Abstract
Objective:
Candida auris has emerged as a health-care-associated and multidrug-resistant fungal pathogen of great clinical concern. As many as 50% of C. auris clinical isolates are reported to be resistant to amphotericin B, but no mechanisms contributing to this resistance have been identified. Here we describe a clinical case in which high-level amphotericin B resistance was acquired in vivo during therapy and undertake molecular and genetic studies to identify and characterize the genetic determinant of resistance.
Methods:
Whole-genome sequencing was performed on four C. auris isolates obtained from a single patient case. Cas9-mediated genetic manipulations were then used to generate mutant strains harbouring mutations of interest, and these strains were subsequently subjected to amphotericin B susceptibility testing and comprehensive sterol profiling.
Results:
A novel mutation in the C. auris sterol-methyltransferase gene ERG6 was found to be associated with amphotericin B resistance, and this mutation alone conferred a >32-fold increase in amphotericin B resistance. Comprehensive sterol profiling revealed an abrogation of ergosterol biosynthesis and a corresponding accumulation of cholesta-type sterols in isolates and strains harbouring the clinically derived ERG6 mutation.
Conclusions:
Together these findings definitively demonstrate mutations in C. auris ERG6 as the first identified mechanism of clinical amphotericin B resistance in C. auris and represent a significant step forward in the understanding of antifungal resistance in this emerging public health threat.
Keywords: Amphotericin B, Candida auris, ERG6, In vivo evolution, Resistance
Introduction
In little over a decade, Candida auris has transformed from a newly identified species of Candida originally associated with infections of the auditory canal, to being recognized by the Centers for Disease Control and Prevention (CDC) as the first fungal pathogen to represent an urgent level of threat to public health [1–4]. Recently found to be the cause of outbreaks of invasive candidiasis on multiple continents and having been isolated in more than 45 countries, C. auris readily colonizes patients and disseminates easily within health-care facilities, changing the paradigm of health-care-associated fungal infections [4,5]. Although limited epidemiological and clinical outcomes data currently preclude the establishment of true clinical breakpoints for the assessment of C. auris antifungal susceptibility, the CDC has defined tentative antifungal breakpoints using susceptibility data from hundreds of clinical C. auris isolates and available pharmacokinetic-pharmacodynamic data. Applying these tentative breakpoints, more than 90% of C. auris isolates are resistant to fluconazole (modal MIC ≥256 mg/L), 30%–50% are resistant to amphotericin B and approximately 5% are resistant to echinocandins [1,4,6,7]. Unfortunately, C. auris has also demonstrated the capacity to rapidly acquire resistance to antifungals in vivo, leaving clinicians with no reliable option for the treatment of infections caused by this emerging public health threat [8]. The fluconazole resistance frequently identified among clinical isolates of C. auris has previously been associated with mutations in both ERG11 and TAC1B, and resistance to echinocandins has been associated with mutations in FKS1, but to date, no mechanisms contributing to clinical amphotericin B resistance have been identified in C. auris [4,7,9–11].
We present here a case where a patient receiving treatment for a fluconazole-resistant C. auris infection subsequently acquired amphotericin B-resistant, and later echinocandin-resistant, disease following multiple courses of antifungal therapy. Leveraging the clinical isolates from this single case, whole-genome sequencing, comprehensive sterol profiling and Cas9-mediated genetic manipulations, we have identified the first known mechanism of clinical amphotericin B resistance in C. auris conferred by mutations in the sterol-methyltransferase gene ERG6. Furthermore, we show that the observed mutations in ERG6 result in abolished biosynthesis of ergosterol, the target of amphotericin B, and these mutations alone abrogate the activity of this antifungal agent.
Materials and methods
Institutional review board statement
The study was approved by the Health Sciences Center Ethical Committee, Kuwait University (approval letter VDR/EC/3724). Clinical samples were obtained after verbal consent only as part of routine patient care and diagnostic workup for the isolation and susceptibility testing of pathogens.
The need for informed consent was waived by the Health Sciences Centre Ethical Committee, Kuwait University.
