Abstract
The ςX and ςW extracytoplasmic function sigma factors regulate more than 40 genes in Bacillus subtilis. ςW activates genes which function in detoxification and the production of antimicrobial compounds, while ςX activates functions that modify the cell envelope. Transposon mutagenesis was used to identify loci which negatively regulate ςW or ςX as judged by up-regulation from the autoregulatory promoter site PW or PX. Fourteen insertions that activate PW were identified. The largest class of insertions are likely to affect transport. These include insertions in genes encoding two multidrug efflux protein homologs (yqgE and yulE), a component of the oligopeptide uptake system (oppA), and two transmembrane proteins with weak similarity to transporters (yhdP and yueF). Expression from PW is also elevated as a result of inactivation of at least one member of the ςW regulon (ysdB), an ArsR homolog (yvbA), a predicted rhamnose isomerase (yulE), and a gene (pksR) implicated in synthesis of difficidin, a polyketide antibiotic. In a parallel screen, we identified seven insertions that up-regulate PX. Remarkably, these insertions were in functionally similar genes, including a multidrug efflux homolog (yitG), a mannose-6-phosphate isomerase gene (yjdE), and loci involved in antibiotic synthesis (srfAB and possibly yogA and yngK). Significantly, most insertions that activate PW have little or no effect on PX, and conversely, insertions that activate PX have no effect on PW. This suggests that these two regulons respond to distinct sets of molecular signals which may include toxic molecules which are exported, cell density signals, and antimicrobial compounds.
The soil bacterium Bacillus subtilis has evolved elaborate regulatory systems to adapt and survive under various environmental conditions. Alternative ς factors provide one means of modulating gene expression in response to changes in environment. Alternative ς factors in B. subtilis control sporulation (ςH, ςE, ςF, ςG, and ςK); chemotaxis, motility, and autolysis (ςD); and general stress responses (ςB) (15). Sequencing of the B. subtilis genome has revealed seven previously unidentified ς factors that are members of the extracytoplasmic function (ECF) subfamily (28).
ECF ς factors are found in a wide variety of gram-positive and gram-negative bacteria and often regulate gene expression in response to extracytoplasmic stimuli (32). For example, ECF ς factors regulate genes involved in ferric citrate uptake and periplasmic protein proteolysis in Escherichia coli (3, 8); nickel and cobalt efflux in Alcaligenes eutrophus (30); antibiotic production, oxidative stress responses, and cell wall modification in Streptomyces spp. (26, 43, 44); and alginate and exotoxin secretion in Pseudomonas aeruginosa (18, 41). ECF ς factors are typically regulated by a cotranscribed anti-ς factor that is targeted to the cell membrane. Thus, expression of the ς factor operon leads to the synthesis of inactive ς–anti-ς complexes that are then regulated by signals that inhibit anti-ς function. These signals are likely to include alterations in the chemical composition or structure of the cell envelope.
The physiological functions of the ECF ς factors of B. subtilis are not well understood, and mutants with mutations in each of the seven ς factors are all viable. Only three of these regulators have been studied in detail (20–24): a sigX mutant is slightly more sensitive to heat and oxidative stress, a sigM mutant is unable to grow in high concentrations of salt, and a sigW mutant is altered in resistance to cell wall biosynthesis inhibitors. The ςX regulon is expressed during late logarithmic growth, while the ςW regulon is activated early in stationary phase (21, 22). Derepression of ς factor regulons, by mutation of the corresponding anti-ς factor, can also lead to phenotypic alterations. Increased expression of the ςX regulon in an anti-ς (rsiX) mutant represses expression of ςW (22) and leads to reduced competence (61).
To investigate the roles of B. subtilis ECF ς factors, we used consensus-based promoter searches to identify genes under the control of ςX and ςW (23, 24). The ςX regulon includes a putative glucosyltransferase (CsbB), a regulator of autolysin expression (LytR), and a response regulator aspartate phosphatase (RapD). Recent results indicate that ςX also regulates the d-alanylation of teichoic acids and membrane phospholipid composition (M. Cao, J. Qiu, and J. D. Helmann, unpublished results). Proteins dependent on ςW for expression include a fosfomycin resistance determinant (FosB), a penicillin binding protein (PBP4*), signal peptide peptidase (YteI), an ATP-binding cassette transporter (YknXYZ), a nonheme bromoperoxidase (YdjP), epoxide hydrolase (YfhM), several small hydrophobic peptides (YvlC, YxzE, and YdjO), and a large number of membrane proteins of unknown function (23). At least four additional genes (abh, divIC, yrhH, and ywbN) are apparently transcribed by both ςX and ςW (22). The characterization of these two regulons suggests that ςX regulates cell envelope modification processes while ςW regulates detoxification responses and the production of antimicrobial compounds.
To further define the physiological roles of ςX and ςW in B. subtilis, we have used mini-Tn10 mutagenesis to identify mutants with increased ςW or ςX activity. The resulting transposon insertions indicate that defects in transport, cell density signaling, sugar metabolism, and antimicrobial production affect the activity of these ς factors. However, the signals that activate ςX appear to be largely distinct from those that activate ςW.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
All the bacterial strains and plasmids used in this study are listed in Table 1. Bacterial cultures were grown at 37°C with aeration in liquid Luria-Bertani medium (LB) (53) or Tris-Spizizen salts (TSS) minimal medium (16) containing either d-glucose or d-mannose as the sugar and auxotrophic requirements. Plates contained 1.25% Bacto Agar (Difco) and 40 μg of X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) per ml. Ampicillin (100 μg/ml) or spectinomycin (SPC) (200 μg/ml) was used for the selection of E. coli strains. Erythromycin (1 μg/ml) and lincomycin (25 μg/ml) (for testing macrolide-lincosamide-streptogramin B [MLS] resistance), SPC (100 μg/ml), neomycin (8 μg/ml), kanamycin (15 μg/ml), and chloramphenicol (2 to 5 μg/ml) were used for the selection of various B. subtilis strains.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Reference or source |
|---|---|---|
| B. subtilis strains | ||
| CU1065 | W168 trpC2 attSPβ | Lab stock |
| HB7070 | CU1065 SPβ7063 [PW-cat-lacZ] | 22 |
| HB0011 | HB7070 rsiW::Km | This work |
| HB7022 | CU1065 SPβ7019 [PX-cat-lacZ] | 21 |
| HB7024 | HB7022 rsiX::pVA29 | 21 |
| OKB105 | pheA1 sfp | 39 |
| HB300 | CU1065 yqgE::pMUTIN (MLSr) | This study |
| HB301 | HB7070 pXT-yshB′ (Spcr MLSr Neor) | This study |
| HB302 | CU1065 yjdD::pMUTIN (MLSr) | This study |
| E. coli strains | ||
| DH5α | supE44 ΔlacU169 (φ80 lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | 53 |
| Jm2r− | mcrAB hsdR recA1 Δ(lac-proAB) thi gyrA96 relA1 srl::Tn10 F′ (proAB lacZ ΔM15) | S. Zahler |
| Plasmids | ||
| pIC333 | pUC replicon, shuttle vector carrying mini-Tn10, thermosensitive replicon for gram-positive hosts (Apr MLSr Spcr) | 57 |
| pMUTIN4 | pBR322-based integration vector for B. subtilis; contains a multiple cloning site downstream of the Pspac promoter (Apr MLSr) | 64 |
| pMUTIN-yqgE | pMUTIN4 derivative carrying DNA from the 5′ end of yqgE | This study |
| pMUTIN-yjdD | pMUTIN4 derivative carrying DNA from within yjdD | This study |
| pXT | Vector which integrates into the thrC locus and contains the xylA xylose-inducible promoter upstream of a multiple cloning site (Apr MLSr Spcr) | T. Msadek |
| pXT-yshB′ | pXT derivative carrying DNA from the 5′ end of yshB | This study |
Construction of mini-Tn10 libraries.
