Abstract
Background.
Neoadjuvant chemotherapy (NAC) is standard for most triple-negative and human epidermal growth factor receptor 2 (HER2)+ breast cancers, and frequently downstages node-positive (cN+) disease, permitting omission of axillary dissection. In estrogen receptor (ER)+/HER2− disease, response rates are lower. Whether Ki67 is associated with axillary downstaging in ER+/HER2− disease is unknown.
Methods.
With institutional review board approval, we queried our institutional database to identify all patients with primary ER+/HER2− biopsy-proven cN+ breast cancer treated with NAC followed by surgery from January 2012 to December 2021. Nodal pathologic complete response (pCR) rates were evaluated by pretreatment Ki67 and patient age.
Results.
315 patients (median age 50 years) were included. Nodal pCR rate was 24.8% (78/315) and was higher in patients aged < 50 years than ≥ 50 years (31.8% versus 17.7%, p = 0.004). Ki67 was available on 236 patients (74.9%). Median Ki67 was 29.0% (range 1–98%) and did not differ by age category (p = 0.23). Patients with nodal pCR had higher Ki67 (median 40.3% versus 25.0%, p < 0.001). Nodal pCR rates were 28.4% (Ki67 ≥ 20%) versus 8.1% (Ki67 < 20%) (p < 0.001). On multivariable analysis, Ki67 and age category were predictive of nodal pCR. Combining these two parameters together, nodal pCR rates in age < 50 years were 35.8% when Ki67 ≥ 20% versus 14.3% with Ki67 < 20% (p = 0.02). In contrast, for age ≥ 50 years, nodal pCR was 21.0% for Ki67 ≥ 20% versus 2.6% with Ki67 < 20% (p = 0.008).
Conclusions.
In ER+/HER2− breast cancer, nodal downstaging with NAC is associated with age (< 50 years) and Ki67 (≥ 20%). Age and Ki67 should be considered for NAC decision-making and can identify patients with high rates of nodal downstaging (36%) who would benefit from NAC to enable axillary preservation.
Chemotherapy is increasingly delivered in the neoadjuvant setting, particularly in patients with biologically aggressive tumors including triple-negative breast cancer (TNBC), HER2+ disease, and those with ER+/HER2− breast cancer who present with clinical and pathological high-risk features.1 Many of these patients present with node-positive (cN+) disease at presentation. Advantages of neoadjuvant chemotherapy (NAC) include downstaging extent of disease in the breast to permit breast conservation and downstaging nodal disease to avoid axillary lymph node dissection (ALND).
While response to neoadjuvant chemotherapy is prognostic in all tumor types,2,3 pCR rates are much higher for TNBC and HER2+ breast cancer compared with the luminal subtypes,2 hence use of NAC has increased significantly in TNBC and in HER2+ breast cancer.1 Further, in these tumor types, additional adjuvant therapies are recommended postoperatively based on response to NAC. In contrast, in ER+/HER2− breast cancer, NAC use is typically reserved for patients with higher-risk clinical pathological characteristics, as pCR rates are much lower (< 10%), with higher rates of axillary dissection.4 Since pCR is uncommon in ER+/HER2− breast cancer, an important aspect in guiding the decision for or against NAC is likelihood of altering surgical management and enabling deescalation of surgical therapy, particularly the opportunity to avoid axillary dissection. For ER+/HER2− breast cancer, the decision regarding whether to proceed with NAC versus surgical resection first is complex as decisions for neoadjuvant chemotherapy are mainly based on extent of disease (tumor size and nodal involvement). Further, given that genomic assays provide data on whether patients with ER+/HER2− breast cancer with 1–3 positive lymph nodes should receive chemotherapy, chemotherapy may be increasingly deferred until following surgery, when genomic assay results are available. However, given that a substantial benefit of NAC relates to nodal downstaging and the avoidance of axillary lymph node dissection, there is a critical need to better understand whether there are a group of patients with luminal breast cancer who derive clinical benefit from NAC (in terms of nodal downstaging) and thus can avoid lymph node dissection. The goal of this study is to evaluate whether readily available clinical and biological factors impact nodal pCR rates in patients with luminal breast tumors.
METHODS
With institutional review board (IRB) approval, we queried our institutional database to identify all patients with ER+/HER2− breast cancer with clinically node-positive disease (stage I-III) treated with NAC followed by surgery from January 2012 through December 2021. ER+ was defined as estrogen receptor > 1%. Patients with ER−/progesterone receptor (PR)+ disease were excluded. All patients underwent percutaneous biopsy of axillary nodes prior to NAC. We excluded patients with stage IV disease, those with recurrent breast cancer, and those without biopsy-proven nodal involvement prior to NAC. Differences in rate of nodal pCR and breast pCR were evaluated by age and pretreatment Ki67. Nodal pCR was defined as ypN0 or ypN0i+ pathologic N category, and breast pCR was defined as ypT0 or ypTis pathologic T category. At our institution, Ki67 is obtained in all ER+/HER2− breast cancer cases diagnosed at our institution and most outside cases with ER+/HER2− disease that are managed at our institution. Ki67 was assessed on the breast core-needle biopsy at initial diagnosis (prior to any NAC).
The Mayo Clinic Ki67 test was performed with the MIB-1 clone from Dako (Glostrup, Denmark) using an optimized staining protocol on the Ventana Benchmark ULTRA autostaining platform. The scoring method using the Aperio Digital Pathology System was developed and validated in the Biomarker and Image Analysis Laboratory at Mayo Clinic. The results are reported as a percentage of tumor cells staining positive for Ki67. The digital data and corresponding glass slides are reviewed by subspecialized breast pathologists with expertise in clinical biomarker interpretation for final interpretation and read out. The cut-point of 20% was chosen based on prospective data demonstrating the prognostic effects in luminal breast cancer.5
We further looked at extent of axillary surgery performed in patients with surgery between January 2017 and December 2021, as this is a more contemporary cohort after ACOSOG Z1071 had been implemented in the practice and sentinel lymph node (SLN) surgery without ALND was being used for patients with node-positive disease treated with NAC who converted to SLN negative. Patients with inflammatory breast cancer were excluded from analysis of breast and axillary surgery decision given that modified radical mastectomy (mastectomy with ALND) is the standard of care regardless of response to neoadjuvant chemotherapy.
