Abstract
This study investigated the effects of acute stress on breast meat quality, redox status, and mitochondrial function in pectoralis major (PM) muscle of broilers. A total of 168 broiler chickens (42-d-old, Ross 308) were randomly divided into control (CON) and preslaughter transport (T) treatments. A broiler was an experimental unit. Each treatment consisted of 84 broilers, and they were put in 12 crates with 7 broilers each. Broilers in the T group were transported according to a designed protocol, and the CON broilers were kept in crates under normal living conditions before slaughtering. Based on the meat quality traits assessed at postmortem 24 h, all PM muscles of the transported broilers were further classified into normal (T-NOR) and pale, soft, and exudative (PSE)-like (T-PSE) groups for the determination of redox status in PM muscle and isolated mitochondria, energy metabolites, mitochondrial electron transport chain complexes activities, as well as mitochondrial function-modulating genes expression. Compared with CON, the extent of lipid peroxidation as well as protein oxidation were significantly increased in both PM muscles and mitochondria in T-PSE (P < 0.05), whereas not in T-NOR. Higher activities of glutathione peroxidase, total superoxide dismutase, and Cu–Zn superoxide dismutase were observed in PM muscle of T-NOR broilers when compared with CON (P < 0.05). Preslaughter transport increased the generation of reactive oxygen species, as well as enhanced antioxidant capacity in PM mitochondria of broilers (P < 0.05). Compared with CON, the ATP content, activities of complexes I and III, as well as relative mitochondrial membrane potential and swelling were significantly decreased in T-PSE (P < 0.05), whereas no significant changes in either ATP content or complex I activity were observed in T-NOR. Preslaughter transport enhanced the mRNA expression of regulators involved in the glutathione system, thioredoxin 2 system, and mitochondrial biosynthesis in PM muscle of broilers (P < 0.05). Moreover, we noticed a more evident enhancement effect in T-NOR than in T-PSE (P < 0.05). Overall, this work indicates that acute stress-induced redox imbalance and mitochondrial dysfunction have significant implications for the development of PSE-like meat.
Keywords: acute stress, broiler chicken, meat quality, mitochondrial function, redox status
1. Acute stress causes redox imbalance and mitochondrial dysfunction in broiler muscle.
2. This study provides new aspects for understanding how acute stress affects broiler meat quality.
Introduction
Chicken meat is popular among global consumers because of its comprehensive nutritional value and its diversified processing characteristics. Therefore, the market demand for broiler chicken has been increasing over the past several decades. This growing demand has led to great advances in genetic selection and more intensive feeding in order to produce fast-growing broilers. However, the modern commercial broilers exhibit greater sensitivities to various stressors, resulting in the decline of meat quality and the high incidence of meat abnormalities such as pale, soft, and exudative (PSE)-like meat, white striping, wooden breast, as well as spaghetti meat (Petracci et al., 2015; Zampiga et al., 2018). The recent meat defects of white striping, wooden breast, and spaghetti meat are major concerns to poultry producers and retailers because of their high incidence, impaired appearance, and reduced nutritional quality (Petracci et al., 2019). Although PSE-like meat has little impact on the approximate composition, whereas it seriously compromises the functional and technological properties, thus decreasing the product yield and causing economic losses (Dong et al., 2020a, b). Therefore, PSE-like myopathy still remains a problem in the poultry processing industry.
Stressors involved in preslaughter handling procedures have been reported to induce changes in the behavioral and physiological status of broilers. A series of preslaughter stressors can and lead to potential changes in energy metabolism, redox status, as well as proteolytic system in skeletal muscle of broilers, thereby affecting meat quality (Scheffler and Gerrard, 2007; Xing et al., 2019). Preslaughter acute stress has been confirmed to accelerate the rate of glycolytic metabolism, and lead to a large accumulation of lactic acid and a rapid decline in muscle pH, which further induces the denaturation of sarcoplasmic and myofibrillar protein, causing a pale appearance and a decrease in water holding capacity of chicken pectoralis major (PM) muscle (Xing et al., 2016). Moreover, multifactor pre-slaughter stress can also lead to oxidative stress and induce the deterioration of meat quality. Wang et al. (2009) indicated that acute heat stress (41 °C) reduced the oxidative stability of broiler PM muscle protein, which had a negative impact on protein functionalities and texture properties of meat gels. Exposure to transport under high environmental temperature can lead to an overproduction of reactive oxygen species (ROS), and a higher extent of lipid peroxidation as well as protein oxidation in PM muscle of broilers, which was closely associated with the development of PSE-like meat (Xing et al., 2017a, b).
As an important organelle in eukaryotic cells, mitochondria provide the overall cellular energy production through oxidative phosphorylation and play a key role in maintaining metabolic homeostasis, in stabilizing signal transduction, as well as in regulating programmed cell death (Vassilios et al., 2014). Meanwhile, as the main site of oxygen metabolism, mitochondria increase the production of superoxide anion, which contributes to oxidative stress (Kowaltowski et al., 2009; Wang et al., 2009). Mitochondria are not only a major source of intracellular ROS but may also suffer from oxidative damage (Tomanek, 2015). Severe acute stress has been reported to induce mitochondrial dysfunction in mammalian skeletal muscle. For example, proteins of mouse skeletal muscle mitochondria are oxidatively damaged, and mitochondrial electron transport chain (ETC) complexes are destroyed under acute severe hypobaric and hypoxic conditions (Magalhães et al., 2005). Similarly, acute heat stress has been indicated to induce overproduction of superoxide anion radicals and hydrogen peroxide in broiler skeletal muscle mitochondria, resulting in oxidative damage of mitochondrial proteins and lipids, which triggers an increase in mitochondrial oxygen consumption rate and a decreased membrane potential (Mujahid et al., 2006; Ahmad et al., 2007). The accumulation of ROS induced by oxidative stress impairs cell membrane and mitochondrial integrity through lipid peroxidation, which greatly increases the risk of oxidation during postmortem ageing and meat processing (Estévez, 2015). To date, studies concerning the effects of preslaughter acute stress on mitochondrial function in the skeletal muscle of broilers and their changes on the development of meat quality are limited.
Therefore, the purpose of this study was to investigate the effects of preslaughter transport under high ambient temperature on the redox status and mitochondrial function in PM muscle of broiler chickens, as well as to explore the changes of key factors regulating mitochondrial function to promote an understanding of how acute stress affects meat quality.
Materials and Methods
All experimental procedures and bird managements were approved by Nanjing Agricultural University Institutional Animal Care and Use Committee under protocol number SYXK 2021-0014.
Experimental broilers and sample collection
A total of 168 broiler chickens (42-d-old, Ross 308) with similar body weight (2.73 kg) were obtained from a commercial farm and randomly divided into control (CON) group and transport (T) group. A broiler was an experimental unit. Each treatment consisted of 84 broilers, and they were put in 12 crates with 7 broilers each. The size of the crates was 760 mm × 480 mm × 380 mm. Broilers in the CON group were placed in crates and kept under a normal rearing condition (ambient temperature was 24.6 °C and humidity was 63.7%) for 0.5 h. Broilers in the T group were labeled and randomly distributed at the rear of a truck and subjected to preslaughter transport for 0.5 h. The average speed of the truck was 45 km/h during transport. The ambient temperature was 31.5 °C, and the temperature and humidity inside the crates was 37.5 °C and 75.7%, respectively. After the transport trail, both the CON and T broilers were electrically stunned (40 V: alternating current, 400 Hz for 5 s each) and slaughtered via exsanguination. Immediately after slaughtering, the whole PM muscle separated within 15 min postmortem, the left PM muscle (~20 g) of each sample was taken, minced, frozen in liquid nitrogen quickly, and stored at −80 °C for further use. The remaining PM muscles were stored at 4 °C for meat quality assessment. At 24 h postmortem, meat quality attributes including meat color, muscle pH, water holding capacity, and shear force value of all muscles were measured. PM muscles were classified into normal (46 < L*24h < 53, 5.7 < pH24h < 6.1) and PSE-like (L*24h ≥ 53, pH24h ≤ 5.7) based on the criteria of Dadgar et al. (2010) and Dong et al. (2020a, b). A total of 34 muscles were found to be PSE-like in the T group, whereas none was found in the CON group. Subsequently, the muscle samples in the T group were categorized as normal (T-NOR) and PSE-like (T-PSE). Finally, PM muscle samples were obtained from 8 broilers randomly selected from each treatment for the subsequent biochemical assessments.
