Abstract
Systemic sclerosis (SSc) is a rare, systemic autoimmune disease of unknown etiology. Among the systemic rheumatic diseases, SSc carries the highest mortality, in part due to the historical lack of disease modifying therapies. Recently, landmark randomized controlled trials (RCTs) have been conducted that have illustrated the heterogeneous nature of SSc and furthered our understanding of the key inflammatory and fibrotic pathways involved in SSc pathogenesis. Although SSc affects various organ systems, RCTs have focused on investigating treatments for diffuse cutaneous sclerosis (dcSSc) and interstitial lung disease (ILD). While recent RCTs for dcSSc have failed to demonstrate a treatment benefit, the outcomes of two RCTs led to the approval of two novel therapies for SSc-ILD: nintedanib and tocilizumab. This review summarizes the salient outcome data from recent SSc trials within a practical clinical framework and points out gaps in knowledge that may help inform the design of future SSc studies.
Keywords: systemic sclerosis; interstitial lung disease; cutaneous sclerosis; nintedanib, tocilizumab; therapeutics
Introduction
Systemic sclerosis (SSc; scleroderma) is a rare, chronic connective tissue disease of unknown etiology that portends the highest case-specific mortality among the systemic rheumatic diseases [1]. While pathologic vasculopathy, immune dysregulation and fibrosis uniquely contribute to organ damage in SSc, there is considerable variability in the phenotypic manifestations, rate of disease progression and response to therapy among affected individuals. Historically, scleroderma renal crisis (SRC) carried the highest risk of mortality; however, the implementation of angiotensin converting enzyme inhibitors (ACE-I) in the early 1980s reduced the frequency of deaths in SRC from 42% to 6% over a 3-decade period [2]. Presently, SSc-associated interstitial lung disease (SSc-ILD) and pulmonary hypertension (SSc-PH) are the leading causes of SSc-related death; although, emerging data suggest that improvements in early detection of ILD and PH, along with the discovery of novel therapeutics for these complications has led to better survival [3,4]. Moreover, there are several novel therapeutics under investigation for the treatment of SSc. The purpose of this article is to review existing and novel emerging therapies for the treatment of SSc, with an emphasis on recent trials targeting the cutaneous and pulmonary manifestations of this disease.
Pathogenesis
Although the exact etiology of SSc is unclear [1], numerous studies have demonstrated that genetic susceptibility and environmental factors play a large role in the development of disease [5,6]. Vascular injury occurs early in the SSc disease course, promoting endothelial activation, inflammation via innate and adaptive immune responses, vascular remodeling and eventual fibrosis [7,8]. Figure 1 summarizes important immunological participants of SSc pathogenesis with an emphasis on therapeutic targets.