Statistical analysis
Groups were compared using Wilcoxon rank-sum tests for continuous or ordinal variables and Chi-square or Fisher’s exact tests, as appropriate, for nominal variables. Multivariable logistic regression was used to evaluate the independent effects of age and Ki67 after adjusting for potential confounding variables. Results are reported as odds ratio (OR) with 95% confidence interval (CI) and p value. The Firth penalized likelihood bias-reduction method and profile-likelihood confidence intervals were utilized to improve performance with sparse data. Variables were selected for inclusion in the multivariable model using a combination of strong univariate association and confounding potential as well as the best subset model selection approach based on the score criterion. Analysis was performed using SAS (version 9.4, SAS Institute Inc., Cary, NC).
RESULTS
We identified 315 patients meeting study criteria. All patients had biopsy-proven node-positive disease, and clinical nodal category prior to NAC was predominantly cN1 (281 patients, 89.2%). Clinical tumor category prior to NAC was predominantly cT2 (127 patients, 40.3%) and cT3 (112 patients, 35.6%) (Table 1). Median patient age was 50 years (range 22–79 years) with 49.8% of patients aged < 50 years and 50.2% aged ≥ 50 years.
TABLE 1.
Demographic, clinical, and pathologic factors of the 315 patients meeting study criteria by nodal pCR
| Residual nodal disease (ypN+) (N = 237) | Nodal pCR (ypN0) (N = 78) | Total (N = 315) | p value | |
|---|---|---|---|---|
| Age, years | 0.03 | |||
| Median (range) | 51 (22–79) | 48 (29–69) | 50 (22–79) | |
| Age category, n (%) | 0.004 | |||
| < 50 years | 107 (45.1%) | 50 (64.1%) | 157 (49.8%) | |
| ≥ 50 years | 130 (54.9%) | 28 (35.9%) | 158 (50.2%) | |
| Sex, n (%) | 0.73 | |||
| Female | 235 (99.2%) | 77 (98.7%) | 312 (99.0%) | |
| Male | 2 (0.8%) | 1 (1.3%) | 3 (1.0%) | |
| Race, n (%) | 0.22 | |||
| White | 212 (89.5%) | 66 (84.6%) | 278 (88.3%) | |
| Black or African American | 4 (1.7%) | 3 (3.8%) | 7 (2.2%) | |
| Asian | 9 (3.8%) | 1 (1.3%) | 10 (3.2%) | |
| American Indian or Alaska Native | 1 (0.4%) | 1 (1.3%) | 2 (0.6%) | |
| Native Hawaiian or Pacific Islander | 1 (0.4%) | 0 | 1 (0.3%) | |
| Unknown/unreported | 10 (4.2%) | 7 (9.0%) | 17 (5.4%) | |
| Ethnicity, n (%) | 0.42 | |||
| Not Hispanic or Latino | 224 (94.5%) | 71 (91.0%) | 295 (93.7%) | |
| Hispanic or Latino | 7 (3.0%) | 3 (3.8%) | 10 (3.2%) | |
| Unknown/unreported | 6 (2.5%) | 4 (5.1%) | 10 (3.2%) | |
| Clinical T category, n (%) | 0.16 | |||
| cT0 | 4 (1.7%) | 1 (1.3%) | 5 (1.6%) | |
| cT1 | 24 (10.1%) | 12 (15.4%) | 36 (11.4%) | |
| cT2 | 92 (38.8%) | 35 (44.9%) | 127 (40.3%) | |
| cT3 | 90 (38.0%) | 22 (28.2%) | 112 (35.6%) | |
| cT4 | 27 (11.4%) | 8 (10.3%) | 35 (11.1%) | |
| Clinical N category, n (%) | 0.30 | |||
| cN1 | 214 (90.3%) | 67 (85.9%) | 281 (89.2%) | |
| cN2 | 6 (2.5%) | 3 (3.8%) | 9 (2.9%) | |
| cN3 | 17 (7.2%) | 8 (10.3%) | 25 (7.9%) | |
| Grade, n (%) | < 0.001 | |||
| Missing | 1 | 1 | 2 | |
| I (well differentiated) | 16 (6.8%) | 1 (1.3%) | 17 (5.4%) | |
| II (moderately differentiated) | 146 (61.9%) | 32 (41.6%) | 178 (56.9%) | |
| III (poorly differentiated) | 74 (31.4%) | 44 (57.1%) | 118 (37.7%) | |
| Multifocal or multicentric, n (%) | 0.01 | |||
| No | 123 (51.9%) | 53 (67.9%) | 176 (55.9%) | |
| Yes | 114 (48.1%) | 25 (32.1%) | 139 (44.1%) | |
| Pretreatment Ki67 | < 0.001 | |||
| Missing | 53 | 26 | 79 | |
| Mean (SD) | 29.0 (19.0) | 44.6 (24.5) | 32.4 (21.3) | |
| Median (range) | 25.0 (1.0–91.6) | 40.3 (2.0–98.0) | 29.0 (1.0–98.0) | |
| Interquartile range | 15.0, 37.2 | 26.2, 67.5 | 17.0, 42.6 | |
| Estrogen receptor staining, n (%) | < 0.001 | |||
| Positive, % not specified | 2 | 2 | 4 | |
| 1–10% | 8 (3.4%) | 11 (14.5%) | 19 (6.1%) | |
| 11–50% | 9 (3.8%) | 9 (11.8%) | 18 (5.8%) | |
| 51–70% | 8 (3.4%) | 5 (6.6%) | 13 (4.2%) | |
| > 70% | 210 (89.4%) | 51 (67.1%) | 261 (83.9%) | |
| Progesterone receptor status, n (%) | <0.001 | |||
| Negative | 26 (11.0%) | 28 (35.9%) | 54 (17.1%) | |
| Positive | 211 (89.0%) | 50 (64.1%) | 261 (82.9%) | |
| Breast pCR, n (%) | <0.001 | |||
| No | 222 (93.7%) | 46 (59.0%) | 268 (85.1%) | |
| Yes | 15 (6.3%) | 32 (41.0%) | 47 (14.9%) | |
| Pathologic T category, n (%) | <0.001 | |||
| ypT0/ypTis | 15 (6.3%) | 32 (41.0%) | 47 (14.9%) | |
| ypT1 | 87 (36.7%) | 28 (35.9%) | 115 (36.5%) | |
| ypT2 | 70 (29.5%) | 15 (19.2%) | 85 (27.0%) | |
| ypT3 | 50 (21.1%) | 2 (2.6%) | 52 (16.5%) | |
| ypT4 | 15 (6.3%) | 1 (1.3%) | 16 (5.1%) | |
| Pathologic N category, n (%) | <0.001 | |||
| ypN0 | 0 (0.0%) | 78 (100.0%) | 78 (24.8%) | |
| ypN1 | 111 (46.8%) | 0 (0.0%) | 111 (35.2%) | |
| ypN2 | 81 (34.2%) | 0 (0.0%) | 81 (25.7%) | |
| ypN3 | 45 (19.0%) | 0 (0.0%) | 45 (14.3%) |
Overall nodal pCR rate was 24.8% (78/315), breast pCR was 14.9% (47/315), and total pCR was 10.2% (32/315) (Fig. 1a). Higher-grade tumors had higher rates of breast pCR (5.9% grade I, 7.9% grade II, 25.4% grade III), nodal pCR (5.9% grade I, 18.0% grade II, 37.3% grade III), and total pCR (0% grade I, 5.1% grade II, 18.6% grade III) on univariate analysis (each p < 0.001).