Meat quality measurements
Muscle pH was measured at 45 min (pH45min) and 24 h (pH24h) postmortem by inserting a probe electrode into the cranial part of the PM muscle using a FiveGo pH Meter F2 (Mettler-Toledo AG, Analytical, Shanghai, China). Meat color including lightness (L*), redness (a*), and yellowness (b*) was assessed at the cranial section of the dorsal surface at 24 h postmortem using a CR410 chroma meter (Konica Minolta Sensing Inc., Osaka, Japan). Muscle pH and meat color of each sample were measured in triplicate, the average value was taken as the result.
The drip loss was measured as previously described (Xing et al., 2017a, b). Briefly, 2 pieces of PM meat were taken and weighted. After suspending on a hook at 4 °C for 24 h, the samples were reweighted to calculate drip loss percentage. Duplicate meat samples from the same PM were taken at 24 h postmortem for cooking loss and shear force value according to Xing et al. (2021a, b) with slight modifications. The cooked samples were further used for shear force value measurement after the cooking loss test. Duplicate strips were cut along the direction of muscle fibers and sheared perpendicularly using a Warner–Bratzler meat shear machine (C-LM3B; Northeast Agricultural University, Harbin, China).
Texture profile analyses
The textural properties were determined using a texture analyzer (TMS-Pro, FTC, Sterling, VA) according to Gurikar et al. (2014) with a modification. A cylinder with a diameter of 20 mm and a height of 20 mm perpendicular to the direction of muscle fiber was trimmed. The test settings of the texture analyzer were as follows: probe model P/50, pretest speed 2.0 mm/s, test speed 1.0 mm/s, post-test speed 5.0 mm/s, compression ratio 50%, time between double compression cycle test 5 s, and trigger force 5 g.
Mitochondrial isolation
Mitochondria were isolated using a commercial kit (Beyotime Biotechnology, Shanghai, China). According to the manufacturer’s instructions, fresh PM muscle was rinsed using phosphate-buffered saline (PBS) and minced into mash. Muscle sample was placed in pre-cooled PBS and centrifuged at 600× g for 15 s at 4 °C, the supernatant was then discarded. Mitochondrial separation reagent was added to the pellet and then homogenized, the obtained homogenate was centrifuged at 600× g for 5 min at 4 °C. The supernatant was collected, and centrifuged again at 11,000× g for 10 min at 4 °C. Then, the supernatant was carefully removed, and the precipitate was the isolated mitochondria. Subsequently, the mitochondrial lysate was added to the precipitate, and a suspension was formed after repeated beating. Protein concentration was determined with the BCA Protein Assay kit (Thermo, RD). Mitochondrial suspension was stored at −80 °C for further use.
Determination of ROS
The ROS levels in PM muscle and isolated mitochondria were measured using a fluorescent probe, 2,7-dichlorofluorescein diacetate (DCFH-DA; Nanjing Jiancheng Bioengineering Institute, Nanjing, China) as previously described (Xing et al., 2017a, b). The results were all presented as the relative level to the control group.
Analysis of redox status
PM muscles were homogenized in chilled saline buffer (0.75%) and centrifuged at 2500× g for 10 min at 4 °C. The supernatant was collected for the determination of parameters related to redox status. The obtained mitochondria suspension was also subjected to redox status assessments. The contents of malondialdehyde (MDA), protein carbonyl and glutathione (GSH), as well as the activities of glutathione peroxidase (GSH-Px), total superoxide dismutase (SOD), copper zinc superoxide dismutase (Cu-ZnSOD), manganese superoxide dismutase (MnSOD) and glutathione reductase (GR) were determined using the corresponding commercial kits obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Determination of energy metabolites
The contents of ATP, ADP, and AMP were determined by high-performance liquid chromatography (HPLC) as previously described with slight modifications (Xing et al., 2015). About 0.3 g of frozen PM muscle sample was homogenized in 1.5 mL of chilled 7% HCLO4 solution for 1 min and placed at 4 °C for 15 min for extraction. Then, the homogenate was centrifuged at 15,000× g at 4 °C for 10 min. The supernatant was collected, neutralized with 1 M KOH, and then centrifuged again at 15,000× g for 10 min. The collected supernatant was filtered using a 0.45-μm filter before HPLC test (Waters-2695 Alliance). Mobile phase A was chromatographically pure methanol. Mobile phase B was sodium dihydrogen phosphate buffer. The proportions of mobile phases A and B were 13.5% and 86.5%. The test settings of the HPLC were as follows: sample loading 10 µL, column temperature 30 °C, flow rate 1 mL/min, UV detection wavelength 245 nm, and detection time 20 min. Results were calculated according to an established standard curve. The standard samples of 5ʹ-ATP disodium salt, 5ʹ-ADP sodium salt and 5ʹ-AMP sodium salt were purchased from Sigma-Aldrich Company (Sigma-Aldrich Co., St. Louis, MO).
Mitochondrial function assay
Mitochondrial ETC complexes I and III activities were assessed using the corresponding commercial kits (Cat. No. D799471-0500 and D799479-0100, Sangon Biotech Co. Ltd., Shanghai, China) per the manufacturer’s instructions.
Mitochondria membrane potential (Δψm) was determined using a JC-1 kit (Beyotime Biotechnology, Shanghai, China). Briefly, mitochondrial suspension (20 µL) was mixed with 180 µL of JC-1 dyeing working solution for 10 min, and the fluorescence intensity was detected using a microplate reader (SPARK; Tecan Austria GmbH Unterabergstr, 1A, Austria) at an excitation wavelength of 485 nm and at an emission wavelength of 620 nm, respectively. The mitochondrial swelling assay was performed based on the method of Xing et al. (2021a, b).
RNA purification and real-time quantitative PCR analysis
Total mRNA was isolated from PM muscles (about 70 mg) using RNAiso plus reagent. The mRNA was reversely transcribed into cDNA using a cDNA synthesis commercial kit (Takara Biotechnology Co. Ltd., Dalian, China) according to the manufacturer’s instructions. Quantitative real-time PCR was performed with SYBR Premix Ex Taq kit (Takara Biotechnology Co. Ltd., Dalian, China) on an ABI PRISM 7500 (Applied Biosystems, Foster City, CA) as previously described (Chen et al., 2022). The primer sequences used for qPCR are exhibited in Table 1. The relative mRNA expression was calculated by the method of 2−∆∆Ct with β-actin as the reference gene.
Table 1.
