Abstract
As a consequence of the outbreak of SARS-CoV-2, the clinical practice of otolaryngologists underwent profound transformations. Non-aerosol-generating procedures have been researched and implemented. Transcutaneous laryngeal ultrasonography (TLUSG) provides a rapid and noninvasive method to assess laryngeal function and can support the management of laryngeal disorders. With the aim of investigating the clinical usefulness of TLUSG in otolaryngology practice, a review of the literature published on PubMed, Cochrane Library and Ovid/ Medline databases was performed up to March 2022. 38 studies were eligible to be included in the review. The selected papers were divided into six topics of interest: evaluation of vocal cords function, diagnosis of laryngeal disorders in infants and children, evaluation of swallowing disorders, assessment of laryngeal cancer and other laryngeal lesions, ultrasound-guided cricothyroidotomy, ultrasound-guided laryngeal electromyography. The results of this review demonstrated that TLUSG, applied to ENT practice, can be a valid method for dynamic laryngeal assessment and airway management, since it is time-efficient, non invasive, well tolerated and easily performed.
Keywords: Transcutaneous laryngeal ultrasonography, TLUSG, COVID-19, Laryngeal ultrasonography, ENT, Otolaryngology
Introduction
The impact of the COVID-19 global pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has profoundly affected the practice of otolaryngology [1]. The virus is highly transmissible through droplets and aerosols created during aerosol generating procedures (AGPs) such as endoscopy. Otolaryngologists are well known at-risk subjects for potential infection, since they are particularly exposed to viral transmission through aerosol particles during ENT clinical examination, endoscopic procedures, head and neck surgery [2]. Current recommendations aim to minimize the generation of aerosols as much as possible; in this perspective, endoscopic examination should be avoided as much as possible, and strictly suggested in both emergent and elective situations, if absolutely needed (risk of malignancy, airway obstruction) [3, 4]. Otolaryngologists are currently required to find alternative diagnostic and therapeutic strategies and to prefer, when possible, non-aerosol-generating procedures [5].
Transcutaneous Laryngeal Ultrasonography (TLUSG) has emerged as a non-invasive and well tolerated procedure for laryngeal assessment and airway management. Expecially during the ongoing COVID-19 pandemic, TLUSG has been proposed as a safer alternative to direct flexible laryngoscopy, due to its non-aerosol producing nature [6, 7]. TLUSG may play a role in multiple aspects of otolaryngological diagnostic and therapeutic procedures as the assessment of vocal cord mobility, the performance of cricothyroidotomy or laryngeal electromyography, the examination of swallowing behavior, the management of pediatric dysphonia and stridor and the assessment of laryngeal cancers.
The goal of the present review of literature is to describe the clinical usefulness of TLUSG in otolaryngology practice, in particular in the COVID-19 era, focusing on its diagnostic efficiency, safety and potential advantages. Our purpose is to provide detailed descriptions of the technique in the various fields of application and to explore ongoing clinical recommendations of these applications, to allow ENT clinicians to consider the use of laryngeal ultrasound in their clinical practice, expecially during emergency situations.
Methods
We reviewed the literature to investigate all the application fields of transcutaneous laryngeal ultrasonography in ENT clinical practice. The PRISMA statement guidelines [8] were used to perform this literature review, with the purpose to analyze the accuracy and potential benefits of TLUSG in comparison with accepted diagnostic standard. PubMed, Cochrane Library and Ovid/ Medline databases were consulted, up to March 2022. The following medical subject heading (MeSH) terms and their possible combinations were searched in Title/Abstract: [(“laryngeal ultrasonography” OR “transcutaneous laryngeal ultrasonography” OR “laryngeal ultrasound” OR “TLUSG”) AND (“otolaryngology” OR “ENT”)]. Some of the relevant studies were searched manually to avoid any omissions. All candidate articles were reviewed by two independent reviewers (P.I.S., R.A). The main inclusion criteria were: english-language articles, randomised and controlled trials in humans and observational studies investigating the clinical features of TLUSG. Exclusion criteria were as follows: case reports, editorials, commentary articles, literature reviews and meta-analyses, clinical guidelines, conference papers, veterinary articles. Studies not focusing on TLUSG as a diagnostic tool or a therapeutic procedure in the field of otolaryngology were excluded. Papers where the methodology was not consistent were also excluded. The following data were extracted and summarized in Table 1: first author, publication year, country, topic of interest, application field, sample, main results.
Table 1.
Features of studies included in the review
| First author | Publication year | Country | Topic of interest | Application field | Sample | Main results | |
|---|---|---|---|---|---|---|---|
| 1 | Amis [9] | 2012 | USA | Evaluation of vocal cords function | Assessment of vocal fold paresis | 16 | Sensitivity: 71%. Specificity: 89% |
| 2 | Alexander [24] | 2021 | USA | Diagnosis of laryngeal disorders in infants and children | Diagnosis of vocal fold movement impairment in infants and children | 20 | Sensitivity: 89.0% (quantitative interpretations), 87.3% (qualitative interpretations). Specificity: 92.6% |
| 3 | Bergeret-Cassagne [21] | 2017 | France | Evaluation of vocal cords function | Definition of quantitative measures to characterize vocal fold motion | 55 | Reproducibility of quantitative measures: excellent correlations (r 0.90) |
| 4 | Cheng [15] | 2012 | Taiwan | Evaluation of vocal cords function | Preoperative assessment of vocal cord movement during thyroid and parathyroid surgery | 114 | Detection of vocal cord movement: 84% |
| 5 | De Miguel [17] | 2017 | Spain | Evaluation of vocal cords function | Diagnosis of vocal cord paralysis in the immediate postoperative period following total thyroidectomy | 93 | Sensitivity: 93.3%. Specificity: 96.1% |
| 6 | Dedecjus [13] | 2010 | Poland | Evaluation of vocal cords function | Evaluation of the functionality of vocal folds before and after thyroidectomy | 50 | Sensitivity: 100%. Specificity: 95.7% |
| 7 | Desai [34] | 2004 | India | Assessment of laryngeal cancer and other laryngeal lesions | Assessment of laryngeal and laryngopharyngeal cancers | 25 | Identification of vocal cord mobility: 92%; thyroid cartilage invasion: 68%; extra-laryngeal spread: 16% |
