Abstract
Lassa virus (LASV) is endemic in the rodent populations of Sierra Leone, Nigeria and other countries in West Africa. Spillover to humans occurs frequently and results in Lassa fever, a viral haemorrhagic fever (VHF) associated with a high case fatality rate. Despite advances, fundamental gaps in knowledge of the immunology, epidemiology, ecology and pathogenesis of Lassa fever persist. More frequent outbreaks, the potential for further geographic expansion of Mastomys natalensis and other rodent reservoirs, the ease of procurement and possible use and weaponization of LASV, the frequent importation of LASV to North America and Europe, and the emergence of novel LASV strains in densely populated West Africa have driven new initiatives to develop countermeasures for LASV. Although promising candidates are being evaluated, as yet there are no approved vaccines or therapeutics for human use. This Review discusses the virology of LASV, the clinical course of Lassa fever and the progress towards developing medical countermeasures.
Subject terms: Viral infection, Arenaviruses
Lassa fever is a viral haemorrhagic fever that spills over from its rodent reservoir. Continued viral evolution and increasing exposure make Lassa virus a high-risk pathogen. In this Review, Garry highlights new insights into the virology, disease presentation and potential countermeasures.
Introduction
Lassa fever is an often-fatal viral haemorrhagic fever (VHF) caused by Lassa virus (LASV). Genetic dating suggests that zoonotic transfers of LASV to humans have been occurring for centuries1. However, it was not until 1969 that the first patient with Lassa fever was described. That patient was a missionary nurse infected while working in a rural clinic near Lassa, Nigeria2 (Fig. 1). She died after being transported to a hospital in the city of Jos more than 600 km away. In Jos, two additional nurses were infected and died. A third nurse, Lily (Penny) Pinneo, survived infection after being transported to New York City3. Blood samples from the infected nurses were analysed at Yale University, where LASV was first isolated. The isolate from Pinneo was designated as the type strain. Two Yale researchers were infected with LASV during these initial studies. Tragically, one of these infections was fatal. The surviving virologist Dr Jordi Casals received blood donated from Pinneo as passive immunotherapy4–6. These early experiences with LASV provided incentives for developing improved measures to study emerging pathogens, including construction of high-biocontainment laboratories operating under stringent regulations and oversight.
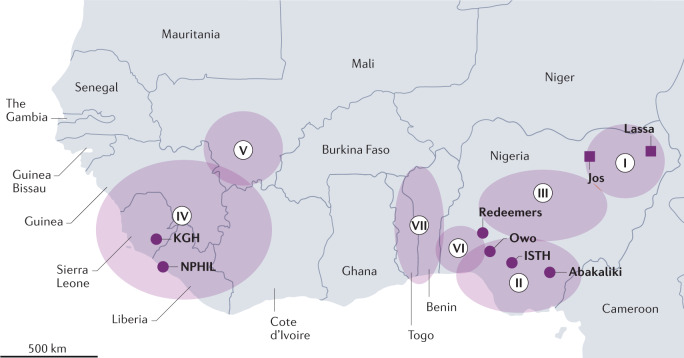
Fig. 1. Lassa fever endemic zone of West Africa.
The first described cases (squares) of Lassa fever were in the town of Lassa, Nigeria. The infected persons were transported to Jos. Seven lineages (I–VII) of Lassa virus (LASV) are present across West Africa. Important centres for Lassa fever research (circles) are located at the Kenema Government Hospital (KGH), the National Public Health Institute of Liberia (NPHIL), the Irrua Specialist Teaching Hospital (ISTH), Owo and Abakaliki. The African Center of Excellence for the Genetics of Infectious Diseases (ACEGID) is located at Redeemers University. The circles with Roman numerals I–VII represent the approximate ranges of the seven different LASV lineages.
Investigation of a 1972 outbreak of Lassa fever in the Eastern Province of Sierra Leone led to the finding that the peri-domestic rodent Mastomys natalensis is the major reservoir of LASV7 (Fig. 2). LASV is transmitted vertically to the offspring of infected rodents and horizontally to humans and other rodents8,9. Lassa fever is endemic in the Mastomys populations of Nigeria, Sierra Leone, Guinea and Liberia. Infected rodents or sporadic human cases have been reported in other West African countries, including Mali, Togo and Benin10,11. The endemic range of LASV appears to be increasing12.
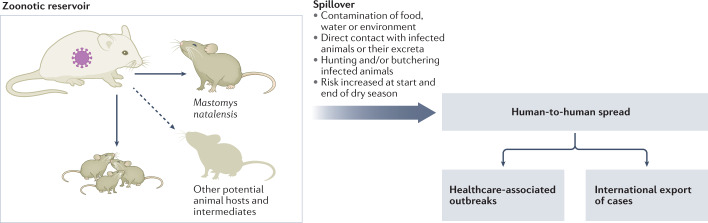
Fig. 2. Lassa virus transmission.
The major reservoir of Lassa virus (LASV) is Mastomys natalensis. LASV spreads among Mastomys via horizontal or vertical (congenital) routes. Other animal species can also be infected with LASV. Spillover of LASV occurs by exposure to excretions of Mastomys or intermediate hosts, or during preparation of infected animals for food. Human-to-human transmission can occur in the home or clinical setting.
Although animal models for Lassa fever have been available since the 1980s, specific and effective countermeasures have not yet been approved for use13,14. Currently, the only available treatment is off-label use of the nucleoside drug ribavirin15. Progress on managing Lassa fever was further slowed by a lengthy civil conflict in Sierra Leone (1991–2002) and, later, a large outbreak of Ebola virus disease, another severe VHF, in the Lassa fever zone (2013–2016)16. More recently, progress has been made towards development of an immunotherapeutic and small-molecule drugs17–20. Although there is no approved Lassa fever vaccine, new initiatives including large-scale epidemiology studies to address this urgent public health issue are now underway21.
Structural biology and replication cycle
The small (80–200 nm) enveloped LASV virion appears to be filled with grains of sand. The grains are host ribosomes and their role, if any, is unknown22 (Fig. 3). Their presence led to the name Arenaviridae from arenos (Latin for sandy) for the virus family that includes LASV and other important human pathogens such as lymphocytic choriomeningitis virus23. Except for the unexplained inclusion of ribosomes, LASV appears to be a minimalist. Each virion contains two single-stranded RNA segments and each segment encodes two proteins24. The four proteins encoded by LASV are multifunctional. The nucleoprotein (NP) encapsulates the viral genome segments and is essential for both transcription of viral mRNAs and replication of genome segments for incorporation into progeny virions25–27. The glycoprotein complex (GPC) mediates viral attachment and cell entry28. The Large (L) protein is an RNA polymerase involved in transcription and replication29,30. L protein has additional functions such as cap-snatching. The Zinc-binding (Z) protein serves as the matrix protein and is sufficient for viral assembly and budding31. Z protein negatively regulates viral replication and transcription and is important for the suppression of both viral and host cell translation.