Primer sequences for gene expression in pectoralis major muscle analyzed by real-time quantitative PCR
| Genes | Prime sequences | Product lengths | Genbank numbers |
|---|---|---|---|
| GCLc | F: TGCGGTTCTGCACAAAATGG | 272 | XM_419910.3 |
| R: TGCTGTGCGATGAATTCCCT | |||
| GCLm | F: CCAGAACGTCAAAGCACACG | 187 | NM_001007953.1 |
| R: TCCTCCCATCCCCCAGAAAT | |||
| GPx | F: AAGTGCTGCTGGTGGTCAACG | 155 | NM_001277853.2 |
| R: GTTGGTGGCGTTCTCCTGGTG | |||
| Trx2 | F: AGTACGAGGTGTCAGCAGTG | 141 | NM_001031410.1 |
| R: CACACGTTGTGAGCAGGAAG | |||
| Trx R2 | F: CCGGGTCCCTGACATCAAA R: TAGCTTCGCTGGCATCAACA |
94 | NM_001122691.1 |
| Prx3 | F: ACCTCGTGCTCTTCTTCTACC R: ACCACCTCGCAGTTCACATC |
110 | XM_004942320.1 |
| MnSOD | F: AGGAGGGGAGCCTAAAGGAGA | 214 | NM_204211.1 |
| R: CCAGCAATGGAATGAGACCTG | |||
| PGC-1α | F: GATTCTTCACCTGGGTGGCA | 142 | NM_001006457.1 |
| R: TCAGCCCGAATTTCCTGGTC | |||
| Nrf1 | F: TCGCTTCCGTTTCTTACCCG | 274 | NM_001030646.1 |
| R: TAAGCTACCATCAGAGGC | |||
| Nrf2 | F: GAGCCCATGGCCTTTCCTAT | 212 | NM_001007858.1 |
| R: CACAGAGGCCCTGACTCAAA | |||
| Tfam | F: GTGAAAGCCTGGCGAAACTG | 228 | NM_204100.1 |
| R: CACAGCTCAGGTTACACCGT | |||
| HO-1 | F: ACGTCGTTGGCAAGAAGCATCC R: TTGAACTTGGTGGCGTTGGAGAC |
181 | NM_205344.1 |
| CAT | F: CACGTATTCAGGCACTGCTGGAC | 86 | NM_001031215.2 |
| R:ACGAGAAGTGGCTTGCGTGTATG | |||
| CuZnSOD | F: CCGGCTTGTCTGATGGAGAT | 124 | NM_205064.1 |
| R: TGCATCTTTTGGTCCACCGT | |||
| avUCP | F: ACAACGTCCCCTGTCACTTC | 231 | AB088685.1 |
| R: ATGAACATCACCACGTTCCA | |||
| β-actin | F: ATCCGGACCCTCCATTGTC | 120 | NM_205518.1 |
| R: AGCCATGCCAATCTCGTCTT |
GCLc, glutamate-cysteine ligase catalytic; GCLm, glutamate-cysteine ligase modifier subunit gene; GPx, glutathione peroxidase; Trx2, thioredoxin 2; Trx R2, thioredoxin reductase 2; Prx3, peroxiredoxin 3; MnSOD, manganese superoxide dismutase; PGC-1α, peroxisome proliferator-activated receptor coactivator 1α; Nrf1, nuclear transcription factor-erythroid 2-related factor 1; Nrf2, nuclear transcription factor-erythroid 2-related factor 2; Tfam, mitochondrial transcription factor A; HO-1, heme oxygenase-1; CAT, catalase; Cu-ZnSOD, copper-zinc superoxide dismutase; avUCP, avian uncoupling protein; β-actin, avian β-actin.
F, forward primer; R, reverse primer.
Statistical analysis
For all variables, broiler was the experiment unit. PM muscle samples were obtained from three treatments (CON, T-PSE, and T-NOR) and each treatment included 8 replicates. Data were analyzed by one-way ANOVA and Duncan’s multiple range tests using the IBM SPSS Statistics 20.0 software (SPSS Inc., Chicago, IL, USA). Data were reported as means ± SE. Significance was considered when P ≤ 0.05.
Results
Meat quality and texture properties
As exhibited in Table 2, preslaughter transport significantly decreased pH45min, pH24h, shear force value and hardness, as well as increased L*, drip loss, and cooking loss of PM muscle of broilers (P < 0.05). In addition, the PM muscle of the T-PSE group showed lesser pH, shear force value, and hardness compared with the T-NOR group (P < 0.05). Meanwhile, meat quality traits of L*, drip loss, and cooking loss in the T-PSE group were significantly greater than those in the T-NOR group (P < 0.05).
Table 2.
Meat quality traits and texture properties of pectoralis major muscles of broilers from control (CON) and transport treatment (T-PSE, T-NOR) used in this study
| Items | Treatments1 | SEM | P-value | ||
|---|---|---|---|---|---|
| CON | T-PSE | T-NOR | |||
| pH45min | 6.53a | 6.25c | 6.37b | 0.03 | <0.001 |
| pH24h | 5.86a | 5.66c | 5.78b | 0.02 | <0.001 |
| L*, lightness | 50.6c | 54.8a | 52.3b | 0.41 | <0.001 |
| a*, redness | 2.75a | 1.88b | 2.32ab | 0.11 | 0.002 |
| b*, yellowness | 2.49 | 3.02 | 2.60 | 0.18 | 0.480 |
| Drip loss, % | 2.85c | 5.07a | 3.75b | 0.21 | <0.001 |
| Cooking loss, % | 11.6c | 16.7a | 14.1b | 0.53 | <0.001 |
| Shear force, N | 32.5a | 23.4c | 27.1b | 0.91 | <0.001 |
| Hardness, N | 32.4a | 21.8c | 25.0b | 0.97 | <0.001 |
| Springiness, mm | 2.65b | 3.22a | 3.06ab | 0.10 | 0.04 |
| Cohesiveness, % | 0.47 | 0.43 | 0.46 | 0.01 | 0.274 |
| Gumminess, N | 16.2 | 13.3 | 14.9 | 0.73 | 0.294 |
| Chewiness, MJ | 42.1 | 36.8 | 37.8 | 1.85 | 0.474 |
The results are presented by mean values and SEM (n = 8). CON, meat samples in the control treatment; T-PSE, PSE-like meat samples in the transport treatment; T-NOR, normal meat samples in the transport treatment.
Means within a row with different superscript lowercase letters differ significantly (P < 0.05).
Redox status in PM muscle
The ROS level, MDA, and carbonyl contents in the T-PSE group were greater than those in the CON group (P < 0.05, Table 3). Nevertheless, there were no significant differences in ROS level, MDA, and carbonyl contents between the CON and T-NOR groups. In addition, we observed that preslaughter transport induced increases in GSH content and GR activity in PM muscle of broilers (P < 0.05). Meanwhile, the activities of GSH-Px, T-SOD, MnSOD, and GR in the T-NOR group were significantly higher than those in the CON group (P < 0.05).
Table 3.
Redox status in pectoralis major muscles of broilers from control (CON) and transport treatment (T-PSE, T-NOR)
| Items | Treatments1 | SEM | P-value | ||
|---|---|---|---|---|---|
| CON | T-PSE | T-NOR | |||
| Relative ROS level | 1.00b | 1.10a | 1.03ba | 0.03 | 0.038 |
| MDA, nmol/mg protein | 0.12b | 0.26a | 0.20ba | 0.02 | 0.004 |
| Carbonyls, nmol/mg protein | 0.41b | 0.63a | 0.50ba | 0.05 | 0.028 |
| GSH, μmol/g protein | 9.07b | 11.6a | 11.4a | 0.43 | 0.019 |
| GSH-Px, U/mg protein | 3.31b | 3.66ba | 4.73a | 0.37 | 0.043 |
| T-SOD, U/mg protein | 16.3b | 18.0ba | 20.4a | 0.71 | 0.047 |
| Cu-ZnSOD, U/mg protein | 13.9b | 14.8ba | 15.9a | 0.34 | 0.039 |
| MnSOD, U/mg protein | 4.45 | 4.77 | 4.38 | 0.68 | 0.950 |
| GR, U/g protein | 2.42c | 3.71b | 4.95a | 0.30 | <0.001 |
The results are presented by mean values and SEM (n = 8). CON, meat samples in the control treatment; T-PSE, PSE-like meat samples in the transport treatment; T-NOR, normal meat samples in the transport treatment.
Means within a row with different superscript lowercase letters differ significantly (P < 0.05).
ROS, reactive oxygen species; MDA, malondialdehyde; GSH, glutathione; GSH-Px, glutathione peroxidase; T-SOD, total superoxide dismutase; Cu-ZnSOD, Copper-Zinc superoxide dismutase; MnSOD, Manganese superoxide dismutase; GR, glutathione reductase.
