ABSTRACT
DLEU2 has been proved to act as an oncogene in a variety of cancers, but its role in cardiovascular diseases is dearth of research. Thus, this study mainly discussed the effect and possible mechanism of DLEU2 on platelet-derived growth factor-BB (PDGF-BB)-triggered vascular smooth muscle cell (VSMC) injury. To obtain authentic results, the expressions of target genes in atherosclerosis serum were determined by reverse transcription quantitative PCR (RT-qPCR) and the protein levels were evaluated by Western blot. PDGF-BB was used to simply simulate the biological characteristics of VSMCs in vitro. The effect of DLEU2 on the biological behavior of PDGF-BB-induced VSMCs was analyzed by gain- and loss-of-function assays. Bioinformatics analysis, dual luciferase reporter assay, and Pearson correlation method were conducted to determine the relationship between target genes. The role of DLEU2/miR-212-5p/ YWHAZ (tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein zeta) axis in PDGF-BB-induced VSMCs was verified by rescue experiments. As a result, DLEU2 and YWHAZ were up-regulated, and miR-212-5p was down-regulated in atherosclerosis serum. Overexpressed DLEU2 facilitated the biological behavior of PDGF-BB-induced VSMCs, whilst siDLEU2 did the opposite. Moreover, overexpressed DLEU2 promoted proliferating cell nuclear antigen (PCNA) expression but repressed α-smooth muscle actin (α-SMA) and Calponin expressions, while it also enhanced YWHAZ expression via suppressing miR-212-5p. MiR-212-5p mimic and siYWHAZ reversed the effects of overexpressed DLEU2 on above biological characteristics and protein expressions in PDGF-BB-induced VSMCs, while the regulatory effect of miR-212-5p mimic was partially offset by overexpressed YWHAZ. Collectively, DLEU2 modulates PDGF-BB-induced VSMC injury via miR-212-5p/YWHAZ axis in atherosclerosis.
KEYWORDS: Atherosclerosis, DLEU2, vascular smooth muscle cells, miR-212-5p, YWHAZ
Introduction
Atherosclerosis, the main cause of disease and death in cardiovascular diseases, is a disease occurring in the large arteries [1,2], which is promoted by dysfunction of endothelial cells (ECs) and abnormal proliferation of vascular smooth muscle cells (VSMCs) together [3]. VSMCs are the main component of blood vessel walls [4]. Emerging evidence shows that the proliferation and migration of VSMCs affect the formation of atherosclerosis [5,6]. To be specific, contractile VSMCs migrate from the vascular media into the vascular intima, transform into synthetic VSMCs and proliferate in large quantities, resulting in thickening of the intima, lipid deposition, and ultimately the formation of atherosclerosis [5]. The phenotypic transition of VSMCs is affected by many factors including platelet-derived growth factor-BB (PDGF-BB) [7]. Studies have found that PDGF-BB regulates the proliferation and migration of VSMCs by activating intracellular signal transduction pathways [8,9]. Therefore, studying the mechanism of biological characteristics changes of PDGF-BB-induced VSMCs may provide strong theoretical supports for the diagnosis and treatment of atherosclerosis.
Increasing evidence has indicated that long non-coding RNAs (lncRNAs) are involved in the development of cardiovascular diseases [10,11]. In practice, lncRNA-p21 can inhibit proliferation of VSMCs and mouse monocyte macrophages and induce cell apoptosis [12]. Incidentally, Shan et al. found that retinal non-coding RNA 3 (RNCR3) is highly expressed in ECs and VSMCs, knockdown of which reduces the proliferation and migration of ECs and VSMCs and accelerates the development of cell apoptosis and atherosclerosis [13]. Arslan et al. pointed out that Cyclin-dependent kinase inhibitor 2B antisense RNA 1 (ANRIL) and myocardial infarction-related transcript (MIAT) expressions were notably increased in atherosclerotic blood vessels [14], indicating that lncRNAs make a profound impact upon the development of atherosclerosis.
In this experiment, after preliminary screening, we uncovered that compared with healthy samples, samples from patients with atherosclerosis have highly expressed lymphocytic leukemia 2 (DLEU2). In the past, lncRNA DLEU2 has only been shown to act as an oncogene in assorted cancers such as pancreatic cancer, laryngeal carcinoma, etc [15,16]. Detailedly, DLEU2 strengthens the malignant biological characteristics of hepatocellular carcinoma (HCC) cells by combining with enhancer of zeste 2 polycomb repressive complex 2 subunit (EZH2), thus aggravating the progression of HCC [17]. However, researches on DLEU2 in cardiovascular diseases are in shortage. Therefore, in this study, we mainly explored the effect of DLEU2 on the proliferation and migration of VSMCs induced by PDGF-BB. Meanwhile, the downstream potential miRNA and targeted genes of lncRNA DLEU2 were analyzed and predicted to study the potential molecular mechanism of DLEU2 involved in atherosclerosis.
Materials and methods
Ethics statement and clinical specimens
Blood samples from patients (n = 34) who were diagnosed as atherosclerosis, and healthy donors (n = 20) were collected from The Second Hospital of Hebei Medical University between May 2020 and August 2020. The clinical trial program had been reviewed and approved by the Ethics Committee of The Second Hospital of Hebei Medical University (AE202004014). Written informed consent was signed by all subjects. The demographic data of patients participated in the study were shown in Table 1. Serum was separated from blood samples by low-speed centrifugation and stored at −80°C. Patients who were diagnosed by clinical symptoms and coronary angiography, and healthy donors without any history of cardiovascular, inflammatory, or other diseases were recruited.
Table 1.
Clinical parameters of atherosclerosis patients.
| Parameters | Normal controls | Atherosclerosis patients |
|---|---|---|
| Number | 20 | 34 |
| Sex (female, %) | 30 | 44 |
| Age | 62.25 ± 6.85 | 61.32 ± 7.52 |
| Total cholesterol (mmol/L) | 3.55 ± 0.90 | 5.65 ± 0.62* |
| LDL-C (mmol/L) | 2.35 ± 0.38 | 2.52 ± 0.35 |
| HDL-C (mmol/L) | 1.71 ± 0.35 | 1.85 ± 0.38 |
| TG (mmol/L) | 1.48 ± 0.30 | 2.12 ± 0.32* |
| Creatinine (mg/dL) | 1.12 ± 0.18 | 1.21 ± 0.15 |
Mean ± S.D.
*P < 0.05
Cells and treatment
VSMCs (CRL-1999), F-12 K Medium (Kaighn’s Modification of Ham’s F-12 Medium) (30–2004), and fetal bovine serum (FBS) (30–2020) were purchased from American Type Culture Collection (USA). Cells were cultivated in F-12 K Medium containing 10% FBS in an incubator (SCO6WE-2, SHELLAB, USA) at 37°C with 5% CO2. The cells were treated with 20 ng/mL PDGF-BB solution for 24 hours (h) as needed [8].