Isolate, strains and growth media used in this study
Isolation and identification of the C. auris strains isolated from the patient case included in this study was performed as described elsewhere [12]. All strains and clinical isolates (see Supplementary material Table S1) were grown in YPD liquid medium (1% yeast extract, 2% peptone, 2% dextrose) at 35°C in a shaking incubator unless otherwise indicated. All stocks were prepared with 50% sterile glycerol and were maintained at −80°C.
Whole-genome sequencing
Isolates were cultured in YPD liquid media at 35°C and genomic DNA was extracted as previously described [13]. Genomic libraries were constructed and barcoded using the NEBNext Ultra DNA Library Prep kit (New England Biolabs, Ipswich, MA, USA) per the manufacturer’s instructions and were sequenced using the Illumina HiSeq 2500 platform as previously described [14]. Read quality and filtering were performed using FastQC v0.11.5 and PRINSEQ v0.20.3 (21,278,185) using ‘-trim_left 15 ‘-trim_qual_left 20 -trim_qual_right 20 -min_len 100 -min_qual_mean 25 -derep 14’. Paired-end reads were aligned to the C. auris B8441 assembly (GenBank accession PEKT00000000.2; (30,559,369)) using BWA mem v0.7.12 (19,451,168) and variants were identified using GATK v3.7 (20,644,199) haploid mode and GATK tools (RealignerTargetCreator, IndelRealigner, HaplotypeCaller for both single nucleotide polymorphisms and insertions/deletions (indels), CombineGVCFs, GenotypeGVCFs, GatherVCFs, SelectVariants, and Variant Filtration). Sites were filtered with Variant Filtration using ‘QD < 2.0 || FS > 60.0 || MQ < 40.0’. Genotypes were filtered if the minimum genotype quality was <50, per cent alternate allele <0.8, or depth <10 (https://github.com/broadinstitute/broad-fungalgroup/blob/master/scripts/SNPs/filterGatkGenotypes.py). Variants were annotated using SnpEff v4.3T (22,728,672).
Plasmid construction and repair template preparation
Cas9-mediated gene editing was performed using the transient episomal plasmid-based system described previously with the plasmid pJMR17v3 [15,16]. Briefly, plasmid pJMR17 was constructed by cloning the autonomously replicating sequence from plasmid BJB-T231 into the ApaI site of plasmid pCP-tRNA [17].The plasmid pJMR17 was digested with BshTI to liberate the Candida parapsilosis TEF1 promoter, and the Meyerozyma guilliermondii TEF1 promoter from plasmid pCT-tRNA was cloned into this locus using the same restriction enzyme generating the plasmid pJMR17v3. Using methods as previously described, guide DNAs consisting of duplexed 23-mer oligos were then introduced into the gRNA expression cassette in pJMR17v3 using the SapI restriction site [15]. Short repair templates were then generated by overlapping PCR as previously described using DNA primers listed in the Supplementary material (Table S2) [15].
Strain construction
Electrocompetent cells were prepared as previously described [10] and were mixed with ~10 mg repair template DNA (containing the desired/introduced ERG6 sequence) and ~10 μg plasmid (cloned with the guide DNA matching the target ERG6 sequence) before electroporation with a BioRad GenePulser (BioRad, Hercules, CA, USA). One millilitre of 1 M sorbitol was used to transfer transformation mixtures to a culture tube containing 1 mL YPD, and transformants were allowed to recover for 4—6 h at 35°C with shaking. Aliquots of the recovered transformants were spread on YPD plates supplemented with 200 mg/mL nourseothricin (Nou200) and incubated at 35°C until colonies formed. Single colonies were picked from transformation plates and patched sequentially on YPD agar. The ERG6 sequence was verified by Sanger sequencing, desired colonies were cultured in 2 mL YPD overnight at 35°C and subsequently single colonies were then replica plated on both YPD agar and Nou200 to confirm nourseothricin susceptibility due loss of the pJMR17v3 plasmid. The GenBank accession numbers for verified strains are listed in the Supplementary material (Table S1).
