Abstract
Background
A comprehensive review and description of the clinical features that impact prognosis for patients with diffuse hemispheric glioma, H3 G34-mutant (G34-DHG) is needed. Understanding survival and prognostic features is paramount for clinical advancements and patient care.
Methods
PubMed, Embase, and Google Scholar were searched for English articles published between January 1, 2012 and June 30, 2021. Eligible studies included patient(s) of any age diagnosed with an H3 G34-mutant brain tumor with at least one measure of survival or progression. Patient-level data were pooled for analyses. This study was prospectively registered in PROSPERO (CRD42021267764) and Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines were followed.
Results
Twenty-seven studies met the criteria with a total of 135 patients included. Median age at diagnosis was 15.8 years (interquartile range [IQR]: 13.3–22.0) with 90% having localized disease. Co-occurring alterations included ATRX mutation in 93%, TP53 mutation in 88%, and MGMT promoter methylation in 70%. Median time-to-progression was 10.0 months (IQR: 6.0–18.0) and median overall survival was 17.3 months (95% CI: 15.0 to 22.9). The median time from progression to death was 5.0 months (IQR: 3.0–11.7). Factors associated with survival duration were age, as patients ≥18 y/o demonstrated longer survival (hazard ratio [HR] =2.05, 95% CI: 1.16 to 3.62), and degree of upfront resection, as near or gross-total resection demonstrated longer survival compared to those with less than near-total resection (HR = 3.75, 95% CI: 2.11 to 6.62).
Conclusion
This systematic review highlights available clinical data for G34-DHG demonstrating poor outcomes and important prognostic features, while serving as a baseline for future research and clinical trials.
Keywords: diffuse hemispheric glioma, H3 G34-mutant, outcomes, survival
Key Points.
Median overall survival (OS) for patients with G34-DHG was found to be 17.3 months.
Time to progression was 10.0 months and time to death after progression was 5.0 months.
Improvements in OS were associated with greater resection and older age.
Importance of the Study.
Though H3 G34-mutant brain tumors (G34-DHG) have been histologically and molecularly studied, further description of the clinical features and factors that may impact prognosis is needed. Understanding survival data and prognostic features is paramount for clinical advancements and informing patient care. This systematic review highlights the poor prognosis of patients with G34-DHG, common clinical and treatment characteristics, and features associated with survival, while also providing a baseline for future outcome measures and clinical trials. Improved survival duration was associated with both increasing ages at diagnosis and the extent of up-front surgical resection. This study highlights the extensive gaps in available clinical data to appropriately evaluate this specific tumor and the need for large-scale collaborative research. Further clinical research is urgently needed for G34-DHG to begin improving outcomes and define relevant biomarkers that stratify clinical behavior.
Diffuse hemispheric glioma, H3 G34-mutant (G34-DHG) was first described in 2012 when two studies reported the first findings of histone mutations in pediatric brain tumors which included point mutations in the H3F3A gene, encoding for the histone variant H3.3.1,2 The H3.3 amino acid substitution at codon 34 from either glycine-to-arginine (G34R) or, more rarely, glycine-to-valine (G34V) ultimately gives rise to this new tumor entity.1 Prior to the 2021 World Health Organization classification, G34-DHGs were categorized based upon their histomorphology. These tumors are reported to occur in less than 1% of all gliomas, but in up to 15% of high-grade gliomas (HGGs) in adolescents and young adults.3 They are generally accompanied by poor prognosis, and are histologically HGGs, tumors of embryonal histology, or a mixture of both.3 Though relatively few studies have published clinical outcome data regarding G34-DHG, these tumors appear to display consistent co-alterations including in ATRX, TP53, and PDGFRA genes, as well as frequent methylation of the MGMT promoter.4,5 Although patients diagnosed with G34-DHG have poor outcomes, generally, they demonstrate a longer overall survival (OS) than HGGs harboring H3 p.K27 mutations or H3/IDH-wildtype HGGs.6
G34-DHGs have been histologically and molecularly studied; however a comprehensive description of clinical features and factors which impact prognosis has not been widely studied.5,7 The literature lacks robust essential information on outcomes measures and common clinical variables for patients with G34-DHG. Of the few studies which have been concerned with the clinical presentations of G34-DHG, many are limited by small sample sizes while other studies seldom apply a clinical lens.5,8,9 We aim to describe the median OS for patients with G34-DHG. Secondary aims include exploration of the median time to progression (TTP), clinical and treatment factors associated with G34-DHG, and clinical features which might influence OS. This systematic review will provide clinicians and researchers with a comprehensive summary of the current literature regarding the common clinical features and outcome characteristics of G34-DHG allowing for an improved understanding of this disease.
Methods
Selection Criteria and Search Strategy
The protocol for this systematic review was prospectively registered with PROSPERO (CRD42021267764)10 and reporting followed PRISMA guidelines.11
This review includes published primary neuro-oncology studies, where patients with diagnosed H3 G34-mutant tumors are identified. Data concerning the patients with the disease of interest were searched for in all types of study designs including randomized control trials to meta-analyses, systematic reviews, retrospective studies, case series, and case reports. Due to the rarity of this tumor type and the low number of available recorded patient information, all types of studies were included to gather sufficient and representative data.
