PURPOSE
To examine the prevalence and dynamics of circulating tumor DNA (ctDNA) and its association with metastatic recurrence in patients with high-risk early-stage hormone receptor–positive breast cancer (HR+ BC) more than 5 years from diagnosis.
METHODS
We enrolled 103 patients with high-risk stage II-III HR+ BC diagnosed more than 5 years prior without clinical evidence of recurrence. We performed whole-exome sequencing (WES) on primary tumor tissue to identify somatic mutations tracked via a personalized, tumor-informed ctDNA test to detect minimal residual disease (MRD). We collected plasma at the time of consent and at routine visits every 6-12 months. Patients were followed for clinical recurrence.
RESULTS
In total, 85 of 103 patients had sufficient tumor tissue; of them, 83 of 85 (97.6%) patients had successful whole-exome sequencing. Personalized ctDNA assays were designed targeting a median of 36 variants to test 219 plasma samples. The median time from diagnosis to first sample was 8.4 years. The median follow-up was 10.4 years from diagnosis and 2.0 years from first sample. The median number of plasma samples per patient was two. Eight patients (10%) had positive MRD testing at any time point. Six patients (7.2%) developed distant metastatic recurrence, all of whom were MRD-positive before overt clinical recurrence, with median ctDNA lead time of 12.4 months. MRD was not identified in one patient (1.2%) with local recurrence. Two of eight MRD-positive patients had not had clinical recurrence at last follow-up.
CONCLUSION
In this prospective study, in patients with high-risk HR+ BC in the late adjuvant setting, ctDNA was identified a median of 1 year before all cases of distant metastasis. Future studies will determine if ctDNA-guided intervention in patients with HR+ BC can alter clinical outcomes.
INTRODUCTION
Breast cancer is the most commonly diagnosed cancer worldwide.1 Most breast cancers, nearly 80%, are hormone receptor–positive (HR+).2 Unlike other subtypes of breast cancers in which risk of recurrence decreases 5 years after diagnosis, the recurrence risk for HR+ breast cancer remains steady from 5 years to at least 20 years with some patients experiencing recurrence three decades after diagnosis.3,4 In some high-risk patients, breast cancer–specific mortality over 5 years after diagnosis exceeds 30%.5 For patients with late recurrences, 10-year breast cancer–specific mortality approaches 50%.6 Although about 6% of patients with breast cancer are diagnosed with de novo metastatic breast cancer, most are diagnosed with earlier-stage disease and treated with curative-intent local and systemic therapy.7 Of more than 150,000 patients currently living with metastatic breast cancer, approximately three quarters initially presented with early-stage disease.8
CONTEXT
Key Objective
Most recurrences of hormone receptor–positive breast cancers (HR+ BCs) occur in the late adjuvant setting. Clinicopathologic tools in routine practice are insufficient to determine who remains at high risk of late recurrence. Detection of minimal residual disease (MRD) via plasma circulating tumor DNA (ctDNA) is promising yet not well understood in this population. To our knowledge, this is the first prospective investigation of plasma ctDNA analysis and clinical outcomes in late adjuvant HR+ BC.
Knowledge Generated
In 83 patients with high-risk HR+ BC at least 5 years after diagnosis, tumor-informed liquid biopsy identified MRD in eight patients (10%). ctDNA identified MRD before all cases of distant metastatic recurrence with a median lead time of 12.4 months.
Relevance
MRD detection in the late adjuvant setting of HR+ BC could inform future study of liquid biopsy to personalize treatment and prevent or delay late recurrence of early-stage breast cancer.
Most distant recurrences of HR+ breast cancers occur more than 5 years after initial diagnosis.4,9 Distant recurrence after resection of primary tumor arises from residual disease not detected in current practice via imaging, laboratory tests, or clinical assessment. The benefits of adjuvant chemotherapy and hormonal therapy in preventing recurrence are well-established, demonstrating the importance of treating residual disease.3,10,11 Adjuvant treatment for patients with HR+, human epidermal growth factor receptor 2–negative (HER2–) breast cancer is tailored on the basis of assessment of recurrence risk using clinical features and tumor molecular testing before or at the time of surgery. In routine clinical practice, recurrence risk is not reliably reassessed after the perioperative period.12
Detection of minimal residual disease (MRD) via plasma circulating tumor DNA (ctDNA) is associated with high risk of breast cancer recurrence, yet little is known about ctDNA in the late adjuvant setting in HR+ breast cancer.13-19 In one cohort of 49 patients up to 4 years after definitive treatment, including 34 with HR+/HER2– disease, investigators identified ctDNA in 16 of 18 patients with relapsed breast cancer with lead time up to 2 years.14 In another cohort of 84 patients from the I-SPY-2 trial, including 29 HR+/HER2– patients, detection of ctDNA after neoadjuvant chemotherapy was associated with similarly high risk of metastatic recurrence.15
Some patients with high-risk HR+ breast cancer remain on adjuvant endocrine therapy (ET) for up to 10 years or longer, although the impact of treatment beyond 5 years is modest.20,21 Accurate detection of MRD has the potential to inform whether to continue, change, or stop adjuvant therapy to maximize duration of disease-free status while minimizing therapy toxicity in patients for whom optimal benefit may have already been achieved.22 As patients with HR+ breast cancer remain at risk for many years, it is possible that ctDNA detection in the late adjuvant setting could facilitate early identification and treatment of MRD, preventing or delaying recurrence. A foundational understanding of ctDNA characteristics in this population is necessary to inform the role of ctDNA in interventions to improve personalized adjuvant treatment and patient outcomes. Here, we report a prospective investigation of ctDNA dynamics and clinical outcomes in 83 patients in the late adjuvant setting—defined here as 5 years or more after diagnosis—of high-risk, early-stage HR+ breast cancer.