Energy metabolites in PM muscle
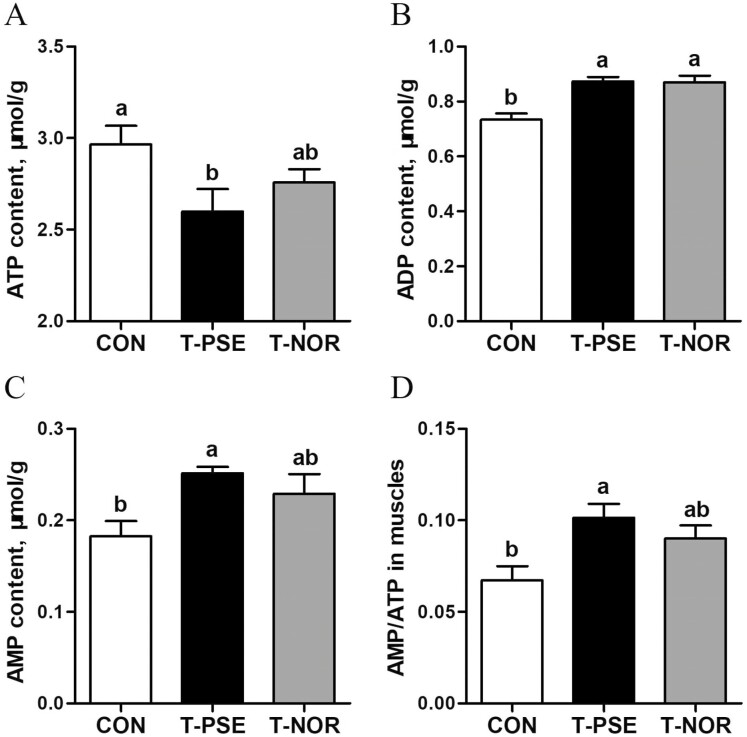
The ATP content in PM muscle of the T-PSE broilers was lower than that of the CON broilers (P < 0.05), whereas the contents of ADP, AMP, and the ratio of AMP to ATP were elevated (P < 0.05; Fig. 1). No significant differences in ATP content, AMP content, and AMP/ATP were observed between the CON and T-NOR groups (P > 0.05).
Figure 1.
Energy status in pectoralis major muscle of broilers from control (CON) and transport group (T-PSE, T-NOR). (A) ATP content (B) ADP content (B) AMP content (D) AMP/ATP ratio. Data are expressed as the mean ± SE (n = 8), with different letters (a, b) indicating a significant difference (P < 0.05).
Redox status in mitochondria
As shown in Table 4, preslaughter transport induced increases in ROS level, GSH content, and the activities of GSH-Px, T-SOD, Cu-ZnSOD, MnSOD as well as GR in mitochondria of PM muscle of broilers (P < 0.05). Whereas the mitochondria of the T-PSE group exhibited a higher ROS level and a lower Cu-ZnSOD activity compared with the T-NOR group (P < 0.05). In addition, MDA and carbonyl contents of the mitochondria in the T-PSE group were significantly higher than those in the CON group (P < 0.05).
Table 4.
Redox status in mitochondria of pectoralis major muscles of broilers from control (CON) and transport treatment (T-PSE, T-NOR)
| Items | Treatments1 | SEM | P-value | ||
|---|---|---|---|---|---|
| CON | T-PSE | T-NOR | |||
| Relative ROS level | 1.00c | 1.54a | 1.25b | 0.08 | <0.001 |
| MDA, nmol/mg protein | 0.30b | 0.49a | 0.29b | 0.04 | 0.007 |
| Carbonyls, nmol/mg protein | 25.7b | 37.5a | 26.5b | 2.19 | 0.037 |
| GSH, μmol/g protein | 7.7b | 9.3a | 10.5a | 0.39 | 0.003 |
| GSH-Px, U/mg protein | 26.6b | 42.2a | 39.3a | 2.61 | 0.024 |
| T-SOD, U/mg protein | 13.7b | 25.7a | 28.1a | 2.34 | 0.013 |
| CuZnSOD, U/mg protein | 7.3c | 12.7b | 16.4a | 1.13 | <0.001 |
| MnSOD, U/mg protein | 9.6b | 13.9a | 12.5a | 0.68 | 0.018 |
| GR, U/g protein | 13.3b | 18.4a | 19.4a | 1.09 | 0.034 |
The results are presented by mean values and SEM (n = 8). CON, meat samples in the control treatment; T-PSE, PSE-like meat samples in the transport treatment; T-NOR, normal meat samples in the transport treatment.
Means within a row with different superscript lowercase letters differ significantly (P < 0.05).
ROS, reactive oxygen species; MDA, malondialdehyde; GSH, glutathione; GSH-Px, glutathione peroxidase; T-SOD, total superoxide dismutase; Cu-ZnSOD, copper-zinc superoxide dismutase; MnSOD, manganese superoxide dismutase; GR, glutathione reductase.
Mitochondrial function
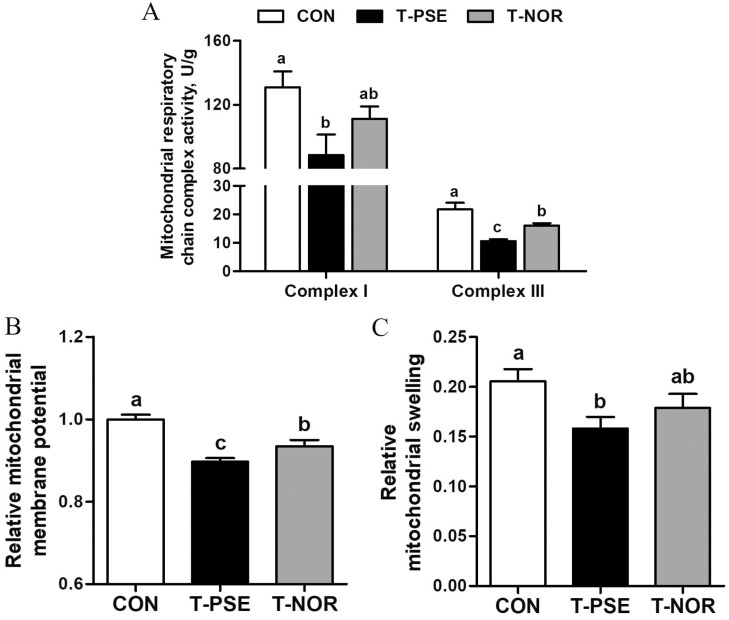
Compared with the CON group, the activities of mitochondrial respiratory chain complexes I and III, as well as the relative mitochondrial membrane potential and mitochondrial swelling were significantly decreased in the T-PSE group (P < 0.05; Fig. 2). No significant differences in mitochondrial respiratory chain complex I activity and relative mitochondrial swelling were observed between the CON and T-NOR groups (P > 0.05). Nevertheless, a higher activity of mitochondrial respiratory chain complex III and a higher relative mitochondrial membrane potential were observed in the T-NOR group when compared with the T-PSE group (P < 0.05).
Figure 2.