Sanger sequencing
Genomic DNA isolated from transformant colonies was used to amplify the ERG6 open reading frame in PCR with primers CAU0013J01m and CAU0014J01m (see Supplementary material, Table S2) and Phusion Green master mix per the manufacturer’s instructions (Thermo Scientific, Waltham, MA, USA). PCR amplicons were then used as templates in sequencing reactions primed with sequencing primers (Table S2) and run on an 3730xl DNA Analyzer (Applied Biosystem, Foster City, CA, USA).
Comprehensive sterol profiling
Laboratory-derived strains and the parental clinical isolates were grown to the exponential growth phase at 35°C in RPMI liquid medium. Alcoholic potassium hydroxide was used to extract nonsaponifiable lipids. Samples were dried in a vacuum centrifuge (Heto) and then derivatized by adding 100 μL 90% N,O-bis (trimethylsilyl)-trifluoroacetamide-10% tetramethylsilane (TMS) (Sigma, St Louis, MO, USA) and 200 μL anhydrous pyridine (Sigma) while heating at 80°C for 2 hours as previously described [10,18]. Gas chromatography-mass spectroscopy (with a Thermo Scientific 1300 gas chromatography system coupled to an ISQ mass spectrometer) was used to identify TMS-derivatized sterols through comparison with known standards, and sterol profiles for each sample were determined using XCALIBUR software (Thermo Scientific). All sterol analyses were performed in biological triplicate. Error bars for each data point represent the standard deviations of results from three technical replicates.
MICs by Etest
Etests were performed in biological duplicate to determine amphotericin B MICs as per the manufacturer’s instructions (Biomérieux USA, Chicago, IL, USA) with modifications as recommended by the CLSI.
Results
Patient case and antifungal susceptibility of clinical C. auris isolates
A 33-year-old woman receiving treatment for Hodgkin’s lymphoma and advanced stage nodular sclerosis was admitted to the hospital after complaints of respiratory distress. High-resolution chest computed tomography revealed bilateral diffuse alveolar disease, ground-glass opacities and mild right-sided pneumothorax. The patient was empirically initiated on broad-spectrum antimicrobials including meropenem, linezolid and liposomal amphotericin B (dosed at 5 mg/kg) (Fig. 1). Bronchoalveolar lavage fluid and endotracheal tube cultures would subsequently grow Candida albicans, and liposomal amphotericin B was continued for a total of 3 weeks. The patient’s condition improved and chemotherapy was resumed approximately 1 month later.
Fig. 1.
High-level amphotericin B resistance develops during treatment of a Candida auris invasive infection. Timeline for the isolation of Candida clinical isolates and antifungal treatment administered to the patient. CAS, caspofungin; ETT, endotracheal tube; L-AMB, liposomal amphotericin B.
Subsequently, the patient developed dyspnoea and alveolar haemorrhage and treatment with meropenem and methylprednisolone was initiated. Cultures of endotracheal tube secretions grew yeast (Isolate 1) initially identified as Candida famata by Vitek2, but later revealed to be C. auris by internal transcribed spacers sequencing. When applying the tentative CDC breakpoints, Isolate 1 was resistant to fluconazole but susceptible to caspofungin and amphotericin B. A 2-week course of caspofungin treatment was initiated, to be followed by a 2-week course of liposomal amphotericin B (5 mg/kg/day) when endotracheal tube cultures remained positive. Following this course of amphotericin B, all respiratory cultures remained negative for C. auris; however, the patient remained on broad-spectrum antibiotics including piperacillintazobactam, amikacin and colistin for a complicated course of treatment for a carbapenem-resistant Pseudomonas aeruginosa respiratory tract infection.
