Abstract
Flagellar motility in Rhodobacter sphaeroides is notably different from that in other bacteria. R. sphaeroides moves in a series of runs and stops produced by the intermittent rotation of the flagellar motor. R. sphaeroides has a single, plain filament whose conformation changes according to flagellar motor activity. Conformations adopted during swimming include coiled, helical, and apparently straight forms. This range of morphological transitions is larger than that in other bacteria, where filaments alternate between left- and right-handed helical forms. The polymorphic ability of isolated R. sphaeroides filaments was tested in vitro by varying pH and ionic strength. The isolated filaments could form open-coiled, straight, normal, or curly conformations. The range of transitions made by the R. sphaeroides filament differs from that reported for Salmonella enterica serovar Typhimurium. The sequence of the R. sphaeroides fliC gene, which encodes the flagellin protein, was determined. The gene appears to be controlled by a ς28-dependent promoter. It encodes a predicted peptide of 493 amino acids. Serovar Typhimurium mutants with altered polymorphic ability usually have amino acid changes at the terminal portions of flagellin or a deletion in the central region. There are no obvious major differences in the central regions to explain the difference in polymorphic ability. In serovar Typhimurium filaments, the termini of flagellin monomers have a coiled-coil conformation. The termini of R. sphaeroides flagellin are predicted to have a lower probability of coiled coils than are those of serovar Typhimurium flagellin. This may be one reason for the differences in polymorphic ability between the two filaments.
Bacteria swim through liquids by means of a propeller-like, rotating flagellum (23). The major component of the flagellum is the long, extracellular filament, a polymer of flagellin protein. The antigenicity of flagellin, its variability, its property of self-assembly into filaments, and the ease with which it may be purified have resulted in an extensive study of these proteins and the gene(s) encoding them in many bacterial genera (reviewed in reference 15). Electron microscopic studies have revealed two distinct types of filaments called “plain” and “complex” (32). Plain filaments have a smooth appearance, whereas complex filaments have ridges and grooves on the surface (32, 38, 39). Most bacteria including Salmonella possess plain filaments. Complex filaments have been observed for three species of soil bacteria: Pseudomonas rhodos (32), Sinorhizobium meliloti (10), and Sinorhizobium lupini (33). Previously, it was suggested that filament type might correlate with the mode of flagellar rotation. Bacteria with plain filaments can switch their rotation from clockwise (CW) to counterclockwise, whereas bacteria with complex filaments can rotate their flagella only in the CW direction and do not switch direction of rotation but stop rotation periodically (so-called unidirectional, intermittent rotation [11]). Complex flagella are brittle and form left-handed helices with little or no structural polymorphism (11). Plain filaments are flexible and have distinct polymorphic forms with different helical characteristics. Filaments rotating in the counterclockwise direction (swimming cells) are normally left-handed helices (normal shape), and a change in direction of rotation to CW (tumbling cells) converts them into right-handed helices (curly shape [24]). This polymorphic ability is required for the swimming and tumbling of bacterial taxis.
Plain filaments can also be induced to change shape in vitro (polymorphic transitions) by changing environmental conditions such as pH, temperature, salt concentration, organic solvent, viscous flow, or sugar concentrations (18, 34). Although it is not clear whether such transitions have a physiological effect on swimming behavior in the wild, they may be a useful way of modulating motility under different conditions in the absence of tactic stimuli. A comparison of the amino acid sequences from plain filament flagellin proteins of many species reveals a relatively high degree of conservation in the amino- and carboxyl-terminal regions, whereas the central regions are very variable even between flagellins from different strains of the same species (8, 14, 15).
A notable deviation from the correlation between filament types and mode of flagellar rotation is seen in the purple, nonsulfur, aquatic bacterium Rhodobacter sphaeroides (37). Each cell has a single plain filament but displays unidirectional, intermittent flagellar rotation. Moreover, the filament can take on at least three distinct polymorphic forms in vivo: a normal structure while rotating, an apparently straight form during fast rotation, and an unusual, loosely coiled conformation during a stop. Detached filaments with the former and latter polymorphic forms can also be observed (2, 3). In light of these interesting differences between R. sphaeroides and other species, we chose to study the filament of this organism in greater detail. In this paper, we describe polymorphic forms of the filament induced in vitro by changes in pH and ionic strength. We also report sequence analysis and expression studies of the fliC gene, which encodes the flagellin protein of this organism.
MATERIALS AND METHODS
Strains, plasmids, and media.