Figure 1. Key pathways involved in SSc pathogenesis and therapeutic targets of current and emerging therapies.
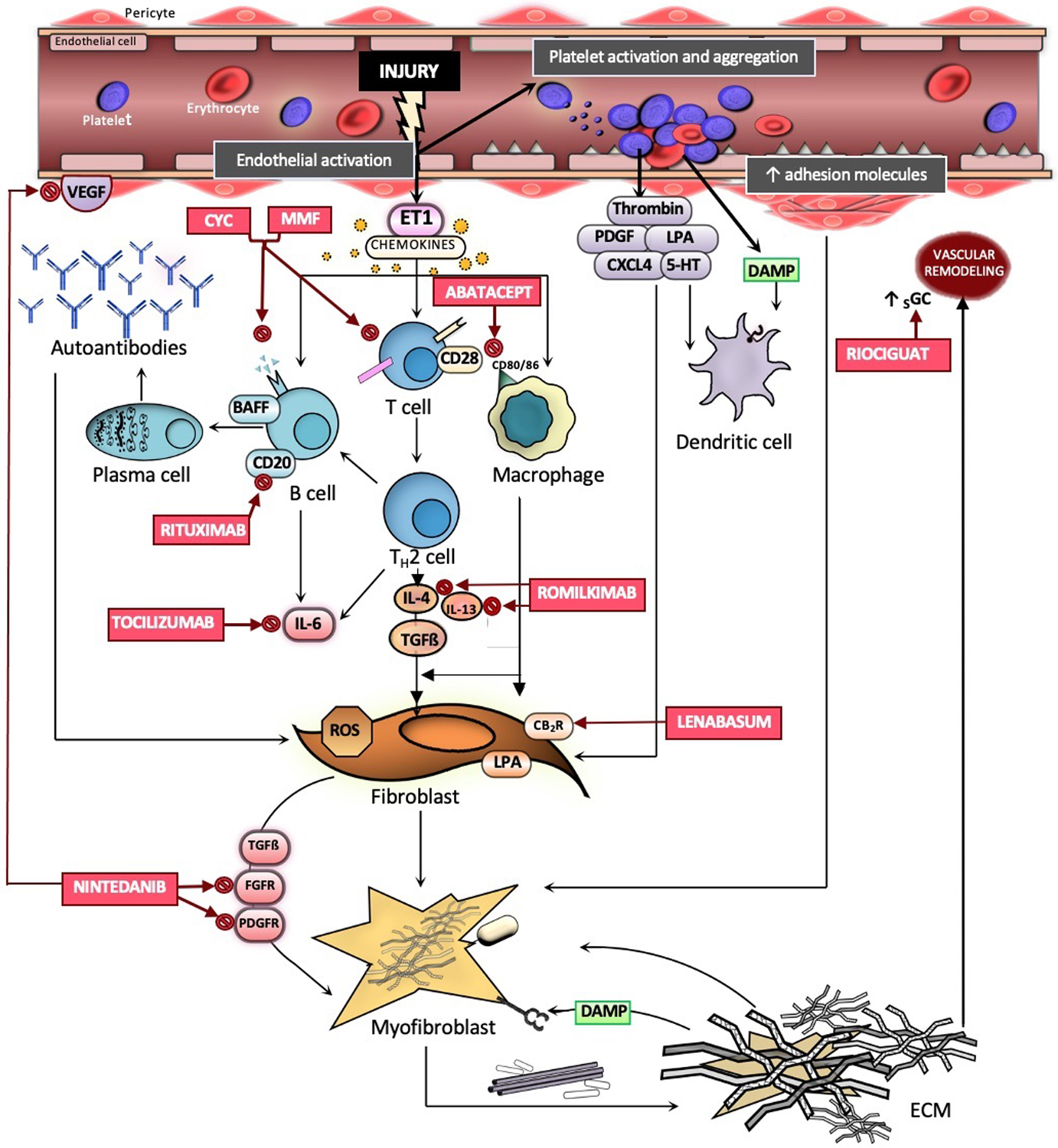
Vascular injury occurs early in the SSc disease course, resulting in endothelial cell activation, expression of adhesion molecules, chemokine production, release of ET1, and platelet activation with subsequent coagulation. Chemokines recruit macrophages from the circulation into the tissue. DAMPs activate dendritic cells via toll-like receptor 4. Activated T cells undergo differentiation to Th2 cells, releasing IL-6, IL-4, and IL-13. B cells are subsequently activated via IL-4 and undergo transformation into plasma cells with subsequent production of autoantibodies. Resident fibroblasts are activated by TGF-B, IL-13, and IL-6, generating ROS and differentiating into myofibroblasts. Myofibroblasts secrete profibrotic growth factors and produce extracellular matrix molecules, forming a fibrotic matrix. Abbreviations: CYC: cyclophosphamide; MMF: mycophenolate mofetil; IL-6: interleukin-6; IL-4 ; interleukin-4; IL-13: interleukin-13; ET1: Endothelin-1; PDGF: Platelet-derived growth factor; LPA: lysophosphatidic acid; CXCL4: chemokine ligand 4; 5-HT: serotonin receptor; BAFF: B-cell activating factor; TGFß: Transforming growth factor beta; FGFR: fibroblast growth factor receptor; PDGFR: Platelet-derived growth factor receptor; VEGF: Vascular endothelial growth factor; CB2: cannabinoid receptor type 2; ROS: reactive oxygen species; sGC: soluble guanylate cyclase; DAMP: Damage-associated molecular patterns; ECM: extracellular matrix.
Current Approach to Management
Treatment of SSc can be challenging owning to its rarity and heterogeneous disease manifestations. Prior to initiating therapy, a pretreatment evaluation is warranted to identify active organ involvement and risk stratify patients (Figure 2). Therapy should be implemented to target active organ-specific complications of disease with a preference for therapies that may target more than one active organ system or target overlap connective tissue disease, if present. Table 1 summarizes the current pharmacologic therapies for SSc by organ system.
Figure 2. Approach to pre-treatment evaluation in patients with SSc.
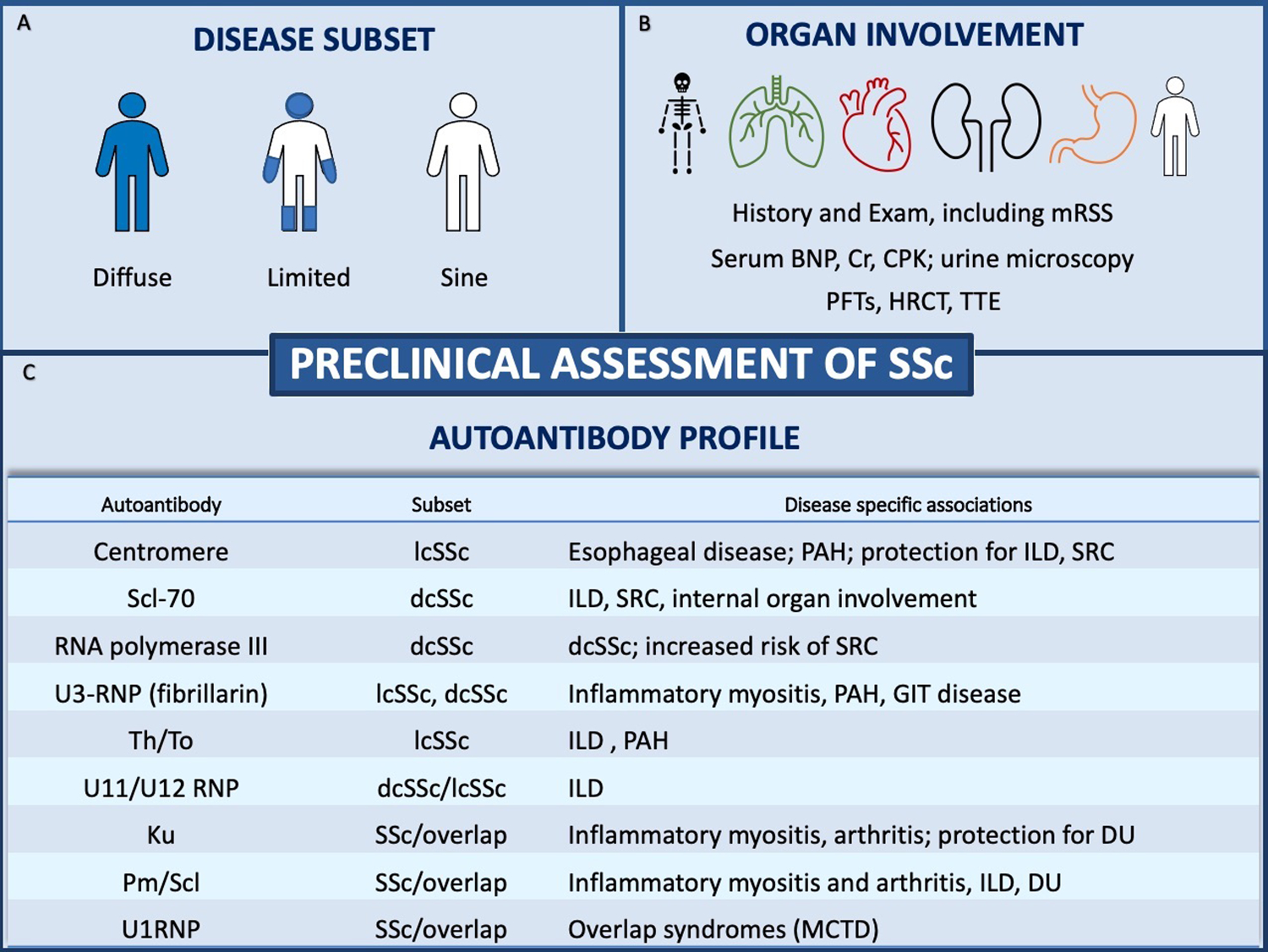
(A) Disease subsets are defined by the extent of skin thickening and include diffuse cutaneous SSc (dcSSc), limited cutaneous SSc (lcSSc) and systemic sclerosis sine scleroderma. (B) Recommended baseline evaluation in SSc. (C) Autoantibodies and their associated subsets and disease-specific associations. Abbreviations: Scl-70: topoisomerase 1; RNA: ribonucleic acid; RNP: ribonuclear protein; DU: digital ulceration; PAH: pulmonary arterial hypertension; SRC: scleroderma renal crisis; GIT: gastrointestinal tract disease; MCTD: mixed connective tissue disease.
