Abstract
Natural biomaterials hold enormous potential for tissue regeneration. The rapid advance of several tissue-engineered biomaterials, such as natural and synthetic polymer-based scaffolds, has led to widespread application of these materials in the clinic and in research. However, biomaterials can have limited repair capacity; obstacles result from immunogenicity, difficulties in mimicking native microenvironments, and maintaining the mechanical and biochemical (i.e., biomechanical) properties of native organs/tissues. The emergence of decellularized extracellular matrix (ECM)-derived biomaterials provides an attractive solution to overcome these hurdles since decellularized ECM provides a nonimmune environment with native three-dimensional structures and bioactive components. More importantly, decellularized ECM can be generated from the tissue of interest, such as the heart, and keep its native macro- and microstructure and tissue-specific composition. These decellularized cardiac matrices/scaffolds can then be reseeded using cardiac cells, and the resulting recellularized construct is considered an ideal choice for regenerating functional organs/tissues. Nonetheless, the decellularization process must be optimized and depends on tissue type, age, and functional goal. Although most decellularization protocols significantly reduce immunogenicity and deliver a matrix that maintains the tissue macrostructure, suboptimal decellularization can change ECM composition and microstructure, which affects the biomechanical properties of the tissue and consequently changes cell-matrix interactions and organ function. Herein, we review methods of decellularization, with particular emphasis on cardiac tissue, and how they can affect the biomechanics of the tissue, which in turn determines success of reseeding and in vivo viability. Moreover, we review recent developments in decellularized ECM-derived cardiac biomaterials and discuss future perspectives.
Keywords: biomechanics, cardiac, decellularization, extracellular matrix
INTRODUCTION
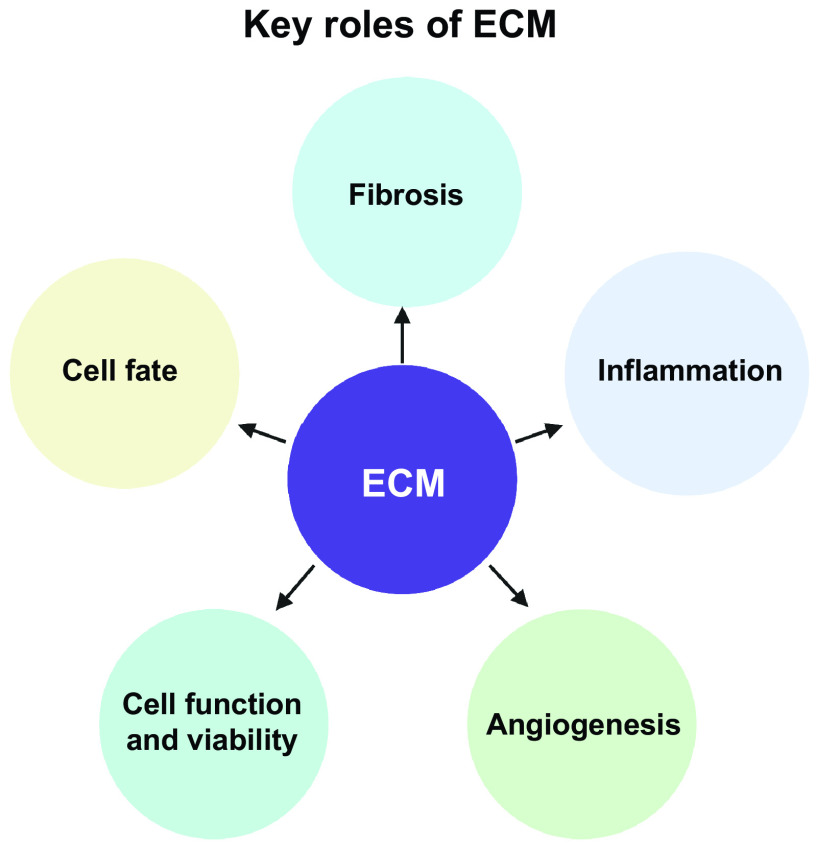
The leading cause of death around the world is ischemic heart disease. Although significant strides were made on disease intervention and management and patient survival after an acute ischemic event has drastically increased, the 5-yr survival after diagnosis of heart failure is still maintained at 40% (1). Due to the heart’s limited regeneration ability, in some cases, transplant is the only solution. There are currently over 100,000 people on the national transplant waiting list and every 9 min that number increases (2). Although much research has gone into improving posttransplant outcomes over the years, there is still no guarantee the organ will be viable in its new environment. Even when viable, organ transplantation still precedes a lifelong immunosuppressant commitment to ensure its survival and productivity. In addition to the lack of viable organs for transplantation, implant rejection or shortened lifespan is also a major concern. Specific to the heart, of the 3,715 patients that receive a donor heart, 23.6% of patients experience acute rejection by 1-yr posttransplant (3). Within the first year, 18% of deaths are due to acute rejections and 22% due to infections resulting from immunosuppression from antirejection medications (4). Among the many issues that can occur after traditional organ transplantation (i.e., from a human donor), it has been concluded that one of the most prominent problems is the lack of a native extracellular matrix (ECM) to support cell function, cell-cell communication, and cell-matrix communication (5). The ECM is responsible for key events necessary for fibrosis, inflammation, angiogenesis, cell function and viability, and resident progenitor cell fate (Fig. 1) (6). The bioengineering of cardiac components, including cardiac ECM, could be the answer to some of the obstacles observed with heart transplantation by providing a dynamic substrate to support cellular function.
Figure 1.
Key roles of the extracellular matrix (ECM). The ECM is responsible for key events necessary for fibrosis, inflammation, angiogenesis, cell function and viability, and resident progenitor cell fate. Images were created with BioRender and published with permission.
In the past decades, techniques involving tissue decellularization, recellularization, on-a-chip methods, and the use of different biomaterials to help generate functional organs and/or cellular material from native cells have gained momentum as techniques to obtain potentially implantable decellularized tissue/organs. These techniques not only could potentially circumvent donor organ transplantation limitations but can also be used to improve tissue regeneration and further our understanding of cell function, cell-cell communication, and cell-matrix communication. Among several biomaterials being investigated for cardiovascular tissue repair are natural/biological matrices. A technique that has gained special interest in the past decade is decellularization of whole hearts; the decellularized ECM is then used as a scaffold for cardiac cells with the goal of developing a whole functional heart. This approach is of particular interest since patient cells, i.e., inducible pluripotent stem cells (iPSCs), can be differentiated and used to repopulate the organ without concerns of immune rejection. This review focuses on the current methods used for organ decellularization, with special emphasis on cardiac tissues, and their effect on ECM biomechanics and subsequent functional outcomes. In addition, we review recent advancements made within this topic and the remaining challenges.
DECONSTRUCTING ECM
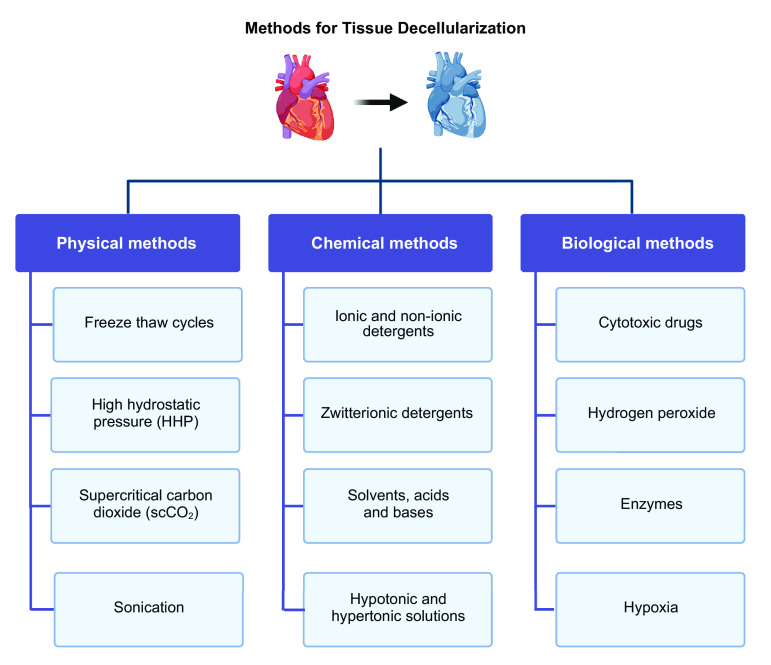
Decellularization is the process of removing cellular material from tissues or whole organs leaving an ECM with its foundational components. Two major steps are needed during decellularization: first, all cells have to be solubilized in a manner that is minimally disruptive to the surrounding ECM; second, the cellular remnants have to be removed from the scaffold to avoid cytotoxicity. Over recent years, several methods and protocols have been developed for matrix/organ decellularization. These are categorized into physical, chemical, and biological (Fig. 2). Some decellularization methods will include a combination of these methods and can be carried out through perfusion of the vasculature or tissue/organ submersion.
Figure 2.
Several methods and protocols have been developed for tissue and organ decellularization. These are categorized into physical, chemical, and biological and can be used individually or in a combination of methods to optimize decellularization. Images were created with BioRender and published with permission.
