accumulate the Q-intermediate octaprenylphenol (compound 2, Fig. 1) (12). However, in this study, we have found that E. coli fre disruption mutants continue to produce CoQ, thereby excluding fre as a candidate for ubiB.
Recent work with the opportunistic pathogen Providencia stuartii identified aarF as a gene required for CoQ biosynthesis (30). The E. coli homologue of aarF was identified as yigR, and the E. coli strain DM123 harboring a disruption in the yigR gene was also found to be CoQ deficient (30). Both the P. stuartii aarF and E. coli yigR mutant strains accumulate compound 2, the same CoQ biosynthetic intermediate found to accumulate in AN59, a ubiB mutant strain of E. coli. In this work, we show that aarF in P. stuartii and yigR in E. coli both correspond to ubiB. Surprisingly, AN59 was found to contain an insertion element (IS1) located within the ubiE gene. The data presented here indicate that the IS1 element impairs the transcription from ubiE and from ubiB (yigR), which corresponds to the third gene in an operon containing ubiE, yigP, and ubiB (yigR). The data presented here identify yigR and aarF as ubiB, a gene required for the first monooxygenase step in CoQ biosynthesis in prokaryotes.
MATERIALS AND METHODS
Strains and growth media.
The E. coli and P. stuartii strains and the plasmids used in this study are listed in Table 1. Sequence analysis (GenBank accession no. 2367309) of the DNA segment containing the ubiE-yigPQR region indicates that the E. coli yigQ and yigR genes actually correspond to one contiguous coding region, now referred to as ubiB. Wild-type and mutant strains were grown in Luria-Bertani (LB) broth overnight at 37°C with vigorous shaking (350 rpm) unless otherwise indicated. Strains harboring plasmids were grown in media supplemented with ampicillin (100 μg/ml). E. coli strain AN59 was grown in brain heart infusion media (Difco), and gentamicin was included in the growth media to select against revertants (2 μg/ml in liquid media; 10 μg/ml in plate media). Succinate defined medium was prepared according to the method of Poole et al. (34). The transformation of strains by electroporation was carried out with the Bio-Rad gene pulser apparatus using 0.1-cm cuvettes as described by Bio-Rad.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Genotype | Source or reference |
|---|---|---|
| E. coli strains | ||
| HW272 | ubiG+ zei::Tn10dTet | 42 |
| GD1 | ubiG::Kan zei::Tn10dTet | 18 |
| RM1734 | (MG1655)1− F−rph-1 (wild type) | 30 |
| DM123 | RM1734 yigR::Kan | 30 |
| AN59 | Hfr, thr-1 leu-6 ubiE409::IS1 | 12a |
| LS1312 | fre::Kan | DiRussob; 11 |
| P. stuartii strains | ||
| PR50 | Wild type | 30 |
| PR54 | PR50 aarF::Cm | 30 |
| Plasmids | ||
| pET21a | T7 expression plasmid, Ap | Novagen |
| pEF1 | pET21a::3.5-kb Sau3A1 fragment containing E. coli ubiE and yigP and ubiB genes | 30 |
| pSK-2.6 | pBluescript SK(−)::2.6-kb SalI fragment from pEF1 (yigP and ubiB genes) | 30 |
| pSK-aarF | pBluescript SK(−)::1.9-kb fragment from pAFM12 (P. stuartii aarF) | 30 |
| pQM | Vector containing the yeast CYC1 promoter | 18 |
| pUE-3 | pQM::ubiE coding region | 28 |
The mutant allele in this strain has been characterized as ubiE409::IS1 and replaces the original designation “ubiB409.”
Department of Biochemistry and Molecular Biology, Albany Medical College, Albany, N.Y.
Lipid extraction.
Aliquots (5 ml) of overnight cultures were used to inoculate 1 liter of the corresponding media, containing 0.45 μCi of p-[U-14C]hydroxybenzoic acid per liter. The synthesis of p-[U-14C]hydroxybenzoic acid (364.8 Ci/mol, 25.2 μCi/ml of ethanol) has been described previously (35). Cultures were harvested (16 to 20 h) and subjected to lipid extraction as described previously (35, 37). The organic layer was reduced to approximately 30 ml by a stream of nitrogen. The extracts were washed thrice (1 ml each time) with distilled water. The samples were then completely evaporated under a stream of N2, and the lipids were resuspended in 0.5 ml of hexane per liter of original culture.
Purification of CoQ8, MK8, and intermediates.
Lipid samples were either subjected to SepPak chromatography or directly analyzed by high-pressure liquid chromatography (HPLC). C18 SepPak cartridges were obtained from Millipore Corporation (Bedford, Mass.). The column was equilibrated with 7 ml of acetonitrile, and 450 μl of extract was applied to the column. The elution solvents were described previously (18): (i) 7 ml of acetonitrile, (ii) 7 ml of acetonitrile-isopropanol (85:15), (iii) 7 ml of acetonitrile-isopropanol (7:3), (iv) 3.5 ml of isopropanol, and (v) 3.5 ml of hexane. All fractions were collected, dried down completely, and then resuspended in 300 μl of hexane. Each fraction was monitored for radioactivity by scintillation counting. Fraction 2 contained greater than 60% of the radioactivity. This fraction was evaporated under a stream of N2 and was resuspended in hexane prior to separation by HPLC. For the samples of E. coli and P. stuartii lipid extracts, the elution profiles of radioactivity were very similar in the total lipid extract and in fraction ii.