Table 1.
Current pharmacological therapies for organ-specific manifestations of SSc
| Therapeutic agent | Benefit in SSc |
|---|---|
| Diffuse cutaneous sclerosis | |
| MMF | Improvement in mRSS |
| CYC | Improvement in mRSS |
| HSCT | Improvement in mRSS |
| Raynaud phenomenon/digital ulcers | |
| Calcium channel blocker | Reduction in frequency and severity of Raynaud phenomenon attacks |
| Fluoxetine | Reduction in frequency and severity of Raynaud phenomenon attacks |
| Sildenafil, tadalafil | Reduction in frequency and severity of Raynaud phenomenon attacks |
| Iloprost | Reduction in frequency and severity of Raynaud phenomenon attacks and digital ulcers |
| Bosentan, ambrisentan | Reduction in development of new digital ulcers |
| Interstitial lung disease | |
| CYC | Improvement in FVC, radiographic fibrosis and dyspnea |
| MMF | Improvement in FVC, radiographic fibrosis and dyspnea |
| Tocilizumab | Stabilization of FVC in early dcSSc with and without ILD |
| Nintedanib | Reduction in annual rate of decline of FVC |
| Pulmonary hypertension | |
| Sildenafil, tadalfil | Improvement in exercise capacity, hemodynamics, functional class |
| Riociguat | Improvement in exercise capacity, hemodynamics, functional class |
| Bosentan | Improvement in 6-minute walk distance; delayed progression to clinical worsening |
| Ambrisentan plus tadalafil | Reduction in risk of clinical failure compared with single agent; Improvement in 6-minute walk distance |
| Selexipag | Reduction in hospitalizations and disease progression |
| Prostanoids (Treprostinil, Epoprostenol, Iloprost, Beraprost) | Improvement in 6-minute walk distance and time to clinical worsening |
| Gastrointestinal disease | |
| Proton-pump inhibitors | Improvement in acid reflux symptoms; decreased risk of upper GI ulcers |
| H2 blockers | Improvement in acid reflux symptoms |
| Pro-kinetic agents | Improvement in symptoms related to GI tract dysmotility |
| Renal disease | |
| ACE inhibitors | Improvement in morbidity and mortality due to scleroderma renal crisis |
| Cardiac disease | |
| Calcium channel blockers | Prevention and treatment of left ventricular systolic dysfunction |
| ACE inhibitors, Calcium channel blockers | Improvement in myocardial perfusion |
| Anti-arrhythmic agents | Improvement in arrhythmias due to SSc-myocardial involvement |
| Immunosuppressive therapy | Improvement in mortality in myocarditis |
Current Therapies for Cutaneous Sclerosis
Cutaneous sclerosis is the cardinal symptom of SSc and is present in most patients, either in a diffuse or limited distribution. The modified Rodnan skin score (mRSS) is routinely used to quantify the extent of cutaneous sclerosis in clinical practice and in research. Most clinical trials have focused on early dcSSc, wherein immunomodulatory therapy may serve to reverse and reduce disease burden.