Transfection
Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein zeta (YWHAZ) overexpression plasmid, DLEU2 overexpression plasmid, and pcDNA3.1-negative control (NC) were purchased from Invitrogen Company (USA). The siRNA targeted against DLEU2 (siDLEU2, A09001, target sequence: TTGCTGAAACTGCACAAAAAATC) and siYWHAZ (A09001, target sequence: CTGCATGAAGTCTGTAACTGAGC), as well as siNC (A06001) were ordered from GenePharma Company (China). MiR-212-5p mimic (M, miR10022695-1-5) and mimic control (miR-NC, miR1N0000002-1-5) were available from RIBOBIO Company (China). VSMCs were transfected with RNAs or plasmids using Lipofectamine 3000 (L3000015, Invitrogen, USA), or Lipofectamine RNAiMAX Transfection Reagent (13778030, Invitrogen, USA), and then treated with PDGF-BB as needed.
Reverse transcription quantitative PCR (RT-qPCR)
Sample RNAs in each group of VSMCs were collected using Trizol reagent (15596018, Invitrogen, USA), followed by measurement of RNA concentration using NanoDrop One machine (Thermo, USA). Total RNAs were reversely transcribed into cDNA as per the instructions of lncRNA cDNA kit (KR202), miRNA cDNA kit (KR211) or RT-PCR kit (KR123). Lastly, RT-qPCR was performed in an ABI Prism 7500 sequence detector (Applied Biosystems, USA) using lnRcute lncRNA SYBR Green premix (FP402), miRcute Plus miRNA qPCR Detection Kit (FP411) and FastFire qPCR PreMix (FP207). The different types of cDNA kits or PCR kits were obtained from TIANGEN Company (China). For lncRNA, RT-qPCR was conducted with 20 μL of mixture, each consisted of 10 μL of lnc lncRNA premix (SYBR Green), 8 μL of RNase-free ddH2O, 1 μL of cDNA (~ 500 ng/μL), and 0.5 μL each of forward and reverse primers (10 μM). The PCR procedure was as follows: an initial denaturation at 95°C for 3 minutes (min), followed by 40 cycles of 95°C for 5 seconds(s) and 60°C for 15s. For miRNA, RT-qPCR was conducted with 20 μL of mixture, each consisted of 10 μL of miRcute plus miRNA premix (SYBR&ROX), 8 μL of RNase-free ddH2O, 1 μL of cDNA (~ 500 ng/μL), and 0.5 μL each of forward and reverse primers (10 μM). The PCR procedure was as follows: an initial denaturation at 95°C for 15 min, followed by 40 cycles of 94°C for 20s and 60°C for 34s. For mRNA, RT-qPCR was conducted with 20 μL of mixture, each consisted of 10 μL of fastfire qPCR premix, 7 μL of RNase-free ddH2O, 1 μL of cDNA (~ 500 ng/μL), 0.4 μL ROX reference dye, and 0.6 μL each of forward and reverse primers (10 μM). The PCR procedure was as follows: an initial denaturation at 95°C for 1 min, followed by 40 cycles of 95°C for 5 s and 60°C for 31s. Results were normalized to U6 or β-actin, and analyzed using 2−ΔΔCt method [17]. Specific primers were as follows (5’-3’): DLEU2: TCCGAGAGTATAGCGCCACT (forward), ACTGCCCTTTGCTCCAAGTA (reverse); miR-212-5p: GGAAACATCCTCGACTG (forward), ATTGAACGTGCCTCCGTGTTGAGG (reverse); Proliferating cell nuclear antigen (PCNA): CCTGCTGGGATATTAGCTCCA (forward), CAGCGGTAGGTGTCGAAGC (reverse); α-smooth muscle actin (α-SMA): AAAAGACAGCTACGTGGGTGA (forward), GCCATGTTCTATCGGGTACTTC (reverse); Calponin 1: CTGTCAGCCGAGGTTAAGAAC (forward), GAGGCCGTCCATGAAGTTGTT (reverse); YWHAZ: CCTGCATGAAGTCTGTAACTGAG (forward), GACCTACGGGCTCCTACAACA (reverse); β-actin: CGAGAAGATGACCCAGATCATG (forward), GTGAAGCTGTAGCCGCGCTCGG (reverse); U6: GGTCGGGCAGGAAAGAGGGC (forward), GCTAATCTTCTCTGTATCGTTCC (reverse).
Cell viability assay
VSMC viability was estimated by cell counting kit-8 (CCK-8) kits (CK04, Solarbio, China). VSMCs (approximately 1 × 103/well) from different groups were incubated for 12, 24 or 36 hours(h), and further cultured with the addition ofCCK-8 solution (10 µL) for 2 h in an incubator at 37°C with 5% CO2. Finally, the absorbance was estimated at 450 nm by a microplate reader (EnVision, PerkinElmer, USA).
Wound healing assay
Wound healing assay was carried out to measure the migration of VSMCs. Briefly, the treated VSMCs (1 × 105/well) were cultured in 6-well plates for 24 h. After VSMCs reached 80% confluence, a straight-line wound was made on cells using a sterilized 200 μL disposable pipette tip. Afterward, VSMCs were cultured for another 24 h. The width of the scratch wounds was acquired with a Nikon ECLIPSE Ts2 microscope (Japan) (magnification × 100).
Transwell assay
The VSMC invasion analysis was performed by Transwell assay. VSMCs (1 × 103/well) were seeded into the upper Transwell chamber (3422, Corning, USA) covered with Matrigel (356234, Solarbio, China). The lower chamber was filled with medium containing 10% FBS. Then, post 24-h incubation, VSMCs transferring to the bottom surface of the chamber were stained with 0.1% crystal violet (C8470, Solarbio, China), the number of which was ultimately counted under a microscope (magnification × 100).
Bioinformatics analysis and dual luciferase reporter assay
The starBase v2.0 (http://starbase.sysu.edu.cn/index.php) was used to assess the targeting relationship between DLEU2 and miR-212-5p. TargetScan v7.2 (http://www.targetscan.org/vert_72/) was introduced to detect the binding sites between miR-212-5p and YWHAZ.
Dual luciferase reporter assay was performed to analyze the relationship between genes. Briefly, the wild-type and mutated sequences of DLEU2 (DLEU2-WT/DLEU2-MUT) or YWHAZ (YWHAZ-WT/YWHAZ-MUT) were synthesized, and reconstituted into luciferase reporter plasmids using pmirGLO vectors (E1330, Promega, USA). Next, wild-type and mutant DLEU2 or YWHAZ recombinant plasmid was co-transfected into 293 T cells (CRL-11268, ATCC, USA) with miR-212-5p mimic or miR-212-5p negative control. Lastly, a dual-luciferase detection kit (E1910, Promega, USA) was adopted to examine relative luciferase activities.
