Abstract
Recent monkeypox (MPX) outbreaks are major ones in non-endemic countries. The present study analyzed molecular phylogenetics, divergence, epidemiology, the geographical distribution, entropy diversity of genome, mutational landscape, and evolution of the monkeypox virus (MPXV) genome and the current MPXV is entitled “hMPXV1.” We used different in-silico and statistical methods to study our objectives. The developed phylogram from molecular phylogenetics describes the origin and evolution of hMPXV1 of A, A.1, A.1.1, A.2, and B.1 lineages. The microevolution of B.1 lineage shows its evolution from May to August 2022. B.1 lineage is further adapting and showing more mutation and sub-lineages. The scatter plot of all lineages shows the clustering pattern of lineages and the divergence. We also developed two statistical models of confirmed cases and a diagram of the age-related pattern of infected cases to illustrate the epidemiology of the MPX outbreaks. The entropy diversity and mutational landscape of the hMPXV1 genome were analyzed in nucleotide and codon contexts. Our study has shown the in-depth evolution pattern of different lineages of the hMPXV1. We found B.1 lineage is associated with the current outbreaks. The mutational landscape informs about the slow mutation of the virus. Finally, the study might assists the new therapeutic development considering all the above points and would help the researcher to set up their future research directions.
Keywords: Monkeypox virus; Outbreaks, evolution; Genome; Mutation; Epidemiology
Introduction
The world has started recovering after over two years of the crucial COVID-19 pandemic. However, emerging variants of SARS-CoV-2 are currently posing significant hurdles to counteract it despite developing vaccines, drugs, and the progressive vaccination campaign and incorporating booster doses [1–3]. The recent spread of the monkeypox virus (MPXV) has created another global health threat when the COVID-19 pandemic wound started healing. The continuous rising cases of MPX have alarmed multiple non-African countries, and the current situation is evolving continuously [4, 5]. According to World Health Organization (WHO), the MPXV has spread over 96 countries worldwide, and about 41,664 infected cases were reported till August 22, 2022 (data published on August 24, 2022) [6]. However, the world has never previously reported many such cases from non-African (non-endemic) countries. In the present outbreak in non-African countries, the first case of MPX was noted in the United Kingdom (UK) on May 13, 2022 [7]. Within a month (around June 15, 2022), the MPX has spread to about 25 countries around Europe. Several health regulatory agencies like WHO and European Centre for Disease Prevention and Control (eCDC) have reported about 1,654 confirmed cases [8]. Other than the UK, the MPX-affected countries in Europe are Germany, Netherlands, Sweden, Spain, France, and Italy. In Europe, MPX has also been reported beyond Europe, Australia, Canada, the USA, and UAE [5, 9, 10]. The disease has spread to numerous populations and geographical areas [11, 12].
The MPX as a zoonotic disease is an uncommon viral disease that sometimes becomes life-threatening. It is a neglected disease, continuously emerging and reemmerging in Africa [4, 13]. The monkeypox virus was first identified in monkey colonies in Copenhagen, Denmark, in 1958. These animal facilities were available in a Danish research laboratory [14]. The pox-like disease symptoms were observed in animals by the researchers. However, it was a reported case of the MPXV in the animal. The first human infection of the MPXV was observed in 1970 in DRC (the Democratic Republic of the Congo) in a 9-month baby suspected of smallpox [15]. Afterward, the virus spread to different parts of Africa, especially western and central African countries. Besides DRC, it was noted that the virus subsequently spread in Sierra Leone, Nigeria, Liberia, Côte d’Ivoire, and Cameroon. However, during the 1970s, about 48 confirmed cases were noted from six different African countries: the DRC, Sierra Leone Liberia, Côte d’Ivoire, Cameroon, and Nigeria. About 38 patients were noted from the DRC [16, 17]. Conversely, several infected cases of MPXV were noted in different African countries. In 2017, the largest outbreak was reported in Nigeria, with 101 confirmed cases [17, 18]. Similarly, the first MPX case was identified outside Africa in 2003 [16]. Afterward, MPXV infected cases were reported from non-African countries from time to time.
Evolution study is a significant area for infection biology. Several researchers have done the evolutionary analysis of pathogens from time to time. Recently, researchers analyzed the evolution of SARS-CoV-2 to understand how quickly the pandemic virus is evolving and the status of the variants creation [19–22]. It illustrated genomic diversity, genetic changes, and the evolutionary epidemiology of this virus. Like SARS-CoV-2, it is also necessary to understand the evolution of the MPXV. Although, very few studies have been conducted to understand the evolution of the neglected virus. Nakazawa et al. have tried to illustrate the probable origin of the Southern Sudan outbreak in 2005 through phylogenetic investigation using the MPXV genome sequences of different isolates [23]. Similarly, another study by Nakazawa et al. reported a phylogenetic analysis using a more extensive MPXV genomes data set of Sub-Saharan Africa [24]. Sadegh-Mba et al. have tried to assess the phylogenetic similarities of isolates of this virus from Nigeria and Cameroon to understand the genetic relatedness [25]. However, it is indispensable to understand the genetic relatedness of the MPXV isolates from the recent outbreaks and the previous outbreaks. Doshi et al. tried to investigate the epidemiology of MPX in DRC during the outbreak of 2017 [26]. Through the systematic review, Beer and Rao have illustrated the epidemiology of hMPXV (human monkeypox virus) outbreaks [27]. Here, they have tried to depict the probable animal-to-human or human-to-human transmission, case fatality, and epidemiology. However, it is also essential to understand the epidemiological pattern of the recent MPX outbreaks. Kraemer et al. have attempted to represent real-time epidemiological data. They have described the total number of confirmed cases in this 2022 outbreak [28]. At the same time, it is necessary to understand the mutation pattern in the human monkeypox virus genome. The mutation pattern can inform us of the frequency of the mutation in the virus's genome. Dumbell and Archard analyzed the variants of MPXV through phenotype and DNA investigation and tried to evaluate the association with the whitepox virus and variola. They also analyzed the mutation pattern of these viruses and the association of the mutation. Finally, the researchers have concluded that the spontaneous mutants present in the whitepox are relatively uncommon in MPXV [29]. At the same time, Karumathil et al. have illustrated the GC3 (GC composition at the third codon position) and the ENC (adequate number of codons) of MPXV. Finally, they have developed the GC3 versus ENC plot to understand the influences of the mutational pressure in the plot [30]. However, more studies are necessary to understand the mutation pattern of the virus.
In this study, we have analyzed the evolution of hMPXV1 through molecular phylogenetics, divergence and the scatter plot of genome cluster of hMPXV1, epidemiology and geographical distribution of the virus infection, entropy diversity of hMPXV1 genome, and mutational landscape of hMPXV1 genome.
Methods and materials
Collection of literature and retrieval of genome sequence
We have collected information about the hMPXV1 and MPX disease from different regulatory health authorities in several countries, such as eCDC [31], WHO [6], and CDC [32]. At the same time, we have performed literature searches from different scientific databases such as PubMed [33, 34], Google Scholar [35], and Web of Science [36].
For the literature search from the literature database, we used different keywords such as “monkeypox virus,” “monkeypox disease,” “evolution,” “epidemiology,” “geographical distribution,” “entropy diversity,” and “mutational landscape.” We have used a single keyword or combination of keywords during our search.
For hMPXV1 genome sequence retrieval, we used NCBI and NCBI virus [37]. We also used the VIRION database [38] to fetch necessary information about the virus.
Data analysis
We used the Nextstrain server for the data analysis and retrieval [39], which uses the metadata and curated sequence data directly fetched by the NCBI virus. Different lineages of the virus were denoted as per the Nextstrain server, and Happi et al. [40]. In this study, we have performed the analysis of hMPXV1 using the server.