Physical Methods
Physical methods often used in combination with another method of decellularization include freeze-thaw cycles (7), high hydrostatic pressure (HHP) (8), vacuums (9), supercritical carbon dioxide (scCO2) (10), and sonication (11). These methods are used to help induce cell lysis but cannot efficiently remove cellular and nuclear content without a chemical or enzymatic agent. For example, at the beginning of the decellularization process, vacuums can use negative pressure to help lyse the cells but must be followed by a chemical or enzymatic reagent. The benefit of using physical methods in combination to chemical or enzymatic methods is to reduce the duration of incubation time in the detergent or enzyme solution to preserve the ECM ultrastructure. Decellularization by hydrostatic pressure is achieved by doing serial washes of varying concentrations of detergents, e.g., Triton X-100 and/or sodium dodecyl sulfate (SDS) while a gravity perfusion system ensures constant hydrostatic pressure (12). Varying detergent concentrations during constant hydrostatic pressure allows for shorter incubation times leading to less cytotoxicity (12). Freeze-thaw cycles are one of the more common physical methods used; this method helps to retain ECM structure and speeds up cell removal time. Like the vacuum, this method is used as a precursor to chemical or enzymatic solution incubation (7). Exposure to scCO2 is a quick method for decellularization, nontoxic, inflammable, relatively inert, and cost-effective; however, the final matrices are found to be too dehydrated for recellularization (13). Therefore, currently, this method has low therapeutic potential. Finally, sonication is another available physical method and has been used successfully to decellularize vascular tissues, such as aorta and arteries. In a study on engineered meniscus tissues, it was used in combination with a low concentration of SDS, and although effective, adding this step lengthened the protocol, which increases the time the ECM is exposed to the detergent, increasing the chance of structural damage to the ECM (14).
Chemical Methods
Ionic detergents, nonionic detergents, zwitterionic detergents, solvents, acids, bases, and hyper- and hypotonic solutions are all examples of chemical agents used for decellularization (15). Commonly used detergents include Triton X-100, a nonionic detergent, and SDS, an ionic detergent. Triton X-100 is popular in decellularization processes because it disrupts the lipid-lipid and protein-lipid interactions without affecting the protein-protein interactions (16). These properties are appealing since it allows for removal of cellular components without affecting the ECM proteins necessary for maintenance of tissue structure as well as successful reseeding. Detergent percentage, during decellularization, can depend on the organ or tissue to be decellularized, and it is one of the variables easily manipulated. Usually, Triton X-100 is used at concentrations lower than 1%; however, because length of exposure time can be also manipulated, there are studies that report using up to 3% Triton X-100 to decellularize porcine annulus fibrosus scaffold, as well as at 2% to decellularize porcine cartilage (17, 18). Unlike Triton X-100, SDS disrupts protein-protein interactions and solubilizes cell membranes; therefore, it removes nuclear matter more rapidly. Because SDS affects protein-protein interactions, the use of this detergent is associated with greater damage to the ECM (16). For example, in a study comparing three decellularization techniques on a porcine anterior cruciate ligament bone, the SDS method showed a significant loss of collagens and glycosaminoglycans (GAG) content as well as a significant increase in tensile stiffness (19). To reduce the loss of biomechanical and biochemical ECM regulators, exposure times and agent concentration can be manipulated. Other detergents recently incorporated into decellularization protocols are sodium deoxycholate (SDC), sodium lauryl ether sulfate (SLES), and potassium laurate (PL). SDC can be used in concentrations up to 4%, but because of its reduced effectiveness at removing cellular material, it is typically combined with deoxyribonuclease (DNase) (20). The only report on successful decellularization with SDC without DNase was in cardiac tissues (21). In this study, 4% SDC was used in combination with 1% Triton X-100 for decellularization of whole intact porcine hearts with serial perfusion. During the biomechanical analysis, it was found that the heart wall stretched to double its original length and the right ventricle showed a higher elastic modulus than the left ventricle (LV) because of increased stiffness. Moreover, measured stresses on both left and right ventricles were significantly higher than in the native heart (21). Cells respond to environmental stimuli; thus, increased matrix stiffness can affect both the rate of cell reseeding of the matrix and change cell function and/or phenotype (22–25). Like SDC, SLES cannot be used by itself; SLES decellularization is followed by either DNase I incubation (26) or the tissue is incubated in dextrose first to help preserve the collagen fibers (27, 28). SLES and SDC both help to preserve collagen and GAG content better than SDS because they are milder (29, 30). Several studies report that decellularized matrices by these detergents show better biocompatibility after recellularization, most likely because of reduced cytotoxicity (26, 27). PL, the other potential ionic detergent, has also shown sufficient cellular and DNA removal with reduced damage to the ECM (31).
Most recently, a new detergent, Tergitol, has been added to the list of chemical methods for decellularization. Faggioli et al. (32) introduced this detergent for decellularization of porcine aortic valves: they reported successful decellularization when using Tergitol in combination with enzyme inhibitors, and hyper- and hypotonic shocks. In their study, native and decellularized porcine aortic valve tissues were compared. Tergitol, having reduced toxicity, was used in place of Triton X-100, and its use in this study showed a nonsignificant loss of GAGs and elastin. Using Tergitol also yielded DNA quantities below threshold; retention of collagen I, collagen IV, laminin and elastin, and collagen and elastin arrangements were intact. These factors led to a successful reseeding of cells; though cell proliferation only occurred until day 7, and by day 14, cell viability was compromised significantly, which questions long-term matrix viability. Another interesting finding from this protocol relates to tissue sterility postdecellularization. The sterilization protocol used followed the guidelines of European Pharmacopoeia where the samples were decontaminated with 70% ethanol followed by incubation in antibiotics and antimycotics. Findings showed native tissues were turbid after 24–72 h, decellularized tissues became turbid on day 7, and sterilized samples had an absence of turbidity up to day 14. Although work needs to be done for successful cell maintenance after reseeding, the use of Tergitol appears to be a promising chemical method for decellularization (32).
Zwitterionic detergents contain both ionic and nonionic properties. Although these detergents have limited ability to remove cellular content, making it necessary to follow-up with enzymatic techniques, they are of interest because they preserve the ECM ultrastructure. 3-[(3-Cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS), an example of these agents, has been concluded to be useful for thinner tissues and contractile tissues like lungs or heart because of the retention of their biomechanical properties such as tensile strength and elasticity (33). Sulfobentaine 10 (SB10) and sulfobetaine 16 (SB16) are two other zwitterionic detergents commonly used in combination with a physical method and followed by an enzymatic technique. The physical aspect of the protocol helps to release the cell material after apoptosis induction caused by the SB10 and SB16. These agents are not as efficient at removing all cellular material, but they show better retention of the ECM basement membrane (34). Finally, solvents such as alcohol, acetone, dimethyl ether (DME), and urea are also used during chemical decellularization. Solvents are typically used as an initiation step to help remove lipid content before incubation with a milder detergent (35). When chemical agents are used, it is important to follow chemical decellularization with several washes to decrease potential cytotoxic effects. Cytotoxicity has been shown to cause issues when reseeding cells as well as future transplantation. Although this makes the method risky, it is appealing because it is often cheap, quick, and efficient.
Biological Methods
Biological methods are aimed to induce apoptosis in the cells using cytotoxic drugs, hydrogen peroxide, enzymes, and hypoxia (36–39). Cytotoxic drugs are usually used in combination with zwitterionic detergents because they are not effective alone (40). In addition to inducing apoptosis, hydrogen peroxide also sterilizes the tissue or organ, which is an essential step for recellularization and transplant processes (41). The use of trypsin, nucleases, proteases, and esterases are examples of types of enzymatic decellularization. Trypsin and ethylenediaminetetraacetic acid (EDTA) are usually paired together since trypsin cleaves at the COOH-terminus of lysine and arginine and EDTA targets the calcium and magnesium ions that maintain the bonds between cells and the ECM (42). EDTA can also be paired with ionic and nonionic detergents. Nucleases, such as DNase I, are used postdecellularization to ensure all nuclear material has been removed. For example, DNase was used in combination with high hydrostatic pressure to help decellularize a porcine radial artery that was successfully transplanted into a rat with no sign of acute rejection after 14 days (43). It is important to note that after decellularization, the tissue was able to withstand 1,500 mmHg, giving evidence that the method did not affect the biomechanical properties of the artery and the tissue demonstrated enough retention strength to endure anastomosis forces. Dispase, collagenase, phospholipase A2, and chondroitinase ABC are examples of proteases and esterases that can be used for decellularization (44–47). Dispase cleaves at fibronectin and collagen IV to dissociate cells from the tissue. Dispase is a good technique for decellularization of the basement membrane as this matrix is primarily made of collagen IV and laminin. Dispase is usually used for thinner tissues but can be used in thicker tissues too in combination with other chemical or enzyme agents (48, 49). Collagenase can be used to metabolize collagens from the ECM in tissues by cleaving collagens I and III (45). An esterase that causes little structural damage to the ECM is phospholipase A2, and it does so by hydrolyzing phospholipids while collagens and proteoglycans are left undisturbed (50). Phospholipase A2 is often used in combination with another form of decellularization to be completely effective (51). Chondroitinase ABC is often used for decellularization of cartilage because of its harsh nature in digesting proteoglycans and resulting reduction of GAGs (39, 52). Accordingly, decellularization of ECM with chondroitinase can increase ECM stiffness making it difficult to reseed. Despite this, studies have shown adequate recellularization of nerve allografts with mesenchymal stem cells (52). All biological treatments involve low concentrations of enzymatic solutions and are followed by washes to rid the tissue of cellular debris and stop cleavage.
When researchers have a choice from the variety of methods available or combinations of these methods, it is important to tailor the protocol to the specific material to be decellularized. For cardiovascular tissues, the most-used methods for decellularization are chemical methods using SDS, SDC, and Triton X-100 detergents. Some studies report optimized protocols for whole hearts, valves, and sectioned cardiovascular tissues (53–55); these protocols typically use a combination of a physical method or an enzymatic method and decellularization with detergents. Sokol et al. (56) conducted multiple single-step decellularization protocols on bovine pericardium, using either detergents or enzyme solutions, and they found that although cellular content was adequately removed, there were negative effects on the collagen structure of the matrix. In the same study, the combination of SDS and trypsin displayed maximum tensile strength when compared with other methods’ showing optimal biomechanical results with the detergent/enzyme combination (56). In another study, Cesur and Laçin found that using the combination of Triton X-100 and scCO2 allowed for reduced exposure to Triton X-100, which helped to avoid structural damage and dehydration of the ECM (57). Although these protocols have shown promising results, the use of detergents is still detrimental due to the effects on GAG content. A combination of solvents, physical methods, and enzymatic methods could be a solution to this. Results from a study by Seo et al. (58) involving a protocol with an ethanol soak, scCO2, and a wash in PBS containing DNase showed better retention of collagens and GAGs when compared with protocols using detergents.