Western blot
Total protein of VSMCs in each group was extracted using RIPA buffer (R0278, Sigma-Aldrich, USA), followed by quantification using bicinchoninic acid (BCA) kit (BCA1, Sigma-Aldrich, USA). After being separated by 8% or 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), the total protein was transferred onto the polyvinylidene fluoride (PVDF) membranes (160–0184, BIO-RAD, USA) which were then blocked for 1 h. Thereafter, the membranes were incubated first with primary antibodies against YWHAZ (ab51129, 28 kDa, 1/1000, Abcam, UK), PCNA (ab92552, 29 kDa, 1/1000, Abcam, UK), α-SMA (ab5694, 42 kDa, 1 µg/mL, Abcam, UK), Calponin 1 (ab46794, 34 kDa, 1/5000, Abcam, UK) and β-actin (ab8226, 42 kDa, 1 µg/mL, Abcam, UK) at 4°C overnight, and then with a horseradish peroxidase-conjugated secondary antibody Goat Anti-Mouse immunoglobulin G (IgG) (ab205719, 1/5000) or Goat Anti-Rabbit IgG (ab205718, 1/5000) for 2 h. Later, protein-antibody complexes were visualized and analyzed using ECL chemiluminescent solution (WP20005, Invitrogen, USA) and ImageJ software (Rawak Software, Germany).
Data analysis
Statistical analysis was performed with GraphPad Prism 8.0 (GraphPad software, USA). Data were described by mean ± standard deviation. Kolmogorov-Smirnov test was used to analyze normal distribution of data. Comparison between two groups and among multiple groups was performed by independent sample t test and one-way analysis of variance, respectively, with P < 0.05 indicating a statistically significant difference.
Results
DLEU2, high-expressed in atherosclerosis, affected PDGF-BB-induced VSMC biological phenotype
The expression of DLEU2 was clearly increased in serum from atherosclerosis patient relative to healthy donor. We applied PDGF-BB to induce VSMC, since PDGF-BB-induced VSMC was reported to be feasibly used in studying the progression of atherosclerosis (P < 0.001, Figure 1(a)). As a result, PDGF-BB raised DLEU expression and overexpressed DLEU evidently promoted DLEU2 expression in PDGF-BB-induced VSMCs, while the effect of siDLEU2 was opposite to that of DLEU overexpression (P < 0.01, Figure 1(b)). Besides, we found that overexpression of DLEU enhanced but siDLEU2 inhibited the viability of PDGF-BB-induced VSMCs (P < 0.01, Figure 1(c)). In addition, PDGF-BB facilitated VSMC migration and invasion, which was reinforced by overexpression of DLEU, yet blocked by siDLEU2 (P < 0.05, Figure 1(d-g)).
Figure 1.
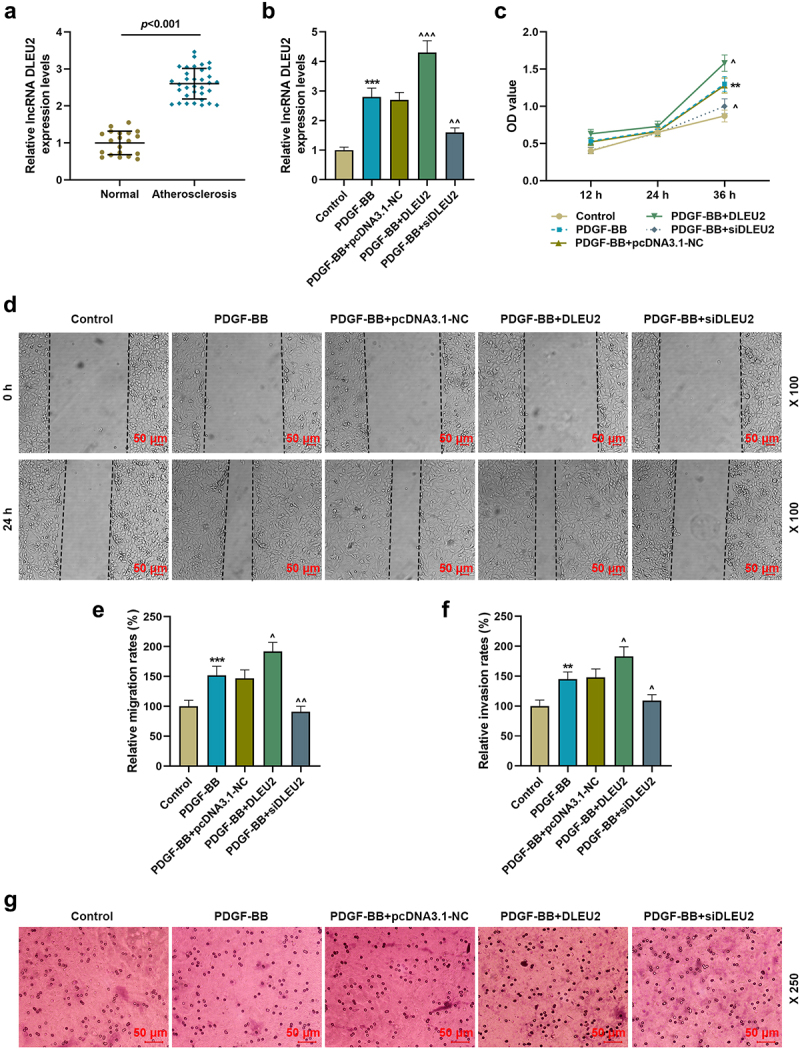
LncRNA DLEU2, high-expressed in atherosclerosis, affected PDGF-BB-induced biological phenotype of VSMCs. (a) The mRNA expression of DLEU2 was analyzed using RT-qPCR in human atherosclerotic serum (n = 34) and normal serum (n = 20). (b) The mRNA expression of DLEU2 was analyzed using RT-qPCR in Control, PDGF-BB, PDGF-BB+pcDNA3.1-NC, PDGF-BB+DLEU2 and PDGF-BB+siDLEU2 groups. (c) Cell Counting Kit-8 (CCK-8) assay was performed to test the cell viability in each group. (d-e) The cell migration ability in each group was analyzed by wound healing assay. (f-g) Transwell assay was applied to determine cell invasion ability. β-actin was used as a control. PDGF-BB: platelet-derived growth factor-BB. RT-qPCR: reverse transcription quantitative PCR. NC: negative control. VSMCs: vascular smooth muscle cells. All experiments were repeated at least three times. **P < 0.01, ***P < 0.001 vs. Control, ^P < 0.05, ^^P < 0.01, ^^^P < 0.001 vs. PDGF-BB+pcDNA3.1-NC. Data were expressed as mean ± standard deviation. Data between two groups or among multiple groups were analyzed by independent sample t test or one-way ANOVA, followed by Tukey’s post hoc test.
DLEU2 competitively bound to miR-212-5p which was low-expressed in atherosclerosis
In order to explore the mechanism of DLEU2 on PDGF-BB-stimulated VSMCs, we predicted and verified the target miRNA of DLEU2. As shown in Figure 2(a,b), there were binding sites between DLEU2 and miR-212-5p, and miR-212-5p mimic reduced the luciferase activity of wild-type DLEU2 (P < 0.001). Additionally, compared with healthy donors, miR-212-5p was confirmed to be lower- expressed in the serum from atherosclerosis patients (P < 0.001, Figure 2(c)). Moreover, a negative interplay was showed in the expressions of miR-212-5p and DLEU2 in the serum of patients with atherosclerosis (r = −0.415, P = 0.015, Figure 2(d)).