METHODS
Patients and Samples
Using detailed tumor and outcomes data collected on patients with newly diagnosed breast cancer (BC) from 1997 to 2012 as part of the National Comprehensive Cancer Network Breast Cancer Outcomes Database, we prospectively identified and consented, between March 2018 and December 2020, patients receiving care at Dana-Farber Cancer Institute with a history of stage II or III HR+/HER2– BC diagnosed at least 5 years prior with no known cancer recurrence at the time of study entry.23 We requested primary tumor tissue for patients determined to be high-risk, specifically staged as T3-T4, N2-N3, T1-T2 with at least three involved lymph nodes, or T2N1 with oncotype DX risk score of 26 or higher, Prosigna (PAM50) high-risk score of 41 or higher, EndoPredict score of 3.3 or higher, Mammaprint high-risk categorization, grade 3 on the Bloom-Richardson grading system, or Ki-67 of 20% or more on central analysis. Pathologic stage was used for patients who received up-front surgery while clinical stage was used for patients who received neoadjuvant systemic therapy. This study was approved by the Dana-Farber/Harvard Cancer Center Institutional Review Board. All patients provided informed consent.
Patients were followed for recurrence by their clinicians using routine follow-up visits and breast imaging per standard of care. Serial blood samples were collected (30 mL in Streck tubes) every 6-12 months at time of scheduled patient visits. The date of clinical recurrence was the date of biopsy-proven metastasis, when available, or the date of imaging confirming metastatic disease. Patient characteristics including age, sex, curative-intent systemic therapy (including neoadjuvant and adjuvant chemotherapy and/or ET), surgeries, pathology, radiation, and imaging were abstracted from the electronic health record with patient consent.
Sequencing and Data Analysis
Archival tumor tissue was obtained from initial breast cancer surgery. Formalin-fixed paraffin-embedded tissue block and Hematoxylin & Eosin slide (or 10-20 unstained slides and Hematoxylin & Eosin slide) were sent to Inivata Inc (Durham, NC) where DNA was extracted and whole-exome sequencing (WES) performed as previously described.24,25 Detection of somatic mutations was used to design, for each patient, a personalized ctDNA RaDaR assay as previously described (Fig 1A).24-26 WES data were reanalyzed as previously described to identify mutations in oncogenes for the comutation plot.27,28