Included in this review are patients of all ages who have been diagnosed with a brain tumor that harbors an H3 G34 mutation and report at least one of the following outcome measures: (1) OS duration, (2) TTP, or (3) follow up duration with known vital status. Reviews of primary studies and non-English written publications were excluded. Duplications of patient data across different published articles were identified by a patient’s assigned ID in their respective publications. Duplications were then removed, ensuring only unique patient data were included in our review.
Electronic searches were conducted using PubMed, Embase, and Google Scholar for articles published between January 1, 2012 and June 30, 2021. January 1, 2012 was chosen as this represents the date the H3 G34 mutation was first described.1,2 This search was conducted using a search strategy, developed with the guidance of a knowledgeable librarian (see Supplementary Data). Covidence, a systematic review production tool, was used for the discarding of duplicate articles, title and abstract screening, and full-text screening.12 The titles and abstracts of all articles identified by the search were independently screened against the eligibility criteria by two reviewers (C.C. and C.E.). In cases of disagreements, inclusion was resolved at a meeting between the two authors through consensus. The authors then independently screened full-text copies of the selected articles to determine final inclusion in the review. This initial screening was then followed by manual reference scanning and snowballing of all initially included studies to capture as many relevant studies as possible.
Bias and Quality Assessment
Given the rarity of G34-DHG, studies that met inclusion criteria were not excluded due to quality, unless striking issues were noted by the reviewing authors. Eligible studies were still assessed for quality through critical appraisal by two reviewers (C.C. and C.E.) to ensure transparency of the review. Studies were assessed using their respective Johanna Briggs Institute critical appraisal checklists.13 Articles regarding molecular meta-analyses and cohort descriptions from randomized trials, which were separate from the original trial study, were appraised as case series. Decisions for ranking studies as low, moderate, and high quality were made a priori based on set scoring criteria. Each of the reviewers assessed the quality of each study independently and those with conflicting assessments were resolved via meeting between the two reviewers.
Data Extraction and Compilation
A standard data collection form (see Supplementary Data) was used to extract data from all included articles and data collection was performed by one reviewer (C.C.). Data were extracted from all available resources included in the selected articles, such as written text, graphs, tables, figures, and supplemental files. Patients within included studies were not included if at least one of the previously mentioned outcome data was unavailable, including OS duration, TTP, or follow-up duration with known vital status. Demographic variables included age and sex, while clinical variables included initial tumor histology, tumor mutation status, radiographic characteristics, disease extent and location, treatment strategies, relapse occurrence, and survival details. Initial tumor histology was classified as either HGG, primitive neuro-ectodermal tumor (PNET), or low-grade glioma (LGG). Tumor location was described as the brain lobe affected or if multiple lobes/structures were affected. Disease extent was categorized as either localized or metastatic at presentation. Tumor hemisphere was described as either the left or right side of the brain, bihemispheric, or midline. From each eligible publication, the individual participant-level data, as well as any potential aggregate data needed to analyze the median TTP or OS was extracted. Common co-alterations were selected based on availability and recognition in the literature including ATRX, TP53, PDGFRA, and MGMT promoter methylation.1,7,14 When immunohistochemistry was used, cut-off percentages for ATRX and TP53 mutation status were set at 10% nuclear staining.15
Data Analysis and Statistics
Descriptive statistics were reported using medians and percentages. Individual patient data were collected, then pooled to generate a Kaplan-Meier plot and survival estimates were obtained. The association between clinical factors and TTP was not performed as insufficient reporting occurred for the time variable to progression. A univariate marginal Cox model was used to estimate the association of regression parameters with OS.16 Cluster random effects methods were used to help account for heterogeneity between studies in the marginal cox model. Variables were included based upon clinical relevance and if less than 20% of the data was missing. A robust sandwich covariance matrix is used to account for intracluster dependence of patients from the same clinical study. Demographic variables of interest included age (<18 vs ≥ 18 years-old), sex (male vs female), tumor histology (HGG vs PNET vs other), tumor mutational subtypes (G34R vs G34V), and extent of up-front tumor resection prior to relapse (Gross Total Resection [GTR]/Near Total Resection [NTR] vs < NTR) were investigated for their impact on the OS of patients with G34-DHG. A P-value of < .05 was used to indicate statistical significance. SAS STAT 14.3 software, version 9.4 was used for all statistical analyses.
Results
Literature Search
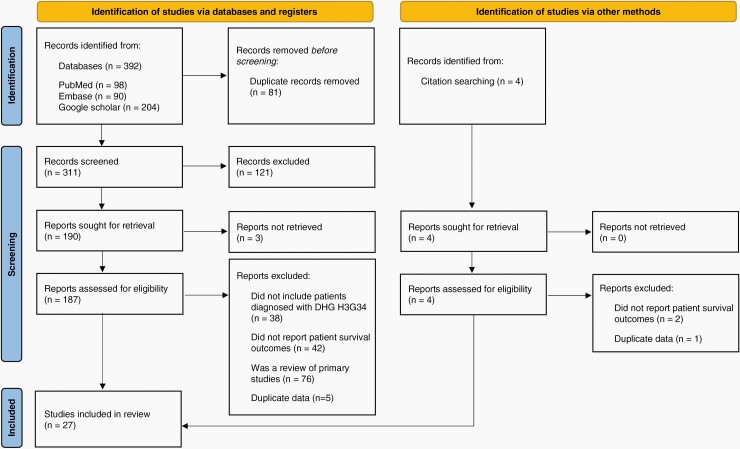
The PRISMA flow chart for our study inclusion is shown in Figure 1. The search identified a total of 392 studies. Twenty-seven studies met inclusion criteria and were included in our review. Reasons for article exclusion can be found in Figure 1. Included studies are detailed in Supplementary Appendix B including the consensus quality appraisal results.