Perfusion decellularization of whole human hearts via antegrade flow, using both detergent and enzymatic digestion, resulted in maintenance of the 3-D organ macro-/microstructure and preservation of the native myocardial ECM structure (59). In this study, a human heart was placed in a pressurized pouch with the aorta cannulated to allow for perfusion, and the pouch was then inverted so pressure could be kept constant throughout the decellularization process. This method is essentially an inverted Langendorff-perfusion decellularization, which previously was performed on porcine hearts (60, 61). The original upright design of this protocol causes an increase in coronary vascular resistance, which results in myocardial wall compression. Therefore, the compression prevents the decellularization fluid to flow consistently through the whole heart leading to inefficient decellularization. In the inverted protocol, a series of detergents were used for decellularization including hyper- and hypotonic solutions, 1% SDS, followed by washes with 1× PBS. Each detergent was infused at different rates to maintain structural integrity while the pressure remained the same. Decellularization of tissues or organs needs to be followed by quality control and validation protocols. Accordingly, in this study, the authors chose to do both a coronary angiogram to confirm that the vasculature was intact and nonlinear optical imaging of thicker areas such as the RV, LV, and septum to validate absence of cells. To further support this, DNA levels were quantified and determined to be less than 10%. Although they did not recellularize the heart from this study, the same group successfully recellularized and transplanted an acellular rat heart (by the same inverted perfusion method), by lining it with rat aortic endothelial cells pretransplantation (62).
Regardless of the methods used, it is important to note that the ECM must be characterized postdecellularization first to validate that decellularization was achieved, then to confirm the native matrix properties of the resultant scaffold construct were preserved, and to ensure cells can be reseeded without negative immune responses. The standards for successful decellularization are the lack of residual nuclear material, biocompatibility, and presence of certain proteins, such as collagens, fibronectin, laminins, and elastin (38). Matrices are visible by phase microscopy immediately after decellularization, and indirect immunofluorescence can be used to analyze the distribution of fibrils and the presence of certain ECM proteins. Nuclear material needs to be below 50 ng of DNA per dry weight, and this can be quantified using a nanodrop, visualized by immunofluorescent DAPI or hematoxylin and eosin staining (15, 63). Most studies show the lack of nuclear material using at least two of these methods. Levels of protein and intracellular contaminants can be determined by immunoblotting or immunofluorescence staining using antibodies specific to the proteins of interest.
DECELLULARIZATION ACROSS ORGANS
The reagents and methods used during tissue decellularization across organs are mostly similar, such as the detergents, washing solutions, and results’ assessment; however, some of the technical aspects will differ, including pressure, rate of flow, concentration of the detergent, and solutions. These differences are imperative to a successful decellularization in specific tissue types; in this section, we illustrate technical considerations associated with decellularization of four distinct organs. For example, the species, age, and lipid content will determine the decellularization protocol for the liver (64). Perfusion decellularization methods of the liver, introduced in 2008, use a combination of SDS, Triton X-100, and PBS. More recently, liver decellularization with detergents such as 3% Triton X-100, 4% SDC, and 8 mM CHAPS was compared with 1% SDS; the report showed the nonionic detergents helped to better maintain the native ECM structure and composition when compared with SDS. In addition, all detergents except 1% SDS retained a soluble collagen content and GAG, and elastin fibers were retained with Triton and SDC treatment only. When biocompatibility was tested by reseeding cells, the scaffold prepared with Triton was comparable with the cellular control (untreated scaffold) (64).
A study to decellularize ECM from whole lungs was conducted on miniature pigs using the following groups: nondecellularized, decellularization with SDS, decellularization with SLES, perfusion with dextrose before decellularization with SDS, and perfusion with dextrose before decellularization with SLES (27). The lungs were perfused serially, multiple times, with double-distilled water, then dextrose, drained, then repeated with fresh dextrose. This was followed by perfusion with the respective group detergent, followed by 1% Triton X-100 treatment to remove residual detergent. The results showed that SDS appeared to be more effective than SLES, which was concluded by earlier swelling and transparency of the lungs. Both SDS and SLES showed similar DNA removal capabilities; however, SLES-treated tissues had fewer ultrastructural disruptions, especially in combination with dextrose. ECM retention was significantly higher in dextrose-treated lungs and protein expression was higher in dextrose-SLES scaffolds than all others. SLES preserved more collagen in ECM while dextrose pretreatment reduced collagen loss during decellularization. Biocompatibility tests showed that both native and decellularized scaffolds degraded over time; however, dextrose-SLES showed fewer signs of degradation. Blood cell analyses posttransplantation showed increased white blood cell (WBC) count and neutrophils in the native group and lower numbers of circulating WBCs, neutrophils, lymphocytes, and monocytes over time in the dextrose-SLES-treated lungs. Finally, cell adhesion and growth of human lung cancer cells and human umbilical vein endothelial cells were supported in the decellularized lung scaffold treated with dextrose-SLES (27).
For kidney decellularization, it is imperial to keep microvascular structures, such as glomeruli and peritubular capillaries, intact and functional. Success depends on detergent choice and concentration, flow rate, decellularization time, and preservation of GAGs and growth factors (65). Presence of growth factors is important for proper location-specific cues when reseeding scaffolds, to guide cell adhesion, proliferation, migration, and differentiation (66). Several studies have reported the use of milder decellularization protocols (i.e., 0.1% SDS instead of 1.0% SDS) to be more effective in kidney decellularization; particularly, to improve reseeding rates and cell differentiation (67, 68).
Decellularized ECM biomaterials that mimic the native cardiac environment are highly promising for heart failure treatment. Heart decellularization can preserve the cardiac 3-D structure; the vasculature, which is vital to ensure blood supply for oxygen and nutrients; and cardiac-specific functions, such as resistance to stretch during the continuous contraction/relaxation of the heart (69). To achieve this, perfusion-decellularization of whole hearts, using a combination of washes followed by serial perfusion with enzymes, ionic, and nonionic detergents has been shown to retain chamber geometry and valve competency, which could act as templates to generate synchronously beating heart tissues (70, 71). Studies also have demonstrated that decellularized cardiac ECM sheets (decellularized with 0.5% SDS, 10 mM Tris, and 25 mM EDTA) retain important topographic properties, such as fiber alignment, which is known to regulate angiogenic growth factors and guide anisotropic microvascular progression, both important during cardiac homeostasis and remodeling (71, 72).
Although most of the protocols for decellularization involve a detergent or enzyme, wash solutions, and validation steps, the above examples demonstrate that these protocols are highly tissue-specific. The concentrations of the reagents, length of incubation time, method of incubation, and physical processes all need to be optimized for the specific organ or tissue. Because of the abundant interest in tissue bioengineering, these protocols are being constantly optimized.
DECELLULARIZATION AND THE BIOMECHANICS OF CARDIAC ECM
The ECM of most organs is composed of a complex network of fibrillar collagens and nonfibrillar components, including the basement membrane, proteoglycans, and glycoproteins (73). In the heart, the fibrillar collagenous matrix is mostly composed of collagen type I (over 80%) and type III (over 10%) (74), and these are anchored to the myocardial cell-basement membranes through collagen type IV and fibronectin (75, 76). More recently, new proteomic approaches revealed that ∼90% of the cardiac ECM is made of only 10 abundant components: serum albumin, collagens (e.g., collagen types I, III, and IV), fibronectin, laminin, proteoglycans, GAGs, and elastin (77). The remaining cardiac ECM works as a reservoir for anchored growth factors, proteases, protease inhibitors, cytokines, chemokines, and noncoding RNAs such as microRNAs (miRNAs) (78–80). Importantly, since decellularization methods include several washes, many of the soluble anchored components are washed away. This intrinsically changes the matrix composition and, therefore, its properties.
The structure, alignment, and composition of the ECM are what will dictate the organ’s physical and biomechanical properties. Physical properties of the ECM can be affected throughout the decellularization process. If decellularization is too harsh, the orientation of the fibrils within the ECM can change leading to increased matrix stiffness. Tissue formation and function are reliant on the fibrils being correctly organized because they act as a highway for the newly introduced cells to spread, migrate, and differentiate (66). The ECM topography and properties can be analyzed by several methods, including confocal microscopy, electron microscopy, Raman spectroscopy, fast-Fourier transform analysis, atomic force microscopy (AFM), and scanning electron microscopy (81, 82). These methods allow for visualization of the fibers and to study the composition, structure, and biomechanical properties of the ECM.