FIG 1.
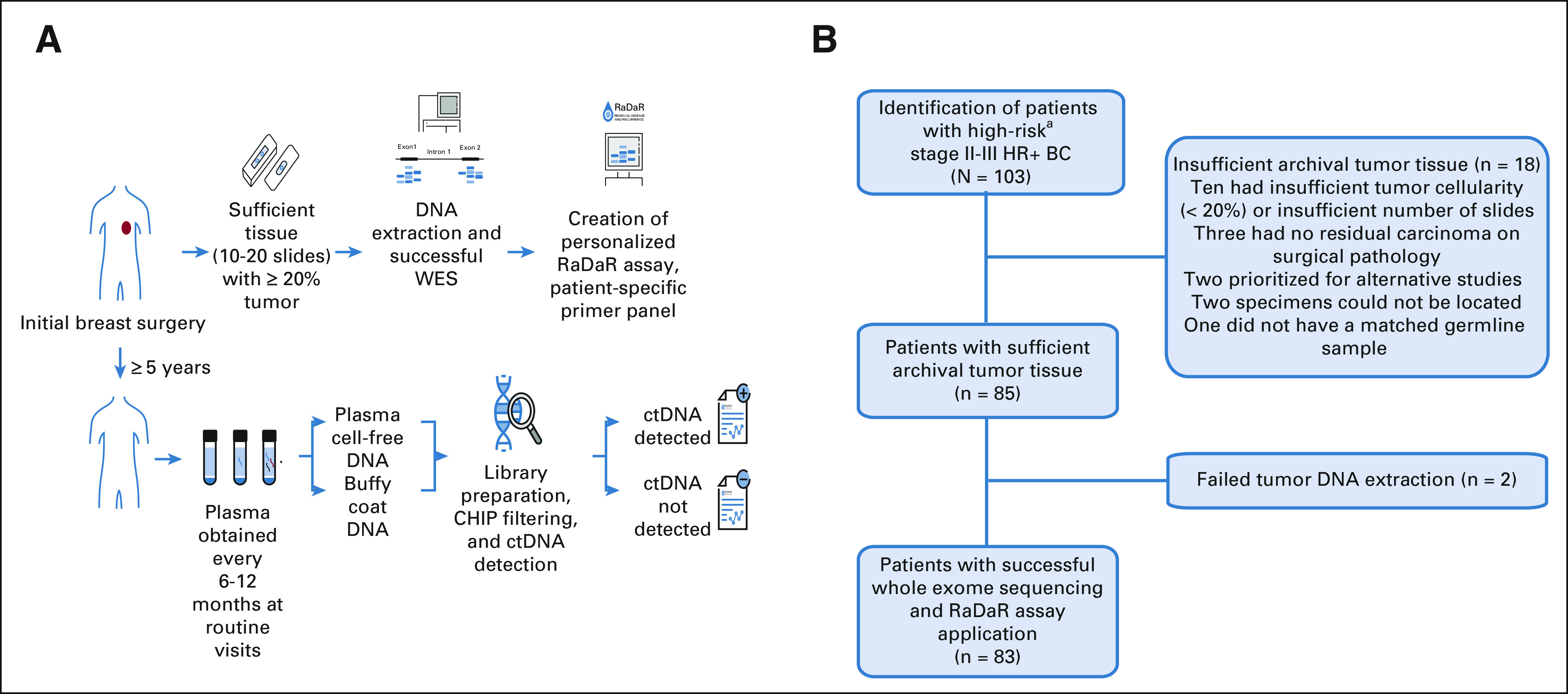
(A) RaDaR assay process. Primary tumor tissue from initial breast cancer surgery was obtained and assessed. For specimens with sufficient tissue (10-20 slides with 20% or higher tumor cellularity), DNA was extracted, and WES was performed to identify somatic mutations used to create personalized RaDaR assays for each patient. Patients were enrolled over 5 years after initial diagnosis, and every 6-12 months at routine follow-up visits, plasma was obtained, and the personalized RaDaR assay for ctDNA detection was applied. (B) CHiRP study flow chart. Patients with high-risk (T3/T4 and/or N2/N3; T1N1 and ≥ 3 involved lymph nodes; or T2N1 if: Ki-67 ≥ 20%, grade 3, or oncotype DX score ≥ 26) HR+ BC provided consent to obtain archival primary tumor tissue, and WES was performed. Patients were excluded from the analysis if there was insufficient primary tumor tissue or if WES was unsuccessful because of failed tumor DNA extraction. aHigh-risk: T3/T4 and/or N2/N3; T1N1 and ≥ 3 involved lymph nodes; or T2N1 if: Ki-67 ≥ 20%, grade 3, or oncotype DX score ≥ 26. CHIP, clonal hematopoiesis of indeterminate potential; CHiRP, circulating tumor DNA (ctDNA) and late recurrence in high-risk hormone receptor-positive, HER2-negative breast cancer; ctDNA, circulating tumor DNA; HR+ BC, hormone receptor–positive breast cancer; WES, whole-exome sequencing.
Blood samples were collected prospectively, spun, and stored at –80°C as plasma and buffy coat. Four milliliter of plasma and 2 mL of buffy coat samples were sent in batches to Inivata Inc. DNA was extracted from buffy coat, and circulating cell-free DNA was extracted from plasma as previously described.24 RaDaR assays were applied retrospectively in a research setting to identify plasma ctDNA (Appendix 1 and Appendix Figs A1 and A2, online only). Because this testing was performed retrospectively for batched samples and designated as research, patients and their care teams were not informed of results.
Statistical Analysis
