Abstract
This study examined the association between maternal smoking during pregnancy and maternal behavior during mother–infant interactions during the neonatal period. Participants included 84 mother–infant dyads (43 cigarette-exposed and 41 nonexposed) who were recruited after birth and assessed at 2 to 4 weeks of infant age. Results indicated that mothers who smoked during pregnancy had higher levels of maternal insensitivity (MI) and lower levels of maternal warmth (MW) during interactions with their infant even after controlling for demographics and pregnancy alcohol use. Maternal anxiety and hostility mediated the association between smoking and MI and maternal anger mediated the association between smoking and reduced MW. In addition, there was an interaction between infant gender and maternal smoking for MW with smokers displaying less warmth to boys during interactions.
A fairly extensive literature examining the teratological impact of prenatal cigarette exposure on various aspects of development currently exists and continues to grow. Smoking during pregnancy increases the risk for adverse perinatal outcomes including preterm delivery and perinatal mortality and has been consistently associated with reduced fetal growth (e.g., Fried & O’Connell, 1987; Haug et al., 2000; Jacobson et al., 1984; Wang, Tager, Van Vunakis, Speizer, & Hanrahan, 1997; Zaren, Lindmark, & Bakketeig, 2000). Prenatal smoking has also been associated with other developmental outcomes that may be the result of either direct (teratogenic) effects of prenatal exposure to cigarettes or indirect or mediated effects via other risk characteristics associated with prenatal exposure to cigarettes. These include outcomes such as altered autonomic nervous system arousal, increased distractability, negative reactivity to environmental challenge, oppositional behavior, and conduct disorder (Cornelius & Day, 2000; Day, Richardson, Goldschmidt, & Cornelius, 2000; Fergusson, Horwood, & Lynskey, 1993; Fried & Makin, 1987; Jacobson, Fein, Jacobson, Schwartz, & Dowler, 1984; Landesman-Dwyer, Ragozin, & Little, 1981; McGee & Stanton, 1994; Olds, 1997; Orlebeke, Knol, & Verhulst, 1997; Schuetze & Eiden, 2006; Schuetze & Zeskind, 2001; Wakschlag & Hans, 2002; Weissman, Warner, Wickramaratne, & Kandel, 1999). Recently, investigators have suggested that one trajectory to later developmental problems among cigarette-exposed infants may be through problematic maternal behavior or parenting difficulties (e.g., Wakschlag & Hans, 2002). However, little is known about the association between maternal smoking and maternal behavior during infancy, or about pathways that may mediate this association.
Maternal smoking has consistently been associated with a constellation of risk characteristics that may impact mother–child interactions. These risk factors include demographic variables such as lower maternal education, lower maternal age, and single motherhood (Baghurst, Tong, Woodward, & McMichael, 1992; Gusella & Fried, 1984; Kleinman & Kopstein, 1987), and environmental variables such as lower quality home environment, higher stress, and lower support (Baghurst et al., 1992; Fried, O’Connell, & Watkinson, 1992). All of these maternal risk factors are interrelated and increase the likelihood of a poor home environment and parenting difficulties (Elder, Eccles, Ardelt, & Lord, 1995). Indeed, studies indicate that pregnancy smoking is associated with less responsive and sensitive parenting behavior such as higher rates of parent–child discord (e.g., Weissman et al., 1999), less maternal nurturing (Fergusson, Goodwin, & Horwood, 2003), and poor parent–child communication, ineffective discipline, and poor supervision (Wakschlag et al., 1997) among older children. However, it is not clear from these studies if these group differences in maternal behavior are due to the direct impact of cigarette use on maternal behavior, or more indirect pathways such as through the associations among maternal cigarette smoking and comorbid psychopathology.
Indeed, maternal psychological functioning, which is associated with both cigarette smoking and parenting behavior, may explain the association between cigarette smoking during pregnancy and maternal behavior during interactions with their children. Although a fairly large literature links smoking with higher levels of psychopathology in the general population (Anda et al., 1990; Breslau, Kilbey, & Andreski, 1990, 1991, 1993; Brooks, Cohen, & Brook, 1998; Fergusson, Goodwin, & Horwood, 2003; Glassman et al., 1990; Johnson et al., 2000; Kandel et al., 1997), only a few studies have systematically examined the psychosocial functioning of pregnant smokers. These studies indicate that pregnant smokers are similar to adult smokers in the general population in terms of their psychological attributes. For example, studies that have prospectively assessed psychosocial functioning during pregnancy have found higher levels of depressive symptomatology among current smokers, compared to nonsmokers, in their second and third trimesters of pregnancy (Pritchard, 1994; Zhu & Valbo, 2002) and higher levels of hostility during the first trimester (Rodriquez, Bohlin, & Lindmark, 2000).
The importance of considering the psychosocial functioning of pregnant smokers is underscored by the rapidly increasing body of literature indicating that both depression and anger or hostility have significant implications for maternal behavior. For instance, depressed mothers are more likely to display flatter affect during mother–child interactions, provide less stimulation, and are less responsive toward their infants (Cohn & Campbell, 1992; Jameson, Gelfand, Kulscar, & Teti, 1997). Similarly, several recent studies have found an association between maternal hostility and harsh or punitive discipline strategies (Kanoy, Ulku-Steiner, Cox, & Burchinal, 2003; Katz & Woodin, 2002; Kim et al., 2003; Nix et al., 1999). Maternal anger or hostility has also been found to exacerbate negative maternal behavior in substance-using populations (Eiden, Chavez, & Leonard, 1999; Hans, Bernstein, & Henson, 1999). Thus, comorbid maternal psychosocial functioning may be one pathway explaining the potential association between pregnancy smoking and maternal behavior during mother–infant interactions.
Finally, there is some evidence from the general developmental literature that infant gender may impact maternal behaviors during interactions (e.g., Stem & Karraker, 1989) although the results are equivocal. Whereas some studies of maternal behaviors during play interactions with their infants indicate that mothers are more likely to respond contingently to displays of affect from their sons than from their daughters (Malatesta & Haviland, 1982; Tronick & Cohn, 1989; Weinberg, Tronick, Cohn, & Olson, 1999), other studies suggest that the infant’s gender does not play a significant role in explaining differences in parenting (Seifer, Sameroff, Anagnostopolou, & Elias, 1992).
There are no studies, to our knowledge, that have examined differences in maternal behavior as a function of infant gender among mothers who smoke during pregnancy. However, there is some consensus in the literature that the male fetus may be more susceptible to teratogenic influences affecting the central nervous system than the female fetus (Flannery & Leiderman, 1994; Hans, 1994; Hynd & Semrud-Clikeman, 1989; Weinberg, Zimmerberg, & Sonderegger, 1992). For instance, data from animal studies suggest that cocaine-exposed male rats are more likely to display aggressive behavior during a stressful task than are females (Wood & Spear, 1998). Similarly, in the human literature, cigarette-exposed boys, but not girls, display lower sociability and negative emotionality as infants and were at an increased risk for conduct problems later in childhood (Wakschlag & Hans, 2002). These gender differences in infant behavior may, in turn, elicit differential maternal behavior. Thus, child gender may be an important moderator of the association between pregnancy smoking and maternal behavior.