Biomechanical properties of the ECM include rigidity/stiffness and viscoelasticity. The first is commonly assessed through the elastic modulus (i.e., Young’s modulus), which can be obtained by AFM and calculates the amount of stretching of a substrate in response to a given level of stress (82). Harsher detergents such as SDS can cause damage to the ECM at certain concentrations and result in increased stiffness. This becomes an issue when reseeding, causing the cells not to proliferate and differentiate properly. Another factor that is known to affect the stiffness of the ECM after decellularization is the use of any type of alcohol as they can dehydrate and dry out the material. Methods to measure stiffness commonly produce a stress versus strain curve. In a recent study, hydrogels were generated from decellularized left ventricle (LV), mitral valve, and aorta from a porcine heart. The authors subjected the hydrogels to compressive loading to determine the material biomechanical properties (83). Specifically, hydrogels were deformed 20%–80% (i.e., strain 0.2–0.8) of their original thickness at a constant strain rate; the resulting slope of the stress-strain curve determines stiffness of the decellularized hydrogels. Although stiffness increased for all tissues with increased ECM concentration, at the higher concentration (20 mg/mL), hydrogels derived from aorta were significantly stiffer than those from LV and mitral valve. Moreover, stress relaxation curves (stress relaxation over time) were used to assess viscoelasticity; these showed that whereas aorta- and mitral-derived hydrogels started with a higher stress value and presented an important relaxation, reaching zero within the follow-up, the LV-derived hydrogels initially displayed low-stress values but kept higher residual stress with time (83). These data indicate that while aorta and mitral hydrogels behave like a viscoelastic liquid, LV hydrogels behave similarly to viscoelastic solid, which is likely a better representation of the native matrix. In a study by Oropeza et al. (84), decellularized cardiac porcine ECM was lyophilized, powdered, and gelled before being used to bioprint a 1:1 scale model of a human ascending aorta and dog-bone-shaped structures. The authors then compared the physiological (cell adhesion, survival, and proliferation) properties of the bioengineered aorta and the biomechanical characteristics of the dog-bone construct, including surface topography and mechanical properties, to those of the native (cellular) tissue (84). With the use of histology, decellularization was confirmed by a lack of cellular structures and nuclear matter. Although all matrices (native, decellularized, and decellularized + bioprinted) showed abundant collagen fibrils. These were randomly oriented in the decellularized ECM compared with both the native and decellularized + bioprinted matrices. Importantly, the decellularized bioprinted ECM exhibited approximately three times greater elasticity, i.e., reduced tensile strength, than the native porcine cardiac tissue and the native matrix displayed two times greater plasticity than the printed decellularized matrix. These results demonstrate significant structural and chemical alterations to the decellularized matrix. Nonetheless, the tested samples contained no cells, thus lacking the additional stability given by the smooth muscle fibers. The authors then incorporated human aortic smooth muscle cells (SMCs) by coprinting and culturing these SMCs in the aortic construct; the cells were visualized by confocal microscopy and indicated normal cell attachment and morphology on the bioprinted matrix (84). This study highlighted some limitations of this approach, which included costs, the availability of bio-ink, and remaining concern for rejection. The need for aggressive treatments to aid in efficient decellularization was also caused for concern, as this resulted in greater structural and chemical alterations to the matrix. The novel aspect of this study is the cells exhibiting normal morphology and no necrotic areas 24-h post coprinting in the decellularized ECM, indicating successful biocompatibility (84).
CARDIAC ECM-BASED BIOMATERIALS—FROM BENCH TO BEDSIDE
Unlike heart valves or blood vessels, there are limited alternatives to replace or regenerate heart muscle. Thus, the most challenging goal for cardiac tissue engineering is the creation of myocardial tissue. Several types of cardiac ECM-derived biomaterials have been developed; those include decellularized ECM in the form of sheets, hydrogels, matrices, lyophilized powders, and whole heart scaffolds (Fig. 3) (21, 53, 54, 83–85). As mentioned in decellularization and the biomechanics of cardiac ecm, decellularized ECM hydrogels were made using porcine LV, mitral valve, and aorta. Hydrogels generated from these three tissues were then compared, and it was found that decellularized ECM allows for the retention of the structure and biochemical composition by retaining the larger macromolecules such as polysaccharides and constructive proteins. However, it is important to note that the smaller molecules such as growth factors, chemokines, and other small signaling molecules were washed away (83). Hydrogels that are derived from decellularized ECM are adaptable since it allows for changes in their stiffness, biochemical parameters, and viscoelasticity. After decellularization, the ECM can be treated differently depending on the end goal. For example, in a study by Becker et al. (85), ECM sheets were processed into a microparticle powder, either by mechanical grinding or pepsin digestion, and self-assembled into ECM hydrogels with preserved bioactivity. In this study, the ECM hydrogel was combined with an acellular amniotic membrane and HL-1 cardiomyocytes were cultured under normoxia or ischemic conditions. The authors reported enhanced cardiomyocyte metabolic activity, proliferation, and cytoprotection and exhibited contractile activity and specific troponin I and cardiac myosin expression. The ECM hydrogel-amniotic membrane patch also supported the proliferation of human cardiac fibroblasts, epicardial progenitor cells, and murine HL-1 cardiomyocytes (85). In a similar study, decellularized mouse hearts were repopulated with human iPSCs-derived cardiovascular progenitor cells. The authors showed that cells migrate, proliferate, and differentiate in situ into cardiomyocytes, smooth muscle cells, and endothelial cells to reconstruct the decellularized hearts (86). Moreover, after 20 days of perfusion, the tissue exhibited spontaneous contractions, generated mechanical force, and was responsive to drugs (86). Advantages of using whole heart decellularization include preservation of the 3-D architecture and natural ECM, which has been shown to be critical for constructing myocardium and vascular structures. Previously, this type of study has been a challenge due to the low amounts of human cardiomyocytes available; however, human pluripotent stem cells are in abundance, have not been exposed to long-term pharmaceutical treatments, and are renewable.
Figure 3.
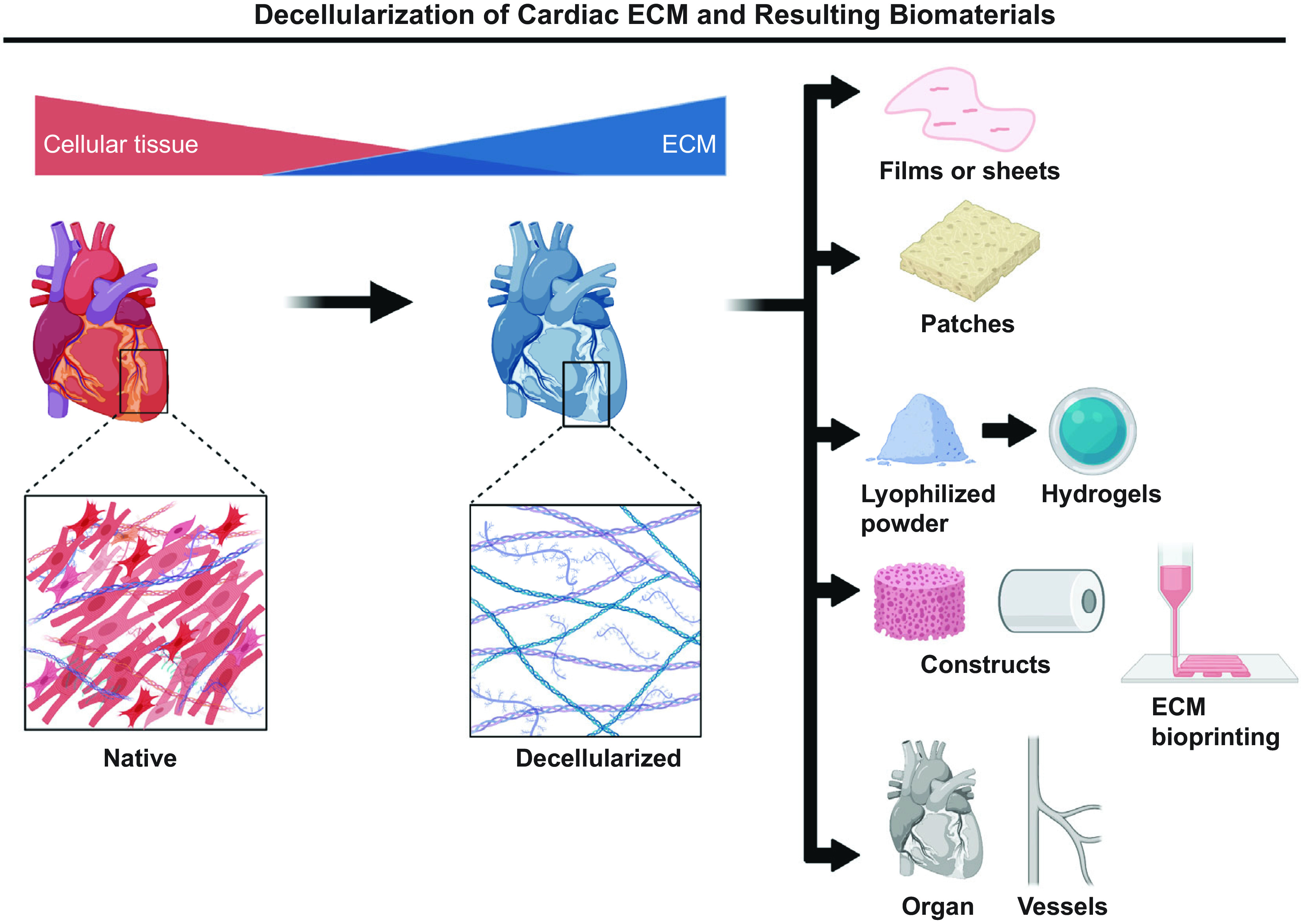
Several types of cardiac ECM-derived biomaterials have been developed, which include decellularized ECM in the form of sheets or films; hydrogels; patches; lyophilized powders; different types of constructs, including bioprinted scaffolds; and whole organs. Images were created with BioRender and published with permission. ECM, extracellular matrix.