The Gilson HPLC system was composed of two 306 pumps, an 811C mixing chamber, a 118 UV-VIS spectrophotometer, a 506C system interface, and a model 203B fraction collector. Data were collected with Gilson 715 system software. The reverse-phase column (Econosphere C18, 5 μm, 4.6 by 250 mm; Alltech) was equilibrated for 1 h (flow rate of 1 ml/min) in acetonitrile-isopropanol (75:25), and upon sample injection, the percentage of isopropanol was increased to a final ratio of 40:60 at 35 min. An aliquot of hexane (“sample blank”) was injected prior to analysis of the lipid extracts. The radioactivity in each 1-ml sample was determined by scintillation counting. Alternatively, if a sample was needed for mass spectrometry, 200 μl of each HPLC fraction was subjected to scintillation counting, and radioactive fractions were evaporated under a stream of nitrogen, resuspended in 25 μl of hexane, and analyzed by high-resolution (electron impact) mass spectrometry.
Determination of CoQ levels.
Levels of CoQ were determined as described before (22) except that bacteria were grown overnight in LB broth (DH5α) or LB broth plus kanamycin (LS1312) or were grown for 3 days to stationary phase (DM123, HW272, and GD1).
PCR amplification, plasmid construction, and DNA sequence analysis.
Segments of genomic DNA from lysates of DH5α and LS1312 were amplified using multiple combinations of the primers FRE1 (5′-GTGACCTCGGTAGAAGCTATCACGGAT-3′), FRE2 (5′-GCTGGTCAGTATTTGATGGTAGTGATG-3′), FRE3 (5′-TCCGGCAATATAGATATCATGCTCTGC-3′), and FRE4 (5′-ACGCTCACTGCAAAACAGATCGCGGGC-3′). The combinations of FRE1 and FRE3, FRE2 and FRE3, and FRE2 and FRE4 in the wild-type strain resulted in PCR products with sizes of 583 nucleotides (nt), 511 nt, and 558 nt, respectively. However, all of these products were approximately 1 kb larger in LS1312, verifying the presence of the kanamycin cassette. DNA sequence analysis of the ubiE open reading frame (ORF) in the mutant strain AN59 was determined by dideoxynucleotide chain termination with a Sequenase kit (U.S. Biochemicals) and α-35S-dATP (1,069 Ci/mmol; NEN Research Products). The DNA was sequenced from a PCR-amplified product of AN59 genomic DNA with the following primers for amplification: pAN70-1 (5′-TTCATCGATGACATGTCCGC-3′, from 71559 to 71578, corresponding to the site 350 bp upstream of the ubiE ATG codon) and pAN70-2 (5′-AATACTTTACCCAGCAGACG-3′, from 72806 to 72787, corresponding to the site 104 bp downstream of the ubiE stop codon). The PCR fragment was gel purified and inserted via blunt-end ligation into the end-filled BamHI site of pBluescript (Stratagene). T3 and T7 primers were used in the initial sequencing reactions; subsequent primers were made based on the sequences obtained from the previous primers, and the amplified segment was sequenced in both directions. The ubiE ORF was sequenced completely, while the insertion element was 90% sequenced.
RESULTS
An E. coli fre disruption mutant continues to produce CoQ8.
The E. coli fre gene, encoding NAD(P)H flavin oxidoreductase (11), had been tentatively identified as ubiB based on its map position and its sequence similarity to subunits of other prokaryotic monooxygenases (13, 32). The involvement of the fre gene in CoQ biosynthesis was investigated in the mutant strain LS1312 containing a disruption in the fre gene (11). The presence of the fre::Kan disruption allele in LS1312 was confirmed by PCR (see Materials and Methods), and total lipid extracts were prepared from this strain and analyzed for the amount of CoQ8 by HPLC and electrochemical detection as described previously (22). The level of CoQ8 in LS1312 (32.6 ± 3.6 ng of Q8 per mg [dry weight] of cells) was not significantly different from that present in a laboratory wild-type strain of E. coli DH5α (40.3 ± 6.2 ng of CoQ8 per mg [dry weight] of cells). The presence of CoQ8 in LS1312, combined with normal growth on succinate defined medium, excludes the fre gene as a candidate gene for ubiB.
Accumulation of octaprenylphenol, a CoQ precursor, in aarF or yigR (ubiB) mutants.