The purpose of this study was to examine the impact of maternal cigarette smoking during pregnancy on maternal behavior in a feeding context during the neonatal period. Feeding interactions were selected for examination because feeding is one of the primary developmental contexts during which mother–infant interactions occur during early infancy. It was hypothesized that mothers who smoked during pregnancy would display lower warmth and higher insensitivity during feeding interactions. Furthermore, we hypothesized that maternal psychological functioning would explain this association. Specifically, we hypothesized that mothers who smoked during pregnancy would have higher levels of psychiatric symptomatology and that these levels would, in turn, be associated with lower maternal warmth and higher insensitivity toward their infants. Finally, we explored the possibility that infant gender may moderate the association between pregnancy smoking and maternal behavior
METHOD
Participants
Participants were mother–infant dyads recruited into a longitudinal study of cigarette exposure and infant development. An outreach worker on the project staff recruited all participants after delivery from one local area inner-city hospital that serves primarily low-income and high-risk women. A total of 111 dyads were recruited into either a smoking group or nonsmoking group. Of these dyads, 62 consisted of mothers who smoked cigarettes during pregnancy and 49 consisted of mothers who reported no smoking during or in the month prior to pregnancy. To minimize the impact of other maternal substance use during pregnancy on the outcome variables of interest, only mothers who reported consuming less than one alcoholic drink (average weekly alcohol consumption of less than .50 ounces of ethanol) and using marijuana less than once per week prior to pregnancy recognition (M = 7.2 weeks gestational age, SD = 2.1) were included in these analyses. Thus, this study included only women who reported consuming less than seven standard drinks and seven joints prior to pregnancy recognition and no alcohol or marijuana use after pregnancy recognition. The final sample size, consequently, consisted of 43 mothers who smoked during pregnancy and 41 mothers who reported not smoking during pregnancy. Maternal report of no illicit substance use during pregnancy was confirmed via urine toxicologies that were available for 90% (n = 76) of the mothers in this final sample. Urine screens were routinely conducted on all mothers who received prenatal care through the hospital’s prenatal clinic and were obtained at birth for the remaining mothers. Urine toxicologies consisted of standard urine screening for drug level or metabolites of cocaine, opiates, benzodiazepines, and tetrahydrocannabinal (THC). Urine was rated positive if the quantity of drug or metabolite was higher than 300 g/ml. All mothers recruited into this study were negative for cocaine, opiates, THC, and benzodiazepines.
All infants in the study were full term (> 36 weeks gestational age) with no major medical problems at birth. Of these infants, 55% (n = 46) were male. Because many of the chemicals in cigarettes can be transmitted to infants via breast milk (Dahlstrom, Ebergsjo, & Lundell, 2004), resulting in additional postnatal cigarette exposure for the infant, only infants who were exclusively formula fed from birth were included in this study. The rates of breastfeeding were extremely low in this sample. Thus, this exclusion criteria resulted in exclusion of only 3 mother–infant dyads.
Mothers ranged in age from 18 to 36 (M = 27.78, SD = 6.61). Of the mothers, 12% were primiparous; the remaining mothers had between two and eight children. The majority of mothers were African American (66%), had high school or less education (75%), and were single (71%). The Hollingshead two-factor index was used to calculate socioeconomic status (SES; Hollingshead, 1975). The average SES was 2.80 (SD = 1.57). Thus, the sample consisted of predominantly low-income families with single mothers.
Procedure
All mothers at one local hospital were approached by study staff after delivery and were invited to participate in a study described as examining maternal health and infant development. Interested and eligible mothers were given detailed information about the study and were then scheduled for their first laboratory visit during the neonatal period, which took place between 2 to 4 weeks postpartum (M = 3.41, SD = 1.24). This visit consisted of a maternal interview used to obtain information on psychosocial functioning and substance use during pregnancy, infant physiological assessments (heart rate and respiratory sinus arrhythmia during sleep) and a 10-min feeding session. Because of concerns about maternal literacy, all measures were administered in an interview format. All assessments were conducted with the primary caregiver of the infant. This was defined as the adult who provided the majority of care of the infant as indicated during a phone interview conducted immediately prior to the scheduled neonatal visit. The biological mother was the primary caregiver for all but one of the infants in the study. This dyad was, therefore, not included in this sample.
The study received approval from the institutional review boards of the hospital as well as the primary institutions with which the authors are affiliated. In addition, informed written consent was obtained from all recruited participants. To meet Health Insurance Portability and Accountability Act guidelines, additional written consent to view the medical records of both the mother and the infant was obtained at the time of recruitment. Participants received $30.00 in the form of gift certificates and a $5.00 infant toy at the neonatal visit for their participation.
Identification of Cigarette Exposure
The Timeline Follow-Back Interview (TLFB; Sobell & Sobell, 1995), administered during the neonatal visit, was used to assess maternal smoking during pregnancy. Participants were provided a calendar and asked to identify events of personal interest (i.e., holidays, birthdays, vacations, etc.) as anchor points to aid recall. This method has been established as a reliable and valid method of obtaining longitudinal data on substance use patterns, has good test–retest reliability, and is highly correlated with other intensive self-report measures and with salivary cotinine (Brown et al., 1998). The use of the TLFB for these substances resulted in the following variables for each of the substances used for the month prior to pregnancy, each trimester of pregnancy and for the 2 to 4 weeks following pregnancy: number of days cigarettes were smoked and average number of cigarettes smoked per week. In addition, the TLFB was used to obtain data on other substance use prior to pregnancy recognition including total number of joints used (for marijuana), total number of standard drinks consumed, and mean standard drinks per drinking day (for alcohol). The average number of cigarettes smoked per week during pregnancy for smokers ranged from .92 to 152.78 (M = 39.92, SD = 39.58).
Assessment of Maternal Psychosocial Functioning
The Brief Symptom Inventory (BSI; Derogatis, 1993) was used as a measure of general psychiatric functioning and was administered during the neonatal assessment. The BSI is a brief form of Symptom Checklist 90–R and is a mental health screening measure widely used in a variety of clinical and research settings. It consists of 53 items rated on a 5-point scale ranging from 0 (not at all) to 4 (extremely). The items are grouped into nine scales: Anxiety (6 items), Hostility (5 items), Somatization (7 items), Obsessive–Compulsive (6 items), Interpersonal Sensitivity (4 items), Depression (6 items), Phobic Anxiety (5 items), Paranoid Ideation (5 items), and Psychoticism (5 items). Test–retest reliabilities for the BSI range from 0.68 to 0.91. BSI normative data indicate that Cronbach’s alpha for global and individual scales ranges from .71 to .83 (Derogatis, 1992). Validity has been demonstrated through the scale’s relation to content scales and cluster scores of other measures of psychological distress (Derogatis, 1992).
The Buss–Perry Aggression Scale (BPA; Buss & Perry, 1992) was administered during the neonatal assessment to measure components of aggression. This scale consists of 29 items rated on a 5-point scale ranging from 0 (extremely uncharacteristic of me) to 4 (extremely characteristic of me). The items are divided among four scales: Physical Aggression (9 items), Verbal Aggression (5 items), Anger (7 items), and Hostility (7 items). Test–retest reliability and internal and construct validity for the scales have been found to be high (Buss & Perry, 1992).
Maternal Behavior During Feeding Interactions
The neonatal laboratory visit was scheduled around a time when the infant was likely to be hungry. Mothers were asked to feed their infants in a comfortable, living room type setting, as they normally would at home. This feeding session was videotaped. The first 10 min of these interactions were coded by two research assistants blind to group status, using the Mother–Infant Feeding Scale (Chatoor et al., 1997). For the purposes of this study, 18 of the original 26 maternal items were coded at this visit. The remaining items were not coded due to low variability during the neonatal period. For the coded items, the scale requires attention to affect (body posture, facial expression), sensitivity to infant cues, nonverbal communication (positioning the infant for eye contact), verbal communication, and feeding behaviors. This scale has been used by several researchers to measure mother–infant feeding interactions among children with eating disorders (Chatoor et al., 2004; Chatoor, Schaefer, Dickson, & Egan, 1984) and in previous studies of mother–infant feeding interactions among substance-abusing mothers (Eiden, 2001). However, little information is available about the factor structure of the scales in high-risk samples not identified as having feeding problems.
