Abstract
Purpose:
The 2017 WHO classification of pituitary adenomas identifies the plurihormonal PIT-1 positive adenoma (PP1) as a distinct subtype. Literature suggests PP1 encompasses the former class of Silent Subtype 3 adenoma (SS3), and may have an aggressive phenotype. This study summarizes the current clinical data on PP1 and SS3 tumors and compares this published data to a single institutional cohort.
Methods:
Medline and Google Scholar were searched from 1990 to 2020 for clinical series of PP1 and SS3 according to PRISMA guidelines. Studies were included with pituitary pathology reported to be either PP1 or SS3 and if they reported clinical outcomes after a surgical intervention. To better define PP1 phenotype in comparison to non-PP1 pituitary adenomas, adenomas treated surgically at the authors’ institution from 2012-2019 were reviewed.
Results:
Of all tumors reported in the literature as PP1 or SS3, 99% were macro-adenomas and 18% were giant (>4cm). 31.8% of patients received radiotherapy. 22.9% of patients underwent multiple surgeries for their pituitary pathology. In the single-center experience reported here, 20 patients met criteria for the category of PP1. Compared to 1146 non-PP1 tumors, PP1 tumors did not show statistically significant differences in extent of resection, size, number of prior surgeries, future re-operations, rates of receiving radiotherapy, p53 staining, or MIB-1 labelling index.
Conclusions:
This large single center study comparing PP1 adenomas to non-PP1 adenomas did not suggest that these tumors are more aggressive. Further work is warranted to identify pathologic subtypes of pituitary adenomas that are consistently more clinically aggressive.
Keywords: Plurihormonal, PIT-1, pituitary adenoma, silent subtype 3 adenoma, atypical pituitary adenoma
Introduction
Pituitary adenomas, also known as pituitary neuroendocrine tumors (PitNETs), are a heterogenous group of intracranial neoplasms that can have starkly different presentations, treatments and clinical courses. Predicting which tumors will be most aggressive as opposed to those that can be treated conservatively is an area of ongoing research1. In 2004, the WHO described the category of atypical pituitary adenoma in terms of markers of proliferation, namely >2 mitoses per 10 HPFs, positive p53 immuno-staining, & Ki-67 index ≥3%2. Since the data supporting the prognostic power of atypical pituitary adenomas was not definitive, this designation was not included in the 2017 WHO classification of pituitary adenomas3,4. The 2017 WHO classifies pituitary adenomas through lineage specific transcription factors and hormone staining3.
One interesting entity described in the 2017 WHO classification of pituitary adenomas is the plurihormonal PIT-1 positive adenoma (PP1)4,5. This subset of neuroendocrine tumor stains for the transcription factor PIT-1 as well as multiple PIT-1 lineage hormones, and is singled out in the literature for its particular aggressiveness6. This new PP1 designation is also suggested by the literature to represent tumors formerly known as Silent Subtype 3 adenomas (SS3)5,7,8. SS3 historically required ultrastructural identification using electron microscopy, but PP1 tumors can be classified based on immunohistochemistry.
The pituitary adenomas classified as SS3 and PP1 are relatively uncommon, making up 1-4% of pituitary adenomas in large, longitudinal series5,9,10. Because of the rarity of these tumors, clinical data on SS3 and PP1 tumors are relatively sparse, limiting evidence-based assertions about these entities. Moreover, the studies currently published in the literature are generally without large matched cohorts of institutional controls. The purpose of this study is to summarize clinical data from the literature on PP1 and SS3 tumors as well as to compare this historical data to a large clinical series. To achieve this, a systematic review of the literature on PP1 and SS3 tumors was carried out and all cases of pituitary adenomas at our large tertiary care pituitary center were reviewed.
Methods
Literature review.