The bacterial strains and plasmids used in this work are described in Table 1. Growth media and antibiotic selection were as described previously (35). Motility analysis was carried out by direct observation of exponentially growing cells by phase-contrast microscopy or by point inoculation of semisolid agar (0.3% [wt/vol] agar, 0.03% [wt/vol] yeast extract, 0.03% [wt/vol] NaCl, 0.03% [wt/vol] tryptone) to test for swarming.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| E. coli | ||
| DH5-α | F′ lacZM15 recA1 | Life Technologies |
| S17-1 | recA integrated plasmid RP4 | 5 |
| YK4146 | fliC mutant | R. M. Macnab |
| YK4516 | fliC mutant | R. M. Macnab |
| R. sphaeroides | ||
| WS8N | Wild-type spontaneous Nalr | 37 |
| nm15b | TnphoA derivative of WS8N, nonmotile, nonflagellate | This study |
| Cosmids or plasmids | ||
| c523 | Cosmid carrying the R. sphaeroides fliC gene; Tcr | This study |
| pUC19 | E. coli-specific cloning vector lacZ Ampr | New England Biolabs |
| pSB390 | luxCDEAB cassette clone | G. S. A. B. Stewart and M. Winson |
| pSB395 | luxCDEAB cassette cloned in pRK415 | 41 |
| pTP11 | luxCDEAB cassette cloned in pRK415 under the control of the promoter region of fliC | This study |
| pRK415-1 | Cloning vector which can replicate in R. sphaeroides; Tcr | 36 |
Isolation of flagella and observation of filament polymorphs.
A modified version of the method of Aizawa and coworkers (1) was used for the large-scale isolation of intact flagella for R. sphaeroides. Photosynthetic culture (1.5 liters) at an optical density at 660 nm of 0.75 was harvested, and the cells were lysed and treated with DNase as described previously (1). The pH was increased to 10 by the dropwise addition of 5 M NaOH in order to eliminate contaminating membrane fragments. Cellular debris was removed by centrifugation at 15,000 rpm for 45 min in Beckman JA 21 rotor. Flagella were collected from the supernatant by centrifugation at 30,000 rpm for 30 min in a Beckman L7 ultracentrifuge. The flagellar pellet was resuspended in TET buffer (10 mM Tris-HCl [pH 8], 1 mM EDTA, 0.1% [wt/vol] Triton X-100). A density gradient was set up by the addition of 0.43 g of CsCl per ml of suspension and centrifugation at 20,000 rpm at 14°C overnight in a Beckman L7 ultracentrifuge. The flagella formed a cloudy white band, which was collected. CsCl was removed by a wash in 4 volumes of TET buffer. The final pellet was resuspended in 0.5 ml of TET buffer. The flagella were observed by high-intensity dark-field microscopy. Broken, detached flagellar filaments were also harvested from stationary-phase cultures by a 30-min 30,000-rpm centrifugation step and CsCl gradient (as detailed above). These were also used in polymorphic studies to preclude any effects of flagellar basal bodies (present in the intact preparation) on filament polymorphisms.
The buffers for observations of filament polymorphism at pH 2 to 8 were prepared with citric acid and Na2HPO4 as described previously (6). A second set of buffers from pH 7 to 11 were prepared by mixing 12.5 ml each of 0.2 M Tris and 0.2 M glycine. The pH was adjusted with either 0.2 M HCl or 0.1 M NaOH before deionized water was added to a final volume of 50 ml. In all cases, an appropriate volume of 5 M NaCl was added to give the desired concentration of NaCl. Equal volumes of buffer and flagellar solution were mixed and incubated at room temperature for 5 min before observation by high-intensity dark-field microscopy. The results were recorded on videotape.
Recombinant DNA techniques.
Recombinant DNA techniques were carried out as described by Sambrook and coworkers (31). Restriction and modification enzymes were obtained from Northumbria Biochemicals, New England Biolabs, and Boehringer Mannheim. DNA fragments were purified using the Geneclean kit (Bio 101), and the Photogene detection kit (Life Technologies) was used for Southern blotting. R. sphaeroides genomic DNA isolations, DNA sequencing, and conjugation protocols for complementation analysis were as described previously (7, 27, 35).
Western blotting.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of whole cells or sheared filaments was carried out according to the method of Laemmli as described by Sambrook and coworkers (31). Sheared filaments were prepared from motile bacterial cultures by 10 passages through a 3FG cannulum (Portex UK) held between two 10-ml syringes. The proteins from the gels were transferred to Hybond-C super nitrocellulose (Amersham) for 1 h at a constant 100 mA. After being blocked overnight in PBS-T (140 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, 0.3% [vol/vol] Tween 20), containing 1% (wt/vol) milk (Marvel nonfat dried milk), the membrane was washed in PBS-T for 5 min. It was incubated in a 1/1,000 dilution of antiserum raised against R. sphaeroides flagellar filaments (36) in PBS-T, containing 1% (wt/vol) milk and 15% (wt/vol) bovine serum albumin for 2 h. The membrane was washed three times with PBS-T, containing 0.3% (wt/vol) milk, and then incubated with a 1/1,000 dilution of anti-rabbit immunoglobulin G-alkaline phosphatase conjugate (Sigma) in PBS-T containing 0.3% (wt/vol) milk. After five washes in phosphate-buffered saline, the blot was developed by incubation in detection buffer (2.5 mg of 5-bromo-4-chloro-3-indolylphosphate [BCIP] ml−1, 5 mg of nitroblue tetrazolium ml−1, 100 mM Tris-HCl [pH 9.5], 100 mM NaCl, 5 mM MgCl2) until color development was observed. The reaction was stopped by washing the blot in excess water.