The primary objective of this study was to characterize the presence and features of MRD in high-risk HR+ breast cancer and variability over time. Secondary objectives included assessment of recurrence-free survival (RFS) in relation to ctDNA detection. All clinical information was deidentified before analysis. Swimmer and comutation plots were created using R technology.29,30 Frequency of commonly mutated oncogenes in advanced HR+ BCs was assessed.31 Lead time was calculated as the time from first positive ctDNA sample to clinical recurrence. RFS was defined as time from surgery to clinical recurrence and was analyzed using the Kaplan-Meier method. To estimate risk of late recurrence, the Clinical Treatment Score post 5 years (CTS5) was calculated using pathological assessment of largest tumor size, tumor grade, and number of involved lymph nodes.32 For patients treated with neoadjuvant chemotherapy, largest size of tumor on imaging, tumor grade from biopsy, and number of lymph nodes with invasive carcinoma or treatment effect were used for CTS5 calculation.
RESULTS
Patient Baseline Characteristics
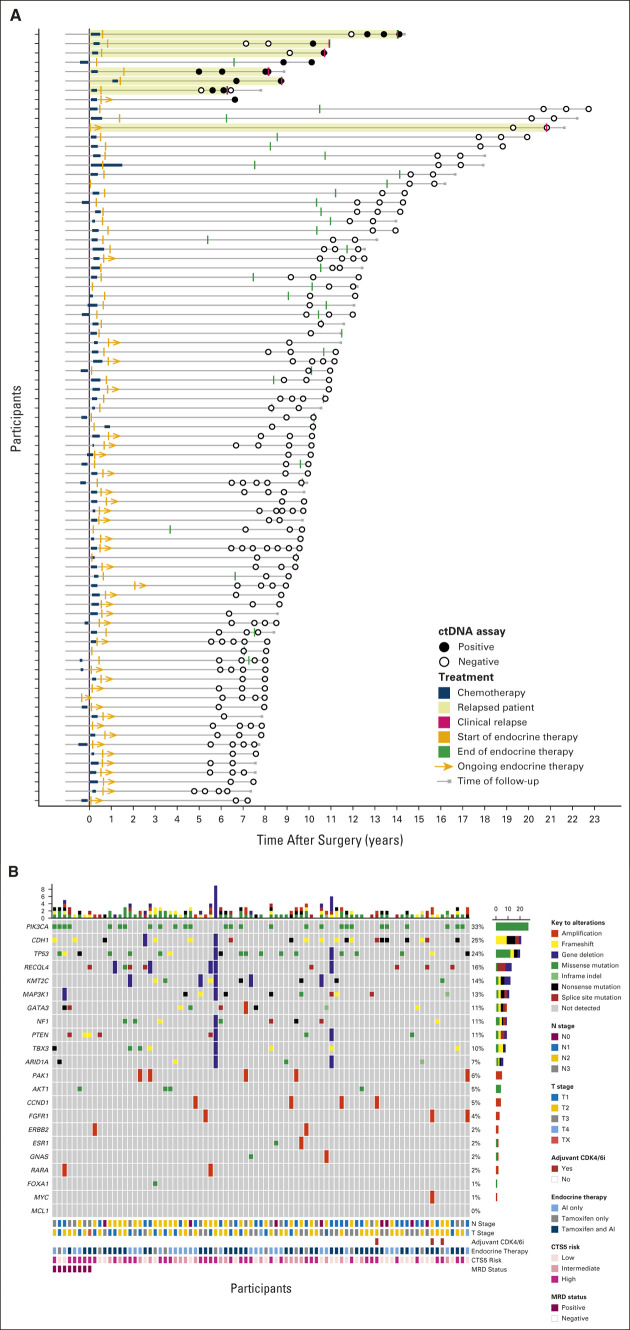
Of 103 patients enrolled, 85 patients had sufficient tissue for sequencing with at least 20% tumor present (Fig 1B). Eighty-three patients had primary tumor tissue that underwent successful WES and comprised the analytic cohort, with a median age of 53 years (range 29-71 years) at initial diagnosis. All were female. Most patients (57, 68.7%) had stage III disease, and most (75, 90.4%) received chemotherapy (Table 1). Of those who received chemotherapy, 17 of 75 (22.7%) had neoadjuvant and 58 of 75 (77.3%) had adjuvant chemotherapy. Thirty-two patients (38.6%) had breast conserving surgery and radiation and 51 (61.4%) had mastectomy. Of the patients who underwent mastectomy, 46 of 51 (90%) also had radiation therapy. All patients in the cohort received ET. Thirty-eight (45.8%) remained on adjuvant ET at the time of last follow-up (Fig 2A). Of 45 patients who had completed adjuvant ET, most (42, 93.3%) received more than 5 years of treatment. The median clinical follow-up was 10.4 years (range 6.7-22.8 years) from diagnosis and 2 years (0-3.9 years) from first plasma sample collected on study.
TABLE 1.
Baseline Characteristics of Patients With High-Risk HR+/HER2– Breast Cancer According to MRD Status
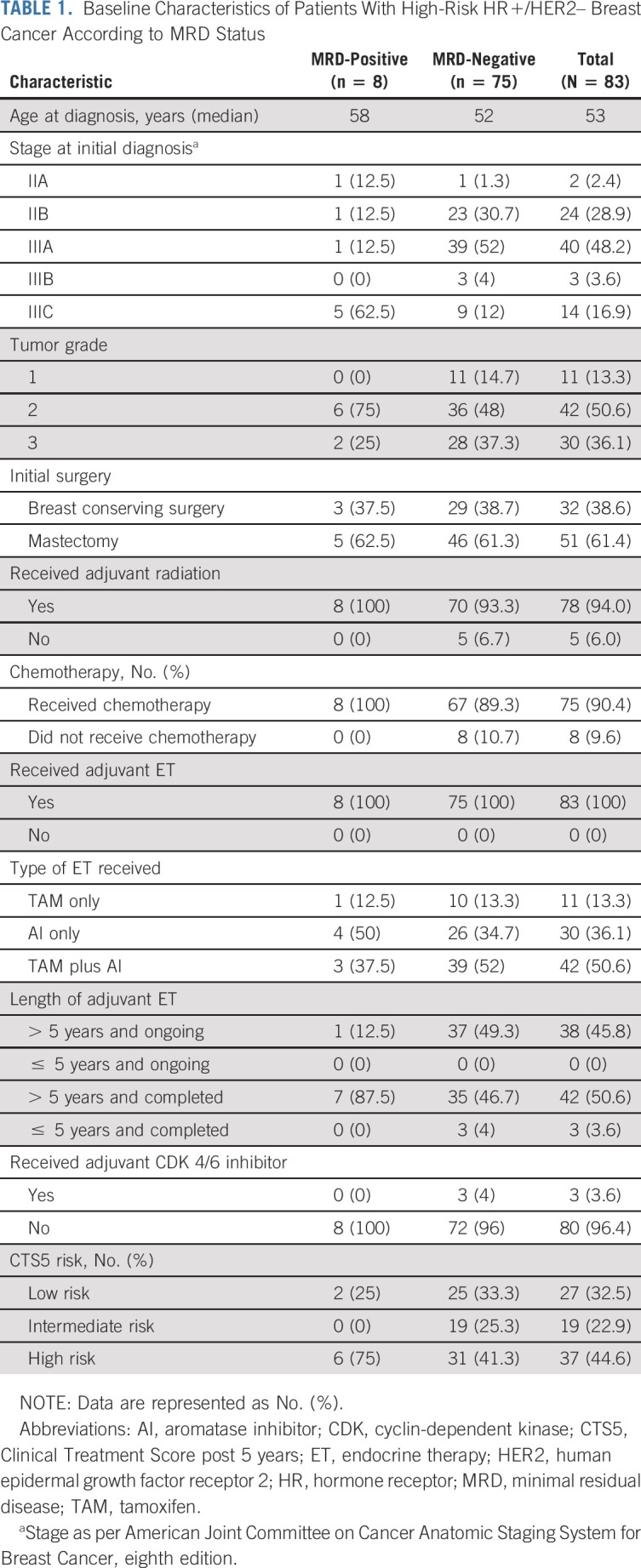
FIG 2.