Two coders blind to group status were trained to code mother–infant interactions by the second author until interrater reliability criterion was reached (percentage agreement = 90% or above). Subsequent interrater reliability was established on 19% of the tapes, with both coders viewing these tapes separately and comparing codes subsequently. If the coders disagreed by more than 1 point on any dimension, they arrived at a final code by viewing the tape again and resolving discrepancies by mutual agreement. Interrater reliability on individual items ranged from Pearson r = .73 (Cohen’s κ = .53) to r = .97 (Cohen’s κ = .95).
To reduce the number of observational variables for parenting and following previous studies (e.g., Eiden et al., 1999; Eiden, Stevens, Schuetze, & Dombkowski, 2006), a principal components analysis with varimax rotation was conducted using the individual maternal behavior items. This analysis indicated high eigenvalues for the first two factors (greater than 3) and lower eigenvalues (between 1 and 2) on six other factors. Thus, this analysis indicated a two-factor solution. The factor loadings for the individual items are displayed in Table 1. The first factor, maternal insensitivity, had an eigenvalue of 5.08 and explained 16.43% of the variance. The second factor, maternal warmth, had an eigenvalue of 3.89 and explained 12.61% of the variance.
TABLE 1.
Factor Loadings for Maternal Behavior During Feeding
| Variables | Factor 1 (Insensitivity) | Factor 2 (Warmth) |
|---|---|---|
| Positions infant for reciprocal exchange | .62 | |
| Talks to infant | .71 | |
| Waits for infant to initiate interactions | .57 | |
| Pleasure toward infant | .66 | |
| Appears cheerful | .74 | |
| Appears sad | −.72 | |
| Appears distressed | −.74 | |
| Appears detached | −.59 | |
| Position without support | .44 | |
| Holds stiffly | .77 | |
| Avoids gaze | .41 | |
| Handles excessively | .54 | |
| Misses infant cues | .53 | |
| Interrupts or terminates feeding, causing infant distress | .45 | |
| Appears angry | .41 | |
| Controls feeding overriding infant cues | .54 | |
| Forces bottle into infant’s mouth | .40 | |
| Handles roughly | .53 |
RESULTS
Sample Characteristics
As shown in Table 2, women in the two groups were similar in terms of parity, age, marital status, and race. However, women in the pregnancy smoking group had a marginally lower SES and significantly less education compared to the nonsmokers. Group differences for infant characteristics are presented in Table 3. Infants of women who smoked during pregnancy weighed less at birth (BW) and at the postnatal visit and had a lower gestational age (GA) than infants of nonsmoking mothers. There were no other significant differences at birth or in growth at the neonatal visit.
TABLE 2.
Group Differences for Maternal Demographic Characteristics and Substance Use During Pregnancy
| Nonsmokersa |
Smokersb |
|||||
|---|---|---|---|---|---|---|
| Variables | M | SD | M | SD | F | |
| Demographic characteristics | ||||||
| Age (years) | 28.63 | 5.53 | 26.85 | 5.79 | 1.49 | .001 |
| Education (years completed) | 12.44 | 1.87 | 11.28 | 1.18 | 11.39* | .21 |
| SES (Hollingshead two-factor) | 3.12 | 1.74 | 2.46 | 1.31 | 3.76+ | .11 |
| Prenatal care (no. of visits) | 15.3 | 13.86 | 15.17 | 17.53 | .02 | .002 |
| Parity | 3.09 | 1.46 | 3.05 | 1.55 | .001 | .04 |
| Marital status (% Single) | 57 | 66 | χ2 = .62 | .01 | ||
| Substance use during pregnancy | ||||||
| Average no. cigarettes per week | 0 | 0 | 39.92 | 39.58 | 44.01* | .37 |
| Average no. standard drinks per week prior to pregnancy recognition | .09 | .19 | .13 | .25 | .86 | .03 |
Note. SES = socioeconomic status.
n = 41.
n = 43.
p < .01.
p < .10.
TABLE 3.
Group Differences for Infant Characteristics
| Nonexposurea |
Prenatal Exposureb |
|||||
|---|---|---|---|---|---|---|
| Variables | M | SD | M | SD | F | |
| Infant characteristics | ||||||
| Age at 2- to 4-week visit (weeks) | 3.14 | 1.10 | 3.22 | .77 | .14 | .02 |
| Gestational age (weeks) | 39.45 | 1.29 | 38.67 | 1.52 | 5.27* | .003 |
| Birth weight (g) | 3458.63 | 523.66 | 3111.53 | 438.48 | 8.48** | .22 |
| Birth length (cm) | 49.77 | 4.70 | 48.21 | 6.16 | 1.35 | .005 |
| Birth head circumference (cm) | 33.80 | 1.43 | 33.79 | 1.81 | .002 | .02 |
| Apgar—1 min | 8.59 | 1.21 | 8.41 | 1.37 | .36 | .01 |
| Apgar—5 min | 8.95 | .22 | 8.97 | .18 | .17 | .02 |
| Weight at 1 month of age (g) | 4588.44 | 712.94 | 4007.95 | 778.54 | 12.57** | .24 |
| Length at 1 month of age (cm) | 52.16 | 4.72 | 52.33 | 3.39 | .03 | .02 |
| Head circumference 1 month (cm) | 36.82 | 2.02 | 36.41 | 1.66 | 1.12 | .04 |
| Sex (% male) | 48% | 53% | ||||
n = 41.
n = 43.
p < .05.
p < .01.
Group Differences in Maternal Psychosocial Functioning
We examined group differences in maternal psychosocial functioning by means of multivariate analyses of variance (MANOVA) with group status as the independent variable and BSI subscale scores as the dependent variable for the first MANOVA and BPA subscales score as the dependent variable for the second MANOVA. The MANOVA for the BSI subscales yielded a significant multivariate effect of group status on maternal functioning, F(9, 74) = 2.14, p < .05, . Univariate analyses indicated that women who smoked during pregnancy had higher scores on the Obsessive–Compulsive, Depression, Interpersonal Sensitivity, Paranoid Ideation, Anxiety, Phobic Anxiety, and Hostility subscales than nonsmokers (see Table 4).
TABLE 4.
Group Differences for Maternal Psychological Functioning and Maternal Behavior
| Nonsmokersa |
Smokersa |
|||||
|---|---|---|---|---|---|---|
| Variables | M | SD | M | SD | F | |
| BSI subscales | ||||||
| Somaticism | .07 | .14 | .17 | .28 | 3.77+ | .03 |
| Obsessive–Compulsive | .08 | .19 | .29 | .40 | 9.73** | .05 |
| Interpersonal Sensitivity | .08 | .22 | .32 | .43 | 10.63** | .10 |
| Depression | .08 | .20 | .23 | .35 | 5.83* | .05 |
| Anxiety | .06 | .16 | .18 | .31 | 4.53* | .04 |
| Hostility | .08 | .16 | .27 | .36 | 9.91** | .09 |
| Phobic Anxiety | .05 | .16 | .18 | .28 | 7.63** | .07 |
| Paranoid Ideation | .23 | .40 | .53 | .63 | 8.54** | .08 |
| Psychoticism | .11 | .23 | .14 | .23 | .42 | .002 |
| BPA subscales | ||||||
| Physical Aggression | 19.49 | 5.71 | 23.1 | 6.93 | 6.82* | .06 |
| Verbal Aggression | 14.55 | 4.23 | 16.05 | 4.81 | 2.35 | .03 |
| Anger | 16.37 | 4.52 | 20.29 | 6.36 | 10.7** | .09 |
| Hostility | 15.21 | 5.19 | 17.98 | 6.83 | 4.4* | .03 |
| Maternal behavior | ||||||
| Maternal warmth | 17.66 | 5.56 | 14.47 | 7.31 | 8.55** | .12 |
| Maternal insensitivity | 6.34 | .88 | 7.66 | 2.83 | 4.25* | .08 |
n = 38.
p < .10.
p < .05.
p < .01.
