Abstract
In recent years, the relevance of diseases associated with fungal pathogens increased worldwide. Members of the Candida genus are responsible for the greatest number of fungal bloodstream infections every year. Epidemiological data consistently indicate a modest shift toward non-albicans species, albeit Candidaalbicans is still the most recognizable species within the genus. As a result, the number of clinically relevant pathogens has increased, and, despite their distinct pathogenicity features, the applicable antifungal agents remained the same. For bloodstream infections, only three classes of drugs are routinely used, namely polyenes, azoles and echinocandins. Antifungal resistance toward all three antifungal drug classes frequently occurs in clinical settings. Compared with the broad range of literature on virulence and antifungal resistance of Candida species separately, only a small portion of studies examined the effect of resistance on virulence. These studies found that resistance to polyenes and echinocandins concluded in significant decrease in the virulence in different Candida species. Meanwhile, in some cases, resistance to azole type antifungals resulted in increased virulence depending on the species and isolates. These findings underline the importance of studies aiming to dissect the connections of virulence and resistance in Candida species.
Keywords: antifungal resistance, virulence, Candida, azoles, echinocandins, amphotericin B
The authors discuss how the rising clinical relevance of fungal infections to the understanding of antifungal resistance development and its effects on the virulence attributes of pathogens could be highly beneficial, as it could lead to more efficient therapeutic practice.
Introduction
Worldwide epidemiological data from recent years suggest that the relevance of fungal infections is increasing over time (Guinea 2014, Astvad et al. 2018, Goemaere et al. 2018, Lamoth et al. 2018). According to estimates, diseases associated with fungal pathogens are responsible for approximately as many fatal cases as tuberculosis and almost three times more than malaria annually (Bongomin et al. 2017, World Health Organization 2020a,b). Generally, Candida infections can range from mild superficial diseases—such as skin, hair and nail infections—to life threatening deep-seated manifestations, such as bloodstream infections. It is long established that species from Candidaspp. are among the most prominent causes of fungal infections (Bongomin et al. 2017). Despite Candida albicans being the most frequently isolated species from patients with candidiasis, the relevance of non-albicans species is increasing globally (Guinea 2014). One of the reasons behind this distribution shift is hypothesized to be related to the characteristically diverse response to antifungals by different Candida species (Lortholary et al. 2011, Arendrup and Patterson 2017). For instance, one of the most frequently isolated non-albicans species, C. glabrata, is less susceptible to azoles than C. albicans. This trait confers a selective advantage to this pathogen in case of azole prophylaxis or treatment (Vale-Silva and Sanglard 2015). Furthermore, C. parapsilosis isolates have reduced susceptibility to echinocandins in vitro. Interestingly, the decreased echinocandin susceptibility of C. parapsilosis does not associate with unsuccessful treatment in clinical circumstances (Lortholary et al. 2011, Fernández-Ruiz et al. 2014).
Antifungal targets are limited due to the structural similarities of fungal and human cells. As a result, antifungal classes capable of efficiently treating systemic fungal infections are relatively low (Cowen and Steinbach 2008). As an effect of modern medical practice like prolonged parenteral nutrition or therapeutic immunosuppression, the size of the sensitive patient population shows a steady increase, whereas treatment options can be further limited by the appearance of antifungal resistance (Cowen and Steinbach 2008, Colombo et al. 2017, Sobel and Akins 2017). This observation further highlights the importance of understanding the mechanisms and effects of resistance, which can lead to more efficient therapeutic approaches.
To underline the clinical relevance of understanding the connection between antifungal resistance and virulence, in 2020, 70 isolates of C. albicans isolated from patients with onychomycosis were studied in Iran. Results suggest that there is a positive correlation between increased azole (fluconazole, itraconazole) MICs and more pronounced biofilm formation as well as phospholipase production. The same pattern was identified between fluconazole resistance and hemolysin activity. These data indicate that the development of antifungal resistance could impact the expression levels of virulence factors (Mohammadi et al. 2020). A similar association was also found in the case of C. glabrata in a study that consisted of the characterization of isolates originated from Brazilian hospital units. The antifungal profile revealed that from the tested 91 isolates, 11 were MDR (multidrug resistant). A correlation was found between the secretion of virulence factors and antifungal resistance. In these strains, phytase production was connected with amphotericin B resistance and the secretion of esterase was linked with micafungin resistance. Additionally, the presence of hemolysin was associated with resistance to both fluconazole and micafungin (Figueiredo-Carvalho et al. 2017). To further strengthen the link between virulence and resistance, experimental studies found that the contact with innate immune system associated cells can promote the expression of characteristic genes involved in antifungal resistance, such as CDR1 and CDR2 in both C. albicans and C. glabrata (Fradin et al. 2007, Walker et al. 2009, Vale-Silva and Sanglard 2015). In some cases, it is more obvious how the selective pressure presented by antifungals has changed the epidemiological landscape of Candida species. In the last decades, vulvovaginal candidiasis (VVC) cases caused by C. glabrata have been rising steadily. To study the reasons behind the increasing incidence, Nakamura-Vasconcelos et al. used 16 C. glabrata strains, isolated from VVC infection. The selected isolates consisted of both fluconazole resistant (nine strains) and susceptible (seven strains) strains. Comparing the two groups, they found a positive correlation between fluconazole resistance and adherence efficiency as well as the biofilm forming ability of the strains. As a result, researchers hypothesized that the extensive use of fluconazole as a first line therapeutic agent and the subsequent increase of virulence capacity can be a factor in the emerging relevance of C. glabrata in VVC cases.(Nakamura-Vasconcelos et al. 2017).
Besides the presence of known virulence factors, microbial pathogenesis highly depends on general fitness attributes of the fungal strains; hence, the development of antifungal resistance often inevitably affects virulence (Ben-Ami et al. 2011, Lewis et al. 2012, Vale-Silva and Sanglard 2015). Depending on the Candida species and the utilized antifungals, these alterations can be both beneficial and disadvantageous to the fungal cells. Along with the increasing interest to understand mechanisms of antifungal resistance, several studies revealed direct or indirect connections between these processes and the pathogenic potential of the microorganisms.
In this review, our aim was to discuss how the development of antifungal resistance could impact virulence in clinically relevant Candida species, according to our current knowledge.