Mitochondrial function of pectoralis major muscles of broilers from control (CON) and transport group (T-PSE, T-NOR). (A) Activities of mitochondrial respiratory chain complexes I and III. (B) Relative mitochondrial membrane potential. (C) Relative mitochondrial swelling. Data are expressed as the mean ± SE (n = 8), with different letters (a, b, c) indicating a significant difference (P < 0.05).
mRNA expression of genes related to the regulation of mitochondrial function
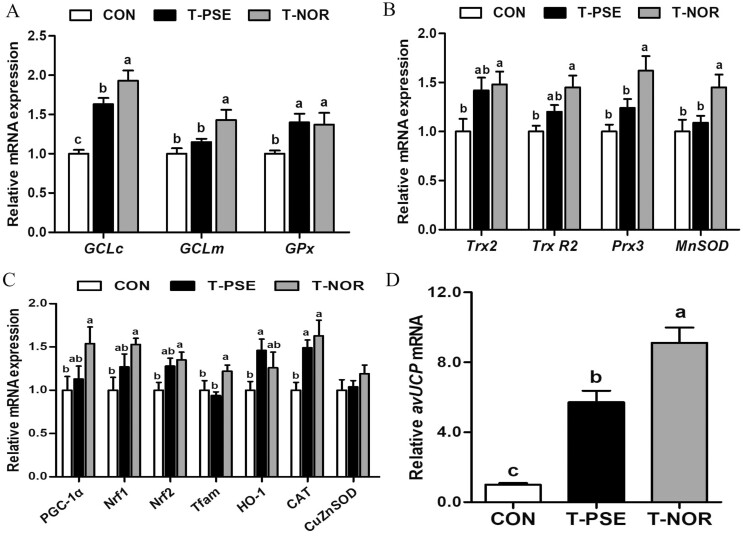
Preslaughter transport enhanced the mRNA expressions of several regulators involved in the glutathione system, thioredoxin 2 system, and mitochondrial biosynthesis in the PM muscle of broilers (P < 0.05; Fig. 3). In addition, the mRNA expressions of GCLc, GPx, CAT, and avUCP in the T-PSE group were lower compared with the T-NOR group (P < 0.05). No significant differences in the mRNA expressions of Trx2, Trx R2, PGC-1α, Nrf1, and Nrf2 were observed between the CON and T-PSE groups (P > 0.05).
Figure 3.
Relative mRNA expression of genes related to mitochondrial function regulation in pectoralis major muscles of broilers from control (CON) and transport group (T-PSE, T-NOR). (A) Relative mRNA expression of glutamate-cysteine ligase catalytic (GCLc), glutamate-cysteine ligase modifier subunit gene (GCLm), glutathione peroxidase (GPx). (B) Relative mRNA expression of thioredoxin 2 (Trx2), thioredoxin reductase 2 (Trx R2), peroxiredoxin 3 (Prx3), manganese superoxide dismutase (MnSOD). (C) Relative mRNA expression of peroxisome proliferator-activated receptor coactivator 1α (PGC-1α), nuclear transcription factor-erythroid 2-related factor 1 (Nrf1), nuclear transcription factor-erythroid 2-related factor 2 (Nrf2), mitochondrial transcription factor A (Tfam), heme alase (CAT), copper-zinc superoxide dismutase (Cu-ZnSOD). (D) Relative mRNA expression of avian uncoupling protein (avUCP). Data are expressed as the mean ± SE (n = 8), with different letters (a, b, c) indicating a significant difference (P < 0.05).
Discussion
Broiler chickens are inevitably subjected to preslaughter handling stress from farm to abattoir. A large number of studies have confirmed that preslaughter stress may compromise welfare and meat quality of broilers (Xing et al., 2019). Preslaughter transport involves a variety of potential factors including mixed group, feed and water deprivation, environmental change, as well as bumpy road condition, which can reduce meat quality and increase the incidence of PSE-like meat in broiler chickens (Xing et al., 2019; Damtew et al, 2018). For example, Jiang et al. (2016) observed a higher incidence of PSE-like chicken meat in broilers subjected to preslaughter transport when the temperature exceeded than 35 °C during summer. Preslaughter stimuli such as temperature fluctuation, food/water deprivation, and handling procedures can before slaughter drives the mobilization of glycogen in skeletal muscle of animals to counteract energy deficiency, thereby accelerating the glycolytic metabolism and resulting in the accumulation of lactic acid, which further decreases postmortem muscle pH (Kim et al., 2014). The current study showed that acute stress-induced PSE-like muscle exhibited a lower ATP content, and a higher AMP content as well as AMP/ATP ratio in PM muscle of broilers. According to Zhang et al. (2017), preslaughter transport accelerated the depletion of energy and promoted the activation of AMP-activated protein kinase, which plays a critical role in mediating postmortem glycolysis and the development of meat quality. Therefore, the impaired meat quality and a high PSE-like meat occurrence of broilers subjected to transport under high environmental temperature were closely associated with the insufficient energy status.
When the homeostasis of pro-oxidants and endogenous antioxidants in organisms is disrupted, excessive ROS can be generated, which leads to oxidative stress (Sies et al., 2017). Chicken muscle is highly susceptible to oxidative stress because of its high contents of unsaturated fatty acids, pigments, and some oxidoreductases (Falowo et al., 2014). Peroxidation of lipids in muscle can lead to poor appearance and impaired hydraulic power, thereby inducing the deterioration of meat color and water holding capacity (Soladoye et al., 2015). Meanwhile, oxidative modification can lead to the denaturation of vital muscle proteins, resulting in changes in protein solubility, which negatively affects meat quality (Zhang et al., 2013). The contents of MDA and protein carbonyl are representative indicators reflecting the degree of lipid peroxidation and protein oxidation, respectively (Falowo et al., 2014). In this study, acute stress induced by preslaughter transport increased ROS levels, as well as MDA and carbonyl contents in PSE-like muscles, suggesting that acute stress-induced oxidative damage of PM muscle of broilers might contribute to the development of PSE-like meat. In addition, we assessed the effects of acute stress on the non-enzymatic (GSH) and enzymatic (GSH-Px, SODs, sand GR) systems to reflect the redox status in PM muscle. The synthesis of intracellular GSH increases upon oxidative stress, which neutralizes ROS and peroxide catalyzed by GSH-Px and transforms itself into GSSH under the catalysis of GR, GSSH can be further restored to GSH (Tomanek, 2015). In addition, T-SOD including Cu-ZnSOD and MnSOD also has the ability to neutralize peroxide and to ameliorate oxidative stress (Zhang et al., 2021). We found that acute stress increased the content of GSH and the activities of GSH-Px, T-SOD, Cu-ZnSOD, and GR in PM muscle of broilers. Similarly, Wang et al. (2015) indicated that preslaughter transport activated antioxidant enzyme defensive system by increasing the activities of T-SOD and GSH-Px in skeletal muscle of broilers. Moreover, the PSE-like muscles exhibited lower antioxidant activity than the normal muscles of broilers subjected to preslaughter transport, indicating the insufficient activation of antioxidant enzymatic system to counteract intracellular free radicals could not alleviate oxidative damage to the PM muscle.
Mitochondria are an important source of ROS and represent an indispensable intracellular redox buffering system (Tang et al., 2014). As could be expected, transport stress induced the overproduction of mitochondrial ROS in PM muscle of broilers. In addition, the contents of MDA and protein carbonyl in mitochondria of PSE-like muscle were increased, whereas not in mitochondria of normal muscle. Numerous studies have evidenced that excessive ROS not only causes oxidative damage to mitochondria but also inhibits mitochondrial antioxidant defense system. Galactosamine/lipopolysaccharide-induced oxidative damage in mice liver stimulated the generation of ROS, which not only caused the oxidative damage and destroyed the structure of mitochondria, but also inhibited the antioxidant defense system of mitochondria by decreasing the activities of GSH-Px, GR, and MnSOD (Zhang et al., 2014). Chronic heat stress-induced oxidative stress led to a massive consumption of GSH, as well as reduction in the activities of GSH-Px and MnSOD in mitochondria of broiler PM muscle (Zhang et al., 2015). However, reports concerning the effects of acute stress on the changes in mitochondrial antioxidant enzymatic activities in broilers are limited. Herein, the content of mitochondrial GSH in PM muscle of broilers was increased upon acute stress. As GSH-Px scavenges hydrogen peroxide by consuming GSH (Galluzzi et al., 2012), its activity was also found to be higher in mitochondria of PM muscle of transported broilers. Manganese superoxide dismutase is one of the important members of the mitochondrial antioxidant defense system and is specifically distributed in the mitochondrial matrix (Shen et al., 2006). Despite the unchanged activity of MnSOD in PM muscle, an increased MnSOD activity in mitochondria of PM muscle of broilers under acute stress was indeed observed. We noted that although acute stress activated mitochondrial antioxidant defense system, the biological oxidation of muscle mitochondria and the disruption of the mitochondrial redox homeostasis still occurred, which might exacerbate the oxidative stress in muscle.