Figure 1.
PRISMA flowchart demonstrating study selection process by C.C and C.E.
Demographic, Clinical, and Radiologic Features
From the eligible studies, a total of 135 patients diagnosed with H3 G34-mutant brain tumors were identified, and their data included for analysis (Table 1). Pooled patient data demonstrates a median age at diagnosis of 15.8 years (interquartile range [IQR]: 13–22). Data regarding sex was available in 126 patients, where 59% were male and 41% were female (1.46:1 ratio). Of the 61 patients with data regarding the extent of disease at diagnosis, 55 patients (90%) presented with localized disease, while 6 patients (10%) presented with metastatic disease at diagnosis including 2 with leptomeningeal disease and 4 not specified. As noted in Table 1, 125 patients had available tumor location data, 36 patients (29%) presented with disease in multiple lobes/structures of the brain.
Table 1.
Demographic and Clinical Characteristics
| Variable | N = | n (%) |
|---|---|---|
| Demographics | ||
| Age at diagnosis (years) | 135 | |
| Median (IQR) | 15.8 (13–22) | |
| Sex | 126 | |
| Male | 74 (59) | |
| Female | 52 (41) | |
| Imaging and Location | ||
| Tumor location | 125 | |
| Frontal lobe | 20 (16) | |
| Parietal lobe | 11 (9) | |
| Temporal lobe | 13 (10) | |
| Occipital lobe | 6 (5) | |
| Multiple lobes/structures | 36 (29) | |
| Cerebrum, not specified | 34 (27) | |
| Deep structure/midline | 5 (4) | |
| Brain lobes involved | 90 | |
| 1 lobe | 49 (54) | |
| 2 lobes | 17 (19) | |
| 3+ lobes | 7 (8) | |
| Lobe(s) + deep structure | 12 (13) | |
| Deep structure only | 5 (6) | |
| Tumor hemisphere | 47 | |
| Right | 14 (30) | |
| Left | 23 (49) | |
| Bihemispheric | 6 (13) | |
| Midline | 4 (8) | |
| Disease extent | 61 | |
| Localized | 55 (90) | |
| Metastatic | 6 (10) | |
| Contrast enhancement | 41 | |
| Yes | 20 (49) | |
| No | 21 (51) | |
| Diffusion restriction | 21 | |
| Yes | 20 (95) | |
| No | 1 (5) | |
| Tumor Characteristics | ||
| Primary tumor histology | 135 | |
| High-grade glioma | 122 (91) | |
| PNET | 10 (7) | |
| Low-grade glioma | 3 (2) | |
| Mutation subtype | 126 | |
| G34R | 118 (94) | |
| G34V | 8 (6) | |
| MGMT hypermethylation | 73 | |
| Yes | 51 (70) | |
| No | 22 (30) | |
| ATRX mutation | 55 | |
| Yes | 52 (95) | |
| No | 3 (5) | |
| TP53 Mutation | 63 | |
| Yes | 55 (87) | |
| No | 8 (13) | |
| PDGFRA alteration | 72 | |
| No alteration | 10 (14) | |
| Mutation present | 11 (15) | |
| Amplification present | 9 (13) | |
| No mutation (amplification not assessed) | 1 (1) | |
| No amplification (mutation not assessed) | 41 (57) |
Abbreviations: IQR, interquartile range; PNET, primitive neuro-ectodermal tumor.
When disease presented in only a single lobe, the most common lobe impacted was the frontal lobe in 20 patients (17%). With respect to further radiologic features, 20 patients (49%) demonstrated significant contrast enhancement and 20 (95%) demonstrated diffusion restriction. Histologically, 122 patients (91%) were initially given a diagnosis of HGG such as glioblastoma or anaplastic astrocytoma, with 10 (7%) PNET, and 3 (2%) having LGGs. Of the 126 patients with data available for their H3G34 mutation subtype, 118 (94%) had p.G34R and the remaining 8 (6%) had p.G34V. The remaining 9 patients had their G34-DHG diagnosed by methylation profiling with the specific G34 mutation not specified. Of the most commonly described co-mutations 95% (52/55) harbored ATRX mutations, and 86% (55/63) harbored TP53 mutations. In addition, PDGFA point mutations were assessed in 24 patients with 11 having a mutation (45%) and 70 patients were assessed for PDGFRA amplifications with 9 being amplified (13%). In our cohort, PDGFRA amplifications and mutations were mutually exclusive. MGMT promoter methylation occurred in 70% (51/73).