Randomized controlled trials (RCTs) have demonstrated that cyclophosphamide (CYC) [9], mycophenolate (MMF) [10,11] and hematopoietic stem cell transplant (HSCT) [12,13] are associated with a statistically significant improvement in cutaneous sclerosis. In general, MMF, is considered first line therapy given its favorable toxicity and side-effect profile compared with CYC. HSCT is generally reserved for patients with severe dcSSc refractory to immunomodulatory therapy given its high morbidity and mortality, although this approach can lead to the most dramatic improvement in cutaneous sclerosis. Although nintedanib and tocilizumab are approved therapies for SSc-ILD, neither of these medications demonstrated a statistically significant benefit in reducing the extent of cutaneous sclerosis compared with placebo (Table 2).
Table 2.
Recent Phase II/III RCTs for dcSSc
| Therapeutic | Sample Size | Skin Outcome Measure | Result of skin outcome measure |
|---|---|---|---|
| Tocilizumab [21] | N=212 | Primary outcome: ΔmRSS at week 48 | Least squares mean ΔmRSS −6.1 in tocilizumab arm and −4.4 in placebo arm; P=0.10 |
| Nintedanib [19] | N=576 | Secondary outcome: ΔmRSS at week 52 | Adjusted mean ΔmRSS −2.17 in the nintedanib arm and −1.96 in the placebo arm; P=0.58 |
| Lenabasum [23] | N=363 | Secondary outcome: ΔmRSS at week 52 | Mean ΔmRSS −8.1 in the placebo arm and −6.7 in the lenabasum arm |
| Abatacept [24] | N=88 | Primary outcome: ΔmRSS at week 52 | Adjusted mean ΔmRSS from baseline −6.24 in abatacept arm and −4.49 in placebo arm; P=0.28 |
| Riociguat [25] | N=109 | Primary outcome: ΔmRSS at week 52 | Mean ΔmRSS −2.09 for riociguat arm and −0.77 for placebo arm; P=0.08 |
Current Therapies for SSc-ILD
Scleroderma Lung Studies
SSc-ILD is recognized as the major cause of disease-related mortality; however, review of contemporary literature suggests improved survival among patients with SSc-ILD due to more aggressive monitoring and treatment [4,14]. In clinical trials of SSc-ILD, change in forced vital capacity (FVC) is commonly used as a primary outcome measure, as low FVC predicts morbidity and mortality [15]. Two landmark clinical trials, SLS-I [9] and SLS-II [10], established CYC and MMF as disease modifying therapies for SSc patients with active ILD. In SLS-I, treatment with CYC resulted in a clinically significant improvement in FVC%-predicted, total lung capacity (TLC)%-predicted and radiographic fibrosis compared with placebo at 12 months [10]. In SLS-II, treatment with MMF for 24 months or CYC for 12 months led to significant improvement in the course of the FVC%-predicted over 24 months [11]. Treatment with MMF and CYC was also associated with improvements in radiographic fibrosis [16] and self-reported dyspnea [17]. Given its favorable tolerability and side effect profile, MMF is currently considered first-line therapy for patients with SSc-ILD. For patients with progressive dcSSc with ILD, refractory to immunosuppressive therapy, autologous HSCT may be considered, as studies have demonstrated improvement in FVC [12] and decreased progression to respiratory failure [13] in patients randomized to HSCT compared with CYC.
Nintedanib
Nintedanib inhibits intracellular tyrosine kinase and fibroblast growth receptor (FGFR) [18]. In a RCT of 576 patients with SSc-ILD, treatment with nintedanib slowed the rate of decline of FVC over 52 weeks compared with placebo; the adjusted annual rate of change in FVC was −52.4 ml per year in the nintedanib group and −93.3 ml per year in the placebo group (P=0.04) [19]. Background immunosuppressive therapy was permitted, and nearly half (48.4%) of patients were receiving MMF for at least 6 months prior to enrollment. Patients who were taking MMF at baseline and randomized to nintedanib had the slowest decline in lung function; however, because patients were not randomized to MMF, confounding factors may account for these outcome disparities. Currently, more data is needed to determine when anti-fibrotic therapy should be initiated in SSc-ILD. Results of the ongoing SLS III study (MMF vs. MMF plus the anti-fibrotic, pirfenidone) may help us understand whether early upfront combination therapy is advantageous in SSc-ILD.