A systematic review was carried out according to PRISMA guidelines. Medline and Google Scholar were searched with the following terms from the period of 1990 to present: "plurihormonal pit-1 positive pituitary" OR "silent subtype III adenoma" OR "silent subtype 3 adenoma". Studies were included if they met the following criteria: 1) were a clinical study involving human patients with pituitary pathology reported to be either plurihormonal pit-1 positive pituitary adenoma or silent subtype 3 adenoma, 2) reported clinical outcomes after a surgical intervention, 3) were written in English. Abstracts were reviewed for inclusion criteria. If criteria were met, the manuscripts were reviewed for clinical characteristics and outcomes reported. Variables and outcomes extracted were: number of patients with either plurihormonal pit-1 positive pituitary adenoma or silent subtype 3 adenoma, achievement of gross total resection after initial surgery, whether the tumors were macro or micro adenomas, whether the tumors were “giant” (>4cm in any dimension), use of radiotherapy, and whether multiple surgeries were necessitated. In addition, Ki-67 staining or MIB-1 labelling index and endocrine remission status was reviewed if reported. These data were gleaned from reading through the manuscripts individually. One risk of bias identified in these studies was the lack of a proper comparison cohort. No studies were eliminated from inclusion because of this risk of bias. A meta-analysis was not performed.
Single center study.
Pituitary adenomas treated surgically at our tertiary care pituitary center from the years 2012-2019 were reviewed and retrospectively categorized as PP1 or non-PP1 pituitary adenomas based on available immunohistochemistry. This study was approved under IRB approval # 11-05901. A large single institution database of all pituitary pathology was constructed, and a series of variables and clinical characteristics were recorded for each patient who underwent surgery for pituitary pathology. Included in these characteristics were staining for pituitary hormones and transcription factors by pathology. Regarding PP1 categorization, the WHO classification denotes plurihormonal PIT-1 positive pituitary adenomas as positive for at least two of the pituitary hormones growth hormone (GH), prolactin (PRL), and the beta subunit of Thyroid stimulating hormone (β-TSH), as well as the transcription factor PIT-14. Of note, the synchronous expression of GH and PRL without evidence of other hormones is not categorized as plurihormonal4.
Diagnostic pathology.
Regarding the PP1 tumors, all cases were reviewed by a senior attending neuropathologist and only cases that were diagnosed as pit-1 plurihormonal adenomas were included in the study as PP1. The criteria for inclusion required diffuse pit-1 positivity in the tumor AND positive staining with at least two of the three PIT-1 lineage hormones GH, Prolactin, or TSH in the tumor. A notable exception is for the synchronous co-expression of only GH and prolactin, whose co-expression without an additional hormone would be indicative of a mammosomatotroph, not meeting criteria for plurihormonal4. WHO 2017 definition of plurihormonal includes expression of more than one adenohypophyseal hormone within a pituitary adenoma (with the exception of the isolated pairing of GH and PRL or β-FSH and β-LH). Positive staining of fewer than 5% of tumor cells were also not considered “positive” to allow for spurious staining and presence of scattered normal acini within the tumor.
Statistical analyses.
T-tests for continuous variables and chi-squared tests for categorical variables were carried out using R version 3.6.1 open source software (R Foundation for Statistical Computing, Vienna, Austria). For the PP1 cohort, Fisher’s exact test was used for comparison of categorical variables, given the small sample size and low number of observed frequencies. All statistical tests were two-tailed, using a threshold of significance of alpha = 0.05.
Results
Literature review.
A systematic review was performed to find clinical series describing plurihormonal PIT-1 positive pituitary adenomas (PP1) and silent subtype 3 adenomas (SS3). Medline and Google Scholar were both searched with the terms outlined in the methods section. 139 studies were returned from the search. 11 of these were clinical series that met inclusion criteria5,7,10-18. These studies were reviewed and select variables are presented in Table 1.
Table 1.