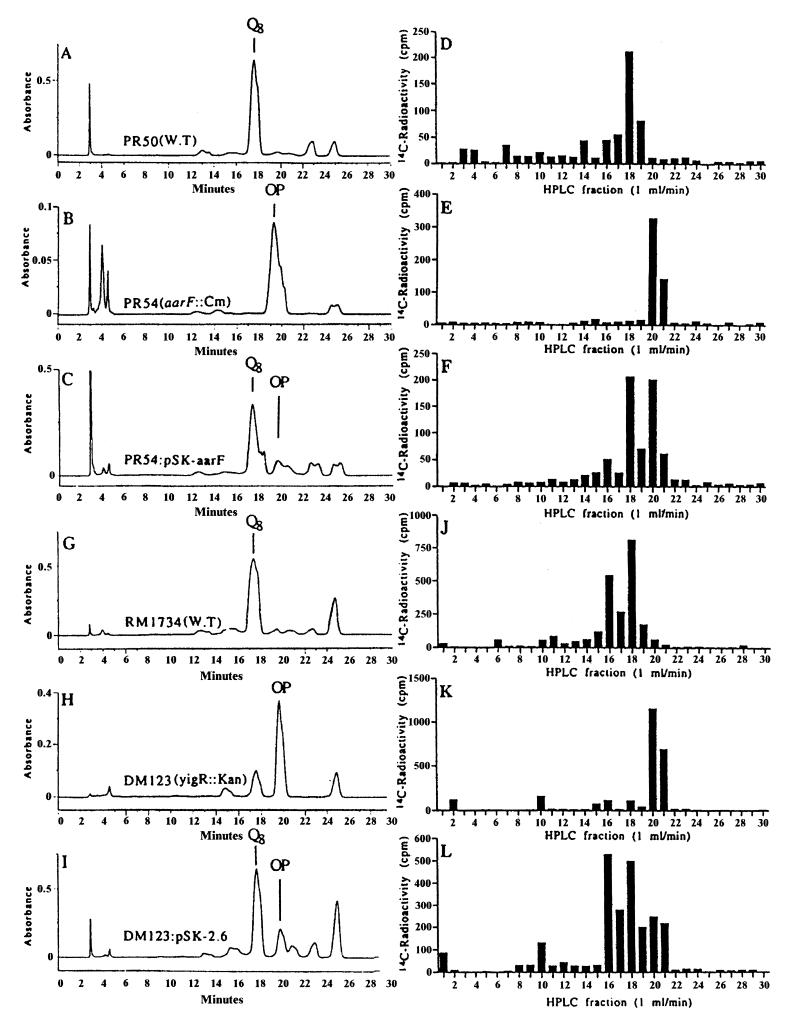
To identify the CoQ intermediates accumulating in the recently identified CoQ-deficient mutant strains of P. stuartii and E. coli (30), growth media were supplemented with p-[U-14C]hydroxybenzoic acid, and lipid extracts were prepared and separated by HPLC as described in Materials and Methods. The UV profiles for the lipid extracts from the wild-type strains demonstrated a major peak eluting at 17 to 18 min and coeluted with a CoQ8 standard (Fig. 2A and G). The subsequent analysis of incorporated radioactivity revealed a large amount of radiolabel that coincided with the UV peak in fraction 18 (Fig. 2D and J). Electron impact mass spectral analysis of fraction 18 from both wild-type strains gave molecular ions which correspond to CoQ8 (M = C49H74O4: 726.558712; Fig. 2D observed mass, 726.556882; ppm, 2.5; Fig. 2J observed mass, 726.560768; ppm, −2.8). Since the separation of the E. coli wild-type lipid extract produced two major radioactive fractions, fraction 16 (Fig. 2J) was also analyzed, and the molecular ion corresponds to CoQ8H2 (M = C49H76O4: 728.574362; observed mass, 728.573189; ppm, 1.6).
FIG. 2.
Disruption of the aarF gene in P. stuartii or the yigR gene in E. coli blocks production of CoQ8 and leads to the accumulation of octaprenylphenol (compound 2). Lipid extracts from P. stuartii (A to F) or E. coli (G to L) strains labeled with p-[U-14C]hydroxybenzoic acid were separated by reverse-phase HPLC. Absorbance (272 nm) is depicted in A to C and G to I, and 14C radioactivity present in each fraction is depicted in D to F and J to L. The profiles of absorbance and radioactivity correspond to (i) designated strains of P. stuartii, namely, PR50, wild-type (A and D); PR54, aarF mutant (B and E); or PR54 rescued with the pSK-aarF plasmid (C and F); and (ii) the E. coli strain RM1734 (G and J), DM123 (H and K), or DM123/pSK-2.6 (I and L).
Both P. stuartii aarF and E. coli yigR mutants (PR54 and DM123, respectively) had lipid profiles that were distinct from wild type, since the large UV peak associated with CoQ8 was absent, and instead a large UV peak eluted at 20 to 21 min (Fig. 2B and H). The radioactivity profile was also different for the mutants, since no radioactivity was detected in the position expected for CoQ8 at fraction 18 (Fig. 2E and K). Analysis of this fraction by mass spectrometry found no molecular ions for either CoQ8 or CoQ8H2. Instead, these mutants accumulated radiolabeled material that eluted at 20 to 21 min, coinciding with the major UV peak (Fig. 2B and H). Electron impact mass spectral analysis of the radiolabeled product in fraction 20 indicated that it was the CoQ precursor, octaprenylphenol (compound 2) (M = C46H70O: 638.542667; Fig. 2E observed mass, 638.544334; ppm, −2.6; and Fig. 2K observed mass, 638.540401; ppm, 3.5). Transformation of PR54 and DM123 with DNA clones containing either aarF or yigR genes (pSK-aarF and pSK-2.6, respectively) restored the production of CoQ8 as determined by HPLC and mass spectral analysis (fraction 18; Fig. 2F observed mass, 726.557703; ppm, 1.4; and Fig. 2L observed mass, 726.557122; ppm, 2.2). The rescue of the mutants harboring the aarF::Cm or ubiB::Kan disruption alleles was inefficient, as high levels of octaprenylphenol were still detected (see Fig. 2F and L). The restoration of CoQ8 biosynthesis is consistent with the finding that plasmids containing the aarF or yigR genes restored the ability of the mutant strains (PR54 and DM123, respectively) to utilize succinate as the sole carbon source (30). The identification of octaprenylphenol as the accumulating intermediate in DM123 indicated a deficiency in the biosynthetic step mediated by the ubiB gene product (12). The rescue of this defect by the yigR gene product suggests that yigR corresponds to ubiB.
Accumulation of both octaprenylphenol and demethylmenaquinone in AN59.
