Abstract
Venous and arterial thromboses, called as cancer-associated thromboembolism (CAT), are common complications in cancer patients that are associated with high mortality. The cell-surface glycoprotein tissue factor (TF) initiates the extrinsic blood coagulation cascade. TF is overexpressed in cancer cells and is a component of extracellular vesicles (EVs). Shedding of TF+EVs from cancer cells followed by association with coagulation factor VII (fVII) can trigger the blood coagulation cascade, followed by cancer-associated venous thromboembolism in some cancer types. Secretion of TF is controlled by multiple mechanisms of TF+EV biogenesis. The procoagulant function of TF is regulated via its conformational change. Thus, multiple steps participate in the elevation of plasma procoagulant activity. Whether cancer cell-derived TF is maximally active in the blood is unclear. Numerous mechanisms other than TF+EVs have been proposed as possible causes of CAT. In this review, we focused on a wide variety of regulatory and shedding mechanisms for TF, including the effect of SARS-CoV-2, to provide a broad overview for its role in CAT. Furthermore, we present the current technical issues in studying the relationship between CAT and TF.
Subject terms: Molecular medicine, Cancer
Background
Cancer is the main risk factor for venous [1, 2] and arterial [2–5] thromboembolism (cancer-associated thromboembolism [CAT]). CAT, especially cancer-associated venous thromboembolism (CA-VTE), is associated with poor patient prognosis [1, 2]. Risk factors for CA-VTE include tumour sites, blood immune cells, and patient characteristics [1, 2].
Tissue factor (TF) is a cell-surface transmembrane protein expressed by various cells in the perivascular space, including fibroblasts and pericytes [6], and immune cells, including monocytes and macrophages [6, 7]. TF triggers the extrinsic blood coagulation cascade by binding to activated blood coagulation factor VII (fVIIa), which is followed by activation of factor X (fX) secreted from the liver [6, 7]. Blood TF levels are low in healthy individuals, but are increased in cancer patients, probably as secretions from cancer and immune cells as a component of extracellular vesicles (EVs) [6]. Formation of the TF–fVIIa complex on EVs as in cell-surface TF is considered a primary determinant of CA-VTE in patients with pancreatic cancer [2, 8], given that the incidence of VTE in these patients is closely associated with blood and tissue TF levels. Neutrophil extracellular traps, increased platelets and cytokines may also contribute to CA-VTE in pancreatic and other cancer types [8].
TF in blood and tumour tissues is evaluated using ELISA [9–11] and immunohistochemistry [10–14], respectively with a limited sample size. TF can also be measured by EV procoagulant activity (PCA), given that CA-VTE is caused by PCA rather than TF antigen [9, 10, 14, 15]. However, these studies did not necessarily show associations between TF tissue and blood levels and the incidence of CAT. Notably, the results of ELISAs may vary according to the specificity of the antibody in each kit. In addition, TF-PCA is regulated by conformational changes in TF, which affects associations with fVII and the generation of fVIIa [7, 16]. Thus, whether or not TF-antigen levels and activity in cells and EVs (TF+EVs) are evaluated appropriately in cancer patients using current experimental methodology and study design is unclear. The shedding mechanics may also affect blood TF+EV levels.
Previous reviews have discussed topics including how TF–fVIIa causes malignant phenotypes through intracellular signalling pathways [17], risk factors, including TF [1, 2] and clinical treatment of CAT [18] and the effect of TF+EVs on the survival of cancer patients [19]. In this review, we discuss how CAT is induced by TF-PCA from molecular biology perspectives including the effect of SARS-CoV-2, which is responsible for the COVID-19 pandemic. We also present the current problems in elucidating this relationship in patients.
TF in CAT
VTE is associated with thrombi, mainly composed of fibrin and red blood cells generated in slow venous blood flow, whereas arterial thromboembolism (ATE) arises in fast arterial blood flow and the thrombi are mainly composed of platelets [3, 20]. Previous studies suggested that TF can be generated from multiple sources, although their roles in CAT have only been partially resolved. First, cancer cell-derived TF+EVs can promote CA-VTE as it activates the extrinsic coagulation cascade [3, 8, 19], resulting in fibrin deposition, as evidenced by in vivo experiments with xenograft models of pancreatic cancer [8, 21, 22]. TF+EVs derived from non-cancer (immune) cells may also contribute to CAT [23]. Indeed, several clinical studies have shown an association between CA-VTE incidence and TF in patients with pancreatic [15, 24, 25] and other cancers [11, 12, 26]. Second, the effect of TF+EVs on the non-extrinsic coagulation cascade might lead to clinical CAT. Indeed, TF+EVs secreted from pancreatic cancer cells were shown to drive platelets to a procoagulant state [21]. Third, TF+ circulating tumour cells may contribute to CAT [27]. Fourth, in addition to full-length TF (hereafter, TF), alternatively spliced soluble TF (sTF) devoind of its transmembrane and cytoplasmic domains may associate with EVs appear on cancer cell surfaces via β1-integrin [28]. Although the role of sTF in CAT is unclear, one study showed that sTF has a procoagulant effect on pancreatic cancer cells and their EVs [29].
ATE is associated with atherosclerotic lesions, which consist of lipids and activated platelets [20]. Although ATE and VTE share common risk factors [20], no direct association between cancer cell-derived TF and ATE has been reported to our knowledge. In ATE, platelets promote arterial thrombus formation by increasing their numbers and activities [3, 4]. TF+EVs released from immune cells, such as monocytes and macrophages, can affect ATE [3, 30]. TF+EVs may bind platelets via interactions between P-selectin and P-selectin glycoprotein ligand-1, which is expressed on immune cell-derived EVs [30]. Thus, TF+EVs can accumulate and cause fibrin deposits at platelet aggregation sites in arteries.
Dyslipidemia also contributes to atherothrombosis in non-cancer individuals [31]. TF may be involved in CA-ATE because oxidised low-density lipoprotein (oxLDL) tends to be high in hyperlipidemic cancer patients [32, 33] and increases TF expression in endothelial cells [31, 34]. In addition, oxLDL enhances TF expression in monocytes and macrophages, thus increasing circulating TF-bearing EVs and accelerating clotting at atherosclerotic plaques [35].
Coagulation factors may be insufficiently supplied within tumour tissues owing to poor vascularisation and high tissue interstitial fluid pressure [36]. Cancer cells might alleviate this disadvantage as they can synthesise coagulation factors [36–38], although the importance of these ectopic procoagulants is unclear.
Regulation of TF procoagulant activity by encryption and decryption
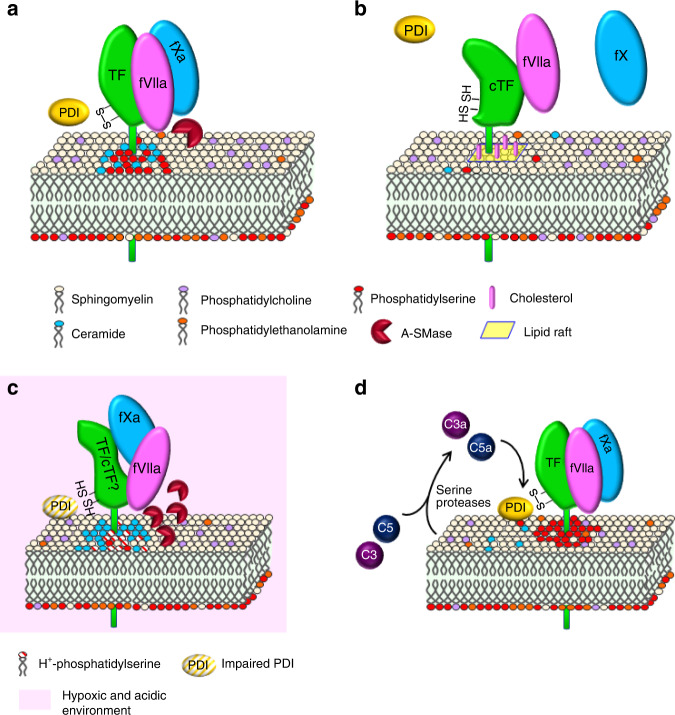
TF activity is partly regulated by its reversible conformational alteration process, “encryption and decryption” (E–D). Thus, the protein level does not necessarily reflect PCA. This mechanism has been studied in various cells including several types of cancer cells (Table 1). It involves many factors but can be attributed to two major mechanisms depending on (1) cell-membrane lipids (phosphatidylserine (PS) and sphingomyelin (SM)) and (2) intramolecular disulfide bonds (Fig. 1a, b). Encrypted TF with inactive procoagulant state (cryptic TF; cTF in Fig. 1b) weakly binds fVIIa [7, 16], but dissociates from coagulation factor X (fX; Fig. 1b), which halts coagulation activation [7, 16]. E–D has been widely studied; however, there are some limitations to understanding its relationship with CAT. First, data in cancer cells are limited. Second, the involvement of E–D in the occurrence of experimental and clinical CAT is unclear. The TF–fVIIa-fXa complex may be unstable in acidic tumour tissue [36] (Fig. 1c), although complex formation would be stabilised in neutral blood flow. Thus, TF-PCA on the cell surface and EVs may vary depending on the surrounding environment of TF in cancer patients.