Treatment and Survival Characteristics
As demonstrated in Table 2, 89 patients had data concerning the degree of up-front surgical resection, where 40 patients (45%) had GTR/NTR, 49 (55%) underwent less than NTR. Seventy-one patients had up-front radiation therapy details available with 69 (97%) of these patients receiving initial radiation therapy. Only 26 patients had detailed the radiation therapy type, with 14 (54%) having focal radiation, 10 (38%) underwent craniospinal radiation, and 2 (8%) not receiving initial radiation. Of the 72 patients that had chemotherapy data available, 69 (96%) patients underwent initial chemotherapy treatment, while 3 (4%) did not. For the 31 patients with detailed chemotherapy agents used, 20 (64%) received Temozolomide-based therapy.
Table 2.
Treatment and Outcome Characteristics
| Variable | N = | n (%) |
|---|---|---|
| Degree of up-front surgical resection | 89 | |
| GTR/NTR | 40 (45) | |
| Less than NTR | 49 (55) | |
| Radiation prior to relapse | 71 | |
| Yes | 69 (97) | |
| No | 2 (3) | |
| Radiation type | 26 | |
| Craniospinal | 10 (38) | |
| Focal | 14 (54) | |
| No radiation | 2 (8) | |
| Chemotherapy prior to relapse | 72 | |
| Yes | 69 (96) | |
| No | 3 (4) | |
| Chemotherapy type | 31 | |
| Temozolomide-based | 20 (64) | |
| Non-temozolomide based | 11 (36) | |
| Site of relapse | 19 | |
| Local | 13 (69) | |
| Distant | 1 (5) | |
| Combined | 5 (26) | |
| Relapse | 93 | |
| Yes | 83 (89) | |
| No | 10 (11) | |
| Time to progression (months) | 83 | |
| Median (IQR) | 10.0 (6.0–18.0) | |
| Time from progression to death (months) | 51 | |
| Median (IQR) | 5.0 (3.0–11.7) | |
| Vital status | 131 | |
| Alive | 38 (29) | |
| Dead | 93 (71) |
Abbreviations: GTR, gross total resection; NTR, near total resection; IQR, interquartile range.
At time of study publications, 93 patients had data reported on disease progression where 83 (89%) experienced progression and 10 (11%) did not. Relapse occurred locally in 13 patients (69%), while 5 (26%) experienced both local and distant relapse and 1 (5%) experiencing only distant relapse. Fifty-one patients who experienced disease progression offered data regarding TTP with a median of 10.0 months (IQR: 6.0–18.0). Of the 131 patients with known survival status in accordance with their last follow-up, 93 patients (71%) had died due to disease, while 38 (29%) were alive at last follow up without known disease status. Of the patients who were alive at last follow up, 37 had data pertaining to the time from diagnosis to follow up, with a median of 22.0 months (IQR: 14–31). For those with both progression and survival data (n = 42), the median time from progression to death was 5.0 months (IQR: 3.0–11.7).
Survival and Associated Variables
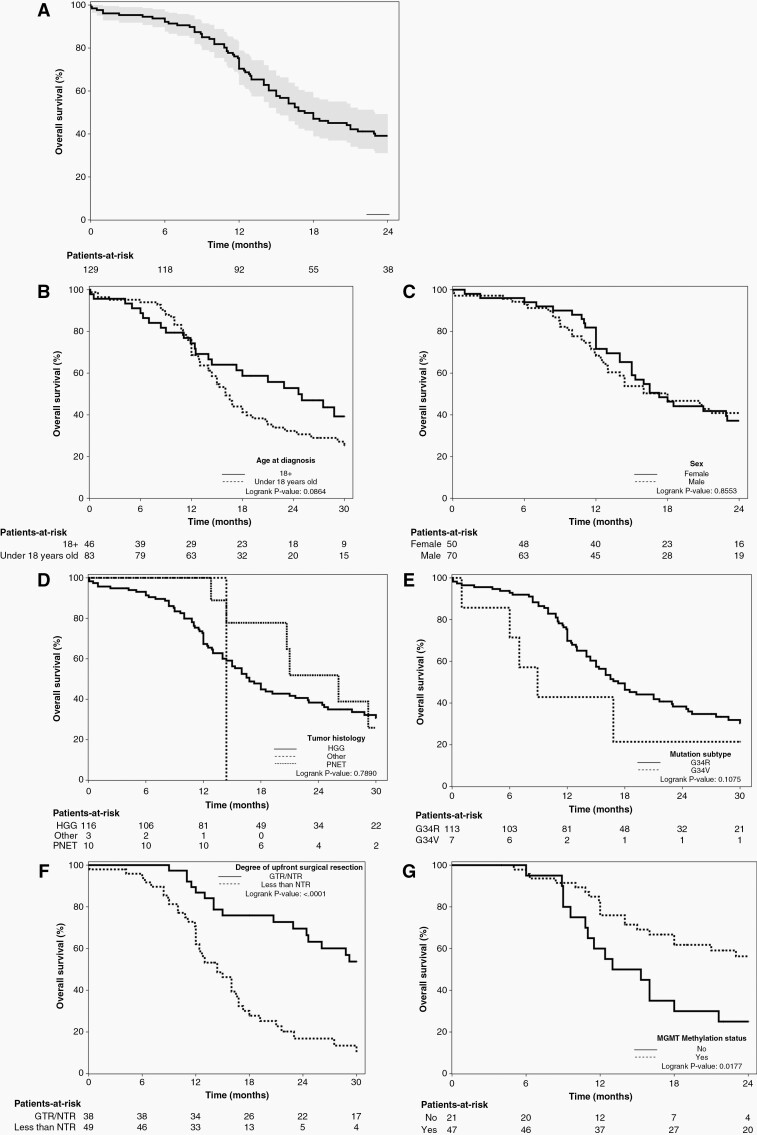
The 1-, 2-, and 3-year survival estimates were found to be 70.4 (95% CI: 62.1 to 78.0)%, 39.1 (95% CI: 30.4 to 48.3)%, and 20.8 (95% CI: 12.9 to 30.0)%, respectively. Five-year survival estimates were not reported as less than 5% of patients had follow-ups longer than 5 years.17 As shown in Figure 2A, Kaplan-Meier survival plots were generated to obtain survival estimates where the median survival time for patients diagnosed with H3 G34-mutant tumors was 17.3 months (95% CI: 15.0 to 22.9). Kaplan-Meier plots using log-rank tests are also shown for age, sex, initial tumor histology, tumor mutation subtypes, degree of up-front surgical resection, and presence of MGMT methylation (Figure 2B–G). Results generated from the univariable Cox model with random cluster effects in Table 3 demonstrate survival is worse with age <18 years old (hazard ratio [HR] = 2.05, 95% CI: 1.16 to 3.62), and degree of up-front surgical resection <NTR (HR = 3.75, 95% CI: 2.11 to 6.62). Patient sex, tumor histology, and histone point mutation type were not associated with survival. Assessment for age, <18 vs ≥18 years of age, showed a cluster random effect P-value of .0315, demonstrating significant variability across studies for this variable. Remaining variables had non-significant P-values, demonstrating no statistically significant evidence of heterogeneity across studies.
Figure 2.
Kaplan-Meier survival plots demonstrating overall survival. (A) Entire cohort with 95% CI. (B) Age: <18 y/o versus ≥ 18 y/o. (C) Sex: female versus male. (D) Tumour histology: HGG versus PNET versus other. (E) Tumor mutation: G34R versus G34V. (F) Degree of up-front surgical resection: <NTR versus GTR/NTR. (G) Presence of MGMT promotor methylation.