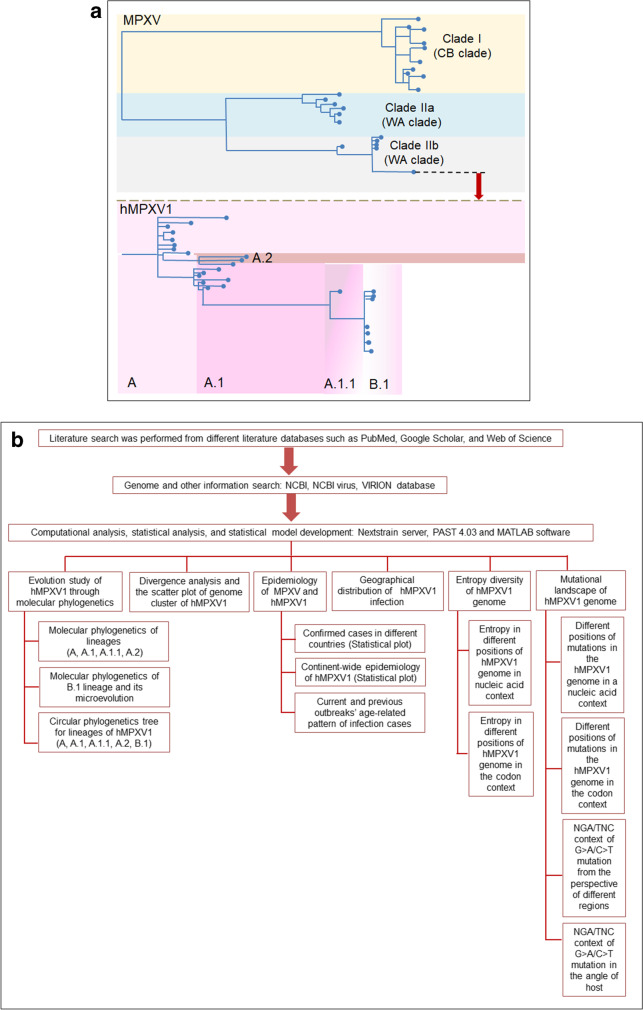
Recently, the WHO announced the clades of MPXV, which comprises Clade I, Clade IIa, and Clade IIb. However, the WHO has not reported the details about the clades yet. Happi et al. illustrated the MPXV clades very nicely [40]. Researchers represented three distinct MPXV clades, and it demonstrated the diversity of MPXV. According to them, Clade I represent the previous “Congo Basin (CB)” Clade. At the same time, Clades IIa and IIb represent the previous “West African (WA)” Clade.
At the same time, Clade 3, which originated from Clade IIb, contains the recent MPXV. It includes the genomes from the 2017 to 2022 human outbreaks and is entitled “hMPXV1.” The “hMPXV1” has several genome diversity, illustrated as lineages/sub-lineages such as A, A.1, A.1.1, and B.1. The genomes belong to the recent outbreak (2017 to 2019) in Singapore, USA, Israel, UK, Nigeria, and the recent outbreak of 2022. Here, we analyzed the ‘hMPXV1’ for evolutionary and mutational study (Fig. 1a).
Fig. 1.
A schematic diagram and the flowchart illustrating the different clades of MPXV and methods of analysis. a A schematic diagram shows the different clades of MPXV. It also depicts the hMPXV1 and its genome diversity. In this study, we have analyzed mainly the evolution and mutational landscape of hMPXV1. b A flowchart illustrates the various methods which are of during our study
For statistical analysis and statistical model development, we have used statistics software (PAST 4.03 software) [41]. At the same time, we have used MATLAB software to analyze further and develop plots and graphs [42]. Finally, we have depicted a flowchart illustrating the different methods used during our study (Fig. 1b).
Result
Evolution of hMPXV1 through molecular phylogenetics
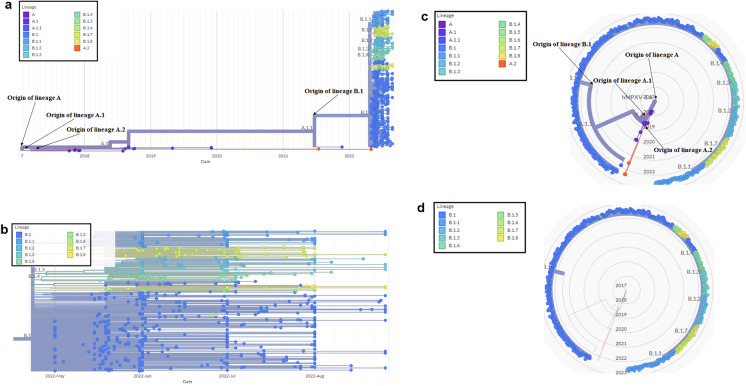
We have analyzed molecular evolution depicted from October 2017 and August 2022, and two types of the phylogenetic tree have been described using 692 genome samples. The first type is rectangular, representing a phylogram (Fig. 2a). Here, we have found reference lineages or sub-lineages which are A, A.1, A.1.1, A.2, B.1, B.1.1, B.1.2, B.1.3, B.1.4, B.1.5, B.1.6, B.1.7, and B.1.8, which are situated in the terminal node of the phylogenetic tree.
Fig. 2.
The phylogenetic tree of hMPXV1 shows the origin of different recent lineages. a The phylogenetic tree of hMPXV1 shows the origin and evolution of different lineages of A, A.1, A.1.1, A.2, B.1, B.1.1, B.1.2, B.1.3, B.1.4, B.1.5, B.1.6, B.1.7, and B.1.8. The phylogenetic tree illustrates the evolution of hMPXV1 with a time scale from October 2017 to August 2022. b The phylogenetic tree of the B.1 lineage shows the microevolution of B.1. It depicts the evolution of the B.1 lineage with a time scale from May to August 2022. c The circular phylogenetic tree of hMPXV1 shows the origin and evolution of different lineages of A, A.1, A.1.1, A.2, B.1, B.1.1, B.1.2, B.1.3, B.1.4, B.1.5, B.1.6, B.1.7, and B.1.8. d The circular phylogenetic tree of the B.1 lineage shows the microevolution of B.1
In the present 2022 hMPXV1 outbreaks, we have noted the involvement of one major lineage, B.1. In this phylogenetic tree, several genomes of the B.1 lineage have aggregated in the tree. The recent B.1 lineage has shown some association with the other four lineages (A, A.1.1, A.2, and B.1) and participated in forming the phylogenetic tree. However, it is apparent that the recent B.1. lineage is associated with the current MPX outbreaks.
Again, we have illustrated the B.1 lineages through an extended phylogenetic tree using 671 genome samples, showing the microevolution of the B.1 lineage (Fig. 2b). The microevolution of the B.1 lineage shows several sub-lineages such as B.1.1, B.1.2, B.1.3, B.1.4, B.1.5, B.1.6, B.1.7, and B.1.8. The microevolution of the B.1 lineage indicates its further evolution and creation of several sub-lineages. The comprehensive phylogenetic tree of the B.1 lineage has demonstrated that the genome sequence is situated between the timeline of May to August 2022. At the same time, we have also developed a circular phylogenetic tree (Fig. 2c). In the circular phylogenetic tree, the two lineages, A and A.1 are situated in different parts of the tree. Lineage A is located in the center, and lineage B.1 is situated in the radius (Fig. 2c). To highlight the B.1 lineage, we developed an extended circular phylogenetic tree (Fig. 2d). However, the circular phylogenetic tree (Fig. 1c and Fig. 1d) did not show the timeline of the evolution of the hMPXV1.
Divergence and the scatter plot of genome cluster of hMPXV1
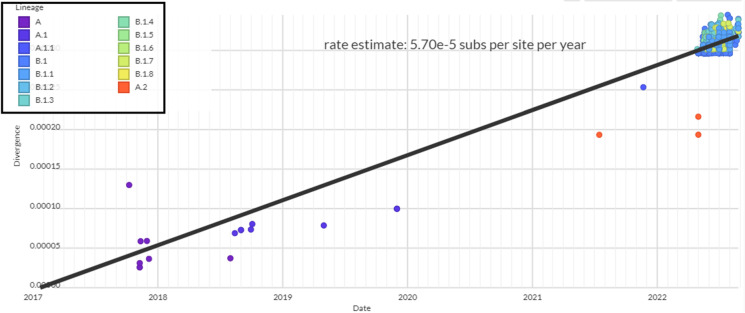
We have tried to assess the divergence of the hMPXV1 evolution and depicted the scatter plot of the genome cluster of hMPXV1 from 2017/2018 to 2022. The cluster analysis was performed, and scattered plot was developed to indicate the clustering of the MPXV genome samples. To show the divergence, we have depicted the regression line (Fig. 3). It intimates the divergence of the 692 hMPXV1 genomes along the regression line. The pattern of the scatter plot with the linear regression model illustrates that the model shows the light and spread distribution pattern of line samples. The model also informed us that 671 genome samples of the B.1 lineage had formed a cluster on the upper side of the regression line. Divergence analysis has shown the result of about 5.70 e-5 or 5.70 × 10–5 substitutions per site per year.