Table 1.
The activation mechanisms of TF.
| Category | Responsible factor | Action mechanism | Examined cell (tissue) type [reference] |
|---|---|---|---|
| Typical decryption | PS in the plasma membrane | Externalisation of PS in plasma membrane | Human leukaemia (THP-1) cells [16, 39] |
| SM in the plasma membrane | Ceramide generation by A-SMase |
• Human monocyte-derived macrophages (MDMs) [46, 47] • Human endothelial cells (HUVECs) [47] • Murine peripheral blood mononuclear cells [47] |
|
| PDI | Disulfide bond (Cys186–Cys209) formation of TF |
• Human keratinocytes (HaCaTs) [52] |
|
| TF de-palmitoylation | Detachment from lipid raft, followed by a conformational change of the transmembrane domain of TF |
• Human endothelial cells [58] • Breast cancer (MDA-MB-231 and MCF-7) cells [58] |
|
| Complements |
• Externalisation of PS in plasma membrane • Enhancement of PDI-mediated disulfide bond formation of TF |
• MDA-MB-231 cells [59] • THP-1 cells [59] • Human and murine monocytes [60] • Human myeloma (MM1) cells [60] |
|
| SARS-CoV-2 | Ceramide formation via A-SMase | Human MDMs [63] | |
| Other mechanisms | Decoupling of Integrins-arf6 association |
• Increase cell-surface availability of TF • Conformational change of TF favourable to bind fVIIa |
• Murine macrophage and smooth muscle cells (SMCs) [77] • Murine breast cancer cells [77] • HaCaT cells [78] • Human melanoma (A7) cells [78] |
| TF glycosylation | Potential facilitation of substrate recognition | Placenta tissue [73] | |
| Pin1 | Maintenance of the active state of TF via its phosphor-Ser258 residue |
• Multiple human endothelial lines and SMCs [74, 75] • MDA-MB-231 cells [75] |
|
| CD248 | Direct allosteric conformational change of TF–fVIIa complex |
• Human and murine vascular SMCs [80] • Human monocytic leukaemia (MM6) and A7 cells [80] |
Fig. 1. Encryption-decryption process of TF associated with multiple regulatory factors.
Schematic representation of the active (a) and cryptic (b) state of TF on the cell surface. Potential E–D states within tumour tissues. Hypoxia and concomitant acidification (c) and cancer cell-driven complement activation (d) might influence the E–D process. c The TF–fVIIa-fXa complex is unstable with an intermediate structure between TF and cTF.
PS
PS is a major component of the cell-membrane lipid bilayer, along with phosphatidylcholine and phosphatidylethanolamine (Fig. 1). PS mainly exists in the inner leaflet and translocates to the outer leaflet (Fig. 1) in response to injury or other stimuli [7, 16]. For example, the generation of reactive oxygen species (ROS) from mitochondria in monocytes (monocytic leukaemia cells), followed by the production of oxidised lipid (4-hydroxynonenal) represses cell-membrane flippase activity to increase the outer leaflet fraction of PS [39]. These mechanisms are regulated by redox enzymes, protein disulfide isomerase (PDI) and thioredoxin/thioredoxin reductase (Trx/TrxR) [16, 40], and therefore should be important for cancer cells, considering the augmented ROS levels in cancer tissues [41, 42]. How PS affects TF decryption is not fully understood, but its interaction with TF ectodomain residues was examined by mutagenesis assay [43]. It was suggested that its interaction with the γ-carboxyglutamic acid domain of fVIIa is thought to help maintain the decrypted state [44]. PS on the cell surface interacts with the TF–fVIIa complex through Lys159 and Gly164 residues of TF [44]. PS exposure likely facilitates binding of fX to the cell membrane to increase TF-PCA. Also, it was suggested that PS interaction with Lys165 and Lys166 residues of TF enables TF-VIIa complex to generate more favourable orientation to associate with fX [44, 45]. Experimentally introduced TF gene (F3) mutations that affect several amino acids predicted to interact with PS reportedly resulted in suppressed TF-PCA [43]. However, mutation of all residues failed to completely impair TF decryption, indicating that the PS-TF interaction alone is insufficient for complete TF decryption [43].
SM and lipid rafts
SM is another major component of the membrane outer leaflet in resting cells. A recent study using endothelial cells, monocytes, and macrophages revealed that SM inhibits TF-PCA. Acid sphingomyelinase (A-SMase; Fig. 1a) transported from lysosomes to the plasma membrane in response to ATP stimuli of prinergic P2rX7 receptor degrades SM to produce ceramide on monocyte surfaces to cause decryption [46]. However, how SM and ceramide contribute to the E–D process is unclear [46]. A-SMase, induced by simultaneous treatment with lipo-polysaccharides and cytokines, also causes TF’s active state in endothelial cells, monocytes and macrophages [46, 47]. A-SMase contributes to cancer progression; the A-SMase–ceramide pathway might be activated under hypoxic and acidic conditions within tumour tissues (Fig. 1c) [48]. In addition, H+ in hypoxic tumour tissue would neutralise negative charges on PS molecules. Thus, the A-SMase pathway might predominate PS-driven decryption (Fig. 1c). Studies showed that ceramide-driven TF activity is suppressed by tricyclic antidepressants, which inhibit A-SMase in vitro and in vivo [47]. Thus, A-SMase inhibitor might be a potent candidate for CAT treatment.
Lipid rafts are plasma membrane microdomains that are associated with high amounts of cholesterol and SM [49]. TF is encrypted by association with lipid rafts, which is mediated by cholesterol [40, 50] (Fig. 1b). Removal of cholesterol from the plasma membrane decrypts TF [50]. Fatty-acid modification of Cys245 in TF affects TF inactivation by association with lipid rafts [51]. Additionally, SM interacts with cholesterol, leading to modulation of raft formation [40]. These data indicate that lipid rafts help maintain the TF cryptic state through multiple mechanisms.
Disulfide bond exchange in TF
Intracellular disulfide bonds between Cys186 and Cys209 cause TF decryption (Fig. 1) [7, 16, 40]. Cell-surface PDI associates with TF and mediates this process, enabling TF to select either the procoagulant or a transducer of signals into endothelial and keratinocyte cells [52]. Although PDI involvement was shown in haematologic cancer cells [53], this was not applicable in breast cancer (MDA-MB-231) cells [54]. This mechanism can contribute to TF association with EVs, as PDI exists on EV surfaces and helps generate decrypted TF [7, 40, 55]. However, PDI might not fully function under tumour hypoxia (Fig. 1c), as the catalytic activity of PDI is O2-dependent [36]. A study with MDA-MB-231 cells revealed that Trx/TrxR modifies the same TF thiol residues, thereby inhibiting the interaction between TF and fVIIa [56]. To date, PDI has been shown to contribute to TF-dependent thrombosis in an FeCl3-driven arterial thrombosis model, but its involvement in CAT is unclear [57].