FIG. 1.
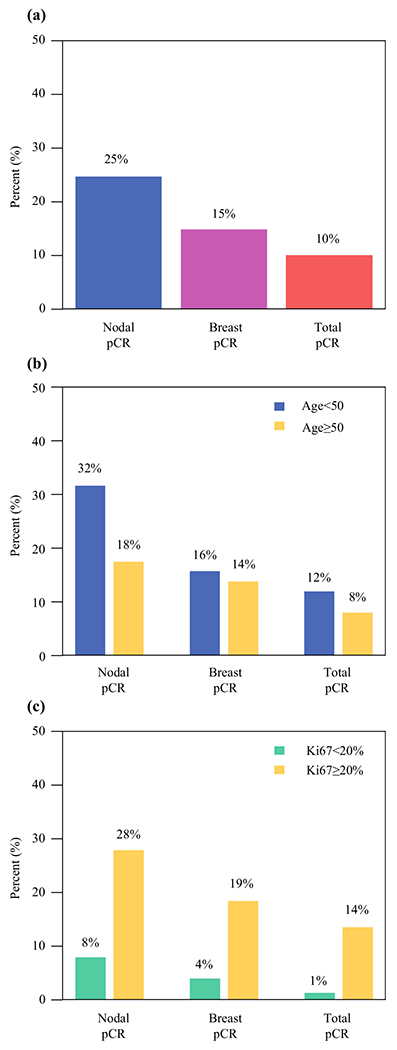
a Percent of patients with ER+/HER2− breast cancer with nodal, breast, and total pCR after neoadjuvant chemotherapy. b Percent with nodal, breast, and total pCR by age category. c Percent with nodal, breast, and total pCR by Ki67 < 20% versus ≥ 20%
Nodal pCR According to Age
Nodal pCR was higher at 31.8% in patients aged < 50 years compared with 17.7% in those aged ≥ 50 years (p = 0.004), but breast and total pCR did not vary significantly between age groups (15.9% versus 13.9%, p = 0.62 and 12.1% versus 8.2%, p = 0.26, respectively) (Fig. 1b).
Nodal pCR According to Ki67
Ki67 was available on 236 patients (74.9%). Ki67 missingness did not vary significantly by clinical factors (age, grade, cT or cN category, degree of ER positivity, or PR status) (Table 2). Among the 236 patients with ER+/HER2− disease with Ki67 data available, median pretreatment Ki67 was 29.0% and ranged from 1.0 to 98.0% (interquartile range 17.0–42.6%). While Ki67 did not differ significantly by age category (p = 0.23) (Fig. 2), it did vary according to grade (p < 0.001), by degree of ER positivity (p < 0.001), and by PR status (p = 0.007).
TABLE 2.
Demographic, clinical, and pathologic factors by availability of Ki67
| Ki67 available (N = 236) | Ki67 missing (N = 79) | Total (N = 315) | p value | |
|---|---|---|---|---|
| Age (years) | 0.49 | |||
| Median (range) | 50 (24–79) | 48 (22–71) | 50 (22–79) | |
| Age category, n (%) | 0.67 | |||
| < 50 years | 116 (49.2%) | 41 (51.9%) | 157 (49.8%) | |
| ≥ 50 years | 120 (50.8%) | 38 (48.1%) | 158 (50.2%) | |
| Gender | 0.10 | |||
| Female | 235 (99.6%) | 77 (97.5%) | 312 (99.0%) | |
| Male | 1 (0.4%) | 2 (2.5%) | 3 (1.0%) | |
| Clinical T category, n (%) | 0.46 | |||
| cT0 | 3 (1.3%) | 2 (2.5%) | 5 (1.6%) | |
| cT1 | 25 (10.6%) | 11 (13.9%) | 36 (11.4%) | |
| cT2 | 97 (41.1%) | 30 (38.0%) | 127 (40.3%) | |
| cT3 | 84 (35.6%) | 28 (35.4%) | 112 (35.6%) | |
| cT4 | 27 (11.4%) | 8 (10.1%) | 35 (11.1%) | |
| Clinical N category, n (%) | 0.78 | |||
| cN1 | 212 (89.8%) | 69 (87.3%) | 281 (89.2%) | |
| cN2 | 5 (2.1%) | 4 (5.1%) | 9 (2.9%) | |
| cN3 | 19 (8.1%) | 6 (7.6%) | 25 (7.9%) | |
| Grade, n (%) | 0.85 | |||
| Missing | 1 | 1 | 2 | |