As the energy factory of the cell, mitochondria provide most of the cellular energy source through oxidative phosphorylation (Vassilios et al., 2014). Mitochondrial oxidative phosphorylation is formed through the ETC on the inner mitochondrial membrane. During the formation of the ETC, some electrons react with oxygen prematurely at the respiratory chain complexes I and III to form superoxide and oxygen radicals (Tang et al., 2014). Attack of mitochondria by these free radicals can trigger abnormal opening of permeability transition pores in the membrane, thereby reducing equivalents within the mitochondria, and neutralizing the mitochondrial membrane potential, as well as inducing mitochondrial swelling (Azzolin et al., 2010). Herein, we took the activities of mitochondrial respiratory chain complexes I and III, mitochondrial membrane potential and mitochondrial swelling as the basic indicators to evaluate the changes of mitochondrial function. Studies have shown that the massive production of mitochondrial ROS can induce mitochondrial dysfunction, resulting in changes in the number and morphology of mitochondria, ETC damage, decline of transmembrane potential, reduction of ATP synthesis and mitochondrial DNA oxidative damage, which leads to the dysregulation of intracellular energy metabolism (Boengler et al., 2017). Meanwhile, when ETC is inhibited, it further induces mitochondrial ROS production and aggravates oxidative stress (Indo et al., 2007). We found that acute stress reduced the activities of mitochondrial respiratory chain complexes I and III, and decreased the relative membrane potential and swelling in PSE-like muscle. Together with the enhanced oxidation of mitochondria in PSE-like muscle, preslaughter transport obviously led to the impairment of mitochondrial function in PM muscle of broilers.
Mitochondrial function changes are synthetically regulated by multiple regulatory pathways. The mitochondrial antioxidant defense system is mainly composed of glutathione (GSH) system and thioredoxin 2 (Trx2) system. The GSH system includes reduced glutathione GSH, GR, and GPx. The Trx system includes Trx, thioredoxin reductase (Trx-R; Koehler et al., 2006). The spontaneous synthesis of endogenous GSH is catalyzed by its rate-limiting enzyme GCL, which consists of two subunits of GCLc and GCLm (Zheng et al., 2007). Trx2 includes both oxidative and reduced forms. Reduced Trx2 can be self-oxidized to form oxidized Trx2 under the catalysis of Prx3, and Trx R2 can reduce oxidized Trx2 (Brown et al., 2008). We observed that the expression of MnSOD, and key factors involved in the GSH system and the Trx2 system were upregulated upon acute stress, indicating that broilers can protect the body from oxidative damage by enhancing the antioxidant defense system of mitochondria. Furthermore, appropriate expression of uncoupling protein on the inner mitochondrial membrane can trigger mitochondrial respiratory chain uncoupling, thereby reducing proton gradient and oxygen concentration, which further reduces ROS production (Mujahid et al., 2006). The activation of peroxisome proliferator-activated γ receptor coactivator-1α (PGC-1α) can promote the transcription of nuclear transcription factors (NRFs) and mitochondrial transcription factor A (Tfam), which contribute to the increase of mitochondrial DNA copy number, the enhancement of mitochondrial biosynthesis and the maintenance of normal metabolism (Manoli et al., 2007). Moreover, PGC-1α and Nrf2 can also improve their own antioxidant capacity by increasing the transcription of HO-1, CAT, and Cu-ZnSOD (Thimmulappa et al., 2002; Valle et al., 2005). Gak et al. (2015) indicated that mitochondrial biogenesis was increased in rat Leydig cells under acute stress as an adaptive mechanism, and this response amplified with the increase of stress severity. Similarly, our results indicated that preslaughter transport enhanced the mRNA expression of PGC-1α together with transcription factors, as well as other mitochondrial synthesis-related genes. Furthermore, the transcriptional levels of almost all these genes were found to be higher in normal muscle than PSE-like muscle, indicating the differences in mitochondrial adaptability of broilers in response to acute stress. Moreover, the insufficient activation of mitochondrial antioxidant defense system might compromise mitochondrial function and contribute to the development of PSE-like meat.
Conclusion
Preslaughter transport under high environmental temperature reduces energy status and induces oxidative stress in PM muscle of broilers, which could contribute to the deterioration of meat quality and the increased occurrence of PSE-like meat. The oxidative damage of mitochondria and the dysregulation of mitochondrial function contribute to the energy deficiency and redox imbalance. Although broilers physiologically respond to acute stress by improving antioxidant capacity, enhancing biosynthesis and respiratory chain decoupling to maintain mitochondrial function, these responses were insufficient to restore mitochondrial homeostasis and redox status when Ross 308 broilers were slaughtered immediately after the transportation stress treatment.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (32001623), the Fundamental Research Funds for the Central Universities (KJQN202128), the Natural Science Foundation of Jiangsu Province in China (BK20190516), the National Natural Science Foundation of China (32072780), the China Postdoctoral Science Foundation Grant (2019T120432), the Jiangsu Postdoctoral Science Foundation (2019K013), and the Earmarked Fund for Jiangsu Agricultural Industry Technology System (JATS[2021]459).
Glossary
Abbreviations
- ADP
adenosine diphosphate
- AMP
adenosine monophosphate
- ATP
adenosine triphosphate
- DCFH-DA
fluorescent probe, 2,7-dichlorofluorescein diacetate
- ETC
electron transport chain
- GR
glutathione reductase
- GSH
glutathione
- GSH-Px
glutathione peroxidase
- HPLC
high-performance liquid chromatography
- MDA
malondialdehyde
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- PM
pectoralis major
- PSE
pale, soft, and exudative
- qPCR
quantitative PCR
- ROS
reactive oxygen species
- T-SOD
total superoxide dismutase
Contributor Information
Hongju Liao, College of Animal Science and Technology; Key Laboratory of Animal Origin Food Production and Safety Guarantee of Jiangsu Province; Jiangsu Collaborative Innovation Center of Meat Production and Processing, Quality and Safety Control, Nanjing Agricultural University, Nanjing 210095, People’s Republic of China.
Lin Zhang, College of Animal Science and Technology; Key Laboratory of Animal Origin Food Production and Safety Guarantee of Jiangsu Province; Jiangsu Collaborative Innovation Center of Meat Production and Processing, Quality and Safety Control, Nanjing Agricultural University, Nanjing 210095, People’s Republic of China.
Jiaolong Li, College of Animal Science and Technology; Key Laboratory of Animal Origin Food Production and Safety Guarantee of Jiangsu Province; Jiangsu Collaborative Innovation Center of Meat Production and Processing, Quality and Safety Control, Nanjing Agricultural University, Nanjing 210095, People’s Republic of China; Institute of Agri-Products Processing, Jiangsu Academy of Agricultural Sciences, Nanjing 210014, People’s Republic of China.
Tong Xing, College of Animal Science and Technology; Key Laboratory of Animal Origin Food Production and Safety Guarantee of Jiangsu Province; Jiangsu Collaborative Innovation Center of Meat Production and Processing, Quality and Safety Control, Nanjing Agricultural University, Nanjing 210095, People’s Republic of China.
Feng Gao, College of Animal Science and Technology; Key Laboratory of Animal Origin Food Production and Safety Guarantee of Jiangsu Province; Jiangsu Collaborative Innovation Center of Meat Production and Processing, Quality and Safety Control, Nanjing Agricultural University, Nanjing 210095, People’s Republic of China.
Conflict of Interest Statement
The authors declare no real or perceived conflicts of interest.