Azoles
Resistance mechanisms
To this day, azoles are the most widely utilized antifungals due to their broad fungistatic activity, sufficient safety profile and relatively low price (Robbins et al. 2016, 2017). Azole type drugs are able to bind and inhibit the function of lanosterol 14-α-demethylase enzyme (encoded by ERG11), which results in the depletion of ergosterol. Additionally, the inhibition of ERG11 activates an alternative ergosterol biosynthetic pathway, and subsequently the buildup of the toxic intermediate product 14-α-Me-3,6-diol (Fig. 1, I-A). These changes are rendering fungal cells unable to grow and divide while inducing membrane stress (Cowen and Steinbach 2008, Ostrosky-Zeichner et al. 2010, Shapiro et al. 2011). As expected, resistance to azoles is mainly associated with the modification of the ergosterol biosynthetic pathway. Point mutations in hot spot regions of ERG11 have already been identified in resistant isolates of clinically relevant Candida species (C. albicans,C. tropicalis,C. parapsilosis,C. glabrata,C. auris). Such mutations (like Y132H, Y132F, K143R in C. albicans) lower the ability of azoles to bind to the target enzyme, decreasing the toxic activity of the antifungals (Xiang et al. 2013, Wang et al. 2015, dos Santos Silva et al. 2016). Another ERG11 related mechanism is the overexpression of target enzymes. For example, in C. albicans, gain-of-function (GOF) mutations in the transcriptional regulator UPC2 lead to constitutively higher expression of ERG11 (Shapiro et al. 2011, Robbins et al. 2017) (Fig. 1, II-A/3). Functional loss of ERG3 is also linked to azole resistance in Candida species, as following to the inhibition of ERG11 by azoles, this enzyme is responsible for the synthesis of the toxic sterol product, which leads to changes of membrane permeability (Cowen and Steinbach 2008, Robbins et al. 2016, 2017, Healey et al. 2018) (Fig. 1, II-A/2). Besides to target alteration, the expulsion of the drug from the cell is a frequent way of Candida species to gain antifungal resistance. This process is associated with the overexpression of ATP-binding cassette (ABC) and major facilitator class (MFS) transporter proteins, such as Cdr1, Cdr2 and MDdr1. Gain-of-function mutations in the transcription activator TAC1 are known to confer the constitutive upregulation of ABC transporters in several Candida species (Cowen and Steinbach 2008, Shapiro et al. 2011, Sanguinetti et al. 2015). In addition, similarly to TAC1, mutations in the regulator MRR1 can be linked to antifungal resistance through the increased expression of MDR1 (Fig. 1, II-A/1). Prominent presence of efflux pumps in the cellular membrane leads to a more effective elimination of the antifungals from the cytoplasm, which results in less azole susceptible cells (Morschhäuser et al. 2007, Robbins et al. 2017). Alteration of various stress response pathways following to antifungal treatment can also indirectly confer resistance. One of the key molecules, Hsp90 has a role in linking together several regulators of resistance. Such regulators include the protein phosphatase calcineurin, as well as the terminal mitogen-activated protein kinase (Mkc1) of the Pkc1 signaling cascade (Cowen and Steinbach 2008, Lafayette et al. 2010). Besides the Hsp90 chaperon, regulators of the target of rapamycin (TOR) pathway, were also shown to have a role in azole resistance. TOR pathway regulates cellular responses to environmental signals, such as changes in nutrient availability. These responses include several cellular processes from protein synthesis to lipid metabolism and cell wall integrity pathways (Hogan and Sundstrom 2009, Khandelwal et al. 2018). Genomic plasticity related sequence changes are also responsible for altered antifungal susceptibility. Development of aneuploidies as a response to environmental distress, like antifungal treatment, is a frequent adaptation mechanism among Candida species (Ahmad et al. 2014, Carreté et al. 2019). For example, the duplication of the left arm of chromosome 5 is rather common in azole-resistant clinical isolates of C. albicans. The formation of the isochromosome i(5L) leads to the increased dosage of ERG11 and TAC1 (Selmecki et al. 2006, Selmecki et al. 2008, 2009). Acquiring an extra copy of chromosome 5 following to antifungal pressure has also been associated with fluconazole resistance in C. auris (Bravo Ruiz et al. 2019). Furthermore, according to a study from 2009, examination of 40 C. glabrata clinical isolates originated from antifungal treated patients, two found to harbor an extra copy of chromosome E and chromosome D, respectively. Both duplicated regions contained known drug resistance associated genes, such as the ortholog of Saccharomyces cerevisiae AUS1 and PDR5 (Poláková et al. 2009).
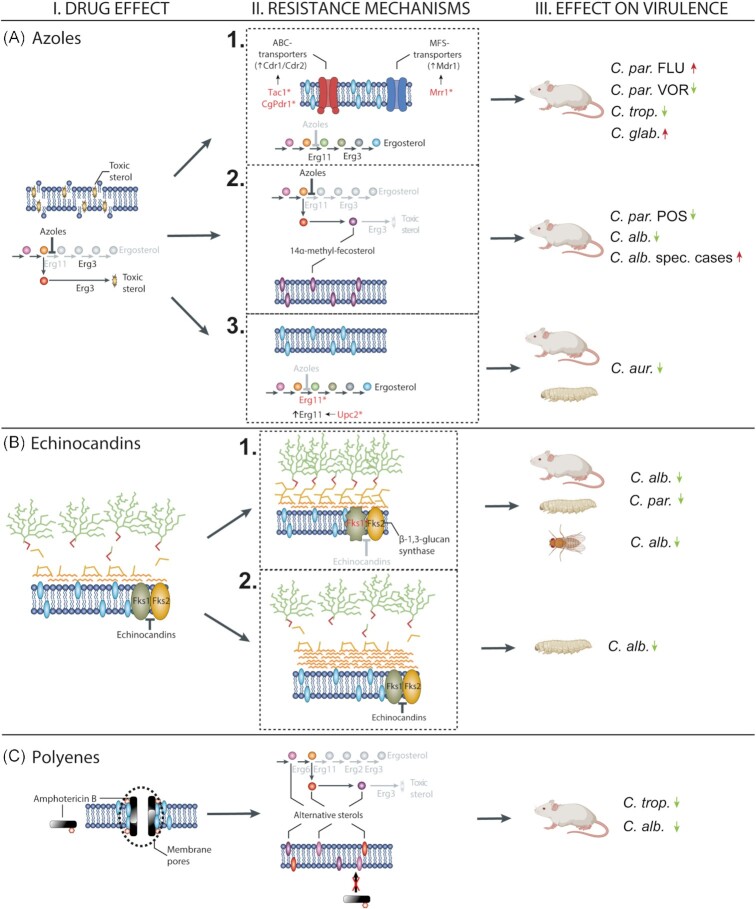
Figure 1.
Effect of antifungal drugs (I), resistance mechanisms against antifungals (II) and the effect of resistance on virulence (III). Azoles inhibit the ergosterol biosynthesis in fungi, which leads to toxic sterol accumulation (I-A). Resistance against azoles can occur via efflux pump overexpression by transportation of drug molecules out of the cell (II-A/1), via ERG3 loss-of-function (LOF) mutation leading to decreased toxic sterol production (II-A/2) or via mutation/overexpression of ERG11 (II-A/3). The effect of overexpression of efflux pumps on the virulence is controversial as it caused increased virulence in C. glabrata and C. parapsilosis fluconazole-resistant strains but led to the reduction of virulence in C. tropicalis and voriconazole-resistant C. parapsilosis strains in mice model (III-A/1). In most of the studies, ERG3 LOF mutation caused decreased virulence in posaconazole-resistant C. parapsilosis and C. albicans strains; however, in some special cases erg3 mutant C. albicans isolates showed increased virulence in mice (III-A/2). Overexpression and point mutation in ERG11 caused decreased virulence in C. auris isolates (III-A/3). Echinocandins block β-glucan synthesis by inhibiting the Fks1p glucan-synthase subunit (I-B). Resistance to echinocandins can occur via point mutations in the FKS1 (II-B/1) or by the accumulation of chitin in the fungal cell wall instead of β-glucan (II-B/2). Both resistance mechanisms against echinocandins caused attenuated virulence in C. parapsilosis and C. albicans in mice, Drosophilamelanogaster, and Galleria mellonella models (III-B/1-2). Polyenes form pores on the plasma membrane on fungal cells as these antifungals couple with ergosterol, which causes cell death (I-C). Resistance to polyenes can occur by LOF mutations in different genes playing role in the ergosterol biosynthetic pathway that leads to accumulation of alternative sterols, which has no toxic effect on the fungal cell membrane (II-C). These alterations in the ergosterol biosynthesis pathway cause attenuated virulence in C. tropicalis and C. albicans, regularly (III-C). Figures were created using images of BioRender and our own graphical elements.