| I (well differentiated) | 12 (5.1%) | 5 (6.4%) | 17 (5.4%) | |
| II (moderately differentiated) | 136 (57.9%) | 42 (53.8%) | 178 (56.9%) | |
| III (poorly differentiated) | 87 (37.0%) | 31 (39.7%) | 118 (37.7%) | |
| Multifocal or multicentric, n (%) | 0.97 | |||
| No | 132 (55.9%) | 44 (55.7%) | 176 (55.9%) | |
| Yes | 104 (44.1%) | 35 (44.3%) | 139 (44.1%) | |
| Estrogen receptor staining, n (%) | 0.63 | |||
| ER positive, % not specified | 4 | 0 | 4 | |
| 1–10% | 12 (5.2%) | 7 (8.9%) | 19 (6.1%) | |
| 11–50% | 15 (6.5%) | 3 (3.8%) | 18 (5.8%) | |
| 51–70% | 10 (4.3%) | 3 (3.8%) | 13 (4.2%) | |
| > 70% | 195 (84.1%) | 66 (83.5%) | 261 (83.9%) | |
| Progesterone receptor status, n (%) | 0.85 | |||
| Negative | 41 (17.4%) | 13 (16.5%) | 54 (17.1%) | |
| Positive | 195 (82.6%) | 66 (83.5%) | 261 (82.9%) | |
| Breast pCR, n (%) | 0.42 | |||
| No | 203 (86.0%) | 65 (82.3%) | 268 (85.1%) | |
| Yes | 33 (14.0%) | 14 (17.7%) | 47 (14.9%) | |
| Nodal pCR, n (%) | 0.05 | |||
| No | 184 (78.0%) | 53 (67.1%) | 237 (75.2%) | |
| Yes | 52 (22.0%) | 26 (32.9%) | 78 (24.8%) | |
| Pathologic T category, n (%) | 0.25 | |||
| ypT0/ypTis | 33 (14.0%) | 14 (17.7%) | 47 (14.9%) | |
| ypT1 | 84 (35.6%) | 31 (39.2%) | 115 (36.5%) | |
| ypT2 | 65 (27.5%) | 20 (25.3%) | 85 (27.0%) | |
| ypT3 | 42 (17.8%) | 10 (12.7%) | 52 (16.5%) | |
| ypT4 | 12 (5.1%) | 4 (5.1%) | 16 (5.1%) | |
| Pathologic N category, n (%) | 0.14 | |||
| ypN0 | 52 (22.0%) | 26 (32.9%) | 78 (24.8%) | |
| ypN1 | 89 (37.7%) | 22 (27.8%) | 111 (35.2%) | |
| ypN2 | 57 (24.2%) | 24 (30.4%) | 81 (25.7%) | |
| ypN3 | 38 (16.1%) | 7 (8.9%) | 45 (14.3%) |
FIG. 2.
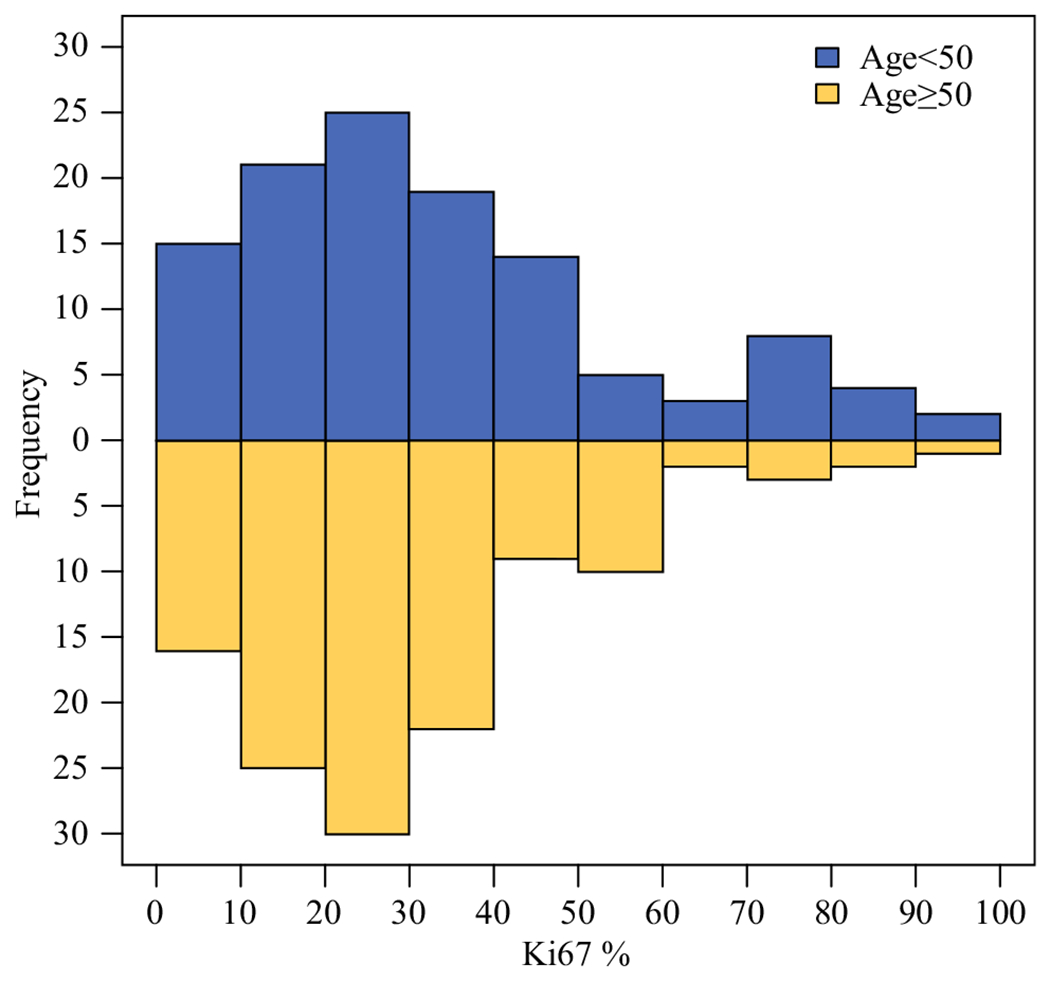
Histograms showing the distribution of Ki67 by age group
Those with breast pCR had significantly higher pre-NAC Ki67 (median 48.0%) compared with those with residual invasive breast disease (median 26.4%, p < 0.001). Similarly, those with nodal pCR had higher median pre-NAC Ki67 (40.3% compared with 25.0% in those with residual nodal disease, p < 0.001), and patients with total pCR had higher median Ki67 compared with those without total pCR (60.0% versus 27.0%, p < 0.001). Each of these findings was also true among patients aged < 50 years and aged ≥ 50 years considered separately.