Literature Cited
- Ahmad, M., Pumford N. R., Walter B., Kiyotaka N., Teruo M., Yukio A., and Masaaki T.. . 2007. Mitochondrial oxidative damage in chicken skeletal muscle induced by acute heat stress. J. Poult. Sci. 44:439–445. doi: 10.2141/jpsa.44.439. [DOI] [Google Scholar]
- Azzolin, L., von Stockum S., Basso E., Petronilli V., Forte M. A., and Bernardi P.. . 2010. The mitochondrial permeability transition from yeast to mammals. FEBS Lett. 584:2504–2509. doi: 10.1016/j.febslet.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boengler, K., Kosiol M., Mayr M., Schulz R., and Rohrbach S.. . 2017. Mitochondria and ageing: role in heart, skeletal muscle and adipose tissue. J. Cachexia Sarcopenia Muscle. 8:349–369. doi: 10.1002/jcsm.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, K. K., Eriksson S. E., Arnér E., and Hampton M. B.. . 2008. Mitochondrial peroxiredoxin 3 is rapidly oxidized in cells treated with isothiocyanates. Free Radic. Biol. Med. 45:494–502. doi: 10.1016/j.freeradbiomed.2008.04.030. [DOI] [PubMed] [Google Scholar]
- Chen, Z. D., Xing T., Li J. L., Zhang L., Jiang Y., and Gao F.. . 2022. Oxidative stress induced by hydrogen peroxide promotes glycolysis by activating CaMKK/LKB1/AMPK pathway in broiler breast muscle. Poult. Sci. 101:101681. doi: 10.1016/j.psj.2021.101681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadgar, S., Lee E. S., Leer T. L., Burlinguette N., Classen H. L., Crowe T. G., and Shand P. J.. . 2010. Effect of microclimate temperature during transportation of broiler chickens on quality of the pectoralis major muscle. Poult. Sci. 89:1033–1041. doi: 10.3382/ps.2009-00248. [DOI] [PubMed] [Google Scholar]
- Damtew, A., Erega Y., Ebrahim H., Tsegaye S., and Msigie D.. . 2018. The effect of long distance transportation stress on cattle: a review. Biomed. J. Sci. Tech. Res. 3:3304–3308. doi: 10.26717/BJSTR.2018.03.000908. [DOI] [Google Scholar]
- Dong, M., Chen H. Q., Zhang Y. M., Xu Y. J., Han M. Y., Xu X. L., and Zhou G. H.. . 2020a. Processing properties and improvement of pale, soft, and exudative-like chicken meat: a review. Food Bioprocess Technol. 13:1280–1291. doi: 10.1007/s11947-020-02464-3. [DOI] [Google Scholar]
- Dong, M., Xu Y. J., Zhang Y. M., Han M. Y., Wang P., Xu X. L., and Zhou G. H.. . 2020b. Physicochemical and structural properties of myofibrillar proteins isolated from pale, soft, exudative (PSE)-like chicken breast meat: Effects of pulsed electric field (PEF). Innov. Food Sci. Emerg. Technol. 59:102277. doi: 10.1016/j.ifset.2019.102277. [DOI] [Google Scholar]
- Estévez, M. 2015. Oxidative damage to poultry: from farm to fork. Poult. Sci. 94:1368–1378. doi: 10.3382/ps/pev094. [DOI] [PubMed] [Google Scholar]
- Falowo, A. B., Fayemi P. O., and Muchenje V.. . 2014. Natural antioxidants against lipid–protein oxidative deterioration in meat and meat products: a review. Food Res. Int. 64:171–181. doi: 10.1016/j.foodres.2014.06.022. [DOI] [PubMed] [Google Scholar]
- Gak, I. A., Radovic S. M., Dukic A. R., Janjic M. M., Tojkov-Mimic N. J. S., Kostic T. S., and Andric S. A.. . 2015. Stress triggers mitochondrial biogenesis to preserve steroidogenesis in Leydig cells. Biochim. Biophys. Acta 1853:2217–2227. doi: 10.1016/j.bbamcr.2015.05.030. [DOI] [PubMed] [Google Scholar]
- Galluzzi, L., Kepp O., and Kroemer G.. . 2012. Mitochondria: master regulators of danger signalling. Nat. Rev. Mol. Cell Biol. 13:780–788. doi: 10.1038/nrm3479. [DOI] [PubMed] [Google Scholar]
- Gurikar, A. M., Lakshmanan V., and Gadekar Y. P.. . 2014. Effect of meat chunk size, massaging time and cooking time on quality of restructured pork blocks. J. Food Sci. Technol. 51:1363–1369. doi: 10.1007/s13197-012-0644-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indo, H. P., Davidson M., Yen H. H., Suenaga S., Tomita K., Nishii T., Higuchi M., Koga Y., Ozawa T., and Majima H. J.. . 2007. Evidence of ROS generation by mitochondria in cells with impaired electron transport chain and mitochondrial DNA damage. Mitochondrion 7:106–118. doi: 10.1016/j.mito.2006.11.026. [DOI] [PubMed] [Google Scholar]
- Jiang, N. N., Wang P., Xing T., Han M. Y., and Xu X. L.. . 2016. An evaluation of the effect of water-misting sprays with forced ventilation on the occurrence of pale, soft, and exudative meat in transported broilers during summer: impact of the thermal microclimate. J. Anim. Sci. 94:2218–2227. doi: 10.2527/jas.2015-9823. [DOI] [PubMed] [Google Scholar]
- Kim, Y. H. B., Warner R. D., and Rosenvold K.. . 2014. Influence of high pre-rigor temperature and fast pH fall on muscle proteins and meat quality: a review. Anim. Prod. Sci. 54:375. doi: 10.1071/an13329. [DOI] [Google Scholar]
- Koehler, C. M., Beverly K. N., and Leverich E. P.. . 2006. Redox pathways of the mitochondrion. Antioxid. Redox Signal. 8:813–822. doi: 10.1089/ars.2006.8.813. [DOI] [PubMed] [Google Scholar]
- Kowaltowski, A. J., Souza-Pinto N., Castilho R. F., and Vercesi A. E.. . 2009. Mitochondria and reactive oxygen species. Free Radical Biol. Med. 47:333–343. doi: 10.1016/j.freeradbiomed.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Magalhães, J., Ascensão A., Soares J. M. C., Ferreira R., Neuparth M. J., Marques F., and Duarte J. A.. . 2005. Acute and severe hypobaric hypoxia increases oxidative stress and impairs mitochondrial function in mouse skeletal muscle. J. Appl. Physiol. 99:1247–1253. doi: 10.1152/japplphysiol.01324.2004. [DOI] [PubMed] [Google Scholar]
- Manoli, I., Alesci S., Blackman M. R., Su Y. A., Rennert O. W., and Chrousos G. P.. . 2007. Mitochondria as key components of the stress response. Trends Endocrinol. Metab. 18:190–198. doi: 10.1016/j.tem.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Mujahid, A., Sato K., Akiba Y., and Toyomizu M.. . 2006. Acute heat stress stimulates mitochondrial superoxide production in broiler skeletal muscle, possibly via downregulation of uncoupling protein content. Poult. Sci. 85:1259–1265. doi: 10.1093/ps/85.7.1259. [DOI] [PubMed] [Google Scholar]
- Petracci, M., Mudalal S., Sogua F., and Cavani C.. . 2015. Meat quality in fast-growing broiler chickens. World’s Poult. Sci. J. 71:363–374. doi: 10.1017/s0043933915000367. [DOI] [Google Scholar]
- Petracci, M., Soglia F., Madruga M., Carvalho L., Ida E., and Estévez M.. . 2019. Wooden-breast, white striping, and spaghetti meat: causes, consequences and consumer perception of emerging broiler meat abnormalities. Compr. Rev. Food Sci. Food Saf. 18:565–583. doi: 10.1111/1541-4337.12431. [DOI] [PubMed] [Google Scholar]
- Scheffler, T. L., and Gerrard D. E.. . 2007. Mechanisms controlling pork quality development: The biochemistry controlling postmortem energy metabolism. Meat Sci. 77:7–16. doi: 10.1016/j.meatsci.2007.04.024. [DOI] [PubMed] [Google Scholar]