Review of Included Studies
| Study | Patients (n) | GTR (n/N) | Macro adenoma (n/N) |
Giant Adenoma (>4 cm; n/N) |
Knosp Grade ≥3 (n/N) |
Multiple Surgeries (n/N) |
RT (n/N) | MIB-1/Ki-67 ≥3% (n/N) |
Endocrine Remission* (%) |
|---|---|---|---|---|---|---|---|---|---|
| Aydin et al.,5 2019 | 9 | 7/9 | 9/9 | 2/9 | 4/9 | NR | NR | 5/9 | 100 |
| Yamaguchi-Okada et al.,17 2012 | 8 | 3/6 | 7/8 | 3/8 | 6/7 | NR | NR | NR† | NR |
| Wang et al.,18 2009 | 13 | NR | 13/13 | 0/13 | NR | NR | NR | 0/2 | NR‡ |
| Nagata et al.,13 2018 | 5 | 0/5 | 5/5 | 2/5 | 3/5 | NR | 2/5 | 1/5 | 20 |
| Wang et al.,12 2010 | 2 | NR | NR | NR | NR | NR | NR | NR | NR |
| Pereira et al.,14 2016 | 1 | 0/1 | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | 0 |
| Erickson et al.,10 2009 | 27 | 19/27 | 27/27 | NR | NR | 7/27 | 14/27 | NR | 70 |
| Mete et al.,7 2016 | 25 | 7/23 | 25/25 | 2/25 | NR | 4/25 | 2/25 | 19/25 | 83 |
| Horvath et al.,15 2005 | 29 | NR | 29/29 | 6/29 | NR | 6/29 | 8/29 | 3/29 | 100 |
| Richardson et al.,16 2010 | 10 | 2/10 | 10/10 | 2/10 | 6/10 | NR | NR | 5/10 | NR§ |
| Raverot et al.,19 2019 | 1 | 0/1 | 1/1 | 1/1 | NR | 1/1 | 1/1 | 0/1 | NR |
| Present study, 2021 | 20 | 16/20 | 18/20 | 1/20 | 13/20 | 3/20 | 3/20 | 5/20 | 94 |
GTR, gross total resection; RT, radiotherapy; NR, not reported.
Percentage of those with evidence of functional hypersecretion.
No consistent trend reported and no specific patient values provided.
No evidence of recurrence reported and no specific endocrine measures provided.
No hypersecretion found.
Of all tumors reported as PP1 or SS3 in the literature, 99% were macro-adenomas, with 18% reported as giant (defined as >4cm in any direction). 31.8% of patients received radiotherapy. 22.9% of patients in this cohort underwent multiple surgeries for their pituitary pathology. These data suggest that these tumors may represent an aggressive phenotype of large tumors that often require multiple surgeries to treat. One marker of risk of bias that was assessed was whether the studies included adequate comparison groups. There were no comparison groups in 5 of the studies included, namely Pereira et al.14, Erickson et al.10, Mete et al.7, Horvath et al.15, and Richardson et al.16. Selective comparison groups, comparing specific cohorts of pituitary adenomas, were available in Aydin et al.,5 Nagata et al.13, Wang et al.19 and Wang et al.12 A large comparison group, but for only a single PP1/SS3 patient of interest to the present study, was present in Raverot et al.20
The endocrine remission status of functioning PP1 tumors in the cohorts of the literature review are also reported in Table 1. Remission rates varied by report. Of the 4 largest series reporting this metric, 100% remission was reported in Horvath et al.15, 83% remission in Mete et al.7, 70% in Erickson et al.10, and 94% remission in the present study.
Single center results.
Pituitary adenomas treated surgically at the authors’ tertiary pituitary referral center between 2012-2019 were reviewed and retrospectively categorized as PP1 or non-PP1 pituitary adenomas based on immunohistochemistry. Tumors were sorted by those that met definitions of PP1 tumors, which then underwent histological review by a senior pathologist (T.T.) blinded to outcomes associated with the tumors. Criteria for hormone positivity was >5% of cells expressing the hormones TSH, GH or PRL. This yielded 20 definitive PP1 tumors. Data on the PP1 cohort are presented in Table 2, compared to a cohort of 1146 functional and non-functional pituitary adenomas that did not meet criteria for PP1. The median clinical follow up for the PP1 cohort was 19 months and for the non-PP1 cohort was 21 months, respectively.