Bioluminescence measurements.
Stationary-phase, nonmotile, photosynthetic cultures (30-ml volume) were diluted by mixing them with 70 ml of fresh medium to introduce flagellar gene expression. Samples were taken immediately and then every hour. Readings for light emission were taken on a Turner luminometer. The light intensity per unit of cell mass was calculated by dividing the luminometer reading by the optical density of the culture sample at 600 nm.
Nucleotide sequence accession number.
The DNA sequence was submitted to EMBL under accession no. Y14687.
RESULTS
Polymorphic ability of detached R. sphaeroides flagella.
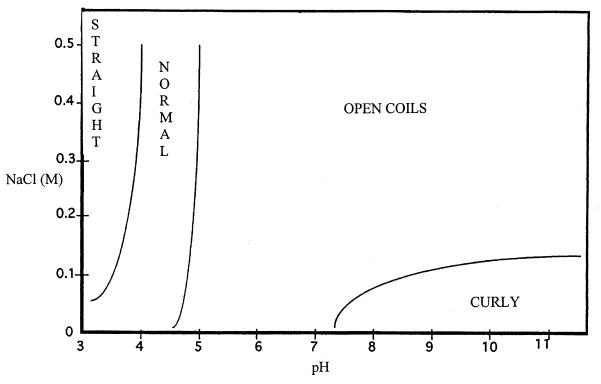
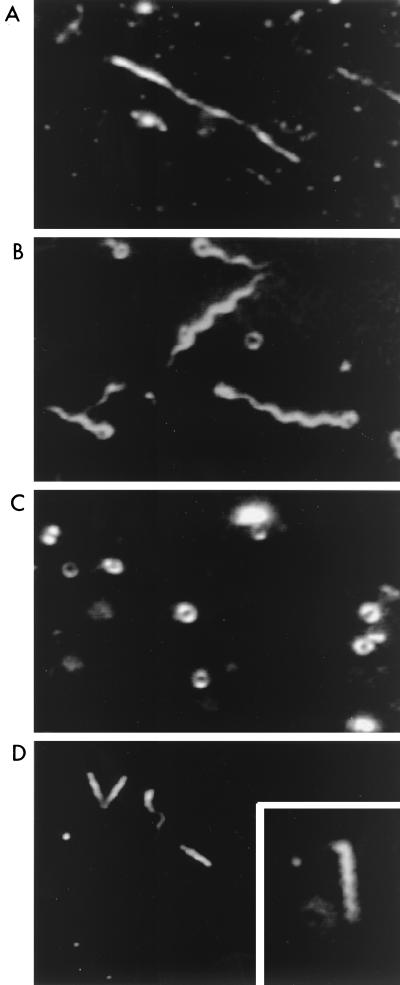
Figure 1 is a phase diagram of the dominant forms observed under different conditions of pH and ionic strength. Four forms for R. sphaeroides filaments could be distinguished: straight, normal, open coils, and curly. Examples are illustrated in Fig. 2. Both intact flagella and broken detached filaments from R. sphaeroides gave the same results. The normal, straight, and curly types resembled morphological types previously observed for the Salmonella filament (17). The coiled structures, seen in this study and by Armitage and Macnab (2), are referred to as “open coils” because they are distinct from the coiled forms of Salmonella filaments observed by Kamiya and Asakura (16). The difference is that the diameter of the R. sphaeroides open coils is greater and the Salmonella coil appears as a long cylinder when viewed from the side whereas no such structures were visible for the R. sphaeroides open coils. They were found to resemble rope lasso structures rather than cylinders.
FIG. 1.
Phase diagram showing the predominant polymorphic forms of isolated R. sphaeroides flagellar filaments in buffers of different pHs and ionic strengths.
FIG. 2.
High-intensity dark-field microscope images of typical polymorphic forms of R. sphaeroides flagella. (A) Straight; (B) normal with some open coils; (C) open coils; (D) curly (the inset shows greater magnification of the curly form). The open coils and the normal forms were faint and highly susceptible to Brownian motion, and therefore it was difficult to obtain good micrographs of these from the videotape; some examples are shown.
Interestingly, the dominant form under physiological conditions is the open-coil form, which was also observed for cells with stopped flagella (2, 3). This is in contrast to Salmonella i-flagella, which have a predominantly normal conformation, which undergoes a transition to curly at pH values lower than 4 in the presence of 0.1 M NaCl (16).
As found previously for Salmonella filaments (16), the lines drawn between the types of Rhodobacter filament conformations in Fig. 1 are not firm boundaries. In many cases, different morphological types could coexist under the same conditions (Fig. 2B), and the lines represent boundaries where one form becomes dominant over all others. When normal filaments were the predominant forms, a small proportion of open coils and straight forms were observed. The curly and straight forms were never observed together. At pH values lower than 3, the filaments were no longer visible owing to the depolymerization of the filament into flagellin monomers. Prolonged incubation (>15 min) at pH 3 also resulted in depolymerization, and this effect was enhanced with increasing NaCl concentration.