Figure 2.
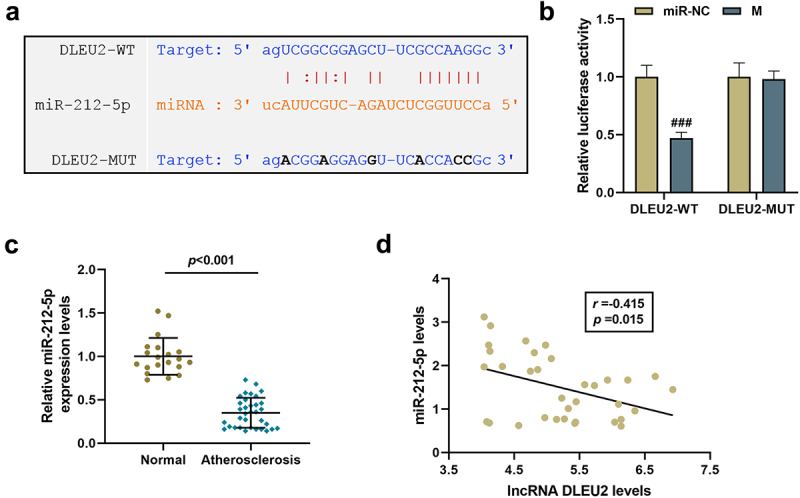
LncRNA DLEU2 competitively bound to miR-212-5p. (a) StarBase v2.0 (http://starbase.sysu.edu.cn/index.php) and (b) dual luciferase reporter assay were adopted to analyze the binding relationship between DLEU2 and miR-212-5p. (c) The expression of miR-212-5p was analyzed using RT-qPCR in human atherosclerotic serum (n = 34) and normal serum (n = 20). (d) Pearson correlation method was applied to analyze the correlation between the expressions of DLEU2 and miR-212-5p in the plasma of patients with atherosclerosis (n = 34). ###P < 0.001 vs. miR-NC. All experiments were repeated at least three times. Data were expressed as mean ± standard deviation. Data between two groups or among multiple groups were analyzed by independent sample t test or one-way ANOVA, followed by Tukey’s post hoc test.
Effect of miR-212-5p mimic on DLEU2 overexpression in promoting PDGF-BB-induced VSMC viability and migration
MiR-212-5p mimic was transfected into PDGF-BB-induced VSMCs as needed. As expected, miR-212-5p mimic up-regulated miR-212-5p and attenuated the inhibitory effect of DLEU2 overexpression on miR-212-5p expression (P < 0.01, Figure 3(a)). Subsequent functional testing unveiled that miR-212-5p mimic dampened the stimulating effect of PDGF-BB on VSMC cell viability, migration and invasion, and partially weakened the promoting effect of overexpressed DLEU2 (P < 0.05, Figure 3(b-f)). Additionally, we tested the expressions of PCNA as well as the myoepithelial cell markers α-SMA and Calponin. MiR-212-5p mimic reversed the up-regulation of PCNA and the down-regulations of α-SMA and Calponin induced by PDGF-BB, whereas overexpression of DLEU2 had the opposite effect on the above-mentioned proteins in PDGF-BB-induced VSMCs. Also, miR-212-5p mimic neutralized the effect of DLEU2 (P < 0.05, Figure 4(a-c)).
Figure 3.
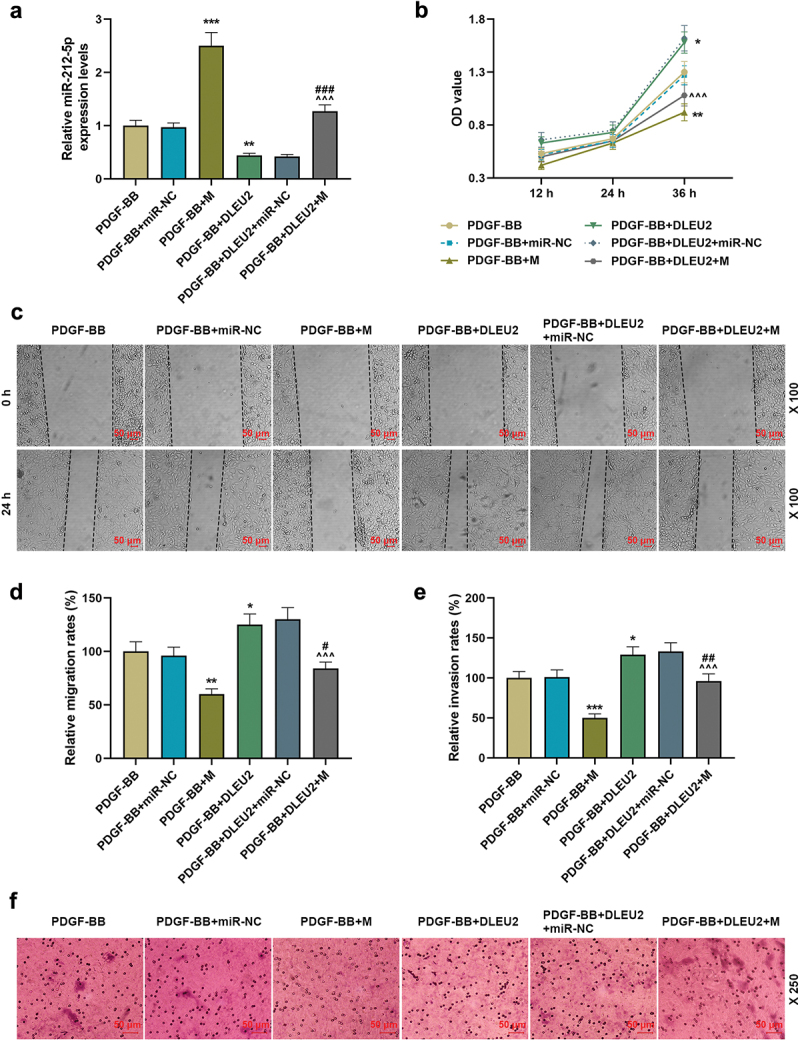
The promoting effect of lncRNA DLEU2 on the biological phenotype of VSMC induced by PDGF-BB was reversed by miR-212-5p mimic. VSMC was induced by PDGF-BB, with or without different RNA transfection. (a) RT-qPCR was conducted to detect the expression of miR-212-5p in each group of cells. U6 was used as a control. (b) CCK-8 assay was used for the determination of cell viability. (c-d) The effect of miR-212-5p mimic on cell migration ability was tested by wound healing assay. (e-f) Transwell assay was performed to determine cell invasion. *P < 0.05, **P < 0.01, ***P < 0.001 vs. PDGF-BB, ^^^P < 0.001 vs. PDGF-BB+DLEU2+ miR-NC, #P < 0.05, ##P < 0.01, ###P < 0.001 vs. PDGF-BB+M. All experiments were repeated at least three times. Data were expressed as mean ± standard deviation. Data among multiple groups were analyzed by one-way ANOVA, followed by Tukey’s post hoc test.
Figure 4.