To better mimic human tissue, several laboratories have tested decellularized porcine, equine, and bovine scaffolds in cardiac repair. Porcine small intestine submucosa (SIS)-ECM has been extensively used in cardiovascular applications such as arterial or venous grafting, valve replacement, and myocardial repair or patching in pigs, sheep, and cows (87). In these studies, thrombosis was the main cause of failure and inflammatory reactions were rarely observed, demonstrating low immunogenicity. The explants had evidence to support matrix repopulation with fibroblasts and smooth muscle cells, neoangiogenesis, and surface endothelialization. However, these studies are limited by small sample sizes and short follow-up times. Dohmen et al. (88) experimentally implanted decellularized equine pericardial scaffolds into the descending aorta of juvenile sheep to determine the efficacy of decellularized tissue at high, systemic, and circulatory pressures. Four months after implantation, gross examination revealed a smooth, elastic surface without evidence of degeneration, thrombus formation, or aneurysmal dilation. Light microscopy and immunohistochemistry demonstrated matrix repopulation with a monolayer of endothelial cell lining the luminal side of the patch and host fibroblasts in all layers of the scaffold. In addition, neovascularization was evident on the outside of the patch, with no evidence of deterioration or calcification of the scaffold. This report showed excellent hemodynamic performance of equine decellularized pericardial tissue at systemic pressures and highlighted the potential of using equine tissues in cardiac repair. CardioCel, a pericardial scaffold manufactured from bovine spongiform encephalopathy-free pericardium, has been studied for its potential use in aortic valve repair and reconstruction of pulmonary and mitral valves (89, 90). With the use of a sheep surgical model, a complete trileaflet reconstruction of the aortic valve was performed with three separate pericardial patches (Ozaki technique). This study was limited by the small sample size of animals (n = 6), further exaggerated by death of three of the sheep due to inability to survive the complicated surgical procedure. Of those that survived, nine leaflets were soft and pliable, with no signs of detachment, thrombosis, tissue failure, or endocarditis. Radiographs demonstrated minimal calcification of two leaflets (from 2 separate sheep), a large commissural calcification on one leaflet of the third sheep, and the sixremaining leaflets were free from calcifications. Echocardiography at 6 mo revealed only minimal regurgitation and no signs of aortic valve insufficiency (89). In a similar study, the posterior leaflet of the mitral valve and of one pulmonary valve cusp was replaced with CardioCel (90). All sheep survived until euthanasia (7-mo postimplantation, n = 6), and valves presented patency at both systemic and pulmonary pressures. In addition, the patches displayed continuous endothelium, a layer of new collagen developed between patch and endothelium, and interstitial cells and SMCs were also present in these layers. Overall, the mechanical properties of CardioCel were preserved and without calcification (90). Several other studies have demonstrated the safety and durability of CardioCel, and this biomaterial is now used in the clinic for heart repair and reconstruction. In addition to the experimental studies of trileaflet aortic valve repair, CardioCel has been clinically studied as a suitable option for repair of congenital cardiac defects such as atrial septal defects, ventricular septal defects, atrioventricular septal defects, aortic root enlargements, and right ventricular outflow tract (RVOT) reconstructions (91–94). In these studies, medium- to long-term performance of the scaffold (up to 10 yr) demonstrates the absence of graft-related adverse events and calcification (95).
Other examples of decellularized ECM-based biomaterials clinically used for cardiac repair are CorMatrix and Matrix Patch. Herein, we will not provide an exhaustive list of commercially available biomaterials; instead, we provide examples of decellularized ECM biomaterials derived from distinct biological sources. CorMatrix is a commercially available and widely used porcine SIS-ECM. It has Food and Drug Administration (FDA) clearance and a Conformitè Europëenne (CE) Mark for pericardial patch repair and reconstruction, cardiac tissue repair, carotid repair, and enveloping implantable electronic devices (96). CorMatrix has been used in congenital cardiac and vascular surgery, valve reconstruction in both adults and children, endocarditis, acquired vascular defects, and to repair damaged myocardium after infarction (87, 96, 97). Although CorMatrix is strong, durable, widely clinically applicable, and easy to manipulate during surgery, uncertainly remains regarding the remodeling process.
Another commercially available ECM-based biomaterial for pediatric cardiovascular use is Autotissue Matrix Patch, a decellularized equine pericardial matrix clinically used for RVOT reconstructions with the Ross procedure (98). This procedure is an alternative to aortic valve replacement and negates the need for anticoagulation long-term treatments, making this more ideal for young patients with either a contraindication to or a wish to avoid oral anticoagulation. The Ross procedure historically treats aortic valve disease using homografts for pulmonary valve replacement, but homografts typically undergo calcification and degeneration over time. The patch has been reported to successfully treat pulmonary artery stenosis and congenital heart defects in children (99, 100). A retrospective clinical study by Christ et al. (98) evaluated the efficacy of Matrix Patch to treat aortic valve disease in adults over a period of 8 yr with up to 10-yr follow-ups. The study demonstrated that Matrix Patch repair of structural pulmonary valve failure in adults is associated with a high rate of reoperation/reintervention and should not be recommended for this population (98).
PERSPECTIVES
The nonregenerative nature of adult hearts makes it a challenge to treat cardiac disease. Depending on the status of disease progression, the ideal treatment would either promote myocardial regeneration or provide a whole functional organ for transplantation. Stem cell therapy for cardiac regeneration has, unfortunately, revealed significant limitations, including cell death and apoptosis, lack of cell engraftment, and limited myocardial regeneration. Cell-free therapies or a combination of cells with the appropriate substrate have become of interest for potential application in regenerative medicine. In particular, the use of decellularized ECM-based biomaterials holds promise as a way of avoiding immunogenicity and providing important microenvironmental cues for cells to adhere, proliferate, and migrate into the injured/damaged area. Functions of the ECM rely on its large multidomain protein components and organization of ECM protein polymers. The complete composition of each tissue ECM is still unknown; as a result, we cannot truly build functional mimetic ECM using individual ECM components or synthetic materials. Thus, the main goal of decellularization is to obtain a balance between clearance of cellular materials and the retention of a close-to-native ECM. The ECM has a highly stable core allowing the cells to be removed and leaving behind an interconnected fibrillar network of ECM polymers. When cells are present, this core interacts with cells and controls the types, amounts, and distributions of many matrixes associated components and modulatory factors. During decellularization, it is important that the ECM retains as much of its native architecture and components as possible to ensure suitable reseeding. Newly introduced cells will require the native ECM microenvironment and signals to proliferate and differentiate properly to generate a viable organ. In the realm of cardiovascular disease, ECM remodeling by resident cells in response to mechanical load, physiological stress, and circulating biomechanical signals is of interest as dysfunctional remodeling is often the underlying cause of many cardiac diseases. Characteristics such as the loss of elastin fibers, aberrant collagen and elastin deposition, and increased ECM stiffness are all outcomes of dysfunctional remodeling that lead to things like hypertension, arterial aging, and arterial calcification. Therefore, to achieve maximal therapeutic outcomes an ideal ECM-derived biomaterial would not only support cell populations and organ function but also resist to disease-triggering stimuli. In conclusion, the creation of decellularized ECM-derived biomaterials has shown progress because of the optimization of decellularization protocols that preserve the native tissue 3-D structure and topography to allow for successful reseeding, to promote cell survival and functionality resulting in a viable tissue or even organs. However, current clinically used ECM biomaterials still present limitations, and work needs to be done to ensure long-term viability.
GRANTS
This work was supported by East Carolina University and National Heart, Lung, and Blood Institute Grant HL152297 (to L. E. de Castro Brás).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.N.C. and L.E.d.C.B. prepared figures; K.M.W., H.K.L.H., S.N.C., and L.E.d.C.B. drafted manuscript; K.M.W., H.K.L.H., S.N.C., and L.E.d.C.B. edited and revised manuscript; K.M.W., H.K.L.H., S.N.C., and L.E.d.C.B. approved final version of manuscript.