The range of forms obtained for the R. sphaeroides filament is different from that obtained for the Salmonella filament by Kamiya and Asakura (16). In order to determine whether this is a result of gross differences between the amino acid sequences of the flagellin proteins of the species, the fliC gene of R. sphaeroides was cloned and sequenced as described below.
Cloning, sequencing, and expression of R. sphaeroides fliC.
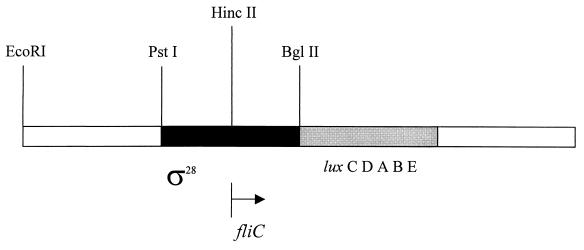
The R. sphaeroides fliC gene was isolated by complementation of a nonmotile, filament-minus TnphoA mutant, R. sphaeroides Nm15 (37), with a clone from a wild-type R. sphaeroides cosmid library. Two kilobases of wild-type DNA from the cosmid clone that flanked the site of TnphoA insertion in the mutant was localized by Southern hybridization and sequenced on both strands. It was found to encode one long open reading frame (ORF) starting at nucleotide 241, just after the HincII site (Fig. 3) and ending at nucleotide 1722. Eleven bases upstream from the ATG start codon was the sequence AGGAGGG, which matches the consensus sequence for ribosome-binding sites in bacteria (9, 20). Upstream from that was a potential promoter region with a version of the ς28 consensus sequence TAAA(N14)GCCGTTGA. The DNA sequence was submitted to EMBL (accession no. Y14687), and the predicted amino acid sequence from the ORF was used to search the EMBL database. The R. sphaeroides ORF showed homology with numerous flagellin proteins, particularly flagellins from the plain flagella of Bacillus subtilis (43% identity), Escherichia coli and Salmonella enterica serovar Typhimurium (36% identity), and Pseudomonas aeruginosa (41% identity) (Fig. 4). The amino acid sequence of R. sphaeroides flagellin, in common with those of other bacteria, lacks cysteine and tryptophan (S. meliloti FlaA and FlaB are the only ones that contain tryptophan), but it is unusual that it also lacks proline. R. sphaeroides flagellin has very low homology with the S. meliloti flagellins FlaA (27% identity) and FlaB (24% identity) from complex flagella. These low levels of homology are not unexpected given that R. sphaeroides has plain flagella and that the unidirectionality of flagella from both species is likely due to motor and not filament properties, although 16S ribosomal DNA phylogenies show that S. meliloti and R. sphaeroides are closely related.
FIG. 3.
Map of the region encoding R. sphaeroides FliC. The putative ς28 promoter region and the site of cloning of the luxCDEAB reporter cassette used in expression studies are shown.
FIG. 4.
Prettybox multiple sequence alignment of R. sphaeroides FliC (flic_rsph) with FliCs from enteric and soil bacteria. For reasons of space, much of the variable central region is not shown. The regions predicted to form coiled coils are shown using serovar Typhimurium coordinates (heavy bars) or R. sphaeroides coordinates (dotted line). Accession numbers are as follows: S. meliloti FlaA (flaa_rhime), P13118; S. meliloti FlaB (flab_rhime), P13119; P. aeruginosa FlaA (flaa_pseae), P21184; B. subtilis FliC (flic_bacsu), P02868; serovar Typhimurium FliC (flic_salty), P06179; E. coli FliC (flic_ecoli), P04949.
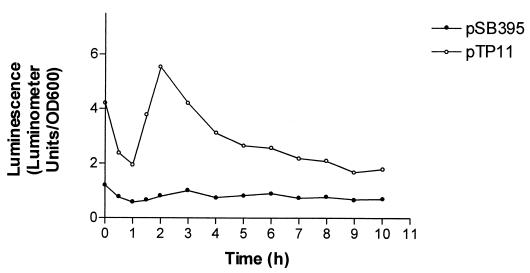
To test for promoter activity, in the 600-bp region upstream from the fliC ORF, a transcriptional fusion containing a promoterless Photorhabdus luminescens luxCDABE cassette (41) was constructed. The cassette from pSB390 (G. S. A. B. Stewart and M. Winson, unpublished data) was cloned as a 5.8-kb BamHI fragment into the BglII site of the fliC gene in plasmid pRK415 to give pTP11. A second plasmid, pSB395 (41), which contains the lux cassette in the same orientation and same vector as pTP11 but without any R. sphaeroides DNA, was used as a background control. The bioluminescence levels of R. sphaeroides WS8N cultures, containing each plasmid, were monitored over a time course after dilution from stationary phase (as detailed in Materials and Methods). The results are shown in Fig. 5. It is clear that the region upstream from the fliC ORF has promoter activity in R. sphaeroides. Interestingly, although the (wild-type) R. sphaeroides WS8N cells containing pSB395 were motile, the cells containing pTP11 were nonmotile throughout the experiment, although the growth rates of the two were similar (data not shown). There are two possible reasons for this: (i) significant overexpression of the products of the lux genes somehow interferes with motility, or (ii) the promoter fragment of the plasmid sequesters some essential transcription factor(s) and prevents or reduces expression of chromosomally encoded fliC. The only identifiable promoter consensus in the upstream 600-bp region is a sequence similar to the ς28-dependent promoters of other species (Fig. 6), and so it may be ς28 that is sequestered by the plasmid-borne promoter. Plasmid pTP11, containing the R. sphaeroides fliC promoter region, did not give luminescence activity in E. coli (data not shown). This may be due to slight differences between the ς28 consensus in R. sphaeroides and that in E. coli (Fig. 6) (n = 14 bases and penultimate base is G in R. sphaeroides, n = 15 bases and penultimate base is A in E. coli).