(A) Swimmer plot depicting patients' disease courses, including treatment and relapse and ctDNA assay samples and results. (B) Comutation plot depicting the frequency of common alterations in hormone receptor–positive breast cancers is presented here along with nodal stage, tumor stage, whether patients received adjuvant CDK 4/6 inhibitor, details regarding type of endocrine therapy received, CTS5 score, and MRD status. AI, aromatase inhibitor; CDK 4/6, cyclin-dependent kinase; ctDNA, circulating tumor DNA; Clinical Treatment Score post-5 years; MRD, minimal residual disease; TAM, tamoxifen.
Detection of ctDNA in the Late Adjuvant Setting and Association With Clinical Recurrence
A median of 33 single nucleotide variants per patient was identified (range, 9-50; Data Supplement, online only). The prevalence of frequent somatic mutations in HR+ BCs, along with clinicopathologic characteristics, was reanalyzed and are included in Figure 2B. All 83 patients provided at least one plasma sample, and 74 patients provided samples at multiple time points, with a median number of samples per patient of 2 (range 1-7) and a total of 219 samples. Time from diagnosis to first sample ranged from 4.9 to 20 years (median 8.4 years). Personalized RaDaR assays were designed and applied with 12-51 variants included (median 36; Data Supplement).
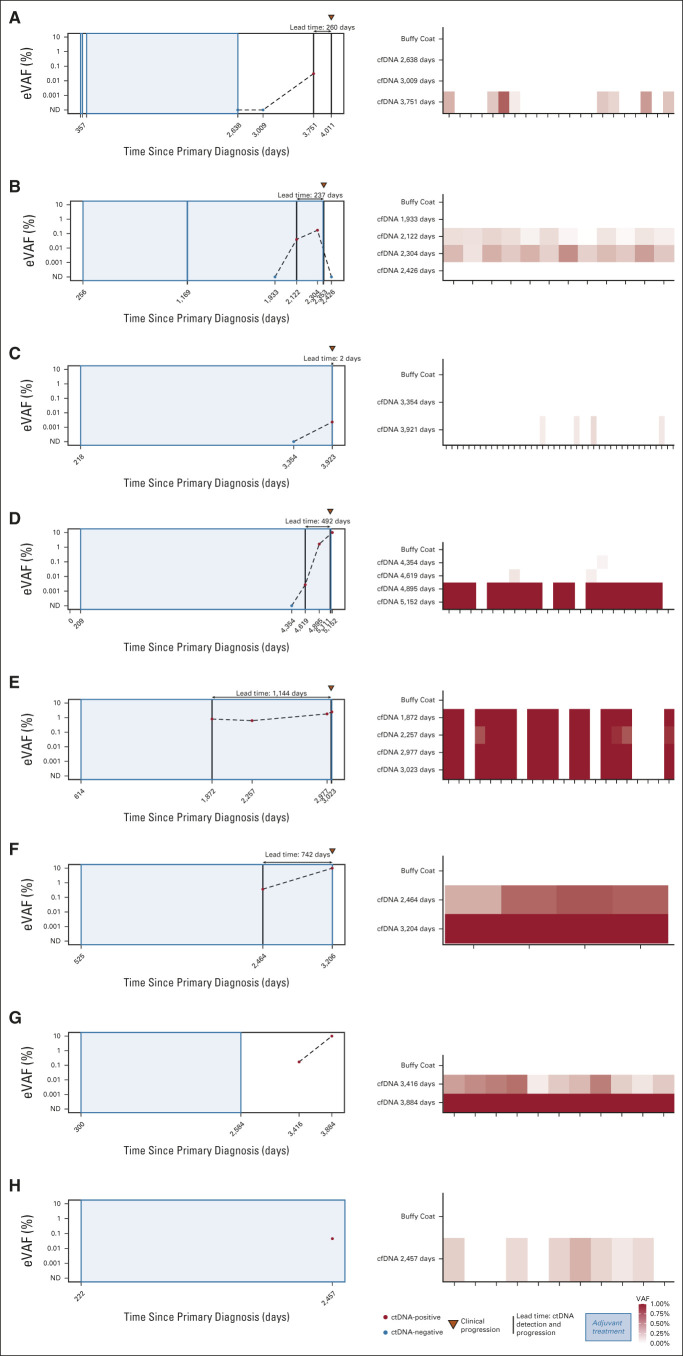
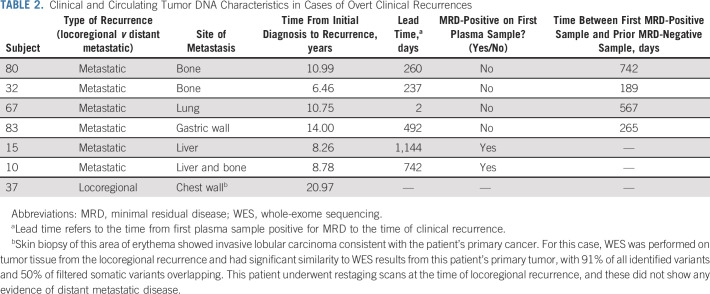
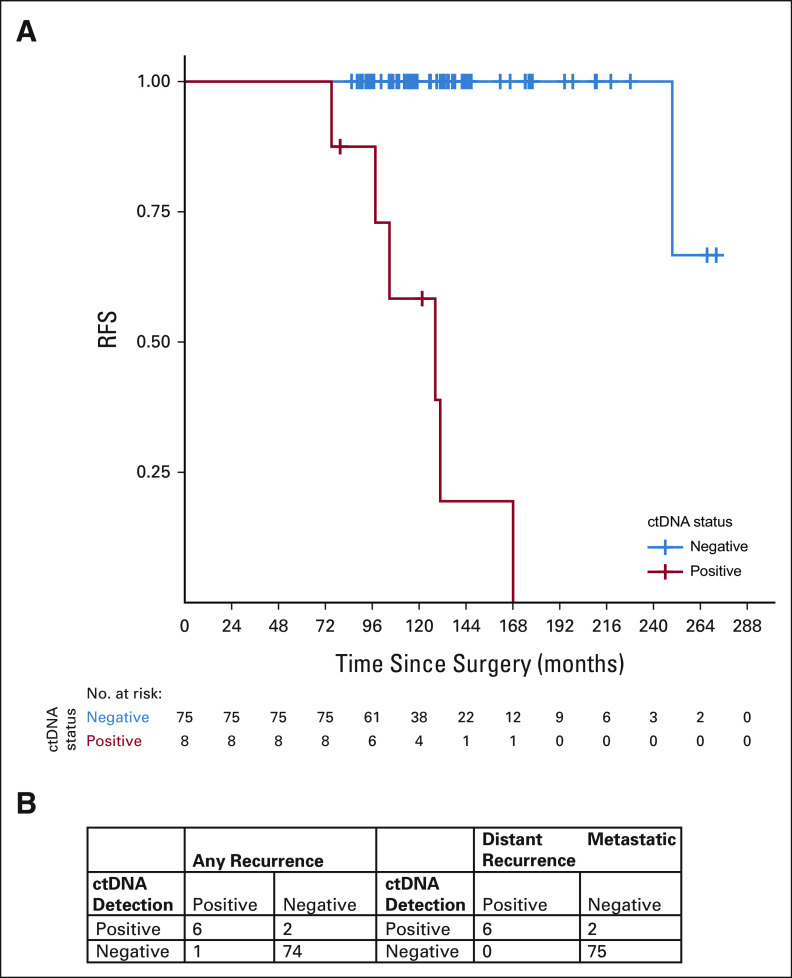
Eight patients (10%) had positive MRD testing at any time point, four of whom (5%) had positive MRD testing at study entry. ctDNA was detected at 0.0023%‐0.8019% (median 0.0425%; Fig 3; Data Supplement). Six patients (7.2%) developed distant metastatic recurrence (with metastatic sites including bone, liver, lung, and gastric wall), and one patient (1.2%) had locoregional recurrence (Table 2). All six patients with distant metastatic recurrence were MRD-positive, with ctDNA lead times of up to 37.6 months (median 12.4 months). One patient had only a 2-day lead time; this patient had not had blood collection in the preceding 567 days. Although 37 of 83 (44.6%) patients in this cohort had high risk of recurrence on the basis of CTS5 risk score, 6 of 8 (75%) patients who were MRD-positive were considered high-risk by CTS5 score. RFS for patients who were MRD-positive was worse compared with RFS of patients who were MRD-negative (Fig 4A). Overall, sensitivity of ctDNA detection for identifying any clinical recurrence was 85.7%, with a negative predictive value (NPV) of 98.7% (Fig 4B). For development of distant metastatic recurrence, the sensitivity was 100%, with a NPV of 100%. For all recurrences and distant metastasis, the specificity was 97.4% and positive predictive value was 75%.