Tocilizumab
Interleukin-6 (IL-6) is a pro-inflammatory cytokine that is frequently overexpressed in systemic sclerosis, promoting inflammation and profibrotic effects via the Janus kinase (JAK) 2/signal transducer and activator of transcription protein (STAT) 3 pathway [20]. Tocilizumab is a humanized monoclonal antibody that functions as an antagonist of the IL-6 receptor, thereby blocking its downstream effects. Tocilizumab in systemic sclerosis (focuSSced) was a phase III trial that enrolled 210 adult patients with relatively early dcSSc with evidence of worsening cutaneous sclerosis and systemic inflammation. Patients were randomized to receive either tocilizumab 162 mg subcutaneous weekly or placebo. While a treatment benefit of tocilizumab on the primary endpoint (change in mRSS) was not observed, the investigators found that treatment with tocilizumab was associated with stabilization of the FVC%-predicted from baseline to 48 weeks [21,22]. While the data from the focuSSced trial resulted in FDA approval of tocilizumab for SSc-ILD, it is unclear whether SSc-ILD patients with lcSSc or SSc-ILD patients without elevated inflammatory markers would also benefit from tocilizumab. Furthermore, in patients with dcSSc-ILD, it is unknown whether tocilizumab can be safely combined with existing immunosuppressive treatments for dcSSc (e.g., MMF).
Emerging Therapies for SSc
A number of novel therapeutic agents have been recently studied for the treatment of dcSSc and SSc-ILD. While some of these therapies, including lenabasum [23], abatacept [24], and riociguat [25], have failed to demonstrated treatment benefit (Table 2), several new agents are currently under investigation as described below.
Rituximab
Dysregulation of adaptive immunity is implicated in the pathogenesis of SSc [8]. Rituximab is a humanized chimeric anti-CD20 monoclonal antibody that depletes peripheral B cells through antibody-dependent cell-mediated cytotoxicity. Although large RCTs studying the efficacy of rituximab in SSc are lacking, a meta-analysis of 24 articles concluded that rituximab improves mRSS, quality of life and may effectively stabilize internal organ involvement in SSc [26]. A small RCT (DESIRES) in Japan randomized patients with both dcSSc and lcSSc to rituximab (375mg/m2) (N=28) or placebo (N=28) once per week for 4 weeks [27]. At 24 weeks, the absolute change in mRSS from baseline in the rituximab arm was −6.30 compared with +2.14 in the placebo arm (P=0.0001). Rituximab was well-tolerated during the study, and the frequency of adverse events were similar between the rituximab and placebo arms. In this study, rituximab demonstrated a treatment benefit regardless of SSc disease duration or subtype. However, a recent post-hoc analysis of the same study found that high CD-19 positive cell count and high mRSS predicted treatment response to rituximab [28].
In the DESIRES trial, the absolute change in FVC%-predicted from baseline to 24 weeks was analyzed as a key secondary endpoint [27]. Among patients randomized to rituximab, 25 patients (89%) and 23 patients (88%) had ILD in the rituximab and placebo arms, respectively. Patients randomized to rituximab experienced an increase in FVC%-predicted of (mean change 0.09), while patients in the placebo arm experienced a decrease (mean change −2.87) (P=0.044). Patients randomized to rituximab also experienced significant radiographic improvement in the percent of lung fields occupied by interstitial changes. An open-label, 24-week RCT in India compared the safety and efficacy of rituximab 1000mg × 2 doses at 0 and 15 days to intravenous CYC 500mg/m2 monthly in patients with dcSSc and SSc-ILD. In this study, patients receiving rituximab (N=30) experienced an improvement in FVC%-predicted at 6 months (+6.22) whereas those in the CYC arm (N=30) experienced decline in FVC%-predicted (−1.19) (P=0.003) [29]. A RCT (RECITAL) assessing the efficacy of rituximab versus CYC for patients with CTD-ILD, including SSc-ILD [30] was recently completed. Although the results are not yet published, this study will undoubtedly provide more insight into rituximab’s efficacy in treating SSc-ILD. Based on current clinical data, rituximab offers a promising role in treating both cutaneous sclerosis and ILD.
Romilkimab
In SSc, endothelial injury prompts activation of T cells, which differentiate into T-helper type 2 (Th2) response with subsequent release of interleukin-4 (IL-4) and interleukin-13 (IL-13). Romilkimab is a humanized IgG4 antibody that binds and neutralizes IL-4/IL-13, halting the promotion of fibrosis. In a recent study, 97 patients with dcSSc, with or without background immunosuppressive therapy, were randomized to receive romilkimab or placebo for 24 weeks. Change in mRSS from baseline was the primary endpoint. At 24 weeks, patients randomized to romilkimab had a greater decline in mRSS (−4.76) compared with placebo (−2.45) (P=0.029) [31]. Among study participants, 18 patients (38%) in the romilkimab group and 18 patients (37%) in the placebo group had a history of SSc-ILD, with a mean baseline FVC%-predicted of 92.8 in both groups. There was a trend towards improvement in FVC in the romilkimab arm compared with placebo. While the aforementioned findings are encouraging, a phase III trial is needed as prior candidate treatments for dcSSc that showed promising phase II trial data ultimately failed to demonstrate a treatment benefit in the phase III trial (e.g., lenabasum, tocilizumab).