| 8 | Dhoot [35] | 2017 | India | Assessment of laryngeal cancer and other laryngeal lesions | Assessment of thyroid cartilage invasion in laryngeal and hypopharyngeal cancers | 62 | Sensitivity: 98%; specificity: 75% |
| 9 | Gadd [49] | 2018 | Australia | Ultrasound-guided cricothyroidotomy | Cricothyroid membrane localization during ultrasound-guided cricothyroidotomy | 80 | Sensitivity: 80%. Specificity: 15% |
| 10 | Gambardella [10] | 2020 | Italy | Evaluation of vocal cords function | Preoperative evaluation of vocal cords function in patients candidates to thyroid surgery | 396 | Sensitivity: 96.8%. Specificity: 95.6% |
| 11 | Gritzmann [33] | 1989 | Austria | Assessment of laryngeal cancer and other laryngeal lesions | Assessment of advanced laryngeal cancer | 37 | Sensitivity: 95%. Specificity: 73% |
| 12 | Hu [37] | 2021 | China | Assessment of laryngeal cancer and other laryngeal lesions | Preoperative assessment of laryngeal carcinoma | 38 | Accuracy rate: 82.9% for conventional US, 87.8% for CEUS, and 90.2% |
| 13 | Huang [31] | 2019 | China | Diagnosis of laryngeal disorders in infants and children | Diagnosis of infant laryngomalacia | 40 | Sensitivity: 96.3%. Specificity: 84.6% |
| 14 | Kandil [23] | 2016 | USA | Evaluation of vocal cords function | Evaluation of preoperative and postoperative vocal fold function in patients undergoing thyroid or parathyroid surgery | 250 | Sensitivity of 53.8%; Specificity of 50.5% |
| 15 | Klinge [28] | 2016 | Germany | Diagnosis of laryngeal disorders in infants and children | Visualization of laryngeal structures and vocal fold movements of children using synchronous video laryngoscopy and laryngeal sonography | 35 | Sonography-only identification rate: 80%. Synchronic laryngoscopy and sonography identification rate: 90% |
| 16 | Klinge [52] | 2019 | Germany | Ultrasound-guided laryngeal electromyography | Ultrasonography-guided electromagnetic needle tracking in laryngeal electromyography | 19 | Adequate LEMG signal: 55% examinations |
| 17 | Kuhl [41] | 2003 | Germany | Evaluation of swallowing disorders | Analysis of laryngeal elevation during swallowing | 42 | Reduction of distance between the hyoid bone and the thyroid cartilage during swallowing in normal subjects: 61%. In the patients with neurogenic dysphagia: 42% |
| 18 | Lazard [19] | 2018 | France | Evaluation of vocal cords function | Laryngeal immobility diagnosis in patients with voice disorders after thyroid/parathyroid surgery | 100 | Subjective interpretation—sensitivity: 100%. Specificity: 96%. Quantitative criteria—Sensitivity and specificity: 82% |
| 19 | Matsuo [42] | 2020 | Japan | Evaluation of swallowing disorders | Evaluation of swallowing movement | 84 | Value of HL motion ratio for normal swallowing: 0.5 |
| 20 | Matsuo [43] | 2021 | Japan | Evaluation of swallowing disorders | Detection of poststroke oropharyngeal dysphagia | 36 |
Laryngeal duration—sensitivity: 72.2%. Specificity: 88.9% HL motion ratio—sensitivity and specificity: 88.9% |
| 21 | Nasr [39] | 2013 | Egypt | Assessment of laryngeal cancer and other laryngeal lesions | Diagnosis of different laryngeal diseases | 54 | Diagnosis of vocal cord nodules: 27.3%, polyps and cysts: 100%, Reinke’s edema: 60%, laryngeal masses: 78.6% |
| 22 | Ongkasuwan [29] | 2017 | USA | Diagnosis of laryngeal disorders in infants and children | Diagnosis of vocal fold nodules in dysphonic children | 46 | Sensitivity: 100%. Specificity: 87% |
| 23 | Park [51] | 2016 | Korea | Ultrasound-guided laryngeal electromyography | Ultrasonography guided laryngeal electromyography | 20 | Cricothyroid muscles identification: 100%. Thyroarytenoid muscles identification: 85% |
| 24 | Sciancalepore [7] | 2021 | Italy | Evaluation of vocal cords function | Assessment of vocal fold movement during COVID-19 | 38 | Sensitivity: 80%; Specificity: 96.42% |
| 25 | Seo [53] | 2014 | Korea | Ultrasound-guided laryngeal electromyography | Measurement and localization of the representative anatomic landmarks for laryngeal electromyography | 518 | The longest dimension of the cricothyroid membrane, height of the arch of the cricoid cartilage, and distance from the superior border of the cricoid cartilage to the midpoint of the vocal fold measured: 1.06 ± 0.33 cm, 0.83 ± 0.24 cm and 1.88 ± 0.48 cm |
| 26 | Shah [16] | 2019 | India | Evaluation of vocal cords function | Assessment of the vocal cord mobility in patients undergoing thyroid surgery | 45 | Sensitivity: 75%. Specificity: 95.1 |
| 27 | Shirley [30] | 2019 | Israel | Diagnosis of laryngeal disorders in infants and children | Assessment of pediatric dysphonia and stridor | 32 | Sensitivity: 87%. Specificity: 100% |
| 28 | Siddiqui [47] | 2018 | Canada | Ultrasound-guided cricothyroidotomy | Identification of the cricothyroid membrane in ultrasound-guided cricothyrotomy in subjects with poorly defined neck landmarks | 223 | Success rate of ultrasound-guided identification of the cricothyroid membrane (CM): 81%. Success rate of palpation identification of the CM: 8% |
| 29 | Vats [27] | 2004 | UK | Diagnosis of laryngeal disorders in infants and children | Assessment of vocal fold paralysis in children | 55 | Concordance rate TLUSG—laryngoscopy: 81.2%. In patients aged over 12 months: 89.5% |
| 30 | Wang [26] | 2011 | China | Diagnosis of laryngeal disorders in infants and children | Assessment of vocal fold paralysis in children using measurement of the maximum glottic angle (MGA) | 45 | Mean value of MGA: 61.4 ± 9.00 in the normal larynx; 42.25 ± 10.41 in the paralyzed larynx |
| 31 | Wang [38] | 2020 | China | Assessment of laryngeal cancer and other laryngeal lesions | Diagnosis of vocal fold polyps | 87 | The rate of detection of vocal fold polyps: 88.0% |
| 32 | Wolff [11] | 2022 | Poland | Evaluation of vocal cords function | Assessment of vocal fold function in patients after thyroid, parathyroid and neck lymph node surgery | 219 | Sensitivity 98.1%. Specificity 100% |
| 33 | Wong [14] | 2017 | China | Evaluation of vocal cords function | Assessment of vocal folds function before thyroidectomy | 1000 | Sensitivity: 88.9% |
| 34 | Wong [18] | 2014 | China | Evaluation of vocal cords function | Vocal cord asymmetry detection after thyroidectomy | 169 | Vocal cord asymmetry seen on TLUSG significantly correlated with grade and roughness components on the GRBAS scale |
| 35 | Wong [20] | 2014 | China | Evaluation of vocal cords function | Assessment of vocal cords post-thyroidectomy: identification of sonographic landmarks | 245 | Visualization rate of false cords, true cords, and arytenoids: 92.7%, 36.7%, 89.8% |
| 36 | Wong [22] | 2015 | China | Evaluation of vocal cords function | Assessment of vocal cord function after thyroidectomy. Evaluation of factors for unassessable vocal cords | 581 |