Effect of resistance on virulence and pathogenicity
LOF mutations in ERG3 have been described as a classical mechanism conferring azole resistance in Candida species. As a result of these mutations, azole treatment cannot trigger the alternative pathway of ergosterol biosynthesis, thus the production of the toxic byproduct, 14-α-Me-3,6-diol (Cowen and Steinbach 2008, Ostrosky-Zeichner et al. 2010, Shapiro et al. 2011). Besides enabling resistance to antifungal drugs, ERG3 mutations in C. albicans were linked to deficiencies in stress tolerance and hyphal growth, resulting in attenuated virulence in a C57BL/6 systemic mouse model. According to the results of in vivo experiments, despite the fact that ERG3 mutant cells were able to persist within the tissues of murine organs, fungal strains were unable to cause lethal infections. In vaginal candidiasis models, the same ERG3 mutant strains showed similar virulence attributes as the azole susceptible parental isolate, implying that certain mutations affect fungal cells in niche-specific ways (Luna-Tapia et al. 2018) (Table 1). Morio et al. studied two fluconazole, voriconazole and amphotericin B-resistant C. albicans strains with azole resistance conferring mutations in ERG3. Similarly to other studies, attenuated virulence of the resistant strains was noted in Swiss mice systemic infection model, hypothetically due to reduced filamentous growth capability (Morio et al. 2012). On the other hand, a work of Vale-Silva et al. suggests that in C. albicans, ERG3 LOF mutations may be present, without the disturbance of normal hyphae formation. In their work, an azole-resistant clinical isolate (VSY2) that harbored a mutation in ERG3 was capable of growing in filamentous form and consequently showed virulence attributes comparable to the parental strains in BALB/c disseminated candidiasis model (Vale-Silva et al. 2012). Taken together, these data and the scarce presence of ERG3 mutant C. albicans strains in clinical settings imply that most of the fungal isolates harboring mutations in ERG3 are unable to grow in filamentous form, which trait results in attenuated virulence. Nevertheless, the established existence of azole-resistant ERG3 mutants with the ability to form hyphae to bypass decreased virulence leaves an alarming opportunity for the emergence of triazole-resistant C. albicans outbreaks (Martel et al. 2010, Vale-Silva et al. 2012) (Fig. 1, III-A/2).
Table 1.
List of studies examining direct connections between antifungal resistance and virulence.
| Mechanism of resistance/cellular process | Drug | Species | Effect | Reference |
|---|---|---|---|---|
| LOF mutation in ERG3 | FLU | C. albicans | Reduced virulence in murine model of systemic infection; no change of virulence in murine model of vaginal infection | (Luna-Tapia et al. 2018) |
| ERG3 mutations | FLU, VOR, AMB | C. albicans | Reduced virulence in murine model of systemic infection | (Morio et al. 2012) |
| LOF mutation in ERG3 | FLU | C. albicans | No change of virulence in murine model of systemic infection | (Vale-Silva et al. 2012) |
| MRR1 mutation | FLU | C. parapsilosis | Increased virulence in murine model of systemic infection | (Papp et al. 2020) |
| ERG3 mutation (POS); MRR1 (VOR) | POS, VOR | C. parapsilosis | Reduced virulence in murine model of systemic infection | (Papp et al. 2020) |
| MDR1,CDR1 overexpression | FLU | C. tropicalis | Reduced virulence in murine model of systemic infection | (Barchiesi et al. 2000) |
| Overexpression of CgCDR1 and CgCDR2 as an effect of CgPDR1 mutation | FLU | C. glabrata | Increased virulence in both immunocompetent and immunosuppressed murine model of systemic infection | (Ferrari et al. 2009) |
| CDR1,CDR2 upregulation; mitochondrial disfunction (absence of BPY41) | FLU | C. glabrata | Reduced virulence in murine model of systemic and vaginal infection | (Ferrari et al. 2011a) |
| Hot spot mutations in ERG11; missense mutations in CDR1,MDR1 and TAC1 | FLU, AMB | C. auris | Attenuated virulence in G. mellonella model; reduced virulence in murine model of systemic infection | (Fan et al. 2021) |
| FKS1 mutation | Echinocandins | C. albicans | Attenuated virulence in Toll-deficient D. melanogaster model; reduced virulence in murine model of systemic infection | (Ben-Ami et al. 2011) |
| FKS1 mutation | CAS | C. albicans | Reduced virulence in murine model of systemic infection | (Wiederhold et al. 2011) |
| PG associated accumulation of chitin | CAS | C. albicans | Attenuated virulence in G. mellonella model | (Rueda et al. 2014) |
| FKS1 mutation | AND, CAS, MICA | C. parapsilosis | Attenuated virulence in G. mellonella model; reduced virulence in murine model of systemic infection | (Papp et al. 2018) |
| Alterations of the ergosterol biosynthetic pathway | AMB | C. albicans | Avirulence in systemic murine infection model | (Vincent et al. 2013) |
| Alterations of the ergosterol biosynthetic pathway | AMB | C. tropicalis | Avirulence in systemic murine infection model | (Vincent et al. 2013) |
In some cases, the development of antifungal resistance can have an incidental effect on several cellular processes. Secreted aspartic proteinases (SAPs) are recognized to be one of the most important hydrolytic enzymes playing a part in the virulence of Candida species. As a result, mutations in SAP genes cause attenuated virulence of C. albicans in mouse models (Naglik et al. 2003). Interestingly, several studies recognized that subinhibitory concentrations of certain antifungals (azoles, amphotericin B) can promote the production and secretion of virulence factors, such as SAPs (Fekete-Forgács et al. 2000, Mores et al. 2011). These findings present an indirect connection between the short-term response to drug treatment and proteolytic activity of the fungal strains, further underlining the importance of understanding cellular processes to improve therapeutic choices during fungal infections (Silva et al. 2014).
The effect of azole resistance on the virulence of Candida strains was also studied in C. parapsilosis. Long-term microevolution was used to generate resistant strains to fluconazole, posaconazole and voriconazole, respectively. Virulence changes of the evolved strains were studied in BALB/c systemic candidiasis model. Compared with the parental isolate, colonization of the kidney and brain by the microevolved strains was significantly altered. Voriconazole- and posaconazole-resistant strains colonized the kidneys less efficiently than both the wild type strains and the strains selected in the presence of fluconazole. Additionally, the fungal burden in the brain was noticeably higher in the case of the fluconazole evolved strain compared with the wild type, while the posaconazole evolved strain colonized this organ less efficiently. These data altogether suggest that virulence attenuation was the most prominent in the posaconazole evolved strain, while the fluconazole challenge was unable to cause the decrease of the pathogenic potential of C. parapsilosis. In line with the literature, ERG3 mutation in the posaconazole evolved strain might be responsible for the severe virulence cost (Papp et al. 2020) (Fig. 1, III-A/1-2). To understand the development of fluconazole resistance in C. tropicalis, Barchiesi et al. also used an in vitro microevolution method to generate fluconazole-resistant strains. Further analysis of the generated resistant C. tropicalis strains revealed that the expression of both MDR1 and CDR1 positively correlated with the increase of MICs toward fluconazole. The generated resistant strain was found to be less virulent than the parental strain, as it was less successful to colonize the kidneys and failed to cause lethal infection in BALB/c mice following venous infection (Barchiesi et al. 2000) (Fig. 1, III-A/1).
In their study, Forastiero et al. used C. tropicalis clinical isolates with differing antifungal resistance profiles. One of the isolates ATCC 200956 showed cross-resistance to azoles and amphotericin B. Sequence analysis revealed major amino acid deletion in ERG11, rendering the protein nonfunctional, therefore the complete depletion of ergosterol was observed in this strain. In in vivo infection model, the resistant strain showed attenuated virulence in G. mellonella model compared with the susceptible isolate, highlighting the importance of the undisturbed sterol composition in the virulence of this species (Forastiero et al. 2013, Mesa-Arango et al. 2013).