The MANOVA for the BPA subscales also yielded a significant multivariate effect of group status on maternal aggression or hostility, F(4,79) = 2.49, p < .05, (see Table 4). Univariate analyses indicated that mothers who smoked during pregnancy had higher scores on the Physical Aggression, Anger, and Hostility subscales.
Association Among Demographics, Psychosocial Functioning, Pregnancy Substance Use, and Maternal Behavior
Correlational analyses were used to examine the association between maternal demographic (maternal age, education, and SES), maternal substance use during pregnancy, and the maternal behavior variables. Maternal age, r(75) = .54, p < .001; education, r(75) = .39, p < .001; and SES, r(75) = .33, p < .01, were positively associated with maternal warmth. In addition, maternal alcohol use during pregnancy prior to pregnancy recognition (average number of drinks per week) was associated with maternal insensitivity, r(76) = .39, p < .001.
Correlational analyses were also conducted to examine the association between psychosocial functioning (BSI and BPA) and the maternal behavior variables. Scores on the BPA Anger, r(76) = −.34, p < .01; Physical Aggression, r(76) = −.23, p < .05; and Verbal Aggression, r(76) = −.23, p < .05, subscales were negatively associated with maternal warmth. None of the psychosocial functioning variables were associated with maternal insensitivity.
Differences in Maternal Behavior and Psychosocial Functioning by Gender
We first examined if maternal behavior varied by infant gender. Results from analysis of variance (ANOVA) indicated that mothers of girls displayed higher levels of warmth compared to mothers of boys, F(1, 74) = 4.25, p < .05, . There were no sex differences for maternal insensitivity, F(1, 74) = 1.89, ns, .
We then examined group differences in maternal psychosocial functioning by means of MANOVA with infant gender as the independent variable and BSI subscale scores as the dependent variable for the first MANOVA and BPA subscale scores as the dependent variable for the second MANOVA. The MANOVA for the BSI subscales indicated no multivariate effect of infant gender, F(9, 74) = .76, ns, . Similarly, the MANOVA for the BPA subscales indicated no multivariate effect of gender, F(4, 79) = 0.87, ns, .
Moderational Analyses
Next, we conducted exploratory analyses to examine if any association between maternal smoking during pregnancy and maternal behavior during mother–infant interactions was moderated by infant gender. Variables that were associated (p < .10) with either cigarette exposure or outcome (maternal warmth and maternal insensitivity) variables were used as covariates. Thus, SES was included as a covariate in analyses of both maternal warmth and maternal insensitivity. In addition, because of their significant association with maternal warmth, maternal age, education, and alcohol use prior to pregnancy recognition (average number of drinks consumed per week) and during pregnancy were included as additional covariates in the analysis of maternal warmth. The covariates were entered in the first step, followed by maternal cigarette smoking during pregnancy and infant gender in the second step, and the interaction term in the third step.
There was a significant interaction term in these analyses for maternal warmth indicating a significant moderation by gender for maternal warmth, F(7, 96) = 9.42 p < .001 (see Figure 1). Mothers who smoked during pregnancy displayed significantly less warmth toward boys than girls during interactions. There were no differences in maternal warmth for boys and girls among the mothers who did not smoke during pregnancy. These analyses, however, indicated that there was no significant moderation by gender for maternal insensitivity. Given prior discussions about the instability of interaction terms in regression equations, the difficulty of finding interaction effects that are generally small in magnitude (see Luthar & Zelazo, 2003), and limitations of sample size in this study, we followed steps outlined by Wakschlag and Hans (2002) to further explore the possibility that infant gender moderated the association between maternal cigarette smoking during pregnancy and maternal insensitivity. Separate regression analyses using the three-step hierarchical approach previously described were conducted to examine the effects of maternal smoking during pregnancy on maternal insensitivity for boys and for girls. There was no relation between maternal smoking during pregnancy for either boys, F(5, 42) = 3.74, t = −0.92, ns, adjusted R2 = 0.23; or girls, F(5, 50) = 2.51, t = 1.36, ns, adjusted R2 = 0.26, indicating that infant gender did not moderate the association between maternal smoking during pregnancy and maternal insensitivity.
FIGURE 1.
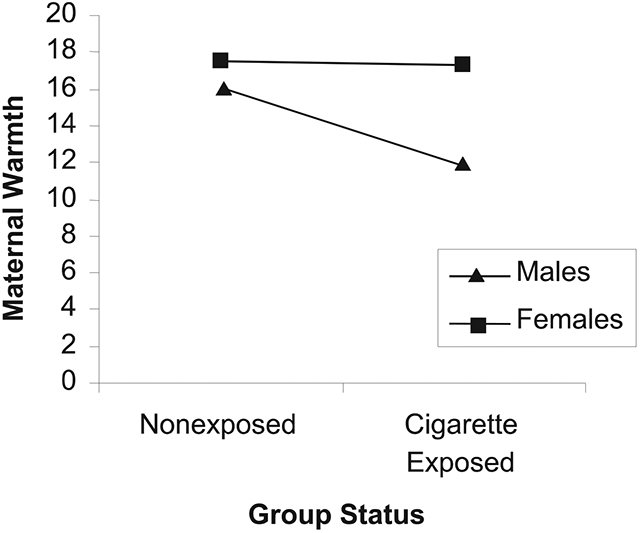
Interaction between infant gender and maternal warmth.
Group Differences in Maternal Behavior
We examined group differences in maternal behavior during feeding interactions by means of two analyses of covariance (ANCOVAs) with group status as the independent variable. Maternal warmth was the dependent variable in the first ANCOVA and maternal insensitivity was the dependent variable in the second ANCOVA. The variables described earlier that were associated with either cigarette exposure or the outcome variables were used as covariates. ANCOVA results indicated that women who smoked during pregnancy had lower levels of maternal warmth than comparison women (see Table 4). For maternal insensitivity, results of ANCOVA indicated that women who smoked during pregnancy had higher levels of maternal insensitivity than comparison women (see Table 4). When these analyses were repeated with BW and GA as additional covariates, the results remained unchanged.
Mediational Analyses
The next step was to examine if there was an indirect association between prenatal exposure to cigarettes and maternal behavior during feeding interactions via maternal psychosocial functioning. First, measures from the BSI were examined as possible mediators of the association between prenatal exposure to cigarettes and maternal behavior. Because of concerns about multicollinearity, each BSI subscale was considered and analyzed separately for each of the two dependent measures (maternal warmth and maternal insensitivity). Two approaches to examining indirect or mediational pathways have been discussed in recent years (e.g., MacKinnon, Lockwood, Hoffman, West, & Sheets, 2002). The first is the widely used causal steps approach to mediation that clearly specifies that to test mediation, the independent (IV), dependent (DV), and mediator variables must all be associated with each other (Baron & Kenny, 1986). The shortcomings of this method have recently been discussed in the literature. The causal steps approach has been faulted because it does not provide a statistical test of the indirect effect of an IV on a DV via a third variable; large sample sizes (n = 500 or more) are required to have adequate power to test mediational effects with small to medium effect sizes; and the condition that IV and DV have to be significantly associated with each other excludes many “inconsistent” intervening variable models in which the direct and indirect effects have opposite signs and may cancel each other out (MacKinnon, Krull, & Lockwood, 2000). Given that we did not have the recommended power because of a moderate sample size (MacKinnon et al., 2002), we chose to analyze the role of mediators using an intervening variable approach discussed by MacKinnon and colleagues (2000; MacKinnon et al., 2002).