FIG 3.
(A-H) For each of the eight patients with ctDNA detected in plasma, longitudinal plots depicting VAFs over time, in addition to lead time and heatmaps showing signal by variant for each patient. cfDNA, cell-free DNA; ctDNA, circulating tumor DNA; eVAF, estimated variant allele frequency; ND, not detected; VAFs, variant allele frequencies.
TABLE 2.
Clinical and Circulating Tumor DNA Characteristics in Cases of Overt Clinical Recurrences
FIG 4.
(A) Kaplan-Meier curve for RFS. (B) Summary of the results from ctDNA assays and clinical recurrences. ctDNA, circulating tumor DNA; RFS, relapse-free survival.
Two of eight (25%) patients who were MRD-positive have not had clinical recurrence at last follow-up. Both patients had stage IIIC disease at diagnosis; neither patient has had restaging scans since initial diagnosis. One patient was MRD-positive at first plasma collection—9.4 years from diagnosis and 2.3 years from ET completion—with a ctDNA estimated variant allele frequency of 0.17% (Fig 3). A second plasma sample obtained at the time of last follow-up, 10.6 years after diagnosis (15.4 months from first ctDNA detection), was also positive with an estimated variant allele frequency of 26.8%. The other patient had a plasma sample positive for MRD at her only plasma collection, approximately 6.7 years after diagnosis, while on adjuvant ET. She was lost to follow-up and has not been seen in the 15 months since the last plasma sample. Because of the research nature of the MRD assay, results were not returned to patients or treating clinicians.
DISCUSSION
In this prospective study of patients with high-risk HR+/HER2– breast cancer 5 or more years from diagnosis, we evaluated plasma ctDNA detection of MRD using a tumor-informed assay tracking multiple somatic mutations. We studied the relationship between MRD detection and patient clinicopathologic characteristics and the association of MRD status with distant and local recurrence in this population. We selected this high-risk population to study MRD status, reasoning that these patients would be the most likely to have MRD.