TF palmitoylation
Palmitoylation of Cys245 in TF is required for the anchoring of TF with lipid rafts enriched with cholesterol and SM. A recent study using breast cancer cells showed that de-palmitoylation by palmitoyl-protein thioestelases enhances TF-PCA through conformational changes of TF favourable to the interaction between TF–fVIIa complex and fXa (Table 1) [58]. The mechanism likely involves lengthening of the transmembrane domain of TF [58]. Palmitoylation precedes the phosphorylation of Ser253 in TF; however, this modification is not responsible for TF activation [58].
Complements-driven decryption
Anti-thymocyte globulin- [59] and anti-phospholipid antibody- [60] driven activation of complements (C3 and C5) causes externalised PS in a PDI-dependent and -independent manner in inflammatory diseases. They can be coupled to thiol-disulfide exchange of PDI to decrypt TF in cancer cells (Table 1). This mechanism likely associates with TF decryption in cancer cells, as cancer cell-derived serine proteases also activate complements [61] (Fig. 1d). Complement can contribute to experimental arterial thrombosis in vivo, although its roles in experimental and clinical CAT are currently unclear [60].
SARS-CoV-2
Cancer patients are more sensitive to SARS-CoV-2 infection and resulting COVID-19 tends to be severer [62]. Recent studies reported that the TF-driven hypercoagulable state is associated with SARS-CoV-2-infected patients [63–67]. Patients with COVID-19 and active cancer are currently recommended to receive pharmacological or mechanical prophylaxis because of their very high thrombotic risk [62]. The SARS-CoV-2 spike protein binds cell-surface angiotensin-converting enzyme-2, leading to viral entry into cells [63]. This results in an increase of TF-PCA through activation of A-SMase, followed by ceramide formation [63] (Table 1). Thus, tricyclic antidepressants such as imipramin may be effective for the prevention of COVID-19-driven hypercoagulopathy [63]. In addition, ambroxol, a mucolytic drug, may be effective in the prevention of TF-driven thromboembolism in patients, because it inhibits both A-SMase activity and SARS-CoV-2 entry into epithelial cells [68].
SARS-CoV-2 infection could also enhance thrombosis because it increases TF expression at the mRNA level in the lung of severe COVID-19 patients associated with acute respiratory distress syndrome [66]. The mechanisms of COVID-19-driven TF increase were not shown; however, tissue hypoxia owing to lung injury likely contributes to this phenomenon given that the F3 (TF) gene can be activated in response to hypoxia [17, 37, 38]. Another study showed that PCA of TF+EVs [64] and an anticoagulant protein, tissue factor pathway inhibitor [67], are significantly increased and decreased, respectively, in the blood of COVID-19 patients, potentially increasing the incidence of thromboembolism. TF+EVs in cancer patients with SARS-CoV-2 infection can be derived from not only cancer cells but also monocytes associated with activated platelets [69]. Importantly, PCA [64] and TF-antigen level [69] are associated with disease severity. Platelet-neutrophil interaction also plays roles in SARS-CoV-2-driven coagulopathy as complements activated in COVID-19 patients enhance neutrophil/monocyte activation, resulting in the release of thrombogenic neutrophil extracellular traps associated with active TF [70].
Additional mechanisms that potentially activate TF
Regulation of TF activity involves many molecular processes and is a complex phenomenon. The mechanisms that contribute to the regulation of TF activity are described below. However, their relation to E–D is currently obscure.
TF glycosylation
Glycosylation as a candidate mechanism of TF regulation has been evaluated in various cell types, including cancer cells (MDA-MB-231) [71, 72]. Mutations at extracellular glycosylation sites within TF did not affect its association to fVIIa or TF-PCA formation [71, 72]. Experiments using HUVEC cells showed that glycosylation also does not affect the TF E–D process [71]. Experiments using cells treated with a pharmacological glycosylation inhibitor, tunicamycin, yielded similar findings [71]. Thus, TF–fVIIa activity is thought to be mainly regulated by membrane lipid components but not by glycosylation. However, an earlier study showed that glycosylation enhances the activity of placenta-derived TF (Table 1) [73]. Thus, different carbohydrate modification patterns, possibly affected by cell type, may influence PCA.
Peptidyl-prolyl isomerase (Pin1)
Pin1 was found to increase TF-PCA in multiple cell types (Table 1) [74]. Although the mechanism is unclear, this effect is likely mediated by Pin1 binding to Ser258–Pro259 in TF’s short cytoplasmic domain (Fig. 2a, b) [75]. Pin1 also enhances TF stability by inhibiting its polyubiquitination and subsequent proteasomal degradation [74, 75]. Pin1 also activates the F3 gene via the pro-inflammatory transcription factor complexes NFκB and AP-1 [74]. Overall, Pin1 likely regulates many TF functions to enhance PCA in normal cells, although whether these mechanisms are present across all cancers is unknown.
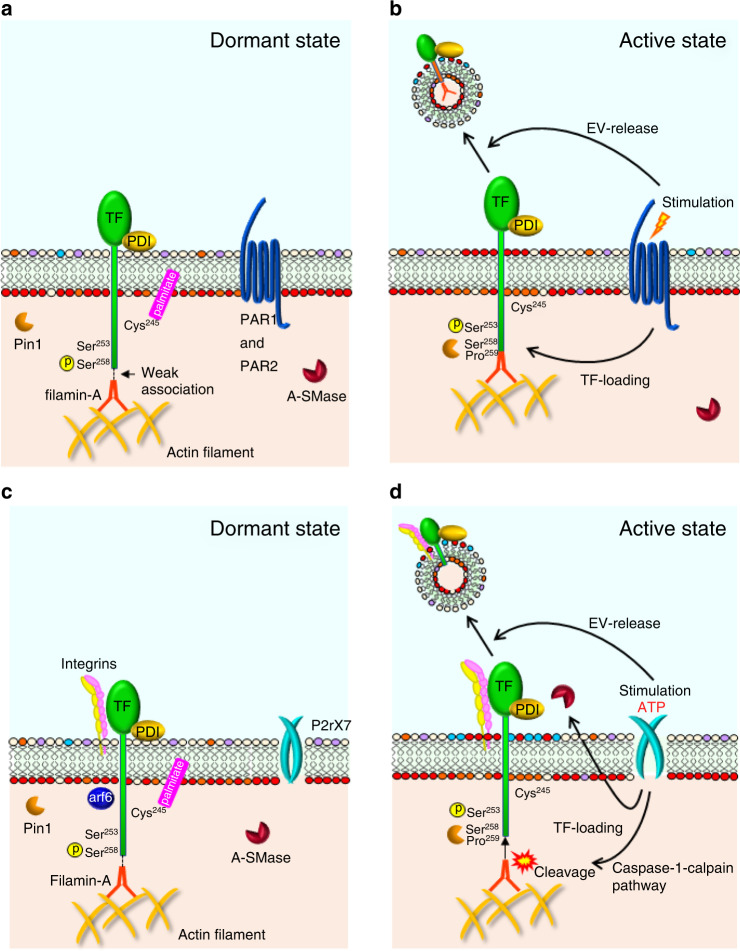
Fig. 2. Shedding of TF-EVs induced by PAR and P2rX7 activation.
Dormant (a) and active (b) states of TF-PCA, TF loading into EVs, and EV shedding in association with PARs. PARs facilitate both incorporation of TF into EVs and the production of EVs in cooperation with filamin-A and Pin1. However, the effects are likely cell-type-dependent. Dormant (c) and active (d) states of TF in relation to P2rX7. P2rX7 functions in cooperation with integrins, A-SMase, and caspase-1-calpain pathway-driven filamin-A cleavage to facilitate TF loading and EV biogenesis.
Integrins
The integrin-β1 heterodimeric complex with its α-subunits associates with TF on the surface (Fig. 2c, d) of various cells in response to fVIIa stimulation, thereby controlling TF-PCA [76, 77]. Stimulation of P2rX7 receptor results in decoupling of the TF–integrin complex from a small GTPase, arf6. This increases cell-surface availability and affinity to fVIIa of TF in association with release of cell-surface PDI (Table 1 and Fig. 2c, d) [77]. TF complexed with integrins also helps activate pro-angiogenic signalling pathways. This process is mediated by arf6, which regulates endosomal trafficking of integrins. Inhibiting arf6 directs TF to a procoagulant status, rather than activating intracellular signals in cancer cells [78]. The TF–integrin interaction in EVs shed from endothelial cells enhances their binding potency to extracellular matrix components, thereby increasing PCA of TF+EVs [79].