Older age: odds ratio (OR) = 1.055 Male sex: OR = 13.657 Taller height: OR = 1.098. Shorter distance from cricoid cartilage to incision: OR = 0.655 |
| 37 | Xia [36] | 2013 | China | Assessment of laryngeal cancer and other laryngeal lesions | Assessment of laryngeal carcinoma | 72 | Detection rate of ultrasonography: 87.5% |
| 38 | Zawadzka-Glos [32] | 2013 | Poland | Diagnosis of laryngeal disorders in infants and children | Evaluation of laryngeal injuries in children | 15 | TLUSG recommended in every case of laryngeal injury as an additional non-invasive complementary diagnostic examination |
Results
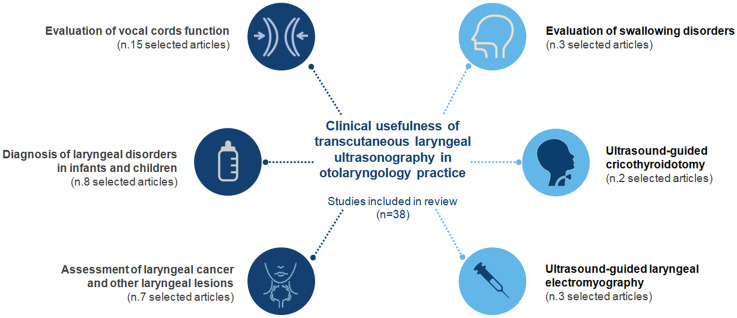
A PRISMA flow chart of the process involved in reference selection is shown in Fig. 1. The initial search returned 8428 citations, using the search strategy and sources listed. After de-duplication 5494 records were screened. Consequently the articles were selected for relevance according to the purpose of this review. Articles that do not fit the eligibility criteria were excluded: non original articles, non-English language articles, non relevant and methodology inconsistent articles, veterinary papers. Finally 38 studies with a total of 5056 participants were eligible to be included in the review. The selected papers were divided into 6 topics of interest, depending on the field of application of TLUSG (Fig. 2): evaluation of vocal cords function (n. 15 selected articles), diagnosis of laryngeal disorders in infants and children (n. 8 selected articles), assessment of laryngeal cancer and other laryngeal lesions (n. 7 selected articles), evaluation of swallowing disorders (n. 3 selected articles), ultrasound-guided cricothyroidotomy (n. 2 selected articles) and ultrasound-guided laryngeal electromyography (n. 3 selected articles). Evaluation of vocal cords function using TLUSG was the most investigated topic.
Fig. 1.

PRISMA (primary reporting items for systematic reviews and meta-analyses) flow diagram
Fig. 2.
Topics of interest of included articles about clinical applications of transcutaneous laryngeal ultrasonography (TLUSG) in otolaryngology practice
Discussion
Evaluation of vocal cords function
Several studies have explored the relevance of ultrasound imaging in the anatomical description of the laryngeal tract. TLUSG represents an interesting alternative for the evaluation of vocal cord function, as compared to endoscopic examination or CT and MRI due to availability, cost-effectiveness, noninvasiveness and high tolerance [9, 10]. During the ultrasound examination, the linear probe was placed transversely, in the midline, over the middle portion of the thyroid cartilage. Symmetry, mobility and position of vocal cords during breathing and phonation were assessed. The diagnosis of vocal fold paralysis is based on the asymmetric abduction and adduction movements of the vocal folds [10]. A recent study [11] showed that the movement of vocal folds are best assessed during whispering the vowel “e”. If the vocal folds are not visible from a midline approach due to acute shape or calcification of the thyroid cartilage, each vocal fold can be assessed laterally to the cartilage [12]. According to Dedecjus et al.[13], by applying Doppler gate to real-time B scan of vocal folds, signals corresponding to tissue motion were observed in the vibratory area of vocal folds during phonation. In contrast, another author [11] considered Doppler wave pattern a not necessary component of the TLUSG evaluation, being unable to replace the observation of vocal folds movement during phonation and respiration. Recently, TLUSG has been proposed as a promising noninvasive tool for routine preoperative and postoperative vocal cord examination in thyroid surgery. According to some authors, TLUSG should be even incorporated as a part of the ultrasound examination of the thyroid [7, 14]. Several researchers recommended TLUSG as a valuable alternative to flexible laryngoscopy for preoperative detection of vocal cord palsy or early identification of postoperative vocal fold dysfunction with its later monitoring, in patients undergoing thyroidectomy or other endocrine-related neck procedures [13–16]. An Italian prospective multicentric study [10] agreed that TLUSG represents a valid alternative method in the preoperatively assessment of vocal cords (sensitivity of 96.8%, a specificity of 95.6%), and it could be useful in identifying patients addressable to second-level examination with laryngoscopy. De Miguel and colleagues [17] proposed this technique for evaluating vocal cord mobility shortly after total thyroidectomy. He found that laryngeal examination using TLUSG has a high sensitivity and specificity (respectively 93.3% and 96.1%) for detecting vocal cord paralysis. The presence of postoperative vocal cord asymmetry assessed by TLUSG correlates with voice quality changes after thyroidectomy, detected with the GRBAS scale and the voice impairment score [18]. In addition to the subjective interpretation of vocal fold motion, two quantitative criteria were proposed: a symmetry index and a mobility index of the two hemi-larynges in adduction and abduction. The quantitative criteria provided a sensitivity and specificity of both 82%, when based on symmetry index. When combining symmetry and mobility index, the sensitivity reached 94%, but the specificity fell to 66% [19]. Current debates deal with the choice of appropriate sonographic landmarks using transcutaneous laryngeal ultrasonography. Wong et al. [20] explored 3 sonographic landmarks: true vocal folds, false vocal folds and arytenoids, in order to discover which landmark provides a more reliable assessment of vocal cords motion. He demonstrated that each sonographic landmark had similar diagnostic accuracy. The visualization of normal movement in one of the sonographic landmarks would be sufficient to exclude vocal cord palsy. Bergeret-Cassagne [21] analyzed vocal fold during free breathing using two-dimensional dynamic ultrasound in axial view. Because of their high echogenicity, arytenoids are proposed as surrogate markers to characterize vocal fold motion. Some studies indicate that there are variables as male gender and calcification of the structures in the advanced age that may limit the performance of TLUSG examination. Wong et al. [22] evaluated what factors affected assessability and accuracy of TLUSG: older age, male sex, tall in height, and incision closer to the thyroid cartilage were independent contributing factors for unassessable vocal cords. The above mentioned study by Wolff [11] showed unsatisfying vocal cords visibility by TLUSG in men (vocal cords visualization in women: 82.0%; men: 7.7%), smokers, patients with higher BMI (32.34 vs. 27.65 kg/m2), secondary surgery, multifocal or T2 cancer, higher left lobe volume and higher free T3 levels.