Fig. 3.
Scatter plot depicting the genome diversity cluster of all recent lineages of hMPXV1
Epidemiology of MPXV and hMPXV1
We have tried to illustrate the infected cases in previous and present outbreaks of MPXV and hMPXV1 infection. From the several literature databases, we described the MPXV and hMPXV1 infection cases reported in different countries during 1970–2021 (Table 1). We found the highest number of cases of DRC, Africa. Here, it has been noted that there were 386 infected cases during 1970–1990 and 511 numbers of infected patients during 1991–1999. Besides DRC, we found Nigeria and the Republic of Congo’s documenting the highest number of cases. In the Republic of Congo, we noted 110 patients (11 confirmed) during 2000–2018 and 39 cases (suspected) during 2019–2021. Again, in Nigeria, we observed 244 (101 confirmed) cases during 2000–2018 and 113 (45 confirmed) from 2019 to 2021.
Table 1.
Monkeypox infection cases were reported in different countries from 1970 to 2021
| Sl. No | Name of the country | Monkeypox infection reported | Reference | ||
|---|---|---|---|---|---|
| 1970–1999 | 2000–2018 | 2019–2021 | |||
| 1 | Cameroon |
2 (1970–1990), 4 (1 confirmed, 1991–1999) |
16 (confirmed) | 3 (confirmed) | [43–46] |
| 2 | USA | No information available | 47 (37 confirmed, 10 probable) | 1 (confirmed) | [19, 47, 48] |
| 3 | Ivory Coast | 2 (confirmed, 1970–1990) | No information available | No information available | [49, 50] |
| 4 | Sierra Leone | 1 (confirmed, 1970–1990) | 2 (confirmed) | 2(confirmed) | [51–53] |
| 5 | Democratic Republic of Congo |
386(1970–1990), 511 (1991–1999) |
Not fully enumerable | 200 (suspected) | [43, 54–59] |
| 6 | Gabon | 10 (one confirmed, 1970–1990) | No information available | No information available | [54, 60, 61] |
| 7 | South Sudan | No information available | 49 (10 confirmed) | No information available | [62] |
| 8 | Liberia | 4 (confirmed, 1970–1990) | 2 (confirmed) | 1 (confirmed) | [51, 63, 64] |
| 9 | Nigeria | 10 (3 confirmed, 1970–1990) | 244 (101 confirmed) | 113 (45 confirmed) | [45, 51, 65, 66] |
| 10 | Central African Republic | 6 (confirmed, 1970–1990) | 72 (29 confirmed) | No information available | [63, 67–69] |
| 11 | Republic of Congo | No information available | 110 (11 confirmed) | 39 (suspected) | [70–73] |
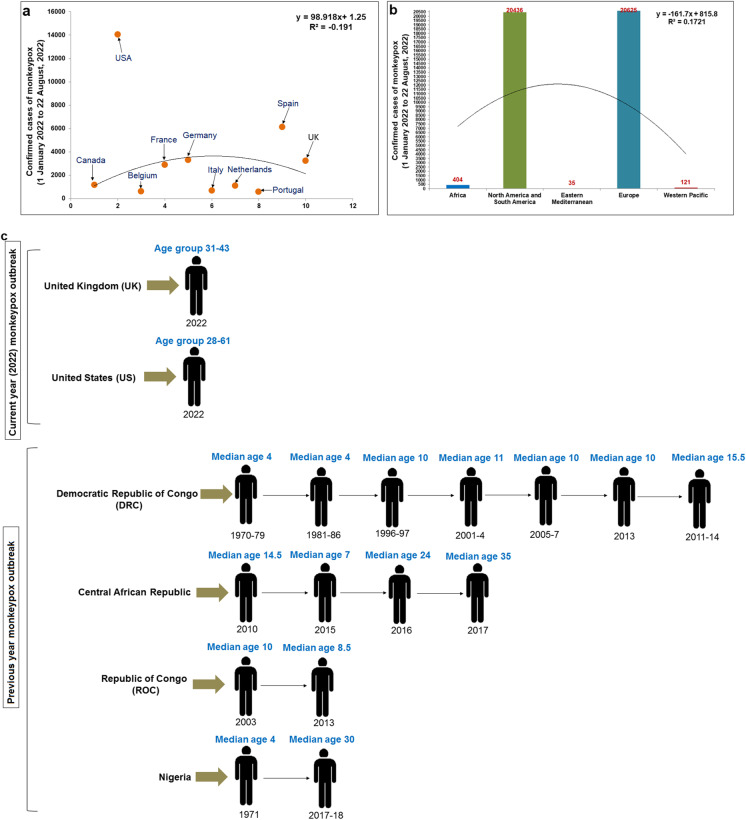
For the present MPX outbreaks, we have plotted the number of confirmed cases in different countries till August 22, 2022, and developed a statistical model (Fig. 4a). In this model, it has been noted that the UK is having highest infection cases (14,049), and the second-highest cases are indicated in Spain (6119 cases). Other significant cases are pointed out in Germany (3295 cases), Portugal (588 cases), and Canada (1168 cases). Again, to understand the continent-wide epidemiology, we have analyzed continent-wise confirmed cases till August 22, 2022 (Fig. 4b). We found Europe to show the highest number of confirmed cases (20,625), and the Western Pacific showed the lowest number of confirmed cases (121). North and South America also revealed several infected cases (20,436 cases), and the cases are more compared to Africa (404 cases).. The result informed us that this hMPXV1 outbreak has spread in high economic countries, especially in Europe and North and South America. At the same time, it has also been noted that the virus has spread in the Eastern Mediterranean like UAE and Morocco. Therefore, this outbreak is a global outbreak. Several scientists have also reported that the cases of this MPX virus infection in Africa were relatively low compared to previous episodes [10].
Fig. 4.
Developed two statistical models and a schematic diagram from our study to illustrate the epidemiology of MPX. a A statistical model shows the number of confirmed cases of MPX in different countries till August 22, 2022. b A statistical model shows continent-wise confirmed cases till August 22, 2022. c A schematic diagram illustrates the current and previous outbreaks' age-related pattern of infection cases of MPX
Again, we have analyzed the current and previous outbreaks' age-related pattern of hMPXV1 infection cases (Fig. 4c). The earlier episodes show the infected individuals' median age of about 4 to 15.5 years. Until 2010 in African countries, the outbreak led to the lower age group of infected individuals' median age (4 to 15.5 years). However, Nigeria’s 2017 and 2018 outbreak showed a higher age group of infected individuals (median age 30 years). Similarly, the outbreak of 2017 in the Central African Republic also showed a higher age group of infected individuals (median age 35 years).
The current MPX outbreaks show the infection among individuals in a higher age group compared to previous episodes. However, the recent outbreak offers the infected individuals a median age range of about 31 to 61 years. In the UK, the age range is 31 to 43 years, and in the USA, the age range is 28 to 61 years. Zumla et al. have also reported that most infection cases in the current outbreak are noted in the age of 20 to 50 years of men [10]. At the same time, WHO has also reported that, in 99% of cases, the observed age group is about 0 to 65 years (median age about 37 years, interquartile range about 32 to 43 years) [6]. Kupferschmidt reported that the MPX spreads among MSM individuals (men who have sex with men) [74]. At the same time, Mahase reported that the virus might be spreading in gay and bisexual men in England and concluded these population at high risk. However, these population might be vaccinated soon [75]. The sexual activity among MSM, gay, and bisexual men might be one reason for higher infection. However, it has been noted that severe cases of the virus occur more usually among children, which is associated with the level of virus exposure [6].