FIG. 5.
Promoter activity of the region upstream from the fliC ORF as indicated by the luxCDEAB reporter system. OD600, optical density at 600 nm.
FIG. 6.
Consensus ς28 recognition sequences compared to the putative promoter sequence of R. sphaeroides fliC. The B. subtilis ς28 is also known as ςD, and that of E. coli is known as ςF (4, 12, 21).
To test whether the polymorphic, plain filament R. sphaeroides flagellin could substitute for the flagellin in E. coli plain filaments, the R. sphaeroides fliC gene was cloned into pUC19 such that it was under the control of the lac promoter. This plasmid, named pKa, was introduced into two nonmotile fliC mutants of E. coli, YK4146 and YK4516. R. sphaeroides fliC failed to complement the E. coli mutations, and although R. sphaeroides FliC protein was detectable by Western blotting in the cytoplasm of E. coli, it was not present in sheared fractions or in the medium (data not shown). This result prevented us from studying any polymorphic properties of hybrid E. coli-R. sphaeroides flagella. It appears that the R. sphaeroides flagellin may not be exported by the E. coli flagellar export system.
DISCUSSION
The flagellar filament of R. sphaeroides shows an interesting range of polymorphic transitions under varying conditions of pH and ionic strength in vitro. Although the curly, normal, and straight shapes are also seen with Salmonella filaments (16, 17), the open-coil form is, as a naturally occurring form, unique to R. sphaeroides filaments. The open-coil form is the most common under physiological conditions and is distributed across a broad range of conditions. Interestingly, in vivo, stopped flagella have an open-coil shape whereas fast-rotating flagella have a normal helical or apparently straight shape (2, 3). It is thought that slow rotation of the coiled flagellum after a stop facilitates cell reorientation (3). This ability to change direction is essential during a tactic response since R. sphaeroides cells are unable to tumble in the manner of bacteria with switching flagella. Thus, it appears that the filament of R. sphaeroides has intrinsic properties that allow polymorphic transitions adapted to the mode of swimming.
In serovar Typhimurium, mutations that affect the polymorphic ability of the filament are found to cause amino acid substitutions in the highly conserved N- and C-terminal regions of flagellin (19) and flagellins with small deletions at either terminus are also affected in their polymorphic ability (40). Direct interactions within the termini of flagella are important for the polymorphic ability of the flagellar filament (25). Thus, terminal portions of flagellin are a determinant for polymorphic ability in serovar Typhimurium. In addition, a deletion in the variable central domain of serovar Typhimurium flagellin also resulted in alteration of polymorphic ability of the filament, possibly because a change in the overall charge of the region altered repulsive or attractive forces between subunits (26, 43). Such studies suggest that a combination of interactions between the ordered termini at the filament core and interactions between the outer domains of flagellin molecules is responsible for polymorphic changes.
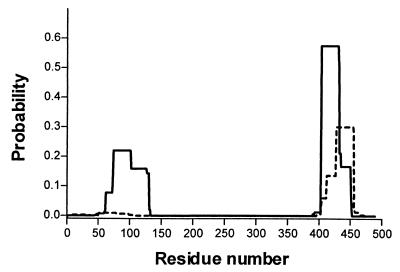
In order to test whether the differences in polymorphic abilities of the filaments from R. sphaeroides and serovar Typhimurium were reflected in differences at the molecular level, the flagellin sequences of these two organisms were compared. The hydropathy profiles of the two proteins are similar throughout, despite the divergence of the R. sphaeroides sequence in the central, nonconserved domain (data not shown). The terminal regions of both proteins are predicted to have an alpha-helical character. The serovar Typhimurium flagellin forms coiled-coil structures at the termini (13, 28, 42). We analyzed both flagellins using the COILS program (22) (Fig. 7). As expected, serovar Typhimurium flagellin is predicted to have coiled coils close to either terminus whereas R. sphaeroides flagellin has a much lower probability of coiled coils in these regions. This difference may be the key reason for the different polymorphic abilities of filaments from these two species. Interestingly, most of the mutations affecting polymorphic ability in serovar Typhimurium flagella (19) map within, or close to, these regions. Furthermore, many of these mutant sequences are predicted to have altered coiled-coil probabilities compared to the wild-type sequence according to the COILS program (data not shown).