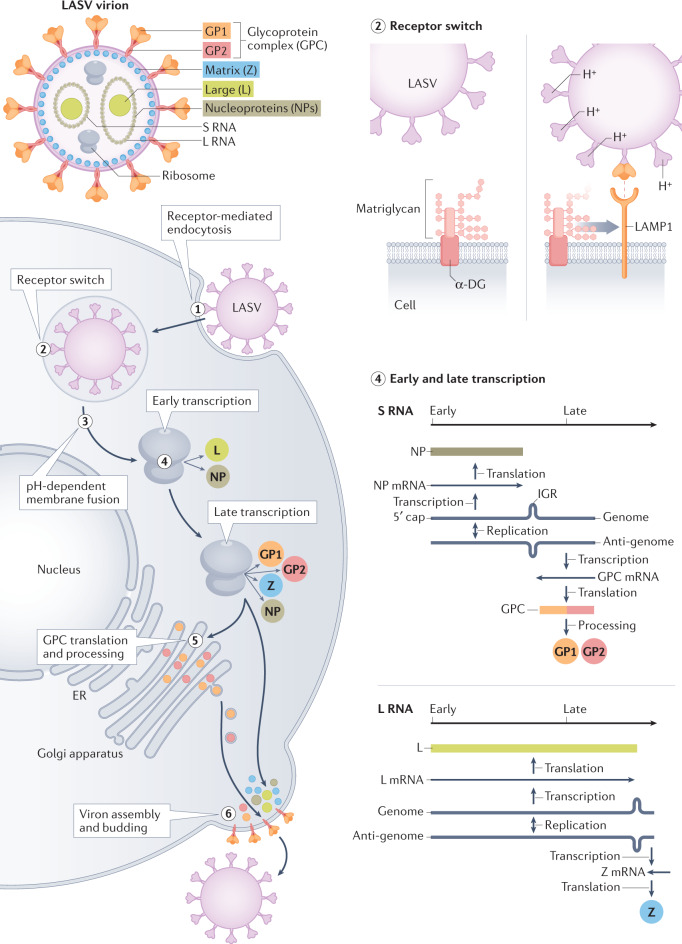
Fig. 3. Lassa virus structure, genome organization and replication strategy.
The Lassa virus (LASV) virion contains host ribosomes and two segments of single-stranded RNA that encode four proteins. Steps in LASV replication include: LASV enters cells via receptor-mediated endocytosis (step 1); LASV binds α-dystroglycan (α-DG) at the cell surface — as the endosomal pH drops, the LASV glycoprotein complex (GPC) undergoes a conformational shift that enables binding to lysosomal-associated membrane protein 1 (LAMP1) (step 2); LASV undergoes pH-dependent membrane fusion releasing the viral genome segments into the cytoplasm (step 3); both segments use an ambisense strategy — early transcription results in the synthesis of the Large (L) protein, an RNA-dependent RNA polymerase and the nucleoprotein (NP), and late transcription additionally involves synthesis of the GPC and the Zinc-binding (Z) protein (step 4); GPC translation and post-translational processing occur in the Golgi apparatus and result in association of GPC with the plasma membrane (step 5); and LASV acquires its membrane by budding at the cell surface (step 6). ER, endoplasmic reticulum; GP1, glycoprotein 1; IGR, inter-gene region; L RNA, large RNA; S RNA, small RNA.
The primary cell surface receptor for LASV entry is matriglycan, an extended oligosaccharide located on a widely distributed peripheral membrane protein named α-dystroglycan (α-DG)32–37 (Fig. 3). After binding, LASV virions are internalized via endocytosis. GPC responds to the acidic endosomal environment by undergoing a conformational shift termed ‘GPC priming’, which causes its dissociation from α-DG. Primed GPC binds to an endosomal receptor, lysosomal-associated membrane protein 1 (LAMP1)38,39. Following LAMP1 binding, GPC undergoes additional conformational changes that mediate virus–endosomal membrane fusion and enable release of the LASV genome segments into the cytosol40.
Both LASV genome segments are ambisense, meaning that one portion is positive sense, the same sense as mRNA, and another portion is negative sense, the complement of mRNA41 (Fig. 3). A stem–loop structure between the positive and negative sense genes terminates the bidirectional transcription. The ambisense strategy aids in regulating LASV gene expression and genome replication. The endonuclease activity of L protein cleaves the 5′ ends of cellular mRNAs, which then prime synthesis of mRNAs transcribed using the negative sense portions of the small and large genome segments as templates42. Thus, the first viral proteins made are NP and L protein. Together, newly synthesized NP and L protein enable synthesis of complementary strands of the genome segments referred to as antigenomes, via RNA-dependent RNA polymerization. The antigenomes then serve as templates for mRNAs encoding the GPC precursor and Z protein. The antigenomes also serve as templates for production of more genome segments that are incorporated into progeny virions.
More than 30 glycans are added to the LASV GPC trimer43. The host cell subtilase SKI-1/S1P proteolytically processes GPC into its components, receptor-binding glycoprotein 1 (GP1), GP2, which is a class I membrane fusion protein, and a myristoylated stable signal peptide (SSP)44. NP encases the viral genome via its amino-terminal domain27. Cleaved glycoproteins are incorporated into the virion envelope. Z protein controls the packing and budding of infectious particles45. NP, Z protein, L protein and the genomic RNA are assembled and released from the cell membrane.
Genetics and epidemiology of Lassa fever
An analysis of LASV using the tools of genetic epidemiology suggests a likely origin in Nigeria more than a thousand years ago1. LASV spread into neighbouring West African countries over the past several hundred years and has undergone substantial genetic divergence (Fig. 1). Many of the mutations have accumulated in epitopes of viral surface proteins, suggesting selection for immune escape, most likely in the rodent reservoirs. Three distinct lineages are found in Nigeria, with the first lineage from the north-east designated lineage I. Lineages II and III are commonly found in the south and central regions, respectively46. Lineage IV is present in Sierra Leone, Guinea and Liberia47. Both the spread and the diversification of LASV appear to be ongoing. In 2015, Nigeria reported a greater incidence of Lassa fever than had previously been reported48. This trend has continued49–53. New LASV lineages, termed lineages V, VI and VII, have emerged in Mali, Côte d’Ivoire, Nigeria, Benin and Togo54,55.
The initial symptoms of Lassa fever are similar to other febrile illnesses, often leading to misdiagnoses as common endemic illnesses such as malaria56. Most persons infected with LASV in West Africa never see health professionals due to limited access to medical care. The true incidence of Lassa fever has not been determined, in part because of the general lack of available diagnostic assays. Extrapolations from prior limited serological studies suggested that there may be as many as 500,000 LASV infections and 5,000 deaths per year in West Africa57,58. Recent imported cases document that Lassa fever is also a threat to non-endemic countries59. Confirmed human-to-human transmission occurred in Germany in 2018 (ref.60), two medical personnel were infected with LASV will working in Sierra Leone in 2019 and were evacuated to The Netherlands61 and, recently, a person contracted Lassa fever in Mali and then transmitted it to family members in the United Kingdom with a fatal fetal outcome62.
Ecology and spillover of Lassa fever
LASV is a prime example of a zoonotic virus, one that circulates in an animal reservoir, or reservoirs, but is capable of infecting humans. A recent ranking based on host, viral and environmental risk factors determined that LASV poses the highest threat for spillover amongs known pathogens63. The main reservoir of LASV is M. natalensis, a peri-domestic rodent that is abundant across sub-Saharan Africa64 (Fig. 2). Although M. natalensis is broadly distributed throughout sub-Saharan Africa, only populations in West Africa are known to carry LASV. Anti-LASV IgG antibodies and LASV itself have been detected in other rodent species, including common rats (Rattus rattus), providing evidence of occasional animal-to-animal spillover11,20,21. LASV and LASV-like viruses have been isolated in Pygmy mice (Mus baoulei) in Benin and Ghana24,25. These newly sequenced viral genomes do not cluster with genomes of viruses in known LASV lineages, revealing a potentially new lineage.
Knowledge regarding habitats, reproduction and movement of rodents that carry LASV is essential for implementing effective preventative measures against Lassa fever. M. natalensis is the most common rodent in sub-Saharan Africa, and a serious agricultural pest64. Lassa fever has a seasonal distribution, although cases can occur year-round65. In West Africa, the rainy season spans from May to November, whereas the hot and dry season extends from January to March4. There is a distinct increase in Lassa fever cases at the beginning and end of the dry season65. Rodents are nocturnal animals that source food during the night. The dry season drives rodents into homes, where food and water containers can become contaminated with rodent excrement66. LASV can also become aerosolized in dust particles or urine droplets. Upon inhalation or handling the infectious material, the virus can enter through mucous membranes or micro-abrasions. Rodents are also consumed as food, which can lead to infection of the persons trapping or cooking the rodents67. Abatement practices, including keeping household cats, protecting food and water and chemical rodenticides, can help control LASV68.