Geographical distribution of hMPXV1 infection
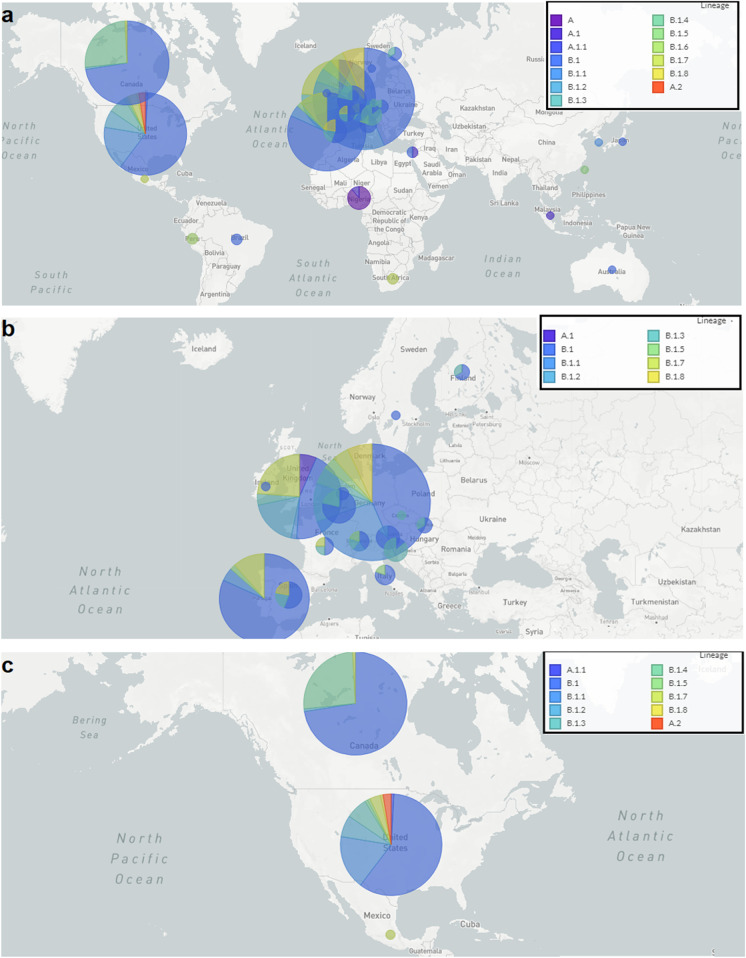
We have analyzed the geographical distribution of hMPXV1 infection for the recent outbreaks, and depicted the distribution of hMPXV1 lineages and their distribution throughout the world (Fig. 5a). We found infection case clusters in different regions of the world. Several disease case clusters have been noted in Europe (Fig. 5b). The countries in the cluster include the UK, France, Germany, Portugal, Spain, Italy, and Finland. However, in the figure, the generated circles for the cases of Germany, Portugal, and the UK are more significant than others. It might be due to the higher number of cases in those countries. At the same time, it has been observed that 433 genome sequences have been submitted from Europe between September 2018 and August 2022.
Fig. 5.
Geographical distribution of recent outbreak of MPX. a The geographical distribution of hMPXV1 lineages and their geographical distribution throughout the world. b Geographical distribution of MPX case clusters observed in Europe. c Geographical distribution of MPX case clusters observed in North America
Again, the country-wise plot was generated for North America (Fig. 5c). The geographical plot shows the infection region of America and Canada. From the figure, it has been noted that the generated circles for the cases of Canada are bigger than the USA. It might be a higher number of cases in Canada than in the USA. However, it has been observed that 237 genome sequences were submitted from North America between September 2018 and August 2022.
Entropy diversity of hMPXV1 genome
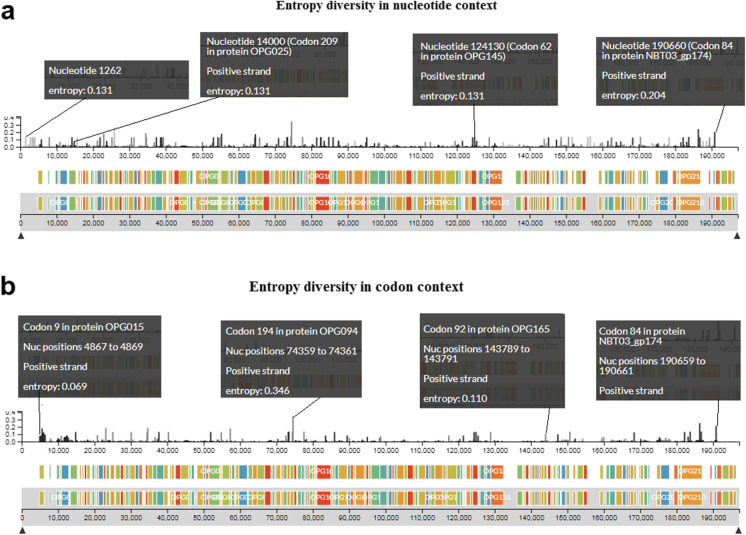
We have analyzed the entropy diversity of the hMPXV1 genome. We illustrated the entropy in different positions of the hMPXV1 genome in the nucleotide (nt) context (Fig. 6a). The hMPXV1 genome is about 198 kbp long. The entropy changes were noted in different positions in the positive strand in the genome. The first positional entropy change was noted in the hMPXV1 genome at 1262 position (entropy: 0.131) and the last positional entropy change was noted at the 190,660 position (entropy: 0.204). Some other significant entropy changes are noted in the different nucleotide positions such as 3818 position (entropy: 0.131), 14,000 position (entropy: 0.131), 38,360 position (entropy: 0.131), 64,426 position (entropy: 0.174), 65,571 position (entropy: 0.131), 74,360 position (entropy: 0.346), 124,130 position (entropy: 0.131), 124,674 position (entropy: 0.131), 150,469 position (entropy: 0.136), 162,331 position (entropy: 0.131), 165,782 position (entropy: 0.036), and 181,980 position (entropy: 0.131).
Fig. 6.
A diagram that illustrates the entropy diversity of the hMPXV1 genome. a The figure illustrates the entropy diversity in several positions of the hMPXV1 genome in the nucleic acid context. b The figure illustrates the entropy diversity in several positions of the hMPXV1 genome in the codon context
Again, we illustrated the entropy in different positions of the hMPXV1 genome in the codon context (Fig. 6b). The first positional entropy change was noted in the MPXV genome at the codon position 9 in OPG015 protein (entropy: 0.069). The last positional entropy change was noted at the codon position 84 NBT03_gp174 protein (entropy: 0.215). Some other significant entropy changes are noted in the different codon positions such as codon position 62 in OPG019 protein (entropy: 0.046), codon position 48 in OPG047 protein (entropy: 0.131), codon position 194 in OPG094 protein (entropy: 0.346), codon position 740 in OPG109 protein (entropy: 0.082), codon position 4 in OPG118 protein (entropy: 0.073), codon position 92 in OPG165 protein (entropy: 0.110), and codon position 1741 in OPG210 protein (entropy: 0.137).
However, we have observed high entropy diversity in the hMPXV1 genome in both the nucleic acid and codon context.