Table 2.
Patient Characteristics Stratified by Adenoma Type
| Characteristic | Non-PP1 Adenoma | PP1 Adenoma | P Value |
|---|---|---|---|
| Patients | 1146 (98.2) | 20 (1.71) | NA |
| Sex | 0.550 | ||
| Female | 593 (51.7) | 9 (45.0) | |
| Male | 553 (48.3) | 11 (55.0) | |
| Age (Years) | 51.0 (37.0–63.0) | 47.5 (35.0–60.8) | 0.821 |
| Size | 0.342 | ||
| Microadenoma | 207 (18.3) | 2 (10.0) | |
| Macroadenoma | 927 (81.7) | 18 (90.0) | |
| Previous Surgery | 0.898 | ||
| No | 962 (83.9) | 17 (85.0) | |
| Yes | 184 (16.1) | 3 (15.0) | |
| Future Reoperation | 0.910 | ||
| No | 1082 (94.4) | 19 (95.0) | |
| Yes | 64 (5.6) | 1 (5.0) | |
| P53 Staining | 0.325 | ||
| No | 97 (28.3) | 1 (12.5) | |
| Yes | 246 (71.7) | 7 (87.5) | |
| Neoadjuvant RT | 0.597 | ||
| No | 1112 (97.0) | 19 (95.0) | |
| Yes | 34 (3.0) | 1 (5.0) | |
| Adjuvant RT | 0.654 | ||
| No | 992 (86.6) | 18 (90.0) | |
| Yes | 154 (13.4) | 2 (10.0) | |
| Resection Extent | 0.139 | ||
| GTR | 725 (64.0) | 16 (80.0) | |
| STR | 408 (36.0) | 4 (20.0) | |
| MiB Index | 2.44 ± 2.29 | 2.34 ± 1.69 | 0.846 |
Data presented as n (%), median (interquartile range), or mean ± standard deviation.
PP1, plurihormonal PIT-1–positive; NA, not applicable; RT, radiotherapy; GTR, gross total resection; STR, subtotal resection.
In this cohort of patients, PP1 did not show statistically significant differences in extent of resection, macro vs microadenoma status, number of prior surgeries, future re-operations, prior surgeries, rates of receiving radiotherapy, p53 staining, or MIB-1 labelling index (N and P-values reported in Table 2). Of the PP1 tumors, seven had a Knosp grade of 4, six had a Knosp grade of 3, five had Knosp grade of 2, and 2 had a Knosp grade of 1.