Table 3.
Univariate Cox Regression for Clinical Factors Influencing Survival
| Variable | Hazard Ratio (95% CI) | P-Value | Cluster Random Effect |
|---|---|---|---|
| P-Value | |||
| Age | |||
| 10-year increase in age (continuous) | 0.714 (0.512–0.995) | .0369 | .0715 |
| Age | |||
| < 18 y/o vs ≥18 y/o | 2.053 (1.16–3.623) | .0086 | .0315 |
| Sex | |||
| Female vs male | 0.971 (0.623–1.513) | .8858 | .1394 |
| Tumor histology | |||
| HGG vs PNET | 1.279 (0.509–3.216) | .6005 | .1064 |
| Other vs PNET | 1.562 (0.173–14.147) | .6914 | |
| Mutation | |||
| G34R vs G34V | 0.440 (0.164–1.185) | .0874 | .1011 |
| Degree up-front surgery | |||
| <NTR vs GTR/NTR | 3.745 (2.114–6.623) | <.001 | .2439 |
Abbreviations: HGG, high-grade glioma; PNET, primitive neuro-ectodermal tumor; GTR, gross total resection; NTR, near total resection.
Discussion
There is currently no standard of care for patients with G34-DHG and our understanding of outcome measures and influencing variables is limited. This systematic review represents a comprehensive assessment of outcome measures, clinical factors, and their influence on survival for 135 patients affected by G34-DHG available in the literature. Our data illustrates the heterogeneity in treatment approaches and the poor prognosis of G34-DHG. Patients with G34-DHG demonstrated a slightly longer OS when compared to the median survival of 15 months for wildtype IDH1 GBM and the median survival of 8–13 months for diffuse midline gliomas (DMGs) harboring p.K27M mutations.18–22 However, nearly 40% of patients with G34-DHG can live more than 2-years compared to approximately 10% of patients with DMGs.22 Concerning disease progression, the median time to disease progression was brisk at 10.0 months (IQR: 6.0–18.0) with a surprisingly high frequency of patients noted to have distant relapse sites (26%). Distant relapses might be overrepresented in our cohort due to missing items and case selection bias for those chosen to be published, but importantly highlights that this disease has the ability to have distant relapse and further robust investigation of relapse patterns is needed. Of note, the date of disease progression indicating data on progression was widely underreported, highlighting the need for better data on progression in future research. It was found that the time from disease progression to death was short with a median of only 5.0 months (IQR: 3.0–11.7), and the vast majority ultimately dying from the disease. The longer survival observed in G34-DHG compared to DMG is likely in part due to the location where surgical resections are possible and about half of G34-DHG were reported to have an NTR or GTR in our review. Our results show that patients who were able to have GTR/NTR during up-front surgical resection had improved OS compared to those who had less than NTR (P < .001). Previous trials of pediatric HGGs also demonstrate that greater initial resections result in better event-free survival and OS.23 However, what constitutes a GTR or NTR was seldom defined in the included studies. Thus, standardized guidelines to designate degree of resection in these tumors to allow for comparative analysis across different studies are also needed.24
Our review demonstrated a wide range of ages affected by G34-DHG. We report a median age of diagnosis of 15.8 years, with an age range of 7 to 66 years old. Given the demographic of patients affected by this disease, it is important to acknowledge the role that age plays in their OS. We found that as age at diagnosis increases, so does the OS of the patient (P = .037). Patients diagnosed with a G34-DHG before the age of 18 years old were found to exhibit a poorer OS compared to those diagnosed after the age of 18 years old (P = .0086). The reason for this is unknown and requires further assessment. One possibility is the difference in adult compared to pediatric management, where adult institutions treat more patients with HGGs. Another possibility is biology may vary depending on age. Similar findings are seen in older patients with diffuse intrinsic pontine glioma suspected due to age-related variations in tumor biology.25 Though age was found to have a significant cluster random effect P-value, this is likely due to the nature of some studies exclusively including pediatric patients, while others included adults only.