One month later, the patient developed a urinary tract infection and cultures grew yeast initially identified as Candida haemulonii, but later confirmed as C. auris by internal transcribed spacer sequencing (Isolate 2). Isolate 2 was highly resistant to both fluconazole and amphotericin B but remained susceptible to caspofungin (Table 1), and a 2-week course of caspofungin was initiated. After the completion of caspofungin treatment, urine cultures again grew C. auris (Isolate 3), and a 3-week course of liposomal amphotericin B (5 mg/kg/day) was initiated. Ten days later, urine cultures again grew C. auris (Isolate 4). Intriguingly, although Isolate 4 was resistant to both fluconazole and caspo-fungin, it had regained susceptibility to amphotericin B (Table 1). Shortly after the completion of amphotericin B therapy, the patient was transferred to another medical facility and was lost to follow up.
Table 1.
Clinical antifungal susceptibilities for Candida auris isolates
| Clinical isolate | Clinical antifungal MIC (mg/L) |
||
|---|---|---|---|
| Amphotericin B | Fluconazole | Caspofungin | |
| Isolate 1 | 0.75 | 256 a | 0.75 |
| Isolate 2 | >32 | 96 | 0.38 |
| Isolate 3 | >32 | 96 | 0.38 |
| Isolate 4 | 0.75 | 256 | 4 |
Minimum inhibitory concentrations shown in bold type exceed tentative CDC breakpoints.
Whole-genome sequencing
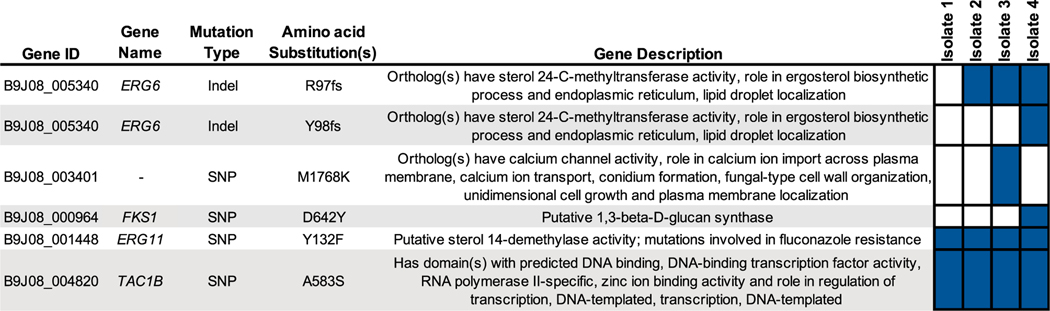
Whole-genome sequencing revealed all four isolates to belong to Clade I (subclade b). Four or fewer single nucleotide polymorphisms or indels were found to separate any two isolates, consistent with all isolates being genetically related (Fig. 2) [11]. All isolates were found to have mutations previously associated with fluconazole resistance in the ERG11 and TAC1B genes, encoding the Y132F and A583S amino acid substitutions, respectively. The two isolates exhibiting high-level (MIC >32 mg/L) amphotericin B resistance, Isolates 2 and 3, were both found to have an indel mutation creating a frame shift at the 97th codon (R97 fs) in the sterol-methyltransferase gene, ERG6, resulting in both an amino acid substitution and an early stop codon and probably nonsense transcript as this alteration occurs upstream of the conserved sterol-methyltransferase domain (the resulting ERG6 allele is represented in this manuscript as ERG6YY98V*). Intriguingly, the terminal amphotericin B-susceptible isolate, Isolate 4, was found to retain this indel mutation in ERG6, but to have also acquired a duplication of two nucleotides creating a second frameshift mutation at codon 98 (Y98 fs), resulting in a full-length ERG6 transcript with three altered amino acid residues encoding RYY97LVS (the resulting ERG6 allele containing both frameshift mutations is represented in this manuscript as ERG6RYY97LVS). Isolate 4 was also found to have a novel mutation (encoding D642Y) in hot-spot 1 of the gene encoding β-d-glucan synthase, FKS1. Importantly, this mutation alters a residue distinct from the three resistance-associated mutations in C. auris FKS1 (encoding the S639F, S639P and S639Y amino acid substitutions) previously reported [7,8,19]. No other non-synonymous mutations were found to differ between the clinical isolates in this study.