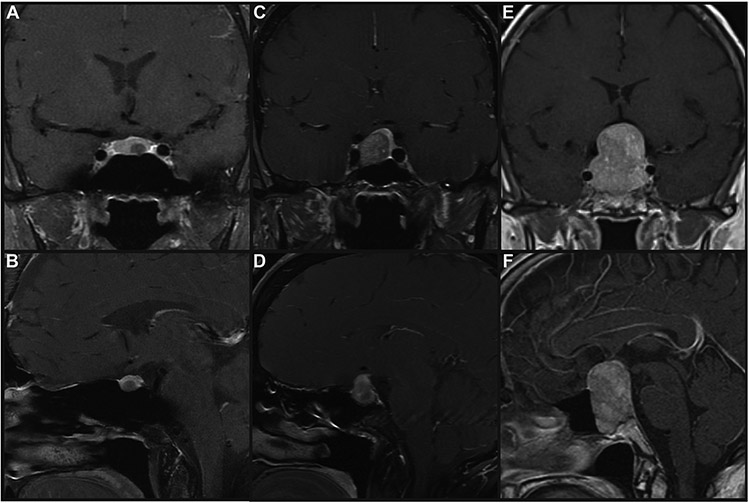
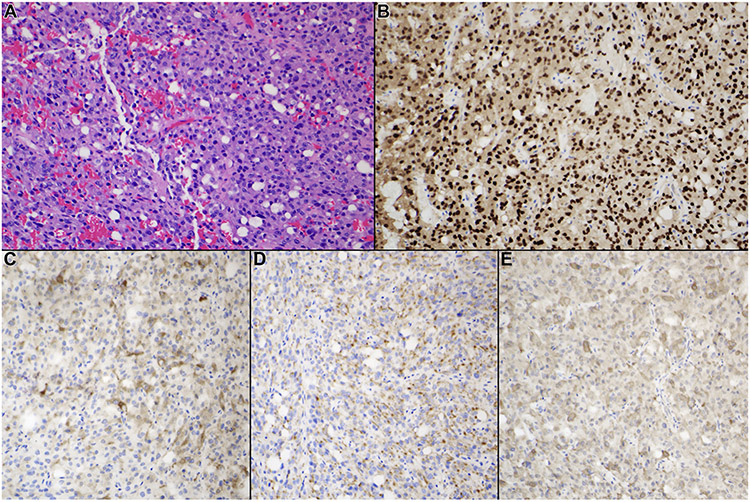
Imaging examples of 3 PP1 tumors are presented in Figure 1. Depicted is an example of a microadenoma (7mm in maximum dimension, 1A and B), a macroadenoma (18mm in maximal dimension, 1C and D) and a giant pituitary adenoma (41mm in maximum dimension, 1E and F), all with >5% staining of GH, TSH and PRL, as well as diffuse Pit-1 positive staining. This variability in size on presentation is representative of the sample, as there was not a clear radiographic characteristic distinguishing PP1 tumors from non-PP1 tumors. Representative histology is shown in Figure 2. All tumors included in this category were monomorphous tumors with no evidence of the acinar pattern of the normal pituitary gland. The figure exhibits A) H&E staining B) PIT-1 staining C) GH staining D) Prolactin staining E) TSH staining of a PP1 tumor.
Figure 1.
T1-weighted magnetic resonance imaging (MRI) scans of plurihormonal PIT-1–positive (PP1) pituitary adenomas. T1-weighted, gadolinium-enhanced MRI scans of a (A and B) microadenoma (maximal dimension, 7 mm), (C and D) macroadenoma (maximal dimension, 18 mm), and (E and F) giant pituitary adenoma (maximal dimension, 41 mm), all with >5% staining of growth hormone, β-Subunit of thyroid-stimulating hormone, and prolactin and diffuse PIT-1–positive staining.
Figure 2.
Typical histological and immunohistochemical features of PIT-1–positive plurihormonal adenoma. All tumors included in this category demonstrated monomorphous features with no evidence of the acinar pattern of the normal pituitary gland. (A) Hematoxylin and eosin staining, (B) PIT-1 staining, (C) growth hormone staining, (D) prolactin staining, and (E) β-subunit of thyroid-stimulating hormone staining (original magnification ×200).
There was no significant difference in MIB-1 labelling index (2.34% in PP1 vs 2.44% non-PP1 adenomas, P=0.846). In contrast to the trend on size observed in the literature review, PP1 tumors in the single center cohort were not on average larger than non-PP1 pituitary adenomas.