Work by Cox et al. (12) characterized the E. coli mutant strain AN59 as accumulating octaprenylphenol (compound 2). Our analysis of the p-[U-14C]hydroxybenzoic acid-radiolabeled lipid extracts from AN59 confirmed this finding. Electron impact mass spectral analysis of radioactive fractions for AN59 (denoted by the asterisk in Fig. 3A) identified the CoQ-intermediate octaprenylphenol (M = C46H70O: 638.542667; observed mass, 638.543099; ppm, −0.7). In the same lipid extract, significant amounts of UV-absorbing material eluted at 22 min but lacked radioactivity. Mass spectral analysis of this UV peak revealed the presence of demethylmenaquinone-8 (DMK8; M = C50H70O2: 702.537582; observed mass, 702.537439; ppm, 0.2). The presence of DMK8 in the AN59 mutant strain suggests a defect in the ubiE gene, since the accumulation of DMK8 was previously observed in the ubiE mutant strain AN70 (28). The presence of a large UV-absorbing peak but a lack of radioactivity is expected here since MK8, unlike CoQ8, is not derived from the CoQ precursor p-hydroxybenzoic acid.
FIG. 3.
AN59 lacks CoQ8 and accumulates octaprenylphenol and DMK8. Lipid extracts prepared from E. coli strains labeled with p-[U-14C]hydroxybenzoic acid were separated by reverse-phase HPLC as described in Materials and Methods. Absorbance (272 nm) is depicted. The major peak of radioactivity for each strain analyzed by mass spectroscopy is depicted (*). The profiles of absorbance correspond to AN59 (A), AN59/pSK-2.6 (B), and AN59/pEF1 (C). OP, octaprenylphenol.
IS1 insertion mutation in the ubiE gene of AN59.
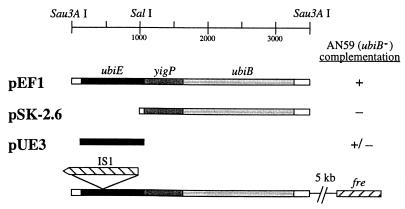
The results of the lipid analysis described above suggested that the mutation in AN59 may lie within the ubiE gene. PCR amplification of the ubiE region from genomic DNA of AN59 consistently produced a single DNA fragment of approximately 2,000 bp, 800 bp larger than the product observed with wild-type genomic DNA. Sequencing of this fragment revealed the presence of IS1 in an inverse orientation at nt 516 (Fig. 4). The presence of this element explains the high rate of reversion observed for AN59 by the ability of insertion sequences to self-excise from the DNA. It follows that the accumulation of octaprenylphenol in AN59 results from polar effects of the IS1 mutation on the downstream ubiB gene in the ubiE operon.
FIG. 4.
Identification of the mutation in AN59 (ubiE409::IS1) and determination of the sequences required for complementation. The shaded bars designate the ORFs in an operon comprised of ubiE, yigP, and ubiB (yigR). The fre gene is located approximately 5 kb from the 3′ end of ubiB. The position of a unique SalI restriction site is shown; the two Sau3A sites represent the ends of the fragment which was generated by partial digestion (30). Complementation of the slow-growth phenotype by plasmids derived from pEF1 is shown. +, restoration of wild-type growth rate and rescue of growth on succinate minimal media; −, failure to restore wild-type growth rate and growth on succinate minimal media; ±, mixed population with a preponderance of small, slow-growth colonies.
Determination of the sequences required for complementation of AN59.
The accumulation of octaprenylphenol in both AN59 (ubiB mutant) and DM123 (ubiB::Kan) suggested that the locus may correspond to ubiB. To examine this, the plasmids shown in Fig. 4 were introduced into AN59, and the resulting transformants were tested for growth on succinate defined media. Transformation of AN59 with pEF1 rescued the slow growth phenotype of AN59 and restored growth on media containing succinate. Transformation with pSK-2.6 generated only small “pinpoint” colonies that do not utilize succinate. However, transformation of AN59 with pUE-3 generated a mixed population of colonies: a majority of small pinpoint colonies and a minority of large wild-type-size colonies. Transfer of the small colonies by restreaking to LB plate media again resulted in a mixed population containing mostly small colonies but with large colonies appearing at a high frequency. These complementation results suggest that the IS1 element is removed by homologous recombination within the ubiE ORF and that such repair produces the large colony (succinate+) phenotype.
Lipid extracts were prepared from AN59 harboring either pEF1 or pSK-2.6 to examine the effects of these plasmids on the synthesis of CoQ8 and MK8. The major radioactive fractions corresponded to the UV peaks labeled in Fig. 3. Mass spectral analysis of radiolabeled lipids from AN59 transformed with pEF1 demonstrated the presence of CoQ8 (M = C49H74O4: 726.558712; Fig. 3C observed mass, 726.555928; ppm, 3.8). In addition, the lipid analysis of AN59 transformed with pEF1 demonstrated the presence of a UV peak in fraction 25 (Fig. 3C) that corresponded to MK8 (M = C51H72O2: 716.553232; Fig. 3C observed mass, 716.552534; ppm, 1.0). Thus, the presence of the pEF1 restores the synthesis of both CoQ8 and MK8. Analysis of the radiolabeled lipids from AN59/pSK-2.6 showed a large radioactive peak at fraction 20 corresponding to the UV peak (Fig. 3B) and was identified as octaprenylphenol (M = C46H70O: 638.542667; observed mass, 638.544103; ppm, −2.2). No other radioactive peak was detected. However, a UV peak was detected in fraction 23 (Fig. 3B) which corresponded to DMK8 when analyzed by mass spectrometry (M = C50H70O2: 702.537582; Fig. 3E observed mass, 702.537407; ppm, 0.2).
ubiB Disruption mutant DM123 produces low levels of CoQ8 in stationary phase.