Mutational landscape of hMPXV1 genome
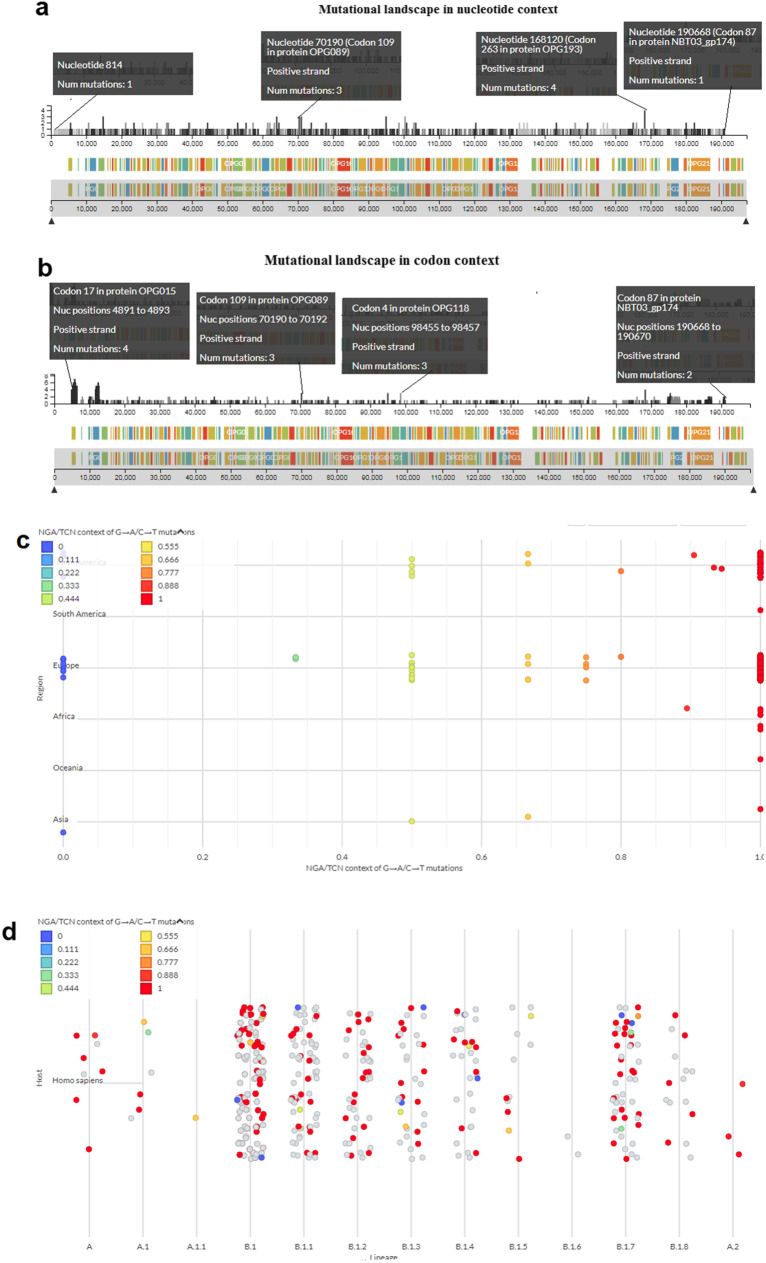
We have analyzed the mutational landscape of the hMPXV1 genome. We have tried to demonstrate the different positions of mutations in the hMPXV1 genome in nucleotide (nt) context (Fig. 7a). The first position of the mutation was observed in the hMPXV1 genome at the 814 nt position with one mutation, and the last mutation was noted at the 190,668 nt position with one mutation. The highest number of mutations was observed as four at the 168,120 nt positions. In most of the genome positions, we found one mutation. However, we have noted two numbers of mutation in some positions, which are nucleotide positions at 39,139, 44,220, 61,844, 63,147, 77,807, 132,625, 148,427, 164,832, 165,782, 170,698, 182,189. We have noted three mutations in some positions, which are nucleotide positions at 14,611, 63,811, 70,190, 70,780, 94,798, and 100,182.
Fig. 7.
A diagram that illustrates the mutational landscape of the hMPXV1 genome. a The figure illustrates the mutations in several positions of the hMPXV1 genome in the nucleic acid context. b The figure illustrates the mutations in several positions of the hMPXV1 genome in the codon context. c A figure demonstrates the NGA /TNC context of G- > A/C- > T mutation from the perspective of different regions. d A figure demonstrates the NGA /TNC context of G- > A/C- > T mutation in the angle of the human host
At the same time, we have tried to demonstrate the different positions of mutations in the hMPXV1 genome in the codon context (Fig. 7b). The first position of the mutation was observed in the hMPXV1 genome codon position 17 in OPG015 protein with four mutations. With two mutations, the last mutation was noted at the codon position 87 in the NBT03_gp174 protein. The most mutations were observed at seven at the codon position 261 in OPG015. The second-highest number of mutations was observed at six at the codon position 740 in OPG023. For most of the parts of the genome at the protein level, we found one mutation. However, we have noted two or more mutations in some positions, which are codon position 343 in OPG015 protein with five mutations, codon position 408 in OPG015 protein with five mutations, codon position 470 in OPG023 protein with five mutations, codon position 525 in OPG023 protein with five mutations, codon position 353 in OPG057 protein with two mutations, codon position 148 in OPG178 protein with two mutations, codon position 689 in OPG205 protein with two mutations, codon position 1622 in OPG210 protein with two mutations.
We have also studied of NGA /TNC context of G- > A/C- > T mutation from the perspective of different regions (Fig. 7c). Here, we found the recent hMPXV1 lineage B.1 has formed two clusters in North America and Europe. In Europe, we found several NGA /TNC contexts of G- > A/C- > T mutations encompass value 1. At the same time, the hMPXV-1 lineage has been noted African region.
We have also studied of NGA /TNC context of G- > A/C- > T mutation in the angle of the host (Fig. 7d). Here, we found the result of the NGA /TNC context of G- > A/C- > T mutation has developed for human (Homo sapiens) host. The recent hMPXV1 lineage B.1 and its sub-lineages have formed several clusters, especially B.1, B.1.1, B.1.2, B.1.3, B.1.4, B.1.7, and B.1.8. At the same time, the A lineage has also developed one cluster. However, the recent lineage B.1 and its sub-lineages clusters are more danced than lineage A and A.1.
Discussion
Monkeypox has emerged and reemerged in Central and West Africa from time to time. It has been noted that the MPXV is endemic in countries like Nigeria, the Democratic Republic of the Congo, and Morocco. The virus causes outbreaks very rarely in other different parts of the world beyond African countries. Presently, the world is experiencing multi-countries episodes of MPX in non-African countries. However, very little research has been done in the direction of the evolutionary biology of hMPXV1. Alakunle et al. have studied this virus's evolution, epidemiology, and infection biology in Nigeria and explained the MPXV evolution in terms of gene loss and gene gain, and the role of recombination [76]. Recently, Babkin et al. have highlighted the molecular evolution of Orthopoxvirus, wherein they have illustrated the separation of deferent species of Orthopoxvirus. At the same time, the researchers have demonstrated the origin and genetic variants of several Orthopoxvirus species [65]. For MPXV, Likos et al. have reported two different clades of MPXV from the incident of emergence and re-emergence, previously reported by scientists. These are the MPXV Congo Basin (CB) and the West African (WA) clade [77, 78]. The observed fatal outcome of the Congo Basin clade was found to be about 10% [26, 78]. At the same time, the reported mortality of West African clade usually displays less than 1% [78, 79]. Here, we have developed the molecular phylogenetics of the hMPXV1, which has been attempted to illustrate every detail of the molecular evolution of this virus and the different lineage creation of this virus from October 2017 and August 2022. The molecular evolution has indicated recently evolved lineage B.1 and the association with the other recent lineages A, A.1, A.1.1, and A.2. Our study also describes the divergence of the different lineages of the hMPXV1 in the context of the phylogenetic tree. We also discuss the particular point of lineages of the hMPXV1 and their point of origin in the phylogeny tree. At the same time, our reset of the micro-evolution of lineage B.1 indicated the origin of the lineage started in May 2022. The microevolution shows the B.1 lineage is further mutating through adaptation and developing numerous sub-lineages. We analyzed our data using the Nextstrain server. Currently, the Nextstrain server used the lineage and sublineage instead of the clade. However, WHO is using the clade for the new naming of MPXV. Recently, the WHO announced the clades of MPXV, which comprises Clade I, Clade IIa, and Clade IIb [80]. A recent study by Isidro et al. has also analyzed the hMPXV1 from a phylogenomic perspective and described the divergence of the phylogenetic tree branches [81]. Our study also corroborates the study of Isidro et al. about the creation of lineage B.1 and its association with the recent hMPXV1 outbreaks.
At the same time, the research also noted the divergence of the genome cluster of MPXV. An in-depth divergence analysis might reveal the virus evolution and creation of lineages for common ancestry and amino acid substitutions [82, 83]. Our research indicates the genome cluster of all lineages, the recent origin, and its clustering of B.1. It has been noted that the DNA virus mutation rate is slow compared to RNA virus. It was noted that the variola virus's evolution rate is about 1 × 10−5 substitutions per site per year [84, 85]. If we calculate in terms of nucleotide changes, it should be related to approximately 1–2 nucleotide changes per year. However, the evolution study of this dsDNA virus is slow compared to the other pandemic ssRNA virus (SARS-CoV-2) [20, 86, 87].