Of those with pretreatment Ki67 ≥ 20%, nodal pCR was 28.4% (46/162) compared with 8.1% (6/74) among those with Ki67 < 20% (p < 0.001). Similarly, breast pCR rates were lower at 4.1% (3/74) in those with Ki67 < 20%, compared with 18.5% (30/162) in those with Ki67 ≥ 20% (p = 0.003). For total pCR, the rates were 1.4% (1/74) in patients with Ki67 < 20% and 13.6% (22/162) in those with Ki67 ≥ 20% (p = 0.003) (Fig. 1c).
Role of Age and Ki67 Combined
In patients aged < 50 years, 35.8% (29/81) with pre-NAC Ki67 ≥ 20% had nodal pCR compared with only 14.3% (5/35) of those with pre-NAC Ki67 < 20% achieving a nodal pCR (p = 0.02). Breast pCR was also significantly higher for patients aged < 50 years with Ki67 ≥ 20% versus < 20% (19.8% versus 2.9%, p = 0.02), and total pCR in this age group by Ki67 level was 14.8% versus 2.9% (p = 0.10). Similar differences were seen in those aged ≥ 50 years, where nodal pCR was 21.0% (17/81) for Ki67 ≥ 20% and extremely low at only 2.6% (1/39) in those with Ki67 < 20% (p = 0.008), while breast pCR was 17.3% versus 5.1% (p = 0.07) and total pCR was 12.3% versus 0% (p = 0.03) in patients aged ≥ 50 years with Ki67 ≥ 20% versus < 20%, respectively (Fig. 3).
FIG. 3.
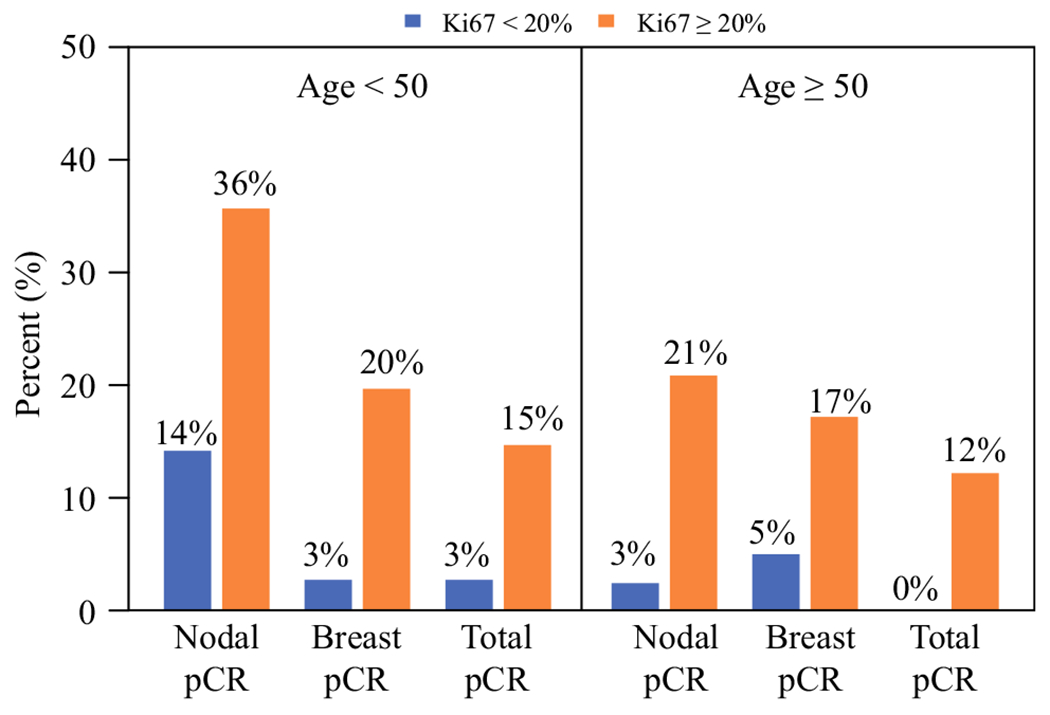
Percent with nodal, breast, and total pCR by age category and pre-NAC Ki67
On multivariable analysis adjusting for degree of ER positivity, PR status, grade III disease, and presence of multifocal or multicentric disease, both Ki67 ≥ 20% (adjusted OR 2.60, 95% CI 1.03–7.42, p = 0.04) and age < 50 years (adjusted OR 2.44, 95% CI 1.20–5.12, p = 0.01) remained significant independent predictors of nodal pCR. For breast pCR, Ki67 ≥ 20% remained significant (adjusted OR 4.67, 95% CI 1.49–19.69, p = 0.006) after adjusting for clinical T category and ER%, but age category was not significantly associated with breast pCR on multivariable analysis (Table 3).
TABLE 3.
Multivariable analysis of factors associated with nodal pCR and breast pCR
| Odds ratio (95% CI) | p value | |
|---|---|---|
| Nodal pCR | ||
| Age < 50 versus ≥ 50 | 2.44 (1.20–5.12) | 0.01 |
| Pre-NAC Ki67 ≥ 20% versus < 20% | 2.60 (1.03–7.42) | 0.04 |
| Estrogen receptor percent ≤ 70% versus > 70% | 2.29 (0.95–5.41) | 0.06 |
| Progesterone receptor negative versus positive | 3.80 (1.63–8.96) | 0.002 |
| Unifocal versus multifocal or multicentric | 2.54 (1.24–5.44) | 0.01 |
| Grade III versus I/II | 1.67 (0.79–3.55) | 0.18 |
| Breast pCR | ||
| Age < 50 versus ≥ 50 | 0.80 (0.34–1.85) | 0.60 |
| Pre-NAC Ki67 ≥ 20% versus < 20% | 4.67 (1.49–19.69) | 0.006 |
| Estrogen receptor percent ≤ 70% versus > 70% | 5.83 (2.35–14.86) | < 0.001 |
| cT0/cT1 versus cT3/cT4 | 8.10 (2.57–26.99) | < 0.001 |
| cT2 versus cT3/cT4 | 1.21 (0.47–3.18) | 0.69 |
Impact of PR Status
PR status was also a strong independent predictor of nodal pCR with adjusted OR 3.80 (p = 0.002). Further stratifying the patients by PR status within age and Ki67 category showed that PR status provided additional discrimination for patients with Ki67 ≥ 20%. Specifically, for patients aged < 50 years with Ki67 ≥ 20%, 75.0% (12/16) of patients with PR-negative tumors had nodal pCR as compared with 26.2% (17/65) for PR-positive tumors (p < 0.001), and for patients aged ≥ 50 years with Ki67 ≥ 20%, 38.9% (7/18) of patients with PR-negative tumors had nodal pCR compared with 15.9% (10/63) for PR-positive tumors (p = 0.049). In patients with Ki67 < 20%, the numbers of PR-negative tumors were too small to adequately assess the separate effect of PR status with 50% (1/2) PR-negative tumors versus 12.1% (4/33) of PR-positive tumors having nodal pCR (p = 0.27) in the subgroup aged < 50 years with Ki67 < 20% and 0% (0/5) PR-negative tumors versus 2.9% (1/34) of PR-positive tumors having nodal pCR (p > 0.99) in the subgroup aged ≥ 50 years with Ki67 < 20%.