It was noted that lipid extracts prepared from DM123 grown to stationary phase contained significant levels of CoQ8 (48.7 ± 2.8 ng of CoQ8 per mg [dry weight] of cells). These levels were lower than those detected in stationary-phase cultures of the wild-type strain HW272 (184.0 ± 5.0 ng of CoQ8 per mg [dry weight] of cells). The production of CoQ8 in DM123 stationary-phase cultures was not accounted for by reversion or suppression mutations because aliquots of the culture were still unable to grow when transferred to succinate plate media. Previous studies on mutant strains defective in one of the monooxygenase steps (ubiB, ubiH, or ubiF) showed that each defect in CoQ8 biosynthesis was bypassed under anaerobic growth conditions (1). E. coli ubi mutants that are blocked at other steps of CoQ biosynthesis (e.g., ubiD, ubiE, and ubiA) do not exhibit a bypass in response to anaerobic culture conditions (1). In the ubiB, ubiH, and ubiF strains, alternate hydroxylases appear to be responsible for anaerobic CoQ8 biosynthesis. It seems likely that this type of bypass is operating under stationary-phase growth conditions of our experiments. Furthermore, the production of CoQ8 at stationary phase was specific for the ubiB defect, since CoQ8 biosynthesis was not detected in stationary-phase cultures of the ubiG deletion strain GD1. Hence, the role of ubiB in the first monooxygenase step in CoQ biosynthesis is specific for aerobically grown log-phase cells, and CoQ synthesis can occur in stationary-phase cells despite the defect in ubiB.
DISCUSSION
The results presented provide strong evidence for the identification of the ubiB gene in E. coli. Disruption mutants of ubiB in E. coli and ubiB (aarF) in P. stuartii each accumulate octaprenylphenol, the sole CoQ intermediate expected to accumulate in E. coli blocked at the first monooxygenase step. The nature of the CoQ biosynthetic defect in AN59 (the original ubiB mutant) was discovered to be an IS1 element located in the ubiE coding region causing a polar mutation that affects the downstream ubiB gene and results in the accumulation of octaprenylphenol.
Lipid analyses of AN59/pSK-2.6 transformants contain the intermediates octaprenylphenol (compound 2 in Fig. 1) and DMK8. This is surprising since we predicted that AN59/pSK-2.6 would produce compound 7, the same intermediate that is observed in ubiE mutant strains (28). We speculate that the rescue provided by the aarF or ubiB plasmid constructs may not be as efficient. This could be due to overexpression resulting in aggregated or inactive protein, or possibly the promoter driving expression of ubiB or aarF is suboptimal relative to the normal chromosomal promoter. This hypothesis also explains the high levels of octaprenylphenol detected in the PR54 and DM123 mutants (harboring the aarF::Cm or ubiB::Kan disruption alleles, respectively) that were rescued with plasmids carrying the wild-type gene. In our previous experience with the ubiE and ubiG mutants, rescue by the corresponding wild-type genes was associated with the disappearance of the characteristic CoQ intermediate peak.
The functional role of the ubiB gene product in the hydroxylation of octaprenylphenol is unknown. However, the presence of a homologue in P. stuartii demonstrates the conservation of function found in nature for this gene product. The ubiB gene does not contain sequence identity corresponding to known monooxygenases in the databases. Examination of ubiB shows that it shares sequence identity with the ABC1 gene in S. cerevisiae, which is required for function of the mitochondrial bc1 complex (5, 8), in which CoQ functions as an essential cofactor. Recently, the Arabidopsis thaliana homologue of ABC1 was identified through functional complementation of a yeast ABC1 deletion mutant (9). Thus, the function of ABC1 in the biosynthesis of CoQ is likely to be conserved. Cardazzo et al. (9) have shown that Abc1 and Abc1-related proteins are part of a large family of proteins in both eukaryotes and prokaryotes, including Mycobacterium tuberculosis, Mycobacterium leprae, and Clostridium pasteurianum. The presence of ABC1 in M. tuberculosis and M. leprae is curious since these gram-positive bacteria are generally considered to produce menaquinone but not CoQ (10). However, inspection of the genomes of M. tuberculosis and M. leprae reveals the presence of other CoQ biosynthetic genes, including ispB/COQ1, ubiA, ubiG, ubiE/COQ5, and a candidate gene for ubiF (W. W. Poon, unpublished observations). Since each of these genes encodes an enzyme catalyzing a biosynthetic step of CoQ biosynthesis (see Fig. 1 and references 21, 32, and 38), it seems likely that both M. tuberculosis and M. leprae do in fact produce CoQ. The presence of a ubiB homologue was also noted for C. pasteurianum (9). This gram-positive unicellular endospore-forming prokaryote is considered to lack both isoprenylated quinones and cytochromes (10). It is not currently possible to determine whether other CoQ biosynthetic genes are present in C. pasteurianum, as the complete genome sequence is not available. For this organism, the putative kinase function of the protein may have been conserved but its role in CoQ synthesis is questionable. It is possible that the homologue in C. pasteurianum corresponds to one of the ABC1-like homologues that are not required for respiration (9).
Along with ABC1, ubiB is part of a large family of proteins that contain motifs found in eukaryotic-type protein kinases (29). Although it is not known if the protein encoded by ubiB contains kinase activity or what substrates it may act on, it is interesting to speculate that UbiB may play a role in ubiquinone biosynthesis-activating proteins necessary for the monooxygenase step(s) via phosphorylation. Previous work had identified a pool of octaprenylphenol that remained bound to a protein complex until a signal such as oxygen activated the complex to continue the ring modifications required in CoQ biosynthesis (24, 25). The rapid conversion of octaprenylphenol to CoQ8 (26) upon transfer from anaerobic to aerobic growth conditions is consistent with a mechanism requiring a kinase(s) to regulate such a response. This may also be the function of the yeast homologue ABC1, since recent analyses of yeast abc1 mutants show that the ABC1 gene is required for CoQ biosynthesis (T. Q. Do and C. F. Clarke, unpublished data).