Consider TLUSG as an alternative to laryngoscopy is still a matter of scientific debate. According to Kandil et al. [23] TLUSG should not be considered as an alternative to the current practice of flexible fiberoptic laryngoscopy. In fact adequate ultrasonographic visualization of the vocal folds and arytenoids is challenging in overweight and obese patients and in the postoperative setting. (Sensitivity and specificity of preoperative TLUSG: 53.8%, 50.5%; postoperative TLUSG: 55.6%, 38.7%). The results regarding the diagnostic accuracy of TLUSG in the assessment of laryngeal function are controversial. Accordingly there is a tendency to consider TLUSG as just a screening method for vocal cord paralysis, followed by diagnostic confirmation with laryngoscopy, if required [11].
We believe that TLUSG can not replace laryngoscopy, that remains the gold standard to confirm the diagnosis of vocal fold paralysis. However we recognize its key role in providing to otolaryngologists a noninvasive method to screen for vocal cord paralysis during COVID-19 pandemic [7]. Especially in the assessment of laryngeal function in non-compliant or high-risk infection patients, TLUSG would avoid the need for an invasive and non strictly necessary procedure.
Diagnosis of laryngeal disorders in infants and children
The management of stridor or dysphonia in infants and children is an important target for otolaryngologists. Laryngoscopy, the current gold standard, can be challenging in infants due to poor compliance, floppy supraglottic structures, risk of laryngospasm. TLUSG is a nonirradiating and noninvasive method for assessing the upper airway in children. It may be a diagnostic alternative for the evaluation of laryngeal disorders, well tolerated by patients and parents and easy to perform. It also decreases aerosolization during COVID-19 pandemic and may serve as an alternative tool of visualizing the larynx in children who can not tolerate laryngoscopy [24]. During TLUGS patients are scanned with a linear probe, in the supine position, with the neck well extended. Transverse scans are performed at the level of the false vocal cords, the true vocal cords and the subglottic area. No sedation or anesthesia is required [25]. Many authors have demonstrated that laryngeal ultrasound can be used to evaluate vocal cord function, allowing early detection of vocal fold paralysis in children [24, 26, 27]. The diagnostic criteria for vocal fold paralysis using TLUGS are: abnormal mobility, hyperechoic air-column band of the glottic rima during phonation, flaccid vocal fold and asymmetry of the glottal structures [26]. Vats and colleagues [27] reported the need to interpret with caution ultrasonographic findings in patients aged below 12 months (Concordance rate of 77.7% vs 89.5% in patients over 12 months). However, Alexander et al. [24] investigated a quantitative laryngeal ultrasound interpretation by vocal fold-arytenoid angle measurement, using a digital protractor. He demonstrated that quantitative measurements may increase LUS interpretation accuracy, sensitivity, and negative predictive value. Quantitative analysis of vocal fold mobility was carried out also by Wang et al. [26] through the measurement of the maximum glottic angle and vocal fold-arytenoid angle, defining them quantitative indicators of vocal fold immobility. Synchronic video laryngoscopy and sonography of the larynx were proposed by Klinge et al. [28] The addition of a laryngoscopic view to sonography image increased investigators’ ability to correctly identify anatomical structures in the larynx (Sonography-only identification rate: 80%; Synchronic laryngoscopy and sonography identification rate: 90%). Some authors suggested TLUSG as a diagnostic method for vocal cord nodules or other space-occupied lesions of the larynx in infant and children, with high sensitivity and substantial agreement with laryngeal stroboscopy [29, 30]. Moreover, Garel [25] showed the possibility to diagnose laryngeal stenosis, laryngeal atresia, anterior synechiae, lymphangioma, hemangioma and laryngeal edema, allowing even subglottic examination, generally difficult using endoscopy, and the assessment of extra-laryngeal components. Main limitations of this technique are: poor cooperation in children, anterior calcifications after surgery, causing an acoustic shadow that makes the examination impossible, and the limitated visualization of a defect of the posterior lamina of the cricoid, in case of laryngeal clefts [25, 30]. A research by Huang et al. [31] evaluated the role of laryngeal ultrasound in diagnosis of infant laryngomalacia: he showed that the presence of an omega-shaped epiglottis and/or arytenoid tissue prolapsing into the airway during inspiration may be suggestive for laryngomalacia with an excellent comparability with flexible fiberoptic laryngoscopy (sensitivity: 96.3%, specificity: 84.6%). TLUSG is also recommended in the case of laryngeal injury in children. In fact the evaluation of vocal folds mobility is more accurate if compared with laryngoscopy under general anesthesia and easily lead to the diagnosis of fractures of cartilages without dislocation [32].
Assessment of laryngeal cancer and other laryngeal lesions
Laryngoscopy, CT and MRI are commonly used to diagnose laryngeal cancers and determine correct staging. TLUSG was proposed as a promising technique in the assessment of laryngeal carcinoma and it could be used as a valuable supplementary imaging method to CT/MRI and laryngoscopy, helping in planning treatment. Studies have confirmed that ultrasonography can accurately investigate laryngeal cartilage infiltration, local extension of laryngeal tumors, vocal cord mobility, nodal staging and extralaryngeal spread into pre-epiglottic space, base of the tongue, hypopharynx, subglottic space and thyroid gland [33, 34]. According to Dhoot et al. [35], ultrasonography has been shown to be more sensitive than CT in diagnosing invasion of the thyroid cartilage. TLUGS would be used to solve the diagnostic uncertainty of the presence of cartilage invasion when CT is inconclusive (For thyroid cartilage invasion, CT sensitivity: 91%, vs US sensitivity: 98%). Structures lying in the larynx anteriorly or superficially as thyroid cartilage, pre-epiglottic space, paraglottic space, thyroid and cervical soft tissues are easy to be assessed with TLUGS. Commonly, these structures are isoechoic or hyperechoic; in contrast the cancer is visualized as a hypoechoic invading mass. Moreover, the paraglottic space normally looks like a thin layer of adipose tissue mobile during respiration in contrast to the motionless of adjacent inner perichondrium of thyroid cartilage. The neoplastic spread to the paraglottic space may be suspected if movement is reduced or ceased. On the other hand, the posterior and bilateral wall of subglottis can be difficultly visualized by TLUGS due to the air influence and the refractive shadowing [36]. Moreover sonography has proved to be reliable in cervical lymph node staging and in the relationship of the tumor or the lymph nodes to the great cervical vessels. Duplex sonography of the great cervical vessels can be performed during the same investigation to exclude stenosis of the carotid artery [33]. In addition Hu et al. [37] proposed contrast-enhanced ultrasound (CEUS) as a complementary modality for the detection and pretherapeutic staging of laryngeal carcinoma, revealing perfusion characteristics of tumors. According to the author, CEUS has a reliable T-staging accuracy and diagnostic power for detecting laryngeal cartilage invasion (Sensitivity, specificity, and accuracy in the evaluation of involvement of thyroid cartilage by CEUS of 92.9%, 87.5%, and 90.0%). The value of transcutaneous laryngeal ultrasonography was explored also in the diagnosis of benign vocal fold lesions because of its cost-effectiveness, non-radiation nature and non-invasiveness. Wang et al. [38] described high-frequency ultrasound images of vocal fold polyps as limited uniform hypo-echoic area between two hyper-echoic regions (superficial lamina propria), with clear boundary and regular shape. Color Doppler blood flow signals could not be detected in most vocal fold polyps. (Rate of detection of vocal fold polyps by TLUGS: 88.0%: 92.0% for circular polyps and 70.6% for non-circular polyps). Current literature revealed concerns regarding TLUGS ability to accurately diagnose benign diseases of the vocal fold, such as polyps, mainly because the thyroid cartilage calcification at the glottic level may affect the vocal fold visualization [23]. In fact the identification of the morphology, location and size of vocal fold polyps may be reliable in patients with thyroid cartilage without calcification or a calcification length percentage < 33% [38]. A comparative cross-sectional study by Nasr et al. [39] evaluated laryngeal ultrasound as an alternative to CT in the diagnosis of different laryngeal diseases: TLUGS was found to be successful in detecting vocal cord nodules (27.3%) polyps and cysts (100%), Reinke’s edema (60%) and laryngeal masses (78.6%). Ultrasonographic findings were comparable with those of the CT scans.