Lassa fever — a variable clinical course
There are a range of possible outcomes following infection with LASV, from asymptomatic to fatal. Despite a lack of reliable epidemiological data, it is likely that only a minority of infections result in severe disease. It has been estimated that as many as 80% of LASV infections are asymptomatic or mild69. The reasons for the variation in disease severity are unknown, but may be related to differences in LASV strain, route and dose of inoculation, host genetic susceptibility, or comorbid infections or conditions. The period between LASV infection and the initial signs and symptoms is also variable, from 1 to 3 weeks. After 4–7 days of mild illness, an estimated 20% of infected individuals develop symptoms of VHF. Fever, sore throat, vomiting and coughing are common, but each case presentation is distinct57,70,71. Approximately 40% of patients with Lassa fever experience bleeding from the nose, mouth, other orifices and mucosal surfaces, which confers a poor prognosis. Case fatality rates (CFRs) vary among countries. Patients who present to the Lassa fever ward in Kenema, Sierra Leone, have a CFR of 69%65. Lassa CFRs vary by location in Nigeria, but are typically lower at 20–30%50,72–74. However, the 2015–2016 surge in Nigeria was associated with 60% case fatality in clinics75.
Death from Lassa fever generally occurs between 10 and 14 days after symptom onset and is attributed to diminished effective circulating volume, shock and multi-organ system failure71. High LASV load is consistently observed as a significant risk factor for fatal infection. Other significant laboratory findings associated with fatality are elevated liver enzymes (aspartate and alanine transamidases (AST and ALT)), as well as elevated levels of the kidney enzyme creatinine65. Clinical laboratory investigations have shown reduced platelet and white cell counts, increased blood urea and proteinuria in patients with acute Lassa fever. Age older than 45 years is a risk factor for death in Nigeria73, whereas in Sierra Leone more fatal cases occur in children, teenagers or young adults65. Central nervous system manifestations and renal failure are strongly associated with a poor outcome of Lassa fever73. Infection is highly lethal to women in the third trimester of pregnancy76,77. Termination of the pregnancy can improve survival. Lassa fever is usually fatal for the developing fetus. Temporary or permanent unilateral or bilateral deafness has been reported to occur in ~30% of those who survive Lassa fever73,78–80. Other long-term sequelae in survivors include neurological and visual defects81–83. The risk of LASV recrudescence, as is well documented for Ebola virus84,85, has yet to be adequately investigated.
Supportive care is the mainstay for management of patients with Lassa fever. Dialysis in patients with evidence of kidney injury is used frequently in Nigeria for the treatment of Lassa fever73. At present, there are no approved drugs for the treatment of Lassa fever. The nucleoside analogue ribavirin (1-β-d-ribofuranosyl-1,2,4-triazole-3-carboxamide) remains the standard treatment for Lassa fever. Its mode of action is not established even though oral and aerosol formulations have been used for treatment of respiratory syncytial virus and hepatitis C virus infections. The efficacy of ribavirin was observed in an early study in Sierra Leone86. Early administration of ribavirin following infection with LASV appears critical for successful recovery. However, considerable uncertainty remains about ribavirin’s efficacy in Lassa fever87,88.
Immune responses to Lassa virus
Both innate and adaptive immunity play important roles in response to LASV infection (Fig. 4).
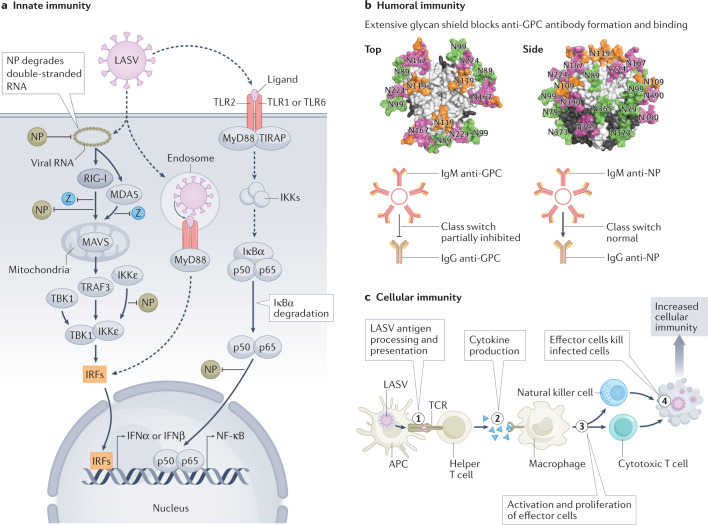
Fig. 4. Immune responses to Lassa virus.
a | Lassa virus (LASV) double-stranded RNA triggers the type I interferon pathway and involves retinoic acid-inducible gene I (RIG-I) and melanoma differentiation-associated protein 5 (MDA5). The exoribonuclease function of nucleoprotein (NP) degrades LASV double-stranded RNA, thereby blunting RIG-I and MDA5 activation. LASV Zinc-binding (Z) protein binds RIG-I and MDA5 and prevents mitochondrial antiviral signalling (MAVS) protein activation of tumour necrosis factor (TNF) receptor-associated factor 3 (TRAF3) and TANK-binding kinase 1 (TBK1), which are also involved in interferon induction via interferon-responsive factors (IRFs). NP binds RIG-I and IκB kinase-ε (IKKε) inhibiting activation of IRFs. NP blocks nuclear factor-κ light-chain enhancer of activated B cells (NF-κB) activation. The NF-κB pathway, which is involved in activating various aspects of innate immunity, is activated by recognition of LASV components via Toll-like receptors (TLRs) and involves Toll-interleukin 1 receptor (TIR) domain containing adaptor protein (TIRAP), myeloid differentiation primary response 88 (MyD88), various IKKs and IκBα. NP interferes with the p65 subunit of NF-κB via an unknown mechanism. In addition to MAVS activating IRFs (via TRAF3/TBK1), MAVS activates NF-κB (via TRAF6/IKK complex) independently of TLR signalling (not shown). Elements of panel a were inspired by ref.131. b | LASV evades humoral immunity by elaborating a dense glycan shield on the glycoprotein complex (GPC) shown modelled onto trimeric LASV GPC [PDB:5VK2]91,113. LASV also blocks or delays class switching from IgM that recognizes GPC to anti-GPC IgG. Class switching of anti-NP IgM to IgG is not affected. c | Antigen presenting cells (APCs), including dendritic cells and macrophages, phagocytose material from LASV-infected cells in local tissues, degrade it and display the resultant peptides (step 1). T cell proliferation requires recognition of the displayed peptides by the T cell receptor (TCR; co-receptor CD28 and co-stimulatory molecule B7 not shown) (step 2). Proliferation of effector cells is dependent on stimulation with cytokines. Direct killing of infected cells by natural killer cells or cytotoxic T cells involves recognition of viral peptide–major histocompatibility complex (MHC) class I complexes (step 3). Effector functions can also involve other immune cells (for example, antibody-dependent cellular cytotoxicity). Humans or experimental animals that fail to develop effective cellular immunity have a higher fatality rate than individuals who develop effective cellular immune responses (step 4).
Innate immunity
The innate immune system is the primary line of defence against invading pathogens. It involves various cell types, including macrophages, neutrophils, dendritic cells, mast cells, eosinophils and natural killer cells. One of the most important innate responses to a virus involves the interferon system. The interferon response in cells can be induced by double-stranded RNA, a by-product of viral transcription and replication. An example of double-stranded RNA in LASV-infected cells would be a complex between a genome and an antigenome (Fig. 4a). The carboxy-terminal domain of NP has similar structure to the DEDD family of exonucleases and functions as a 3′,5′-exoribonuclease that specifically and rapidly digests double-stranded RNA25,26,89. LASV NP and Z protein strongly inhibit human retinoic acid-inducible gene I (RIG-I) and RIG-I-like proteins as well as other pathways to suppress host innate immunity90 (Fig. 4a).