Random mini-Tn10 libraries of B. subtilis strains were contructed using the plasmid pIC333 (57). This plasmid contains a ColE1 origin and a thermosensitive origin of replication for gram-positive bacteria (inactive at temperatures greater than 35°C). B. subtilis strains HB7070 (PW-cat-lacZ) and HB7022 (PX-cat-lacZ) were transformed with pIC333 with selection for Spcr on LB plates incubated overnight at 28°C. Single colonies were inoculated into 2 ml of LB-SPC and incubated at 28°C overnight. Following a 1:100 dilution in the same medium, the cultures were grown for 3 h at 28°C, then shifted to 37°C, and grown for at least another 5 h. Diluted aliquots of these cultures were plated onto LB and LB-SPC plates and incubated overnight at 37°C. The rest of the culture was collected by centrifugation and resuspended in LB containing 15% glycerol for storage at −80°C. The transposition frequency was estimated from the ratio of the number of colonies on LB-SPC to that on LB and was in the general range (0.01 to 1%) reported for this system.
DNA manipulations and sequencing.
Isolation of B. subtilis chromosomal DNA and transformations were done by standard procedures (16). Restriction endonucleases and DNA ligase (New England Biolabs, Inc., Beverly, Mass.) were used according to the manufacturer's instructions. Plasmid rescue experiments were performed as previously described (6). PCR experiments used for cloning DNA into plasmids were performed by using the Expand High Fidelity PCR System (Boehringer Mannheim) according to the manufacturer's instructions. DNA was purified by using the QIAprep Spin Miniprep and PCR purification and gel extraction kits (Qiagen Inc., Chatsworth, Calif.). DNA sequencing was performed with AmpliTaq-FS DNA polymerase and dye terminator chemistry at the DNA Services Facility of the Cornell New York State Center for Advanced Technology-Biotechnology.
Screening and identification of mini-Tn10 mutants up-regulated in ςW and ςX activity.
Initially, several mini-Tn10 libraries of B. subtilis HB7070 were plated onto LB-SPC at a density of approximately 150 transposants per plate. The majority of colonies were light blue after 1 day of growth at 37°C; however, some colonies exhibited enhanced β-galactosidase (β-Gal) activity. Three colonies (W1, W2, and W3) from different mini-Tn10 libraries with enhanced β-Gal activity were further characterized. Also, several mini-Tn10 libraries of B. subtilis HB7070 and HB7022 were plated onto LB-SPC containing chloramphenicol (2 to 5 μg/ml) at a density of approximately 10,000 transposants per plate. Several mutants which grew faster on these plates and had elevated β-Gal activity were further characterized. The phenotypes were linked to the mini-Tn10 Spcr marker by transformation. Plasmids containing the mini-Tn10 element with a ColE1 origin, the ampicillin resistance gene, and flanking B. subtilis chromosomal DNA were recovered from selected mutant strains. DNA sequence upstream and downstream of the transposon was obtained using two primers corresponding to the left and right ends of the mini-Tn10, as described previously (4).
Generation of B. subtilis mutants HB300, HB301, and HB302.
DNA upstream of and including the 5′ end of the yqgE gene was amplified from B. subtilis chromosomal DNA using primers 425 (5′-TTGAATTCTTCTTTTTACATATCTCGG-3′) and 426 (5′-CAGGATCCTGTCTATTTTTTTGGCTAACCG-3′). The ∼320-bp PCR product was digested with EcoRI and BamHI (sites underlined) and cloned into pMUTIN4 (64) to generate pMUTIN-yqgE. This plasmid was then transformed into strain CU1065 to generate strain HB300. DNA upstream of and including the truncated yshB gene (caused by the mini-Tn10 insertion) from strain W14 was amplified using primers 508 (5′-ATGGATCCGCCGGGCGGTTTTGCCTG-3′) and 509 (5′-ATCAGAATTCAAGATGTGTATCCACC-3′). The ∼640-bp PCR product encoding a hydrophobic peptide from the 5′ sequence of yshB was digested with BamHI and EcoRI and cloned into pXT, a derivative of pDG1731 allowing gene expression from the PxylA xylose-inducible promoter and integration by a double-crossover event at the thrC locus (T. Msadek, unpublished data). The resulting plasmid, pXT-yshB′, was linearized with ScaI and transformed into strain CU1065 to generate strain HB301. To generate a yjdD mutant, an internal fragment of the yjdD gene was amplified from B. subtilis chromosomal DNA using primers 511 (5′-AGGAATTCTGCAAAAAGCTGCTGACAGAC-3′) and 512 (5′-AAGGATCCAGTCGCCGCAATATAACCGC-3′). The ∼590-bp PCR product was digested with EcoRI and BamHI and cloned into pMUTIN4 to generate pMUTIN-yjdD. This plasmid was then transformed into strain CU1065 to generate HB302.
β-Gal assays.
To determine the β-Gal activities of various strains, cells were diluted 1:100 from an overnight culture grown in LB containing the necessary antibiotics into LB. Samples were then collected from the phase of growth when the relevant ς factor is most active. For strains containing the PW-cat-lacZ fusion, samples were taken at T1 (1 h after the end of exponential growth), and for strains containing the PX-cat-lacZ fusion, samples were taken at T−2. The assay used for determining β-Gal levels by the method of Miller has been described previously (6, 34). All assays were performed on duplicate samples, and the values were averaged.
Computer analysis.
To determine the loci in which the mini-Tn10 had been inserted, the sequence of chromosomal DNA flanking the mini-Tn10 was compared with the B. subtilis genome using the BLAST program (2) available on the SubtiList website (37) at http://www.pasteur.fr/Bio/SubtiList.html. Searches of B. subtilis protein sequences in other databases were performed using BLASTP (2) at http://www.ncbi.nlm.nih.gov/BLAST/ using the unfiltered setting. Protein localization and transmembrane domains were predicted using both the PSORT (38) and TMPred (19) programs available at http://psort.nibb.ac.jp:8800/form.html and http://www.isrec.isb-sib.ch/software/TMPRED_form.html, respectively.
RESULTS
Rationale for mutant isolation.
To assess the activity of ςW and ςX in vivo, we used strains [HB7070 (PW-cat-lacZ) and HB7022 (PX-cat-lacZ)] containing operon fusions to the autoregulatory promoters, PW and PX. These promoters are specifically recognized by each ς factor in vivo and in vitro (21, 22). Since each promoter drives expression of an operon encoding both chloramphenicol resistance (cat) and β-Gal (lacZ), this system is suitable for both genetic screens and selections for increased promoter activity (56).
Isolation and analysis of mini-Tn10 mutants with increased ςW activity.
Initially, several HB7070 mini-Tn10 libraries were plated onto LB plates containing SPC and X-Gal. From approximately 9,000 transposants, three mutants (W1, W2, and W3 [Table 2]) with obviously elevated β-Gal activity were identified. All three have a mini-Tn10 insertion at the same position within the yvbA gene. Since these mutants were isolated from independent mini-Tn10 libraries, and one has the mini-Tn10 inserted in the opposite orientation from that of the other two, they are not siblings. YvbA is an uncharacterized member of the ArsR family of transcriptional regulators (Table 3). ArsR-like proteins (including ArsR, SmtB, ZiaR, and CadC) regulate resistance to arsenic, zinc, and cadmium (10, 25, 60, 67). Since resistance is often associated with metal ion efflux, it is possible that YvbA may regulate efflux from B. subtilis. Unlike other members of this family, YvbA does not contain cysteine residues, which have been implicated in metal binding (55).
TABLE 2.