In conclusion TLUGS can be used as a non-invasive, easily reproducible and effective supplement to laryngoscopy or complementary to CT scans for the screening and evaluation of vocal fold polyps and other laryngeal lesions. Its clinical usefulness is evident especially when endoscopy or CT scanning are not available or their use carries a risk to the patient.
Evaluation of swallowing disorders
Swallowing disorders are common conditions in neurological diseases like stroke, amyotrophic lateral sclerosis, parkinsonism, dementia, myasthenia and myopathy. Videofluoroscopy (VFS) and fibreoptic endoscopy evaluation of swallowing (FEES) are so far considered gold standard for instrumental assessment of swallowing. However, videofluoroscopy has many limitations such as availability problems, radiation exposure and the use of radiological contrast media. FEES is an invasive procedure that does not allow visualization of each stage of swallowing and quantification of swallowing movements. Moreover the COVID-19 pandemic has restricted access to VFS and FEES leading otolaryngologists to explore alternative diagnostic tools to perform swallowing assessment, like ultrasound techniques [40].
TLUSG is a non-invasive, repeatable and low cost diagnostic method for the investigation of laryngeal motion during swallowing, without risk of radiation exposure. Relevant information can be obtained in clinical practice by evaluating dynamic laryngeal ultrasonography during swallowing: the movement of soft tissue, such as muscles and tendons, and the coordinated movement of the hyoid bone and the larynx. Kuhl [41] suggested to analyse vertical laryngeal excursion during swallowing using TLUSG. The linear probe was placed in a longitudinal position above the larynx to allow visualization of the hyoid bone and the thyroid cartilage. Laryngeal elevation was measured by identification of the distance between the hyoid bone and the upper end of the thyroid cartilage: in healthy subjects the reduction of distance during swallowing was approximately 61%; the mean relative laryngeal elevation in the patients with neurogenic dysphagia was reduced to 42%. The relationship between movements of the hyoid bone and the larynx while swallowing was further investigated using ultrasonography. The hyoid bone–laryngeal (HL) motion ratio, defined as the hyoid bone displacement divided by the laryngeal displacement during the elevation phase, was proposed as an index that evaluates swallowing movement, independent of physiological changes associated with height and age. The value of HL motion ratio for normal swallowing is approximately 0.5 [42]. Laryngeal displacement is an important index of swallowing function: when the larynx is elevated, the anterior larynx closes protecting the respiratory tract against aspiration. The objective evaluation of hyoid bone and larynx movements during swallowing by ultrasonography facilitated the detection of neurogenic oropharyngeal dysphagia (HL motion ratio: sensitivity of 88.9%; specificity of 88.9%). When the cut-off value of the HL motion ratio was 0.56 or higher, it predicted dysphagia. Laryngeal duration was a less effective index than the HL motion ratio, but may also be used to predict dysphagia (laryngeal duration: sensitivity of 72.2%; specificity of 88.9%) [43].
Ultrasound-guided cricothyroidotomy
Cricothyroidotomy is a life-saving procedure for a “cannot intubate-cannot oxygenate” situation, performed on patients with severe respiratory distress. The technique involves making an incision in the cricothyroid membrane, followed by inserting a breathing tube which allows ventilation [44]. Misidentification of the cricothyroid membrane (CM) by inspection and palpation can be the reason for tube misplacements, leading to cricothyroidotomy failures and serious complications. Ultrasound guidance can identify the cricothyroid membrane more accurately than external palpation, particularly in patients with difficult palpable neck landmarks. According to the current evidence, ultrasound-guided identification of CM may improve correct tube insertion and successful cricothyroidotomy. Ultrasonography of neck landmarks in airway management has been proven decrease complications like injuries to the larynx and trachea and enhance patient safety, compared with conventional digital palpation technique [45, 46]. In subjects with poorly defined neck landmarks, ultrasonography is more accurate than conventional approach in localizing the CM, and it is comparable to computed-tomography scan as the accepted standard (success rate of ultrasound-guided identification of the CM: 81% vs success rate of palpation identification of the CM: 8%) [47]. These results support the use of TLUSG for the prelocalization of the cricothyroid membrane in patients with neck pathology such as previous neck surgery, irradiation and neck mass, in anticipation of difficult airways, before airway management. There is still no consensus about the best ultrasound technique for the performance of cricothyroidotomy. A structured method for ultrasonographic identification of the CM was previously described in literature. This stepwise approach involves the ultrasonographic identification of the trachea in the transverse plane, the rotation of the transducer to the longitudinal plane and the subsequent identification of the anterior parts of the tracheal rings and the cricothyroid cartilage [48]. Gadd et al. [49] studied the transverse laryngeal ultrasonography for the identification of the inverted-V shapes of the thyroid cartilage which allows cricothyroid membrane localization. He proved that ultrasonography identified the inverted-V shapes of the thyroid cartilage images with high sensitivity, but with low specificity, potentially confusing membrane localization. Further knowledge is required to improve the accuracy of ultrasound-guided cricothyroidotomy.