In C. glabrata fluconazole resistance can be often traced back to the upregulation of ABC transporters such as Cdr1 and Cdr2. The role of the increased expression of these transporters is highlighted by the fluconazole hypersensitivity of mutants with disrupted CDR1 and CDR2 expression. Studying 122 C. glabrata isolates, Ferrari et al. found that azole resistance typically correlated with point mutations in the zinc finger containing transcription factor, PDR1. Further examination of this connection revealed that GOF mutations in three distinct ‘hot spot’ regions of PDR1 can induce azole resistance by promoting the increase in the expression of CDR1,CDR2 and occasionally SNQ2. To evaluate how mutations in PDR1 affect the virulence of the isolates, both immunocompetent and immunosuppressed BALB/c mice models were used. According to their results, regardless of the utilized animal model, higher fungal loads were registered from the organs of mice infected intravenously with the azole-resistant isolate compared with susceptible strain. This data suggests that mutations conferring antifungal resistance can also be beneficial for the pathogen during host invasion (Fig. 1, III-A/1). It is important to note, that this in vivo fitness gain, associated with increased virulence, was absent in in vitro competition experiments, where PDR1 mutants had no selective advantage compared with azole susceptible isolates (Ferrari et al. 2009). In a later study, Ferrari et al. found upregulated expression of both CDR1 and PUP1 (PDR1 UPregulated gene) in C. glabrata strains harboring point mutations in PDR1. Constant overexpression of these genes in the more virulent PDR1 GOF strains strongly indicates their connection to the enhanced pathogenic properties of these strains (Ferrari et al. 2011b). According to the work of Moran et al., hyperactivation of PDR1 could also partake in antifungal resistance by promoting the increased recruitment of the Mediator complex and other coactivators, to the promoters of transporter coding genes, such as CDR1 and CDR2. Mediator, as an RNA polymerase II associated activator of transcription, has a role in the antifungal drug resistance in pathogenic fungi, while it also affects the virulence attributes of Candida species. The complex consists of several subunits, each responsible for distinct functions, therefore mutations in these subunits can impact genes responsible for the filamentous growth or biofilm formation of the pathogens, as well as the expression of efflux pumps. These findings indicate that Mediator could play a central role in connecting the acquisition of antifungal resistance to altered pathogenic potential in Candidaspp. (Moran et al. 2019). Ferrari et al. examined two sequential isolates of C. glabrata, both recovered from the same patient treated with fluconazole. According to antifungal tests, the earlier isolate showed low MIC to fluconazole, while the isolate recovered after fluconazole therapy was resistant. The azole-resistant strain showed the upregulation of ABC transporters, such as Cdr1 and Cdr2 and also exhibited mitochondrial dysfunction as a result of the absence of BPY41 in mitochondrial DNA. Despite these mutations, the azole-resistant strain was able to colonize the host organism more efficiently in both systemic and vaginal BALB/c models, suggesting fitness gain despite severe respiratory deficiency. Previous studies reported the presence of major virulence attenuation due to mitochondrial dysfunction. This data points out, that these mutations can positively and negatively affect virulence as well, depending on the fungal strains and experimental setup (Ferrari et al. 2011a).
As mentioned above one of the well-known mechanism of antifungal resistance development is the emergence of aneuploidy under antifungal pressure. Rapid changes in the genomic organization in clinical C. glabrata isolates have been reported in many cases, suggesting that chromosomal rearrangements occur in high frequency in this non-albicans members of the genus (Poláková et al. 2009, Ahmad et al. 2014, Carreté et al. 2019). A study from 2009 analyzed C. glabrata isolates with additional small chromosomes and segmental aneuploidy of the left arms of chromosome E and F. Genome mapping revealed that several genes in this region have roles in the pathogenicity and antifungal susceptibility of C. glabrata. For example, duplicated segments of ABC transporter coding genes (CDR1,PDH1,CAGL0F01419g paralog of S. cerevisiae AUS1) were reported. Further analysis of the changes in chromosome E noted duplicated segments of virulence related genes (phospholipase B, extracellular glycosyl phosphatidylinositol-linked aspartyl proteases). They also identified a minichromosome derived from chromosome F, with a clear role in antifungal resistance. This minichromosome was stable in most cases under antifungal pressure and was lost in the absence of the drug. The presence of this drug responsive chromosomal dynamism indicates that in C. glabrata this mechanism may also have an essential role, linking pathogenic attributes and antifungal susceptibility, while similar broad genomic alterations could also have considerable importance in species frequently displaying high level of genome plasticity (Poláková et al. 2009).
Understanding the connection between antifungal resistance and virulence is especially crucial in the case of the recently emerging member of the Candidaspp.,Candida auris. This species quickly became an alarming threat, mainly in clinical units, because of its remarkable ability to acquire resistance toward the most widely used antifungal drugs (Pristov and Ghannoum 2019, Chakrabarti and Singh 2020). Nonetheless, to this day the number of studies linking resistance to virulence is scarce in this emerging pathogen. A recent study from China compared two isolates, one of them susceptible to antifungals, while the other showed resistance to fluconazole and amphotericin B. Although the resistant isolate showed elevated SAPs production at higher temperatures in vitro, it was found to be less virulent than the susceptible strain in both Galleria mellonella and in systemic infection model (BALB/c) (Fan et al. 2021) (Fig. 1, III-A/3). It is also important to note, that the two studied isolates originated from different clades, making the direct comparison difficult due to the general variability of the pathogenic abilities of C. auris isolates from differing origins (Forgács et al. 2020).
When comparing studies, there are inconsistencies regarding the identified azole resistance mechanisms in different Candida species, which implies major variances in the process of resistance development even in a strain-dependent manner. Based on that we cannot rule out the clinical importance of nonconventional resistance mechanisms, especially when they are known to affect the virulence attributes of the pathogens. Stress response pathways have an essential role indirectly connecting the antifungal drug initiated cellular reactions to pathogenesis by regulating diverse processes. For example, Hsp90 manages cellular stress circuits by stabilizing several downstream proteins, such as calcineurin. Inhibition of this key chaperon reduces the azole and echinocandin resistance of Candida isolates (Robbins et al. 2017a). Similarly, calcineurin inhibition by cyclosporin A decreases fluconazole tolerance in resistant C. albicans isolates. The presence of calcineurin is also essential in response to other stress conferring agents, such as voriconazole, caffeine, calcofluor white and congo red. Furthermore, it also determines pathogenesis in mouse models, as calcineurin affects colony morphology and hyphal transition of fungal cells (Sanglard et al. 2003a). As a result of its pivotal role in antifungal resistance, targeted inhibition of calcineurin can be a potential option for improved antifungal therapy in the future. In 2019, Hans et al. through several experiments proved that magnesium deprivation affects the calcineurin pathway in C. albicans leading to increased susceptibility to fluconazole. Besides that, decreased virulence of C. albicans was also noted in magnesium restricted environment, hypothetically due to the disruption of hyphae formation, supporting the idea that magnesium chelation could improve the activity of membrane-targeting antifungals (Hans et al. 2019). It is also known that calcineurin and its potential target Crz1 have an important role in the antifungal susceptibility and virulence of C. glabrata and C. tropicalis (Miyazaki et al. 2010, Chen et al. 2014).
Vacuolar proton-translocating ATPase (V-ATPase) also functions as a key factor in both antifungal resistance and virulence in C. glabrata. The deletion of VPH2—a gene that codes an assembly factor of the V-ATPases—confers decreased susceptibility of FLU (fluconazole), VOR (voriconazole) and AMB (amphotericin B), also responsible for reduced virulence in murine models of disseminated candidiasis. The reason behind the decreased virulence can be connected to the impaired vacuolar function of mutant strains that is responsible for increased susceptibility to environmental stress factors like the immune response of the host organism (Minematsu et al. 2019).
Echinocandins
Resistance mechanisms
Echinocandins are the newest antifungal drugs, which were developed in the late ’90s. This group of antifungal drugs inhibits the catalytic subunit of β-glucan synthase enzyme (encoded by FKS complex), therefore perturbing the homeostasis of the cell wall (Fig. 1, I-B). Despite echinocandins having a relatively short application history in the clinical environment, infections caused by resistant Candida isolates regularly occur (Robbins et al. 2016). Generally, most of the clinically important Candida species are susceptible to echinocandins, however C. parapsilosis isolates bear the highest MIC values of this class of antifungals (Lortholary et al. 2011). Experimental data suggest that the development of echinocandin resistance in Candida species is starkly similar. Compared with azole resistance, target enzyme modifications are even more prominent in the case of acquired echinocandin resistance. Mutations in hot spot regions of the FKS complex coding regions are known to confer antifungal resistance by inhibiting the binding of the drug (Cowen and Steinbach 2008, Robbins et al. 2017a, Sobel and Akins 2017). Isolates harboring point mutations in FKS1 and FKS2 are frequently found in clinical settings, suggesting that these modifications have an in vivo role as they develop following to echinocandin therapy (Pham et al. 2014, Berrio et al. 2018, Chowdhary et al. 2018).