Characterization of genes which affect ςW and ςX activity
| Straina | Gene | Mini-Tn10 insertion siteb | Presumed function and/or featuresc | Downstream gene(s) possibly affected by insertiond | LB plate phenotypee |
|---|---|---|---|---|---|
| HB7070 | NAf | NA | NA | NA | −/+ |
| HB0011 | rsiW | NA | Anti-ςW | NA | ++ |
| W1, W2, and W3 | yvbA | 244 | Regulator of export | yvaZ | +++ |
| W4 | yqgE | 240 | Multidrug efflux | pbpA | −/+g |
| W5 | yheH | 1640 | Multidrug efflux | + | |
| W6 | yhdP | 260 | Metal ion efflux | + | |
| W7 | yueF | 931 | Unknown; transport? | yueG | + |
| W8 | oppA | 391 | Oligopeptide uptake | oppBCDF | ++ |
| W9 | yulE | 1058 | Rhamnose isomerase | + | |
| W10 | pksR | 6772 | Difficidin synthesis | + | |
| W11 | ysdB | −43 | Unknown; ςW- and possibly ςB-regulated membrane protein | + | |
| W12 | yqfD | 71 | Unknown; possibly ςW regulated | phoH | + |
| W13 | yodE | 240 | Aromatic ring cleavage | yodD | + |
| W14 | yshB | 47 | Unknown | yshCDE | ++ |
| W15 | yshD | 1862 | DNA mismatch repair | yshE | + |
| W16 | yopH | 331 | Unknown | yopIJKL | + |
| HB7022 | NA | NA | NA | NA | − |
| HB7024 | rsiX | NA | Anti-ςX | NA | ++ |
| X1 | yitG | 631 | Multidrug efflux | yitFE | + |
| X2 | yjdE | 60 | Mannose-6-phosphate isomerase | yjdF | + |
| X3 | srfAB | 10064 | Surfactin synthesis | srfAC, srfAD | + |
| X4 | yogA | 933 | Polyketide synthesis | −/+ | |
| X5 | yngK | 1517 | Unknown | −/+ | |
| X6 | ytxJ | 227 | Unknown | −/+ | |
| X7 | ywpH | −74 | DNA replication | glcR, ywpJ | + |
Mini-Tn10 mutants up-regulated in ςW and ςX activity begin with W and X, respectively.
Relative to the first nucleotide of the gene.
The majority of predicted functions are based on those of homologous proteins (see Table 3).
In most cases, operon structures have not been characterized, but inspection of the genome sequence indicates that expression of these downstream genes would also likely be affected by the transposon insertion. We have also not ruled out possible effects of the transposon insertion on expression of upstream genes.
Expression of the PW and PX promoters in each strain was measured by growth on 15-ml LB plates containing 40 μg of X-Gal per ml. Colony color was observed following overnight growth at 37°C and is indicated as follows: +++ (strong blue) > ++ (blue) > + (light blue) > −/+ (very light blue) > − (white). The parent (HB7070) and rsiW mutant (HB0011) strains containing the PW-cat-lacZ fusion are included. The parent (HB7022) and rsiX mutant (HB7024) strains containing the PX-cat-lacZ fusion are also included.
NA, not applicable.
After 2 days of growth, the yqgE mutant had significantly greater ςW activity than did the parent strain HB7070.
TABLE 3.
Database homologies
| Protein of unknown function | No. of amino acids | B. subtilis paralog(s) of interest (% identity/no. of amino acids) | Homologous protein(s) of known or predicted function (% identity/no. of amino acids) | Reference for functionally known protein |
|---|---|---|---|---|
| YvbA | 90 | Staphylococcus xylosus ArsR (40/87) | 51 | |
| YqgE | 430 | Staphylococcus aureus NorA (22/304) | 68 | |
| YheH | 673 | Homo sapiens MDR1 (33/517) | 5 | |
| Pasteurella haemolytica HylB (31/509) | 59 | |||
| Lactococcus lactis LcnC (27/532) | 58 | |||
| YhdP | 444 | YrkA (62/434) | Rickettsia typhi TylC (33/214) | 48 |
| YhdT (60/430) | S. enterica serovar Typhimurium CorC (27/277) | 12 | ||
| YugS (60/427) | S. enterica serovar Typhimurium CorB (24/224) | 12 | ||
| YqhB (59/433) | ||||
| YueF | 369 | E. coli PerM (27/315) | ||
| YsdB | 130 | None | ||
| YqfD | 398 | Bacillus megaterium SpoIV (43/395) | 66 | |
| YodE | 303 | YdfO (48/296) | Sphingomonas paucimobilis LinE (30/306) | 35 |
| YkcA (37/313) | Sphingomonas chlorophenolica PcpA (28/299) | 42 | ||
| YulE | 424 | E. coli RhaA (58/411) | 36 | |
| YshB | 177 | None | ||
| YshD | 785 | MutS (24/520) | 13 | |
| YopH | 178 | None | ||
| YitG | 422 | Blt (23/407) | 1 | |
| Bmr (22/383) | ||||
| YjdE | 315 | Pmi (56/316) | Streptococcus mutans ManA (53/311) | 54 |
| YdhS (55/313) | ||||
| YogA | 329 | Streptomyces cinnamonensis CCR (26/364) | 31 | |
| Pseudomonas syringae Cfa8 (25/356) | 49 | |||
| YngK | 510 | None | ||
| YtxJ | 108 | None | ||
| YwpH | 113 | SSB (63/106) |
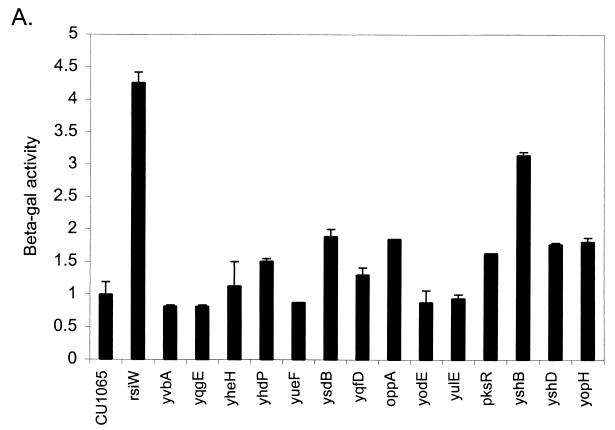
To expand our collection of mutants with altered ςW activity (Table 2), we selected for upregulation of the PW-cat-lacZ fusion using chloramphenicol. Mini-Tn10 libraries were plated onto LB containing SPC, X-Gal, and growth-inhibitory levels (2 to 5 μg per ml) of chloramphenicol. Mutants with elevated β-Gal activity were isolated following 2 days of incubation at 37°C. Those transposon insertions that were genetically linked to the derepressed phenotype were further characterized. For quantification, β-Gal activities were determined for mutants grown in LB to early stationary phase (T1), when PW activity is maximal (Fig. 1). Most mutants had only slightly elevated ςW activity in liquid medium, despite an obvious effect on solid medium (Table 2 and Fig. 1). This is reminiscent of the observation that PW can be strongly induced by cell wall biosynthesis inhibitors on plates but not in liquid medium (M. Cao and J. D. Helmann, unpublished data).
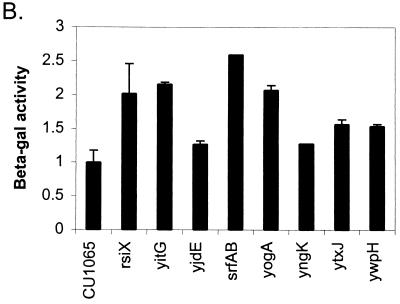
FIG. 1.
Expression of PW-cat-lacZ (A) and PX-cat-lacZ (B) during growth in liquid culture. Strains containing PW-cat-lacZ and PX-cat-lacZ were grown to T1 and T−2, respectively, and β-Gal levels were determined. Results are normalized to those for wild-type strains, in which the PW-cat-lacZ and PX-cat-lacZ strains produced 28.5 and 30.5 Miller units, respectively. Error bars represent standard deviations from the mean, with n = 2. Note that, in many cases, up-regulation is not apparent in liquid culture, despite obvious effects on plates.