Humoral immunity
Humoral immunity involves production of antibodies that bind with a high degree of specificity to various pathogen components. In some cases, antibody binding to viral surface proteins blocks, and thus neutralizes, virus infectivity. Antibodies can also direct effector functions, such as antibody-dependent cellular cytotoxicity, which kills virus-infected cells. Infected humans develop high levels of antibodies in response to LASV infection; however, those antibodies mostly recognize non-neutralizing epitopes28. Many of the antibodies formed recognize linear epitopes exposed only in the post-fusion conformation of the glycoprotein trimer present after the virus has entered the cell28. Such antibodies cannot block viral infectivity. The numerous glycans on LASV GPC form a dense shield that blocks antibody binding to the glycoprotein trimer, leaving only a few vulnerable areas91 (Fig. 4b). Neutralizing epitopes of LASV require the glycoprotein to be in its prefusion (virion) configuration and usually recognize complex epitopes. LASV neutralizing antibodies, if detected at all, are usually only present after recovery. Most neutralizing antibodies are of the IgG class. LASV interferes with antibody class switching via an unknown mechanism, which limits or delays production of LASV neutralizing antibodies92.
Cellular immunity
The adaptive immune response leads to recognition of specific ‘non-self’ molecules on viruses or other pathogens. It is divided into cellular (T cell) and humoral (B cell) responses. Cytotoxic T cells specifically kill infected cells that express short viral peptides (typically 8–11 amino acids in length) displayed by major histocompatibility complex (MHC) class I molecules. Protective immunity has been correlated with cellular immune responses to LASV, rather than humoral responses93 (Fig. 4c). T cell responses directed to both GPC and NP have been detected. Some T cell epitopes are restricted to certain LASV lineages, and others are pan-lineage94,95. Severe or fatal LASV infection appears to involve low or delayed T cell responses96–98.
Lassa fever medical countermeasures
Because of its alarming CFR, pervasive socio-economic impacts in endemic regions, wide geographic range and frequent importation to other countries, LASV has been targeted for accelerated development of medical countermeasures.
Diagnostics
Because Lassa fever is difficult to recognize clinically, especially in the early stages, prompt laboratory diagnosis is essential to enable the initiation of specific treatments. Laboratory-made and commercial assays are now available, but LASV testing is still confined to central laboratories in West Africa. PCR testing is sensitive and specific, but requires advanced laboratory infrastructure72,99. A qualitative rapid diagnostic test that is visually interpreted by the end user, requiring no instrumentation, is also available but in limited use100,101. In principle, rapid tests could be disseminated to peripheral clinics and laboratories and reliably and safely performed by persons with limited laboratory training. Likewise, LASV antigen-capture and IgM and IgG-capture enzyme-linked immunosorbent assays (ELISAs) using recombinant LASV proteins have been produced and characterized101,102. IgM antibodies appear not to be a reliable indicator of recent LASV infection, as this class of anti-LASV antibody can persist for months or years after infection92.
Therapeutics
Ribavirin is ineffective when administered late in the course of disease after viraemia has peaked and physiological dysregulation has progressed into severe and often irreversible stages86. Although side effects of ribavirin are manageable and acceptable in the life-threatening situation of acute LASV infection, these toxicities preclude the use of the drug to prevent infection. Additional small-molecule drugs are under evaluation to treat Lassa fever20. Favipiravir (T-705), a small-molecule purine analogue103, has greater efficacy than ribavirin in treating LASV infection in several animal models104–109. Two patients with Lassa fever survived after a combination treatment with favipiravir and ribavirin, but viral RNA was detected in their blood and semen for a prolonged period110. The novel drug LHF-535 (Kineta, Seattle, WA, USA), a viral entry inhibitor of LASV, has proven safe in a phase Ia human clinical trial. It is an enhanced analogue of ST-193 (a benzimidazole derivative; SIGA, Corvallis, OR, USA) and acts to inhibit LASV entry by targeting the envelope glycoprotein of the virus111.
Immunotherapeutics
Although humoral immune responses play a minor role, if any, in recovery from LASV infection, immunotherapy may provide a significant therapeutic benefit. Although neutralizing antibodies against LASV GPC are rare, 16 neutralizing human monoclonal antibodies from survivors — some of whom had long-term, multiple LASV exposures — have been cloned and expressed112. These antibodies have various mechanisms of action, including competition with cellular entry factors and inhibiting fusion of viral and host cell membranes28,43,113,114. A monoclonal antibody cocktail, termed Arevirumab-3 and comprising three neutralizing antibodies, has been extensively evaluated in guinea pigs and non-human primate models of Lassa fever17,18. Arevirumab-3 completely protected non-human primates from lethal Lassa fever even when first administered as late as 8 days post challenge18. Protection has been achieved against challenge with representative viral strains from lineages II, III (Nigeria) and IV (Sierra Leone). Pharmacokinetic studies suggest that Arevirumab-3 should also be evaluated as a prophylactic immunotherapeutic drug for Lassa fever. The high genetic diversity of LASV may be problematic for creating a broadly reactive immunotherapeutic. Antibodies that are highly potent against LASV of lineage IV were ineffective against the divergent lineage I115. Synergistic drug combinations may be needed to achieve therapeutic levels116. Combinations of drugs from different classes and mechanisms of action, such as an immunotherapeutic combined with a small-molecule inhibitor, should be evaluated.
Vaccines
There are currently no LASV vaccines approved for use. However, several vaccine platforms have been developed that show efficacy in animal models, and some of these have recently entered phase I human clinical trials21. Among platforms that have been evaluated as potential LASV vaccines are a ML29 MOPV/LASV live reassortant117, a DNA vaccine118 and recombinant vesicular stomatitis virus119,120, rabies virus121, measles virus122, vaccinia virus123 and adenovirus vectored vaccines124,125. Additional vaccine candidates include LASV virus-like particles126,127 and a virus replicon particle vaccine128.
The road ahead and recommendations
Improved diagnostic assays based on recombinant LASV proteins, and the potential of vaccines and therapeutics in or about to enter human clinical trials, raise the possibility of greatly improving clinical management of Lassa fever and preventing the disease in at-risk populations. Important considerations for LASV vaccines and drugs stem from their use in Africa, where cost, stability and accessibility are key factors. Seroprevalence and incidence studies using recombinant antigen ELISAs and incidence studies based on PCR with reverse transcription of LASV in West Africa are ongoing. Such studies will be essential for conducting clinical trials to determine vaccine or therapeutic efficacy in patients with Lassa fever129. The seroprevalence and incidence studies will also provide important information for managing Lassa fever in endemic countries.
Although development of LASV vaccines and drugs has taken a promising turn, this does not mean that research on potential countermeasures should be suspended or stopped. Multiple vaccines may be required for use in different situations or populations. Travellers to endemic regions may benefit from different vaccines to people living in endemic areas. Short-term efficacy will be a more important consideration for travellers, whereas considerations of long-term durability of protective responses would be more important for people living in the Lassa fever zone. If a vaccine is proven safe and effective, public health experts in endemic countries in consultation with the World Health Organization (WHO) must formulate equitable distribution mechanisms.
Developing effective drugs to treat Lassa fever should continue apace with vaccine development. Combinations of drugs with different mechanisms of action may prove to be more effective than any one drug alone. Furthermore, the pathway to approval of drugs is uncertain and alternative candidates should be available for testing if existing candidates fail. This will be a challenge, in part, because non-human primate resources are becoming increasingly scarce, which will make future pathogenesis studies and critical evaluations of countermeasures more difficult. Investment in broader distribution of LASV diagnostics as well as in non-pharmaceutical interventions should continue. Point-of-care diagnostics are of particular value in remote settings and in hospitals where patients who are acutely ill, including pregnant women, are at high risk for Lassa fever.