The first step in this process was to estimate the association between maternal cigarette use and the individual BSI subscales using linear regression with the BSI subscale as the criterion variable and maternal cigarette use as the predictor. Prenatal exposure to cigarettes was associated with higher scores on the BSI Obsessive–Compulsive (β = .20, SE = .07, p < .01), Interpersonal Sensitivity (β = .22, SE = .08, p < .01), Depression (β = .13, SE = .06, p < .05), Hostility (β = .17, SE = .06, p < .01), Anxiety (β = .22, SE = .08, p < .05), Phobic Anxiety (β = .12, SE = .05, p < .05), and Paranoid Ideation (β = .30, SE = .12, p < .01) subscales. There was no significant association between prenatal exposure to cigarettes and scores on the BSI Somaticism or Psychoticism subscales. Consequently, these were not considered further as potential intervening variables. We then estimated the association between maternal cigarette use during pregnancy and the individual BPA subscales. Prenatal exposure to cigarettes was associated with higher scores on the Physical Aggression (β = 3.30, SE = 1.39, p < .05) and Anger (β = 3.51, SE = 1.21, p < .01) subscales of the BPA but not with scores on the Verbal Aggression or Hostility subscales. Thus, BPA Verbal Aggression and BPA Hostility were not considered further as potential intervening variables.
In the next step, we examined if the BSI or BPA subscales and cigarette smoking during pregnancy predicted maternal behavior during feeding. First, separate linear regression analyses were used with maternal insensitivity as the criterion variable for each BSI and BPA subscale score already identified. The BSI Anxiety (β = 3.25, SE = 1.25, p < .05) and Hostility (β = 2.4, SE = .86, p < .05) subscales were both associated with maternal insensitivity. None of the other BSI or BPA subscales predicted maternal insensitivity. The product of coefficients test for the intervening variable effect was used to calculate the significance of any indirect effect (see MacKinnon et al., 2002). The significance of the intervening variable effect was tested by dividing the estimate of the intervening variable effect by its standard error, which was then compared to the values of the normal distribution (MacKinnon et al., 2002). Both of the intervening variable effects for the BSI Anxiety (Z = 2.01) and Hostility subscales (Z = 2.55) were significant, indicating that symptomatology in these areas mediated the association between smoking during pregnancy and maternal insensitivity.
The same procedure was used to evaluate BSI and BPA subscale scores as potential mediators of the association between maternal cigarette smoking during pregnancy and maternal warmth during interactions. The BPA Anger subscale was significantly associated with maternal warmth (β = −.256, SE = .09, p < .01). None of the BSI or other BPA subscales was associated with maternal warmth. The intervening variable effect for the BPA Anger subscale was significant (Z = −2.14), indicating that maternal anger mediated the association between smoking during pregnancy and maternal warmth.
DISCUSSION
Recent studies have suggested that parenting behavior may be one pathway through which behavioral problems develop among children prenatally exposed to cigarettes. As such, one of the major goals of this study was to examine if women who smoked during pregnancy displayed higher insensitivity and lower warmth during mother–infant interactions compared to those who did not. Although results indicated an association between pregnancy smoking and maternal behavior, they also indicated that this association was not direct. Instead, the pathway to less optimal maternal behaviors during interactions appeared to be indirect through maternal characteristics. These findings have implications for attributing negative child outcomes among cigarette-exposed children solely to the teratological impact of prenatal cigarette exposure. Because of theoretical and empirical associations between parenting behavior and child outcomes that are well established in the literature, these findings lend support to the idea that parenting behavior may be another pathway to adverse developmental outcomes among cigarette-exposed children.
Women who smoked during pregnancy had higher levels of maternal insensitivity during interactions with their infant. These results lend further support to previous studies that have found an association between smoking during pregnancy and less responsive and sensitive parenting behavior (e.g., Fergusson, Woodward, & Horwood, 1998; Wakschlag et al., 1997; Weissman et al., 1999). However, our data extend these findings by suggesting that the association between maternal smoking during pregnancy and maternal behavior can be explained, at least in part, by maternal psychosocial functioning. Specifically, we found that mothers who smoked during pregnancy and had more symptoms of anxiety and hostility had higher levels of insensitivity during interactions with their infants. These findings are particularly important because low maternal sensitivity is a significant predictor of poor parent–child relationships or attachment quality in infancy (e.g., Belsky, Fish, & Isabella, 1991). Furthermore, insecure attachment during infancy is a predictor of higher externalizing behavior problems and conduct disorder among older children (e.g., Lyons-Ruth, Alpern, & Repacholi, 1993). Thus, an intervention designed to promote sensitive maternal responsiveness to their infants and reduce maternal symptoms of psychopathology in addition to reducing or eliminating maternal smoking may be very effective for this population. In fact, studies by van den Boom (1994, 1995) have demonstrated the efficacy of such interventions in promoting mother–infant attachment security and positive infant outcomes.
Second, results indicated that women who smoked during pregnancy had lower levels of maternal warmth during interactions with their infant and that this association was mediated by maternal anger or hostility. A more detailed examination of the items in the maternal warmth scale indicated that the majority of the included behaviors, such as making positive remarks to their infant, showing pleasure toward their infant, showing less negative affect, and appearing less detached during the feeding interaction, are behaviors that are associated with increased levels of positive affect. Thus, mothers who have higher levels of anger or hostility may have more difficulty engaging in behaviors during mother–child interactions that are associated with positive affect. This combination of increased hostility and decreased warmth has the potential for significant implications for continuing development. As described earlier, maternal hostility is associated with harsh or punitive discipline strategies with older children that, in turn, have been linked to externalizing behavior problems in the children. In fact, two consistent and significant predictors of externalizing behavior problems are the combination of low warmth and high hostility, characteristics of an authoritarian style of parenting (Baumrind, 1971). Thus, these may explain, at least in part, the consistent link that has been found between prenatal exposure to cigarettes and externalizing behavior problems in older children (e.g., Day et al., 2000; Orlebeke et al., 1997; Wakschlag & Hans, 2002). Future studies are needed to further explore if maternal hostility is one of the mechanisms underlying the development of externalizing behavior problems in exposed children. However, given the established link between maternal hostility and the development of externalizing behaviors in children, intervention programs for pregnancy smokers should consider addressing hostility and anger as part of their intervention strategy in an attempt to improve parenting and optimize development for the children. Furthermore, future studies should control for maternal psychosocial functioning and hostility and anger, in particular, when examining the association between prenatal smoking and child behavior problems.
The large group differences in GA and weight, both at birth and at the neonatal visit, suggest that these infant characteristics may have influenced maternal behavior. For example, there is evidence that even slight impairments in fetal growth are associated with a range of behavioral and cognitive difficulties in infancy and in early childhood (Behnke et al., 1999; Breslau et al., 1994; Horwood, Mogridge, & Darlow, 1998; Johnston, Low, de Baess, & Mac Vean, 1987; Lester, Garcia-Coll, Valcarcel, Hoffman, & Brazelton, 1986; McCormick, Workman-Daniels, & Brooks-Gunn, 1996; Rose, 1994; Sigman, McDonald, Neumann, & Bwibo, 1991; Taylor, Klein, Schatschneider, & Hack, 1998) that, in turn, could elicit altered parenting behaviors. In fact, one recent study found an association between low BW and lower levels of maternal sensitivity and fewer maternal positive vocalizations in a sample of substance-exposed toddlers (Uhlhorn, Messinger, & Bauer, 2005). It is unclear, however, if slight reductions in infant weight and GA would be associated with maternal behavior as early as the first weeks of life. Although controlling for these variables in our study did not change the findings, future studies should explore the influence that infant characteristics have on maternal behavior in this population.