Our epidemiological study has revealed previous and present outbreaks of MPXV infection. This outbreak is the first multi-countries outbreak of MPX in non-African countries. Cohen has also expressed a similar view [88]. Several researchers have also tried to assess the country-wise epidemiology of MPX. Miura et al. have tried to predict the incubation period for infected patients from the Netherlands. They found that the mean incubation period of the virus was 8.5 days. The estimation was performed using 18 cases (n = 18) [89]. Vivancos et al. discussed the community transmission of this virus in the UK. Here, researchers have tried to describe the monkeypox community and healthcare cases from the 588 patients [8]. Kraemer et al. have tried to illustrate the real-time epidemiological data and illustrated where they depicted the step by step of increase of the cumulative number of confirmed cases after the first observed case in the outbreak [29]. Similarly, Quarleri et al. described the spread, basic reproduction number (R0), and clinical symptoms of the virus [85]. Adler et al. have illustrated the clinical features of MPX infection and treatment outcomes of Brincidofovir and Tecovirimat. Brincidofovir was provided orally with a dose of 200 mg to three patients once a week. Tecovirimat treatment was provided orally with a dose of 600 mg to one patient twice daily for 2 weeks. Tecovirimat treatment to the patent shows a shorter duration of viral shedding [90]. However, this study reveals two significant points for the current multi-countries outbreaks. First, the episode informs the higher age group of infected individuals compared to the previous outbreaks. Second, the MPX spread among the MSM individuals. However, early detection and vaccination are the two essential criteria to stop the spread of MPX. Real-time PCR is one of the significant assays for the detection of the virus [91]. At the same time, we noted another central point from the epidemiological survey: the infection cases show low fatal rates compared to the Congo Basin (CB) clade and the West African (WA) clade. Graham also reported that the recent strain of the virus causes milder disease and the infection with the virus lesser death rate, which shows approximately 1% in rural poor populations [92]. It has been noted that the orthopox group of viruses is serologically cross-reactive. Therefore, antigen and antibody detection-based detection methods for all virus species might not be suitable for detection. So, the virus might not provide a specific confirmation antigen, and antibody-based detection (serology and antigen detection) methods might not be recommended. The WHO also does not recommend diagnosis or case investigation with this method [93].
Similarly, it has been noted that smallpox vaccination programs finished worldwide in 1980, after the eradication of smallpox. However, the protection of vaccinated individuals may have waned over time. Simultaneously, first-generation, original smallpox vaccines are no longer available from the smallpox-eradication program to the general public. It might be one cause of the present MPX outbreaks.
Finally, we also studied this virus’s entropy diversity and mutational landscape in terms of nucleic acid and codon contexts. The entropy range throughout the genome was found as approximately from 0 to 0.346 levels. At the same time, the mutational landscape throughout the hMPXV1 genome was noted as about 0 to 4 in the nucleic acid context and 0 to 7 in the codon context. We also studied the NGA/TNC context of G- > A/C- > T mutation in the angle of the human and found clustering of recent B.1 lineage. Isidro et al. have also analyzed the hMPXV1 mutational analysis and concluded the role of the APOBEC3 host in viral evolution. They also concluded that possible hMPXV1 human adaptation is ongoing [81]. However, our mutational result might help researchers to calculate the mutation of hMPXV1 throughout the genome in terms of nucleic acid and codon context to know more about the mutation in the genomic landscape of this virus.
Conclusion
Monkeypox is a neglected disease after it was first reported as an infection of monkeys in 1958. Very few researches have been studied the evolution of the hMPXV1, entropy diversity of genome, and mutational landscape of this virus. Therefore, there is a vast knowledge gap in most areas of the virus, starting from the evolution and mutation of this virus. Cohen describes that the neglected virus fetched the scientific community’s attention due to a recent outbreak [94]. Our study has shown the clues of the different lineages, especially the cluster of B.1 lineage and sub-lineages for the current MPX outbreak. The cluster formation also informs us about the ongoing viral evolution.
Monkeypox is a zoonotic disease, emerging and remerging in different parts of the world. The reservoir of the virus is yet to be known. Recently, we urged the scientific community for long-term research on zoonotic viruses such as the monkeypox virus and SARS-CoV-2 [95]. Our study on evolution, epidemiology, geographical distribution, entropy diversity, and mutational landscape will solve some knowledge gaps in that area of the virus and this viral disease during the current outbreak. The study might help the researchers to set up their research directions for the recent MPX episodes toward control measures considering epidemiology, therapeutics discovery, and vaccine research taking into account the evolution and mutational landscape of this important virus.
Acknowledgements
We are thankful to the researchers of the Nextstrain server for evolutionary analysis and distribution map generation. The sequence data has been fetched from Genbank. Therefore, we are also thankful to NCBI.
Author contribution
Conceptualization, data curation, data analysis, and manuscript writing were done by CC. MB developed the table and some figures. ARS did the validation. Manuscript reviewing and editing was done by KD. All authors have read and approved the manuscript.
Data availability
All data generated or analysed during this study are included in this published article.
Declarations
Ethics approval
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brust KB, Papineni V, Columbus C, Arroliga AC. COVID-19-from emerging global threat to ongoing pandemic crisis. Proc (Bayl Univ Med Cent). 2022;35(4):468–475. doi: 10.1080/08998280.2022.2068940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohapatra RK, et al. The recombinant variants of SARS-CoV-2: concerns continues amid COVID-19 pandemic. J Med Virol. 2022;94(8):3506–3508. doi: 10.1002/jmv.27780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohapatra RK, et al. The recently emerged BA. 4 and BA. 5 lineages of Omicron and their global health concerns amid the ongoing wave of COVID-19 pandemic–Correspondence. Int J Surg. 2022;103:106698. doi: 10.1016/j.ijsu.2022.106698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Lancet Infectious D. Monkeypox: a neglected old foe. Lancet Infect Dis. 2022;22(7):913 [DOI] [PMC free article] [PubMed]
- 5.Mohapatra RK, et al. Unexpected sudden rise of human monkeypox cases in multiple non-endemic countries amid COVID-19 pandemic and salient counteracting strategies: another potential global threat? Int J Surg. 2022;103:106705–106705. doi: 10.1016/j.ijsu.2022.106705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO. Multi-country monkeypox outbreak: situation update. 2022. https://www.who.int/publications/m/item/multi-country-outbreak-of-monkeypox--external-situation-report--4---24-august-2022 (Acessed on 27 August, 2022).
- 7.Vivancos R, et al. Community transmission of monkeypox in the United Kingdom, April to May 2022. Eurosurveillance. 2022;27(22):2200422. doi: 10.2807/1560-7917.ES.2022.27.22.2200422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kluge H, Ammon A. Monkeypox in Europe and beyond–tackling a neglected disease together. Eurosurveillance. 2022;27(24):2200482. doi: 10.2807/1560-7917.ES.2022.27.24.2200482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.León-Figueroa DA, et al. The never ending global emergence of viral zoonoses after COVID-19? The rising concern of monkeypox in Europe, North America and beyond. Travel med infect dis. 2022;49:102362. doi: 10.1016/j.tmaid.2022.102362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zumla A, et al. Monkeypox outbreaks outside endemic regions: scientific and social priorities. Lancet Infect Dis. 2022;22:929–931. doi: 10.1016/S1473-3099(22)00354-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Z. Monkeypox: a potential global threat? J Med Virol. 2022;94(9):4034–4036. doi: 10.1002/jmv.27884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferraro F, et al. multiple introductions of MPX in Italy from different geographic areas. Eurosurveillance. 2022;27(23):2200456. doi: 10.2807/1560-7917.ES.2022.27.23.2200456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhattacharya M, Dhama K, Chakraborty C. Recently spreading human monkeypox virus infection and its transmission during COVID-19 pandemic period: a travelers’ prospective. Travel Med Infect Dis. 2022;49:102398. doi: 10.1016/j.tmaid.2022.102398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parker S, Buller RM. A review of experimental and natural infections of animals with monkeypox virus between 1958 and 2012. Future virol. 2013;8(2):129–157. doi: 10.2217/fvl.12.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Velavan TP, Meyer CG. Monkeypox 2022 outbreak: an update. Trop Med Int Health. 2022;27(7):604–605. doi: 10.1111/tmi.13785. [DOI] [PubMed] [Google Scholar]