Allogenic bone marrow-derived multipotent mesenchymal stromal cells
Mesenchymal stromal cells (MSC) contain immunomodulatory, proangiogenic, and antifibrotic therapeutic benefits and represent a novel intervention for patients with SSc [32]. In a recent open-label phase 1/2 study, 20 patients with severe dcSSc received a single infusion of allogenic bone marrow-derived MSC [33]. All 20 patients enrolled had evidence of SSc-ILD. Single infusions of both 1×106 bone marrow-derived MSC per kg body weight and 3×106 bone marrow-derived MSC per kg body weight were safe and well tolerated during short and long-term follow-up. Following the first infusion of MSC, an improvement in mRSS was appreciated and sustained at 12 months, while FVC remained stable at 12 months. Unlike HSCT, which is associated with high risk of complications and mortality, MSC may represent a safer option for treating severe dcSSc. Future studies are needed to understand which patients may benefit from this therapeutic strategy over conventional immunosuppression for dcSSc.
Conclusions
With improved understanding of the important pathways that drive the pathogenesis of SSc, a number of new therapies are currently available to treat cutaneous and pulmonary manifestations of SSc. Future studies are needed to determine the optimal time to introduce these therapies, to understand whether combing therapies leads to improved outcomes and to develop improved strategies for personalizing treatment for patients based on molecular profiling.
Acknowledgements
The National Heart, Lung, and Blood Institute (K23 HL150237-01 to ERV) provided funding to support this work. The National Heart, Lung, and Blood Institute had no involvement in the writing of this manuscript.
Conflict of interest statement:
None of the authors received any financial support or other benefits from commercial sources for the work reported in this manuscript, nor do any of the authors have any financial interests, which could create a potential conflict of interest or appearance thereof. ERV has received consulting fees from Boehringer Ingelheim and grant support for systemic sclerosis research and clinical trials from Kadmon, Horizon, Forbius, and Boehringer Ingelheim.
References
- 1.Denton CP, Khanna D. Systemic sclerosis. Lancet. 2017;390(10103):1685–1699. doi: 10.1016/S0140-6736(17)30933-9 [DOI] [PubMed] [Google Scholar]
- 2.Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis. 2007. Jul;66(7):940–4. doi: 10.1136/ard.2006.066068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung L, Domsic RT, Lingala B, Alkassab F, Bolster M, Csuka ME, Derk C, Fischer A, Frech T, Furst DE, et al. Survival and predictors of mortality in systemic sclerosis-associated pulmonary arterial hypertension: outcomes from the pulmonary hypertension assessment and recognition of outcomes in scleroderma registry. Arthritis Care Res (Hoboken). 2014;66(3):489–95. doi: 10.1002/acr.22121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Volkmann ER, Fischer A. Update on Morbidity and Mortality in Systemic Sclerosis-Related Interstitial Lung Disease. J Scleroderma Relat Disord. 2021;6(1):11–20. doi: 10.1177/2397198320915042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bossini-Castillo L, López-Isac E, Mayes MD, Martín J. Genetics of systemic sclerosis. Semin Immunopathol. 2015;37(5):443–51. doi: 10.1007/s00281-015-0499-z. [DOI] [PubMed] [Google Scholar]
- 6.Ferri C, Arcangeletti MC, Caselli E, Zakrzewska K, Maccari C, Calderaro A, D’Accolti M, Soffritti I, Arvia R, Sighinolfi G, et al. Insights into the knowledge of complex diseases: Environmental infectious/toxic agents as potential etiopathogenetic factors of systemic sclerosis. J Autoimmun. 2021;124:102727. doi: 10.1016/j.jaut.2021.102727. [DOI] [PubMed] [Google Scholar]
- 7.Truchetet ME, Brembilla NC & Chizzolini C Current Concepts on the Pathogenesis of Systemic Sclerosis. Clinic Rev Allerg Immunol (2021). 10.1007/s12016-021-08889-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Volkmann ER, Varga J. Emerging targets of disease-modifying therapy for systemic sclerosis. Nat Rev Rheumatol. 2019;15(4):208–224. doi: 10.1038/s41584-019-0184-z. [DOI] [PubMed] [Google Scholar]
- 9.Tashkin DP, Elashoff R, Clements PJ, Goldin J, Roth MD, Furst DE, Arriola E, Silver R, Strange C, Bolster M, et al. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med. 2006;354(25):2655–66. doi: 10.1056/NEJMoa055120. [DOI] [PubMed] [Google Scholar]