Effect of resistance on virulence and pathogenicity
The connection between virulence and echinocandin resistance seems more obvious compared with the data observed in the case of azoles. The main reason for that is that echinocandin resistance is usually associated with the mutations in certain hot spots of the FKS1 gene (Fig. 1, II-B/1). As an effect of the deficient glucan synthesis and therefore the reduction of β-glucan in the cell wall, increased chitin content was noted as a compensatory mechanism. Several studies revealed how this compensatory effect relates to virulence changes. In 2011, Ben-Ami et al. conducted experiments with echinocandin-resistant clinical and laboratory C. albicans strains harboring homozygous mutations in their FKS1 genes. Virulence properties of the strains were tested in Toll-deficient D. melanogaster model. In this model, the increase of the echinocandin MICs highly correlated with attenuated virulence compared with the susceptible wild-type strain. To confirm the virulence changes, the FKS1 mutant strains were further tested in a systemic candidiasis model using BALB/c mice. Consistent with the results of the fly model, hypovirulence was also evident in mice infected intravenously with echinocandin-resistant strains. Phenotype analysis of the FKS1 mutants revealed that both impaired growth rate and defective filamentation generally accompanied the reduced catalytic capacity of the glucan synthase complex. Additionally, increased chitin content in mutant cells can be linked to attenuated Dectin-1 mediated inflammatory response, and therefore decreased virulence (Ben-Ami et al. 2011, Lee et al. 2012). Similarly, Wiederhold et al. compared three C. albicans clinical isolates. One of them was susceptible to echinocandins, while the other two strains—harboring FKS1 mutation—were resistant to caspofungin treatment. They found that the isolate with the lowest echinocandin MIC was the most virulent in a murine model of invasive candidiasis. While the two FKS1 mutant strains showed differences in pathogenicity, they were both less capable of causing lethal infections than the susceptible isolate (Wiederhold et al. 2011).
In response to echinocandin therapy, several Candida species are capable of paradoxical growth (PG) in vitro. Relevance of this phenomenon in clinical practice is still unclear, but it cannot be ruled out as a virulence modifying factor. Paradoxical growth was found to be associated with changes in phenotype, such as the increase of cell size, clump formation and defects in cell separation. Similarly, PG was also associated with the accumulation of chitin, but it was not sufficient for the cells to escape the fungicidal activity of caspofungin. Fungal cells showing PG upon challenge with caspofungin, were used to infect G. mellonella larvae. Forty % of the larvae infected with the CAS treated C. albicans cells survived the experiment, whereas without drug treatment 100% mortality was noted, suggesting attenuated virulence of PG strains. Further examination revealed that in the case of PG, hyphae formation of fungal cells was significantly lower than in untreated control cells. Further investigation of immune responses pointed out that compared with untreated cells, caspofungin challenged PG cells induced a significantly stronger pro-inflammatory response (TNF-α; IL-17, IL-12) and lower anti-inflammatory response (IL-10) in primary murine peritoneal macrophages. Besides the defective hyphae formation, alterations in the cell wall due to the increased chitin content can potentially explain the change of virulence properties in PG cells (Rueda et al. 2014) (Fig. 1, III-B/2). Chitin itself is known to have diverse immunoregulatory functions depending on its fragment length and interactions with fungal proteins (Ben-Ami et al. 2011, Lee et al. 2012). In addition, echinocandin treatment can also cause the unmasking of β-1,3-glucan, allowing more effective immune recognition by the host organism (Lee et al. 2012, Lewis et al. 2012).
The work of Choudhary et al further straightens the role of chitin as a key component linking virulence with echinocandin resistance in C. glabrata. It has been previously shown that phosphoinositide signaling pathways have a role in resistance development to azoles by regulating various cellular processes such as protein trafficking, autophagy, cell cycle progression and hyphal morphogenesis (Choudhary et al. 2019). Additionally, phosphatidylinositol-4-phosphate is vital for the trafficking of the major multidrug transporter Cdr1, to the plasma membrane (Ghugtyal et al. 2015). Another component of the pathway phosphatidylinositol-3,5-bisphosphate (PI(3,5)P2) kinase (Fab1) is necessary for the reorganization of the actin cytoskeleton and azole stress survival (Bhakt et al. 2018). Further two components of the pathway, Vac7 and Vac14 are responsible for the regulation of the protein synthesis and turnover of PI(3,5)P2, also partake in azole resistance by controlling the quantity of the protein in C. glabrata (McCartney et al. 2014). Upon deletion of VAC7 and VAC14,C. glabrata cells showed growth defects, reduced stress tolerance and sensitivity to caspofungin. Mutant strains were also shown to have attenuated virulence in BALB/c mice as both were less able to colonize the kidneys of the animals after intravenous infection. The change in the pathogenic potential of the mutant cells can be traced back to the impaired trafficking of the cell wall proteins, resulting in the increase of the chitin content similarly to previous findings (Choudhary et al. 2019). In a study from Papp et al. in vitro microevolution was used for the generation of echinocandin-resistant C. parapsilosis strains. An echinocandin susceptible isolate was incubated in the presence of increasing concentrations of anidulafungin, caspofungin and micafungin respectively. The virulence of the generated resistant strains was evaluated both in G. mellonella and in a murine model (BALB/c) of systemic candidiasis. In both models, all three of the evolved strains showed attenuated virulence compared with the echinocandin susceptible initial isolate (Fig. 1, III-B/1). Further analysis of the cell wall of the evolved strains revealed severe alterations in the surface exposure of β-1,3-glucan and especially chitin compared with the parental strain. The altered structure of the cell wall was presumably due to the effect of FKS1 mutations in all three of the evolved strains. This study further affirms that the acquisition of echinocandin resistance correlates with virulence attenuation in Candida species (Papp et al. 2018).
Amphotericin B
Resistance mechanisms
Despite polyenes having been in clinical use in the last 70 years, resistance to these agents is relatively rare. Polyenes, like amphotericin B, bind to ergosterol in the fungal membrane, forming channels that cause the disruption of the cellular homeostasis (Fig. 1, I-C). Recently, another mechanism of action was also noted in the case of amphotericin B. Polyenes may extract ergosterol from the membrane, forming sponge-like structures and disturbing normal membrane function (Kamiński 2014, Carolus et al. 2020). Additionally, amphotericin B can also induce an oxidative stress response and therefore the death of fungal cells (Belenky et al. 2013, Mesa-Arango et al. 2014). Despite their remarkable fungicidal effect, their use in the clinical setting is limited, due to the structural similarities between ergosterol and cholesterol. During the treatment of fungal infections with amphotericin B, nephrotoxicity in the host is a common side effect (Robbins et al. 2017b). Amphotericin B resistance is generally associated with alternations in the ergosterol biosynthesis pathway due to LOF mutations in certain genes encoding key enzymes of sterol synthesis. For example, combined loss of ERG3 and ERG11 function in C. albicans leads to the depletion of ergosterol, hence the inability of polyenes to attach to their target (Sanglard et al. 2003b, Cowen and Steinbach 2008, Vincent et al. 2013, Carolus et al. 2020). Similarly, combined alteration of ERG11 and ERG5, as well as ERG6 and ERG2 also prompt amphotericin B resistance in C. albicans (Fig. 1, I-C). Compared with C. albicans, Candida species with alternative membrane sterol profiles, like C. haemulonii species complex, also show differing susceptibility to polyenes (Carolus et al. 2020). Interestingly, amphotericin B resistance is more common in C. auris, as ∼30% of known isolates were found to have reduced polyene susceptibility. The study of resistant strains originating from both the clinical setting and experimental microevolution revealed point mutations in FLO8 that, among other downstream functions, regulate the expression of ERG11. Although no direct correlation was found between amphotericin B MICs and altered FLO8, the appearance of these mutations suggests a key role for this gene in polyene resistance development in C. auris (Carolus et al. 2020, 2021).