CD248
The cell-surface glycoprotein CD248 contributes to TF activation (Table 1) [80]. CD248 binds TF and changes the conformation of the TF–fVIIa-fX complex to activate fX in various cells in a decryption-independent manner [80]. Furthermore, CD248 facilitated ATE and VTE in CD248-knockout mouse models [80]. The importance of this mechanism in CAT is currently unclear.
Regulation of TF+EV production
TF loading into EVs
Studies of TF release into EVs based on experiments using human endothelial cell lines with forced TF expression found that phosphorylated-Ser253 facilitates and phosphorylated-Ser258 suppresses TF incorporation in EVs upon cell-surface protease-activated receptor 2 (PAR2) activation (Fig. 2a, b) [81]. Protein kinase Cα and p38α phosphorylate cytoplasmic Ser253 and Ser258 residues, respectively [82]. The two adjacent serine residues reciprocally regulate TF release into EVs. In addition, de-palmitoylation of Cys245 promotes TF incorporation, as this process precedes the phosphorylation of Ser253 (Fig. 2a, b) [58].
Investigations of how the cytoplasmic domain controls TF release into EVs found that filamin-A, an actin-binding protein that connects between the cytoskeleton (actin filament) and cell-membrane proteins, is essential for TF release into EVs in endothelial and cancer cell lines (Fig. 2a, b) [37, 83]. Filamin-A binds to cytoplasmic immunoglobulin-like repeats in TF, which likely helps incorporate TF into EVs in MDA-MB-231 cells when PAR2 is activated [83]. Another study using ovarian cancer cells in hypoxic conditions found that filamin-A is also crucial for release of TF-filamin-A complex-positive EVs [37]. However, neither PAR1 nor PAR2 mediate TF loading to EVs, which suggests that cell type and context affect PAR2 function in TF release into EVs (Fig. 2a, b). Follow-up experiments with MDA-MB-231 cells showed the TF-filamin-A interaction was enhanced by TF Ser253 phosphorylation, whereas phosphorylated-Ser258 alleviated this interaction (Fig. 2a, b) [84]. This is consistent with findings that phosphorylating Ser253 and Ser258 facilitates and diminishes TF release into EVs, respectively [75]. Furthermore, disrupting lipid rafts by extracting cholesterol from plasma membranes impaired cell-surface TF activity and TF+EV release [84, 85]. The authors showed that cellular TF released into the cytoplasm might translocate to cell-surface lipid rafts in a filamin-A-dependent manner. However, the role of cholesterol in TF regulation is controversial, as another study suggested that the association with lipid rafts (cholesterol) generates cryptic TF (see SM and lipid rafts section).
A previous study showed that P2rX7 receptor signalling is responsible for TF incorporation into EVs in macrophages (Fig. 2c, d) [57, 86]. The P2rX7 receptor uncouples the Trx/TrxR system to increase cellular ROS, leading to activation of caspase-1-calpain pathway and filamin-A cleavage [86]. This cleavage releases TF from the cytoskeleton, thereby facilitating its incorporation into EVs (Fig. 2c, d). Thus, the role of filamin-A in TF release into EVs may be cell-type-dependent. P2rX7 receptor signalling also contributes to integrin-TF complex incorporation into EVs on the surface of macrophages, smooth muscle cells, and breast cancer cells by inhibiting arf6 activity, which controls cellular integrin trafficking (Fig. 2c, d) [77]. Thus, P2rX7 signalling promotes TF release by multiple mechanisms.
Pin1 interacts with the cytoplasmic tail of TF by interacting with phospho-Ser258 and Pro259 in human endothelial and MDA-MB-231 cells (Fig. 2) [74]. The stability of TF within the cytoplasm is increased by the Pin1–TF interaction, followed by proline isomerisation [74, 75]. This alteration prevents polyubiquitination of TF [75]. Thus, protection of TF from proteasomal degradation promotes prolonged and efficient TF loading into EVs.
Generation of EVs
Filamin-A also affects the generation of EVs from ovarian cancer cells, at least under hypoxic conditions (Fig. 2a, b) [37]. This contrasts with a study that showed filamin-A inhibition increased PAR2-driven EV shedding from MDA-MB-231 cells cultured under normoxia [83]. In macrophages, caspase-1 activation associated with filamin-A cleavage, followed by TF incorporation into EVs, also contributed to EV shedding [7, 86]. ATP-driven P2rX7 receptor signalling facilitates TF loading, TF-PCA, and EV secretion (Fig. 2c, d) [7, 16, 40, 57, 86]. These findings suggest that active TF release is coupled to EV shedding. Lipid rafts have been reported to be a significant factor in EV shedding from MDA-MB-231 [84] and THP-1 [85] cells, as described above. A recent study provided evidence of TF signalling in EV biogenesis from MDA-MB-231 cells, in which TF was abolished by genome editing [87].
Evaluating TF produced in cancer specimens
Prediction of CAT by TF-PCA
Given that PCA, rather than TF level, causes thrombosis, the evaluation of PCA seems more important for the prediction of CAT compared with that of TF antigen [15, 24, 25, 88–90]. EVs are widely used to evaluate TF-PCA, as most cancer cell-derived TF is likely secreted as an EV component. Indeed, PCA has been shown to positively correlate with VTE incidence in patients with pancreatic cancer [2, 15, 24, 25]. They are generally isolated from plasma samples by high-speed centrifugation (~20,000×g) [9, 10, 15, 88, 89]. However, plasma preparation, centrifugation force and duration for EV isolation varied among these studies. Thus, the recovery rates for cancer cell-derived EVs may differ by study design. One study of EV recovery from cultured medium of various cancer cell lines found that centrifugation at 20,000×g and 100,000×g resulted in 26% and 67% EV recovery, respectively [91]. Thus, isolation using 20,000 × g might miss a considerable fraction of TF+EVs, leading to underestimated PCA. However, 20,000 × g has been suggested to be qualitatively sufficient to recover TF+EVs [92]. A centrifugation procedure with an EV washing step also reportedly affects results [93]. Thus, improved techniques may enhance understanding of the relationship between CAT and TF. TF levels likely do not necessarily correlate with CAT incidence because TF is not fully decrypted in blood samples. However, TF level may be important as it predicts PCA at the onset of CAT, as shown in our recent study [94].
Prediction of CAT by TF-antigen level
ELISA and immunohistochemistry [10–13, 15] are used to evaluate TF levels in plasma and cancer tissues. Indeed, the specificity and reactivity of anti-TF antibodies in ELISA affect their precision as previously suggested [9, 90]. In addition, current ELISA kits mostly use sandwich-ELISA methodology with two different antibodies. However, the reactivity of anti-TF antibodies might be influenced by glycosylation and the TF folding pattern [71, 72]. In addition, the identities of TF antibodies in commercial ELISA kits are uncertain. Thus, whether ELISA systems precisely quantitate TF levels is unclear. Table 2 lists the TF-ELISA kits used in published reports and highlights that the specific kits and their application differ by study.
Table 2.