A total of 13 additional insertion mutations were identified in this genetic selection (Table 2). These insertions define genes that cluster into three functional classes: (i) transport, (ii) sugar metabolism, and (iii) antibiotic biosynthesis. Insertions were also obtained in genes of unknown function including at least one known member of the ςW regulon.
Transport functions.
Mutants W4 and W5 possess mini-Tn10 insertions in genes yqgE and yheH which encode transmembrane proteins with similarity to multidrug efflux proteins. YheH is a putative ATP-binding protein which has been classified into subfamily 6 of the B. subtilis ATP-binding proteins (47). Subfamily 6 includes proteins which are similar to multidrug resistance proteins of eukaryotes and bacterial proteins involved in bacteriocin and hemolysin export (Table 3). YqgE has 10 to 12 potential hydrophobic domains and is similar to drug efflux proteins (Table 3). Although yqgE is located downstream of sodA (superoxide dismutase) in several Bacillus species, it has been previously shown not to be involved in SodA activity (17).
Multidrug efflux proteins export a variety of structurally unrelated toxic chemicals including ethidium bromide, chloramphenicol, and puromycin (1). However, the yqgE::Tn10 mutant is no more sensitive than the wild type to a variety of toxic chemicals (including ethidium bromide, chloramphenicol, or tetracycline). Since B. subtilis possesses a number of multidrug efflux proteins, it is possible that they are functionally redundant (1). Next, we placed yqgE under the control of the inducible Pspac promoter by integration of pMUTIN-yqgE into the chromosome. Induction of the resulting strain with IPTG (isopropyl-β-d-thiogalactopyranoside), to potentially elevate YqgE levels, failed to reveal any increase in resistance to ethidium bromide, tetracycline, or puromycin (data not shown). Thus, the role of this transporter and identification of its substrates await further study.
Mutant W6 contains a mini-Tn10 in yhdP which encodes one of five highly similar B. subtilis paralogs (Table 3). YdhP has significant sequence similarity to proteins from Salmonella enterica serovar Typhimurium involved in magnesium uptake (Table 3). A potential role of YhdP in transport is bolstered by the presence of a gene (yhdQ) encoding a MerR homolog immediately upstream of yhdP. Some MerR homologs regulate gene expression in response to metal ions, whereas others are known to regulate multidrug efflux proteins (1). Mutant W7 has a mini-Tn10 inserted in the yueF gene which encodes a protein of unknown function that has eight potential membrane-spanning domains. YueF is similar to putative integral membrane proteins including E. coli PerM (Table 3).
Mutant W8 has a mini-Tn10 inserted in the oppA gene which encodes an oligopeptide binding lipoprotein that is part of an ATP-binding cassette transport system (52). This system is required for sporulation and competence (52) and transports peptides that act as cell density signals (29). As predicted, mutant W8 has a sporulation-negative phenotype on Difco sporulation medium plates and is reduced in competence compared to the parent strain (data not shown). Identification of an oppA::Tn10 insertion suggests that ςW may be negatively regulated by some of the same cell density signals that positively regulate sporulation and competence.
Sugar metabolism.
Mutant W9 has a mini-Tn10 in the yulE gene which encodes a protein highly similar to rhamnose isomerases (Table 3). yulE is located in an operon with other genes encoding enzymes involved in rhamnose metabolism, and YulE appears to be the only rhamnose isomerase homolog in B. subtilis. Rhamnose isomerase catalyzes the interconversion of l-rhamnose and l-rhamnulose and is required for the first step in the metabolism of l-rhamnose (36). After several days of growth on minimal medium plates containing rhamnose as the sole carbon source, colonies of the yulE::Tn10 mutant become translucent, in contrast to the parent, which remains opaque. This indicates that yulE is likely to be involved in rhamnose metabolism, since this phenotype was not observed when the yulE::Tn10 mutant was grown on glucose or mannose as the sole carbon source. It is not yet clear why a defect in rhamnose metabolism might lead to increased ςW activity.
Antimicrobial synthesis.
Mutant W10 has a mini-Tn10 insertion in the pksR gene implicated in the synthesis of the broad-spectrum antimicrobial compound difficidin (28). Difficidin is a highly unsaturated 22-membered macrolide phosphate compound first identified in B. subtilis strains ATCC 39320 and ATCC 39374 (69). The mini-Tn10 has been inserted into the carboxyl-terminal thioesterase domain of PksR.
Insertions in genes of unknown function.
Several additional insertions identify genes of unknown function. Interestingly, at least one (and possibly two) of these genes is under ςW control. First, a mini-Tn10 insertion (W11) was identified 43 bp upstream of the ςW-controlled ysdB gene, which encodes a predicted membrane protein (23). A recent study indicates that ysdB is also partially transcribed from an upstream promoter recognized by the general stress ς factor, ςB (45). Since the mini-Tn10 was inserted into the gene-proximal ςW promoter, it is predicted that ysdB is not expressed in this mutant. Since this insertion increases ςW activity, we envision a feedback process whereby the loss of this protein leads to an unidentified signal that leads to up-regulation of ςW.
The second example is provided by mutant W12. The yqfD gene encodes a protein with similarity to a putative sporulation protein of Bacillus megaterium (Table 3). Interestingly, the genomic organization in the vicinity of yqfD suggests a possible operon structure including yqeZ yqfABCD phoH. The phoH gene encodes a homolog of an ATP-binding protein from E. coli which is induced under phosphate starvation conditions (27). The yqeZ gene encodes a paralog of the ςW-dependent YteI (signal peptide peptidase) and has recently been shown to also depend on ςW for expression (23; J. Qiu and J. D. Helmann, unpublished results). Thus, it is possible that yqfD is also under ςW control.
Additional genes of unknown function include yodE, yshB, yshD, and yopH. The yodE gene (W13) encodes a homolog of aromatic ring cleavage dioxygenases from Sphingomonas spp., which are involved in degradation of organic insecticides such as pentachlorophenol (Table 3). YodE also has high similarity to the B. subtilis YdfO and YkcA proteins, also of unknown function. Two independent mutants (W14 and W15) identify genes in the ysh locus. The yshB gene encodes a protein with four potential membrane-spanning domains with no significant similarity to other proteins in databases. The yshB::Tn10 strain could potentially express the 16 amino-terminal residues of YshB: MLDIIILILLLMGTLL. Since two known members of the ςW regulon are signal peptide peptidase homologs, we speculated that production of this hydrophobic peptide might be the signal leading to up-regulation of ςW. However, when we expressed this peptide using a xylose-inducible promoter we did not observe an increase in PW activity (data not shown). The yshD gene encodes a protein similar to the DNA mismatch repair MutS protein family (Table 3). Since the yshABCDE locus is probably an operon (Table 2), it is yet not known which particular gene or genes influence ςW activity. The last insertion isolated (W16) is in the yopH gene located on the SPβ prophage. The function of yopH is unknown, although the product of yopH is predicted to have two membrane-spanning regions.
Isolation and analysis of mini-Tn10 mutants with increased ςX activity.
In parallel with the above studies, we identified seven mini-Tn10 insertions that led to an up-regulation of a PX-cat-lacZ operon fusion. Unexpectedly, the resulting insertions defined a distinct group of genes that are nevertheless implicated in the same general set of cellular functions: transport, sugar metabolism, and antimicrobial biosynthesis.
Transport functions.
Mutant X1 has a mini-Tn10 in the yitG gene which encodes a putative transmembrane protein similar to multidrug efflux proteins. It has similarity to several characterized B. subtilis multidrug efflux proteins (Table 3) which mediate the efflux of a variety of structually diverse toxic compounds (1). Immediately downstream of the yitG gene is yitF, which encodes a protein similar to muconate cycloisomerases and mandelate racemases. These enzymes are involved in the catabolism of aromatic compounds (40). It is possible that yitG may be involved in the export of an aromatic-like compound in B. subtilis.
Sugar metabolism.