Discussion
In this study we compiled the available literature documenting clinical course and characteristics associated with plurihormonal PIT-1 positive pituitary adenomas and compared the results to outcomes at a tertiary referral center with a large volume of pituitary tumors. No other study has compared their PP1 subset to the whole of their non-PP1 tumors operated on during the same time period. Taken together, the literature review and institutional series present divergent pictures of PP1 tumors, with the literature suggesting a more aggressive picture of PP1 tumors, while the single-center series showed no objective signs of different clinical characteristics associated with PP1 tumors. The intra-institutional control is critical in differentiating clinical course, as this study does not refute the presence of differing genetics, mutational etiology or precursor cells for PP1 tumors. Rather, these data show that in a single center with multiple expert pituitary surgeons, PP1 tumors do not take highly divergent courses from their non-PP1 counterparts. These findings underscore the importance using reserve when prognosticating based on case series that lack contemporaneous controls and are therefore potentially subject to selection bias. The 2017 WHO classification of pituitary adenomas describe PP1 tumors as a specific subset of plurihormonal pituitary adenoma, which may represent a more aggressive subtype3. A systematic review of the literature supports the view that these tumors may portend a more aggressive course, by suggesting they may require multiple surgeries and radiation therapy, and that gross total resection is often not achieved7,10,15. A single-institution series of histologically defined PP1 tumors from a large database of pituitary adenomas is also presented for comparison with the published literature. Neither p53 staining, nor MIB-1 labelling index showed a statistically significant difference that would suggest these tumors proliferate at a faster or more aggressive rate. The majority of pooled clinical data suggest little to no difference in the course of PP1 from non-PP1 pituitary adenomas. In the single center data reported here, PP1 do not have significantly higher rates of repeat surgical procedures, radiation therapy, or higher rates of pituitary macroadenomas. Taken together, these data weigh against the idea that PP1 tumors are a significantly more aggressive phenotype.
Prognostication of pituitary neuroendocrine tumors continues to evolve. While most of these tumors respond well to conventional therapies, it is clear that some PitNETs act clinically more aggressive, with faster growth rates and invasion into nearby structures17,21,22. The European Society of Endocrinology provides guidance regarding aggressive PitNETs, but leaves some diagnostic criteria to generalizations23. These guidelines recommend consideration of the aggressive pituitary tumor diagnosis in those with radiographic evidence of invasiveness, unusually rapid tumor growth, or growth despite optimal clinical therapy23. The definitions of each of these features, however, is subjective and somewhat nuanced22. Having biomarkers or pathologic indicators predictive of aggressiveness is an important clinical goal, so that patients can receive the full spectrum of clinical therapies available, whether that be radiation, chemotherapy or surgical intervention17,24,25.
Regarding pathologic characteristics of PitNETs that predict more aggressive tumor behavior, mitotic count, p53 positivity and MIB-1/Ki-67 are generally regarded as relevant. The exact parameters of these is a topic of ongoing debate1,26. The cut-offs of >2 mitoses per 10 high power fields on microscopy, and Ki-67/MIB-1 labelling of ≥3% have been proposed23. Retrospective studies do tend to show that these markers are associated with measures of recurrence27. However, these features were previously used in the formulation of the entity of the atypical pituitary adenoma, which has been removed from the WHO categorization for lack of evidence for prognostic value. Assessment of these pathologic characteristics independent of the “atypical” label, is nonetheless still encouraged4,9,28. The exact mechanisms of what in the PP1 molecular profile would make it more aggressive than other transcription factor or histologic subtypes is also still a point of speculation.
Weaknesses of the current study are its level of granularity. Some of the literature on PP1 and SS3 tumors reviewed here rely on detailed re-counting of individual patients’ clinical courses. That level of detail is one step removed here, instead being replaced by a higher N such that generalities can be presented. Another aspect that may be interpreted as a weakness here is the reliance on immunohistochemistry alone to make the PP1 diagnosis. While this is indeed the stipulation and intent of the classification by WHO, the supposition that SS3 and PP1 pituitary adenomas represent the same entity still has yet to be proven unequivocally7.