Fig. 2.
Whole genome sequencing reveals an association between mutations in Candida auris ERG6 and high-level amphotericin B resistance. Non-synonymous mutations differing between C. auris clinical isolates in this study, and those previously associated with antifungal resistance are shown with gene identifier, gene name, mutation type, encoded amino acid substitution(s) and gene description (as listed on the Candida Genome Database; candidagenome.org). Boxes filled in blue indicate the presence of the listed mutation in the corresponding clinical isolate. Isolate 4 possesses both the R97 fs and Y98 fs mutations in ERG6, resulting in the allele containing the amino acid substitutions RYY97LVS in the native reading frame.
Impact of ERG6 mutations on amphotericin B susceptibility
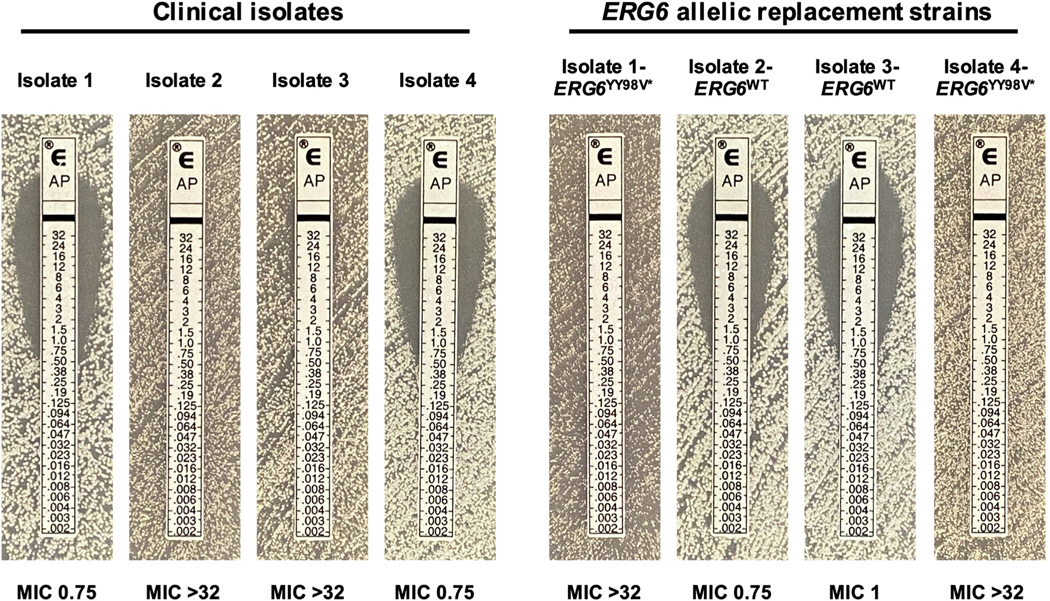
To test the influence of the identified mutations in C. auris ERG6 on amphotericin B susceptibility, a transient Cas9-mediated C. auris genetic manipulation system was used to perform allelic exchange. Transformations were performed by electroporation, two independent transformants were obtained for each allele exchange, and all transformants were confirmed by Sanger sequencing as previously described [10,11]. Introduction of the YY98V* encoding indel mutation into the ERG6 gene of Isolates 1 and 4 resulted in a ≥32-fold increase in amphotericin B MIC in both backgrounds (Fig. 3) and no difference was observed between biological replicate transformants (data not shown). Conversely, introduction of the wild-type (matching the B8441 reference sequence) ERG6 sequence into both Isolates 2 and 3 resulted in a complete restoration of amphotericin susceptibility (≥32-fold reduction in MIC), confirming the role of this mutation in the high-level resistance observed.
Fig. 3.
Mutations in Candida auris ERG6 confer high-level amphotericin B resistance. Amphotericin B MIC as determined by Etest for each of the clinical isolates and the ERG6 allelic replacement strains constructed in these studies are shown.