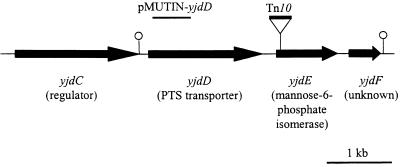
Mutant X2 has a mini-Tn10 in the yjdE gene which encodes one of the three mannose-6-phosphate isomerase homologs in B. subtilis (Table 3). Mannose-6-phosphate isomerase catalyzes the interconversion of mannose-6-phosphate and fructose-6-phosphate. Located upstream of yjdE are genes encoding a putative transcriptional activator (yjdC) and a phosphoenolpyruvate:sugar phosphotransferase (PTS) enzyme II of the fructose-mannitol family of PTS permeases (yjdD) (Fig. 2). It has been recently hypothesized, from sequence comparisons, that this locus may be involved in mannose metabolism in B. subtilis (50); however, no direct experimental evidence has confirmed this.
FIG. 2.
Diagram of genes surrounding yjdE and mutant strains used in this study. The proposed function of the genes is based on homologies only. The mini-Tn10 insertion in yjdE identified from mutant X2 is indicated as a triangle with Tn10. The pMUTIN-yjdD plasmid used to disrupt yjdD contains DNA from within yjdD indicated with a line above the gene. Potential stem-loops are indicated as lollipops.
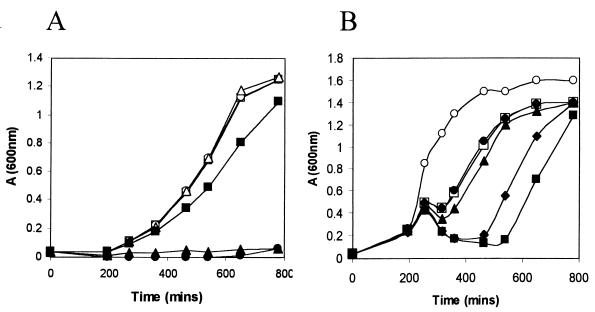
We observed that the yjdE mutant is unable to grow in minimal medium with mannose as the sole carbon source (Fig. 3A), demonstrating that this isomerase is essential for mannose catabolism. A yjdD mutant is also unable to grow on mannose (Fig. 3A). Since the yjdD mutation may be polar on downstream genes (Fig. 2), we induced the expression of yjdE from the Pspac promoter in the integrated pMUTIN-yjdD plasmid using IPTG. However, this induced strain was still unable to grow on mannose minimal medium plates. Thus, this operon appears to encode both a mannose-specific PTS enzyme and mannose isomerase.
FIG. 3.
Growth of B. subtilis strains in minimal medium containing glucose or mannose as the sugar (A) or in LB with varying concentrations of mannose (B). (A) B. subtilis CU1065 (squares) and yjdE (circles) and yjdD (triangles) mutant were grown in Tris-Spizizen salts minimal medium containing either glucose (open symbols) or mannose (filled symbols) as the sugar. (B) Effect of mannose on growth of the yjdE mutant. Cells were grown in LB (open squares) or LB with 1.4 μM (filled circles), 14 μM (triangles), 0.14 mM (diamonds), or 1.4 mM (filled squares) mannose. Growth of B. subtilis CU1065 (open circles) was unaffected by mannose addition, and all growth curves were essentially superimposable.
Interestingly, the yjdE mutant was impaired in growth in LB and more so in LB supplemented with greater than 1.4 mM mannose (Fig. 3B). This suggests that (i) YjdE may be needed for mannose synthesis from glucose during growth in LB and (ii) the accumulation of mannose-6-phosphate in a B. subtilis yjdE mutant grown on mannose inhibits growth.
Antimicrobial and polyketide synthesis.
Three mutant strains with increased ςX activity were affected in genes known or suggested to be involved in the synthesis of antimicrobial compounds. Mutant X3 has a mini-Tn10 insertion in the srf locus near the end of the srfAB gene. The srf operon encodes subunits of the surfactin synthetase (7). Located within the srfAB gene is a small gene termed comS which encodes a protein which is involved in competence (9). However, the mini-Tn10 insertion in srfAB is located downstream of comS.
Additional insertions were recovered in yogA (X4) and yngK (X5). YogA is similar to oxidoreductase enzymes which synthesize butyryl coenzyme A, used as a carbon extender in polyketide synthesis (31). yngK is the second gene located downstream of and in the same direction as the pps operon required for the synthesis of the antifungal cyclic decapeptide antibiotic pliplastin (63). Downstream of yngK is a putative operon encoding proteins involved in fatty acid metabolism (yngJIHGFE), and it has been suggested previously that they may be required for the synthesis of the lipopeptide (62). YngK may therefore play a role in the synthesis of pliplastin.
Proteins with unclear functions.
Mutant X6 has a mini-Tn10 inserted in the ytxJ gene which encodes a protein of unknown function. ytxJ has been previously termed csb40, is controlled by ςB and ςH, and is strongly induced by the addition of salt to the cells (65). Upstream of ytxJ, and located in the same operon, ytxH encodes a product with similarity to plant proteins induced by desiccation stress (65).
Mutant X7 has a mini-Tn10 insertion upstream of the ywpH gene. It is predicted that this insertion will affect the expression of ywpH, glcR, and ywpJ. ywpH encodes a product similar to single-stranded DNA binding proteins that are involved in DNA replication (Table 3). GlcR is similar to the DeoR family of transcriptional regulators which regulate genes involved in sugar utilization. Interestingly, GlcR is most similar to YulB (35% identity over 231 amino acids), which is encoded upstream of, and most likely in the same operon as, the putative rhamnose isomerase gene, yulE (mentioned above). ywpJ encodes a conserved protein of unknown function.
DISCUSSION
In this study, we sought to identify genes affecting the activity of ςW or ςX. We reasoned that mutations causing deficiencies in aspects of cell metabolism controlled by either ςX or ςW might lead to up-regulation of the corresponding regulons and aid in the identification of the molecular signals controlling ς factor activity. Since each of these ς factors is negatively regulated by a specific anti-ς, we anticipated that at least one class of mutations would be insertions in the anti-ς gene. However, we did not recover insertions in the anti-ς genes in this screen. This may reflect a low frequency of transposition in these genes by the Tn10 derivative employed in these studies or may simply reflect the fact that we have not saturated this screen. However, we have obtained multiple insertions in the same gene (yvbA) or in different genes in the same operon (yshB and yshD), and our collection of mutants defines several discrete functional groups: export, sugar metabolism, and antimicrobial synthesis.
Although ςW and ςX activity is affected by mutations affecting similar functions, only the yvbA::Tn10 mutation up-regulated both ςW and ςX (ςW more so than ςX). For the 16 other mutants tested (yopH::Tn10 was not included), the isolated transposon insertion affected expression of one reporter fusion, but not the other, as determined by measurements of β-Gal activity on solid medium. It has been previously shown that ςW and ςX coregulate several B. subtilis genes (22), and so these two ς factors do overlap in function. However, our results suggest that ςW and ςX respond to distinct stimuli, consistent with the observation that these two regulons are generally induced at different growth phases (21–24).
Many of the mini-Tn10 insertions identified in this study affect genes encoding transport proteins, including several with homology to multidrug efflux proteins. For the majority of these transporters, substrates have not yet been identified. Up-regulation of ς factor activity in these transport mutants may result from the inability to export toxic compounds from the cell. Another class of mutants affects genes involved in sugar metabolism. Interestingly, both rhamnose and mannose are components of cell surface polysaccharides of some gram-negative and gram-positive bacteria (14, 33). It is possible that the yulE and yjdE mutants may be affected in the synthesis of sugar-containing cell envelope components. Although N-acetyl-mannosamine is present in the linkage unit of cell wall teichoic acid, mannose-6-phosphate does not appear to be an intermediate in its synthesis (11). Further work will be needed to examine the cell envelope constituents in wild type and sigX, sigW, yulE, and yjdE mutants.