Notably, other clinical factors like sex, tumor histology, and H3 G34 mutational subtype did not statistically influence OS of patients. However, the number of patients with H3 G34V tumors is small occurring in 6% of our cohort. Therefore, further study as to whether this point mutation has prognostic value is needed.
The influence of treatment strategies on OS, such as chemotherapy regimen and radiotherapy, were difficult to analyze as almost all patients described in this study underwent initial chemotherapy and radiotherapy prior to relapse with limited information regarding the specifics of these treatments. There was no statistically significant difference found regarding OS for temozolomide-based chemotherapy compared to non-temozolomide-based, as well as focal compared to craniospinal radiotherapy, although substantial missing and incomplete data hampered robust analysis.
In addition to clinical outcomes, we aimed to summarize the common radiological features associated with G34-DHG. In the studies included in this review, tumor location was not well characterized and was most often described as affecting multiple lobes and/or structures, or tumors were noted as affecting the cerebrum, not otherwise specified. Where literature documented tumor location, G34-DHGs were most often located in the frontal lobe of the brain and more often in the left hemisphere. Interestingly, our review describes a small number of G34-DHGs impacting midline structures, including G34-DHGs with co-occurring H3 K27M mutations, with both alterations having been confirmed via sequencing, suggesting importance of tumor location.26,27 Also notable, this study captured patients who presented with metastatic disease at diagnosis, demonstrating that this tumor is not restricted to localized disease and full imaging of the neuraxis at diagnosis should be considered. Other radiological features at diagnosis such as contrast enhancement and diffusion restriction were poorly represented in the included literature. When described, however, they often had evidence of diffusion restriction while only half had evidence of significant contrast enhancement. The substantial portion of G34-DHGs lacking significant contrast enhancement is perhaps not surprising given similar findings seen in other histone mutant gliomas such as DMG, and also in subset of previously reported G34-DHGs.28,29 Our data on contrast enhancement and diffusion restriction variables are consistent with a recent review on imaging features of G34-DHG.30
This study describes several important molecular co-alterations. MGMT promoter methylation occurred in 70% of tumors tested and though its presence demonstrated a more favorable median survival from Kaplan Meier analysis, this result although clinically meaningful remains descriptive as over 20% of patients were missing this MGMT data in our review. MGMT promoter methylation has a favorable prognosis in adult HGGs, but its use as a biomarker in pediatrics remains controversial.31,32 Studies suggest that MGMT promoter methylation can predict a beneficial response to temozolomide chemotherapy treatment in older patients.33,34TP53 and ATRX mutations are key players in the epigenetic dysregulation and pathogenesis G34-DHG, and were the most commonly described co-mutations. Aside from these common mutations, a study investigating activating mutations of the PDGFRA gene occurs at a high frequency, with co-occurrence of G34R/V-mutant tumors and PDGFRA gene mutations being 7- to 8-fold higher than K27M DMGs, IDH-mutant, and H3/IDH-wildtype HGGs.5PDGFRA mutations can act as a glioma driver overtaking the pathogenic role of G34R/V, likely due to downstream activation of mitogen-activated protein kinase signaling.5 It has been found that alterations in the PDGFRA gene might indicate poor prognosis and potentially represent a therapeutic target for patients with G34-DHG.14 One study exploring biomarkers of survival in 15 patients with G34-DHG demonstrated that patients exhibiting PDGFRA mutations tended to exhibit shorter OS, while those that had MUC16 alterations might have a more favorable survival, although patient numbers were limited and neither result was statistically significant.14 Our study did not assess outcomes based on tumor mutational burden where hypermutation has been reported in some patients with G34-DHG at the time of relapse.8 Tumor mutational burden might also be an important biomarker for future assessment on outcome and treatment.
A study recently published by Vuong et al. aimed to highlight the role of genetic events and molecular alterations in prognosis for G34-DHG.35 Our studies demonstrated different median OS with theirs being slightly lower at 14.4 months compared to 17.3 months in our study. Of note, their study also determined that G34V-mutant tumors had significantly worse OS when compared to G34R-mutant tumors. However, our study had determined no difference in survival when comparing the two types of G34-DHG subtypes. Given very few patients with G34V-mutant tumors, in both our study and the Vuong et al. study, we urge caution in making conclusions about differences in survival outcomes based on current available data for this variable. Also, their study demonstrated the role of EGFR amplification on reducing patient survival, not assessed in our study, and should be further evaluated.35
Our systematic review has several limitations. First, many of the studies included in our review are case reports and series, which are often criticized for presenting non-generalizable findings. However, considering the rarity G34-DHG, and resulting scarce literature, these important studies represent the bulk of available clinical data making them a useful starting point for clinical consideration and outcome evaluations. Another limitation is the quantity of missing data for many variables. Therefore, to minimize this limitation in analyses and meaningfully assess their impact on survival, we limited our analysis to variables with less than 20% missing data. These missing data points may also lead to inaccuracies in the represented prevalence for some of the described clinical features. The quantity of missing data, alongside the heterogenous nature of the data, restricted any reliable multivariate analyses. Though this study review intended to capture other important clinical variables, such as relapse treatment strategies, there was insufficient data for appropriate data description. This again only highlights the need for large-scale collaborative clinical research for this disease. Lastly, heterogeneity in study design and reporting is a limitation for this review although attempts to abrogate this was undertaken in survival analysis using a cluster random effects assessment.