Finally, our data indicate that infant gender was associated with maternal warmth among pregnancy smokers but not among nonsmokers. The absence of gender differences among nonsmoking mothers is consistent with recent studies that find no differences in the way in which parents behave toward their infant as a function of infant gender (e.g., Seifer et al., 1992). However, among mothers who smoked during pregnancy, mothers had lower levels of maternal warmth during feeding interactions with their sons than with their daughters. It is possible that this gender-related difference in maternal responsiveness is in response to gender variations in temperament. During infancy, boys tend to have higher levels of negative affect and more difficulty regulating their emotions than girls (Carter, Mayes, & Pajer, 1990; Kohnstamm, 1989; Osofsky & O’Connell, 1977; Rosen, Adamson, & Bakeman, 1992; Weinberg et al., 1999). This gender difference may be exacerbated among cigarette-exposed infants. Thus, mothers may display less positive affect and warmth during interactions with their sons in response to increased levels of negativity in the infant. Future studies should explore the interaction of infant temperament, affect, and gender with maternal behavior during interactions among cigarette-exposed mother–infant dyads.
There are a number of limitations of this study. The first limitation concerns the self-report nature of both the assessment of cigarette exposure and psychosocial functioning in these women. The accurate assessment of substance use is always difficult. Pregnant women are often hesitant to divulge information regarding the use of substances during pregnancy. Furthermore, because our measure of smoking during pregnancy was based on maternal self-report, it is possible that a smoker may have been misidentified as a nonsmoker. However, in spite of this possibility that some women in the control group may have misreported their smoking exposure, it is important to note that there were consistent group differences in both reported psychosocial functioning and observed maternal behavior. Similarly, both measures of psychosocial functioning are based on maternal self-report, whereas the measures of maternal behavior are observational in nature. Consequently, women may have misrepresented their levels of symptomatology. However, it is noteworthy that there are significant group differences in almost all aspects of psychosocial functioning and that many of these domains of psychosocial functioning are associated with the observed maternal behaviors.
Second, the measures of maternal behavior were limited to a single context (i.e., feeding) and were limited to a brief, 10-min period. We chose to focus on feeding interactions because they provide a naturalistic context in which a majority of mother–infant interactions in the first month of life occur. However, future studies measuring maternal behavior in multiple contexts would be better able to address the issue of generalizability of results to contexts other than feeding.
Finally, it is unclear from this study whether the group differences in maternal behavior will persist beyond the first month of life and whether they indicate the potential for a different developmental trajectory for child outcomes. Thus, longitudinal studies should be conducted to look at maternal behavior and its association with behavioral outcomes throughout infancy and into early childhood among children who were prenatally exposed to cigarettes.
Despite these limitations, these findings highlight the importance of examining maternal behavior among mothers who smoked during pregnancy. Furthermore, these results suggest that maternal psychological functioning should be examined as a possible predictor of parenting in this high-risk population.
ACKNOWLEDGMENTS
This study was made possible by a grant from the National Institute of Child Health and Human Development (R15 HD039645-01A2). We thank the mothers and infants who participated in this study, and the research staff responsible for recruiting and conducting the mother–infant assessments. Special thanks are due to Dr. Amol Lele and the staff of the Women’s and Children’s Hospital of Buffalo who collaborated with regard to data collection in this study.
Contributor Information
Pamela Schuetze, Department of Psychology, State University of New York College at Buffalo, Research Institute on Addictions and Department of Pediatrics, State University of New York at Buffalo.
Rina D. Eiden, Research Institute on Addictions and Department of Pediatrics, State University of New York at Buffalo
Laura Dombkowski, Department of Psychology, State University of New York College at Buffalo.
REFERENCES
- Anda RF, Williamson DF, Escobedo LG, Mast EE, Giovino GA, & Remington PL. (1990). Depression and the dynamics of smoking: A national perspective. Journal of the American Medical Association, 264, 1541–1545. [PubMed] [Google Scholar]
- Baghurst PA, Tong SL, Woodward A, & McMichael AJ (1992). Effects of maternal smoking upon neuropsychological development in early childhood: Importance of taking account of social and environmental factors. Paediatric and Perinatal Epidemiology, 6, 403–415. [DOI] [PubMed] [Google Scholar]
- Baron RM, & Kenny DA (1986). The moderator–mediatory variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology, 51, 1173–1182. [DOI] [PubMed] [Google Scholar]
- Baumrind D (1971). Current patterns of parental authority. Developmental Psychology Monographs, 4, 1–103. [Google Scholar]
- Behnke M, Eyler FD, Garvan CW, Tenholder MJ, Wobie K, Woods NS, et al. (1999). Cranial ultrasound abnormalities identified at birth: Their relationship to perinatal risk and neurobehavioral outcome. Pediatrics, 103, E41. [DOI] [PubMed] [Google Scholar]
- Belsky J, Fish M, & Isabella R (1991). Continuity and discontinuity in infant negative and positive emotionality: Family antecedents and attachment consequences. Developmental Psychology, 27, 421–431. [Google Scholar]
- Breslau N, DelDotto JE, Brown GG, Kumar S, Ezhuthachan S, & Hufnagle KG (1994). A gradient relationship between low birth weight and IQ at age 6 years. Archives of Pediatric and Adolescent Medicine, 148, 377–383. [DOI] [PubMed] [Google Scholar]
- Breslau N, Kilbey MM, & Andreski P (1990). DSM–III–R nicotine dependence in young adults: Prevalence, correlates and associated psychiatric disorders. Addiction, 89, 743–754. [DOI] [PubMed] [Google Scholar]
- Breslau N, Kilbey MM, & Andreski P (1991). Nicotine dependence, major depression, and anxiety in young adults. Archives of General Psychiatry, 48, 1069–1074. [DOI] [PubMed] [Google Scholar]
- Breslau N, Kilbey MM, & Andreski P (1993). Nicotine dependence, major depression: New evidence from a prospective investigation. Archives of General Psychiatry, 50, 31–35. [DOI] [PubMed] [Google Scholar]
- Brooks JS, Cohen P, & Brook DW (1998). Longitudinal study of co-occurring psychiatric disorders and substance use. Journal of the American Academy of Child and Adolescent Psychiatry, 37, 322–330. [DOI] [PubMed] [Google Scholar]
- Brown RA, Burges ES, Sales SD, Whiteley JA, Evans DM, & Miller I (1998). Reliability and validity of a smoking timeline follow-back interview. Psychology of Addictive Behaviors, 12, 101–112. [Google Scholar]
- Buss AH, & Perry M (1992). The Aggression Questionnaire. Journal of Personality and Social Psychology, 63, 452–459. [DOI] [PubMed] [Google Scholar]
- Carter AS, Mayes LC, & Pajer KA (1990). The role of dyadic affect in play and infant sex in predicting infant response to the still-face situation. Child Development, 61, 764–773. [PubMed] [Google Scholar]