The initiation of clinical trials for vaccine and drugs should be seen as an opportunity to further understanding of Lassa fever pathobiology. Clinical markers, such as high virus load and elevated transamidases, can predict outcome. Early markers of developing severe disease or death are not available, but would be useful for patient management. Clinical studies on managing central nervous system manifestations and renal failure could also improve patient outcomes. Further characterization of cell-mediated and humoral immune responses to LASV infection, and identifying immune correlates of survival following infection, can also benefit patient management. Post-Lassa fever syndromes beyond deafness have not been well studied and should be further evaluated in well-defined survivor cohorts.
Rodent control is an effective means to prevent outbreaks of Lassa fever across endemic countries in West Africa, and should continue to be a point of emphasis. Studies are needed to determine best practices for the use of rodenticides that are effective against Mastomys and intermediate hosts of LASV. Likewise, methods to prevent invasions of Mastomys into homes should be tested and optimized. Well-sealed metal roofs and fortified walls potentially provide a barrier to Mastomys that are seeking food or water. Additional studies on the ecology of Mastomys and their interactions with other rodent species, such as Rattus, should also be prioritized. Careful attention to the considerable genetic diversity of this species is important. It will be important to conduct controlled studies on the rodent-to-rodent transmission of LASV, including immune responses and intra-host and inter-host genetic diversity. Although such studies should involve wild-caught animals, establishing breeding colonies of Mastomys will facilitate determining the immunology and molecular genetics of LASV infection.
In addition to its original Lassa fever facility at the Irrua Specialist Teaching Hospital (ISTH), Nigeria has recently added new facilities for LASV basic and clinical research in Owo and Abakaliki (Fig. 1). These centres join the Kenema Government Hospital (KGH) in Sierra Leone and the National Public Health Institute of Liberia (NPHIL) as leading centres for LASV research in West Africa. Strengthening the capacity of these and other African institutions to conduct clinical trials will benefit not only Lassa fever research but also research on other understudied diseases. It is vital to build on progress by the African Center of Excellence for the Genetics of Infectious Diseases (ACEGID) at Redeemers University in Nigeria, which leads viral sequencing efforts in Nigeria. The AGEGID is also training scientists from other African countries and worldwide (Fig. 1). The ability to deeply characterize genetic diversity of LASV is possible with new accessible technologies, such as nanopore sequencing. Steps should be taken to ensure that this capacity becomes available in all endemic countries. To reduce the burden of Lassa fever, government policies that strengthen fragile health systems, including facilitating heath-seeking behaviours, must continue to be implemented across the Lassa fever endemic zone130.
Peer review
Peer review information
Nature Reviews Microbiology thanks Judith White and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Competing interests
R.F.G. is the co-founder of Zalgen Labs, a biotechnology company developing countermeasures for Lassa virus (LASV) and other emerging viruses.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Andersen KG, et al. Clinical sequencing uncovers origins and evolution of Lassa virus. Cell. 2015;162:738–750. doi: 10.1016/j.cell.2015.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pinneo L, Pinneo R. Mystery virus from Lassa. Am. J. Nurs. 1971;71:1352–1355. [PubMed] [Google Scholar]
- 3.Watts GM. Lily Lyman Pinneo. Lancet. 2013;380:1552. doi: 10.1016/S0140-6736(12)61871-6. [DOI] [Google Scholar]
- 4.Leifer E, Gocke DJ, Bourne H. Lassa fever, a new virus disease of man from West Africa. II. Report of a laboratory-acquired infection treated with plasma from a person recently recovered from the disease. Am. J. Trop. Med. Hyg. 1970;19:677–679. doi: 10.4269/ajtmh.1970.19.677. [DOI] [PubMed] [Google Scholar]
- 5.Buckley SM, Casals J, Downs WG. Isolation and antigenic characterization of Lassa virus. Nature. 1970;227:174. doi: 10.1038/227174a0. [DOI] [PubMed] [Google Scholar]
- 6.Buckley SM, Casals J. Lassa fever, a new virus disease of man from West Africa. 3. Isolation and characterization of the virus. Am. J. Trop. Med. Hyg. 1970;19:680–691. doi: 10.4269/ajtmh.1970.19.680. [DOI] [PubMed] [Google Scholar]
- 7.Monath TP, Newhouse VF, Kemp GE, Setzer HW, Cacciapuoti A. Lassa virus isolation from Mastomys natalensis rodents during an epidemic in Sierra Leone. Science. 1974;185:263–265. doi: 10.1126/science.185.4147.263. [DOI] [PubMed] [Google Scholar]
- 8.Fichet-Calvet E, Becker-Ziaja B, Koivogui L, Gunther S. Lassa serology in natural populations of rodents and horizontal transmission. Vector Borne Zoonotic Dis. 2014;14:665–674. doi: 10.1089/vbz.2013.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fichet-Calvet E, Lecompte E, Koivogui L, Daffis S, ter Meulen J. Reproductive characteristics of Mastomys natalensis and Lassa virus prevalence in Guinea, West Africa. Vector Borne Zoonotic Dis. 2008;8:41–48. doi: 10.1089/vbz.2007.0118. [DOI] [PubMed] [Google Scholar]
- 10.Kakaī CG, et al. Improving cross-border preparedness and response: lessons learned from 3 lassa fever outbreaks across Benin, Nigeria, and Togo, 2017–2019. Health Secur. 2020;18:S105–S112. doi: 10.1089/hs.2019.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patassi AA, et al. Emergence of lassa fever disease in northern Togo: report of two cases in Oti district in 2016. Case Rep. Infect. Dis. 2017;2017:8242313. doi: 10.1155/2017/8242313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sogoba N, Feldmann H, Safronetz D. Lassa fever in West Africa: evidence for an expanded region of endemicity. Zoonoses Public Health. 2012;59:43–47. doi: 10.1111/j.1863-2378.2012.01469.x. [DOI] [PubMed] [Google Scholar]
- 13.Jahrling PB, et al. Lassa virus infection of rhesus monkeys: pathogenesis and treatment with ribavirin. J. Infect. Dis. 1980;141:580–589. doi: 10.1093/infdis/141.5.580. [DOI] [PubMed] [Google Scholar]
- 14.Peters CJ, et al. Experimental studies of arenaviral hemorrhagic fevers. Curr. Top. Microbiol. Immunol. 1987;134:5–68. doi: 10.1007/978-3-642-71726-0_2. [DOI] [PubMed] [Google Scholar]
- 15.Hartnett JN, et al. Current and emerging strategies for the diagnosis, prevention and treatment of Lassa fever. Future Virol. 2015;10:559–584. doi: 10.2217/fvl.15.41. [DOI] [Google Scholar]
- 16.Goba A, et al. An outbreak of Ebola virus disease in the Lassa fever zone. J. Infect. Dis. 2016 doi: 10.1093/infdis/jiw239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cross RW, et al. Treatment of Lassa virus infection in outbred guinea pigs with first-in-class human monoclonal antibodies. Antivir. Res. 2016;133:218–222. doi: 10.1016/j.antiviral.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mire CE, et al. Human-monoclonal-antibody therapy protects nonhuman primates against advanced Lassa fever. Nat. Med. 2017;23:1146–1149. doi: 10.1038/nm.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cashman KA, et al. Evaluation of Lassa antiviral compound ST-193 in a guinea pig model. Antivir. Res. 2011;90:70–79. doi: 10.1016/j.antiviral.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansen F, Jarvis MA, Feldmann H, Rosenke K. Lassa virus treatment options. Microorganisms. 2021 doi: 10.3390/microorganisms9040772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salami K, Gouglas D, Schmaljohn C, Saville M, Tornieporth N. A review of Lassa fever vaccine candidates. Curr. Opin. Virol. 2019;37:105–111. doi: 10.1016/j.coviro.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 22.Gale TV, Horton TM, Hoffmann AR, Branco LM, Garry RF. Host proteins identified in extracellular viral particles as targets for broad-spectrum antiviral inhibitors. J. Proteome Res. 2019;18:7–17. doi: 10.1021/acs.jproteome.8b00204. [DOI] [PubMed] [Google Scholar]