Effect of resistance on virulence and pathogenicity
The observation that amphotericin B-resistant Candida strains are rarely found in patients treated for fungal infections, suggests that the development of resistance to this drug either comes with fitness loss or attenuated virulence (Lewis et al. 2012, Robbins et al. 2017a). Adaptation to this antifungal drug might probably require the collective accumulation of several resistance mechanisms (alteration of ergosterol biosynthetic pathway, acquired resistance to oxidative stress), which can lead to reduced fitness therefore the decrease of pathogenic potential (Vincent et al. 2013). To further understand this phenomenon, Vincent et al. studied amphotericin B-resistant C. albicans and C. tropicalis strains, generated artificially by microevolution process. Whole genome sequencing of the generated stains pointed out alterations in the ergosterol biosynthetic pathway (ERG2 and ERG3/11 mutations) in both strains. Additionally, resistant strains were proved to be avirulent in systemic mouse infection models (BALB/c), due to failure in resisting the host environment and to successfully colonize the organs of animals (Fig. 1, III-C). The lack of ability to withstand the host immune response can be traced back to the constitutive activation of Hsp90-dependent stress response pathways, compensating the hypersensitiveness of the resistant strains to several host modeling stress conditions (Vincent et al. 2013). In C. albicans, the presence of enolase presumably represents a more indirect connection between amphotericin B resistance and virulence. ENO1 null mutants, unable to catalyze the conversion of 2-phospho-d-glycarate to phosphoenolpyruvate, were found to be more susceptible to stress conferring agents, such as AMB, FLU, VOR and calcofluor white. The fact that these deletion mutants showed reduced hyphal growth and reduced virulence in the systemic candidiasis model (BALB/c) suggests that the unhindered progress of glycolysis and gluconeogenesis could also be an important link between antifungal resistance and virulence in Candida species (Ko et al. 2013) (Fig. 1, III-C).
Conclusion
Understanding the virulence attributes of Candida species is an essential task for researchers due to the rising number of candidaemia cases worldwide (Guinea 2014, Astvad et al. 2018, Goemaere et al. 2018, Lamoth et al. 2018). In parallel with bacteria, the frequency of resistance development to antibiotic agents is also pronounced among fungal pathogens. Resistant isolates of the most relevant members of the Candida genus have already been identified to all three routinely used antifungal classes, namely azoles, echinocandins and polyenes. The low number of available antifungals further complicates the selection of the most suitable treatment option (Cowen and Steinbach 2008, Colombo et al. 2017, Sobel and Akins 2017). Besides limiting therapeutic choices, it cannot be ignored that the acquisition of antifungal resistance also affects the pathogen, often impacting several vital cellular processes. Generally, as pathogens use these changes as compensatory mechanisms during antifungal treatment, in the absence of the drugs they confer negative effects on the fitness of the fungal cells (Lewis et al. 2012, Sanguinetti et al. 2015). For example, the relatively low abundance of amphotericin B-resistant Candida isolates in clinical settings indicates that in these cases the fitness tradeoff is so severe that resistant cells are unable to effectively infect the host organisms (Lewis et al. 2012). The target protein of echinocandin type antifungals, Fks1, is an integral part of the fungal cell wall biosynthetic pathway. Since the most definite mechanism of antifungal resistance to echinocandins is the alteration of the target enzyme via point mutations in the hot spot regions of the coding gene, as a consequence of the impaired synthesis, cell wall alterations are expected (Cowen and Steinbach 2008, Robbins et al. 2017, Sobel and Akins 2017). Along with the decreasing content of β-glucan, the ratio of other components such as chitins is known to rise in resistant strains (Vale-Silva and Sanglard 2015). Chitin is recognized to have diverse immunoregulatory functions. The increase of this polymer in the fungal cell wall was previously identified as an anti-inflammatory signal associated with attenuated Dectin-1 mediated inflammatory response. That connection can explain the attenuation of virulence upon echinocandin resistance. It is also important to note that despite echinocandin resistance in most cases being caused by a single point mutation, analysis of the gene expression of the resistant isolates strongly suggests complex genome-wide alterations (Ben-Ami et al. 2011). Based on that the appearance of resistant isolates with increased pathogenic capabilities cannot be ruled out in the future. Candida species can acquire azole resistance in diverse ways. Similarly, to other antifungal classes, the resistance to azole type drugs is often correlated with the loss of fitness in Candida species, although several studies indicate, that even the increase of pathogenic capability is possible as a response to antifungal treatment. For example, in C. albicans certain resistance conferring ERG3 mutations have been identified, that did not result in impaired filamentous growth. Fungal cells harboring this mutation were able to infect the host organism as successfully as nonmutant strains (Vale-Silva et al. 2012). The analysis of CDR1 and CDR2 overexpressing C. glabrata cells reached similar results. In this case, the presence of the resistance conferring mutation in PDR1 even increased the virulence of the strains, in both immunocompetent and immunosuppressed murine models (Ferrari et al. 2009). In certain situations, resistance conferring changes can have niche-specific effects. For example, C. albicans cells with LOF mutation in the ERG3 can display attenuated virulence in systemic infection models but be as capable as the nonmutant cells to infect vaginal tissue. Niche-specific effects can drive attention to the possibility that antifungal treatment can fundamentally modify the epidemiological distribution of Candida (Luna-Tapia et al. 2018).
Taken together, with the rising clinical relevance of fungal infections the understanding of the development of antifungal resistance and its effects on the virulence attributes of the pathogens could be highly beneficial as it could lead to more efficient therapeutic practice.
Contributor Information
Flora Bohner, HCEMM-USZ Fungal Pathogens Research Group, Department of Microbiology, Faculty of Science and Informatics, University of Szeged, Szeged, H-6726, Hungary.
Csaba Papp, HCEMM-USZ Fungal Pathogens Research Group, Department of Microbiology, Faculty of Science and Informatics, University of Szeged, Szeged, H-6726, Hungary.
Attila Gácser, HCEMM-USZ Fungal Pathogens Research Group, Department of Microbiology, Faculty of Science and Informatics, University of Szeged, Szeged, H-6726, Hungary; MTA-SZTE “Lendület” Mycobiome Research Group, University of Szeged, Szeged, H-6726, Hungary.
Conflict of interest statement
None declared.