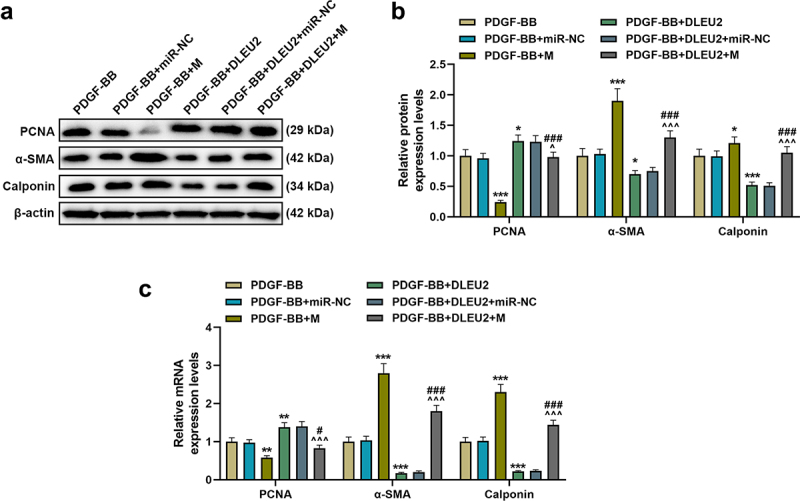
The effects of lncRNA DLEU2 and miR-212-5p mimic on the expressions of proliferating cell nuclear antigen (PCNA), α-smooth muscle actin (α-SMA) and Calponin in PDGF-BB-induced VSMCs were evaluated by western blot and RT-qPCR. β-actin was used as a control. *P < 0.05, **P < 0.01, ***P < 0.001 vs. PDGF-BB, ^P < 0.05, ^^^P < 0.001 vs. PDGF-BB+DLEU2+ miR-NC, #P < 0.05, ###P < 0.001 vs. PDGF-BB+M. All experiments were repeated at least three times. Data were expressed as mean ± standard deviation. Data among multiple groups were analyzed by one-way ANOVA, followed by Tukey’s post hoc test.
DLEU2-miR-212-5p-YWHAZ axis was in PDGF-BB-induced VSMCs
According to the predicted information on the website, there were 7 base complementary pairing sites between YWHAZ and miR-212-5p, and subsequent dual luciferase reporter analysis verified the binding of YWHAZ to miR-212-5p (P < 0.001, Figure 5(a-b)). Next, the expression of YWHAZ in atherosclerosis patient serum and healthy donor serum was measured, and the results identified that YWHAZ level was elevated in atherosclerosis patient serum (P < 0.001, Figure 5(c)). Furthermore, YWHAZ was positively correlated with DLEU2 (r = 0.379, P = 0.027, Figure 5(d)), while YWHAZ was greatly negatively correlated with miR-212-5p (r = −0.531, P = 0.001, Figure 5(e)). MiR-212-5p mimic obviously inhibited the expression of YWHAZ in PDGF-BB-induced VSMCs, and also overly reduced the promoting effects of DLEU2 and PDGF-BB on the expression of YWHAZ (P < 0.05, figure 5(f-h)).
Figure 5.
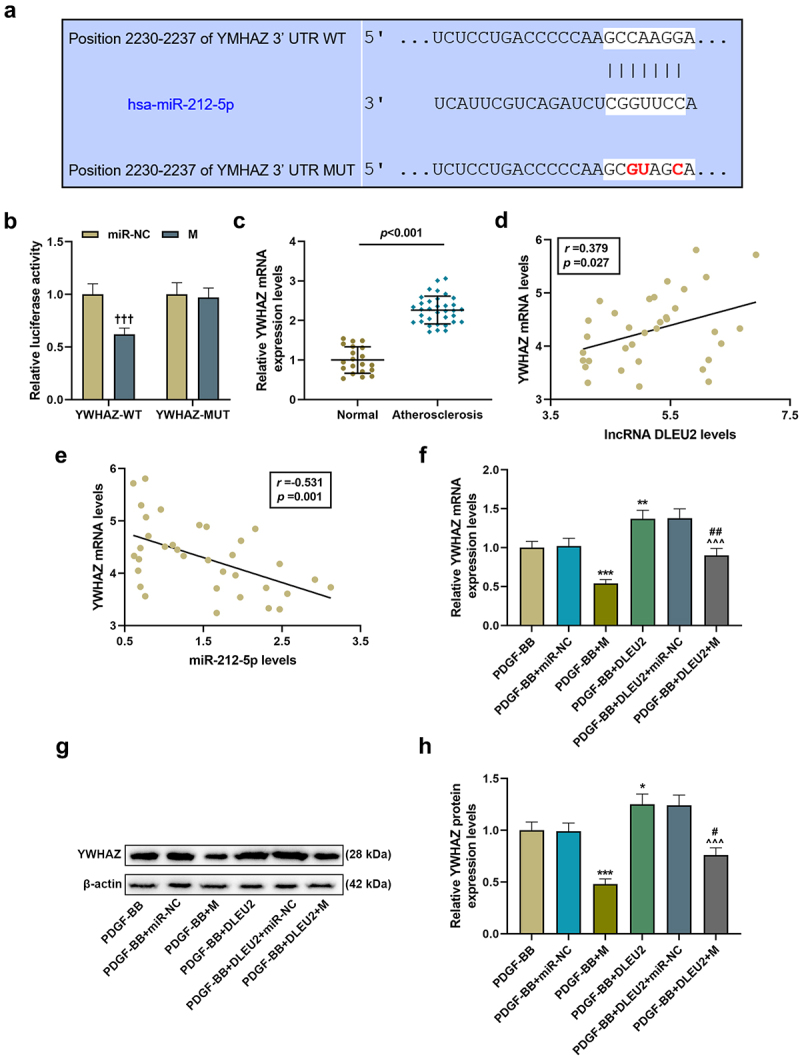
LncRNA DLEU2 up-regulated YWHAZ by competitively binding to miR-212-5p. (a) TargetScan v7.2 (http://www.targetscan.org/vert_72/) and (b) dual luciferase reporter assay were performed to analyze the targeting relationship between miR-212-5p and YWHAZ. (c) YWHAZ was up-regulated in atherosclerotic serum (n = 34) compared to normal serum (n = 20). (d-e) Pearson correlation method was used to analyze the correlation between YWHAZ and DLEU2 or miR-212-5p in atherosclerotic serum (n = 34). (f-h) Western blot and RT-qPCR were carried out to determine YWHAZ expression. β-actin was used as a control. *P < 0.05, **P < 0.01, ***P < 0.001 vs. PDGF-BB, ^^^P < 0.001 vs. PDGF-BB+DLEU2+ miR-NC, #P < 0.05, ##P < 0.01 vs. PDGF-BB+M; †††P < 0.001 vs. miR-NC. All experiments were repeated at least three times. Data were expressed as mean ± standard deviation. Data between two groups or among multiple groups were analyzed by independent sample t test or one-way ANOVA, followed by Tukey’s post hoc test.