- 10.Tashkin DP, Roth MD, Clements PJ, Furst DE, Khanna D, Kleerup EC, Goldin J, Arriola E, Volkmann ER, Kafaja S, et al. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. Lancet Respir Med. 2016;4(9):708–719. doi: 10.1016/S2213-2600(16)30152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Namas R, Tashkin DP, Furst DE, Wilhalme H, Tseng CH, Roth MD, Kafaja S, Volkmann E, Clements PJ, Khanna D; Participants in the Scleroderma Lung Study I and members of the Scleroderma Lung Study II Research Group. Efficacy of Mycophenolate Mofetil and Oral Cyclophosphamide on Skin Thickness: Post Hoc Analyses From Two Randomized Placebo-Controlled Trials. Arthritis Care Res (Hoboken). 2018;70(3):439–444. doi: 10.1002/acr.23282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Laar JM, Farge D, Sont JK, Naraghi K, Marjanovic Z, Larghero J, Schuerwegh AJ, Marijt EW, Vonk MC, Schattenberg AV, et al. EBMT/EULAR Scleroderma Study Group. Autologous hematopoietic stem cell transplantation vs intravenous pulse cyclophosphamide in diffuse cutaneous systemic sclerosis: a randomized clinical trial. JAMA. 2014; 311(24):2490–2498. 10.1001/jama.2014.6368 [DOI] [PubMed] [Google Scholar]
- 13.Sullivan KM, Goldmuntz EA, Keyes-Elstein L, McSweeney PA, Pinckney A, Welch B, Mayes MD, Nash RA, Crofford LJ, Eggleston B, et al. ; SCOT Study Investigators. Myeloablative Autologous Stem-Cell Transplantation for Severe Scleroderma. N Engl J Med. 2018;378(1):35–47. doi: 10.1056/nejmoa1703327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubio-Rivas M, Royo C, Simeón CP, Corbella X, Fonollosa V. Mortality and survival in systemic sclerosis: systematic review and meta-analysis. Semin Arthritis Rheum. 2014;44(2):208–219. doi: 10.1016/j.semarthrit.2014.05.010 [DOI] [PubMed] [Google Scholar]
- 15.Steen VD, Conte C, Owens GR, Medsger TA Jr.., Severe restrictive lung disease in systemic sclerosis. Arthritis Rheum. 1994;37(9):1283–9. doi: 10.1002/art.1780370903 [DOI] [PubMed] [Google Scholar]
- 16.Goldin JG, Kim GH, Volkmann ER, Clements P, Tseng C-H, Furst DE, Brown M, Roth M, Tashkin DP. Longitudinal changes in quantitative lung disease on CT after immunosuppression in the Scleroderma Lung Study II. Ann Am Thorac Soc 2018;15:1286–95. doi: 10.1513/AnnalsATS.201802-079OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- *17.Volkmann ER, Tashkin DP, LeClair H, Roth MD, Kim G, Goldin J, Clements PJ, Furst DE, Khanna D. Treatment with mycophenolate mofetil and cyclophosphamide leads to clinically meaningful improvements in patient-reported outcomes in scleroderma lung disease: Results of Scleroderma Lung Study II. ACR Open Rheumatology 2020;2:362–70. 10.1002/acr2.11125 [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrated that treatment with either cyclophosphamide and mycophenolate lead to improvements in patient reported outcomes in patients with SSc-ILD. Trials of other agents for SSc-ILD have failed to demonstrated improvements in these important patient-centered outcomes.
- 18.Wollin L, Maillet I, Quesniaux V, Holweg A, Ryffel B. Antifibrotic and anti-inflammatory activity of the tyrosine kinase inhibitor nintedanib in experimental models of lung fibrosis. J Pharmacol Exp Ther 2014;349:209–220. doi: 10.1124/jpet.113.208223 [DOI] [PubMed] [Google Scholar]
- **19.Distler O, Highland KB, Gahlemann M, Azuma A, Fischer A, Mayes MD, Raghu G, Sauter W, Girard M, Alves M, et al. ; SENSCIS Trial Investigators. Nintedanib for Systemic Sclerosis-Associated Interstitial Lung Disease. N Engl J Med. 2019;380(26):2518–2528. doi: 10.1056/NEJMoa1903076. [DOI] [PubMed] [Google Scholar]; This was the largest RCT conducted for SSc and was the first to demonstrate the efficacy of using an anti-fibrotic agent to treat SSc-ILD. This study also demonstrated that nintedanib can be safely combined with mycophenolate.
- 20.Khan K, Xu S, Nihtyanova S, Derrett-Smith E, Abraham D, Denton CP, Ong VH. Clinical and pathological significance of interleukin 6 overexpression in systemic sclerosis. Ann Rheum Dis. 2012;71(7):1235–1242. doi: 10.1136/annrheumdis-2011-200955 [DOI] [PubMed] [Google Scholar]
- **21.Khanna D, Lin CJF, Furst DE, Goldin J, Kim G, Kuwana M, Allanore Y, Matucci-Cerinic M, Distler O, Shima Y, et al. ; focuSSced investigators. Tocilizumab in systemic sclerosis: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2020. Oct;8(10):963–974. doi: 10.1016/S2213-2600(20)30318-0. [DOI] [PubMed] [Google Scholar]; Using a unique trial enrichment design, this study illustrated that IL-6 inhibition leads to stabilization of lung function in patients with early, progressive diffuse cutaneous SSc with increased inflammatory markers.