An interesting class of mutants identified in this study were affected in genes implicated in the synthesis of antimicrobials. Insertions in these genes were particularly surprising since surfactin and pliplastin are not thought to be synthesized by B. subtilis 168 (39, 63). This is due to a mutated sfp gene which encodes a phosphopantetheinyl transferase required for conversion of the peptidyl carrier domains within the multidomain synthetase enzymes from inactive apo-forms to active holo-forms (46). We suggest that another holo-acyl carrier protein synthase homolog (perhaps YdcB) may, albeit less efficiently, activate the antimicrobial synthetase subunits and allow a low level of antimicrobial production. Indeed, Sfp phosphopantetheinylates, with varying efficiency, a wide substrate spectrum, including acyl carrier protein domains of fatty acid synthases (46). If correct, it is possible that up-regulation reflects an inability to synthesize these antibiotics or, alternatively, the presence of a covalent antibiotic-synthetase complex resulting from insertions inactivating the thioesterase domain of the synthetase which is needed for release of the antibiotic. To test this latter idea, we hypothesized that transfer of these insertions into sfp+ strains (known to produce active synthase) might further elevate ς factor activity. However, when the srfAB::Tn10 mutation was transferred to the sfp+ strain B. subtilis OKB105 containing the PX-cat-lacZ fusion, no further up-regulation in ςX activity was observed.
Our results suggest that ςW and ςX respond to a variety of signals related to extracellular functions. By analogy with other ECF ς factors, the increase in ς factor activity is likely mediated by the corresponding anti-ς factors. We hypothesize that RsiW and RsiX sense, either directly or indirectly, molecules which are exported from the cell including cell density signal peptides, sugar-containing cell envelope components, and secondary metabolites such as antimicrobial compounds.
ACKNOWLEDGMENTS
We thank Tarek Msadek for providing pIC333 and pXT together with detailed instructions for their use and Peter Zuber for the sfp+ strain OKB105 and for helpful discussions.
This work was supported by Public Health Service grant GM47446 from the National Institutes of Health.
REFERENCES
- 1.Ahmed M, Lyass L, Markham P N, Taylor S S, Vazquez-Laslop N, Neyfakh A A. Two highly similar multidrug transporters of Bacillus subtilis whose expression is differentially regulated. J Bacteriol. 1995;177:3904–3910. doi: 10.1128/jb.177.14.3904-3910.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul S F, Madden T L, Schäffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angerer A, Enz S, Ochs M, Braun V. Transcriptional regulation of ferric citrate transport in Escherichia coli K-12. FecI belongs to a new subfamily of sigma-70-type factors that respond to extracytoplasmic stimuli. Mol Microbiol. 1995;18:163–174. doi: 10.1111/j.1365-2958.1995.mmi_18010163.x. [DOI] [PubMed] [Google Scholar]
- 4.Bsat N, Chen L, Helmann J D. Mutation of the Bacillus subtilis alkyl hydroperoxide reductase (ahpCF) operon reveals compensatory interactions among hydrogen peroxide stress genes. J Bacteriol. 1996;178:6579–6586. doi: 10.1128/jb.178.22.6579-6586.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen C J, Chin J E, Ueda K, Clark D P, Pastan I, Gottesman M M, Roninson I B. Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell. 1986;47:381–389. doi: 10.1016/0092-8674(86)90595-7. [DOI] [PubMed] [Google Scholar]
- 6.Chen L, James L P, Helmann J D. Metalloregulation in Bacillus subtilis: isolation and characterization of two genes differentially regulated by metal ions. J Bacteriol. 1993;175:5428–5437. doi: 10.1128/jb.175.17.5428-5437.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cosmina P, Rodriguez F, de Ferra F, Grandi G, Perego M, Venema G, van Sinderen D. Sequence and analysis of the genetic locus responsible for surfactin synthesis in Bacillus subtilis. Mol Microbiol. 1993;8:821–831. doi: 10.1111/j.1365-2958.1993.tb01629.x. [DOI] [PubMed] [Google Scholar]
- 8.Danese P N, Silhavy T J. The ςE and the Cpx signal transduction systems control the synthesis of periplasmic protein-folding enzymes in Escherichia coli. Genes Dev. 1997;11:1183–1193. doi: 10.1101/gad.11.9.1183. [DOI] [PubMed] [Google Scholar]
- 9.D'Souza C, Nakano M M, Zuber P. Identification of comS, a gene of the srfA operon that regulates the establishment of genetic competence in Bacillus subtilis. Proc Natl Acad Sci USA. 1994;91:9397–9401. doi: 10.1073/pnas.91.20.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Endo G, Silver S. CadC, the transcriptional regulatory protein of the cadmium resistance system of Staphylococcus aureus plasmid pI258. J Bacteriol. 1995;177:4437–4441. doi: 10.1128/jb.177.15.4437-4441.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghosh G, Roseman S. The sialic acids. IV. N-Acyl-d-glucosamine-6-phosphate 2-epimerase. J Biol Chem. 1965;240:1525–1530. [PubMed] [Google Scholar]
- 12.Gibson M M, Bagga D A, Miller C G, Maguire M E. Magnesium transport in Salmonella typhimurium: the influence of new mutations conferring Co2+ resistance on the CorA Mg2+ transport system. Mol Microbiol. 1991;5:2753–2762. doi: 10.1111/j.1365-2958.1991.tb01984.x. [DOI] [PubMed] [Google Scholar]
- 13.Ginetti F, Perego M, Albertini A M, Galizzi A. Bacillus subtilis mutS mutL operon: identification, nucleotide sequence and mutagenesis. Microbiology. 1996;142:2021–2029. doi: 10.1099/13500872-142-8-2021. [DOI] [PubMed] [Google Scholar]
- 14.Glushka J, Cassels F J, Carlson R W, van Halbeek H. Complete structure of the adhesin receptor polysaccharide of Streptococcus oralis ATCC 55229 (Streptococcus sanguis H1) Biochemistry. 1992;31:10741–10746. doi: 10.1021/bi00159a014. [DOI] [PubMed] [Google Scholar]
- 15.Haldenwang W G. The sigma factors of Bacillus subtilis. Microbiol Rev. 1995;59:1–30. doi: 10.1128/mr.59.1.1-30.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harwood C R, Cutting S M. Molecular biological methods for Bacillus. Chichester, England: John Wiley and Sons, Ltd.; 1990. [Google Scholar]
- 17.Henriques A O, Melsen L R, Moran C P., Jr Involvement of superoxide dismutase in spore coat assembly in Bacillus subtilis. J Bacteriol. 1998;180:2285–2291. doi: 10.1128/jb.180.9.2285-2291.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hershberger C D, Ye R W, Parsek M R, Xie Z D, Chakrabarty A M. The algT (algU) gene of Pseudomonas aeruginosa, a key regulator involved in alginate biosynthesis, encodes an alternative sigma factor ςE. Proc Natl Acad Sci USA. 1995;92:7941–7945. doi: 10.1073/pnas.92.17.7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hofmann K, Stoffel W. TMbase—a database of membrane spanning protein segments. Biol Chem Hoppe-Seyler. 1993;347:166. [Google Scholar]
- 20.Horsburgh M J, Moir A. ςM, an ECF RNA polymerase sigma factor of Bacillus subtilis 168, is essential for growth and survival in high concentrations of salt. Mol Microbiol. 1999;32:41–50. doi: 10.1046/j.1365-2958.1999.01323.x. [DOI] [PubMed] [Google Scholar]