Impact on Surgery
Excluding patients with inflammatory breast cancer, who all underwent mastectomy, still a large majority of patients (82.9%, 242/292) had mastectomy, with only 17.1% having breast-conserving surgery after NAC. The percent undergoing mastectomy was slightly lower for patients aged ≥ 50 years (78.2%) compared with patients aged ≥ 50 years (87.3%) (p = 0.04), but did not differ by Ki67 < 20% versus Ki67 ≥ 20% (p = 0.31).
Axillary surgery was assessed also excluding patients with inflammatory breast cancer and further restricted to the period 2017–2021, after the implementation of the Z1071 trial results at our institution. In this subset, 36.0% (63/175) underwent SLN surgery only, 44.0% (77/175) underwent SLN surgery and completion ALND, and 20.0% (35/175) had ALND only. Avoidance of ALND was significantly associated (p < 0.001) with nodal pCR [89.4% (42/47) with nodal pCR underwent SLN surgery only versus 16.4% (21/128) without nodal pCR undergoing SLN surgery only (5 ypN1mi, 15 ypN1a–c, 1 ypN2)]. Patients aged < 50 years were more likely to have SLN surgery only (47.4%) compared with those aged ≥ 50 years (22.5%, p < 0.001). Patients with Ki67 ≥ 20% did not have significantly higher percent with SLN surgery only compared with patients with Ki67 < 20% when assessed overall (37.0% versus 25.0%, p = 0.15). However, when assessed in combinations of age and Ki67, patients aged < 50 years with Ki67 ≥ 20% were able to preserve their axilla and undergo SLN surgery only in 49.0% (25/51), followed by 36.0% (9/25) undergoing SLN surgery only in the group aged < 50 years with Ki67 < 20%, 22.0% (9/41) in patients aged ≥ 50 years with Ki67 ≥ 20%, and only 13.0% (3/23) in patients aged ≥ 50 years with Ki67 < 20% (p < 0.001) (Fig. 4).
FIG. 4.
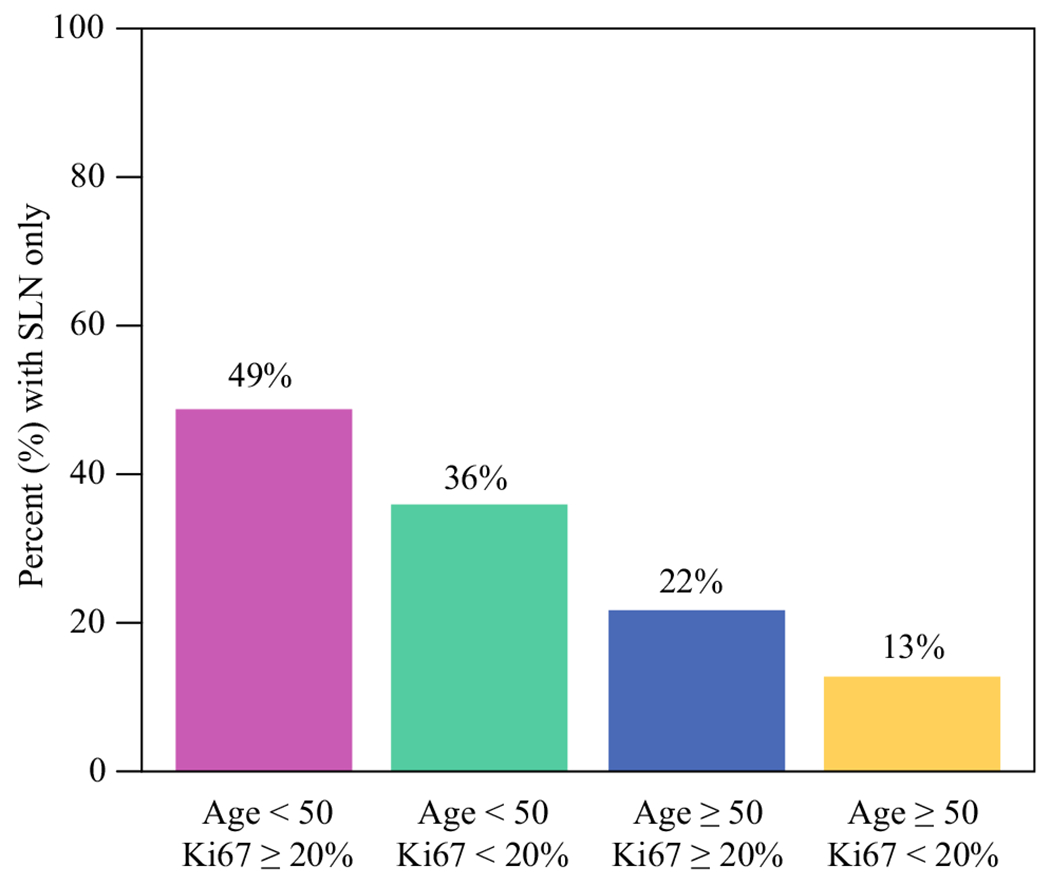
Percent undergoing SLN surgery only by combinations of age category and pre-NAC Ki67 category during the period 2017–2021 and excluding patients with inflammatory breast cancer
DISCUSSION
In this study evaluating nodal response in neoadjuvant chemotherapy for ER+/HER2− breast cancer, we demonstrated that nodal downstaging is associated with age (< 50 years) and proliferation as indicated by Ki67 (≥ 20%). Rates of nodal pCR in patients aged ≥ 50 years with Ki67 < 20% are very low (< 3%). In contrast, for those aged < 50 years with Ki67 ≥ 20%, nodal downstaging rates were much higher at > 35%. Of note, in patients aged < 50 years with Ki67 < 20% 14.3% (5/35) still achieved nodal pCR, suggesting that additional factors may be driving nodal pCR for young patients.