- 23.Rowe WP, et al. Arenoviruses: proposed name for a newly defined virus group. J. Virol. 1970;5:651–652. doi: 10.1128/jvi.5.5.651-652.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oldstone MB. Arenaviruses. I. The epidemiology molecular and cell biology of arenaviruses. Introduction. Curr. Top. Microbiol. Immunol. 2002;262:V–XII. [PubMed] [Google Scholar]
- 25.Hastie KM, Kimberlin CR, Zandonatti MA, MacRae IJ, Saphire EO. Structure of the Lassa virus nucleoprotein reveals a dsRNA-specific 3′ to 5′ exonuclease activity essential for immune suppression. Proc. Natl Acad. Sci. USA. 2011;108:2396–2401. doi: 10.1073/pnas.1016404108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hastie KM, King LB, Zandonatti MA, Saphire EO. Structural basis for the dsRNA specificity of the Lassa virus NP exonuclease. PloS ONE. 2012;7:e44211. doi: 10.1371/journal.pone.0044211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hastie KM, et al. Crystal structure of the Lassa virus nucleoprotein–RNA complex reveals a gating mechanism for RNA binding. Proc. Natl Acad. Sci. USA. 2011;108:19365–19370. doi: 10.1073/pnas.1108515108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hastie KM, Saphire EO. Lassa virus glycoprotein: stopping a moving target. Curr. Opin. Virol. 2018 doi: 10.1016/j.coviro.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kouba T, et al. Conformational changes in Lassa virus L protein associated with promoter binding and RNA synthesis activity. Nat. Commun. 2021;12:7018. doi: 10.1038/s41467-021-27305-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu X, et al. Cryo-EM structures of Lassa and Machupo virus polymerases complexed with cognate regulatory Z proteins identify targets for antivirals. Nat. Microbiol. 2021;6:921–931. doi: 10.1038/s41564-021-00916-w. [DOI] [PubMed] [Google Scholar]
- 31.Hastie KM, et al. Crystal structure of the oligomeric form of Lassa virus matrix protein Z. J. Virol. 2016;90:4556–4562. doi: 10.1128/JVI.02896-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joseph S, Campbell KP. Lassa fever virus binds matriglycan-A polymer of alternating xylose and glucuronate-on α-dystroglycan. Viruses. 2021 doi: 10.3390/v13091679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brouillette RB, et al. TIM-1 mediates dystroglycan-independent entry of Lassa virus. J. Virol. 2018 doi: 10.1128/jvi.00093-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fedeli C, Moreno H, Kunz S. The role of receptor tyrosine kinases in lassa virus cell entry. Viruses. 2020 doi: 10.3390/v12080857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fedeli C, et al. Axl can serve as entry factor for Lassa virus depending on the functional glycosylation of dystroglycan. J. Virol. 2018 doi: 10.1128/jvi.01613-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goncalves AR, et al. Role of DC-SIGN in Lassa virus entry into human dendritic cells. J. Virol. 2013;87:11504–11515. doi: 10.1128/JVI.01893-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cao W, et al. Identification of α-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science. 1998;282:2079–2081. doi: 10.1126/science.282.5396.2079. [DOI] [PubMed] [Google Scholar]
- 38.Li S, et al. Acidic pH-induced conformations and LAMP1 binding of the Lassa virus glycoprotein spike. PloS Pathog. 2016;12:e1005418. doi: 10.1371/journal.ppat.1005418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Israeli H, Cohen-Dvashi H, Shulman A, Shimon A, Diskin R. Mapping of the Lassa virus LAMP1 binding site reveals unique determinants not shared by other old world arenaviruses. PloS Pathog. 2017;13:e1006337. doi: 10.1371/journal.ppat.1006337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Radoshitzky, S. R., Buchmeier, M. J. & de la Torre, J. C. in Field’s Virology 7th edn Vol. 1 Ch. 18 (eds Howley, P. M. & Knipe, D. M.) (Lippincott Williams & Wilkins, 2020).
- 41.Auperin DD, Sasso DR, McCormick JB. Nucleotide sequence of the glycoprotein gene and intergenic region of the Lassa virus S genome RNA. Virology. 1986;154:155–167. doi: 10.1016/0042-6822(86)90438-1. [DOI] [PubMed] [Google Scholar]
- 42.Reguera J, et al. Comparative structural and functional analysis of Bunyavirus and Arenavirus cap-snatching endonucleases. PloS Pathog. 2016;12:e1005636. doi: 10.1371/journal.ppat.1005636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hastie KM, et al. Structural basis for antibody-mediated neutralization of Lassa virus. Science. 2017;356:923–928. doi: 10.1126/science.aam7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lenz O, ter Meulen J, Klenk HD, Seidah NG, Garten W. The Lassa virus glycoprotein precursor GP-C is proteolytically processed by subtilase SKI-1/S1P. Proc. Natl Acad. Sci. USA. 2001;98:12701–12705. doi: 10.1073/pnas.221447598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yasuda SU, Jiro Molecular mechanism of arenavirus assembly and budding. Viruses. 2012;4:2049–2079. doi: 10.3390/v4102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ehichioya DU, et al. Phylogeography of Lassa virus in Nigeria. J. Virol. 2019 doi: 10.1128/jvi.00929-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bowen MD, et al. Genetic diversity among Lassa virus strains. J. Virol. 2000;74:6992–7004. doi: 10.1128/JVI.74.15.6992-7004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mofolorunsho KC. Outbreak of lassa fever in Nigeria: measures for prevention and control. Pan Afr. Med. J. 2016;23:210. doi: 10.11604/pamj.2016.23.210.8923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Akhiwu HO, et al. Lassa fever outbreak in adolescents in north central Nigeria: report of cases. J. Virus Erad. 2018;4:225–227. doi: 10.1016/S2055-6640(20)30306-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akpede GO, Asogun DA, Okogbenin SA, Okokhere PO. Lassa fever outbreaks in Nigeria. Expert Rev. Anti Infect. Ther. 2018;16:663–666. doi: 10.1080/14787210.2018.1512856. [DOI] [PubMed] [Google Scholar]
- 51.Ilori EA, et al. Epidemiologic and clinical features of Lassa fever outbreak in Nigeria, January 1–May 6, 2018. Emerg. Infect. Dis. 2019;25:1066–1074. doi: 10.3201/eid2506.181035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maxmen A. Deadly Lassa-fever outbreak tests Nigeria’s revamped health agency. Nature. 2018;555:421–422. doi: 10.1038/d41586-018-03171-y. [DOI] [PubMed] [Google Scholar]
- 53.Siddle KJ, et al. Genomic analysis of Lassa virus during an increase in cases in Nigeria in 2018. N. Engl. J. Med. 2018;379:1745–1753. doi: 10.1056/NEJMoa1804498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Manning JT, Forrester N, Paessler S. Lassa virus isolates from Mali and the Ivory Coast represent an emerging fifth lineage. Front. Microbiol. 2015;6:1037. doi: 10.3389/fmicb.2015.01037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Whitmer SLM, et al. New lineage of Lassa virus, Togo, 2016. Emerg. Infect. Dis. 2018;24:599–602. doi: 10.3201/eid2403.171905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boisen ML, et al. Multiple circulating infections can mimic the early stages of viral hemorrhagic fevers and possible human exposure to filoviruses in Sierra Leone prior to the 2014 outbreak. Viral Immunol. 2015;28:19–31. doi: 10.1089/vim.2014.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Richmond JK, Baglole DJ. Lassa fever: epidemiology, clinical features, and social consequences. BMJ. 2003;327:1271–1275. doi: 10.1136/bmj.327.7426.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Macher AM, Wolfe MS. Historical Lassa fever reports and 30-year clinical update. Emerg. Infect. Dis. 2006;12:835–837. doi: 10.3201/eid1205.050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wolf T, Ellwanger R, Goetsch U, Wetzstein N, Gottschalk R. Fifty years of imported Lassa fever–a systematic review of primary and secondary cases. J. Travel Med. 2020 doi: 10.1093/jtm/taaa035. [DOI] [PubMed] [Google Scholar]
- 60.Ehlkes L, et al. Management of a Lassa fever outbreak, Rhineland-Palatinate, Germany, 2016. Eur. Surveill. 2017 doi: 10.2807/1560-7917.Es.2017.22.39.16-00728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Njuguna C, et al. A challenging response to a Lassa fever outbreak in a non endemic area of Sierra Leone in 2019 with export of cases to The Netherlands. Int. J. Infect. Dis. 2022;117:295–301. doi: 10.1016/j.ijid.2022.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.WHO. Lassa fever–United Kingdom of Great Britain and Northern Ireland. World Health Organizationhttps://www.who.int/emergencies/disease-outbreak-news/item/lassa-fever-united-kingdom-of-great-britain-and-northern-ireland (2022).