REFERENCES
- 1. Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW. et al. Heart Disease and Stroke Statistics-2021 Update: a Report From the American Heart Association. Circulation 143: e254–e743, 2021. doi: 10.1161/cir.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 2.HRSA. Organ Donation Statistics (Online). Health Resources & Services Administration. https://www.organdonor.gov/learn/organ-donation-statistics [2022 July 11]. [Google Scholar]
- 3. Colvin M, Smith JM, Ahn Y, Skeans MA, Messick E, Bradbrook K, Gauntt K, Israni AK, Snyder JJ, Kasiske BL. OPTN/SRTR 2020 Annual Data Report: Heart. Am J Transplant 22, Suppl 2: 350–437, 2022. doi: 10.1111/ajt.16977. [DOI] [PubMed] [Google Scholar]
- 4. Eisen HJ. Patient Education: Heart Transplantation (Beyond the Basics) (Online). UpToDate.com. https://www.uptodate.com/contents/heart-transplantation-beyond-the-basics [2022 July 11]. [Google Scholar]
- 5. Taha IN, Naba A. Exploring the extracellular matrix in health and disease using proteomics. Essays Biochem 63: 417–432, 2019. doi: 10.1042/EBC20190001. [DOI] [PubMed] [Google Scholar]
- 6. Singh P, Carraher C, Schwarzbauer JE. Assembly of fibronectin extracellular matrix. Annu Rev Cell Dev Biol 26: 397–419, 2010. doi: 10.1146/annurev-cellbio-100109-104020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li N, Li Y, Gong D, Xia C, Liu X, Xu Z. Efficient decellularization for bovine pericardium with extracellular matrix preservation and good biocompatibility. Interact Cardiovasc Thorac Surg 26: 768–776, 2018. doi: 10.1093/icvts/ivx416. [DOI] [PubMed] [Google Scholar]
- 8. Zemmyo D, Yamamoto M, Miyata S. Efficient decellularization by application of moderate high hydrostatic pressure with supercooling pretreatment. Micromachines (Basel) 12: 1486, 2021. doi: 10.3390/mi12121486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang Z, Sun F, Lu Y, Zhang B, Zhang G, Shi H. Rapid preparation method for preparing tracheal decellularized scaffolds: vacuum assistance and optimization of DNase I. ACS Omega 6: 10637–10644, 2021. doi: 10.1021/acsomega.0c06247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chaschin IS, Britikov DV, Khugaev GA, Salokhedinova RR, Zubko AV, Abramchuk SS, Petlenko AA, Muratov RM, Bakuleva NP. Decellularization of the human donor aortic conduit by a new hybrid treatment in a multicomponent system with supercritical CO2 and Tween 80. J Supercrit Fluids 180: 105452, 2022. doi: 10.1016/j.supflu.2021.105452. [DOI] [Google Scholar]
- 11. Fitriatul N, Sha'ban M, Azhim A. Evaluation of recellularization on decellularized aorta scaffolds engineered by ultrasonication treatment. Annu Int Conf IEEE Eng Med Biol Soc 2017: 2072–2075, 2017.doi: 10.1109/EMBC.2017.8037261. [DOI] [PubMed] [Google Scholar]
- 12. Morales-Guerrero NA, Varela-Echavarría A, Lozano-Flores C, Vázquez-Cuevas FG, Velázquez-Miranda E, Reyes-López JV, García-Solís P, Solís- SJ, Hernandez-Montiel HL. A new strategy for the decellularization of whole organs by hydrostatic pressure. Biotechnol Prog 38: e3248, 2022. doi: 10.1002/btpr.3248. [DOI] [PubMed] [Google Scholar]
- 13. Sawada K, Terada D, Yamaoka T, Kitamura S, Fujisato T. Cell removal with supercritical carbon dioxide for acellular artificial tissue. J Chem Technol Biotechnol 83: 943–949, 2008. doi: 10.1002/jctb.1899. [DOI] [Google Scholar]
- 14. Mardhiyah A, Sha'ban M, Azhim A. Evaluation of histological and biomechanical properties on engineered meniscus tissues using sonication decellularization. Annu Int Conf IEEE Eng Med Biol Soc 2017: 2064–2067, 2017. doi: 10.1109/EMBC.2017.8037259. [DOI] [PubMed] [Google Scholar]
- 15. Gilpin A, Yang Y. Decellularization strategies for regenerative medicine: from processing techniques to applications. BioMed Res Int 2017: 9831534, 2017. doi: 10.1155/2017/9831534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. White LJ, Taylor AJ, Faulk DM, Keane TJ, Saldin LT, Reing JE, Swinehart IT, Turner NJ, Ratner BD, Badylak SF. The impact of detergents on the tissue decellularization process: a ToF-SIMS study. Acta Biomater 50: 207–219, 2017. doi: 10.1016/j.actbio.2016.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Luo Z, Bian Y, Su W, Shi L, Li S, Song Y, Zheng G, Xie A, Xue J. Comparison of various reagents for preparing a decellularized porcine cartilage scaffold. Am J Transl Res 11: 1417–1427, 2019. [PMC free article] [PubMed] [Google Scholar]
- 18. Xu H, Xu B, Yang Q, Li X, Ma X, Xia Q, Zhang Y, Zhang C, Wu Y, Zhang Y. Comparison of decellularization protocols for preparing a decellularized porcine annulus fibrosus scaffold. PLoS One 9: e86723, 2014. doi: 10.1371/journal.pone.0086723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Woods T, Gratzer PF. Effectiveness of three extraction techniques in the development of a decellularized bone–anterior cruciate ligament–bone graft. Biomaterials 26: 7339–7349, 2005. doi: 10.1016/j.biomaterials.2005.05.066. [DOI] [PubMed] [Google Scholar]
- 20. McCrary MW, Vaughn NE, Hlavac N, Song YH, Wachs RA, Schmidt CE. Novel sodium deoxycholate-based chemical decellularization method for peripheral nerve. Tissue Eng Part C Methods 26: 23–36, 2020. doi: 10.1089/ten.tec.2019.0135. [DOI] [PubMed] [Google Scholar]
- 21. Methe K, Bäckdahl H, Johansson BR, Nayakawde N, Dellgren G, Sumitran-Holgersson S. An alternative approach to decellularize whole porcine heart. Biores Open Access 3: 327–338, 2014. doi: 10.1089/biores.2014.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sun M, Chi G, Li P, Lv S, Xu J, Xu Z, Xia Y, Tan Y, Xu J, Li L, Li Y. Effects of matrix stiffness on the morphology, adhesion, proliferation and osteogenic differentiation of mesenchymal stem cells. Int J Med Sci 15: 257–268, 2018. doi: 10.7150/ijms.21620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Trappmann B, Chen CS. How cells sense extracellular matrix stiffness: a material’s perspective. Curr Opin Biotechnol 24: 948–953, 2013. doi: 10.1016/j.copbio.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hu Y, Huang G, Tian J, Qiu J, Jia Y, Feng D, Wei Z, Li S, Xu F. Matrix stiffness changes affect astrocyte phenotype in an in vitro injury model. NPG Asia Mater 13: 35, 2021. doi: 10.1038/s41427-021-00304-0. [DOI] [Google Scholar]
- 25. Wells RG. The role of matrix stiffness in regulating cell behavior. Hepatology 47: 1394–1400, 2008. doi: 10.1002/hep.22193. [DOI] [PubMed] [Google Scholar]
- 26. Hassanpour A, Talaei-Khozani T, Kargar-Abarghouei E, Razban V, Vojdani Z. Decellularized human ovarian scaffold based on a sodium lauryl ester sulfate (SLES)-treated protocol, as a natural three-dimensional scaffold for construction of bioengineered ovaries. Stem Cell Res Ther 9: 252, 2018. doi: 10.1186/s13287-018-0971-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li Y, Wu Q, Li L, Chen F, Bao J, Li W. Decellularization of porcine whole lung to obtain a clinical-scale bioengineered scaffold. J Biomed Mater Res A 109: 1623–1632, 2021. doi: 10.1002/jbm.a.37158. [DOI] [PubMed] [Google Scholar]
- 28. Zhu X, Li Y, Yang Y, He Y, Gao M, Peng W, Wu Q, Zhang G, Zhou Y, Chen F, Bao J, Li W. Ordered micropattern arrays fabricated by lung-derived dECM hydrogels for chemotherapeutic drug screening. Mater Today Bio 15: 100274, 2022. doi: 10.1016/j.mtbio.2022.100274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Keshvari MA, Afshar A, Daneshi S, Khoradmehr A, Baghban M, Muhaddesi M, Behrouzi P, Miri MR, Azari H, Nabipour I, Shirazi R, Mahmudpour M, Tamadon A. Decellularization of kidney tissue: comparison of sodium lauryl ether sulfate and sodium dodecyl sulfate for allotransplantation in rat. Cell Tissue Res 386: 365–378, 2021. doi: 10.1007/s00441-021-03517-5. [DOI] [PubMed] [Google Scholar]
- 30. Emami A, Talaei-Khozani T, Vojdani Z, Zarei Fard N. Comparative assessment of the efficiency of various decellularization agents for bone tissue engineering. J Biomed Mater Res B Appl Biomater 109: 19–32, 2021. doi: 10.1002/jbm.b.34677. [DOI] [PubMed] [Google Scholar]
- 31. Obata T, Tsuchiya T, Akita S, Kawahara T, Matsumoto K, Miyazaki T, Masumoto H, Kobayashi E, Niklason LE, Nagayasu T. Utilization of natural detergent potassium laurate for decellularization in lung bioengineering. Tissue Eng Part C Methods 25: 459–471, 2019. doi: 10.1089/ten.tec.2019.0016. [DOI] [PubMed] [Google Scholar]
- 32. Faggioli M, Moro A, Butt S, Todesco M, Sandrin D, Borile G, Bagno A, Fabozzo A, Romanato F, Marchesan M, Imran S, Gerosa G. A new decellularization protocol of porcine aortic valves using Tergitol to characterize the scaffold with the biocompatibility profile using human bone marrow mesenchymal stem cells. Polymers (Basel) 14: 1226, 2022. doi: 10.3390/polym14061226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Qiu X, Lee BL, Wong SY, Ding X, Xu K, Zhao W, Wang D, Sochol R, Dong N, Li S. Cellular remodeling of fibrotic conduit as vascular graft. Biomaterials 268: 120565, 2021. doi: 10.1016/j.biomaterials.2020.120565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Philips C, Campos F, Roosens A, Sánchez-Quevedo MDC, Declercq H, Carriel V. Qualitative and quantitative evaluation of a novel detergent-based method for decellularization of peripheral nerves. Ann Biomed Eng 46: 1921–1937, 2018. doi: 10.1007/s10439-018-2082-y. [DOI] [PubMed] [Google Scholar]