- Chatoor I, Getson P, Menvielle E, Brasseaux C, O’Donnell R, Rivera Y, & Mrazek D (1997). A feeding scale for research and clinical practice to assess mother-infant interactions in the first three years of life. Infant Mental Health Journal, 18, 76–91. [Google Scholar]
- Chatoor I, Sartes J, Ganiban J, Beker L, Paez LM, & Kerzner B (2004). Failure to thrive and cognitive development in toddlers with infantile anorexia. Pediatrics, 113, 440–447. [DOI] [PubMed] [Google Scholar]
- Chatoor I, Schaefer S, Dickson L, & Egan J (1984). Non-organic failure to thrive: A developmental perspective. Pediatric Annals, 13, 829–835. [PubMed] [Google Scholar]
- Cohn JF, & Campbell SB (1992). Influence of maternal depression on infant affect regulation. In Cicchetti D & Toth S (Eds.), Developmental perspectives on depression (4th ed., pp. 103–130). Rochester, NY: University of Rochester Press. [Google Scholar]
- Cornelius M, & Day NL (2000). The effects of tobacco use during and after pregnancy on exposed children and the relevance of these findings for alcohol research. Alcohol Health and Research World, 24, 242–249. [PMC free article] [PubMed] [Google Scholar]
- Dahlstrom A, Ebergsjo C, & Lundell B (2004). Nicotine exposure in breastfed infants. Acta Paediatrica, 93, 810–816. [PubMed] [Google Scholar]
- Day NL, Richardson GA, Goldschmidt L, & Cornelius MD (2000). Effects of prenatal tobacco exposure on preschoolers’ behavior. Journal of Developmental and Behavioral Pediatrics, 21, 180–188. [PubMed] [Google Scholar]
- Derogatis LR (1992). The Brief Symptom Inventory manual. Baltimore: Clinical Psychometric Research. [Google Scholar]
- Derogatis LR (1993). The Brief Symptom Inventory (BSI) administration, scoring, and procedures manual. Minneapolis, MN: National Computer Systems. [Google Scholar]
- Eiden RD (2001). Maternal substance use and mother–infant feeding interactions. Infant Mental Health Journal, 22, 497–511. [Google Scholar]
- Eiden RD, Chavez F, & Leonard KE (1999). Parent–infant interactions in alcoholic and control families. Development and Psychopathology, 11, 745–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden RD, Stevens A, Schuetze P, & Dombkowski LE (2006). A conceptual model for maternal behavior among poly-drug cocaine using mothers. Psychology of Addictive Behaviors, 20, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder GH, Eccles JS, Ardelt M, & Lord S (1995). Inner-city parents under economic pressure: Perspective on the strategies of parenting. Journal of Marriage and the Family, 57, 771–784. [Google Scholar]
- Fergusson DM, Goodwin RD, & Horwood LJ (2003). Major depression and cigarette smoking: Results of a 21-year longitudinal study. Psychological Medicine, 33, 1357–1367. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, & Lynskey MT (1993). Maternal smoking before and after pregnancy: Effects on behavioral outcomes in middle childhood. American Academy of Pediatrics, 92, 815–822. [PubMed] [Google Scholar]
- Fergusson DM, Woodward LJ, & Horwood LJ (1998). Maternal smoking during pregnancy and psychiatric adjustment in late adolescence. Archives of General Psychiatry, 55, 721–727. [DOI] [PubMed] [Google Scholar]
- Flannery KA, & Leiderman J (1994). A re-examination of the sex ratios of families with a neurodevelopmentally disordered child. Journal of Child Psychology and Psychiatry, 37, 621–623. [DOI] [PubMed] [Google Scholar]
- Fried PA, & Makin JE (1987). Neonatal behavioral correlates of prenatal exposure to marihuana, cigarettes, and alcohol in a low risk population. Neurobehavioral Toxicology and Teratology, 9, 1–7. [DOI] [PubMed] [Google Scholar]
- Fried PA, & O’Connell CM (1987). A comparison of the effects of prenatal exposure to tobacco, alcohol, cannabis, and caffeine on birth size and subsequent growth. Neurobehavioral Toxicology and Teratology, 9, 79–85. [DOI] [PubMed] [Google Scholar]
- Fried PA, O’Connell CM, & Watkinson B (1992). 60- and 72-month follow-up of children prenatally exposed to marijuana, cigarettes, and alcohol: Cognitive and language assessment. Developmental and Behavioral Pediatrics, 13, 383–391. [PubMed] [Google Scholar]
- Glassman AH, Helzer JE, Covey LA, Cottier LB, Stetner F, Tipp JE, et al. (1990). Smoking, smoking cessation, and major depression. Journal of the American Medical Association, 264, 1546–1549. [PubMed] [Google Scholar]
- Gusella JL, & Fried PA (1984). Effects of maternal social drinking and smoking on offspring at 13 months. Neurobehavioral Toxicology and Teratology, 6, 13–17. [PubMed] [Google Scholar]
- Hans SL (1994). Sex differences in children of substance-abusing parents. Drug and Alcohol Abuse Reviews, 5, 475–490. [Google Scholar]
- Hans SL, Bernstein VJ, & Henson LG (1999). The role of psychopathology in the parenting of drug-dependent women. Development and Psychopathology, 11, 957–977. [DOI] [PubMed] [Google Scholar]
- Haug K, Irgens LM, Skjaerven R, Markestad T, Baste V, & Schreuder P (2000). Maternal smoking and birthweight: Effect modification of period, maternal age and paternal smoking. Acta Obstetricia et Gynecologica Scandinavica, 79, 485–489. [PubMed] [Google Scholar]
- Hollingshead AB (1975). Four Factor Index of Social Status. Unpublished manuscript, Yale University, New Haven, CT. [Google Scholar]
- Horwood LJ, Mogridge N, & Darlow BA (1998). Cognitive, educational, and behavioural outcomes at 7 to 8 years in a national very low birthweight cohort. Archives of Disease in Childhood: Fetal and Neonatal Edition, 79, F12–F20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynd GW, & Semrud-Clikeman M (1989). Dyslexia and brain morphology. Psychological Bulletin, 106, 447–482. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Fein GG, Jacobson JL, Schwartz PM, & Dowler JK (1984). Neonatal correlates of prenatal exposure to smoking, caffeine, and alcohol. Infant Behavior and Development, 7, 253–265. [Google Scholar]
- Jameson PB, Gelfand D, Kulscar E, & Teti DM (1997). Mother–toddler interaction patterns associated with maternal depression. Developmental Psychopathology, 9, 557–590. [PubMed] [Google Scholar]
- Johnson JG, Cohen P, Pine DS, Klein DF, Kasen S, & Brook JS (2000). Association between cigarette smoking and anxiety disorders during adolescence and early adulthood. Journal of the American Medical Association, 284, 2348–2351. [DOI] [PubMed] [Google Scholar]
- Johnston FE, Low SM, de Baess Y, & Mac Vean RB (1987). Interaction of nutritional and socioeconomic status as determinants of cognitive development in disadvantaged urban Guatemalan children. American Journal of Physical Anthropology, 73, 501–506. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Johnson JG, Bird HR, Canino G, Goodman SH, Lahey B, et al. (1997). Psychiatric disorders associated with substance use among children and adolescents: Findings from the Methods for the Epidemiology of Child and Adolescent Mental Disorders (MECA) study. Journal of Abnormal Child Psychology, 25, 121–132. [DOI] [PubMed] [Google Scholar]
- Kanoy K, Ulku-Steiner B, Cox M, & Burchinal M (2003). Marital relationship and individual psychological characteristics that predict physical punishment of children. Journal of Family Psychology, 17, 20–28. [DOI] [PubMed] [Google Scholar]
- Katz LF, & Woodin EM (2002). Hostility, hostile detachment, and conflict engagement in marriages: Effects on child and family functioning. Child Development, 73, 636–651. [DOI] [PubMed] [Google Scholar]
- Kim IJ, Ge X, Brody GH, Conger RD, Gibbons FX, & Simons RL (2003). Parenting behaviors and the occurrence and co-occurrence of depressive symptoms and conduct problems among African American children. Journal of Family Psychology, 17, 571–583. [DOI] [PubMed] [Google Scholar]