- 21.Huang X, Decatur A, Sorokin A, Helmann J D. The Bacillus subtilis ςX protein is an extracytoplasmic function sigma factor contributing to the survival of high temperature stress. J Bacteriol. 1997;179:2915–2921. doi: 10.1128/jb.179.9.2915-2921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang X, Fredrick K L, Helmann J D. Promoter recognition by Bacillus subtilis ςW: autoregulation and partial overlap with the ςX regulon. J Bacteriol. 1998;180:3765–3770. doi: 10.1128/jb.180.15.3765-3770.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang X, Gaballa A, Cao M, Helmann J D. Identification of target promoters for the Bacillus subtilis extracytoplasmic function ς factor, ςW. Mol Microbiol. 1999;31:361–371. doi: 10.1046/j.1365-2958.1999.01180.x. [DOI] [PubMed] [Google Scholar]
- 24.Huang X, Helmann J D. Identification of target promoters for the Bacillus subtilis ςX factor using a consensus-directed search. J Mol Biol. 1998;279:165–173. doi: 10.1006/jmbi.1998.1765. [DOI] [PubMed] [Google Scholar]
- 25.Huckle J W, Morby A P, Turner J S, Robinson N J. Isolation of a prokaryotic metallothionein locus and analysis of transcriptional control by trace metal ions. Mol Microbiol. 1993;7:177–187. doi: 10.1111/j.1365-2958.1993.tb01109.x. [DOI] [PubMed] [Google Scholar]
- 26.Jones G H, Paget M S B, Chamberlin L, Buttner M J. Sigma-E is required for the production of the antibiotic actinomycin in Streptomyces antibioticus. Mol Microbiol. 1997;23:169–178. doi: 10.1046/j.1365-2958.1997.2001566.x. [DOI] [PubMed] [Google Scholar]
- 27.Kim S K, Makino K, Amemura M, Shinagawa H, Nakata A. Molecular analysis of the phoH gene, belonging to the phosphate regulon in Escherichia coli. J Bacteriol. 1993;175:1316–1324. doi: 10.1128/jb.175.5.1316-1324.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kunst F, Ogasawara N, Moszer I, Albertini A M, Alloni G, Azevedo V, Bertero M G, Bessieres P, Bolotin A, Borchert S, Borriss R, Boursier L, Brans A, Braun M, Brignell S C, Bron S, Brouillet S, Bruschi C V, Caldwell B, Capuano V, Carter N M, Choi S K, Codani J J, Connerton I F, Cummings N J, Daniel R A, Denizot F, Devine K M, Duesterhoeft A, Ehrlich S D, Emmerson P T, Entian K D, Errington J, Fabret C, Ferrari E, Foulger D, Fritz C, Fujita M, Fujita Y, Fuma S, Galizzi A, Galleron N, Ghim S Y, Glaser P, Goffeau A, Golightly E J, Grandi G, Guiseppi G, Guy B J, Haga K, Haiech J, Harwood C R, Henaut A, Hilbert H, Holsappel S, Hosono S, Hullo M F, Itaya M, Jones L, Joris B, Karamata D, Kasahara Y, Klaerr-Blanchard M, Klein C, Kobayashi Y, Koetter P, Koningstein G, Krogh S, Kumano M, Kurita K, Lapidus A, Lardinois S, Lauber J, Lazarevic V, Lee S M, Levine A, Liu H, Masuda S, Mauel C, Medigue C, Medina N, Mellado R P, Mizuno M, Moestl D, Nakai S, Noback M, Noone D, O'Reilly M, Ogawa K, Ogiwara A, Oudega B, Park S H, Parro V, Pohl T M, Portetelle D, Porwollik S, Prescott A M, Presecan E, Pujic P, Purnelle B, Rapoport G, Rey M, Reynolds S, Rieger M, Rivolta C, Rocha E, Roche B, Rose M, Sadaie Y, Sato T, Scanlan E, Schleich S, Schroeter R, Scoffone F, Sekiguchi J, Sekowska A, Seror S J, Serror P, Shin B S, Soldo B, Sorokin A, Tacconi E, Takagi T, Takahashi H, Takemaru K, Takeuchi M, Tamakoshi A, Tanaka T. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 29.Lazazzera B A, Solomon J M, Grossman A D. An exported peptide functions intracellularly to contribute to cell density signaling in B. subtilis. Cell. 1997;89:917–925. doi: 10.1016/s0092-8674(00)80277-9. [DOI] [PubMed] [Google Scholar]
- 30.Liesegang H, Lemke K, Siddiqui R A, Schlegel H G. Characterization of the inducible nickel and cobalt resistance determinant cnr from pMOL28 of Alcaligenes eutrophus CH34. J Bacteriol. 1993;175:767–778. doi: 10.1128/jb.175.3.767-778.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu H, Reynolds K A. Role of crotonyl coenzyme A reductase in determining the ratio of polyketides monensin A and monensin B produced by Streptomyces cinnamonensis. J Bacteriol. 1999;181:6806–6813. doi: 10.1128/jb.181.21.6806-6813.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lonetto M A, Brown K L, Rudd K E, Buttner M J. Analysis of the Streptomyces coelicolor sigE gene reveals the existence of a subfamily of eubacterial ς factors involved in the regulation of extracytoplasmic functions. Proc Natl Acad Sci USA. 1994;91:7573–7577. doi: 10.1073/pnas.91.16.7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marolda C L, Valvano M A. Identification, expression, and DNA sequence of the GDP-mannose biosynthesis genes encoded by the O7 rfb gene cluster of strain VW187 (Escherichia coli O7:K1) J Bacteriol. 1993;175:148–158. doi: 10.1128/jb.175.1.148-158.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. pp. 352–355. [Google Scholar]
- 35.Miyauchi K, Adachi Y, Nagata Y, Takagi M. Cloning and sequencing of a novel meta-cleavage dioxygenase gene whose product is involved in degradation of gamma-hexachlorocyclohexane in Sphingomonas paucimobilis. J Bacteriol. 1999;181:6712–6719. doi: 10.1128/jb.181.21.6712-6719.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moralejo P, Egan S M, Hidalgo E, Aguilar J. Sequencing and characterization of a gene cluster encoding the enzymes for l-rhamnose metabolism in Escherichia coli. J Bacteriol. 1993;175:5585–5594. doi: 10.1128/jb.175.17.5585-5594.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moszer I, Glaser P, Danchin A. SubtiList: a relational database for the Bacillus subtilis genome. Microbiology. 1995;141:261–268. doi: 10.1099/13500872-141-2-261. [DOI] [PubMed] [Google Scholar]
- 38.Nakai K, Kanehisa M. Expert system for predicting protein localization sites in gram-negative bacteria. Proteins. 1991;11:95–110. doi: 10.1002/prot.340110203. [DOI] [PubMed] [Google Scholar]
- 39.Nakano M M, Marahiel M A, Zuber P. Identification of a genetic locus required for biosynthesis of the lipopeptide antibiotic surfactin in Bacillus subtilis. J Bacteriol. 1988;170:5662–5668. doi: 10.1128/jb.170.12.5662-5668.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neidhart D J, Kenyon G L, Gerlt J A, Petsko G A. Mandelate racemase and muconate lactonizing enzyme are mechanistically distinct and structurally homologous. Nature. 1990;347:692–694. doi: 10.1038/347692a0. [DOI] [PubMed] [Google Scholar]
- 41.Ochsner U A, Johnson Z, Lamont I L, Cunliffe H E, Vasil M L. Exotoxin A production in Pseudomonas aeruginosa requires the iron-regulated pvdS gene encoding an alternative sigma factor. Mol Microbiol. 1996;21:1019–1028. doi: 10.1046/j.1365-2958.1996.481425.x. [DOI] [PubMed] [Google Scholar]
- 42.Ohtsubo Y, Miyauchi K, Kanda K, Hatta T, Kiyohara H, Senda T, Nagata Y, Mitsui Y, Takagi M. PcpA, which is involved in the degradation of pentachlorophenol in Sphingomonas chlorophenolica ATCC39723, is a novel type of ring-cleavage dioxygenase. FEBS Lett. 1999;459:395–398. doi: 10.1016/s0014-5793(99)01305-8. [DOI] [PubMed] [Google Scholar]