Use of chemotherapy in the neoadjuvant setting has evolved substantially over the last 10 years. Initially, the main indication for neoadjuvant chemotherapy was to downsize the primary tumor in the breast to permit breast conservation for larger tumors or enable mastectomy in locally advanced disease.6 Early studies demonstrated no difference in survival based on whether chemotherapy was delivered in the adjuvant or neoadjuvant setting, and the regimens used were the same.7 Over the last 10 years, further advantages of chemotherapy in the neoadjuvant setting have unfolded. First, because pathologic complete response (total pCR: absence of disease in the breast or lymph nodes) is prognostic for breast cancer mortality, new clinical trial strategies have focused on those patients with residual disease after NAC at risk for distant recurrence.2,3 Specifically, the addition of postsurgical adjuvant chemotherapy (capecitabine in triple-negative breast cancer)8 and trastuzumab emtansine (T-DM1) in HER2+ disease9 is now well established and incorporated into practice guidelines. Furthermore, the neoadjuvant setting is commonly used as a testing ground for new drug development. Finally, in addition to downstaging disease in the breast to permit breast conservation, neoadjuvant chemotherapy can also eradicate disease from the axillary lymph nodes in 21–65% of patients with node-positive disease at presentation.4 Rates are highest in TNBC (50%) and HER2+ disease (65%).4 This results in lower rates of axillary node dissection and thus decreases the potential morbidity from lymphedema. All these factors have led to the increasing use of chemotherapy in the neoadjuvant setting in patients with TNBC and those with HER2+ disease.1 The most recent American Society of Clinical Oncology (ASCO) guidelines recommend consideration of chemotherapy in the neoadjuvant setting for T1c and greater TNBC and HER2+ disease.10
While chemotherapy in the neoadjuvant setting is a well-established standard for TNBC and HER2+ breast cancer, the role of NAC for ER+/HER2− breast cancer is less straightforward. Given the widespread use of multigene tests and evidence that these assays (e.g., 21-gene assay) are both prognostic and predictive of chemotherapy benefit, decisions regarding the use of chemotherapy are often deferred until after surgery. Additionally, for ER+/HER2− breast cancer patients treated with NAC, rates of overall pCR are significantly lower (at 10%) than in TNBC or HER2+ disease and rates of nodal pCR are also low (around 21%).4 Finally, presence of residual disease in the breast or axilla does not alter adjuvant systemic therapy recommendations, and adjuvant endocrine therapy is generally felt to provide the greatest therapeutic benefit to many patients with ER+/HER2− breast cancer. While neoadjuvant endocrine therapy can also downsize the extent of disease in the breast and allow assessment of responsiveness to endocrine therapy, which may allow omission of chemotherapy, the rates of pCR and nodal pCR to neoadjuvant endocrine therapy are low.
For patients with ER+/HER2− breast cancer treated with upfront surgery and found to have low-volume axillary disease (one or two positive nodes), omission of axillary dissection is the standard of care. However, for patients with node-positive disease treated with neoadjuvant chemotherapy who have any positive lymph nodes at surgery, axillary lymph node dissection is recommended. Thus, some have advocated for upfront surgery for patients with ER+/HER2− breast cancer with cN1 disease to avoid axillary overtreatment, as the number of positive nodes cannot be reliably determined preoperatively based on imaging.11 Thus, additional information to identify which patients are most likely to achieve pathologic nodal response can further help decision-making regarding strategy for use of neoadjuvant chemotherapy.
Prior studies have demonstrated that tumors with higher proliferation rate are more likely to respond to neoadjuvant chemotherapy.12,13 In this study, our findings confirm those previous studies. However, we additionally focused specifically on nodal pCR, and showed that nodal pCR rates are higher in ER+/HER2− disease with elevated Ki67 (≥ 20%) compared with those with low Ki67 (< 20%). Thus, this is the first study we are aware of that demonstrates that baseline tumor Ki67 may be helpful to differentiate which patients are more likely to convert positive nodes to pathologic negative nodes with neoadjuvant chemotherapy and thus receive one of the beneficial effects of NAC (namely a reduction in axillary surgery). Furthermore, age was also identified as an independent predictor of nodal response (but not breast response). Combining age with Ki67 provides a simple but powerful way to identify patients with higher nodal response rates (as high as 36%) and those with low nodal response rates (3%). For those patients aged < 50 years with Ki67 ≥ 20%, the nodal response rates was 36%, and for these patients, neoadjuvant chemotherapy may enable deescalation of axillary surgery, with one in three patients being able to avoid axillary lymph node dissection due to negative SLNs. This is much higher than the overall anticipated pCR rate in ER+/HER2− breast cancer and thus identifies a subgroup of patients where neoadjuvant chemotherapy can truly impact axillary surgery. This is potentially practice-changing and important information for multidisciplinary teams to consider when deciding on optimal treatment sequencing (neoadjuvant chemotherapy, neoadjuvant endocrine therapy, or upfront surgery). In contrast, patients aged > 50 years with Ki67 < 20%, who have a very low nodal response rate of 3%, are unlikely to be able to preserve their axilla with neoadjuvant chemotherapy and use of upfront surgery or neoadjuvant endocrine therapy may be more beneficial.
While negative PR status was also associated with nodal pCR, the relative rarity of ER+/PR− disease (accounting for less than 20% of ER+/HER2− tumors) and the fact that PR status is correlated to some degree with Ki67 limit the clinical applicability of PR status, thus we focused on Ki67 and age.