Commercial human TF-ELISA kits used in previous studies.
| Supplier | Kit name (current availability) | Sample [reference] |
|---|---|---|
| Biomedica Diagnostics | IMUBIND Tissue Factor ELISA (yes) |
• PPP (healthy volunteer and patients with various cancer types) [88] • PPP and PFP (healthy volunteer) [92] • EVs secreted from endothelial cells [98] • Serum of epithelial ovarian cancer patients [99] • Cell lysate [94] |
| HYPHEN BioMed | ZYMUTEST Tissue Factor (yes) |
• PPP and PFP (healthy volunteer) [92] • Tumour tissue (epithelial ovarian cancer patients) [11] • Cell lysate [94] |
| R&D Systems | Human Tissue Factor Quantikine ELISA (yes) |
• Plasma (unclear whether PPP or PFP) (patients of various cancer types) [26] • PPP and EVs (healthy volunteer and epithelial ovarian cancer patients) [9] • EVs secreted from MDA-MB-231 cells [84] • Cell lysate and PFP (healthy volunteer and pancreatic cancer patients) [94] |
| Boster Bio | Human Tissue Factor PicoKine ELISA (yes) | • PPP (epithelial ovarian cancer patients) [10] |
| Abcam | Tissue Factor (CD142) Human ELISA (yes) |
• Serum (healthy volunteers and patients with essential and renovascular hypertension) [100] • Cell lysate [94] |
| Affinity Biologicals | (No) |
• EVs isolated from multiple human endothelial cells [81, 83] • Lysate of MDA-MB-231 cells [83] |
PPP platelet-poor plasma, PFP platelet-free plasma.
To address this problem, we recently tested whether the TF levels in various cancer cell lines examined by western blotting using 10H10 antibody corresponded with those evaluated by TF-ELISA kits [94]. TF levels measured by the Quantikine kit showed a good proportional correlation and is suitable for the detection of cancer cell-derived TF. ELISA using clinical samples revealed that TF level but not PCA at patient registration is an important predictive factor of CAT in pancreatic cancer patients [94]. Our study indicated that the evaluation of TF level as well as PCA is also important for the prediction of CAT.
Plasma TF levels and PCA-associated with EVs may be irrelevant to VTE incidence in patients with ovarian cancer [9, 10]. In one study [9], ELISA failed to detect TF+EVs because the estimated TF levels in plasma samples were similar to those of supernatant fractions after high-speed centrifugation. The study thus claimed that only the TF fraction not associated with EVs must have been detected by the ELISA kit. Whether this phenomenon applies to other experimental systems is unclear, as other studies have detected TF+EVs using the same ELISA kit [84]. Thus, we should carefully estimate TF in relation to its association with EVs. In addition, ELISA may not discern differences between TF from cancer cells, sTF, and TF from non-cancer cells (such as monocytes).
Recently, Mackman et al. [95] and our group [96] discussed the detection of TF antigen in plasma samples by ELISA (Quantikine). On the basis of these arguments, the reactivity of the Quantkine system to TF can be affected depending on the sample (e.g., cell lysate or plasma), source of TF (e.g., immune cells or cancer cells), and lipid environment around the TF antigen. In addition, unknown epitope masking mechanism(s) might restrict the precise detection of TF antigen in plasma. It may involve the high affinity of TF to plasma- and cancer cell-derived FVII as association with FVII can hide TF antibody epitopes [97]. Indeed, the Quantikine system failed to recognise TF–EVs released in blood treated with lipopolysaccharide [95]. However, this kit recognised higher TF-antigen levels in the plasma of CAT + pancreatic cancer patients compared with that in healthy volunteers and CAT − patients [94, 96]. In any case, current experimental methodologies have limited abilities for evaluating TF in biological samples, as suggested in a previous review [8]. Thus, the evaluation of TF antigen and activity through multiple viewpoints should improve our understanding of the relationship between TF and CAT.
Summary
TF+EV secretion can cause VTE, and potentially ATE, in cancer patients. TF-PCA and TF+EV-shedding are regulated by many mechanisms and modulated in response to conditions in tumours. The predominant mechanisms in cancer tissues may thus vary depending on the cancer type and tumour conditions. As PCA of TF–EVs directly contributes to VTE, the relationships between TF-PCA and CA-VTE incidence have been analysed. PCA is estimated by isolating TF+EVs. However, the most commonly used centrifugation condition might not recover the full proportion of TF+EVs. TF level is also important because a higher TF level enables greater PCA in cancer patients. TF in patient blood has been determined by many commercial ELISA kits; however, the kits use different anti-TF antibodies with potential different substrate reactivity. Current experimental methodologies may thus underestimate TF, with potential impacts on study findings. Examining TF by multiple means would lead to a more precise understanding of the effects of TF on CAT incidence and the generation of new therapeutics targeting TF-PCA. The recent spread of SARS-CoV-2 worldwide is a new risk factor for CAT. Further studies are required for better treatment of forthcoming patients with thrombotic disorders.
Acknowledgements
We thank Gabrielle White Wolf, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Author contributions
SK conceived and wrote the manuscript. YM contributed to drafting the manuscript. SK and YM approved the final version of the manuscript.
Funding
None.
Data availability
None.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent to publish
Not applicable.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood. 2013;5:1712–23. doi: 10.1182/blood-2013-04-460121. [DOI] [PubMed] [Google Scholar]
- 2.Campello E, Llich A, Simioni P, Key NS. The relationship between pancreatic cancer and hypercoagulability: a comprehensive review on epidemiological and biological issues. Br J Cancer. 2019;121:359–71. doi: 10.1038/s41416-019-0510-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldin M, Koulas I, Weitz J, Spyropoulos AC. State-of-Art mini review: dual-pathway inhibition to reduce arterial and venous thromboembolism. Thromb Haemost. 2022; 10.1055/a-1778-1083. [DOI] [PubMed]
- 4.Tuzovic M, Hermann J, Iliescu C, Marmagkiolis K, Ziaeian B, Yang EH. Arterial thrombosis in patients with cancer. Curr Treat Options Med. 2018;20:40. doi: 10.1007/s11936-018-0635-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grilz E, Königsbrügge O, Posch F, Schmidinger M, Pirker R, Lang IM, et al. Frequency, risk factors, and impact on mortality of arterial thromboembolism in patients with cancer. Haematologica. 2018;103:1549–56. doi: 10.3324/haematol.2018.192419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grover SP, Mackman N. Tissue factor an essential mediator of hemostasis and trigger of thrombosis. Arterioscler Thromb Vasc Biol. 2018;38:709–25. doi: 10.1161/ATVBAHA.117.309846. [DOI] [PubMed] [Google Scholar]
- 7.Zelaya H, Rothmeier AS, Ruf W. Tissue factor at the crossroad of coagulation and cell signaling. J Thromb Haemost. 2018;16:1941–52. doi: 10.1111/jth.14246. [DOI] [PubMed] [Google Scholar]
- 8.Hisada Y, Mackman N. Cancer-associated pathways and biomarkers of venous thrombosis. Blood. 2017;130:1499–506. doi: 10.1182/blood-2017-03-743211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Claussen C, Rausch A-V, Lezius S, Amirkhosravi A, Davila M, Francis JL, et al. Microvesicle-associated tissue factor procoagulant activity for the preoperative diagnosis of ovarian cancer. Thromb Res. 2016;141:39–48. doi: 10.1016/j.thromres.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Cohen JG, Prendergast E, Geddings JE, Walts AE, Agadjanian H, Hisada Y, et al. Evaluation of venous thrombosis and tissue factor in epithelial ovarian cancer. Gynecol Oncol. 2017;146:146–52. doi: 10.1016/j.ygyno.2017.04.021. [DOI] [PubMed] [Google Scholar]