Comprehensive sterol profiling
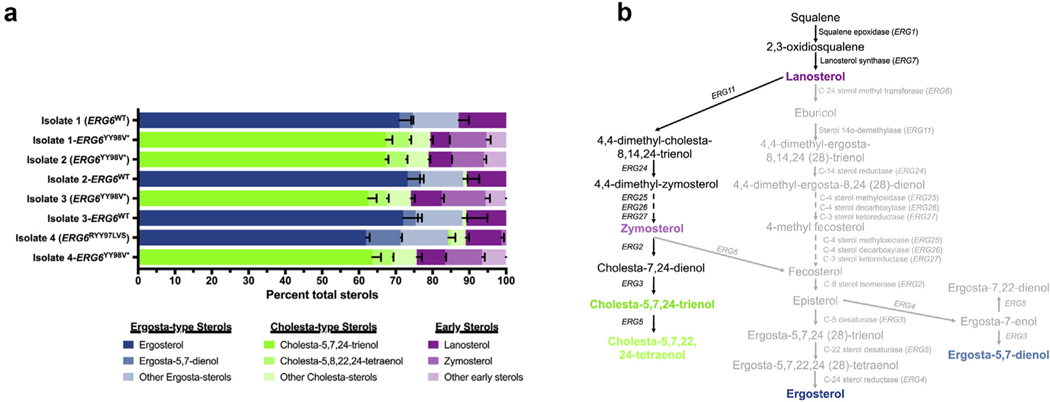
As amphotericin B is known to act by directly binding to the predominant sterol of most medically relevant fungi, ergosterol and the YY98V* encoding mutation in ERG6 was hypothesized to result in an abrogation of sterol-methyltransferase activity, we next performed comprehensive sterol profiling to investigate changes in sterol composition [11]. Consistent with previously reported C. auris sterol compositions, all amphotericin B-susceptible isolates and strains were observed to have ergosterol as the predominant sterol (>60%), with ergosta-type sterols (such as ergosta-5,7-dienol and ergosta-5,8,22,24(28)-tetraenol), and early sterols (such as lanosterol and zymosterol) comprising the majority of remaining sterols (Fig. 4). Conversely, all isolates and strains with the amphotericin B resistance-conferring ERG6YY98V* allele were found to have sterol profiles completely devoid of ergosta-type sterols. Instead, cholesta-5,7,24-trienol was found to be the most abundant sterol (>60%) in these samples, with the remainder of sterols comprised of other cholesta-type sterols (such as cholesta5,8,22,24-tetraenol and cholesta-7,24-dienol) and early sterols. Hence, the observed resistance to amphotericin B correlates directly with the abrogation of ergosterol production.
Fig. 4.
Mutations in Candida auris ERG6 result in an abrogation of ergosterol biosynthesis and accumulation of cholesta-type sterols. (a) Comprehensive sterol profiles of each of the clinical isolates and the ERG6 allelic replacement strains constructed in these studies. Sterol profiles are shown with each sterol represented as the proportion of total cell sterols. Error bars represent the standard deviation from three independent biological samples. (b) Putative C. auris sterol biosynthesis pathway demonstrating diversion of sterol production towards cholesta-type sterols upon loss of Erg6 activity. Arrows between sterols are labelled with the specific ergosterol biosynthesis genes encoding the enzymes predicted to catalyse this reaction. Sterols shown in green, blue and purple correspond to cholesta-type, ergosta-type and early sterols as shown in comprehensive sterol profiling. Loss of Erg6 activity is predicted to abrogate production of ergosterol, ergosta-5,7-dienol and intermediary sterols shown in grey.
