We appreciate the efforts of Cosentino and Marino in reviewing and commenting [1] on our recent opinion article on the putative role of the anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mRNA vaccines-produced antigen – that is, SARS-CoV-2 Wuhan-Hu-1 strain spike (S) protein – in reported adverse effects (AEs) of vaccination [2]. Cosentino and Marino attempted to answer one of our ‘outstanding questions’ (sixth question in [2]), and they have introduced an additional issue while also providing a plausible answer. We are responding here to the discussion by adding further perspectives to their arguments.
As stated by the commenters, the systemic distribution of mRNA-containing lipid nanoparticles (LNPs) of current coronavirus disease 2019 (COVID-19) mRNA vaccines was considered rather negligible until mid–late 2021, when the first reports showed the existence of the S protein in the circulation of vaccine recipients [3]; admittedly, however, this was to be expected given previous reports showing that LNPs can be found in the liver and other tissues in animal models [2]. Nonetheless, beyond the unquestionably useful theoretical calculations of the S protein concentration needed to bind half of the available angiotensin-converting enzyme 2 (ACE2), we remain largely ignorant of the concentration and half-life of S protein in different tissues and/or in the circulation; also, we have no accurate information for the rate of possible tissue spillover of S (or related peptides) in the plasma. In the absence of this critical information, it is premature to allude to toxic concentrations of S protein in tissues and organs postvaccination. If recent findings revealing the existence of vaccine mRNA in lymph nodes, and of S antigen in lymph nodes and blood, for 2 months post-SARS-CoV-2 mRNA vaccination [4] are confirmed, then the mechanistic details of S mRNA persistence and protein production for such a prolonged period in human tissues should be prioritized for investigation. In that context, we would agree with Cosentino and Marino that the period for causality assessment of suspect vaccination-related AEs should be significantly extended. Parallel to this line of research, the development of alternative LNP chemistries that avoid the liver and circulation – and ideally allow on-demand tissue distribution of the antigen, especially at immunogenic sites such as the muscle – may also decisively increase mRNA vaccines tolerability without affecting their potency.
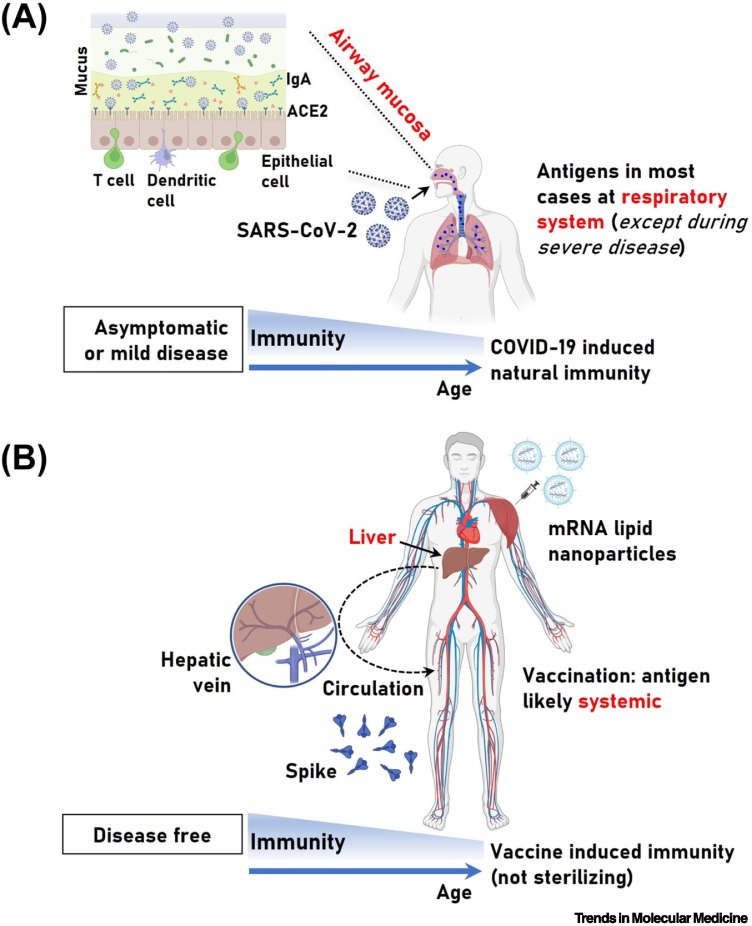
Regarding the question asked by Cosentino and Marino: “How does production of S protein compare between COVID-19 mRNA vaccines and SARS-CoV-2 infection”, we argue that, contrary to the likely systemic distribution of S protein following vaccination (Figure 1 ), SARS-CoV-2 is a respiratory virus, and thus in the majority of infected individuals with no prior health problems, it will be contained in the respiratory system (RS), resulting in either no (asymptomatic) or mild symptoms [5]. Consistently, SARS-CoV-2 RNA detected in blood products from patients with COVID-19 is not associated with infectious virus [6], as was also confirmed in hospitalized patients [7]. Therefore, the detection of viral RNA or related antigens (e.g., S protein) in the blood does not indicate the presence of actively replicating virus; in fact, even the detection of viral particles in autopsy tissues post-lethal COVID-19 by electron microscopy is (in some cases) debatable [8].
Figure 1.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection- and mRNA vaccine-induced immunity and the likely distribution of the related antigens.