Ultrasound-guided laryngeal electromyography
Laryngeal electromyography (LEMG) is a diagnostic method to detect laryngeal movement disorders including vocal fold paralysis and make a differential diagnosis with cricoarytenoid joint fixation and other neuromuscular dysfunctions [50]. The technical execution of LEMG requires specific skills: the target intralaryngeal muscles are very small, thus often hard to locate with the needle, especially in patients with short or obese necks or former surgery or radiation. The application of ultrasonography in LEMG, through electromagnetic needle tracking, may facilitate the needle placement to the target muscle in addition to being painless, well tolerated and radiation free. Laryngeal ultrasonography is used to identify cricothyroid and thyroarytenoid muscles during LEMG, by a transverse/midline and transverse/oblique approach, also in case of calcified thyroid cartilage or anatomic variations [51]. Studies showed that ultrasound-guided LEMG can potentially shorten the examination time, especially in difficult anatomy, and enables the exact insertion of the needle electrode. The use of ultrasonography to locate laryngeal structures in LEMG would overcome the technical difficulties of LEMG and improve the accuracy, preventing complications [52, 53].
Conclusions
COVID-19 pandemic brought to the attention of otolaryngologists the need to practice diagnostic and treatment strategies alternative to aerosol generating endoscopic procedures. The results of this review demonstrated that transcutaneous laryngeal ultrasonography has proven to be a valid method for dynamic laryngeal assessment and airway management. It is time-efficient, non-invasive, well tolerated and easily performed also in a out-patient setting. These properties make laryngeal ultrasonography promising in various fields of application, in the context of ENT clinical practice. In this regard, TLUSG has emerged as an alternative modality to assess vocal cord mobility, even in non-compliant or high-risk infection patients, especially in subjects undergoing thyroid surgery. This topic is the most investigated in literature according to our review. Diagnosis of laryngeal disorders in infants and children using TLUSG has also attracted considerable interest among the scientific community. It represents a non invasive diagnostic tool to detect pediatric laryngeal diseases as vocal cord paralysis, laryngomalacia or space-occupied lesions. In addition, laryngeal ultrasonography is found to be a highly sensitive test for the investigation of swallowing disorders, providing quantification of laryngeal elevation during swallowing. TLUSG has been also proposed for the assessment of laryngeal carcinoma, allowing detection of laryngeal cartilage infiltration, extralaryngeal extension, vocal cord mobility and nodal staging. TLUSG could be a screening tool for other laryngeal lesions as vocal cord nodules, polyps and cysts. Laryngeal ultrasound may improve successful cricothyroidotomy thanks to ultrasound-guided identification of cricothyroid membrane. It can also facilitate the exact insertion of the needle electrode during an ultrasound-guided laryngeal electromyography, especially in difficult neck anatomy. Although TLUSG is easy performed and readily available, it requires growth of expertise, further knowledge and practical experience to achieve high levels of accuracy. We expect transcutaneous laryngeal ultrasonography will be more widely applied among otolaryngologists, with the potential for becoming a gold standard for ENT specific fields in the future.
Declarations
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Pasqua Irene Sciancalepore and Roberta Anzivino. The first draft of the manuscript was written by Pasqua Irene Sciancalepore. Critical revision of the article was performed by Paolo Petrone, Domenico Petrone and Nicola Quaranta. All authors read and approved the final manuscript.
Ethics approval
All review procedures have been performed in accordance with the requirements of the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cho RHW, Yeung ZWC, Ho OYM, et al. Pearls of experience for safe and efficient hospital practices in otorhinolaryngology—head and neck surgery in Hong Kong during the 2019 novel coronavirus disease (COVID-19) pandemic. J Otolaryngol Head Neck Surg. 2020;49:30. doi: 10.1186/s40463-020-00427-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anagiotos A, Petrikkos G. Otolaryngology in the COVID-19 pandemic era: the impact on our clinical practice. Eur Arch Oto-rhino-laryngol. 2021;278(3):629–636. doi: 10.1007/s00405-020-06161-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CEORL-HNS (2020) Confederation of the European Otorhinolaryngology and Head and Neck Surgery CEORL-HNS Statement to COVID-19. https://www.ceorlhns.org/covid-19. Accessed 31 Mar 2022.
- 4.Reddy PD, Nguyen SA, Deschler D. Bronchoscopy, laryngoscopy, and esophagoscopy during the COVID-19 pandemic. Head Neck. 2020;42(7):1634–1637. doi: 10.1002/hed.26221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balakrishnan K, Schechtman S, Hogikyan ND, Teoh A, McGrath B, Brenner MJ. COVID-19 pandemic: what every otolaryngologist-head and neck surgeon needs to know for safe airway management. Otolaryngology Head Neck Surg. 2020;162(6):804–808. doi: 10.1177/0194599820919751. [DOI] [PubMed] [Google Scholar]
- 6.Noel JE, Orloff LA, Sung K. Laryngeal evaluation during the COVID-19 pandemic: transcervical laryngeal ultrasonography. Otolaryngology Head Neck Surg. 2020;163(1):51–53. doi: 10.1177/0194599820922984. [DOI] [PubMed] [Google Scholar]
- 7.Sciancalepore PI, Anzivino R, Petrone P, Petrone D, Quaranta N. Transcutaneous laryngeal ultrasonography: a promising tool for otolaryngologists during COVID-19. Am J Otolaryngol. 2021;42(1):102772. doi: 10.1016/j.amjoto.2020.102772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Moher D, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical research ed) 2021;372:n71 . doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amis RJ, Gupta D, Dowdall JR, Srirajakalindini A, Folbe A. Ultrasound assessment of vocal fold paresis: a correlation case series with flexible fiberoptic laryngoscopy and adding the third dimension (3-D) to vocal fold mobility assessment. Middle East J Anaesthesiol. 2012;21(4):493–498. [PubMed] [Google Scholar]