- 11.Saadeh FA, Norris L, O’Toole S, Mohamed BM, Langhe R, O’Leary J, et al. Tumor expression of tissue factor and tissue factor pathway inhibitor in ovarian cancer-relationship with venous thrombosis risk. Thromb Res. 2013;132:627–34. doi: 10.1016/j.thromres.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 12.Uno K, Homma S, Satoh T, Nakanishi K, Abe D, Matsumoto K, et al. Tissue factor expression as a possible determinant of thromboembolism in ovarian cancer. Br J Cancer. 2007;96:290–5. doi: 10.1038/sj.bjc.6603552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thaler J, Preusser M, Ay C, Kaider A, Marosi C, Zielinski C, et al. Intratumoral tissue factor expression and risk of venous thromboembolism in brain tumor patients. Thromb Res. 2013;131:162–5. doi: 10.1016/j.thromres.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 14.Thaler J, Ay C, Mackman N, Bertina RM, Kaider A, Marosi C, et al. Microparticle-associated tissue factor activity, venous thromboembolism and mortality in pancreatic, gastric, colorectal, and brain cancer patients. J Thromb Haemost. 2012;10:1363–70. doi: 10.1111/j.1538-7836.2012.04754.x. [DOI] [PubMed] [Google Scholar]
- 15.Woei-A-Jin FJSH, Tesselaar ME, Garcia Rodriguez P, Romijin FP, Bertina RM, Osanto S. Tissue factor-bearing microparticles and CA19.9: two players in pancreatic cancer-associated thrombosis? Br J Cancer. 2016;115:332–8. doi: 10.1038/bjc.2016.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langer F, Ruf W. Synergies of phosphatidylserine and protein disulfide isomerase in tissue factor activation. Thromb Haemost. 2014;111:590–7. doi: 10.1160/TH13-09-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Unruh D, Horbinski C. Beyond thrombosis: the impact of tissue factor signaling in cancer. J Hematol Oncol. 2020;13:93. doi: 10.1186/s13045-020-00932-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ay C, Beyer-Westendorf J, Pabinger I. Treatment of cancer-associated venous thromboembolism in the age of direct oral anticoagulants. Ann Oncol. 2019;30:897–907. doi: 10.1093/annonc/mdz111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hisada Y, Mackman N. Cancer-cell derived tissue factor-positive extracellular vesicles: biomarkers of thrombosis and survival. Curr Opin Hematol. 2019;26:349–56. doi: 10.1097/MOH.0000000000000521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poredoš P. Interrelationship between venous and arterial thrombosis. Int Angiol. 2017;36:295–8. doi: 10.23736/S0392-9590.17.03820-2. [DOI] [PubMed] [Google Scholar]
- 21.Geddings JE, Hisada Y, Boulaftali Y, Getz TM, Whelihan M, Fuentes R, et al. Tissue factor-positive tumor microvesicles activates platelets and enhance thrombosis in mice. J Thromb Haemost. 2016;14:153–66. doi: 10.1111/jth.13181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hisada Y, Ay C, Auriemma AC, Coorey BC, Mackman N. Human pancreatic tumors grown in mice release tissue factor-positive microvesicles that increase venous clot size. J Thromb Haemost. 2017;15:22082217. doi: 10.1111/jth.13809. [DOI] [PubMed] [Google Scholar]
- 23.Von Bruhl M-L, Stark K, Steinhart A, Chandraratne S, Konrad I, Lorenz M, et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med. 2012;209:819–35. doi: 10.1084/jem.20112322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khorana AA, Francis CW, Menzies KE, Wang JG, Hyrien O, Hathcock J, et al. Plasma tissue factor may be predictive of venous thromboembolism in pancreatic cancer. J Thromb Haemost. 2008;6:1983–5. doi: 10.1111/j.1538-7836.2008.03156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Es N, Hisada Y, Nisio MD, Cesarman G, Kleinjan A, Mahe I, et al. Extracellular vesicles exposing tissue factor for the prediction of venous thromboembolism in patients with cancer: a prospective cohort study. Thromb Res. 2018;166:54–9. doi: 10.1016/j.thromres.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Khorana AA, Kamphuisen PW, Meyer G, Bauersachs R, Janas MS, Jarner MF, et al. Tissue factor as a predictor of recurrent venous thromboembolism in malignancy: biomarker analyses of the CATCH trial. J Clin Oncol. 2017;35:1078–85. doi: 10.1200/JCO.2016.67.4564. [DOI] [PubMed] [Google Scholar]
- 27.Mitrugno A, Tormoen GW, Kuhn P, McCarty OJT. The prothrombotic activity of cancer cells in the circulation. Blood Rev. 2016;30:11–19. doi: 10.1016/j.blre.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kocatürk B, Versteeg H. Tissue factor-integrin interactions in cancer and thrombosis: every Jack has his Jill. J Thromb Haemost. 2013;11:285–93. doi: 10.1111/jth.12222. [DOI] [PubMed] [Google Scholar]
- 29.Unruh D, Turner K, Srinivasan R, Kocatürk B, Qi X, Chu Z, et al. Alternatively spliced tissue factor contributes to tumor spread and activation of coagulation in pancreatic ductal adenocarcinoma. Int J Cancer. 2014;134:9–20. doi: 10.1002/ijc.28327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Es,N, Blecker S, Sturk A, Nieuwland R. Clinical Significance of tissue factor-exposing microparticles in arterial and venous thrombosis. Semin Thromb Haemost. 2015;41:718–27. doi: 10.1055/s-0035-1556047. [DOI] [PubMed] [Google Scholar]
- 31.Ouweneel AB, Eck MV. Lipoproteins as modulators of atherothrombosis: From endothelial function to primary and secondary coagulation. Vasc Pharm. 2016;82:1–10. doi: 10.1016/j.vph.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 32.Delimaris I, Faviou E, Antonakos G, Stathopoulou E, Zachari A, Dionyssiou-Asteriou A. Oxidized LDL, serum oxidizability and serum lipid levels in patients with breast or ovarian cancer. Clin Biochem. 2007;40:1129–34. doi: 10.1016/j.clinbiochem.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 33.Khaidakov M, Mitra S, Kang B-Y, Wang X, Kadlubar S, Novelli G, et al. Oxidized LDL receptor 1 (OLR1) as a possible link between obesity, dyslipidemia and cancer. PLoS ONE. 2011;6:e20227. doi: 10.1371/journal.pone.0020277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drake TA, Hannani K, Fei H, Lavi S, Berliner JA. Minimally oxidized low-density lipoprotein induces tissue factor expression in cultured human endothelial cells. Am J Pathol. 1991;138:601–7. [PMC free article] [PubMed] [Google Scholar]
- 35.Tatsumi K, Mackmann N. Tissue factor and atherothrombosis. J Atheroscler Thromb. 2015;22:543–9. doi: 10.5551/jat.30940. [DOI] [PubMed] [Google Scholar]
- 36.Koizume S, Miyagi Y. Potential coagulation factor-driven pro-inflammatory responses in ovarian cancer tissues associated with insufficient O2 and plasma supply. Int J Mol Sci. 2017;18:809. doi: 10.3390/ijms18040809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koizume S, Ito S, Yoshioka Y, Kanayama T, Nakamura Y, Yoshihara M, et al. High-level secretion of tissue factor-rich extracellular vesicles from ovarian cancer cells mediated by filamin-A and protease-activated receptors. Thromb Haemost. 2016;115:299–310. doi: 10.1160/th15-03-0213. [DOI] [PubMed] [Google Scholar]
- 38.Koizume S, Takahashi T, Yoshihara M, Nakamura Y, Ruf W, Takenaka K, et al. Cholesterol starvation and hypoxia activate the FVII gene via the SREBP1-GILZ pathway in ovarian cancer cells to produce procoagulant microvesicles. Thromb Haemost. 2019;119:1058–71. doi: 10.1055/s-0039-1687876. [DOI] [PubMed] [Google Scholar]