- 43.Paget M S, Kang J G, Roe J H, Buttner M J. ςR, an RNA polymerase sigma factor that modulates expression of the thioredoxin system in response to oxidative stress in Streptomyces coelicolor A3(2) EMBO J. 1998;17:5776–5782. doi: 10.1093/emboj/17.19.5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paget M S B, Chamberlin L, Atrih A, Foster S J, Buttner M J. Evidence that the extracytoplasmic function sigma factor ςE is required for normal cell wall structure in Streptomyces coelicolor A3(2) J Bacteriol. 1999;181:204–211. doi: 10.1128/jb.181.1.204-211.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petersohn A, Bernhardt J, Gerth U, Hoper D, Koburger T, Volker U, Hecker M. Identification of ςB-dependent genes in Bacillus subtilis using a promoter consensus-directed search and oligonucleotide hybridization. J Bacteriol. 1999;181:5718–5724. doi: 10.1128/jb.181.18.5718-5724.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quadri L E, Weinreb P H, Lei M, Nakano M M, Zuber P, Walsh C T. Characterization of Sfp, a Bacillus subtilis phosphopantetheinyl transferase for peptidyl carrier protein domains in peptide synthetases. Biochemistry. 1998;37:1585–1595. doi: 10.1021/bi9719861. [DOI] [PubMed] [Google Scholar]
- 47.Quentin Y, Fichant G, Denizot F. Inventory, assembly and analysis of Bacillus subtilis ABC transport systems. J Mol Biol. 1999;287:467–484. doi: 10.1006/jmbi.1999.2624. [DOI] [PubMed] [Google Scholar]
- 48.Radulovic S, Troyer J M, Beier M S, Lau A O, Azad A F. Identification and molecular analysis of the gene encoding Rickettsia typhi hemolysin. Infect Immun. 1999;67:6104–6108. doi: 10.1128/iai.67.11.6104-6108.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rangaswamy V, Mitchell R, Ullrich M, Bender C. Analysis of genes involved in biosynthesis of coronafacic acid, the polyketide component of the phytotoxin coronatine. J Bacteriol. 1998;180:3330–3338. doi: 10.1128/jb.180.13.3330-3338.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reizer J, Bachem S, Reizer A, Arnaud M, Saier M H, Jr, Stulke J. Novel phosphotransferase system genes revealed by genome analysis—the complete complement of PTS proteins encoded within the genome of Bacillus subtilis. Microbiology. 1999;145:3419–3429. doi: 10.1099/00221287-145-12-3419. [DOI] [PubMed] [Google Scholar]
- 51.Rosenstein R, Peschel A, Wieland B, Götz F. Expression and regulation of the antimonite, arsenite, and arsenate resistance operon of Staphylococcus xylosus plasmid pSX267. J Bacteriol. 1992;174:3676–3683. doi: 10.1128/jb.174.11.3676-3683.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rudner D Z, LeDeaux J R, Ireton K, Grossman A D. The spo0K locus of Bacillus subtilis is homologous to the oligopeptide permease locus and is required for sporulation and competence. J Bacteriol. 1991;173:1388–1398. doi: 10.1128/jb.173.4.1388-1398.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1990. [Google Scholar]
- 54.Sato Y, Yamamoto Y, Kizaki H, Kuramitsu H K. Isolation and sequence analysis of the pmi gene encoding phosphomannose isomerase of Streptococcus mutans. FEMS Microbiol Lett. 1993;114:61–66. doi: 10.1111/j.1574-6968.1993.tb06551.x. [DOI] [PubMed] [Google Scholar]
- 55.Shi W, Wu J, Rosen B P. Identification of a putative metal binding site in a new family of metalloregulatory proteins. J Biol Chem. 1994;269:19826–19829. [PubMed] [Google Scholar]
- 56.Slack F J, Mueller J P, Sonenshein A L. Mutations that relieve nutritional repression of the Bacillus subtilis dipeptide permease operon. J Bacteriol. 1993;175:4605–4614. doi: 10.1128/jb.175.15.4605-4614.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Steinmetz M, Richter R. Easy cloning of mini-Tn10 insertions from the Bacillus subtilis chromosome. J Bacteriol. 1994;176:1761–1763. doi: 10.1128/jb.176.6.1761-1763.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stoddard G W, Petzel J P, van Belkum M J, Kok J, McKay L L. Molecular analyses of the lactococcin A gene cluster from Lactococcus lactis subsp. lactis biovar diacetylactis WM4. Appl Environ Microbiol. 1992;58:1952–1961. doi: 10.1128/aem.58.6.1952-1961.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Strathdee C A, Lo R Y. Cloning, nucleotide sequence, and characterization of genes encoding the secretion function of the Pasteurella haemolytica leukotoxin determinant. J Bacteriol. 1989;171:916–928. doi: 10.1128/jb.171.2.916-928.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thelwell C, Robinson N J, Turner-Cavet J S. An SmtB-like repressor from Synechocystis PCC 6803 regulates a zinc exporter. Proc Natl Acad Sci USA. 1998;95:10728–10733. doi: 10.1073/pnas.95.18.10728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tortosa P, Albano M, Dubnau D. Characterization of ylbF, a new gene involved in competence development and sporulation in Bacillus subtilis. Mol Microbiol. 2000;35:1110–1119. doi: 10.1046/j.1365-2958.2000.01779.x. [DOI] [PubMed] [Google Scholar]
- 62.Tosato V, Albertini A M, Zotti M, Sonda S, Bruschi C V. Sequence completion, identification and definition of the fengycin operon in Bacillus subtilis 168. Microbiology. 1997;143:3443–3450. doi: 10.1099/00221287-143-11-3443. [DOI] [PubMed] [Google Scholar]
- 63.Tsuge K, Ano T, Hirai M, Nakamura Y, Shoda M. The genes degQ, pps, and lpa-8 (sfp) are responsible for conversion of Bacillus subtilis 168 to plipastatin production. Antimicrob Agents Chemother. 1999;43:2183–2192. doi: 10.1128/aac.43.9.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vagner V, Dervyn E, Ehrlich S D. A vector for systematic gene inactivation in Bacillus subtilis. Microbiology. 1998;144:3097–3104. doi: 10.1099/00221287-144-11-3097. [DOI] [PubMed] [Google Scholar]
- 65.Varon D, Brody M S, Price C W. Bacillus subtilis operon under the dual control of the general stress transcription factor ςB and the sporulation transcription factor ςH. Mol Microbiol. 1996;20:339–350. doi: 10.1111/j.1365-2958.1996.tb02621.x. [DOI] [PubMed] [Google Scholar]
- 66.Wittchen K D, Strey J, Bultmann A, Reichenberg S, Meinhardt F. Molecular characterization of the operon comprising the spoIV gene of Bacillus megaterium DSM319 and generation of a deletion mutant. J Gen Appl Microbiol. 1998;44:317–326. doi: 10.2323/jgam.44.317. [DOI] [PubMed] [Google Scholar]
- 67.Wu J, Rosen B P. Metalloregulated expression of the ars operon. J Biol Chem. 1993;268:52–58. [PubMed] [Google Scholar]
- 68.Yoshida H, Bogaki M, Nakamura S, Ubukata K, Konno M. Nucleotide sequence and characterization of the Staphylococcus aureus norA gene, which confers resistance to quinolones. J Bacteriol. 1990;172:6942–6949. doi: 10.1128/jb.172.12.6942-6949.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zimmerman S B, Schwartz C D, Monaghan R L, Pelak B A, Weissberger B, Gilfillan E C, Mochales S, Hernandez S, Currie S A, Tejera E, et al. Difficidin and oxydifficidin: novel broad spectrum antibacterial antibiotics produced by Bacillus subtilis. I. Production, taxonomy and antibacterial activity. J Antibiot (Tokyo) 1987;40:1677–1681. doi: 10.7164/antibiotics.40.1677. [DOI] [PubMed] [Google Scholar]