To our knowledge, these are the first data on plasma ctDNA analysis for MRD detection in late adjuvant HR+ breast cancer, a major and understudied cause of more than 40,000 annual breast cancer–related deaths in the United States.7 Here, 10% of patients were MRD-positive more than 5 years from diagnosis despite no clinical evidence of metastatic recurrence at the time of first plasma sample. Importantly, ctDNA analysis identified MRD in all cases of distant recurrence. ctDNA analysis did not identify MRD in the case of local recurrence in this study, consistent with previous reports.13,15 Additionally, ctDNA was detected in two patients who had not experienced clinical recurrence at the time of last follow-up, although imaging had not been obtained in these cases.
Here, we prospectively identified, enrolled, and followed patients and collected biospecimens. We retrospectively applied a tumor-informed, custom MRD assay allowing for a greater diversity of alterations to be tracked across patients with the goal of increasing sensitivity and specificity.13 Although the cohort is relatively small (n = 83) and distant recurrence was infrequent (n = 6), prospective evaluation allows for the first estimation of prevalence of ctDNA positivity in this important population (10%). This finding is consistent with studies of late recurrence in HR+ breast cancer for which the annual rate of late recurrence in the highest risk group is between 1.7% and 4.5% per year.3 The prospective collection also affords the opportunity to estimate NPV (100%) and positive predictive value (75%) for distant recurrence. Despite a relatively short follow-up of 24 months, the median lead time from a positive test to clinical recurrence was 12.4 months. This time frame—if confirmed in patients who were scan-negative, MRD-positive—could allow for testing in clinical trials whether intervention to treat MRD improves patient outcomes.13
One limitation of this study is the low absolute number of recurrences, likely related to (1) the relatively short follow-up time relative to the setting and (2) the low but steady rate of breast cancer distant recurrences seen more than 5 years from diagnosis. This low number of events limits nuanced, statistically robust analyses to further assess relationships between clinicopathologic and molecular features, ctDNA positivity, and recurrence. Larger studies are needed to better understand these relationships. Many recently completed and ongoing studies in HR+ breast cancer collect plasma for ctDNA evaluation—some at or beyond 5 years from diagnosis—and we eagerly anticipate these analyses.
Another limitation of our study and of all MRD studies in HR+ breast cancer thus far is lack of concordant body imaging. Importantly, per current guidelines, patients here were followed clinically but did not undergo routine body imaging in the absence of concerning symptoms. Some patients in this study may have had clinically occult but scan-detectable metastatic disease. Therefore, although MRD detection preceded presentation of clinically overt metastatic disease by up to 37.6 months with a median of 12.4 months, it is not known whether patients had radiographically identifiable metastases at the time of MRD detection. Recent data from the cTRAK TN trial—albeit in triple-negative BCs and with a significantly less sensitive ctDNA assay—illustrate this as most patients with identified MRD had radiographically visible metastatic disease at time of positive ctDNA sample.33 Future studies should include concurrent imaging of all—both MRD-positive and MRD-negative—patients at study entry to determine the overlap in time between imaging and MRD assessment. Imaging of patients who were MRD-negative is critical to understanding the proportion of patients who were MRD-negative with clinically occult, scan-detectable disease.
Additionally, in our study, plasma samples were timed with routine follow-up, every 6-12 months. Because sampling was infrequent, lead time assessments may be less accurate than more densely timed sampling would allow. More frequent sampling of large populations in this setting may be challenging, given the cost, and because patient follow-up schedules—recommended annually after more than 5 years from diagnosis—typically occur no more than every 6 months in routine practice.
Several clinical trials are underway to investigate the efficacy of potential interventions after MRD detection. For example, in the ongoing TRAK-ER (ClinicalTrials.gov identifier: NCT04985266), DARE (ClinicalTrials.gov identifier: NCT04567420), and LEADER (ClinicalTrials.gov identifier: NCT03285412) trials, patients with high-risk HR+/HER2– early-stage BC who are ctDNA-positive in the adjuvant setting are treated in the intervention arm with ET and a CDK 4/6 inhibitor compared with ET alone in the control arm.
Although most patients with early-stage HR+ breast cancer do not experience recurrence, because it is the most commonly diagnosed cancer in women, over 600,000 women worldwide die from breast cancer each year.1 Current clinical, pathologic, and molecular tools are insufficient—particularly in the late adjuvant setting—to determine who is at higher risk of developing metastatic disease. For example, in our study, MRD analysis identified two patients—assessed as low risk by CTS5—at increased risk and both went on to experience recurrence. In response to the important problem of late recurrence in breast cancer, the Breast Cancer Steering Committee of the NCI convened a Clinical Trial Planning Meeting in May 2019.34 At that time, regarding blood-based biomarkers, the group concluded that liquid biopsy is promising but additional data are needed to determine the validity and clinical utility of this potential biomarker. REFINE-BrCa (Refining Adjuvant Therapy Through Identification and Escalation) evolved from this discussion as a collaboration across National Clinical Trials Network group members developing clinical trials to address key questions about late recurrence in HR+ breast cancer. The findings presented here are an important step toward characterization and incorporation of ctDNA into prospective clinical trials in this setting. Importantly, the personalized RaDaR assays used here were performed in a research setting, so patients and treating clinicians were not informed of testing results. Before there is a known effective intervention for MRD-positive patients without scan-detectable disease, MRD testing may contribute to unintentional harm if incorporated into routine clinical practice.35 Our institution is studying patient understanding of and attitudes toward late recurrence in a survey study on Patient-reported Outcomes in Women with ER+/PR+ breast cancer (POWER). Additionally, many patients with early-stage HR+ breast cancer in the late adjuvant setting ultimately die of non–breast cancer causes including cardiovascular, cerebrovascular, and neurodegenerative diseases.36 If MRD screening becomes part of future routine care, the decision to screen for MRD should incorporate traditional factors predictive of breast cancer recurrence (eg, stage and nodal status) and patient life expectancy on the basis of age and other comorbidities, to maximize its clinical impact.37 Overall, the clinical utility of MRD assays in breast cancer has not yet been established. We look forward to results of upcoming clinical trials in the late adjuvant setting that use MRD detection to guide therapy.