- 22.Roofeh D, Lin CJF, Goldin J, Kim GH, Furst DE, Denton CP, Huang S, Khanna D; focuSSced Investigators. Tocilizumab Prevents Progression of Early Systemic Sclerosis-Associated Interstitial Lung Disease. Arthritis Rheumatol. 2021;73(7):1301–1310. doi: 10.1002/art.41668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spiera R, Kuwana M, Khanna D, Hummers L, Frech T, Stevens W, Gordon J, Kafaja S, Matucci-Cerinic M, Distler O, et al. Phase 3 trial of lenabasum, a CB2 agonist, for the treatment of diffuse cutaneous systemic sclerosis. Ann Rheum Dis 2021; 80(1): 102. 10.1136/annrheumdis-2021-eular.1795 [DOI] [Google Scholar]
- 24.Khanna D, Spino C, Johnson S, Chung L, Whitfield ML, Denton CP, Berrocal V, Franks J, Mehta B, Molitor J, et al. Abatacept in Early Diffuse Cutaneous Systemic Sclerosis: Results of a Phase II Investigator-Initiated, Multicenter, Double-Blind, Randomized, Placebo-Controlled Trial. Arthritis Rheumatol. 2020; 72(1):125–136. 10.1002/art.41055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khanna D, Allanore Y, Denton CP, Kuwana M, Matucci-Cerinic M, Pope JE, Atsumi T, Becvar R, Czirjak L, Hachulla E, et al. Riociguat in patients with early diffuse cutaneous systemic sclerosis (RISE-SSc): randomised, double-blind, placebo-controlled multicentre trial. Ann Rheum Dis. 2020; 79: 618–625. doi: 10.1136/annrheumdis-2019-216823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moradzadeh M, Aghaei M, Mehrbakhsh Z, Arab-Bafrani Z, Abdollahi N. Efficacy and safety of rituximab therapy in patients with systemic sclerosis disease (SSc): systematic review and meta-analysis. Clin Rheumatol. 2021;40:3897–3918. 10.1007/s10067-021-05698-4 [DOI] [PubMed] [Google Scholar]
- **27.Ebata S, Yoshizaki A, Oba K, Kashiwabara K, Ueda K, Uemura Y, Watadani T, Fukasawa T, Miura S, Yoshizaki-Ogawa A, et al. Safety and efficacy of rituximab in systemic sclerosis (DESIRES): a double-blind, investigator-initiated, randomised, placebo-controlled trial. Lancet Rheumatol. 2021;3(7):E489–E497 10.1016/S2665-9913(21)00107-7 [DOI] [PubMed] [Google Scholar]; This is the first randomized controlled trial to demonstrate a treatment benefit for rituximab in SSc-ILD and cutaneous sclerosis.
- 28.Ebata S, Oba K, Kashiwabara K, Ueda K, Uemura Y, Watadani T, Fukasawa T, Miura S, Yoshizaki-Ogawa A, Yoshihide A, Yoshizaki A, Sato S. Predictors of Rituximab Effect on Modified Rodnan Skin Score in Systemic Sclerosis: a machine learning analysis of the DESIRES trial. Rheumatology (Oxford). 2022. Feb 8:keac023. doi: 10.1093/rheumatology/keac023. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 29.Sircar G, Goswami RP, Sircar D, Ghosh A, Ghosh P. Intravenous cyclophosphamide vs rituximab for the treatment of early diffuse scleroderma lung disease: open label, randomized, controlled trial. Rheumatology (Oxford). 2018. Dec 1;57(12):2106–2113. doi: 10.1093/rheumatology/key213. [DOI] [PubMed] [Google Scholar]
- 30.Saunders P, Tsipouri V, Keir GJ, Ashby D, Flather MD, Parfrey H, Babalis D, Renzoni EA, Denton CP, Wells AU, Maher TM. Rituximab versus cyclophosphamide for the treatment of connective tissue disease-associated interstitial lung disease (RECITAL): study protocol for a randomised controlled trial. Trials. 2017. Jun 15;18(1):275. doi: 10.1186/s13063-017-2016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allanore Y, Wung P, Soubrane C, Esperet C, Marrache F, Bejuit R, Lahmar A, Khanna D, Denton CP; On behalf of the investigators. A randomised, double-blind, placebo-controlled, 24-week, phase II, proof-of-concept study of romilkimab (SAR156597) in early diffuse cutaneous systemic sclerosis. Ann Rheum Dis. 2020. Dec;79(12):1600–1607. doi: 10.1136/annrheumdis-2020-218447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peltzer J, Aletti M, Frescaline N, Busson E, Lataillade JJ, Martinaud C. Mesenchymal Stromal Cells Based Therapy in Systemic Sclerosis: Rational and Challenges. Front Immunol. 2018; 9:2013. doi: 10.3389/fimmu.2018.02013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *33.Farge D, Loisel S, Resche-Rigon M, Lansiaux P, Colmegna I, Langlais D, Charles C, Pugnet G, Thibault Jacquez Maria A, et al. Safety and preliminary efficacy of allogeneic bone marrow-derived multipotent mesenchymal stromal cells for systemic sclerosis: a single-centre, open-label, dose-escalation, proof-of-concept, phase 1/2 study. Lancet Rheumatol 2022;4: e91–104. 10.1016/S2665-9913(21)00326-X. [DOI] [PubMed] [Google Scholar]; This forward-thinking study evaluated the disease-modifying potential of allogeneic mesenchymal stromal cells for the treatment of SSc, and found that this intervention was safe and well tolerated. If future studies demonstrated efficacy in larger cohorts, this could emerge as a potentially safer intervention than autologous hematopoietic stem cell transplantation.