- 39.Ansari SA, Pendurthi UR, Rao LVM. The lipid peroxidation product 4-hydroxy-2-nonenal induces tissue factor decryption via ROS generation and thioredoxin. Blood Adv. 2017;1:2399–413. doi: 10.1182/bloodadvances.2017010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ansari SA, Pendurthi UR, Rao LVM. Role of cell surface lipids and thiol-disulphide exchange pathways in regulating the encryption and decryption of tissue factor. Thromb Haemost. 2019;119:860–70. doi: 10.1055/s-0039-1681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moloney JN, Cotter TG. ROS signaling in the biology of cancer. Semin Cell Dev Biol. 2018;80:50–64. doi: 10.1016/j.semcdb.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 42.Zhong H. & Yin, Huiyong. Role of lipid peroxidation derived 4-hydroxynonenal (4-HNE) in cancer: focusing on mitochondria. Redox Biol. 2015;4:193–9. doi: 10.1016/j.redox.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ke K, Yuan J, Morrissey JH. Tissue factor residues that putatively interact with membrane phospholipids. PLoS ONE. 2014;9:e88675. doi: 10.1371/journal.pone.0088675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ansari SA, Pendurthi UR, Sen P, Rao LVM. The role of putative phosphatidylserine-interactive residues of tissue factor on its coagulant activity at the cell surface. PLoS ONE. 2016;11:e0158377. doi: 10.1371/journal.pone.0158377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rao LV, Ruf W. Tisssue factor residues Lys165 and Lys166 are essential for rapid formation of the quaternary complex of tissue factor.VIIa with Xa.tissue factor pathway inhibitor. Biochemistry. 1995;34:10867–71. doi: 10.1021/bi00034a020. [DOI] [PubMed] [Google Scholar]
- 46.Wang J, Pendurthi UR, Rao VM. Sphingomyelin encrypts tissue factor: ATP-induced activation of A-SMase leads to tissue factor decryption and microvesicle shedding. Blood Adv. 2017;13:849–62. doi: 10.1182/bloodadvances.2016003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang J, Pendurthi UR, Rao LVM. Acid sphingomyelinase plays a critical role in LPS- and cytokine-induced tissue factor procoagulant activity. Blood. 2019;134:645–55. doi: 10.1182/blood.2019001400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Glaser UG, Fandrey J. Sphingolipids in inflammatory hypoxia. Biol Chem. 2018;399:1169–74. doi: 10.1515/hsz-2018-0173. [DOI] [PubMed] [Google Scholar]
- 49.Lingwood B, Kaiser H-J, Levental I, Simons K. Lipid rafts as functional heterogeneity in cell membranes. Biochem Soc Trans. 2009;37:955–60. doi: 10.1042/BST0370955. [DOI] [PubMed] [Google Scholar]
- 50.Dietzen DJ, Page KL, Tetzloff TA. Lipid rafts are necessary for tonic inhibition of cellular tissue factor procoagulant activity. Blood. 2004;103:3038–44. doi: 10.1182/blood-2003-07-2399. [DOI] [PubMed] [Google Scholar]
- 51.Rao LVM, Pendurthi UR. Regulation of tissue factor coagulant activity on cell surfaces. J Thromb Haemost. 2012;10:2242–53. doi: 10.1111/jth.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ahamed J, Versteeg HH, Kerver M, Chen VM, Mueller BM, Hogg PJ, et al. Disulfide isomerization switches tissue factor from coagulation to cell signaling. Proc Natl Acad Sci USA. 2006;103:13932–7. doi: 10.1073/pnas.0606411103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beckman L, Rolling CC, Voigtländer M, Mäder J, Klingler F, Schulenkorf A, et al. Bacitracin and Rutin regulate tissue factor production in inflammatory monocytes and acute myeloid leukemia blasts. Cancers. 2021;13:3941. doi: 10.3390/cancers13163941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pendurthi UR, Ghosh S, Mandal SK, Rao LVM. Tissue factor activation: is disulfide bond switching a regulatory mechanism? Blood. 2007;110:3900–8. doi: 10.1182/blood-2007-07-101469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Versteeg HH, Ruf W. Tissue factor coagulant function is enhanced by protein-disulfide isomerase independent of oxidoreductase activity. J Biol Chem. 2007;35:25416–24. doi: 10.1074/jbc.M702410200. [DOI] [PubMed] [Google Scholar]
- 56.Wang P, Wu Y, Li X, Ma X, Zhong L. Thioredoxin reductase control tissue factor activity by thiol redox-dependent mechanism. J Biol Chem. 2013;288:3346–58. doi: 10.1074/jbc.M112.418046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Furlan-Freguia C, Marchese P, Gruber A, Ruggeri ZM, Ruf W. P2X7 receptor signaling contributes to tissue factor-dependent thrombosis in mice. J Clin Investig. 2011;121:2932–44. doi: 10.1172/JCI46129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ettelaie C, Featherby S, Rondon AM, Greenman J, Versteeg HH, Maraveyas A. De-palmitoylation of tissue factor regulates its activity, phosphorylation and cellular functions. Cancers. 2021;13:3837. doi: 10.3390/cancers13153837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Langer F, Spath B, Fischer C, Stolz M, Ayuk FA, Kröger N, et al. Rapid activation of monocyte tissue factor by antithymocyte globulin is dependent on complement and protein disulfide isomerase. Blood. 2013;21:2324–35. doi: 10.1182/blood-2012-10-460493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muller-Calleja N, Ritter S, Hollerbach A, Falter T, Lackner KJ, Ruf W. Complement C5 but not C3 is expendable for tissue factor activation by cofactor-independent antiphospholipid antibodies. Blood Adv. 2018;2:979–86. doi: 10.1182/bloodadvances.2018017095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nitta H, Murakami Y, Wada Y, Eto M, Baba H, Imamura T. Cancer cells release anaphylatoxin C5a from C5 by serine protease to enhance invasiveness. Oncol Rep. 2014;32:1715–9. doi: 10.3892/or.2014.3341. [DOI] [PubMed] [Google Scholar]
- 62.Horowitz NA, Brenner B. Thrombosis and Hemostasis issues in cancer patients with COVID-19. Semin Thromb Hemost. 2020;46:785–8. doi: 10.1055/s-0040-1714275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang J, Pendurthi UR, Yi G, Rao LVM. SARS-CoV-2 infection induces the activation of tissue factor-mediated coagulation via activation of acid sphingomyelinase. Blood. 2021;138:344–9. doi: 10.1182/blood.2021010685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rosell A, Havervall S, von Meijenfeldt F, Hisada Y, Aguilera K, Grover SP, et al. Patients with COVID-19 have elevated levels of circulating extracellular vesicle tissue factor activity that is associated with severity and mortality-Brief report. Arterioscler Thromb Vasc Biol. 2021;41:878–82. doi: 10.1161/ATVBAHA.120.315547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hess CN, Capell WH, Bristow MR, Ruf W, Szarek M, Morrow DA, et al. Rationale and design of a study to assess the safety and efficacy of rNAPc2 in COVID-19: the phase 2b ASPEN-COVID-19 trial. Am Heart J. 2022;246:136–43. doi: 10.1016/j.ahj.2021.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Subrahmanian S, Borczuk A, Salvatore S, Fung K-M, Merrill JT, Laurence J, et al. Tissue factor upregulation is associated with SARS-CoV-2 in the lungs of COVID-19 patients. J Thromb Haemost. 2021;19:2268–74. doi: 10.1111/jth.15451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Al-Tamimi AO, Yusuf AM, Jayakumar MN, Ansari AW, Elhassan M, AbdulKarim F, et al. SARS-CoV-2 infection induces soluble platelet activation markers and PAI-1 in the early moderate stage of COVID-19. Int J Lab Hematol. 2022;00:1–10. doi: 10.1111/ijlh.13829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carpinteiro A, Gripp B, Hoffmann M, Pohlmann S, Hoertel N, Edwards MJ, et al. Inhibition of acid sphingomyelinase by ambroxol prevents SARS-CoV-2 entry into epithelial cells. J Biol Chem. 2021;296:10071. doi: 10.1016/j.jbc.2021.100701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hottz ED, Azevedo-Quintanilha I, Palhinha L, Teixeira L, Barreto EA, Pão CRR, et al. Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19. Blood. 2020;136:1330–41. doi: 10.1182/blood.2020007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Skendros P, Mitsios A, Chrysanthopoulou A, Mastellos DC, Metallidis S, Rafailidis P, et al. Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J Clin Investig. 2020;130:6151–7. doi: 10.1172/JCI141374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kothari H, Rao LVM, Pendurthi UR. Glycosylation of tissue factor is not essential for its transport or functions. J Thromb Haemost. 2011;9:1511–20. doi: 10.1111/j.1538-7836.2011.04332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kothali H, Pendurthi UR, Rao LVM. Tissue factor purified from different cellular sources and non-glycosylated tissue factor show similar procoagulant activity. J Thromb Haemost. 