- 63.Grange Z, et al. Ranking the risk of animal-to-human spillover for newly discovered viruses. Proc. Natl Acad. Sci. USA. 2021;118:e2002324118. doi: 10.1073/pnas.2002324118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smither AR, Bell-Kareem AR. Ecology of Lassa virus. Curr. Top. Microbiol. Immunol. 2021 doi: 10.1007/82_2020_231. [DOI] [PubMed] [Google Scholar]
- 65.Shaffer JG, et al. Lassa fever in post-conflict Sierra Leone. PloS Negl. Trop. Dis. 2014;8:e2748. doi: 10.1371/journal.pntd.0002748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bonwitt J, et al. At home with Mastomys and Rattus: human–rodent interactions and potential for primary transmission of Lassa virus in domestic spaces. Am. J. Trop. Med. Hyg. 2017;96:935–943. doi: 10.4269/ajtmh.16-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bonwitt J, et al. Rat-atouille: a mixed method study to characterize rodent hunting and consumption in the context of Lassa fever. EcoHealth. 2016;13:234–247. doi: 10.1007/s10393-016-1098-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mari Saez A, et al. Rodent control to fight Lassa fever: evaluation and lessons learned from a 4-year study in Upper Guinea. PloS Negl. Trop. Dis. 2018;12:e0006829. doi: 10.1371/journal.pntd.0006829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.WHO. Lassa fever. World Health Organizationhttps://www.who.int/health-topics/lassa-fever#tab=tab_1 (2022).
- 70.Knobloch J, et al. Clinical observations in 42 patients with Lassa fever. Tropenmed Parasitol. 1980;31:389–398. [PubMed] [Google Scholar]
- 71.McCormick JB, Fisher-Hoch SP. Lassa fever. Curr. Top. Microbiol. Immunol. 2002;262:75–109. doi: 10.1007/978-3-642-56029-3_4. [DOI] [PubMed] [Google Scholar]
- 72.Akhuemokhan OC, et al. Prevalence of Lassa virus disease (LVD) in Nigerian children with fever or fever and convulsions in an endemic area. PloS Negl. Trop. Dis. 2017;11:e0005711. doi: 10.1371/journal.pntd.0005711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Okokhere P, et al. Clinical and laboratory predictors of Lassa fever outcome in a dedicated treatment facility in Nigeria: a retrospective, observational cohort study. Lancet Infect. Dis. 2018;18:684–695. doi: 10.1016/S1473-3099(18)30121-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ehichioya DU, et al. Hospital-based surveillance for Lassa fever in Edo State, Nigeria, 2005–2008. Trop. Med. Int. Health. 2012;17:1001–1004. doi: 10.1111/j.1365-3156.2012.03010.x. [DOI] [PubMed] [Google Scholar]
- 75.Buba MI, et al. Mortality among confirmed lassa fever cases during the 2015–2016 outbreak in Nigeria. Am. J. Public Health. 2018;108:262–264. doi: 10.2105/AJPH.2017.304186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Branco LM, et al. Lassa hemorrhagic fever in a late term pregnancy from northern Sierra Leone with a positive maternal outcome: case report. Virol. J. 2011;8:404. doi: 10.1186/1743-422X-8-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Okogbenin S, et al. Retrospective cohort study of Lassa fever in pregnancy, southern Nigeria. Emerg. Infect. Dis. 2019 doi: 10.3201/eid2508.181299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ilori EA, et al. Increase in Lassa fever cases in Nigeria, January–March 2018. Emerg. Infect. Dis. 2019;25:1026–1027. doi: 10.3201/eid2505.181247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ficenec SC, et al. Lassa fever induced hearing loss: the neglected disability of hemorrhagic fever. Int. J. Infect. Dis. 2020;100:82–87. doi: 10.1016/j.ijid.2020.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ficenec SC, Schieffelin JS, Emmett SD. A review of hearing loss associated with Zika, Ebola, and Lassa fever. Am. J. Trop. Med. Hyg. 2019;101:484–490. doi: 10.4269/ajtmh.18-0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Duvignaud A, et al. Delayed-onset paraparesis in Lassa fever: a case report. Int. J. Infect. Dis. 2020;92:49–52. doi: 10.1016/j.ijid.2019.12.022. [DOI] [PubMed] [Google Scholar]
- 82.Ezeomah C, et al. Sequelae of Lassa fever: postviral cerebellar ataxia. Open Forum Infect. Dis. 2019;6:ofz512. doi: 10.1093/ofid/ofz512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li AL, et al. Ophthalmic manifestations and vision impairment in Lassa fever survivors. PloS ONE. 2020;15:e0243766. doi: 10.1371/journal.pone.0243766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Garry RF. Ebola virus can lie low and reactivate after years in human survivors. Nature. 2021;597:478–480. doi: 10.1038/d41586-021-02378-w. [DOI] [PubMed] [Google Scholar]
- 85.Lee H, Nishiura H. Recrudescence of Ebola virus disease outbreak in West Africa, 2014–2016. Int. J. Infect. Dis. 2017;64:90–92. doi: 10.1016/j.ijid.2017.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McCormick JB, et al. Lassa fever. Effective therapy with ribavirin. N. Engl. J. Med. 1986;314:20–26. doi: 10.1056/NEJM198601023140104. [DOI] [PubMed] [Google Scholar]
- 87.Eberhardt KA, et al. Ribavirin for the treatment of Lassa fever: a systematic review and meta-analysis. Int. J. Infect. Dis. 2019 doi: 10.1016/j.ijid.2019.07.015. [DOI] [PubMed] [Google Scholar]
- 88.Salam AP, et al. Time to reconsider the role of ribavirin in Lassa fever. PloS Negl. Trop. Dis. 2021;15:e0009522. doi: 10.1371/journal.pntd.0009522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hastie KM, Bale S, Kimberlin CR, Saphire EO. Hiding the evidence: two strategies for innate immune evasion by hemorrhagic fever viruses. Curr. Opin. Virol. 2012;2:151–156. doi: 10.1016/j.coviro.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang Q, Liu X, Brisse M, Ly H, Liang Y. Effect of strain variations on Lassa virus Z protein-mediated human RIG-I inhibition. Viruses. 2020 doi: 10.3390/v12090907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Watanabe Y, et al. Structure of the Lassa virus glycan shield provides a model for immunological resistance. Proc. Natl Acad. Sci. USA. 2018;115:7320–7325. doi: 10.1073/pnas.1803990115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Branco LM, et al. Emerging trends in Lassa fever: redefining the role of immunoglobulin M and inflammation in diagnosing acute infection. Virol. J. 2011;8:478. doi: 10.1186/1743-422X-8-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Prescott JB, et al. Immunobiology of Ebola and Lassa virus infections. Nat. Rev. Immunol. 2017;17:195–207. doi: 10.1038/nri.2016.138. [DOI] [PubMed] [Google Scholar]
- 94.Sakabe S, et al. Identification of common CD8+ T cell epitopes from Lassa fever survivors in Nigeria and Sierra Leone. J. Virol. 2020 doi: 10.1128/jvi.00153-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sullivan BM, et al. High crossreactivity of human T cell responses between Lassa virus lineages. PloS Pathog. 2020;16:e1008352. doi: 10.1371/journal.ppat.1008352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Botten J, et al. Identification of protective Lassa virus epitopes that are restricted by HLA-A2. J. Virol. 2006;80:8351–8361. doi: 10.1128/JVI.00896-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Flatz L, et al. T cell-dependence of Lassa fever pathogenesis. PloS Pathog. 2010;6:e1000836. doi: 10.1371/journal.ppat.1000836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Port JR, et al. Severe human Lassa fever is characterized by nonspecific T-cell activation and lymphocyte homing to inflamed tissues. J. Virol. 