DLEU2 modulated PDGF-BB-induced VSMC viability and migration through miR-212-5p/YWHAZ axis
Overexpressed YWHAZ partially offset the inhibitory effects of miR-212-5p on YWHAZ expression, cell viability, migration and invasion, and siYWHAZ overtly neutralized the promoting effects of DLUE2 on the same aspects (P < 0.001, Figure 6(a-c); p < 0.05, Figure 6(d,f), Figure 7(a,b)). Additionally, the regulatory effects of miR-212-5p mimic and DLUE2 on PCNA, α-SMA and Calponin levels in PDGF-BB-induced VSMCs were reversed by overexpressed YWHAZ and siYWHAZ, respectively (P < 0.05, Figure 7(c-e)).
Figure 6.
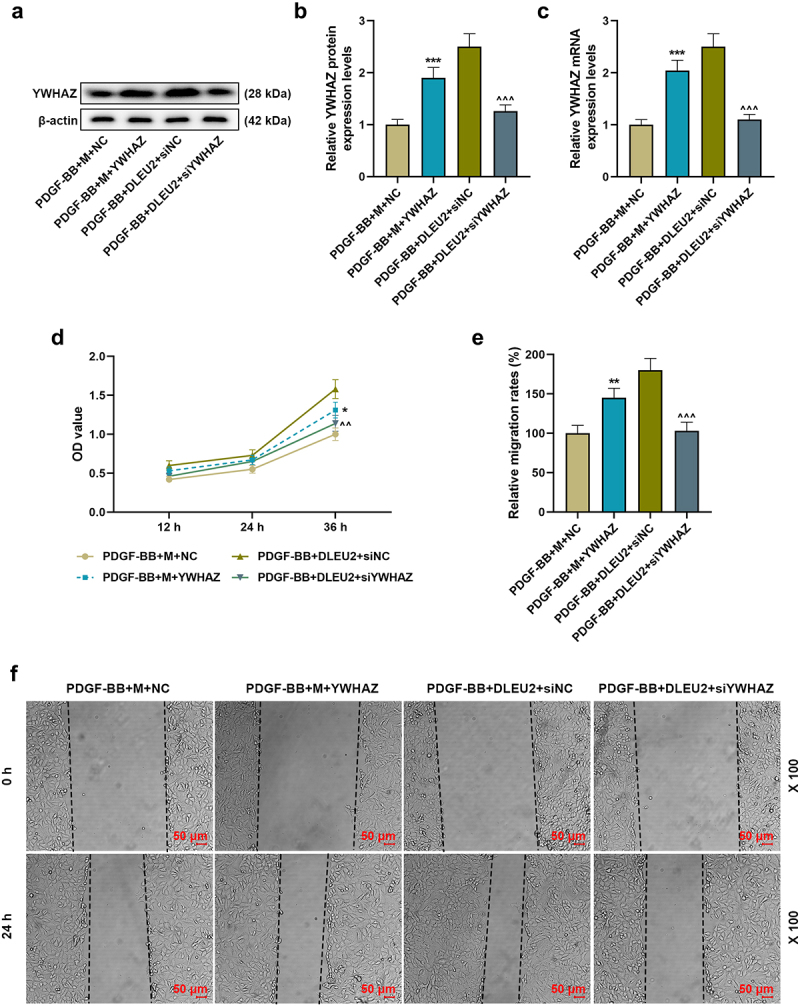
YWHAZ attenuated the effects of miR-212-5p mimic on the viability and migration of PDGF-BB-induced VSMCs, while siYWHAZ reversed the effects of overexpressed DLEU2. (a-c) The expression of YWHAZ in PDGF-BB+mimic (m) +NC, PDGF-BB+M+ YWHAZ, PDGF-BB+DLEU2+ siNC and PDGF-BB+DLEU2+ siYWHAZ groups was detected by RT-qPCR and Western blot. β-actin was used as a control. (d) YWHAZ overexpression partially reversed the inhibitory effect of miR-212-5p on cell viability, and siYWHAZ offset the promoting effect of DLUE2 on cell viability, which was confirmed by CCK-8 assay. (e-f) Wound healing assay was performed to measure the migration ability of cells in each group. *P < 0.05, **P < 0.01, ***P < 0.001 vs. PDGF-BB+M+ NC; ^^P < 0.01, ^^^P < 0.001 vs. PDGF-BB+DLEU2+ siNC. All experiments were repeated at least three times. Data were expressed as mean ± standard deviation. Data among multiple groups were analyzed by one-way ANOVA, followed by Tukey’s post hoc test.
Figure 7.
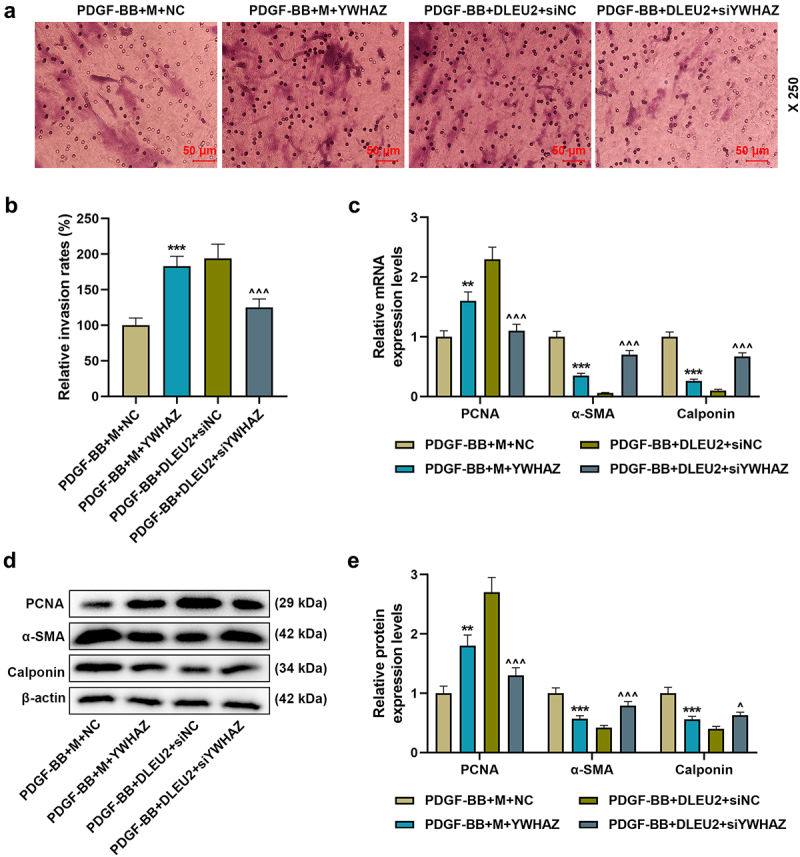
DLEU2 affected PDGF-BB-induced VSMC migration and the expressions of PCNA, α-SMA and Calponin through miR-212-5p/YWHAZ axis. (a-b) The invasion rate of cells in PDGF-BB+M+ NC, PDGF-BB+M+ YWHAZ, PDGF-BB+DLEU2+ siNC, and PDGF-BB+DLEU2+ siYWHAZ groups was assessed by Transwell assay. (c-e) The expressions of PCNA, α-SMA and Calponin were analyzed by RT-qPCR and Western blot. β-actin was used as a control. **P < 0.01, ***P < 0.001 vs. PDGF-BB+M+ NC; ^P < 0.05, ^^^P < 0.001 vs. PDGF-BB+DLEU2+ siNC. All experiments were repeated at least three times. Data were expressed as mean ± standard deviation. Data among multiple groups were analyzed by one-way ANOVA, followed by Tukey’s post hoc test.