(A) The risk for severe coronavirus disease 2019 (COVID-19) increases significantly with age and pre-existing comorbidities, whereas healthy adults are largely spared from severe COVID-19. SARS-CoV-2 is a respiratory virus, and during asymptomatic infection or mild COVID-19 innate and adaptive immunity will efficiently contain SARS-CoV-2 in the respiratory system (RS), resulting in no systemic spread of replicating virus and/or related antigens. Viral antigen leakage in the circulation and/or viremia may occur during severe COVID-19, possibly correlating with ‘long COVID-19’. (B) Vaccination at the deltoid muscle triggers robust and durable anti-SARS-CoV-2 spike (S) protein systemic immune responses in the absence of disease; however, intramuscular vaccine administration may not optimally activate local mucosal responses, thus providing limited protection (compared with what would be desirable) at the main site of infection (i.e., the respiratory mucosa). Given that the mRNA-containing nanoparticles also localize in the liver (among other tissues), it is likely that the antigen(s) eventually enters the circulation (e.g., via the hepatic vein) and is distributed systemically. Both COVID-19- and mRNA vaccine-triggered immune responses are weaker in the elderly and in the presence of certain pre-existing comorbidities and/or medication (A and B, lower parts). Abbreviation: ACE2, angiotensin-converting enzyme 2.
We remain optimistic that the trends in common with other respiratory viruses – also seen in the evolution of SARS-CoV-2 emerging variants of concern (VoCs) (e.g., Omicron), namely, that despite being more transmissible, new VoCs so far appear less pathogenic [9] – will result in accelerated long-lasting natural or hybrid immunity in the community. Given also that the virus will likely not be eradicated, we are seemingly transitioning away from ‘pandemic’ and toward an ‘endemic’ phase. Indeed, in a recent update of a study of US blood donations, the combined seroprevalence from infection or vaccination had reached 94.7% by December 2021 [10]. The ability of the emerged new SARS-CoV-2 VoCs to cause reinfections and/or breakthrough infections in this setting of high seroprevalence clearly illustrates the significant evolutionary leaps of the evolving VoC antigenic sites and not the waning of immunity against the ancestral antigen (i.e., Wuhan-Hu-1 S protein). In fact, the idea of ‘fading’ protection (increased risk of serious COVID-19 outcomes) from boosting doses should be viewed (excluding conditions of immunosuppression) as a significant paradox given the durable extremely high titers of anti-S antibodies post boosting vaccination [11].
Overall, given the wide range of ‘unknowns’ discussed in this and previous articles [1,2], further boosting with the ‘extinct’ (since early 2020) Wuhan-Hu-1 S antigen in healthy individuals should be administered only if the benefit–risk profile is unambiguously demonstrated. As for the vulnerable individuals, and especially those who – despite repeated vaccine doses – do not mount appropriate immune responses [12], their removal from the vaccination program and instead their guidance to increase prophylactic measures, or their treatment (if needed) with therapeutics such as COVID-19 monoclonal antibodies and/or antiviral agents should be considered.
Our multidisciplinary research team is actively investigating these topics, including interindividual variability and subjects at high risk for developing AEs. Unwinding the unknowns of COVID-19 mRNA vaccine-induced AEs will eventually contribute not only to guaranteeing safety and directing health policies regarding the anti-COVID-19 vaccination campaign, but also to future applications of these technological platforms (mRNA-containing LNPs) against various infectious diseases and beyond.
References
- 1.Cosentino M., Marino F. The spike hypothesis in vaccine-induced adverse effects: questions and answers. Trends Mol. Med. 2022;28:797–799. doi: 10.1016/j.molmed.2022.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trougakos I.P., et al. Adverse effects of COVID-19 mRNA vaccines: the spike hypothesis. Trends Mol. Med. 2022;28:542–554. doi: 10.1016/j.molmed.2022.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogata A.F., et al. Circulating severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine antigen detected in the plasma of mRNA-1273 vaccine recipients. Clin. Infect. Dis. 2022;74:715–718. doi: 10.1093/cid/ciab465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Röltgen K., et al. Immune imprinting, breadth of variant recognition, and germinal center response in human SARS-CoV-2 infection and vaccination. Cell. 2022;185:1025–1040. doi: 10.1016/j.cell.2022.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Driscoll M., et al. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature. 2021;590:140–145. doi: 10.1038/s41586-020-2918-0. [DOI] [PubMed] [Google Scholar]
- 6.Andersson M.I., et al. SARS-CoV-2 RNA detected in blood products from patients with COVID-19 is not associated with infectious virus. Wellcome Open Res. 2020;5:181. doi: 10.12688/wellcomeopenres.16002.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wölfel R., et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 8.Dittmayer C., et al. Why misinterpretation of electron micrographs in SARS-CoV-2-infected tissue goes viral. Lancet. 2020;396:e64–e65. doi: 10.1016/S0140-6736(20)32079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewnard J.A., et al. Clinical outcomes associated with SARS-CoV-2 Omicron (B.1.1.529) variant and BA.1/BA.1.1 or BA.2 subvariant infection in southern California. Nat. Med. 2022 doi: 10.1038/s41591-022-01887-z. Published online June 8, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones J.M., et al. Updated US infection- and vaccine-induced SARS-CoV-2 seroprevalence estimates based on blood donations, July 2020–December 2021. JAMA. 2022;328:298–301. doi: 10.1001/jama.2022.9745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ntanasis-Stathopoulos I., et al. Third dose of the BNT162b2 vaccine results in sustained high levels of neutralizing antibodies against SARS-CoV-2 at 6 months following vaccination in healthy individuals. Hemasphere. 2022;6 doi: 10.1097/HS9.0000000000000747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terpos E., et al. Booster BNT162b2 optimizes SARS-CoV-2 humoral response in patients with myeloma: the negative effect of anti-BCMA therapy. Blood. 2022;139:1409–1412. doi: 10.1182/blood.2021014989. [DOI] [PMC free article] [PubMed] [Google Scholar]