- 10.Gambardella C, Offi C, Romano RM, De Palma M, Ruggiero R, Candela G, Puziello A, Docimo L, Grasso M, Docimo G. Transcutaneous laryngeal ultrasonography: a reliable, non-invasive and inexpensive preoperative method in the evaluation of vocal cords motility-a prospective multicentric analysis on a large series and a literature review. Updates Surg. 2020;72(3):885–892. doi: 10.1007/s13304-020-00728-3. [DOI] [PubMed] [Google Scholar]
- 11.Wolff S, Gałązka A, Borkowski R, Gorzelnik A, Dedecjus M. Application of translaryngeal ultrasound (TLUS) in patients with neck surgery-a single-centre, prospective cohort study on technique evaluation. J Clin Med. 2022;11(6):1691. doi: 10.3390/jcm11061691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woo JW, Suh H, Song RY, Lee JH, Yu HW, Kim SJ, Chai YJ, Choi JY, Lee KE. A novel lateral-approach laryngeal ultrasonography for vocal cord evaluation. Surgery. 2016;159(1):52–56. doi: 10.1016/j.surg.2015.07.043. [DOI] [PubMed] [Google Scholar]
- 13.Dedecjus M, Adamczewski Z, Brzeziński J, Lewiński A. Real-time, high-resolution ultrasonography of the vocal folds–a prospective pilot study in patients before and after thyroidectomy. Langenbecks Arch Surg. 2010;395(7):859–864. doi: 10.1007/s00423-010-0694-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong KP, Au KP, Lam S, Lang BH. Lessons learned after 1000 cases of transcutaneous laryngeal ultrasound (TLUSG) with laryngoscopic validation: is there a role of TLUSG in patients indicated for laryngoscopic examination before thyroidectomy? Thyroid. 2017;27(1):88–94. doi: 10.1089/thy.2016.0407. [DOI] [PubMed] [Google Scholar]
- 15.Cheng SP, Lee JJ, Liu TP, Lee KS, Liu CL. Preoperative ultrasonography assessment of vocal cord movement during thyroid and parathyroid surgery. World J Surg. 2012;36(10):2509–2515. doi: 10.1007/s00268-012-1674-1. [DOI] [PubMed] [Google Scholar]
- 16.Shah MK, Ghai B, Bhatia N, Verma RK, Panda NK. Comparison of transcutaneous laryngeal ultrasound with video laryngoscope for assessing the vocal cord mobility in patients undergoing thyroid surgery. Auris Nasus Larynx. 2019;46(4):593–598. doi: 10.1016/j.anl.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 17.de Miguel M, Peláez EM, Caubet E, González Ó, Velasco M, Rigual L. Accuracy of transcutaneous laryngeal ultrasound for detecting vocal cord paralysis in the immediate postoperative period after total thyroidectomy. Minerva Anestesiol. 2017;83(12):1239–1247. doi: 10.23736/S0375-9393.17.11755-4. [DOI] [PubMed] [Google Scholar]
- 18.Wong KP, Lang BH, Ng SH, Cheung CY, Chan CT, Chan MY. Is vocal cord asymmetry seen on transcutaneous laryngeal ultrasonography a significant predictor of voice quality changes after thyroidectomy? World J Surg. 2014;38(3):607–613. doi: 10.1007/s00268-013-2337-6. [DOI] [PubMed] [Google Scholar]
- 19.Lazard DS, Bergeret-Cassagne H, Lefort M, Leenhardt L, Russ G, Frouin F, Trésallet C. Transcutaneous laryngeal ultrasonography for laryngeal immobility diagnosis in patients with voice disorders after thyroid/parathyroid surgery. World J Surg. 2018;42(7):2102–2108. doi: 10.1007/s00268-017-4428-2. [DOI] [PubMed] [Google Scholar]
- 20.Wong KP, Woo JW, Youn YK, Chow FC, Lee KE, Lang BH. The importance of sonographic landmarks by transcutaneous laryngeal ultrasonography in post-thyroidectomy vocal cord assessment. Surgery. 2014;156(6):1590–1596. doi: 10.1016/j.surg.2014.08.061. [DOI] [PubMed] [Google Scholar]
- 21.Bergeret-Cassagne H, Lazard DS, Lefort M, Hachi S, Leenhardt L, Menegaux F, Russ G, Trésallet C, Frouin F. Sonographic dynamic description of the laryngeal tract: definition of quantitative measures to characterize vocal fold motion and estimation of their normal values. J Ultrasound Med. 2017;36(5):1037–1044. doi: 10.7863/ultra.16.05014. [DOI] [PubMed] [Google Scholar]
- 22.Wong KP, Lang BH, Chang YK, Wong KC, Chow FC. Assessing the validity of transcutaneous laryngeal ultrasonography (TLUSG) after thyroidectomy: what factors matter? Ann Surg Oncol. 2015;22(6):1774–1780. doi: 10.1245/s10434-014-4162-z. [DOI] [PubMed] [Google Scholar]
- 23.Kandil E, Deniwar A, Noureldine SI, Hammad AY, Mohamed H, Al-Qurayshi Z, Tufano RP. Assessment of vocal fold function using transcutaneous laryngeal ultrasonography and flexible laryngoscopy. JAMA Otolaryngol Head Neck Surg. 2016;142(1):74–78. doi: 10.1001/jamaoto.2015.2795. [DOI] [PubMed] [Google Scholar]
- 24.Alexander NL, Tran B, Zhu H, Ongkasuwan J. Learning to interpret pediatric vocal fold mobility: a laryngeal ultrasound training module. Laryngoscope. 2021;131(11):2545–2549. doi: 10.1002/lary.29582. [DOI] [PubMed] [Google Scholar]
- 25.Garel C, Contencin P, Polonovski JM, Hassan M, Narcy P. Laryngeal ultrasonography in infants and children: a new way of investigating. Normal and pathological findings. Int J Pediatr Otorhinolaryngol. 1992;23(2):107–115. doi: 10.1016/0165-5876(92)90046-r. [DOI] [PubMed] [Google Scholar]
- 26.Wang LM, Zhu Q, Ma T, Li JP, Hu R, Rong XY, Xu W, Wang ZC. Value of ultrasonography in diagnosis of pediatric vocal fold paralysis. Int J Pediatr Otorhinolaryngol. 2011;75(9):1186–1190. doi: 10.1016/j.ijporl.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 27.Vats A, Worley GA, de Bruyn R, Porter H, Albert DM, Bailey CM. Laryngeal ultrasound to assess vocal fold paralysis in children. J Laryngol Otol. 2004;118(6):429–431. doi: 10.1258/002221504323219545. [DOI] [PubMed] [Google Scholar]
- 28.Klinge K, Guntinas-Lichius O, Axtmann K, Mueller AH. Synchronous video laryngoscopy and sonography of the larynx in children. Eur Arch Oto-rhino-laryngol. 2016;273(2):439–445. doi: 10.1007/s00405-015-3788-1. [DOI] [PubMed] [Google Scholar]