This systematic review highlights the poor prognosis of patients with G34-DHG, common clinical and treatment characteristics, and features associated with survival, while also providing a critical baseline for future outcome measures and clinical trials. Improved survival duration was associated with both increasing ages at diagnosis and extent of up-front surgical resection. This review highlights the paucity of data available to study biomarkers in G34-DHG, and further clinical research is urgently needed for G34-DHG to begin improving outcomes and to define relevant biomarkers that stratify clinical behavior.
Supplementary Material
Acknowledgments
We would like to thank librarian Gwendolyn MacNairn for her help in the development of our search strategy, as well as Conrad Fernandez for his editorial support.
Contributor Information
Cameron Crowell, Division of Hematology and Oncology, IWK Health Centre, Halifax, Nova Scotia, Canada; Faculty of Science, Dalhousie University, Halifax, Nova Scotia, Canada.
Daddy Mata-Mbemba, Department of Diagnostic Imaging, IWK Health Centre, Halifax, Nova Scotia, Canada.
Julie Bennett, Division of Hematology and Oncology, The Hospital for Sick Children, Toronto, Ontario, Canada.
Kara Matheson, Research Methods Unit, Nova Scotia Health Authority, Halifax, Nova Scotia, Canada.
Michael Mackley, Division of Clinical and Metabolic Genetics, The Hospital for Sick Children, Toronto, Ontario, Canada.
Sébastien Perreault, Department of Pediatrics, Centre Hospitalier Universitaire de Sainte-Justine , Montreal, Quebec, Canada.
Craig Erker, Division of Hematology and Oncology, IWK Health Centre, Halifax, Nova Scotia, Canada; Department of Pediatrics, Dalhousie University, Halifax, Nova Scotia, Canada.
Funding
This project was funded through an IWK Establishment Grant from the IWK Health Centre (Grant Award #1026354 to C.E.)
Conflict of Interest
There are no conflicts of interest to disclose.
Authorship
Study design: All authors; Data collection: C.C., C.E.; Data analysis: C.C., K.M., C.E.; Manuscript writing: All authors; Final manuscript approval: All authors.
Prior Presentation
This project was selected for presentation at the 20th International Symposium on Pediatric Neuro-Oncology. This project was submitted in partial requirement for an undergraduate honors degree at Dalhousie University by C.C.
References
- 1. Schwartzentruber J, Korshunov A, Liu XY, et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482(7384):226–231. [DOI] [PubMed] [Google Scholar]
- 2. Wu G, Broniscer A, McEachron TA, et al. Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet. 2012;44(3):251–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Picart T, Barritault M, Poncet D, et al. Characteristics of diffuse hemispheric gliomas, H3 G34-mutant in adults. Neurooncol Adv. 2021;3(1):vdab061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haase S, Nunez FM, Gauss JC, et al. Hemispherical pediatric high-grade glioma: molecular basis and therapeutic opportunities. Int J Mol Sci. 2020;21(24):9654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen CCL, Deshmukh S, Jessa S, et al. Histone H3.3G34-Mutant interneuron progenitors co-opt PDGFRA for gliomagenesis. Cell. 2020;183(6):1617–1633.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sturm D, Witt H, Hovestadt V, et al. Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell. 2012;22(4):425–437. [DOI] [PubMed] [Google Scholar]
- 7. Korshunov A, Capper D, Reuss D, et al. Histologically distinct neuroepithelial tumors with histone 3 G34 mutation are molecularly similar and comprise a single nosologic entity. Acta Neuropathol. 2016;131(1):137–146. [DOI] [PubMed] [Google Scholar]
- 8. Wood MD, Neff T, Nickerson JP, et al. Post-treatment hypermutation in a recurrent diffuse glioma with H3.3 p.G34 Mutation. Neuropathol Appl Neurobiol. 2021;47(3):460–463. [DOI] [PubMed] [Google Scholar]
- 9. Lim KY, Won JK, Park CK, et al. H3 G34-mutant high-grade glioma. Brain Tumor Pathol. 2021;38(1):4–13. [DOI] [PubMed] [Google Scholar]
- 10. Crowell C, Erker C. A Systematic review of diffuse hemispheric Glioma, H3 G34-mutant and clinical factors influencing outcomes. PROSPERO 2021 CRD42021267764. Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021267764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Covidence Systematic Review Software. Melbourne, Australia: Veritas Health Innovation. Available from www.covidence.org. [Google Scholar]
- 13. Moola S, Munn Z, Tufanaru C, et al. Systematic reviews of etiology and risk. In: Joanna Briggs Institute Reviewer’s Manual. Adelaide, Australia: The Joanna Briggs Institute; 2017. [Google Scholar]