Discussion
The proliferation and migration of VSMCs are crucial events in the pathogenesis of intimal hyperplasia, a key factor of atherosclerosis [8]. Thus, the proliferation and migration of VSMCs are important pathological changes of atherosclerosis [5,6]. In the present study, DLEU2 modulates PDGF-BB-induced proliferation and migration of VSMCs via miR-212-5p/YWHAZ axis. DLEU2 may be considered as a promising therapeutic target in atherosclerosis treatment.
In recent years, the involvement of some lncRNAs has been identified in regulating the proliferation and migration of VSMCs induced by PDGF-BB, such as growth arrest-specific transcript 5 (GAS5), predicting cardiac remodeling (LIPCAR), colorectal neoplasia differentially expressed (CRNDE) and so on [18–20]. Specifically, the overexpression of CAMK2D-associated transcript 1 (C2dat1) accelerated the growth and migration of VSMCs and enhanced the expression of PCNA [21]. In the present study, we first revealed that DLEU2 expression was abnormally elevated in the serum of atherosclerosis patients and PDGF-BB-induced VSMCs. Moreover, overexpressed DLEU2 enhanced the effects of PDGF-BB on promoting viability, migration and invasion of VSMCs, while silent DLEU2 produced the opposite effects. DLEU2 is an oncogene in most cancers such as non-small cell lung cancer, and has the effect of accelerating the malignant phenotype of cells [22]. Our research demonstrated that the role of DLEU2 in PDGF-BB-induced VSMCs was similar to its role in cancer, both enhancing the biological characteristics of cells.
MiR-212-5p inhibited proliferation and migration of angiotensin II–induced VSMCs [23]. Zhang et al. analyzed the role of YWHAZ in PDGF-BB treated VSMCs [8], unveiling that overexpression of YWHAZ partially weakened the inhibitory effect of miR-451 up-regulation on PDGF-BB-induced VSMC damage, which implied that YWHAZ could promote PDGF-BB-induced VSMC injury in atherosclerosis [8]. Existing studies revealed that lncRNAs could act as miRNA molecular sponges, inhibit the binding of miRNA and mRNA, and ultimately lead to up-regulation of mRNA [24,25]. DLEU2 can act as a competing endogenous RNA (ceRNA) to promote cancer progression by regulating miRNA/mRNA expression in non-small cell lung cancer [22], human acute myeloid leukemia [26] and gastric cancer [27]. Interestingly, we discovered that DLEU2 could serve as a ceRNA to competitively bind to miR-212-5p, thereby regulating YWHAZ expression. The present results indicated that DLEU2 can act as a ceRNA to promote cell viability, migration and invasion of PDGF-BB-stimulated VSMCs by regulating miR-212-5p/ YWHAZ expression.
PCNA is closely related to DNA synthesis in cells, and is a good indicator of cell proliferation [28]. As mentioned in the introduction, the phenotypic transition of VSMCs is a pathological process in atherosclerotic events. α-SMA and Calponin are typical markers of myoepithelial cells, which are usually highly expressed in normal adult blood vessels [29,30]. When blood vessels are damaged, the phenotype of VSMCs changes rapidly, leading to the inhibition of α-SMA and Calponin [31]. Our study proved that miR-212-5p mimic can restore DLEU2 overexpression-caused down-regulations of α-SMA and Calponin and up-regulation of PCNA in PDGF-BB-induced VSMCs. Our study found that YWHAZ was targeted by miR-212-5p, and proved that DLEU2 up-regulated YWHAZ through suppressing miR-212-5p, thereby triggering the proliferation and biological phenotype of VSMCs. In our study, the effect of YWHAZ on the biological characteristics of PDGF-BB-treated VSMCs was consistent with the findings from the report of Zhang et al. [8], but the difference lied in that DLEU2-miR-212-5p-YWHAZ axis was reported for the first time.
Conclusion
In summary, DLEU2 is high-expressed in atherosclerosis and PDGF-BB-induced VSMCs, and accelerates PDGF-BB-induced VSMC viability, migration and invasion, at least in part, through suppressing miR-212-5p to up-regulate YWHAZ level. Therefore, DLEU2 may function as a biomarker for the diagnosis and treatment of atherosclerosis. In the future, we will further study the impact of this pathway on the animal models.
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Authors’ contributions
Substantial contributions to conception and design: Zhiying Zhao
Data acquisition, data analysis and interpretation: Guangming Zhang, Jing Yang, Rui Lu, Haijuan Hu
Drafting the article or critically revising it for important intellectual content: Zhiying Zhao
Final approval of the version to be published: Zhiying Zhao, Guangming Zhang, Jing Yang, Rui Lu, Haijuan Hu
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of the work are appropriately investigated and resolved: Zhiying Zhao, Guangming Zhang, Jing Yang, Rui Lu, Haijuan Hu
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
References
- [1].Hansson GK, Hermansson A.. The immune system in atherosclerosis. Nat Immunol. 2011. Mar;12(3):204–212. PubMed PMID: 21321594. [DOI] [PubMed] [Google Scholar]
- [2].Frostegard J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013. May 1;11:117. PubMed PMID: 23635324; PubMed Central PMCID: PMCPMC3658954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bennett MR, Sinha S, Owens GK. Vascular smooth muscle cells in atherosclerosis. Circ Res. 2016. Feb 19;118(4):692–702. PubMed PMID: 26892967; PubMed Central PMCID: PMCPMC4762053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Majesky MW. Vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2016. Oct;36(10):e82–6. PubMed PMID: 27655780; PubMed Central PMCID: PMCPMC5102260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Grootaert MOJ, Moulis M, Roth L, et al. Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis. Cardiovasc Res. 2018. Mar 15;114(4):622–634. PubMed PMID: 29360955. [DOI] [PubMed] [Google Scholar]
- [6].Hu D, Yin C, Luo S, et al. Vascular smooth muscle cells contribute to atherosclerosis immunity. Front Immunol. 2019;10:1101. PubMed PMID: 31164888; PubMed Central PMCID: PMCPMC6534067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Togliatto G, Dentelli P, Rosso A, et al. PDGF-BB carried by endothelial cell-derived extracellular vesicles reduces vascular smooth muscle cell apoptosis in diabetes. Diabetes. 2018. Apr;674:704–716. PubMed PMID: 29386225 [DOI] [PubMed] [Google Scholar]