- 29.Ongkasuwan J, Devore D, Hollas S, Jones J, Tran B. Laryngeal ultrasound and pediatric vocal fold nodules. Laryngoscope. 2017;127(3):676–678. doi: 10.1002/lary.26209. [DOI] [PubMed] [Google Scholar]
- 30.Shirley F, Oshri W, Ari D, Gad F. The role of laryngeal ultrasound in the assessment of pediatric dysphonia and stridor. Int J Pediatr Otorhinolaryngol. 2019;122:175–179. doi: 10.1016/j.ijporl.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 31.Huang H, Xia C, Hu M, Ma T, Zhu Q, Zhao H. The role of laryngeal ultrasound in diagnosis of infant laryngomalacia. Int J Pediatr Otorhinolaryngol. 2019;124:111–115. doi: 10.1016/j.ijporl.2019.05.043. [DOI] [PubMed] [Google Scholar]
- 32.Zawadzka-Glos L, Jakubowska A, Frackiewicz M, Brzewski M. External laryngeal injuries in children–comparison of diagnostic methods. Int J Pediatr Otorhinolaryngol. 2013;77(9):1582–1584. doi: 10.1016/j.ijporl.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 33.Gritzmann N, Traxler M, Grasl M, Pavelka R. Advanced laryngeal cancer: sonographic assessment. Radiology. 1989;171(1):171–175. doi: 10.1148/radiology.171.1.2648469. [DOI] [PubMed] [Google Scholar]
- 34.Desai AA, Pandya VK, Bhalani DB, Desai S, Parikh BD. Value of ultrasonography in laryngeal and laryngopharyngeal cancers. Indian J Otolaryngol Head Neck Surg. 2004;56(3):191–195. doi: 10.1007/BF02974348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dhoot NM, Choudhury B, Kataki AC, Kakoti L, Ahmed S, Sharma J. Effectiveness of ultrasonography and computed tomography in assessing thyroid cartilage invasion in laryngeal and hypopharyngeal cancers. J Ultrasound. 2017;20(3):205–211. doi: 10.1007/s40477-017-0259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xia CX, Zhu Q, Zhao HX, Yan F, Li SL, Zhang SM. Usefulness of ultrasonography in assessment of laryngeal carcinoma. Br J Radiol. 2013;86(1030):20130343. doi: 10.1259/bjr.20130343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu Q, Zhu SY, Liu RC, Zheng HY, Lun HM, Wei HM, Weng JJ. Contrast-enhanced ultrasound for the preoperative assessment of laryngeal carcinoma: a preliminary study. Acta Radiol (Stockholm, Sweden: 1987) 2021;62(8):1016–1024. doi: 10.1177/0284185120950108. [DOI] [PubMed] [Google Scholar]
- 38.Wang H, Yang X, Hou J, Li X, Sun L, Jiang J, Zhou Q. Application of transcutaneous laryngeal ultrasonography in the diagnosis of vocal fold polyps. Ultrasound Med Biol. 2020;46(9):2293–2302. doi: 10.1016/j.ultrasmedbio.2020.05.014. [DOI] [PubMed] [Google Scholar]
- 39.Nasr WF, Amer HS, Askar SM, et al. Laryngeal ultrasound as effective as CT scans for the diagnosis of various laryngeal lesions. Egypt J Otolaryngol. 2013;29:93–98. doi: 10.7123/01.EJO.0000426360.69695.f1. [DOI] [Google Scholar]
- 40.Allen JE, Clunie GM, Winiker K. Ultrasound: an emerging modality for the dysphagia assessment toolkit? Curr Opin Otolaryngol Head Neck Surg. 2021;29(3):213–218. doi: 10.1097/MOO.0000000000000708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuhl V, Eicke BM, Dieterich M, Urban PP. Sonographic analysis of laryngeal elevation during swallowing. J Neurol. 2003;250(3):333–337. doi: 10.1007/s00415-003-1007-2. [DOI] [PubMed] [Google Scholar]
- 42.Matsuo T, Matsuyama M, Nakatani K, Mori N. Evaluation of swallowing movement using ultrasonography. Radiol Phys Technol. 2020;13(1):62–68. doi: 10.1007/s12194-019-00547-1. [DOI] [PubMed] [Google Scholar]
- 43.Matsuo T, Matsuyama M. Detection of poststroke oropharyngeal dysphagia with swallowing screening by ultrasonography. PLoS ONE. 2021;16(3):e0248770. doi: 10.1371/journal.pone.0248770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Libert N, Leclerc T, De Rudnicki S. Cricothyroidotomy. N Engl J Med. 2008;359(10):1073–1074. doi: 10.1056/NEJMc081375. [DOI] [PubMed] [Google Scholar]
- 45.Kristensen MS. Ultrasonography in the management of the airway. Acta Anaesthesiol Scand. 2011;55(10):1155–1173. doi: 10.1111/j.1399-6576.2011.02518.x. [DOI] [PubMed] [Google Scholar]
- 46.Siddiqui N, Arzola C, Friedman Z, Guerina L, You-Ten KE. Ultrasound improves cricothyrotomy success in cadavers with poorly defined neck anatomy: a randomized control trial. Anesthesiology. 2015;123(5):1033–1041. doi: 10.1097/ALN.0000000000000848. [DOI] [PubMed] [Google Scholar]
- 47.Siddiqui N, Yu E, Boulis S, You-Ten KE. Ultrasound is superior to palpation in identifying the cricothyroid membrane in subjects with poorly defined neck landmarks: a randomized clinical trial. Anesthesiology. 2018;129(6):1132–1139. doi: 10.1097/ALN.0000000000002454. [DOI] [PubMed] [Google Scholar]
- 48.Kristensen MS, Teoh WH, Rudolph SS, Tvede MF, Hesselfeldt R, Børglum J, Lohse T, Hansen LN. Structured approach to ultrasound-guided identification of the cricothyroid membrane: a randomized comparison with the palpation method in the morbidly obese. Br J Anaesth. 2015;114(6):1003–1004. doi: 10.1093/bja/aev123. [DOI] [PubMed] [Google Scholar]
- 49.Gadd KJ, Ganguly A, Mauldon EC. The inverted-V shape during transverse laryngeal ultrasonography for cricothyroid membrane localisation. Anaesthesia. 2018;73(12):1572–1573. doi: 10.1111/anae.14485. [DOI] [PubMed] [Google Scholar]
- 50.Sataloff RT, Praneetvatakul P, Heuer RJ, Hawkshaw MJ, Heman-Ackah YD, Schneider SM, Mandel S. Laryngeal electromyography: clinical application. J Voice. 2010;24(2):228–234. doi: 10.1016/j.jvoice.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 51.Park HS, Jung SY, Yoo JH, Park HJ, Lee CH, Kim HS, Chung SM. Clinical usefulness of ultrasonography-guided laryngeal electromyography. J Voice. 2016;30(1):100–103. doi: 10.1016/j.jvoice.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 52.Klinge K, Guntinas-Lichius O, Naumann A, Mueller AH. Ultrasonography-guided electromagnetic needle tracking in laryngeal electromyography. Eur Arch Oto-rhino-laryngol. 2019;276(4):1109–1115. doi: 10.1007/s00405-019-05360-5. [DOI] [PubMed] [Google Scholar]
- 53.Seo HG, Jang HJ, Oh BM, Kim W, Han TR. Use of ultrasonography to locate laryngeal structures for laryngeal electromyography. PM R. 2014;6(6):522–527. doi: 10.1016/j.pmrj.2013.11.008. [DOI] [PubMed] [Google Scholar]