FIG. 7.
Prediction of coiled-coil structures at the termini of flagellin proteins. The predictions were carried out using the COILS program (22) with a window size of 28 residues. Solid line, serovar Typhimurium FliC; dashed line, R. sphaeroides FliC.
The overall amino acid compositions of the nonconserved central regions of FliCs (corresponding to amino acids 204 to 292 of serovar Typhimurium), which comprise the outer domains in the assembled flagellar filament, are similar in flagellins of R. sphaeroides and Salmonella. Therefore, the intersubunit interactions in the outer parts of the filaments of these species might be expected to be similar. This general assertion does not seem to be borne out by the differences that we observed in filament polymorphisms for the two bacteria. Mimori-Kiyosue and coworkers (26) have found that the outer domain (D3) plays a significant role in determining the polymorphic abilities of serovar Typhimurium flagellar filament. It is possible that the central domain plays no role in the differences in polymorphic ability seen here, or subtle changes involving just a few amino acids in the D3 domain of R. sphaeroides flagellin, rather than the overall amino acid composition, are responsible for altered intersubunit interactions that account for the unusual polymorphic abilities of R. sphaeroides flagellar filaments. It would be interesting to exchange the central domains of Salmonella and R. sphaeroides flagellins and test whether the resulting filaments had altered polymorphic abilities.
We have discussed some features that may be responsible for the polymorphic properties of the R. sphaeroides filament. The R. sphaeroides FliC is predicted to differ in its secondary structure at the termini from the serovar Typhimurium flagellin. It is also possible that a few key amino acid changes in the nonconserved region of R. sphaeroides FliC cause changes in intersubunit interactions. It would be interesting to see if these predictions are borne out by a detailed structural study of the R. sphaeroides filament.
It is difficult to speculate on the biological significance of the unusual properties of the R. sphaeroides filaments. It is likely that the properties of the filament are adapted to its mode of swimming motility, which in turn is adapted to its environment. R. sphaeroides inhabits aquatic or terrestrial environments, whereas serovar Typhimurium is found mainly in intestinal mucous surfaces. It may be that tumbling facilitates directional changes in highly viscous mucous surfaces whereas in relatively low-viscosity aquatic environments a combination of Brownian motion and slow rotation of the filament open coil is sufficient to achieve a change in orientation. Thus, the flagellar filaments of the two species may be adapted to these different requirements. Additionally, for R. sphaeroides, having a single flagellum, which forms a coil close to the cell body when stopped, may reduce the risk of being caught by protozoal predators in the wild. The single flagellum presents only a single receptor site for protozoa like Acanthamoeba which bind to bacterial flagella (30). There could be other explanations for the unusual properties of R. sphaeroides FliC that will be illuminated by a greater understanding of its natural history in the wild.
This study has provided some interesting insights into the properties of the R. sphaeroides flagellum and a comparison of it with the well-studied serovar Typhimurium flagellum. Understanding polymorphic transitions in different flagella and how they vary with the chemical environment sheds light on the nature of FliC subunit interactions in filaments. Such knowledge may have implications for the design of nanofilaments with predictable physical properties.
ACKNOWLEDGMENTS
This work was supported by grant no. F.114L from the Leverhulme Trust to R.E.S. and by a Royal Society study visit grant to D.S.H.S. and a BBSRC ISIS study visit grant to R.E.S. S.M.S. was supported by an EC technical training scheme.
We thank Takuya Gotou for assistance with image processing and members of the Aizawa and Sockett labs for useful discussions.