Discussion
The patient case presented here exemplifies the challenges that antifungal resistance poses to clinicians treating patients with C. auris infections, and for the first time identifies mutations in the C. auris sterol-methyltransferase gene, ERG6, as a mechanism conferring high-level amphotericin B resistance. These findings were supported by allelic replacement studies. The ERG6 mutations described here resulted in a dramatic shift in C. auris cell sterol profiles, diverting sterol production towards cholesta-type sterols more like those found in mammalian cell membranes. Furthermore, a decreased growth rate was observed among all isolates or strains harbouring the amphotericin B resistance-associated ERG6 allele (ERG6YY98V*), suggesting a possible selective pressure that may have led to the mutation restoring ERG6 activity in Isolate 4 (Fig. S1; see supplementary methods and results Appendix S1). Intriguingly, although we have previously reported the combination of mutations in Candida glabrata ERG2 and ERG6 as being associated with both sterol profiles favouring cholesta-type sterols and increased amphotericin B resistance, the level of amphotericin B resistance observed in C. glabrata clinical isolates was significantly lower (MICs of 1–4 mg/L) than that which we observed in the C. auris clinical isolates in these studies (MIC >32 mg/L) [20]. It remains to be seen whether this stark difference in the magnitude of amphotericin B resistance is due to differences in other cell membrane lipids or corresponds to how these distantly related species of Candida respond to the stress induced by amphotericin B. Moreover, although mutations in C. auris ERG6 were not identified as associated with amphotericin B resistance in our previous analysis of 92 clinical isolates (including 22 amphotericin B-resistant isolates), multiple studies have recently described C. auris clinical isolates with high-level amphotericin B resistance, similar to the resistance observed in the isolates studied in this work, and ERG6 sequences were not reported [21–23].Further research is needed to determine the prevalence of ERG6 mutations among amphotericin B-resistant clinical isolates of C. auris.
Transparency declaration
All authors have stated that there are no reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed. This work was supported through the Society of Infectious Diseases Pharmacists Young Investigator Research Award granted to JMR, and NIH grant R01 A1058145 awarded to PDR. CAC and JFM were supported by NIAID award U19AI110818 to the Broad Institute. CAC is a CIFAR fellow in the Fungal Kingdom Programme. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Supplementary Material
Acknowledgements
Preliminary results of these studies were presented on 9 July 2021 at the ‘News in the antifungal world: new drugs and resistance mechanism’ oral session as part of the 31st European Congress of Clinical Microbiology & Infectious Diseases with the support of the 31st ECCMID 2021 Travel Grant.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2021.11.024.
References
- [1].Lockhart SR, Etienne KA, Vallabhaneni S, Farooqi J, Chowdhary A, Govender NP, et al. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis 2017;64:134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Anonymous. CDC’s antibiotic resistance threats in the United States. Available at: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threatsreport-508.pdf; 2019.
- [3].Satoh K, Makimura K, Hasumi Y, Nishiyama Y, Uchida K, Yamaguchi H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol Immunol 2009;53: 41–4. [DOI] [PubMed] [Google Scholar]
- [4].Ahmad S, Alfouzan W. Candida auris: epidemiology, diagnosis, pathogenesis, antifungal susceptibility, and infection control measures to combat the spread of infections in healthcare facilities. Microorganisms 2021;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sexton DJ, Bentz ML, Welsh RM, Derado G, Furin W, Rose LJ, et al. Positive correlation between Candida auris skin-colonization burden and environmental contamination at a ventilator-capable skilled nursing facility in Chicago. Clin Infect Dis 2021;73:1142–8. 10.1093/cid/ciab327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Anonymous. Candida auris: antifungal susceptibility testing and interpretation. Atlanta, GA: Centers for Disease Control and Prevention; 2020. Available at: https://www.cdc.gov/fungal/candida-auris/c-auris-antifungal.html. [Google Scholar]