- 35. Ventura RD, Padalhin AR, Park CM, Lee BT. Enhanced decellularization technique of porcine dermal ECM for tissue engineering applications. Mater Sci Eng C Mater Biol Appl 104: 109841, 2019. doi: 10.1016/j.msec.2019.109841. [DOI] [PubMed] [Google Scholar]
- 36. Giordano S, Lee J, Darley-Usmar VM, Zhang J. Distinct effects of rotenone, 1-methyl-4-phenylpyridinium and 6-hydroxydopamine on cellular bioenergetics and cell death. PLoS One 7: e44610, 2012. doi: 10.1371/journal.pone.0044610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Qing Q, Zhang YJ, Yang JL, Ning LJ, Zhang YJ, Jiang YL, Zhang Y, Luo JC, Qin TW. Effects of hydrogen peroxide on biological characteristics and osteoinductivity of decellularized and demineralized bone matrices. J Biomed Mater Res A 107: 1476–1490, 2019. doi: 10.1002/jbm.a.36662. [DOI] [PubMed] [Google Scholar]
- 38. Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials 32: 3233–3243, 2011. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Moffat D, Ye K, Jin S. Decellularization for the retention of tissue niches. J Tissue Eng 13: 20417314221101151, 2022. doi: 10.1177/20417314221101151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Novoseletskaya E, Grigorieva O, Nimiritsky P, Basalova N, Eremichev R, Milovskaya I, Kulebyakin K, Kulebyakina M, Rodionov S, Omelyanenko N, Efimenko A. Mesenchymal stromal cell-produced components of extracellular matrix potentiate multipotent stem cell response to differentiation stimuli. Front Cell Dev Biol 8: 555378, 2020. doi: 10.3389/fcell.2020.555378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gosztyla C, Ladd MR, Werts A, Fulton W, Johnson B, Sodhi C, Hackam DJ. A comparison of sterilization techniques for production of decellularized intestine in mice. Tissue Eng Part C Methods 26: 67–79, 2020. doi: 10.1089/ten.tec.2019.0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Giraldo-Gomez DM, Leon-Mancilla B, Del Prado-Audelo ML, Sotres-Vega A, Villalba-Caloca J, Garciadiego-Cazares D, Piña-Barba MC. Trypsin as enhancement in cyclical tracheal decellularization: morphological and biophysical characterization. Mater Sci Eng C Mater Biol Appl 59: 930–937, 2016. doi: 10.1016/j.msec.2015.10.094. [DOI] [PubMed] [Google Scholar]
- 43. Negishi J, Funamoto S, Kimura T, Nam K, Higami T, Kishida A. Porcine radial artery decellularization by high hydrostatic pressure. J Tissue Eng Regen Med 9: E144–E151, 2015. doi: 10.1002/term.1662. [DOI] [PubMed] [Google Scholar]
- 44. Asadi M, Khalili M, Lotfi H, Vaghefi Moghaddam S, Zarghami N, André H, Alizadeh E. Liver bioengineering: Recent trends/advances in decellularization and cell sheet technologies towards translation into the clinic. Life Sci 276: 119373, 2021. doi: 10.1016/j.lfs.2021.119373. [DOI] [PubMed] [Google Scholar]
- 45. Kuljanin M, Brown CFC, Raleigh MJ, Lajoie GA, Flynn LE. Collagenase treatment enhances proteomic coverage of low-abundance proteins in decellularized matrix bioscaffolds. Biomaterials 144: 130–143, 2017. doi: 10.1016/j.biomaterials.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 46. Gessner RC, Hanson AD, Feingold S, Cashion AT, Corcimaru A, Wu BT, Mullins CR, Aylward SR, Reid LM, Dayton PA. Functional ultrasound imaging for assessment of extracellular matrix scaffolds used for liver organoid formation. Biomaterials 34: 9341–9351, 2013. doi: 10.1016/j.biomaterials.2013.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Natoli RM, Revell CM, Athanasiou KA. Chondroitinase ABC treatment results in greater tensile properties of self-assembled tissue-engineered articular cartilage. Tissue Eng Part A 15: 3119–3128, 2009. doi: 10.1089/ten.tea.2008.0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chen RN, Ho HO, Tsai YT, Sheu MT. Process development of an acellular dermal matrix (ADM) for biomedical applications. Biomaterials 25: 2679–2686, 2004. doi: 10.1016/j.biomaterials.2003.09.070. [DOI] [PubMed] [Google Scholar]
- 49. Oh JY, Kim MK, Lee HJ, Ko JH, Wee WR, Lee JH. Processing porcine cornea for biomedical applications. Tissue Eng Part C Methods 15: 635–645, 2009. doi: 10.1089/ten.TEC.2009.0022. [DOI] [PubMed] [Google Scholar]
- 50. Burke JE, Dennis EA. Phospholipase A2 biochemistry. Cardiovasc Drugs Ther 23: 49–59, 2009. doi: 10.1007/s10557-008-6132-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wu Z, Zhou Y, Li N, Huang M, Duan H, Ge J, Xiang P, Wang Z. The use of phospholipase A2 to prepare acellular porcine corneal stroma as a tissue engineering scaffold. Biomaterials 30: 3513–3522, 2009. doi: 10.1016/j.biomaterials.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 52. Bautista CA, Park HJ, Mazur CM, Aaron RK, Bilgen B. Effects of chondroitinase ABC-mediated proteoglycan digestion on decellularization and recellularization of articular cartilage. PLoS One 11: e0158976, 2016. doi: 10.1371/journal.pone.0158976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Di Meglio F, Nurzynska D, Romano V, Miraglia R, Belviso I, Sacco AM, Barbato V, Di Gennaro M, Granato G, Maiello C, Montagnani S, Castaldo C. Optimization of human myocardium decellularization method for the construction of implantable patches. Tissue Eng Part C Methods 23: 525–539, 2017. doi: 10.1089/ten.TEC.2017.0267. [DOI] [PubMed] [Google Scholar]
- 54. Perea-Gil I, Uriarte JJ, Prat-Vidal C, Gálvez-Montón C, Roura S, Llucià-Valldeperas A, Soler-Botija C, Farré R, Navajas D, Bayes-Genis A. In vitro comparative study of two decellularization protocols in search of an optimal myocardial scaffold for recellularization. Am J Transl Res 7: 558–573, 2015. [PMC free article] [PubMed] [Google Scholar]
- 55. Tenreiro MF, Almeida HV, Calmeiro T, Fortunato E, Ferreira L, Alves PM, Serra M. Interindividual heterogeneity affects the outcome of human cardiac tissue decellularization. Sci Rep 11: 20834, 2021. doi: 10.1038/s41598-021-00226-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sokol AA, Grekov DA, Yemets GI, Yu GA, Shchotkina NV, Dovghaliuk AA, Telehuzova OV, Rudenko NM, Romaniuk OM, Yemets IM. Comparison of bovine pericardium decellularization protocols for production of biomaterial for cardiac surgery. Biopolym. Cell 36: 392–403, 2020. doi: 10.7124/bc.000A3C. [DOI] [Google Scholar]
- 57. Cesur NP, Laçin NT. Decellularization of ram cardiac tissue via supercritical CO2. J Supercrit Fluids 180: 105453, 2022. doi: 10.1016/j.supflu.2021.105453. [DOI] [Google Scholar]
- 58. Seo Y, Jung Y, Kim SH. Decellularized heart ECM hydrogel using supercritical carbon dioxide for improved angiogenesis. Acta Biomater 67: 270–281, 2018. doi: 10.1016/j.actbio.2017.11.046. [DOI] [PubMed] [Google Scholar]
- 59. Taylor DA, Sampaio LC, Cabello R, Elgalad A, Parikh R, Wood RP, Myer KA, Yeh AT, Lee PF. Decellularization of whole human heart inside a pressurized pouch in an inverted orientation. J Vis Exp e58123, 2018. doi: 10.3791/58123. [DOI] [PubMed] [Google Scholar]
- 60. Bell RM, Mocanu MM, Yellon DM. Retrograde heart perfusion: the Langendorff technique of isolated heart perfusion. J Mol Cell Cardiol 50: 940–950, 2011. doi: 10.1016/j.yjmcc.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 61. Lee P-F, Chau E, Cabello R, Yeh AT, Sampaio LC, Gobin AS, Taylor DA. Inverted orientation improves decellularization of whole porcine hearts. Acta Biomater 49: 181–191, 2017. doi: 10.1016/j.actbio.2016.11.047. [DOI] [PubMed] [Google Scholar]
- 62. Robertson MJ, Dries-Devlin JL, Kren SM, Burchfield JS, Taylor DA. Optimizing recellularization of whole decellularized heart extracellular matrix. PLoS One 9: e90406, 2014. doi: 10.1371/journal.pone.0090406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bruyneel AAN, Carr CA. Ambiguity in the presentation of decellularized tissue composition: the need for standardized approaches. Artif Organs 41: 778–784, 2017. doi: 10.1111/aor.12838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Faulk DM, Wildemann JD, Badylak SF. Decellularization and cell seeding of whole liver biologic scaffolds composed of extracellular matrix. J Clin Exp Hepatol 5: 69–80, 2015. doi: 10.1016/j.jceh.2014.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zambon JP, Ko IK, Abolbashari M, Huling J, Clouse C, Kim TH, Smith C, Atala A, Yoo JJ. Comparative analysis of two porcine kidney decellularization methods for maintenance of functional vascular architectures. Acta Biomater 75: 226–234, 2018. doi: 10.1016/j.actbio.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 66. de Haan MJA, Witjas FMR, Engelse MA, Rabelink TJ. Have we hit a wall with whole kidney decellularization and recellularization: a review. Curr Opin Biomed Eng 20: 100335, 2021. doi: 10.1016/j.cobme.2021.100335. [DOI] [Google Scholar]
- 67. Ullah I, Busch JF, Rabien A, Ergün B, Stamm C, Knosalla C, Hippenstiel S, Reinke P, Kurtz A. Adult tissue extracellular matrix determines tissue specification of human iPSC-derived embryonic stage mesodermal precursor cells. Adv Sci (Weinh) 7: 1901198, 2020. doi: 10.1002/advs.201901198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ullah I, Abu-Dawud R, Busch JF, Rabien A, Erguen B, Fischer I, Reinke P, Kurtz A. VEGF – supplemented extracellular matrix is sufficient to induce endothelial differentiation of human iPSC. Biomaterials 216: 119283, 2019. [Erratum in Biomaterials 230: 119660, 2020]. doi: 10.1016/j.biomaterials.2019.119283. [DOI] [PubMed] [Google Scholar]