The lack of objective data supporting a PP1 as more aggressive phenotype of pituitary adenoma is surprising. It is certainly possible that nuances of the clinical courses of patients with this entity are lost in this study. That being said, the PP1 entity in some sense unseated the designation of atypical adenoma as the new aggressor of pituitary adenomas2. The characterization of pituitary adenomas by immunohistochemistry and lineage transcription factors is an intuitive and useful framework through which to approach these diverse tumors. Regarding the prognostic strength of PP1 as an aggressive subtype, though, if the data presented here are replicated in other institutional experiences, PP1 may follow closely in the footsteps of its predecessor, the atypical adenoma.
Funding:
funded in part by NIH grant 5R01NS079697-07
Footnotes
Conflicts of interest/Competing interests: None
References
- 1.Di Ieva A, Rotondo F, Syro LV, Cusimano MD, Kovacs K. Aggressive pituitary adenomas—diagnosis and emerging treatments. Nature Reviews Endocrinology. 2014;10(7):423. [DOI] [PubMed] [Google Scholar]
- 2.Rutkowski MJ, Alward RM, Chen R, et al. Atypical pituitary adenoma: a clinicopathologic case series. Journal of neurosurgery. 2018;128(4):1058–1065. [DOI] [PubMed] [Google Scholar]
- 3.Inoshita N, Nishioka H. The 2017 WHO classification of pituitary adenoma: overview and comments. Brain tumor pathology. 2018;35(2):51–56. [DOI] [PubMed] [Google Scholar]
- 4.Mete O, Lopes MB. Overview of the 2017 WHO classification of pituitary tumors. Endocrine Pathology. 2017;28(3):228–243. [DOI] [PubMed] [Google Scholar]
- 5.Aydin S, Comunoglu N, Ahmedov M, Korkmaz O, … Clinicopathologic Characteristics and Surgical Treatment of Plurihormonal Pituitary Adenomas. World neurosurgery. 2019. [DOI] [PubMed] [Google Scholar]
- 6.Tortosa F Pituitary tumors: Update on histopathological diagnosis. Current Opinion in Endocrine and Metabolic Research. 2018;1:13–18. [Google Scholar]
- 7.Mete O, Gomez-Hernandez K, Kucharczyk W, et al. Silent subtype 3 pituitary adenomas are not always silent and represent poorly differentiated monomorphous plurihormonal Pit-1 lineage adenomas. Modern Pathology. 2016;29(2):131. [DOI] [PubMed] [Google Scholar]
- 8.Mete O, Asa S. Therapeutic implications of accurate classification of pituitary adenomas. Seminars in diagnostic pathology. 2013. [DOI] [PubMed] [Google Scholar]
- 9.Mete O, Cintosun A, Pressman I, Asa S. Epidemiology and biomarker profile of pituitary adenohypophysial tumors. nature.com; 2018. [DOI] [PubMed] [Google Scholar]
- 10.Erickson D, Scheithauer B, Atkinson J, … Silent subtype 3 pituitary adenoma: a clinicopathologic analysis of the Mayo Clinic experience. Clinical …. 2009. [DOI] [PubMed] [Google Scholar]
- 11.Yamaguchi-Okada M, Inoshita N, Nishioka H, … Clinicopathological analysis of nonfunctioning pituitary adenomas in patients younger than 25 years of age. Journal of …. 2012. [DOI] [PubMed] [Google Scholar]
- 12.Wang E, Qian Z, Yamada S, Rahman M, … Clinicopathological characterization of TSH-producing adenomas: special reference to TSH-immunoreactive but clinically nonfunctioning adenomas. Springer; 2009. [DOI] [PubMed] [Google Scholar]
- 13.Nagata Y, Inoshita N, Fukuhara N, et al. Growth hormone-producing pituitary adenomas in childhood and young adulthood: clinical features and outcomes. Pituitary. 2018;21(1):1–9. [DOI] [PubMed] [Google Scholar]