References
- Ahmad KM, Kokošar J, Guo Xet al. Genome structure and dynamics of the yeast pathogen Candida glabrata. FEMS Yeast Res. 2014;14:529–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendrup MC, Patterson TF. Multidrug-resistant Candida: epidemiology, molecular mechanisms, and treatment. J Infect Dis. 2017;216:S445–51. [DOI] [PubMed] [Google Scholar]
- Astvad KMT, Johansen HK, Røder BLet al. Update from a 12-year nationwide fungemia surveillance: increasing intrinsic and acquired resistance causes concern. J Clin Microbiol. 2018;56:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barchiesi F, Calabrese D, Sanglard Det al. Experimental induction of fluconazole resistance in Candida tropicalis ATCC 750. Antimicrob Agents Chemother. 2000;44:1578–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenky P, Camacho D, Collins JJ. Fungicidal drugs induce a common oxidative-damage cellular death pathway. Cell Rep. 2013;3:350–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ami R, Garcia-Effron G, Lewis REet al. Fitness and virulence costs of Candida albicans FKS1 hot spot mutations associated with echinocandin resistance. J Infect Dis. 2011;204:626–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrio I, Maldonado N, De Bedout Cet al. Comparative study of Candidaspp. isolates: identification and echinocandin susceptibility in isolates obtained from blood cultures in 15 hospitals in Medellín, Colombia. J Glob Antimicrob Resist. 2018;13:254–60. [DOI] [PubMed] [Google Scholar]
- Bhakt P, Shivarathri R, Choudhary DKet al. Fluconazole-induced actin cytoskeleton remodeling requires phosphatidylinositol 3-phosphate 5-kinase in the pathogenic yeast Candida glabrata. Mol Microbiol. 2018;110:425–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongomin F, Gago S, Oladele ROet al. Global and multi-national prevalence of fungal diseases: estimate precision. J Fungi. 2017;3:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo Ruiz G, Ross ZK, Holmes Eet al. Rapid and extensive karyotype diversification in haploid clinical Candida auris isolates. Curr Genet. 2019;65:1217–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carolus H, Pierson S, Lagrou Ket al. Amphotericin B and other polyenes: discovery, clinical use, mode of action and drug resistance. J Fungi. 2020;6:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carolus H, Pierson S, Muñoz JFet al. Genome-wide analysis of experimentally evolved Candida auris reveals multiple novel mechanisms of multidrug resistance. mBio. 2021;12:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreté L, Ksiezopolska E, Gómez-Molero Eet al. Genome comparisons of Candida glabrata serial clinical isolates reveal patterns of genetic variation in infecting clonal populations. Front Microbiol. 2019;10:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti A, Singh S.. Multidrug-resistant Candida auris: an epidemiological review. Expert Rev Anti Infect Ther. 2020;18:551–62. [DOI] [PubMed] [Google Scholar]
- Chen YL, Yu SJ, Huang HYet al. Calcineurin controls hyphal growth, virulence, and drug tolerance of Candida tropicalis. Eukaryotic Cell. 2014;13:844–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary DK, Bhakt P, Kaur R.. Essential role for the phosphatidylinositol 3,5-bisphosphate synthesis complex in caspofungin tolerance and virulence in Candida glabrata. Antimicrob Agents Chemother. 2019;63:e00886–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhary A, Prakash A, Sharma Cet al. A multicentre study of antifungal susceptibility patterns among 350 Candida auris isolates (2009-17) in India: role of the ERG11 and FKS1 genes in azole and echinocandin resistance. J Antimicrob Chemother. 2018;73:891–9. [DOI] [PubMed] [Google Scholar]
- Colombo AL, de Almeida Júnior JN, Guinea J. Emerging multidrug-resistant Candida species. Curr Opin Infect Dis. 2017;30:528–38. [DOI] [PubMed] [Google Scholar]
- Cowen LE, Steinbach WJ. Stress, drugs, and evolution: the role of cellular signaling in fungal drug resistance. Eukaryotic Cell. 2008;7:747–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos, Silva DB, Carbonera Rodrigues LMet al. Novel point mutations in the ERG11 gene in clinical isolates of azole resistant Candida species. Mem Inst Oswaldo Cruz. 2016;111:192–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan S, Zhan P, Bing Jet al. A biological and genomic comparison of a drug-resistant and a drug-susceptible strain of Candida auris isolated from Beijing, China. Virulence. 2021;12:1388–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete-Forgács K, Gyüre L, Lenkey B. Changes of virulence factors accompanying the phenomenon of induced fluconazole resistance in Candida albicans. Mycoses. 2000;43:273–9. [DOI] [PubMed] [Google Scholar]
- Fernández-Ruiz M, Aguado JM, Almirante Bet al. Initial use of echinocandins does not negatively influence outcome in Candida parapsilosis bloodstream infection: a propensity score analysis. Clin Infect Dis. 2014;58:1413–21. [DOI] [PubMed] [Google Scholar]
- Ferrari S, Ischer F, Calabrese Det al. Gain of function mutations in CgPDR1 of Candida glabrata not only mediate antifungal resistance but also enhance virulence. PLoS Pathog. 2009;5:e1000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S, Sanguinetti M, De Bernardis Fet al. Loss of mitochondrial functions associated with azole resistance in Candida glabrata results in enhanced virulence in mice. Antimicrob Agents Chemother. 2011a;55:1852–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S, Sanguinetti M, Torelli Ret al. Contribution of CgPDR1-regulated genes in enhanced virulence of azole-resistant Candida glabrata. PLoS One. 2011b;6:e17589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo-Carvalho MHG, Ramos L de S, Barbedo LSet al. Relationship between the antifungal susceptibility profile and the production of virulence-related hydrolytic enzymes in Brazilian clinical strains of Candida glabrata. Mediators Inflamm. 2017;2017:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forastiero A, Mesa-Arango AC, Alastruey-Izquierdo Aet al. Candida tropicalis antifungal cross-resistance is related to different azole target (Erg11p) modifications. Antimicrob Agents Chemother. 2013;57:4769–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgács L, Borman AM, Prépost Eet al. Comparison of in vivo pathogenicity of four Candida auris clades in a neutropenic bloodstream infection murine model. Emerg Microbes Infect. 2020;9:1160–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fradin C, Mavor AL, Weindl Get al. The early transcriptional response of human granulocytes to infection with Candida albicans is not essential for killing but reflects cellular communications. Infect Immun. 2007;75:1493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghugtyal V, Garcia-Rodas R, Seminara Aet al. Phosphatidylinositol-4-phosphate-dependent membrane traffic is critical for fungal filamentous growth. Proc Natl Acad Sci USA. 2015;112:8644–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goemaere B, Becker P, Van Wijngaerden Eet al. Increasing candidaemia incidence from 2004 to 2015 with a shift in epidemiology in patients preexposed to antifungals. Mycoses. 2018;61:127–33. [DOI] [PubMed] [Google Scholar]
- Guinea J. Global trends in the distribution of Candida species causing candidemia. Clin Microbiol Infect. 2014;20:5–10. [DOI] [PubMed] [Google Scholar]
- Hans S, Fatima Z, Hameed S.. Magnesium deprivation affects cellular circuitry involved in drug resistance and virulence in Candida albicans. J Glob Antimicrob Resist. 2019;17:263–75. [DOI] [PubMed] [Google Scholar]
- Healey KR, Kordalewska M, Ortigosa CJet al. Limited ERG11 mutations identified in isolates of candida auris directly contribute to reduced azole susceptibility. Antimicrob Agents Chemother. 2018;62:e01427–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan DA, Sundstrom P. The Ras/cAMP/PKA signaling pathway and virulence in Candida albicans. Future Microbiol. 2009;4:1263–70. [DOI] [PubMed] [Google Scholar]
- Kamiński DM. Recent progress in the study of the interactions of amphotericin B with cholesterol and ergosterol in lipid environments. Eur Biophys J. 2014;43:453–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandelwal NK, Chauhan N, Sarkar Pet al. Azole resistance in a Candida albicans mutant lacking the ABC transporter CDR6/ROA1 depends on TOR signaling. J Biol Chem. 2018;293:412–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko H-C, Hsiao T-Y, Chen C-Tet al. Candida albicans ENO1 null mutants exhibit altered drug susceptibility, hyphal formation, and virulence. J Microbiol. 2013;51:345–51. [DOI] [PubMed] [Google Scholar]
- Lafayette SL, Collins C, Zaas AKet al. PKC signaling regulates drug resistance of the fungal pathogen candida albicans via circuitry comprised of mkc1, calcineurin, and hsp90. PLoS Pathog. 2010;6:79–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamoth F, Lockhart SR, Berkow ELet al. Changes in the epidemiological landscape of invasive candidiasis. J Antimicrob Chemother. 2018;73:i4–13. [DOI] [PubMed] [Google Scholar]