2013;11:2066–8. doi: 10.1111/jth.12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Krudysz-Amblo J, Jennings ME, II, Mann KG, Butenas S. Carbohydrates and activity of natural and recombinant tissue factor. J Biol Chem. 2010;285:3371–82. doi: 10.1074/jbc.M109.055178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kurakula K, Koenis D, Herzik MA, Jr, Liu Y, Craft JW, Jr, van Loenen PB, et al. Structural and cellular mechanisms of peptidyl-prolyl isomerase Pin1-mediated enhancement of tissue factor gene expression, protein half-life, and pro-coagulant activity. Haematologica. 2018;103:1073–82. doi: 10.3324/haematol.2017.183087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ettelaie C, Collier MEW, Featherby S, Greenman J, Maraveyas A. Peptidyl-prolyl isomerase 1 (Pin1) preserves the phosphorylation state of tissue factor and prolongs its release within microvesicles. Biochim Biophys Acta Moll Cell Res. 2018;1865:12–24. doi: 10.1016/j.bbamcr.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 76.Versteeg HH, Schaffner F, Kerver M, Petersen HH, Ahamed J, Felding-Habermann B, et al. Inhibition of tissue factor signaling suppresses tumor growth. Blood. 2008;111:190–9. doi: 10.1182/blood-2007-07-101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rothmeier AS, Marchese P, Langer F, Kamikubo K, Schaffner F, Cantor J, et al. Tissue factor prothrombotic activity is regulated by integrin-arf6 trafficking. Arterioscler Thromb Vasc Biol. 2017;37:1323–31. doi: 10.1161/ATVBAHA.117.309315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rothmeier AS, Liu E, Chakrabarty S, Disse J, Mueller BM, Østergaard H, et al. Identification of the integrin-binding site on coagulation factor VIIa required for proangiogenic PAR2 signaling. Blood. 2018;131:674–85. doi: 10.1182/blood-2017-02-768218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ettelaie C, Collier MEW, Mei MP, Xiao YP, Maraveyas A. Enhanced binding of tissue factor-microparticles to collagen-IV and fibronectin leads to increased tissue factor activity in vitro. Thromb Haemost. 2013;109:61–71. doi: 10.1160/TH12-05-0279. [DOI] [PubMed] [Google Scholar]
- 80.Kapopara PR, Safikhan NS, Huang J, Meixner SC, Gonzalez K, Loghmani H, et al. CD248 enhances tissue factor procoagulant function, promoting arterial and venous thrombosis in mouse models. J Thromb Haemost. 2021;19:1932–47. doi: 10.1111/jth.15338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Collier MEW, Ettelaie C. Regulation of the incorporation of tissue factor into microparticles by serine phosphorylation of the cytoplasmic domain of tissue factor. J Biol Chem. 2011;286:11977–84. doi: 10.1074/jbc.M110.195214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ettelaie C, Elkeeb AM, Maraveyas A, Collier MEW. p38a phosphorylates serine 258 within the cytoplasmic domain of tissue factor and prevents its incorporation into cell-derived microparticles. Biochim Biophys Acta Moll Cell Res. 2013;1833:613–21. doi: 10.1016/j.bbamcr.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 83.Collier MEW, Maraveyas A, Ettelaie C. Filamin-A is required for the incorporation of tissue factor into cell-derived microvesicles. Thromb Haemost. 2014;111:647–55. doi: 10.1160/TH13-09-0769. [DOI] [PubMed] [Google Scholar]
- 84.Collier MEW, Ettelaie C, Goult BT, Maraveyas A, Goodall AH. Investigation of the filamin A-dependent mechanisms of tissue factor incorporation into microvesicles. Thromb Haemost. 2017;117:2034–44. doi: 10.1160/TH17-01-0009. [DOI] [PubMed] [Google Scholar]
- 85.del Conde I, Shrimpton CN, Thiagarajan P, López JA. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood. 2005;106:1604–11. doi: 10.1182/blood-2004-03-1095. [DOI] [PubMed] [Google Scholar]
- 86.Rothmeier AS, Marchese P, Petrich BG, Furlan-Freguia C, Ginsberg MH, Ruggeri ZM, et al. Caspase-1-mediated pathway promotes generation of thromboinflammatory microparticles. J Clin Investig. 2015;125:1471–84. doi: 10.1172/JCI79329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rondon AMR, de Almeida VH, Gomes T, Verçoza BRF, Carvalho RS, König S, et al. Tissue factor mediates microvesicles shedding from MDA-MB-231 breast cancer cells. Biochem Biophys Res Commun. 2018;502:137–44. doi: 10.1016/j.bbrc.2018.05.136. [DOI] [PubMed] [Google Scholar]
- 88.Van Doormaal F, Kleinjan A, Berckmans RJ, Mackman N, Manly D, Kamphuisen PW, et al. Coagulation activation and microparticle-associated coagulant activity in cancer patients. Thromb Haemost. 2012;108:160–5. doi: 10.1160/TH12-02-0099. [DOI] [PubMed] [Google Scholar]
- 89.Ender F, Freund A, Quecke T, Steidel C, Zamzow P, von Bubnoff N, et al. Tissue factor activity on microvesicles from cancer patients. J Cancer Res Clin Oncol. 2020;146:467–75. doi: 10.1007/s00432-019-03073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ay C, Mackman N. Tissue factor: Catch me if you can! J Clin Oncol. 2017;35:1128–30. doi: 10.1200/JCO.2016.70.6788. [DOI] [PubMed] [Google Scholar]
- 91.Ettelaie C, Collier MEW, Maraveyas A, Ettelaie R. Characterization of physical properties of tissue factor containing microvesicles and comparison of ultracentrifuge-based recovery procedures. J Extracell Vesicles. 2014;3:23592. doi: 10.3402/jev.v3.23592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee RD, Barcel DA, Williams JC, Wang JG, Boles JC, Manly DA, et al. Pre-analytical and analytical variables affecting the measurement of plasma-derived microparticle tissue factor activity. Thromb Res. 2012;129:80–5. doi: 10.1016/j.thromres.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nielsen T, Kristensen AF, Pedersen S, Christiansen G, Kristensen SR. Investigation of procoagulant activity in extracellular vesicles isolated by differential ultracentrifugation. J Extracell Vesicles. 2018;7:1454777. doi: 10.1080/20013078.2018.1454777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kobayashi S, Koizume S, Takahashi T, Ueno M, Oishi R, Nagashima S, et al. Tissue factor and its procoagulant activity on cancer-associated thromboembolism in pancreatic cancer. Cancer Sci. 2021;112:4679–91. doi: 10.1111/cas.15106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mackman N, Hisada Y, Archibald SJ. Tissue factor and its procoagulant activity on cancer-associated thromboembolism in pancreatic cancer: comment by Mackman et al. Cancer Sci. 2022;113:1885–7. doi: 10.1111/cas.15276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Koizume S, Kobayashi S, Ruf W, Miyagi Y. Author’s reply to the letter to the editor: Tissue factor and its procoagulant activity on cancer-associated thromboembolism in pancreatic cancer. Cancer Sci. 2022;113:1888–90. doi: 10.1111/cas.15324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ruf W, Edgington TS. An anti-tissue factor monoclonal antibody which inhibits TF-FVIIa complex is a potent anticoagulant in plasma. Thromb Haemost. 1991;66:529–33. doi: 10.1055/s-0038-1646454. [DOI] [PubMed] [Google Scholar]
- 98.Lechner D, Kollas M, Gleiss A, Kyrle PA, Weltermann A. Chemotherapy-induced thrombin generation via procoagulant endothelial microparticles is independent of tissue factor activity. J Thromb Haemost. 2007;5:2445–52. doi: 10.1111/j.1538-7836.2007.02788.x. [DOI] [PubMed] [Google Scholar]
- 99.Han LY, Landen CN, Jr, Kamat AA, Lopez A, Bender DP, Mueller P, et al. Preoperative serum tissue factor levels are an independent prognostic factor in patients with ovarian carcinoma. J Clin Oncol. 2006;24:755–670. doi: 10.1200/JCO.2005.02.9181. [DOI] [PubMed] [Google Scholar]
- 100.Park MY, Herrmann SM, Saad A, Eirin A, Tang H, Lerman A, et al. Biomarkers of kidney injury and Klotho in patients with atherosclerotic renovascular disease. Clin J Am Soc Nephrol. 2015;10:443–51. doi: 10.2215/CJN.07290714. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
None.