- [8].Zhang W, Liu D, Han X, et al. MicroRNA-451 inhibits vascular smooth muscle cell migration and intimal hyperplasia after vascular injury via Ywhaz/p38 MAPK pathway. Exp Cell Res. 2019. Jun 15;379(2):214–224. PubMed PMID: 30930138. [DOI] [PubMed] [Google Scholar]
- [9].Dong X, Hu H, Fang Z, et al. CTRP6 inhibits PDGF-BB-induced vascular smooth muscle cell proliferation and migration. Biomed Pharmacother. 2018. Jul;103:844–850. PubMed PMID: 29710500 [DOI] [PubMed] [Google Scholar]
- [10].Poller W, Dimmeler S, Heymans S, et al. Non-coding RNAs in cardiovascular diseases: diagnostic and therapeutic perspectives. Eur Heart J. 2018. Aug 1;39(29):2704–2716. PubMed PMID: 28430919; PubMed Central PMCID: PMCPMC6454570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wang Y, Song X, Li Z, et al. Long non-coding RNAs in coronary atherosclerosis. Life Sci. 2018. Oct 15;211:189–197. PubMed PMID: 30195033. [DOI] [PubMed] [Google Scholar]
- [12].Wu G, Cai J, Han Y, et al. LincRNA-p21 regulates neointima formation, vascular smooth muscle cell proliferation, apoptosis, and atherosclerosis by enhancing p53 activity. Circulation. 2014. Oct 21;130(17):1452–1465. PubMed PMID: 25156994; PubMed Central PMCID: PMCPMC4244705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Shan K, Jiang Q, Wang XQ, et al. Role of long non-coding RNA-RNCR3 in atherosclerosis-related vascular dysfunction. Cell Death Dis. 2016. Jun 2;7(6)e2248. PubMed PMID: 27253412; PubMed Central PMCID: PMCPMC5143375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Arslan S, Berkan O, Lalem T, et al. Long non-coding RNAs in the atherosclerotic plaque. Atherosclerosis. 2017. Nov;266:176–181. PubMed PMID: 29035780 [DOI] [PubMed] [Google Scholar]
- [15].Xu B, Gong X, Zi L, et al. Silencing of DLEU2 suppresses pancreatic cancer cell proliferation and invasion by upregulating microRNA-455. Cancer Sci. 2019. May;110(5):1676–1685. PubMed PMID: 30838724; PubMed Central PMCID: PMCPMC6501038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Xie ZZ, Xiao ZC, Song YX, et al. Long non-coding RNA Dleu2 affects proliferation, migration and invasion ability of laryngeal carcinoma cells through triggering miR-16-1 pathway. Eur Rev Med Pharmacol Sci. 2018. Apr;227:1963–1970. PubMed PMID: 29687850 [DOI] [PubMed] [Google Scholar]
- [17].Guo Y, Bai M, Lin L, et al. LncRNA DLEU2 aggravates the progression of hepatocellular carcinoma through binding to EZH2. Biomed Pharmacother. 2019. Oct;118:109272. PubMed PMID: 31376657 [DOI] [PubMed] [Google Scholar]
- [18].Liu K, Liu C, Zhang Z. lncRNA GAS5 acts as a ceRNA for miR-21 in suppressing PDGF-bb-induced proliferation and migration in vascular smooth muscle cells. J Cell Biochem. 2019. Sep;120(9):15233–15240. PubMed PMID: 31069831. [DOI] [PubMed] [Google Scholar]
- [19].Wang X, Li D, Chen H, et al. Expression of long noncoding RNA LIPCAR promotes cell proliferation, cell migration, and change in phenotype of vascular smooth muscle cells. Med Sci Monit. 2019. Oct 11;25:7645–7651. PubMed PMID: 31603865; PubMed Central PMCID: PMCPMC6800467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhou Y, He X, Liu R, et al. LncRNA CRNDE regulates the proliferation and migration of vascular smooth muscle cells. J Cell Physiol. 2019. Feb 10; PubMed PMID: 30740670. DOI: 10.1002/jcp.28284. [DOI] [PubMed] [Google Scholar]
- [21].Wang H, Jin Z, Pei T, et al. Long noncoding RNAs C2dat1 enhances vascular smooth muscle cell proliferation and migration by targeting MiR-34a-5p. J Cell Biochem. 2019. Mar;120(3):3001–3008. PubMed PMID: 30474870 [DOI] [PubMed] [Google Scholar]
- [22].Zhou Y, Shi H, Du Y, et al. lncRNA DLEU2 modulates cell proliferation and invasion of non-small cell lung cancer by regulating miR-30c-5p/SOX9 axis. Aging (Albany NY). 2019. Sep 20;11(18):7386–7401. PubMed PMID: 31541993; PubMed Central PMCID: PMCPMC6781974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Deng JH, Zheng GY, Li HZ, et al. MiR-212-5p inhibits the malignant behavior of clear cell renal cell carcinoma cells by targeting TBX15. Eur Rev Med Pharmacol Sci. 2019. Dec;2324:10699–10707. PubMed PMID: 31858538 [DOI] [PubMed] [Google Scholar]
- [24].Qi X, Zhang DH, Wu N, et al. ceRNA in cancer: possible functions and clinical implications. J Med Genet. 2015. Oct;5210:710–718. PubMed PMID: 26358722 [DOI] [PubMed] [Google Scholar]
- [25].Ballantyne MD, McDonald RA, Baker AH. lncRNA/MicroRNA interactions in the vasculature. Clin Pharmacol Ther. 2016. May;99(5):494–501. PubMed PMID: 26910520; PubMed Central PMCID: PMCPMC4881297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wu DM, Wen X, Han XR, et al.Role of Circular RNA DLEU2 in Human Acute Myeloid Leukemia. Mol Cell Biol. 2018. Oct 15; 38(20). PubMed PMID: 30037980; PubMed Central PMCID: PMCPmc6168983. eng. DOI: 10.1128/mcb.00259-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Han S, Qi Y, Xu Y, et al. lncRNA DLEU2 promotes gastric cancer progression through ETS2 via targeting miR-30a-5p. Cancer Cell Int. 2021. Jul 14;21(1):376. PubMed PMID: 34261460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Choe KN, Moldovan GL. Forging ahead through darkness: PCNA, still the principal conductor at the replication fork. Mol Cell. 2017. Feb 2;65(3):380–392. PubMed PMID: 28157503; PubMed Central PMCID: PMCPMC5302417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Arnoldi R, Hiltbrunner A, Dugina V, et al. Smooth muscle actin isoforms: a tug of war between contraction and compliance. Eur J Cell Biol. 2013. Jun-Jul;926–7:187–200. PubMed PMID: 23915964 [DOI] [PubMed] [Google Scholar]
- [30].Salabei JK, Cummins TD, Singh M, et al. PDGF-mediated autophagy regulates vascular smooth muscle cell phenotype and resistance to oxidative stress. Biochem J. 2013. May 1;451(3):375–388. PubMed PMID: 23421427; PubMed Central PMCID: PMCPMC4040966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wang TM, Chen KC, Hsu PY, et al. microRNA let-7g suppresses PDGF-induced conversion of vascular smooth muscle cell into the synthetic phenotype. J Cell Mol Med. 2017. Dec;2112:3592–3601. PubMed PMID: 28699690; PubMed Central PMCID: PMCPMC5706591 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.