- 16.Bunge EM, et al. The changing epidemiology of human monkeypox—a potential threat? A systematic review. PLoS negl trop dis. 2022;16(2):e0010141. doi: 10.1371/journal.pntd.0010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sklenovska N, Van Ranst M. Emergence of monkeypox as the most important orthopoxvirus infection in humans. Front pub health. 2018;6:241. doi: 10.3389/fpubh.2018.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fenollar F, Mediannikov O. Emerging infectious diseases in Africa in the 21st century. New Microbes New Infect. 2018;26:S10–S18. doi: 10.1016/j.nmni.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chakraborty C, et al. Evolution, mode of transmission, and mutational landscape of newly emerging SARS-CoV-2 variants. MBio. 2021;12(4):e01140–e1221. doi: 10.1128/mBio.01140-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chakraborty C, et al. SARS-CoV-2 and other human coronaviruses: mapping of protease recognition sites, antigenic variation of spike protein and their grouping through molecular phylogenetics. Inf Genet Evol. 2021;89:104729. doi: 10.1016/j.meegid.2021.104729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Attwood SW, Hill SC, Aanensen DM, Connor TR, Pybus OG. Phylogenetic and phylodynamic approaches to understanding and combating the early SARS-CoV-2 pandemic. Nat Rev Genet. 2022;23(9):547–562. doi: 10.1038/s41576-022-00483-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chakraborty C, et al. Comparative genomics, evolutionary epidemiology, and RBD-hACE2 receptor binding pattern in B. 1.1. 7 (Alpha) and B. 1.617. 2 (Delta) related to their pandemic response in UK and India. Inf Genet Evol. 2022;101:105282. doi: 10.1016/j.meegid.2022.105282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakazawa Y, et al. Phylogenetic and ecologic perspectives of a monkeypox outbreak, southern Sudan, 2005. Emerg infect dis. 2013;19(2):237. doi: 10.3201/eid1902.121220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakazawa Y, et al. A phylogeographic investigation of African monkeypox. Viruses. 2015;7(4):2168–2184. doi: 10.3390/v7042168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sadeuh-Mba SA, et al. Monkeypox virus phylogenetic similarities between a human case detected in Cameroon in 2018 and the 2017–2018 outbreak in Nigeria. Infect Genet Evol. 2019;69:8–11. doi: 10.1016/j.meegid.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doshi RH, et al. Epidemiologic and ecologic investigations of monkeypox, Likouala Department, Republic of the Congo, 2017. Emerg infect dis. 2019;25(2):273. doi: 10.3201/eid2502.181222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beer EM, Rao VB. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLoS negl trop dis. 2019;13(10):e0007791. doi: 10.1371/journal.pntd.0007791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kraemer MU, et al. Tracking the 2022 monkeypox outbreak with epidemiological data in real-time. Lancet Infect Dis. 2022;22(7):941–942. doi: 10.1016/S1473-3099(22)00359-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dumbell K, Archard L. Comparison of white pock (h) mutants of monkeypox virus with parental monkeypox and with variola-like viruses isolated from animals. Nature. 1980;286(5768):29–32. doi: 10.1038/286029a0. [DOI] [PubMed] [Google Scholar]
- 30.Karumathil S, et al. Evolution of synonymous codon usage bias in west African and central African strains of monkeypox virus. Evol Bioinformatics. 2018;14:1176934318761368. doi: 10.1177/1176934318761368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.ECDC, Monkeypox multi-country outbreak. 2022. https://www.ecdc.europa.eu/en/monkeypox-outbreak (Acessed on 27 July, 2022).
- 32.CDC, U.S. Monkeypox Outbreak 2022: Situat Summ. 2022. https://www.cdc.gov/poxvirus/monkeypox/response/2022/index.html#:~:text=CDC%20is%20tracking%20an%20outbreak,with%20someone%20who%20has%20monkeypox. (Acessed on 27 July, 2022).
- 33.White J. PubMed 2.0. Medical Reference Services Quarterly. 2020;39(4):382–387. [DOI] [PubMed]
- 34.Kang P, Kalloniatis M, Doig GS. Using updated PubMed: new features and functions to enhance literature searches. JAMA. 2021;326(6):479–480. doi: 10.1001/jama.2021.12021. [DOI] [PubMed] [Google Scholar]
- 35.Thoma B, Chan TM. Using Google Scholar to track the scholarly output of research groups. Perspect med educ. 2019;8(3):201–205. doi: 10.1007/s40037-019-0515-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang L, Fang X, Zhu J. Knowledge mapping analysis of public health emergency management research based on web of science. Front Public Health. 2022;10:755201. doi: 10.3389/fpubh.2022.755201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao M, et al. A landscape analysis on virus: based on NCBI database. China CDC Weekly. 2022;4(7):120. doi: 10.46234/ccdcw2022.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carlson CJ, et al. The Global Virome in One Network (VIRION): an atlas of vertebrate-virus associations. MBio. 2022;13(2):e02985–e3021. doi: 10.1128/mbio.02985-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hadfield J, et al. Nextstrain: real-time tracking of pathogen evolution. Bioinformatics. 2018;34(23):4121–4123. doi: 10.1093/bioinformatics/bty407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Happi C, et al. Urgent need for a non-discriminatory and non-stigmatizing nomenclature for monkeypox virus. https://virological.org/t/urgent-need-for-a-non-discriminatory-and-non-stigmatizing-nomenclature-for-monkeypox-virus/853. (Acessed on 20 July, 2022). [DOI] [PMC free article] [PubMed]
- 41.Hammer Ø, Harper DA, Ryan PD. PAST: Paleontological statistics software package for education and data analysis. Palaeontol electron. 2001;4(1):9. [Google Scholar]
- 42.MathWorks I. MATLAB, High-performance numeric computation and visualization software: user’s guide: for UNIX Workstations 1992: MathWorks. Publishers the University of Michigan. pp. 1–90.
- 43.Jezek Z, et al. Human monkeypox: a study of 2,510 contacts of 214 patients. J Infect Dis. 1986;154(4):551–555. doi: 10.1093/infdis/154.4.551. [DOI] [PubMed] [Google Scholar]
- 44.Eozenou P. Enquête rétrospective sur un cas de monkeypox en République Unie du Cameroun. Bull OCEAC. 1980;2(3):23–26. [Google Scholar]
- 45.WHO. Weekly Bulletin on Outbreak and other Emergencies: Week 27: 30 June-06 July 2018. Health Emergency Information and Risk Assessment. https://apps.who.int/iris/handle/10665/273028. (Acessed on 20 July, 2022).
- 46.Metuge A, et al. Humanitarian led community-based surveillance: case study in Ekondo-titi, Cameroon. Confl Heal. 2021;15(1):17. doi: 10.1186/s13031-021-00354-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reed KD, et al. The detection of monkeypox in humans in the Western Hemisphere. N Engl J Med. 2004;350(4):342–350. doi: 10.1056/NEJMoa032299. [DOI] [PubMed] [Google Scholar]
- 48.Hobson G, et al. Family cluster of three cases of monkeypox imported from Nigeria to the United Kingdom, May 2021. Eurosurveillance. 2021;26(32):2100745. doi: 10.2807/1560-7917.ES.2021.26.32.2100745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Merouze F, JJ L. Monkeypox: second cas humain observé en Côte d'Ivoire (secteur de santé rurale de Daloa) 1983;43(2):145-7 [PubMed]
- 50.Breman JG, et al. Human monkeypox, 1970–79. Bull World Health Organ. 1980;58(2):165. [PMC free article] [PubMed] [Google Scholar]
- 51.Lourie B, et al. Human infection with monkeypox virus: laboratory investigation of six cases in West Africa. Bull World Health Organ. 1972;46(5):633. [PMC free article] [PubMed] [Google Scholar]
- 52.Li D, et al. Evaluation of the GeneXpert for human monkeypox diagnosis. Am J Trop Med Hyg. 2017;96(2):405. doi: 10.4269/ajtmh.16-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reynolds MG, et al. Human monkeypox in Sierra Leone after 44-year absence of reported cases. Emerg Infect Dis. 2019;25(5):1023. doi: 10.3201/eid2505.180832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heymann DL, Szczeniowski M, Esteves K. Re-emergence of monkeypox in Africa: a review of the past six years. Br Med Bull. 1998;54(3):693–702. doi: 10.1093/oxfordjournals.bmb.a011720. [DOI] [PubMed] [Google Scholar]
- 55.Breman JG. Monkeypox: an emerging infection for humans? Emerg Infect. 2000;4:45–67. doi: 10.1128/9781555816971.ch5. [DOI] [Google Scholar]
- 56.Rimoin AW, Mulembakani PM, Johnston SC, Lloyd Smith JO, Kisalu NK, Kinkela TL, et al. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc Natl Acad Sci U S A. 2010;107(37):16262–7. doi: 10.1073/pnas.1005769107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mwamba D, et al. Profil épidemiologique du monkeypox en RDC, 2010–2014. Ann Afr Med. 2014;8:1855–1860. [Google Scholar]
- 58.Nolen LD, et al. Extended human-to-human transmission during a monkeypox outbreak in the Democratic Republic of the Congo. Emerg Infect Dis. 2016;22(6):1014. doi: 10.3201/eid2206.150579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heymann DL, Simpson K. The evolving epidemiology of human monkeypox: questions still to be answered. 2021. Oxford University Press US. 1839–1841. [DOI] [PubMed]
- 60.Meyer A, Esposito JJ, Gras F, Kolakowski T, Fatras M, Muller G. Première apparition au Gabon de monkey-pox chez l’homme [First appearance of monkey pox in human beings in Gabon] Med Trop (Mars). 1991;51(1):53–7. [PubMed] [Google Scholar]
- 61.Organization WH. Technical Advisory Group on Human Monkeypox: report of a WHO meeting, Geneva, Switzerland, 11–12 January 1999. in Technical Advisory Group on Human Monkeypox: report of a WHO meeting, Geneva, Switzerland, 11–12 January 1999. 1999.