A major question remains regarding the prognostic effects of nodal response in ER+/HER2− breast cancer. As previously noted, decisions regarding use of chemotherapy are often deferred until after surgery and are commonly based upon multigene tests. However, a major question remains regarding the use of the 21-gene assay for the selection of adjuvant chemotherapy in women aged < 50 years, given that this assay, while predictive of chemotherapy benefit in postmenopausal women, was not predictive in premenopausal women from either TAILORx or RxPONDER.14,15 In our study, nodal pCR was higher at 31.8% in patients aged < 50 years compared with 17.7% in those aged ≥ 50 years (p = 0.004). However, breast and total pCR did not vary significantly between age groups (15.9% versus 13.9% and 12.1% versus 8.2%, respectively). Given these findings, a major question in the neoadjuvant chemotherapy treatment of ER+/HER2− breast cancer is whether nodal pCR or total pCR is a better surrogate for distant metastasis-free survival. To answer this question, studies are ongoing at our institution to evaluate the long-term prognostic role of total breast pCR versus nodal pCR according to tumor subtype.
There are several limitations to this study. In particular, this is a single-institution study and would benefit from further evaluation in a larger cohort of patients. Not all institutions perform Ki67 evaluation on breast tumors as evaluation of Ki67 is highly variable across laboratories and lacks standardization. This can limit the applicability in the clinical setting. However, the recent US Food and Drug Administration (FDA) approval of abemaciclib along with Ki67 IHC MIB-1 pharmDx (Dako Omnis) assay,16 as a companion diagnostic, will lead to standardization and more widespread availability of Ki67 testing.
The ASCO guidelines recommend that, in HR+/HER2− disease, neoadjuvant chemotherapy can be used when a treatment decision can be made without surgical information. Therefore, having more information to predict response prior to deciding management strategy can help inform decision-making. These data suggest that simple clinicopathological factors of age and Ki67 should be considered for NAC decision-making and can identify a group of patients with high rates of nodal downstaging who may benefit from NAC to enable axillary preservation and potentially avoid an axillary dissection. Rates of nodal pCR in cases with Ki67 < 20% are very low, especially in patients ≥ 50 years. Use of NAC to enable preservation of the axilla should not be considered in patients ≥ 50 years of age with ER+/HER2− disease and low Ki67. For patients aged < 50 years with Ki67 ≥ 20%, use of chemotherapy in the neoadjuvant setting has a nodal response rate of 36% for conversion of node-positive to node-negative disease, and thus use of neoadjuvant chemotherapy should be considered to enable axillary conservation.
DISCLOSURE
Dr. Goetz and Dr. Boughey have a research collaboration with SymBioSis unrelated to this work. Dr. Goetz has grant funding to Mayo Clinic from Lilly, Pfizer, and Sermonix. Dr. Boughey has grant funding paid to Mayo Clinic from Lilly. This work was supported in part by National Institutes of Health Mayo Clinic Breast SPORE grant P50CA116201 (to M.P.G.). Dr. Goetz has personal fees for CME activities from: Research to Practice (6/2021), Clinical Education Alliance (2/2021), Medscape (12/2021), and Curio Science Moderator (1/2022); personal fees for serving as a panelist for a panel discussion for: Total Health Conferencing (2021); consulting fees to Mayo Clinic from: ARC Therapeutics (3/2022), AstraZeneca (1/2021), Biovica (12/2019), Biotheranostics (7/2020), Blueprint Medicines (1/2021), Eagle Pharmaceuticals (6/2020), Lilly (11/2021), Novartis (6/2020), Pfizer (7/2019), Sermonix (7/2020), and Sanofi Genzyme (12/2021).
REFERENCES
- 1.Murphy BL, Day CN, Hoskin TL, Habermann EB, Boughey JC. Neoadjuvant chemotherapy use in breast cancer is greatest in excellent responders: triple-negative and HER2+ subtypes. Ann Surg Oncol. 2018;25(8):2241–8. [DOI] [PubMed] [Google Scholar]
- 2.Yau C, Osdoit M, van der Noordaa M, et al. Residual cancer burden after neoadjuvant chemotherapy and long-term survival outcomes in breast cancer: a multicentre pooled analysis of 5161 patients. Lancet Oncol. 2022;23(1):149–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–72. [DOI] [PubMed] [Google Scholar]
- 4.Boughey JC, McCall LM, Ballman KV, et al. Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: findings from the ACOSOG Z1071 (Alliance) prospective multicenter clinical trial. Ann Surg. 2014;260(4):608–14 (discussion 614-6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harbeck N, Rastogi P, Martin M, et al. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: updated efficacy and Ki-67 analysis from the MonarchE study. Ann Oncol. 2021;32(12):1571–81. [DOI] [PubMed] [Google Scholar]
- 6.Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001;30:96–102. [DOI] [PubMed] [Google Scholar]
- 7.Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project protocols B-18 and B-27. J Clin Oncol. 2008;26(5):778–85. [DOI] [PubMed] [Google Scholar]
- 8.Masuda N, Lee S-J, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376(22):2147–59. [DOI] [PubMed] [Google Scholar]
- 9.von Minckwitz G, Huang C-S, Mano MS, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617–28. [DOI] [PubMed] [Google Scholar]
- 10.Korde LA, Somerfield MR, Carey LA, et al. Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. J Clin Oncol. 2021;39(13):1485–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pilewskie M, Zabor EC, Mamtani A, Barrio AV, Stempel M, Morrow M. The optimal treatment plan to avoid axillary lymph node dissection in early-stage breast cancer patients differs by surgical strategy and tumor subtype. Ann Surg Oncol. 2017;24(12):3527–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim KII, Lee KH, Kim TR, Chun YS, Lee TH, Park HK. Ki-67 as a predictor of response to neoadjuvant chemotherapy in breast cancer patients. J Breast Cancer. 2014;17(1):40–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jain P, Doval DC, Batra U, et al. Ki-67 labeling index as a predictor of response to neoadjuvant chemotherapy in breast cancer. Jpn J Clin Oncol. 2019;49(4):329–38. [DOI] [PubMed] [Google Scholar]
- 14.Kalinsky K, Barlow WE, Gralow JR, et al. 21-Gene assay to inform chemotherapy benefit in node-positive breast cancer. N Engl J Med. 2021;385(25):2336–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sparano JA, Gray RJ, Makower DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018;379(2):111–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.FDA Approves Abemaciclib with Endocrine Therapy for Early Breast Cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-abemaciclib-endocrine-therapy-early-breast-cancer. Accessed 3 March 2021.