- Shen, X., Zheng S., Metreveli N. S., and Epstein P. N.. . 2006. Protection of cardiac mitochondria by overexpression of MnSOD reduces diabetic cardiomyopathy. Diabetes 55:798–805. doi: 10.2337/diabetes.55.03.06.db05-1039. [DOI] [PubMed] [Google Scholar]
- Sies, H., Berndt C., and Jones D. P.. . 2017. Oxidative stress. Annu. Rev. Biochem. 86:715–748. doi: 10.1146/annurev-biochem-061516-045037. [DOI] [PubMed] [Google Scholar]
- Soladoye, O. P., Juárez M. L., Aalhus J. L., Shand P., and Estévez M.. . 2015. Protein oxidation in processed meat: mechanisms and potential Implications on human health. Compr. Rev. Food Sci. Food Saf. 14:106–122. doi: 10.1111/1541-4337.12127. [DOI] [PubMed] [Google Scholar]
- Tang, X. Q., Luo Y. X., Chen H. Z., and Liu D. P.. . 2014. Mitochondria, endothelial cell function, and vascular diseases. Front. Physiol. 5:175. doi: 10.3389/fphys.2014.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimmulappa, R. K., Mai K. H., Srisuma S., Kensler T. W., Yamamoto M., and Biswal S.. . 2002. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 62:5196–5203. [PubMed] [Google Scholar]
- Tomanek, L. 2015. Proteomic responses to environmentally induced oxidative stress. J. Exp. Biol. 218:1867–1879. doi: 10.1242/jeb.116475. [DOI] [PubMed] [Google Scholar]
- Valle, I., Alvarez-Barrientos A., Arza E., Lamas S., and Monsalve M.. . 2005. PGC-1α regulates the mitochondrial antioxidant defense system in vascular endothelial cells. Cardiovasc. Res. 66:562–573. doi: 10.1016/j.cardiores.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Vassilios, N. K., Michael R. D., and Laura D. O.. . 2014. Mitochondrial quality control and communications with the nucleus are important in maintaining mitochondrial function and cell health. Biochim. Biophys. Acta 1840:1254–1265. doi: 10.1016/j.bbagen.2013.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, R. R., Pan X. J., and Peng Z. Q.. . 2009. Effects of heat exposure on muscle oxidation and protein functionalities of pectoralis majors in broilers. Poult. Sci. 88:1078–1084. doi: 10.3382/ps.2008-00094. [DOI] [PubMed] [Google Scholar]
- Wang, X. F., Zhu X. D., Li Y. J., Liu Y., Li J. L., Gao F., Zhou G. H., and Zhang L.. . 2015. Effect of dietary creatine monohydrate supplementation on muscle lipid peroxidation and antioxidant capacity of transported broilers in summer. Poult. Sci. 94:2797–2804. doi: 10.3382/ps/pev255. [DOI] [PubMed] [Google Scholar]
- Xing, T., Chen X., Li J., Zhang L., and Gao F.. . 2021a. Dietary taurine attenuates hydrogen peroxide-impaired growth performance and meat quality of broilers via modulating redox status and cell death signaling. J. Anim. Sci. 99:skab089. doi: 10.1093/jas/skab089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing, T., Gao F., Tume R. K., Zhou G. H., and Xu X. L.. . 2019. Stress effects on meat quality: a mechanistic perspective. Stress effects on meat quality: a mechanistic perspective. Compr. Rev. Food Sci. Food Saf. 18:380–401. doi: 10.1111/1541-4337.12417. [DOI] [PubMed] [Google Scholar]
- Xing, T., Pan X. N., Zhang L., and Gao F.. . 2021b. Hepatic oxidative stress, apoptosis, and inflammation in broiler chickens with wooden breast myopathy. Front. Physiol. 12:659777. doi: 10.3389/fphys.2021.659777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing, T., Wang M. F., Han M. Y., Zhu X. S., Xu X. L., and Zhou G. H.. . 2017a. Expression of heat shock protein 70 in transport-stressed broiler pectoralis major muscle and its relationship with meat quality. Animal 11:1599–1607. doi: 10.1017/S1751731116002809. [DOI] [PubMed] [Google Scholar]
- Xing, T., Xu X. L., Jiang N. N., and Deng S. L.. . 2016. Effect of transportation and pre-slaughter water shower spray with resting on AMP-activated protein kinase, glycolysis and meat quality of broilers during summer. Anim. Sci. J. 87:299–307. doi: 10.1111/asj.12426. [DOI] [PubMed] [Google Scholar]
- Xing, T., Xu X. L., Zhou G. H., Wang P., and Jiang N. N.. . 2015. The effect of transportation of broilers during summer on the expression of heat shock protein 70, postmortem metabolism and meat quality. J. Anim. Sci. 93:62–70. doi: 10.2527/jas.2014-7831. [DOI] [PubMed] [Google Scholar]
- Xing, T., Zhao X., Wang P., Chen H., Xu X. L., and Zhou G. H.. . 2017b. Different oxidative status and expression of calcium channel components in stress-induced dysfunctional chicken muscle. J. Anim. Sci. 95:1565–1573. doi: 10.2527/jas.2016.0868. [DOI] [PubMed] [Google Scholar]
- Zampiga, M., Laghi L., Petracci M., Zhu C., and Sirri F.. . 2018. Effect of dietary arginine to lysine ratios on productive performance, meat quality, plasma and muscle metabolomics profile in fast-growing broiler chickens. J. Anim. Sci. Biotechnol. 9:79. doi: 10.1186/s40104-018-0294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. F., Hu Z. P., Lu C. H., Yang M. X., Zhang L. L., and Wang T.. . 2015. Dietary curcumin supplementation protects against heat-stress-impaired growth performance of broilers possibly through a mitochondrial pathway. J. Anim. Sci. 93:1656–1665. doi: 10.2527/jas.2014-8244. [DOI] [PubMed] [Google Scholar]
- Zhang, W. G., Marwan A. H., Samaraweera H., Lee E. J., and Ahn D. L.. . 2013. Breast meat quality of broiler chickens can be affected by managing the level of nitric oxide. Poult. Sci. 92:3044–3049. doi: 10.3382/ps.2013-03313. [DOI] [PubMed] [Google Scholar]
- Zhang, L., Wang X. F., Li J. L., Zhu X. D., Gao F., and Zhou G. H.. . 2017. Creatine monohydrate enhances energy status and reduces glycolysis via Inhibition of AMPK pathway in pectoralis major muscle of transport-stressed broilers. J. Agric. Food Chem. 65:6991–6999. doi: 10.1021/acs.jafc.7b02740. [DOI] [PubMed] [Google Scholar]
- Zhang, J. F., Xu L., Zhang L. L., Ying Z. X., Su W. P., and Wang T.. . 2014. Curcumin attenuates D-galactosamine/lipopolysaccharide-induced liver injury and mitochondrial dysfunction in mice. J. Nutr. 144:1211–1218. doi: 10.3945/jn.114.193573. [DOI] [PubMed] [Google Scholar]
- Zhang, J. F., Yang Y. X., Han H. L., Zhang L. L., Wang T., and Lillig C. H.. . 2021. Bisdemethoxycurcumin protects small intestine from lipopolysaccharide-induced mitochondrial dysfunction via activating mitochondrial antioxidant systems and mitochondrial biogenesis in broiler chickens. Oxid. Med. Cell Longev. 2021:9927864. doi: 10.1155/2021/9927864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, S. Z., Yumei F., and Chen A.. . 2007. De novo synthesis of glutathione is a prerequisite for curcumin to inhibit hepatic stellate cell (HSC) activation. Free Radic. Biol. Med. 43:444–453. doi: 10.1016/j.freeradbiomed.2007.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]