- [7].Chowdhary A, Prakash A, Sharma C, Kordalewska M, Kumar A, Sarma S, et al. A multicentre study of antifungal susceptibility patterns among 350 Candida auris isolates (2009–17) in India: role of the ERG11 and FKS1 genes in azole and echinocandin resistance. J Antimicrob Chemother 2018;73:891–9. [DOI] [PubMed] [Google Scholar]
- [8].Biagi MJ, Wiederhold NP, Gibas C, Wickes BL, Lozano V, Bleasdale SC, et al. Development of high-level echinocandin resistance in a patient with recurrent Candida auris candidemia secondary to chronic candiduria. Open Forum Infect Dis 2019;6:ofz262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Healey KR, Kordalewska M, Jimenez Ortigosa C, Singh A, Berrio I, Chowdhary A, et al. Limited ERG11 mutations identified in isolates of Candida auris directly contribute to reduced azole susceptibility. Antimicrob Agents Chemother 2018;62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rybak JM, Doorley LA, Nishimoto AT, Barker KS, Palmer GE, Rogers PD. Abrogation of triazole resistance upon deletion of cdr1 in a clinical isolate of Candida auris. Antimicrob Agents Chemother 2019;63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rybak JM, Munoz JF, Barker KS, Parker JE, Esquivel BD, Berkow EL, et al. Mutations in TAC1B: a novel genetic determinant of clinical fluconazole resistance in Candida auris. mBio 2020;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ahmad S, Khan Z, Al-Sweih N, Alfouzan W, Joseph L. Candida auris in various hospitals across Kuwait and their susceptibility and molecular basis of resistance to antifungal drugs. Mycoses 2020;63:104–12. [DOI] [PubMed] [Google Scholar]
- [13].Rybak JM, Dickens CM, Parker JE, Caudle KE, Manigaba K, Whaley SG, et al. Loss of C-5 sterol desaturase activity results in increased resistance to azole and echinocandin antifungals in a clinical isolate of Candida parapsilosis. Antimicrob Agents Chemother 2017;61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Munoz JF, Gade L, Chow NA, Loparev VN, Juieng P, Berkow EL, et al. Genomic insights into multidrug-resistance, mating and virulence in Candida auris and related emerging species. Nat Commun 2018;9:5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lombardi L, Oliveira-Pacheco J, Butler G. Plasmid-based CRISPR-Cas9 gene editing in multiple Candida species. mSphere 2019;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lombardi L, Oliveira-Pacheco J, Butler G, et al. Plasmid-Based CRISPR-Cas9 Gene Editing in Multiple Candida Species. mSphere 2020;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bijlani S, Thevandavakkam MA, Tsai HJ, Berman J. Autonomously replicating linear plasmids that facilitate the analysis of replication origin function in Candida albicans. mSphere 2019;Anonymous. [DOI] [PMC free article] [PubMed]
- [18].Kelly SL, Lamb DC, Kelly DE, Manning NJ, Loeffler J, Hebart H, et al. Resistance to fluconazole and cross-resistance to amphotericin B in Candida albicans from AIDS patients caused by defective sterol delta5,6-desaturation. FEBS Lett 1997;400:80–2. [DOI] [PubMed] [Google Scholar]
- [19].Rhodes J, Abdolrasouli A, Farrer RA, Cuomo CA, Aanensen DM, ArmstrongJames D, et al. Genomic epidemiology of the UK outbreak of the emerging human fungal pathogen Candida auris. Emerg Microbe. Infect 2018;7:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ahmad S, Joseph L, Parker JE, Asadzadeh M, Kelly SL, Meis JF, et al. ERG6 and ERG2 are major targets conferring reduced susceptibility to amphotericin B in clinical Candida glabrata isolates in Kuwait. Antimicrob Agents Chemother 2019;63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Maphanga TG, Naicker SD, Kwenda S, Munoz JF, van Schalkwyk E, Wadula J, et al. In Vitro antifungal resistance of Candida auris isolates from bloodstream infections, South Africa. Antimicrob Agents Chemother 2021;65:e0051721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Barantsevich NE, Vetokhina AV, Ayushinova NI, Orlova OE, Barantsevich EP. Candida auris bloodstream infections in Russia. Antibiotics (Basel) 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Shastri PS, Shankarnarayan SA, Oberoi J, Rudramurthy SM, Wattal C, Chakrabarti A. Candida auris candidaemia in an intensive care uni-t-dprospective observational study to evaluate epidemiology, risk factors, and outcome. J Crit Care 2020;57:42–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.