- 14. Hu W, Duan H, Zhong S, Zeng J, Mou Y. High frequency of PDGFRA and MUC family gene mutations in diffuse hemispheric glioma, H3 G34-mutant: a glimmer of hope? J Transl Med. 2022;20(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Onishi S, Amatya VJ, Karlowee V, et al. Radiological and Immunostaining Characteristics of H3.3 G34R-Mutant Glioma: a report of 3 cases and review of the literature. Pediatr Neurosurg. 2020;55(5):319–325. [DOI] [PubMed] [Google Scholar]
- 16. Lee EW, Wei L, Amato DA, Leurgans S. Cox-type regression analysis for large numbers of small groups of correlated failure time observations. In Survival Analysis: State of the Art. Dordrecht, Netherlands: Springer; 1992:237–247. [Google Scholar]
- 17. Pocock SJ, Clayton TC, Altman DG. Survival plots of time-to-event outcomes in clinical trials: good practice and pitfalls. Lancet. 2002;359(9318):1686–1689. [DOI] [PubMed] [Google Scholar]
- 18. Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang Y, Feng LL, Ji PG, et al. Clinical features and molecular markers on diffuse midline gliomas with H3K27M mutations: a 43 cases retrospective cohort study. Front Oncol. 2020;10:602553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang L, Li Z, Zhang M, et al. H3 K27M-mutant diffuse midline gliomas in different anatomical locations. Hum Pathol. 2018;78:89–96. [DOI] [PubMed] [Google Scholar]
- 21. Hassan U, Latif M, Yousaf I, et al. Morphological spectrum and survival analysis of diffuse midline glioma with H3K27M mutation. Cureus 2021;13(8):e17267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hoffman LM, Veldhuijzen van Zanten SEM, Colditz N, et al. Clinical, radiologic, pathologic, and molecular characteristics of long-term survivors of Diffuse Intrinsic Pontine Glioma (DIPG): a collaborative report from the international and European society for pediatric oncology DIPG registries. J Clin Oncol. 2018;36(19):1963–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jakacki RI, Cohen KJ, Buxton A, et al. Phase 2 study of concurrent radiotherapy and temozolomide followed by temozolomide and lomustine in the treatment of children with high-grade glioma: a report of the Children’s Oncology Group ACNS0423 study. Neuro Oncol. 2016;18(10):1442–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Karschnia P, Vogelbaum MA, van den Bent M, et al. Evidence-based recommendations on categories for extent of resection in diffuse glioma. Eur J Cancer. 2021;149:23–33. [DOI] [PubMed] [Google Scholar]
- 25. Erker C, Lane A, Chaney B, et al. Characteristics of patients >/=10 years of age with diffuse intrinsic pontine glioma: a report from the International DIPG/DMG Registry. Neuro Oncol. 2022;24(1):141–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kleinschmidt-DeMasters BK, Mulcahy Levy JM. H3 K27M-mutant gliomas in adults vs. children share similar histological features and adverse prognosis. Clin Neuropathol. 2018;37(2):53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Morris M, Driscoll M, Henson JW, et al. Low-Grade gemistocytic morphology in H3 G34R-Mutant gliomas and concurrent K27M mutation: clinicopathologic findings. J Neuropathol Exp Neurol. 2020;79(10):1038–1043. [DOI] [PubMed] [Google Scholar]
- 28. Puntonet J, Dangouloff-Ros V, Saffroy R, et al. Historadiological correlations in high-grade glioma with the histone 3.3 G34R mutation. J Neuroradiol. 2018;45(5):316–322. [DOI] [PubMed] [Google Scholar]
- 29. Leach JL, Roebker J, Schafer A, et al. MR imaging features of diffuse intrinsic pontine glioma and relationship to overall survival: report from the International DIPG Registry. Neuro Oncol. 2020;22(11):1647–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kurokawa R, Baba A, Kurokawa M, et al. Neuroimaging features of diffuse hemispheric glioma, H3 G34-mutant: a case series and systematic review. J Neuroimaging. 2022;32(1):17–27. [DOI] [PubMed] [Google Scholar]
- 31. Donson AM, Addo-Yobo SO, Handler MH, Gore L, Foreman NK. MGMT promoter methylation correlates with survival benefit and sensitivity to temozolomide in pediatric glioblastoma. Pediatr Blood Cancer. 2007;48(4):403–407. [DOI] [PubMed] [Google Scholar]
- 32. Lee JY, Park CK, Park SH, et al. MGMT promoter gene methylation in pediatric glioblastoma: analysis using MS-MLPA. Childs Nerv Syst. 2011;27(11):1877–1883. [DOI] [PubMed] [Google Scholar]
- 33. Zhao YH, Wang ZF, Cao CJ, et al. The clinical significance of O6-methylguanine-DNA methyltransferase promoter methylation status in adult patients with glioblastoma: a meta-analysis. Front Neurol. 2018;9:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 35. Vuong HG, Le HT, Dunn IF. The prognostic significance of further genotyping H3G34 diffuse hemispheric gliomas. Cancer. 2022;128(10):1907–1912. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.