- Lee KK, MacCallum DM, Jacobsen MDet al. Elevated cell wall chitin in Candida albicans confers echinocandin resistance in vivo. Antimicrob Agents Chemother. 2012;56:208–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RE, Viale P, Kontoyiannis DP.. The potential impact of antifungal drug resistance mechanisms on the host immune response to Candida. Virulence. 2012;3:368–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lortholary O, Desnos-Ollivier M, Sitbon Ket al. Recent exposure to caspofungin or fluconazole influences the epidemiology of candidemia: a prospective multicenter study involving 2,441 patients. Antimicrob Agents Chemother. 2011;55:532–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna-Tapia A, Willems HME, Parker JEet al. Loss of Upc2p-inducible ERG3 transcription is sufficient to confer niche-specific azole resistance without compromising Candida albicans pathogenicity. mBio. 2018;9:e00225–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel CM, Parker JE, Bader Oet al. Identification and characterization of four azole-resistant erg3 mutants of Candida albicans. Antimicrob Agents Chemother. 2010;54:4527–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney AJ, Zhang Y, Weisman LS.. Phosphatidylinositol 3,5-bisphosphate: low abundance, high significance. Bioessays. 2014;36:52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesa-Arango AC, Forastiero A, Bernal-Martínez Let al. The non-mammalian host Galleria mellonella can be used to study the virulence of the fungal pathogen Candida tropicalis and the efficacy of antifungal drugs during infection by this pathogenic yeast. Med Mycol. 2013;51:461–72. [DOI] [PubMed] [Google Scholar]
- Mesa-Arango AC, Trevijano-Contador N, Román Eet al. The production of reactive oxygen species is a universal action mechanism of amphotericin B against pathogenic yeasts and contributes to the fungicidal effect of this drug. Antimicrob Agents Chemother. 2014;58:6627–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minematsu A, Miyazaki T, Shimamura Set al. Vacuolar proton-translocating ATPase is required for antifungal resistance and virulence of Candida glabrata. PLoS One. 2019;14:e0210883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki T, Yamauchi S, Inamine Tet al. Roles of calcineurin and Crz1 in antifungal susceptibility and virulence of Candida glabrata. Antimicrob Agents Chemother. 2010;54:1639–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi F, Ghasemi Z, Familsatarian Bet al. Relationship between antifungal susceptibility profile and virulence factors in Candida albicans isolated from nail specimens. Rev Soc Bras Med Trop. 2020;53:e20190214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran GP, Anderson MZ, Myers LCet al. Role of mediator in virulence and antifungal drug resistance in pathogenic fungi. Curr Genet. 2019;65:621–30. [DOI] [PubMed] [Google Scholar]
- Mores AU, Souza RD, Cavalca Let al. Enhancement of secretory aspartyl protease production in biofilms of Candida albicans exposed to sub-inhibitory concentrations of fluconazole. Mycoses. 2011;54:195–201. [DOI] [PubMed] [Google Scholar]
- Morio F, Pagniez F, Lacroix Cet al. Amino acid substitutions in the Candida albicans sterol 5,6-desaturase (Erg3p) confer azole resistance: characterization of two novel mutants with impaired virulence. J Antimicrob Chemother. 2012;67:2131–8. [DOI] [PubMed] [Google Scholar]
- Morschhäuser J, Barker KS, Liu TTet al. The transcription factor Mrr1p controls expression of the MDR1 efflux pump and mediates multidrug resistance in Candida albicans. PLoS Pathog. 2007;3:1603–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naglik JR, Challacombe SJ, Hube B.. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol Mol Biol Rev. 2003;67:400–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura-Vasconcelos SS, Fiorini A, Zanni PDet al. Emergence of Candida glabrata in vulvovaginal candidiasis should be attributed to selective pressure or virulence ability?. Arch Gynecol Obstet. 2017;296:519–26. [DOI] [PubMed] [Google Scholar]
- Ostrosky-Zeichner L, Casadevall A, Galgiani JNet al. An insight into the antifungal pipeline: selected new molecules and beyond. Nat Rev Drug Discovery. 2010;9:719–27. [DOI] [PubMed] [Google Scholar]
- Papp C, Bohner F, Kocsis Ket al. Triazole evolution of Candida parapsilosis results in cross-resistance to other antifungal drugs, influences stress responses, and alters virulence in an antifungal drug-dependent manner. mSphere. 2020;5:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp C, Kocsis K, Tóth Ret al. Echinocandin-induced microevolution of Candida parapsilosis influences virulence and abiotic stress tolerance. mSphere. 2018;3:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham CD, Iqbal N, Bolden CBet al. Role of FKS mutations in Candida glabrata: MIC values, echinocandin resistance, and multidrug resistance. Antimicrob Agents Chemother. 2014;58:4690–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poláková S, Blume C, Zárate JÁet al. Formation of new chromosomes as a virulence mechanism in yeast Candida glabrata. Proc Natl Acad Sci. 2009;106:2688–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pristov KE, Ghannoum MA.. Resistance of Candida to azoles and echinocandins worldwide. Clin Microbiol Infect. 2019;25:792–8. [DOI] [PubMed] [Google Scholar]
- Robbins N, Caplan T, Cowen LE. Molecular evolution of antifungal drug resistance. Annu Rev Microbiol. 2017a;71:753–75. [DOI] [PubMed] [Google Scholar]
- Robbins N, Wright GD, Cowen LE. Antifungal drugs: the current armamentarium and development of new agents. Microbiol Spectr. 2016;4. DOI: 10.1128/microbiolspec.FUNK-0002-2016. [DOI] [PubMed] [Google Scholar]
- Rueda C, Cuenca-Estrella M, Zaragoza O.. Paradoxical growth of Candida albicans in the presence of caspofungin is associated with multiple cell wall rearrangements and decreased virulence. Antimicrob Agents Chemother. 2014;58:1071–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanglard D, Ischer F, Marchetti Oet al. Calcineurin A of Candida albicans: involvement in antifungal tolerance, cell morphogenesis and virulence. Mol Microbiol. 2003a;48:959–76. [DOI] [PubMed] [Google Scholar]
- Sanglard D, Ischer F, Parkinson Tet al. Candida albicans mutations in the ergosterol biosynthetic pathway and resistance to several antifungal agents. Antimicrob Agents Chemother. 2003b;47:2404–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinetti M, Posteraro B, Lass-Flörl C. Antifungal drug resistance among Candida species: mechanisms and clinical impact. Mycoses. 2015;58:2–13. [DOI] [PubMed] [Google Scholar]
- Selmecki A, Forche A, Berman J.. Aneuploidy and isochromosome formation in drug-resistant Candida albicans. Science. 2006;313:367–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmecki A, Gerami-Nejad M, Paulson Cet al. An isochromosome confers drug resistance in vivo by amplification of two genes, ERG11 and TAC1. Mol Microbiol. 2008;68:624–41. [DOI] [PubMed] [Google Scholar]
- Selmecki AM, Dulmage K, Cowen LEet al. Acquisition of aneuploidy provides increased fitness during the evolution of antifungal drug resistance. PLos Genet. 2009;5:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro RS, Robbins N, Cowen LE.. Regulatory circuitry governing fungal development, drug resistance, and disease. Microbiol Mol Biol Rev. 2011;75:213–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva NC, Nery JM, Dias ALT.. Aspartic proteinases of Candidaspp.: role in pathogenicity and antifungal resistance. Mycoses. 2014;57:1–11. [DOI] [PubMed] [Google Scholar]
- Sobel JD, Akins RA. The role of resistance in Candidainfections: epidemiology and treatment. Antimicrobial Drug Resistance. Cham: Springer International Publishing, 2017, 1075–97. [Google Scholar]
- Vale-Silva LA, Coste AT, Ischer Fet al. Azole resistance by loss of function of the sterol Δ5,6-desaturase gene (ERG3) in Candida albicans does not necessarily decrease virulence. Antimicrob Agents Chemother. 2012;56:1960–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale-Silva LA, Sanglard D.. Tipping the balance both ways: drug resistance and virulence in Candida glabrata. FEMS Yeast Res. 2015;15:25. [DOI] [PubMed] [Google Scholar]
- Vincent BM, Lancaster AK, Scherz-Shouval Ret al. Fitness trade-offs restrict the evolution of resistance to amphotericin B. PLoS Biol. 2013;11:e1001692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LA, MacCallum DM, Bertram Get al. Genome-wide analysis of Candida albicans gene expression patterns during infection of the mammalian kidney. Fungal Genet Biol. 2009;46:210–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Huang LH, Zhao JXet al. ERG11 mutations associated with azole resistance in Candida albicans isolates from vulvovaginal candidosis patients. Asian Pac J Trop Biomed. 2015;5:909–14. [Google Scholar]
- Wiederhold NP, Najvar LK, Bocanegra RAet al. Caspofungin dose escalation for invasive candidiasis due to resistant Candida albicans. Antimicrob Agents Chemother. 2011;55:3254–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . Global Tuberculosis Report 2020. Geneva: World Health Organization, 2020a. Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- World Health Organization . World Malaria Report 2020: 20 Years of Global Progress and Challenges. Geneva: World Health Organization, 2020b. Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- Xiang MJ, Liu JY, Ni PHet al. Erg11 mutations associated with azole resistance in clinical isolates of Candida albicans. FEMS Yeast Res. 2013;13:386–93. [DOI] [PubMed] [Google Scholar]