The possibility of regulation of CoQ biosynthesis by phosphorylation is intriguing. The synthesis of CoQ, an essential component of the electron transport chain, would constitute a potential site for kinase regulation of energy metabolism. There is ample precedent for the involvement of kinases in energy metabolism (e.g., phosphorylation control of glycogen and fatty acid metabolism) (39). In E. coli mutants that are defective in one of the monooxygenase steps, CoQ is synthesized in the absence of oxygen, suggesting that an alternative hydroxylase functions under anaerobic conditions (1). These questions arise: how is CoQ synthesis regulated in aerobic versus anaerobic cultures, and does ubiB play a role in such regulation? Furthermore, is this mechanism also present when E. coli shift from early log phase to stationary-phase growth conditions? The data presented here show that CoQ is synthesized differently in log phase and stationary phase, since the defect in ubiB seems to affect only CoQ biosynthesis in log-phase cultures and is bypassed in stationary phase, similar to the bypass mechanism found in anaerobically grown cells. This bypass could be the result of differential phosphorylation by the ubiB gene product during growth, and therefore it will be important to investigate the possibility of ubiB kinase activity. Additional studies on ubiB and its exact role in CoQ biosynthesis will further examine this scenario.
ACKNOWLEDGMENTS
We thank Richard L. Stevens of the UCLA Mass Spectrometry Laboratory for performing mass spectrometry analysis. We thank Concetta DiRusso and Ian G. Young for generously providing the LS1312 and AN59 E. coli strains, respectively.
This work was supported by National Institutes of Health grant GM45952 (to C.F.C.) and United States Public Health Service National Research Service Awards GM07185 (to T.J. and W.W.P.) and GM08496 (to D.E.D.).
REFERENCES
- 1.Alexander K, Young I G. Alternative hydroxylases for the aerobic and anaerobic biosynthesis of ubiquinone in Escherichia coli. Biochemistry. 1978;17:4750–4755. doi: 10.1021/bi00615a024. [DOI] [PubMed] [Google Scholar]
- 2.Andersson S G, Zomorodipour A, Andersson J O, Sicheritz-Ponten T, Alsmark U C, Podowski R M, Naslund A K, Eriksson A S, Winkler H H, Kurland C G. The genome sequence of Rickettsia prowazekii and the origin of mitochondria. Nature. 1998;396:133–140. doi: 10.1038/24094. [DOI] [PubMed] [Google Scholar]
- 3.Bader M, Muse W, Ballou D P, Gassner C, Bardwell J C A. Oxidative protein folding is driven by the electron transport system. Cell. 1999;98:217–227. doi: 10.1016/s0092-8674(00)81016-8. [DOI] [PubMed] [Google Scholar]
- 4.Barkovich R J, Shtanko A, Shepherd J A, Lee P T, Myles D C, Tzagaloff A, Clarke C F. Characterization of the COQ5 gene from Saccharomyces cerevisiae. Evidence for a C-methyltransferase in ubiquinone biosynthesis. J Biol Chem. 1997;272:9182–9188. doi: 10.1074/jbc.272.14.9182. [DOI] [PubMed] [Google Scholar]
- 5.Bousquet I, Dujardin G, Slonimski P P. ABC1, a novel yeast nuclear gene, has a dual function in mitochondria: it suppresses a cytochrome b mRNA translation defect and is essential for the electron transfer in the bc1 complex. EMBO J. 1991;10:2023–2031. doi: 10.1002/j.1460-2075.1991.tb07732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowry V W, Mohr D, Cleary J, Stocker R. Prevention of tocopherol-mediated peroxidation in ubiquinol-10-free human low density lipoprotein. J Biol Chem. 1995;270:5756–5763. doi: 10.1074/jbc.270.11.5756. [DOI] [PubMed] [Google Scholar]
- 7.Brandt U, Trumpower B. The protonmotive Q cycle in mitochondria and bacteria. Crit Rev Biochem Mol Biol. 1994;29:165–197. doi: 10.3109/10409239409086800. [DOI] [PubMed] [Google Scholar]
- 8.Brasseur G, Tron P, Dujardin G, Slonimski P P, Brivet-Chevillotte P. The nuclear ABC1 gene is essential for the correct conformation and functioning of the cytochrome bc1 complex and the neighboring complexes II and IV in the mitochondrial respiratory chain. Eur J Biochem. 1997;246:103–111. doi: 10.1111/j.1432-1033.1997.t01-1-00103.x. [DOI] [PubMed] [Google Scholar]
- 9.Cardazzo B, Hamel P, Sakamoto W, Wintz H, Dujardin G. Isolation of an Arabidopsis thaliana cDNA by complementation of a yeast abc1 deletion mutant deficient in complex III respiratory activity. Gene. 1998;221:117–125. doi: 10.1016/s0378-1119(98)00417-x. [DOI] [PubMed] [Google Scholar]