- Kleinman JC, & Kopstein A (1987). Smoking during pregnancy, 1967–1980. American Journal of Public Health, 77, 823–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohnstamm GA (1989). Temperament in childhood: Cross-cultural and sex differences. In Kohnstamm GA & Bates JE (Eds.), Temperament in childhood (pp. 483–508). New York: Wiley. [Google Scholar]
- Landesman-Dwyer S, Ragozin AS, & Little RE (1981). Behavioral correlates of prenatal alcohol exposure: A four-year follow-up study. Neurobehavioral Toxicology, 3, 187–193. [PubMed] [Google Scholar]
- Lester BM, Garcia-Coll C, Valcarcel M, Hoffman J, & Brazelton TB (1986). Effects of atypical patterns of fetal growth on newborn (NBAS) behavior. Child Development, 57, 11–19. [PubMed] [Google Scholar]
- Luthar SS, & Zelazo LB (2003). Resilience and vulnerability: Adaptation in the context of childhood adversities. New York: Cambridge University Press. [Google Scholar]
- Lyons-Ruth K, Alpern L, & Repacholi B (1993). Disorganized infant attachment classification and maternal psychosocial problems as predictors of hostile-aggressive behavior in the preschool classroom. Child Development, 64, 572–585. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Krull JL, & Lockwood CM (2000). Equivalence of the mediation, confounding, and suppression effect. Prevention Science, 1, 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, Hoffman JM, West SG, & Sheets V (2002). A comparison of methods to test mediation and other intervening variable effects. Psychological Methods, 7, 83–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malatesta CZ, & Haviland JM (1982). Learning display rules: The socialization of emotion expression in infancy. Child Development, 53, 991–1003. [PubMed] [Google Scholar]
- McCormick MC, Workman-Daniels K, & Brooks-Gunn J (1996). The behavioral and emotional well-being of school-age children with different birth weights. Pediatrics, 97, 18–25. [PubMed] [Google Scholar]
- McGee R, & Stanton WR (1994). Smoking in pregnancy and child development to age 9 years. Journal of Pediatric Child Health, 30, 263–268. [DOI] [PubMed] [Google Scholar]
- Nix RL, Pinderhughes EE, Dodge KA, Bates JE, Pettit GS, & McFadyen-Ketchum SA (1999). The relation between mothers’ hostile attribution tendencies and children’s externalizing behavior problems: The mediating role of mothers’ harsh discipline practices. Child Development, 70, 896–909. [DOI] [PubMed] [Google Scholar]
- Olds D (1997). Tobacco exposure and impaired development: A review of the evidence. Mental Retardation and Developmental Disabilities Research Reviews, 3, 257–269. [Google Scholar]
- Orlebeke JF, Knol DL, & Verhulst FC (1997). Increase in child behavior problems resulting from maternal smoking in pregnancy. Archives of Environmental Health, 52, 317–321. [DOI] [PubMed] [Google Scholar]
- Osofsky JD, & O’Connell EJ (1977). Patterning of newborn behavior in an urban population. Child Development, 48, 532–536. [Google Scholar]
- Pritchard CW (1994). Depression and smoking in pregnancy in Scotland. Journal of Epidemiology and Community Health, 48, 377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriquez A, Bohlin G, & Lindmark G (2000). Psychosocial predictors of smoking and exercise during pregnancy. Journal of Reproductive and Infant Psychology, 18, 203–223. [Google Scholar]
- Rose SA (1994). Relation between physical growth and information processing in infants born in India. Child Development, 65, 889–902. [PubMed] [Google Scholar]
- Rosen WD, Adamson LB, & Bakeman R (1992). An experimental investigation of infant social referencing: Mothers’ messages and gender differences. Developmental Psychology, 28, 1172–1178. [Google Scholar]
- Schuetze P, & Eiden RD (2006). The association between maternal smoking and secondhand exposure and autonomic functioning at 2-4 weeks of age. Infant Behavior and Development, 29, 32–43. [DOI] [PubMed] [Google Scholar]
- Schuetze P, & Zeskind PS (2001). Relation between prenatal exposure to cigarettes and behavioral and physiological measures of autonomic regulation in neonates. Infancy, 2, 371–383. [DOI] [PubMed] [Google Scholar]
- Seifer R, Sameroff AJ, Anagnostopolou R, & Elias PK (1992). Mother–infant interaction during the first year: Effects of situation, maternal mental illness, and demographic factors. Infant Behavior and Development, 15, 405–26. [Google Scholar]
- Sigman M, McDonald MA, Neumann C, & Bwibo N (1991). Prediction of cognitive competence in Kenyan children from toddler nutrition, family characteristics and abilities. Journal of Child Psychology and Psychiatry and Allied Disciplines, 32, 307–320. [DOI] [PubMed] [Google Scholar]
- Sobell LC, & Sobell MB (1995). Alcohol Timeline Followback users’ manual. Toronto: Addiction Research Foundation. [Google Scholar]
- Stem M, & Karraker KH (1989). Sex stereotyping of infants. Sex Roles, 20, 501–522. [Google Scholar]
- Taylor HG, Klein N, Schatschneider C, & Hack M (1998). Predictors of early school age outcomes in very low birth weight children. Journal of Behavioral Pediatrics, 19, 235–243. [DOI] [PubMed] [Google Scholar]
- Tronick EZ, & Cohn JF (1989). Infant–mother face to face interaction: Age and gender differences in coordination and the occurrence of miscoordination. Child Development, 60, 85–92. [PubMed] [Google Scholar]
- Uhlhorn SB, Messinger DS, & Bauer CR (2005). Cocaine exposure and mother–toddler social play. Infant Behavior and Development, 28, 62–73. [Google Scholar]
- van den Boom DC (1994). The influence of temperament and mothering on attachment and exploration: An experimental manipulation of sensitive responsiveness among lower-class mothers with irritable infants. Child Development, 65, 1457–1477. [DOI] [PubMed] [Google Scholar]
- van den Boom DC (1995). Do first year intervention effects endure? Follow-up during toddlerhood of a sample of Dutch irritable infants. Child Development, 66, 1798–1816. [PubMed] [Google Scholar]
- Wakschlag L, & Hans SL (2002). Maternal smoking during pregnancy and conduct problems in high-risk youth: A developmental framework. Development and Psychopathology, 14, 351–369. [DOI] [PubMed] [Google Scholar]
- Wakschlag L, Lahey B, Loeber R, Green S, Gordon R, & Leventhal B (1997). Maternal smoking during pregnancy and the risk of conduct disorders in boys. Archives of General Psychiatry, 54, 670–676. [DOI] [PubMed] [Google Scholar]
- Wang X, Tager IB, Van Vunakis H, Speizer FE, & Hanrahan JP (1997). Maternal smoking during pregnancy, urine cotinine concentrations, and birth outcomes: A prospective cohort study. International Journal of Epidemiology, 26, 978–988. [DOI] [PubMed] [Google Scholar]
- Weinberg J, Zimmerberg B, & Sonderegger T (1992). Gender-specific effects of perinatal exposure to alcohol and other drugs. In Sonderegger T (Ed.), Perinatal substance abuse: Research and clinical findings (pp. 51–89). Baltimore: Johns Hopkins University Press. [Google Scholar]
- Weinberg MK, Tronick EZ, Cohn JF, & Olson KL (1999). Gender differences in emotional expressivity and self-regulation during early infancy. Developmental Psychology, 35, 175–188. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Warner V, Wickramaratne PJ, & Kandel DB (1999). Maternal smoking during pregnancy and psychopathology in offspring followed to adulthood. Journal of the American Academy of Child and Adolescent Psychiatry, 38, 892–899. [DOI] [PubMed] [Google Scholar]
- Wood RD, & Spear LP (1998). Prenatal cocaine alters social competition of infant, adolescent, and adult rats. Behavioral Neuroscience, 112, 419–131. [DOI] [PubMed] [Google Scholar]
- Zaren B, Lindmark G, & Bakketeig L (2000). Maternal smoking affects fetal growth more in the male fetus. Paediatric and Perinatal Epidemiology, 14, 118–126. [DOI] [PubMed] [Google Scholar]
- Zhu S, & Valbo A (2002). Depression and smoking during pregnancy. Addictive Behaviors, 27, 649–658. [DOI] [PubMed] [Google Scholar]