- 62.Damon IK, Roth CE, Chowdhary V. Discovery of monkeypox in Sudan. N Engl J Med. 2006;355(9):962–963. doi: 10.1056/NEJMc060792. [DOI] [PubMed] [Google Scholar]
- 63.Durski KN, et al. Emergence of monkeypox—west and central Africa, 1970–2017. Morb Mortal Wkly Rep. 2018;67(10):306. doi: 10.15585/mmwr.mm6710a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gweh DD, et al. Monkeypox Outbreak, Harper District, Maryland County, Liberia, December 2017. J Interv Epidemiol Publ Health. 2021;4(5).
- 65.Babkin IV, Babkina IN, Tikunova NV. An update of orthopoxvirus molecular evolution. Viruses. 2022;14(2):388. doi: 10.3390/v14020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Faye O, et al. Genomic characterisation of human monkeypox virus in Nigeria. Lancet Infect Dis. 2018;18(3):246. doi: 10.1016/S1473-3099(18)30043-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Khodakevich L, et al. Orthopoxvirose simienne de l'homme en République centrafricaine. Bulletin de la Société de pathologie exotique. 1985;78(3):311–320. [PubMed] [Google Scholar]
- 68.Berthet N, et al. Maculopapular lesions in the Central African Republic. The Lancet. 2011;378(9799):1354. doi: 10.1016/S0140-6736(11)61142-2. [DOI] [PubMed] [Google Scholar]
- 69.Kalthan E, et al. Twelve cases of monkeypox virus outbreak in Bangassou District (Central African Republic) in December 2015. Bulletin de la Societe de Pathologie Exotique (1990) 2016;109(5):358–363. doi: 10.1007/s13149-016-0516-z. [DOI] [PubMed] [Google Scholar]
- 70.Boumandouki P, et al. Orthopoxvirose simienne (ou variole du singe): étude de 8 cas observés à l’hôpital d’Impfondo de la République du Congo. Bull Soc Pathol Exot. 2007;100(1):17–21. [PubMed] [Google Scholar]
- 71.Learned LA, et al. Extended interhuman transmission of monkeypox in a hospital community in the Republic of the Congo, 2003. Am J Trop Med Hyg. 2005;73(2):428–434. doi: 10.4269/ajtmh.2005.73.428. [DOI] [PubMed] [Google Scholar]
- 72.Reynolds MG, et al. Detection of human monkeypox in the Republic of the Congo following intensive community education. Am J Trop Med Hyg. 2013;88(5):982. doi: 10.4269/ajtmh.12-0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guagliardo SAJ, et al. Do Monkeypox exposures vary by Ethnicity? Comparison of Aka and Bantu suspected monkeypox cases. Am J Trop Med Hyg. 2020;102(1):202–205. doi: 10.4269/ajtmh.19-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kupferschmidt K. Why monkeypox is mostly hitting men who have sex with men. Sci (New York, NY) 2022;376(6600):1364–1365. doi: 10.1126/science.add5966. [DOI] [PubMed] [Google Scholar]
- 75.Mahase E. Monkeypox: Gay and bisexual men with high exposure risk will be offered vaccine in England. Bri Med J Publ Group. 2022;377:o1542. doi: 10.1136/bmj.o1542. [DOI] [PubMed] [Google Scholar]
- 76.Alakunle E, et al. Monkeypox virus in Nigeria: infection biology, epidemiol evolution. Viruses. 2020;12(11):1257. doi: 10.3390/v12111257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Likos AM, et al. A tale of two clades: monkeypox viruses. J Gen Virol. 2005;86(10):2661–2672. doi: 10.1099/vir.0.81215-0. [DOI] [PubMed] [Google Scholar]
- 78.Simpson K, et al. Human monkeypox–After 40 years, an unintended consequence of smallpox eradication. Vaccine. 2020;38(33):5077–5081. doi: 10.1016/j.vaccine.2020.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ogoina D, et al. The 2017 human monkeypox outbreak in Nigeria—report of outbreak experience and response in the Niger Delta University Teaching Hospital, Bayelsa State, Nigeria. PLoS ONE. 2019;14(4):e0214229. doi: 10.1371/journal.pone.0214229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.WHO. Monkeypox: experts give virus variants new names. 2022. https://www.who.int/news/item/12-08-2022-monkeypox--experts-give-virus-variants-new-names (Acessed on 27 August, 2022).
- 81.Isidro J, et al. Phylogenomic characterization and signs of microevolution in the multi-country outbreak of monkeypox virus. Nat Med 2022:1-1 [DOI] [PMC free article] [PubMed]
- 82.Virk RK, et al. Divergent evolutionary trajectories of influenza B viruses underlie their contemporaneous epidemic activity. Proc Natl Acad Sci. 2020;117(1):619–628. doi: 10.1073/pnas.1916585116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu A, et al. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27(3):325–328. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Firth C, Kitchen A, Shapiro B, Suchard MA, Holmes EC, Rambaut A. Using time-structured data to estimate evolutionary rates of double-stranded DNA viruses. Mol Biol Evol. 2010;27(9):2038–51. doi: 10.1093/molbev/msq088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Quarleri J, Delpino M, Galvan V. Monkeypox: considerations for the understanding and containment of the current outbreak in non-endemic countries. GeroScience. 2022:1–9 [DOI] [PMC free article] [PubMed]
- 86.Chakraborty C, et al. D614G mutation eventuates in all VOI and VOC in SARS-CoV-2: Is it part of the positive selection pioneered by Darwin? Elsevier. 2021;237–241. [DOI] [PMC free article] [PubMed]
- 87.Voskarides K. SARS-CoV-2: tracing the origin, tracking the evolution. BMC Med Genomics. 2022;15(1):62. doi: 10.1186/s12920-022-01208-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cohen J. Monkeypox outbreak questions intensify as cases soar. Science. 2022;376(6596):902–903. doi: 10.1126/science.add1583. [DOI] [PubMed] [Google Scholar]
- 89.Miura F, et al. Estimated incubation period for monkeypox cases confirmed in the Netherlands, May 2022. Eurosurveillance. 2022;27(24):2200448. doi: 10.2807/1560-7917.ES.2022.27.24.2200448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Adler H, et al. Clinical features and management of human monkeypox: a retrospective observational study in the UK. The Lancet Infect Dis. 2022. [DOI] [PMC free article] [PubMed]
- 91.Li Y, et al. Real-time PCR assays for the specific detection of monkeypox virus West African and Congo Basin strain DNA. J Virol Methods. 2010;169(1):223–227. doi: 10.1016/j.jviromet.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Graham F. Daily briefing: Why scientists are worried about monkeypox. Nature. [DOI] [PubMed]
- 93.WHO. Monkeypox: Key facts. 2022. https://www.who.int/news-room/fact-sheets/detail/monkeypox. (Acessed on 20 July, 2022).
- 94.Cohen J. Global outbreak puts spotlight on neglected virus. Sci (New York, NY) 2022;3(376):1032–1033. doi: 10.1126/science.add2701. [DOI] [PubMed] [Google Scholar]
- 95.Chakraborty C, et al. Appearance and re-appearance of zoonotic disease during the pandemic period: long-term monitoring and analysis of zoonosis is crucial to confirm the animal origin of SARS-CoV-2 and monkeypox virus. Veterinary Quarterly, 2022(just-accepted): p. 1–11. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.