REFERENCES
- 1.Aizawa S-I, Dean G E, Jones C J, Macnab R M, Yamaguchi S. Purification and characterization of the flagellar hook-basal body complex of Salmonella typhimurium. J Bacteriol. 1985;161:836–849. doi: 10.1128/jb.161.3.836-849.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armitage J P, Macnab R M. Unidirectional, intermittent rotation of the flagellum of Rhodobacter sphaeroides. J Bacteriol. 1987;169:514–518. doi: 10.1128/jb.169.2.514-518.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armitage J P, Pitta T P, Vigeant M A-S, Packer H L, Ford R M. Transformations in flagellar structure of Rhodobacter sphaeroides and possible relationship to changes in swimming speed. J Bacteriol. 1999;181:4825–4833. doi: 10.1128/jb.181.16.4825-4833.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnosti D N, Chamberlin M J. Secondary ς factor controls transcription of flagellar and chemotaxis genes in Escherichia coli. Proc Natl Acad Sci USA. 1989;86:830–834. doi: 10.1073/pnas.86.3.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis J, Donohue T J, Kaplan S. Construction, characterization, and complementation of a Puf mutant of Rhodobacter sphaeroides. J Bacteriol. 1988;170:320–329. doi: 10.1128/jb.170.1.320-329.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dawson R M C, Elliott D C, Elliott W H, Jones K M. Data for biochemical research. 3rd ed. Oxford, United Kingdom: Clarendon Press; 1986. [Google Scholar]
- 7.Donohue T J, McEwan A G, Kaplan S. Cloning, DNA sequence, and expression of the Rhodobacter sphaeroides cytochrome c2 gene. J Bacteriol. 1986;168:962–972. doi: 10.1128/jb.168.2.962-972.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dons L, Rasmussen O F, Olesen J E. Cloning and characterisation of a gene encoding flagellin of Listeria monocytogenes. Mol Microbiol. 1992;6:2919–2929. doi: 10.1111/j.1365-2958.1992.tb01751.x. [DOI] [PubMed] [Google Scholar]
- 9.Gold L, Pribnow D, Schneider T, Shinedling S, Singer B S, Stormo G. Translation initiation in prokaryotes. Annu Rev Microbiol. 1981;35:365–403. doi: 10.1146/annurev.mi.35.100181.002053. [DOI] [PubMed] [Google Scholar]
- 10.Gotz R, Limmer N, Ober K, Schmitt R. Motility and chemotaxis in two strains of Rhizobium with complex flagella. J Gen Microbiol. 1982;128:789–798. [Google Scholar]
- 11.Gotz R, Schmitt R. Rhizobium meliloti swims by unidirectional, intermittent rotation of right-handed flagellar helices. J Bacteriol. 1987;169:3146–3150. doi: 10.1128/jb.169.7.3146-3150.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helmann J D. Alternative sigma factors and the regulation of flagellar gene expression. Mol Microbiol. 1991;5:2875–2882. doi: 10.1111/j.1365-2958.1991.tb01847.x. [DOI] [PubMed] [Google Scholar]
- 13.Homma M, DeRosier D J, Macnab R M. Flagellar hook and hook-associated proteins of Salmonella and their relationship to other axial components of the flagellum. J Mol Biol. 1990;213:819–832. doi: 10.1016/S0022-2836(05)80266-9. [DOI] [PubMed] [Google Scholar]
- 14.Joys T M. The covalent structure of the phase-1 flagellar filament protein of Salmonella typhimurium and its comparison with other flagellins. J Biol Chem. 1985;260:15758–15761. [PubMed] [Google Scholar]
- 15.Joys T M. The flagellar filament protein. Can J Microbiol. 1988;34:452–458. doi: 10.1139/m88-078. [DOI] [PubMed] [Google Scholar]
- 16.Kamiya R, Asakura S. Helical transformations of Salmonella flagella in vitro. J Mol Biol. 1976;106:167–186. doi: 10.1016/0022-2836(76)90306-5. [DOI] [PubMed] [Google Scholar]
- 17.Kamiya R, Asakura S, Yamaguchi S. Formation of helical filaments by copolymerization of two types of ‘straight’ filaments. Nature (London) 1980;286:628–630. doi: 10.1038/286628a0. [DOI] [PubMed] [Google Scholar]
- 18.Kamiya R, Hotani H, Asakura S. Polymorphic transition in bacterial flagella. In: Amos W B, Duckett J D, editors. Prokaryotic and eukaryotic flagella. Cambridge, United Kingdom: Cambridge University Press; 1982. pp. 53–76. [PubMed] [Google Scholar]
- 19.Kanto S, Okino H, Aizawa S-I, Yamaguchi S. Amino acids responsible for flagellar shape are distributed in terminal regions of flagellin. J Mol Biol. 1991;219:471–480. doi: 10.1016/0022-2836(91)90187-b. [DOI] [PubMed] [Google Scholar]
- 20.Kozak M. Comparison of initiation of protein synthesis in prokaryotes, eukaryotes and organelles. Microbiol Rev. 1983;47:1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kutsukake K, Ohya Y, Iino T. Transcriptional analysis of the flagellar regulon of Salmonella typhimurium. J Bacteriol. 1990;172:741–747. doi: 10.1128/jb.172.2.741-747.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lupas A, Van Dyke M, Stock J. Predicting coiled coils from protein sequences. Science. 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- 23.Macnab R M. Flagella and motility. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 123–145. [Google Scholar]