- 10.Collins M D, Jones D. Distribution of isoprenoid quinone structural types in bacteria and their taxonomic implication. Microbiol Rev. 1981;45:316–354. doi: 10.1128/mr.45.2.316-354.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Covès J, Nivière V, Eschenbrenner M, Fontecave M. NADPH-sulfite reductase from Escherichia coli. A flavin reductase participating in the generation of the free radical of ribonucleotide reductase. J Biol Chem. 1993;268:18604–18609. [PubMed] [Google Scholar]
- 12.Cox G B, Young I G, McCann L M, Gibson F. Biosynthesis of ubiquinone in Escherichia coli K-12: location of genes affecting the metabolism of 3-octaprenyl-4-hydroxybenzoic acid and 2-octaprenylphenol. J Bacteriol. 1969;99:450–457. doi: 10.1128/jb.99.2.450-458.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daniels D L, Punkett III G, Burland V, Blattner F R. Analysis of the Escherichia coli genome: DNA sequence of the region from 84.5 to 86.5 minutes. Science. 1992;257:771–778. doi: 10.1126/science.1379743. [DOI] [PubMed] [Google Scholar]
- 14.Dibrov E, Robinson K M, Lemire B D. The COQ5 gene encodes a yeast mitochondrial protein necessary for ubiquinone biosynthesis and the assembly of the respiratory chain. J Biol Chem. 1997;272:9175–9181. doi: 10.1074/jbc.272.14.9175. [DOI] [PubMed] [Google Scholar]
- 15.Do T Q, Schultz J R, Clarke C F. Enhanced sensitivity of ubiquinone-deficient mutants of Saccharomyces cerevisiae to products of autoxidized polyunsaturated fatty acids. Proc Natl Acad Sci USA. 1996;93:7534–7539. doi: 10.1073/pnas.93.15.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ernster L, Forsmark-Andree P. Ubiquinol: an endogenous antioxidant in aerobic organisms. Clin Investig. 1993;71:s60–s65. doi: 10.1007/BF00226842. [DOI] [PubMed] [Google Scholar]
- 17.Ewbank J J, Barnes T M, Lakowski B, Lussier M, Bussey H, Hekimi S. Structural and functional conservation of the Caenorhabditis elegans timing gene clk-1. Science. 1997;275:980–983. doi: 10.1126/science.275.5302.980. [DOI] [PubMed] [Google Scholar]
- 18.Hsu A Y, Poon W W, Shepherd J A, Myles D C, Clarke C F. Complementation of coq3 mutant yeast by mitochondrial targeting of the Escherichia coli UbiG polypeptide: evidence that UbiG catalyzes both O-methylation steps in ubiquinone biosynthesis. Biochemistry. 1996;35:9797–9806. doi: 10.1021/bi9602932. [DOI] [PubMed] [Google Scholar]
- 19.Jonassen T, Marbois B N, Kim L, Chin A, Xia Y R, Lusis A J, Clarke C F. Isolation and sequencing of the rat Coq7 gene and the mapping of mouse Coq7 to chromosome 7. Arch Biochem Biophys. 1996;330:285–289. doi: 10.1006/abbi.1996.0255. [DOI] [PubMed] [Google Scholar]
- 20.Jonassen T, Proft M, Randez-Gil F, Schultz J R, Marbois B N, Entian K-D, Clarke C F. Yeast Clk-1 homologue (Coq7/Cat5) is a mitochondrial protein in coenzyme Q synthesis. J Biol Chem. 1998;273:3351–3357. doi: 10.1074/jbc.273.6.3351. [DOI] [PubMed] [Google Scholar]
- 21.Jonassen T, Clarke C F. Genetic analysis of coenzyme Q biosynthesis. In: Kagan V E, Quinn P J, editors. Coenzyme Q: from molecular mechanisms to nutrition and health. Boca Raton, Fla: CRC Press; 2000. pp. 185–208. [Google Scholar]
- 22.Jonassen T, Clarke C F. Isolation and functional expression of human COQ3, a gene encoding a methyltransferase required for ubiquinone synthesis. J Biol Chem. 2000;275:12381–12387. doi: 10.1074/jbc.275.17.12381. [DOI] [PubMed] [Google Scholar]
- 23.Kagan V, Serbinova E, Packer L. Antioxidant effects of ubiquinones in microsomes and mitochondria are mediated by tocopherol recycling. Biochem Biophys Res Commun. 1990;169:851–857. doi: 10.1016/0006-291x(90)91971-t. [DOI] [PubMed] [Google Scholar]
- 24.Knoell H-E, Kraft R, Knappe J. Dioxygen and temperature dependence of ubiquinone formation in Escherichia coli: studies of cells charged with 2-octaprenylphenol. Eur J Biochem. 1978;90:107–112. doi: 10.1111/j.1432-1033.1978.tb12580.x. [DOI] [PubMed] [Google Scholar]
- 25.Knoell H-E. Isolation of a soluble enzyme complex comprising the ubiquinone-8 synthesis apparatus from the cytoplasmic membrane of Escherichia coli. Biochem Biophys Res Commun. 1979;91:919–925. doi: 10.1016/0006-291x(79)91967-3. [DOI] [PubMed] [Google Scholar]
- 26.Knoell H-E. Stand-by position of the dioxygen-dependent ubiquinone-8 synthesis apparatus in anaerobically grown Escherichia coli K-12. FEMS Microbiol Lett. 1981;10:59–62. [Google Scholar]
- 27.Kobayashi T, Kishigami S, Sone M, Inokuchi H, Mogi T, Ito K. Respiratory chain is required to maintain oxidized states of the DsbA-DsbB disulfide bond formation system in aerobically growing Escherichia coli cells. Proc Natl Acad Sci USA. 1997;94:11857–11862. doi: 10.1073/pnas.94.22.11857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee P T, Hsu A Y, Ha H T, Clarke C F. A C-methyltransferase involved in both ubiquinone and menaquinone biosynthesis: isolation and identification of the Escherichia coli ubiE gene. J Bacteriol. 1997;179:1748–1754. doi: 10.1128/jb.179.5.1748-1754.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leonard C J, Aravind L, Koonin E V. Novel families of putative protein kinases in bacteria and archaea: evolution of the “eukaryotic” protein kinase superfamily. Genome Res. 1998;8:1038–1047. doi: 10.1101/gr.8.10.1038. [DOI] [PubMed] [Google Scholar]