- 14.Pereira BD, Raimundo L, Mete O, Oliveira A, Portugal J, Asa SL. Monomorphous plurihormonal pituitary adenoma of Pit-1 lineage in a giant adolescent with central hyperthyroidism. Endocrine pathology. 2016;27(1):25–33. [DOI] [PubMed] [Google Scholar]
- 15.Horvath E, Kovacs K, Smyth H, Cusimano M, Singer W. Silent adenoma subtype 3 of the pituitary—immunohistochemical and ultrastructural classification: a review of 29 cases. Ultrastructural pathology. 2005;29(6):511–524. [DOI] [PubMed] [Google Scholar]
- 16.Richardson TE, Mathis DA, Mickey BE, et al. Clinical outcome of silent subtype III pituitary adenomas diagnosed by immunohistochemistry. Journal of neuropathology and experimental neurology. 2015;74(12):1170–1177. [DOI] [PubMed] [Google Scholar]
- 17.Raverot G, Vasiljevic A, Jouanneau E, Lasolle H. Confirmation of a new therapeutic option for aggressive or dopamine agonist-resistant prolactin pituitary neuroendocrine tumors. European Journal of Endocrinology. 2019;181(2):C1–C3. [DOI] [PubMed] [Google Scholar]
- 18.Wang EL, Qian ZR, Rahman MM, et al. Increased expression of HMGA1 correlates with tumour invasiveness and proliferation in human pituitary adenomas. Histopathology. 2010;56(4):501–509. [DOI] [PubMed] [Google Scholar]
- 19.Wang E, Qian Z, Rahman M, Yoshimoto K, … Increased expression of HMGA1 correlates with tumour invasiveness and proliferation in human pituitary adenomas. …. 2010. [DOI] [PubMed] [Google Scholar]
- 20.Raverot G, Dantony E, Beauvy J, et al. Risk of recurrence in pituitary neuroendocrine tumors: a prospective study using a five-tiered classification. The Journal of Clinical Endocrinology & Metabolism. 2017;102(9):3368–3374. [DOI] [PubMed] [Google Scholar]
- 21.Kasuki L, Raverot G. Definition and diagnosis of aggressive pituitary tumors. Reviews in Endocrine and Metabolic Disorders. 2019:1–6. [DOI] [PubMed] [Google Scholar]
- 22.Ilie MD, Jouanneau E, Raverot G. Aggressive pituitary adenomas and carcinomas. Endocrinology and Metabolism Clinics. 2020;49(3):505–515. [DOI] [PubMed] [Google Scholar]
- 23.Raverot G, Burman P, McCormack A, et al. European Society of Endocrinology Clinical Practice Guidelines for the management of aggressive pituitary tumours and carcinomas. European journal of endocrinology. 2018;178(1):G1–G24. [DOI] [PubMed] [Google Scholar]
- 24.Lasolle H, Cortet C, Castinetti F, et al. Temozolomide treatment can improve overall survival in aggressive pituitary tumors and pituitary carcinomas. Eur J Endocrinol. 2017;176(6):769–777. [DOI] [PubMed] [Google Scholar]
- 25.Mehta GU, Lonser RR. Management of hormone-secreting pituitary adenomas. Neuro-oncology. 2017;19(6):762–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manojlovic-Gacic E, Bollerslev J, … Pathology of pituitary neuroendocrine tumours: present status, modern diagnostic approach, controversies and future perspectives from a neuropathological and …. Neuropathology and …. 2019. [DOI] [PubMed] [Google Scholar]
- 27.Hasanov R, Aydoğan Bİ, Kiremitçi S, Erden E, Güllü S. The prognostic roles of the Ki-67 proliferation index, P53 expression, mitotic index, and radiological tumor invasion in pituitary adenomas. Endocrine pathology. 2019;30(1):49–55. [DOI] [PubMed] [Google Scholar]
- 28.Nishioka H, Inoshita N. New WHO classification of pituitary adenomas: assessment of pituitary transcription factors and the prognostic histological factors. Brain tumor pathology. 2018;35(2):57–61. [DOI] [PubMed] [Google Scholar]