- 24.Macnab R M, Ornston M K. Normal-to-curly flagellar transitions and their role in bacterial tumbling. Stabilization of an alternative quaternary structure by mechanical force. J Mol Biol. 1977;112:1–30. doi: 10.1016/s0022-2836(77)80153-8. [DOI] [PubMed] [Google Scholar]
- 25.Mimori-Kiyosue Y, Vonderviszt F, Yamashita I, Fujiyoshi Y, Namba K. Direct interaction of flagellin termini essential for the polymorphic ability of the flagellar filament. Proc Natl Acad Sci USA. 1996;93:15108–15113. doi: 10.1073/pnas.93.26.15108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mimori-Kiyosue Y, Yamashita I, Fujiyoshi Y, Yamaguchi S, Namba K. Role of the outermost subdomain of Salmonella flagellin in the filament structure revealed by electron cytomicroscopy. J Mol Biol. 1998;284:521–530. doi: 10.1006/jmbi.1998.2184. [DOI] [PubMed] [Google Scholar]
- 27.Moore M D, Kaplan S. Construction of TnphoA gene fusions in Rhodobacter sphaeroides: isolation and characterization of a respiratory mutant unable to utilize dimethyl sulfoxide as a terminal electron acceptor during anaerobic growth in the dark on glucose. J Bacteriol. 1989;171:4385–4394. doi: 10.1128/jb.171.8.4385-4394.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Namba K, Yamashita I, Vonderviszt F. Structure of the core and central channel of flagella. Nature (London) 1989;342:648–654. doi: 10.1038/342648a0. [DOI] [PubMed] [Google Scholar]
- 29.Pleier E, Schmitt R. Identification and sequence analysis of two related flagellin genes in Rhizobium meliloti. J Bacteriol. 1989;171:1467–1475. doi: 10.1128/jb.171.3.1467-1475.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Preston T M, King C A. Binding sites for bacterial flagella at the surface of the soil amoeba Acanthamoeba. J Gen Microbiol. 1984;130:1449–1458. [Google Scholar]
- 31.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 32.Schmitt R, Raska I, Mayer F. Plain and complex flagella of Pseudomonas rhodos: analysis of fine structure and composition. J Bacteriol. 1974;117:844–857. doi: 10.1128/jb.117.2.844-857.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmitt R, Bamberger I, Acker G, Mayer F. Fine structure analysis of the complex flagella of Rhizobium lupini H13-3. Arch Microbiol. 1974;100:145–162. [Google Scholar]
- 34.Seville M, Ikeda T, Hotani H. The effects of sugar on the morphology of the bacterial flagellum. FEBS Lett. 1993;322:260–262. doi: 10.1016/0014-5793(93)80645-b. [DOI] [PubMed] [Google Scholar]
- 35.Shah D S H, Sockett R E. Analysis of the motA flagellar motor gene from Rhodobacter sphaeroides, a bacterium with a unidirectional, stop-start flagellum. Mol Microbiol. 1995;17:961–969. doi: 10.1111/j.1365-2958.1995.mmi_17050961.x. [DOI] [PubMed] [Google Scholar]
- 36.Sockett R E, Armitage J P. Isolation, characterization, and complementation of a paralyzed flagellar mutant of Rhodobacter sphaeroides WS8. J Bacteriol. 1991;173:2786–2790. doi: 10.1128/jb.173.9.2786-2790.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sockett R E, Foster J C A, Armitage J P. Molecular biology of the Rhodobacter sphaeroides flagellum. FEMS Symp. 1990;53:473–479. [Google Scholar]
- 38.Trachtenberg S, DeRosier D J, Aizawa S-I, Macnab R M. Pairwise perturbation of flagellin subunits. The structural differences between plain and complex bacterial flagellar filaments. J Mol Biol. 1986;190:569–576. doi: 10.1016/0022-2836(86)90242-1. [DOI] [PubMed] [Google Scholar]
- 39.Trachtenberg S, DeRosier D J, Macnab R M. Three-dimensional structure of the complex flagellar filament of Rhizobium lupini and its relation to the structure of the plain filament. J Mol Biol. 1987;195:603–620. doi: 10.1016/0022-2836(87)90185-9. [DOI] [PubMed] [Google Scholar]
- 40.Voderviszt F, Uedaira H, Kidokoro S, Namba K. Structural organisation of flagellin. J Mol Biol. 1990;214:97–104. doi: 10.1016/0022-2836(90)90149-g. [DOI] [PubMed] [Google Scholar]
- 41.Winson M K, Swift S, Hill P J, Sims C M, Griesmayr G, Bycroft B W, Williams P, Stewart G S A B. Engineering the luxCDEAB genes from Photorhabdus luminescens to provide a bioluminescent reporter for constitutive and promoter probe plasmids and mini-Tn5 constructs. FEMS Microbiol Lett. 1998;163:193–202. doi: 10.1111/j.1574-6968.1998.tb13045.x. [DOI] [PubMed] [Google Scholar]
- 42.Yamashita I, Vonderviszt F, Mimori Y, Suzuki H, Oosawa K, Namba K. Radial mass analysis of the flagellar filament of Salmonella: implications for the subunit folding. J Mol Biol. 1995;253:547–588. doi: 10.1006/jmbi.1995.0572. [DOI] [PubMed] [Google Scholar]
- 43.Yoshioka K, Aizawa S-I, Yamaguchi S. Flagellar filament structure and cell motility of Salmonella typhimurium mutants lacking part of the outer domain of flagellin. J Bacteriol. 1995;177:1090–1093. doi: 10.1128/jb.177.4.1090-1093.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]