- 69. Zhang X, Chen X, Hong H, Hu R, Liu J, Liu C. Decellularized extracellular matrix scaffolds: recent trends and emerging strategies in tissue engineering. Bioact Mater 10: 15–31, 2022. doi: 10.1016/j.bioactmat.2021.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, Taylor DA. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med 14: 213–221, 2008. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 71. Rajabi S, Pahlavan S, Ashtiani MK, Ansari H, Abbasalizadeh S, Sayahpour FA, Varzideh F, Kostin S, Aghdami N, Braun T, Baharvand H. Human embryonic stem cell-derived cardiovascular progenitor cells efficiently colonize in bFGF-tethered natural matrix to construct contracting humanized rat hearts. Biomaterials 154: 99–112, 2018. doi: 10.1016/j.biomaterials.2017.10.054. [DOI] [PubMed] [Google Scholar]
- 72. Qian Z, Sharma D, Jia W, Radke D, Kamp T, Zhao F. Engineering stem cell cardiac patch with microvascular features representative of native myocardium. Theranostics 9: 2143–2157, 2019. doi: 10.7150/thno.29552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Chute M, Aujla P, Jana S, Kassiri Z. The non-fibrillar side of fibrosis: contribution of the basement membrane, proteoglycans, and glycoproteins to myocardial fibrosis. J Cardiovasc Dev Dis 6: 35, 2019. doi: 10.3390/jcdd6040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Weber KT. Cardiac interstitium in health and disease: the fibrillar collagen network. J Am Coll Cardiol 13: 1637–1652, 1989. doi: 10.1016/0735-1097(89)90360-4. [DOI] [PubMed] [Google Scholar]
- 75. Bashey RI, Martinez-Hernandez A, Jimenez SA. Isolation, characterization, and localization of cardiac collagen type VI. Associations with other extracellular matrix components. Circ Res 70: 1006–1017, 1992. doi: 10.1161/01.res.70.5.1006. [DOI] [PubMed] [Google Scholar]
- 76. McCurdy S, Baicu CF, Heymans S, Bradshaw AD. Cardiac extracellular matrix remodeling: fibrillar collagens and Secreted Protein Acidic and Rich in Cysteine (SPARC). J Mol Cell Cardiol 48: 544–549, 2010. doi: 10.1016/j.yjmcc.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lindsey ML, Jung M, Hall ME, DeLeon-Pennell KY. Proteomic analysis of the cardiac extracellular matrix: clinical research applications. Expert Rev Proteomics 15: 105–112, 2018. doi: 10.1080/14789450.2018.1421947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hynes RO. The extracellular matrix: not just pretty fibrils. Science 326: 1216–1219, 2009. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Jourdan-Lesaux C, Zhang J, Lindsey ML. Extracellular matrix roles during cardiac repair. Life Sci 87: 391–400, 2010. doi: 10.1016/j.lfs.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Fan D, Creemers EE, Kassiri Z. Matrix as an interstitial transport system. Circ Res 114: 889–902, 2014. doi: 10.1161/circresaha.114.302335. [DOI] [PubMed] [Google Scholar]
- 81. Votteler M, Carvajal Berrio DA, Pudlas M, Walles H, Stock UA, Schenke-Layland K. Raman spectroscopy for the non-contact and non-destructive monitoring of collagen damage within tissues. J Biophotonics 5: 47–56, 2012. doi: 10.1002/jbio.201100068. [DOI] [PubMed] [Google Scholar]
- 82. Kular JK, Basu S, Sharma RI. The extracellular matrix: structure, composition, age-related differences, tools for analysis and applications for tissue engineering. J Tissue Eng 5: 2041731414557112, 2014. doi: 10.1177/2041731414557112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Liguori GR, Liguori TTA, Moraes SR, Sinkunas V, Terlizzi V, Dongen JA, Sharma PK, Moreira LFP, Harmsen MC. Molecular and biomechanical clues from cardiac tissue decellularized extracellular matrix drive stromal cell plasticity. Front Bioeng Biotechnol 8: 520, 2020. doi: 10.3389/fbioe.2020.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Oropeza BP, Adams JR, Furth ME, Chessa J, Boland T. Bioprinting of decellularized porcine cardiac tissue for large-scale aortic models. Front Bioeng Biotechnol 10: 855186, 2022. doi: 10.3389/fbioe.2022.855186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Becker M, Maring JA, Schneider M, Herrera Martin AX, Seifert M, Klein O, Braun T, Falk V, Stamm C. Towards a novel patch material for cardiac applications: tissue-specific extracellular matrix introduces essential key features to decellularized amniotic membrane. Int J Mol Sci 19: 1032, 2018. doi: 10.3390/ijms19041032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lu T-Y, Lin B, Kim J, Sullivan M, Tobita K, Salama G, Yang L. Repopulation of decellularized mouse heart with human induced pluripotent stem cell-derived cardiovascular progenitor cells. Nat Commun 4: 2307, 2013. doi: 10.1038/ncomms3307. [DOI] [PubMed] [Google Scholar]
- 87. Mosala Nezhad Z, Poncelet A, Kerchove L, Gianello P, Fervaille C, El Khoury G. Small intestinal submucosa extracellular matrix (CorMatrix) in cardiovascular surgery: a systematic review. Interact Cardiovasc Thorac Surg 22: 839–850, 2016. doi: 10.1093/icvts/ivw020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Dohmen PM, Costa F, Lopes SV, Vilani R, Bloch O, Konertz W. Successful implantation of a decellularized equine pericardial patch into the systemic circulation. Med Sci Monit Basic Res 20: 1–8, 2014. doi: 10.12659/MSMBR.889915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Meuris B, Ozaki S, Neethling W, De Vleeschauwer S, Verbeken E, Rhodes D, Verbrugghe P, Strange G. Trileaflet aortic valve reconstruction with a decellularized pericardial patch in a sheep model. J Thorac Cardiovasc Surg 152: 1167–1174, 2016. doi: 10.1016/j.jtcvs.2016.05.024. [DOI] [PubMed] [Google Scholar]
- 90. Brizard CP, Brink J, Horton SB, Edwards GA, Galati JC, Neethling WM. New engineering treatment of bovine pericardium confers outstanding resistance to calcification in mitral and pulmonary implantations in a juvenile sheep model. J Thorac Cardiovasc Surg 148: 3194–3201, 2014. doi: 10.1016/j.jtcvs.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 91. Patukale A, Shikata F, Marathe SS, Patel P, Marathe SP, Colen T, Venugopal P, Alphonso N. A single centre, retrospective study of mid-term outcomes of aortic arch repair using a standardized resection and patch augmentation technique. Interact Cardiovasc Thorac Surg 35: ivac135, 2022. doi: 10.1093/icvts/ivac135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. van Beynum IM, Kurul S, Krasemann T, Dalinghaus M, de Woestijne PV, Etnel JR, Bogers A. Reconstruction of the aortic arch in neonates and infants: the importance of patch material. World J Pediatr Congenit Heart Surg 12: 487–491, 2021. doi: 10.1177/21501351211003502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Mohamed S, Patel AJ, Mazhar K, Jeeji R, Ridley PD, Balacumaraswami L. Anterior leaflet replacement and reconstruction with Admedus Cardiocel™ decellularized pericardial patch in tricuspid valve endocarditis. J Surg Case Rep 2021: rjab106, 2021. doi: 10.1093/jscr/rjab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Marathe SP, Chávez M, Sleeper LA, Marx GR, Friedman K, Feins EN, Del Nido PJ, Baird CW. Single-leaflet aortic valve reconstruction utilizing the Ozaki technique in patients with congenital aortic valve disease. Semin Thorac Cardiovasc Surg S1043-0679(21)00468-8, 2021. doi: 10.1053/j.semtcvs.2021.10.009. [DOI] [PubMed] [Google Scholar]
- 95. Neethling W, Rea A, Forster G, Bhirangi K. Performance of the ADAPT-treated cardiocel scaffold in pediatric patients with congenital cardiac anomalies: medium to long-term outcomes. Front Pediatr 8: 198, 2020. doi: 10.3389/fped.2020.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Yanagawa B, Rao V, Yau TM, Cusimano RJ. Initial experience with intraventricular repair using CorMatrix extracellular matrix. Innovations (Phila) 8: 348–352, 2013. doi: 10.1097/imi.0000000000000014. [DOI] [PubMed] [Google Scholar]
- 97. Ashfaq A, Brown T, Reemtsen B. Repair of complete atrioventricular septal defects with decellularized extracellular matrix: initial and midterm outcomes. World J Pediatr Congenit Heart Surg 8: 310–314, 2017. doi: 10.1177/2150135116684797. [DOI] [PubMed] [Google Scholar]
- 98. Christ T, Paun AC, Grubitzsch H, Holinski S, Falk V, Dushe S. Long-term results after the Ross procedure with the decellularized AutoTissue Matrix P bioprosthesis used for pulmonary valve replacement. Eur J Cardiothorac Surg 55: 885–892, 2019. doi: 10.1093/ejcts/ezy377. [DOI] [PubMed] [Google Scholar]
- 99. Murin P, Weixler VHM, Kuschnerus K, Romanchenko O, Lorenzen V, Nordmeyer J, Cho M-Y, Sigler M, Photiadis J. Pulmonary artery augmentation using decellularized equine pericardium (Matrix Patch™): initial single-centre experience. Eur J Cardiothorac Surg 60: 1094–1101, 2021. doi: 10.1093/ejcts/ezab183. [DOI] [PubMed] [Google Scholar]
- 100. Elassal AA, Al-Radi OO, Zaher ZF, Dohain AM, Abdelmohsen GA, Mohamed RS, Fatani MA, Abdelmotaleb ME, Noaman NA, Elmeligy MA, Eldib OS. Equine pericardium: a versatile alternative reconstructive material in congenital cardiac surgery. J Cardiothorac Surg 16: 110, 2021. doi: 10.1186/s13019-021-01494-y. [DOI] [PMC free article] [PubMed] [Google Scholar]