2020 doi: 10.1128/jvi.01367-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Asogun DA, et al. Molecular diagnostics for lassa fever at Irrua Specialist Teaching Hospital, Nigeria: lessons learnt from two years of laboratory operation. PloS Negl. Trop. Dis. 2012;6:e1839. doi: 10.1371/journal.pntd.0001839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Boisen ML, et al. Field validation of recombinant antigen immunoassays for diagnosis of Lassa fever. Sci. Rep. 2018;8:5939. doi: 10.1038/s41598-018-24246-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Boisen ML, et al. Field evaluation of a pan-Lassa rapid diagnostic test during the 2018 Nigerian Lassa fever outbreak. Sci. Rep. 2020;10:8724. doi: 10.1038/s41598-020-65736-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Heinrich ML, et al. Antibodies from Sierra Leonean and Nigerian Lassa fever survivors cross-react with recombinant proteins representing Lassa viruses of divergent lineages. Sci. Rep. 2020;10:16030. doi: 10.1038/s41598-020-72539-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Furuta Y, Komeno T, Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2017;93:449–463. doi: 10.2183/pjab.93.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Safronetz D, et al. The broad-spectrum antiviral favipiravir protects guinea pigs from lethal Lassa virus infection post-disease onset. Sci. Rep. 2015;5:14775. doi: 10.1038/srep14775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Oestereich L, et al. Efficacy of favipiravir alone and in combination with ribavirin in a lethal, immunocompetent mouse model of Lassa fever. J. Infect. Dis. 2016;213:934–938. doi: 10.1093/infdis/jiv522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lingas G, Rosenke K, Safronetz D, Guedj J. Lassa viral dynamics in non-human primates treated with favipiravir or ribavirin. PloS Comput. Biol. 2021;17:e1008535. doi: 10.1371/journal.pcbi.1008535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rosenke K, et al. Use of favipiravir to treat Lassa virus infection in macaques. Emerg. Infect. Dis. 2018;24:1696–1699. doi: 10.3201/eid2409.180233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mendenhall M, et al. Effective oral favipiravir (T-705) therapy initiated after the onset of clinical disease in a model of arenavirus hemorrhagic fever. PloS Negl. Trop. Dis. 2011;5:e1342. doi: 10.1371/journal.pntd.0001342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gowen BB, et al. Assessing changes in vascular permeability in a hamster model of viral hemorrhagic fever. Virol. J. 2010;7:240. doi: 10.1186/1743-422X-7-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Raabe VN, et al. Favipiravir and ribavirin treatment of epidemiologically linked cases of Lassa fever. Clin. Infect. Dis. 2017;65:855–859. doi: 10.1093/cid/cix406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Larson RA, et al. Identification of a broad-spectrum arenavirus entry inhibitor. J. Virol. 2008;82:10768–10775. doi: 10.1128/JVI.00941-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Robinson JE, et al. Most neutralizing human monoclonal antibodies target novel epitopes requiring both Lassa virus glycoprotein subunits. Nat. Commun. 2016;7:11544. doi: 10.1038/ncomms11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cross RW, et al. Antibody therapy for Lassa fever. Curr. Opin. Virol. 2019;37:97–104. doi: 10.1016/j.coviro.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 114.Hastie KM, et al. Convergent structures illuminate features for germline antibody binding and pan-Lassa virus neutralization. Cell. 2019;178:1004–1015.e14. doi: 10.1016/j.cell.2019.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Buck TK, et al. Neutralizing antibodies against lassa virus lineage I. mBio. 2022 doi: 10.1128/mbio.01278-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.White JM, et al. Drug combinations as a first line of defense against coronaviruses and other emerging viruses. mBio. 2021;12:e0334721. doi: 10.1128/mbio.03347-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Johnson DM, Cubitt B, Pfeffer TL, de la Torre JC, Lukashevich IS. Lassa virus vaccine candidate ML29 generates truncated viral RNAs which contribute to interfering activity and attenuation. Viruses. 2021 doi: 10.3390/v13020214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cashman KA, et al. A DNA vaccine delivered by dermal electroporation fully protects cynomolgus macaques against Lassa fever. Hum. Vaccines Immunother. 2017;13:2902–2911. doi: 10.1080/21645515.2017.1356500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cross RW, et al. Quadrivalent VesiculoVax vaccine protects nonhuman primates from viral-induced hemorrhagic fever and death. J. Clin. Invest. 2020;130:539–551. doi: 10.1172/JCI131958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Stein DR, et al. A recombinant vesicular stomatitis-based Lassa fever vaccine elicits rapid and long-term protection from lethal Lassa virus infection in guinea pigs. NPJ Vaccines. 2019;4:8. doi: 10.1038/s41541-019-0104-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Abreu-Mota T, et al. Non-neutralizing antibodies elicited by recombinant Lassa–rabies vaccine are critical for protection against Lassa fever. Nat. Commun. 2018;9:4223. doi: 10.1038/s41467-018-06741-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mateo M, et al. A single-shot Lassa vaccine induces long-term immunity and protects cynomolgus monkeys against heterologous strains. Sci. Transl Med. 2021 doi: 10.1126/scitranslmed.abf6348. [DOI] [PubMed] [Google Scholar]
- 123.Salvato MS, et al. A single dose of modified vaccinia ankara expressing Lassa virus-like particles protects mice from lethal intra-cerebral virus challenge. Pathogens. 2019 doi: 10.3390/pathogens8030133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang M, et al. Construction and immunological evaluation of an adenoviral vector-based vaccine candidate for Lassa fever. Viruses. 2021 doi: 10.3390/v13030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fischer RJ, et al. ChAdOx1-vectored Lassa fever vaccine elicits a robust cellular and humoral immune response and protects guinea pigs against lethal Lassa virus challenge. NPJ Vaccines. 2021;6:32. doi: 10.1038/s41541-021-00291-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Müller H, et al. Adjuvant formulated virus-like particles expressing native-like forms of the Lassa virus envelope surface glycoprotein are immunogenic and induce antibodies with broadly neutralizing activity. NPJ Vaccines. 2020;5:71. doi: 10.1038/s41541-020-00219-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Branco LM, et al. Lassa virus-like particles displaying all major immunological determinants as a vaccine candidate for Lassa hemorrhagic fever. Virol. J. 2010;7:279. doi: 10.1186/1743-422X-7-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kainulainen MH, et al. Protection from lethal Lassa disease can be achieved both before and after virus exposure by administration of single-cycle replicating Lassa virus replicon particles. J. Infect. Dis. 2019;220:1281–1289. doi: 10.1093/infdis/jiz284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gouglas D, Christodoulou M, Plotkin SA, Hatchett R. CEPI: driving progress toward epidemic preparedness and response. Epidemiol. Rev. 2019;41:28–33. doi: 10.1093/epirev/mxz012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Koch MR, et al. Health seeking behavior after the 2013–16 Ebola epidemic: Lassa fever as a metric of persistent changes in Kenema District, Sierra Leone. PloS Negl. Trop. Dis. 2021;15:e0009576. doi: 10.1371/journal.pntd.0009576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Meyer B, Ly H. Inhibition of innate immune responses is key to pathogenesis by arenaviruses. J. Virol. 2016;90:3810–3818. doi: 10.1128/JVI.03049-15. [DOI] [PMC free article] [PubMed] [Google Scholar]