- 30.Macinga D R, Cook G M, Poole R K, Rather P N. Identification and characterization of aarF, a locus required for production of ubiquinone in Providencia stuartii and Escherichia coli and for expression of 2′-N-acetyltransferase in P. stuartii. J Bacteriol. 1998;180:128–135. doi: 10.1128/jb.180.1.128-135.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marbois B N, Clarke C F. The COQ7 gene encodes a protein in Saccharomyces cerevisiae necessary for ubiquinone biosynthesis. J Biol Chem. 1996;271:2995–3004. doi: 10.1074/jbc.271.6.2995. [DOI] [PubMed] [Google Scholar]
- 32.Meganathan R. Biosynthesis of the isoprenoid quinones menaquinone (vitamin K2) and ubiquinone (coenzyme Q) In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C.: ASM Press; 1996. pp. 642–656. [Google Scholar]
- 33.Nakahigashi K, Miyamoto K, Nishimura K, Inokuchi H. Isolation and characterization of a light-sensitive mutant of Escherichia coli K-12 with a mutation in a gene that is required for the biosynthesis of ubiquinone. J Bacteriol. 1992;174:7352–7359. doi: 10.1128/jb.174.22.7352-7359.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poole R K, Williams H D, Downie J A, Gibson F. Mutations affecting the cytochrome d-containing oxidase complex of Escherichia coli K12: identification and mapping of a fourth locus, cydD. J Gen Microbiol. 1989;135:1865–1874. doi: 10.1099/00221287-135-7-1865. [DOI] [PubMed] [Google Scholar]
- 35.Poon W W, Marbois B N, Faull K F, Clarke C F. 3-Hexaprenyl-4-hydroxybenzoic acid forms a predominant intermediate pool in ubiquinone biosynthesis in Saccharomyces cerevisiae. Arch Biochem Biophys. 1995;320:305–314. doi: 10.1016/0003-9861(95)90014-4. [DOI] [PubMed] [Google Scholar]
- 36.Poon W W, Barkovich R J, Hsu A Y, Frankel A, Lee P T, Shepherd J N, Myles D C, Clarke C F. Yeast and rat Coq3p and Escherichia coli UbiG polypeptides catalyze both O-methyltransferase steps in coenzyme Q biosynthesis. J Biol Chem. 1999;274:21665–21672. doi: 10.1074/jbc.274.31.21665. [DOI] [PubMed] [Google Scholar]
- 37.Radin N S. Extraction of tissue lipids with a solvent of low toxicity. Methods Enzymol. 1981;72:5–7. doi: 10.1016/s0076-6879(81)72003-2. [DOI] [PubMed] [Google Scholar]
- 38.Soballe B, Poole R K. Microbial ubiquinones: multiple roles in respiration, gene regulation and oxidative stress management. Microbiology. 1999;145:1817–1830. doi: 10.1099/13500872-145-8-1817. [DOI] [PubMed] [Google Scholar]
- 39.Stroud R M. Mechanisms of biological control by phosphorylation. Curr Opin Struct Biol. 1991;1:826–835. [Google Scholar]
- 40.Vajo Z, King L M, Jonassen T, Wilkin D J, Ho N, Munnich A, Clarke C F, Francomano C A. Conservation of the Caenorhabditis elegans timing gene clk-1 from yeast to human: a gene required for ubiquinone biosynthesis with potential implications for aging. Mamm Genome. 1999;10:1000–1004. doi: 10.1007/s003359901147. [DOI] [PubMed] [Google Scholar]
- 41.Villalba J M, Lopez-Lluch G, Santos-Ocana C, Rodriguez-Aguilera J C, Navas P. Extramitochondrial functions of coenzyme Q. In: Kagan V E, Quinn P J, editors. Coenzyme Q: from molecular mechanisms to nutrition and health. Boca Raton, Fla: CRC Press; 2000. pp. 83–98. [Google Scholar]
- 42.Wu G, Williams H D, Zamanian M, Gibson F, Poole R. Isolation and characterization of Escherichia coli mutants affected in aerobic respiration: the cloning and nucleotide sequence of ubiG. Identification of an S-adenosylmethionine-binding motif in protein, RNA, and small-molecule methyltransferases. J Gen Microbiol. 1992;138:2101–2112. doi: 10.1099/00221287-138-10-2101. [DOI] [PubMed] [Google Scholar]
- 43.Young I G, McCann L M, Stroobant P, Gibson F. Characterization and genetic analysis of mutant strains of Escherichia coli K-12 accumulating the ubiquinone precursors 2-octaprenyl-6-methoxy-1,4-benzoquinone and 2-octaprenyl-3-methyl-6-methoxy-1,4-benzoquinone. J Bacteriol. 1971;105:769–778. doi: 10.1128/jb.105.3.769-778.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Young I G, Stroobant P, MacDonald C G, Gibson F. Pathway for ubiquinone biosynthesis in Escherichia coli K-12: gene-enzyme relationships and intermediates. J Bacteriol. 1973;114:42–52. doi: 10.1128/jb.114.1.42-52.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]