In conclusion, we evaluated ctDNA prevalence and dynamics in the late adjuvant setting in HR+/HER2– breast cancer. Detection of MRD was strongly associated with distant metastatic recurrences more than 5 years from breast cancer diagnosis, with favorable test characteristics including sensitivity, specificity, and a median lead time of approximately 1 year to clinical recurrence. These data suggest that there may be a period in which MRD is detectable via ctDNA before overt, late breast cancer recurrences. This will inform future studies of liquid biopsy to personalize treatment and prevent or delay late recurrence of early-stage breast cancer.
APPENDIX 1. RaDaR Minimal Residual Disease Assay Methodology
Assay Overview
RaDaR is a personalized next generation sequencing (NGS) assay for the sensitive detection of circulating tumor DNA (ctDNA) in a patient's blood using a tumor-informed approach. The presence or absence of ctDNA was used as a proxy for the presence of tumor cells after therapy in this study.
Tumor-specific variants were identified through whole-exome sequencing (WES) of DNA extracted from a formalin-fixed paraffin-embedded (FFPE) tumor tissue sample. Custom software was used to prioritize tumor variants and then design a patient-specific primer panel to interrogate up to 48 of the prioritized tumor variants. Leukocyte DNA and cell-free DNA (cfDNA) were extracted from buffy coat and plasma derived from peripheral whole-blood samples collected by venipuncture. Multiplex polymerase chain reaction (PCR) and NGS was used to assess tumor variants in cfDNA. Custom software was used to analyze sequencing data, to statistically determine the presence of ctDNA, to quantify its relative level, and to generate a physician report. The assay was performed at Inivata's Clinical Laboratory Improvement Amendments/College of American Pathologists–accredited facility in Research Triangle Park, NC. The assay workflow is depicted in Appendix Figure A1. The individual steps of the workflow are described in the following sections.
WES
DNA was extracted from provided 10-micron FFPE tumor tissue sections using Maxwell RSC DNA FFPE kits. The extracted DNA was converted into a sequencing library using the KAPA Hyper Prep Kit and indexed uniquely. Resulting precapture libraries were quantified using the Quant-iT dsDNA High Sensitivity assay. Each library proceeded to exome enrichment using the xGen Exome Research Panel v1.0 (IDT, Coralville, IA), and following capture was analyzed on a fragment analyzer and quantified using the Quant-iT dsDNA High Sensitivity assay. Sequencing was performed on the HiSeq4000 platform (Illumina Inc, San Diego, CA). Raw sequencing data were transferred to Inivata in FASTQ format. An aliquot of DNA isolated from FFPE tumor tissue was transferred to Inivata for use in the RaDaR test procedure to confirm the accuracy of the variant selection and RaDaR sequencing panel design and to enable identification and filtering of any nontumor variants.
Somatic Variant Identification, Prioritization, and Primer Design
A custom analytical pipeline was used to process the WES data identifying variants, ranking them, and then designing each a patient-specific RaDaR panel. The WES fastq files were first aligned to the human genome and had duplicates marked, followed by copy number analysis and somatic variant calling. Each sample was assessed for key metrics including total reads, usable % reads, usable reads, percent duplicates, alignment %, and mean sequencing coverage (Data Supplement Table 1). PhiX was included in each sequencing run to quality control cluster generation, sequencing, and alignment. Tumor variants (single nucleotide variants and indels) from WES were prioritized using a custom algorithm using criteria aimed at assembling the variant set best suited to detecting the ctDNA specific to the tumor for which the assay was devised. After variant prioritization, custom software was used to design up to 48 primer pairs targeting the tumor variants for use in subsequent multiplex PCR reactions. The software was optimized to select primers that would work well in multiplex and efficiently amplify the cfDNA.
Primer Qualification and Material Release
Each personalized NGS primer panel was synthesized (IDT), pooled, and then combined with a standard panel of primers used for quality control. After this, the panels were functionally qualified before release for the RaDaR assay. Qualification includes NGS testing against a reference DNA standard, the patient's FFPE tumor DNA, and an amplification-negative control. Libraries were sequenced on an iSeq100 system (Illumina Inc). Primer panels passed quality control if sufficient variants were detected in the tumor DNA and were read at sufficient depth.
DNA Extraction and Quantitation
cfDNA was extracted from the provided plasma using an automated platform using solid phase reversible immobilization magnetic bead isolation carried out on a Hamilton Microlab STAR robot. Each batch of extractions was performed with a negative extraction control, which was used to confirm the absence of contamination during the extraction process. The amount of amplifiable cfDNA was determined using a custom digital droplet PCR (ddPCR) assay where a segment of the RPP30 gene (ribonuclease P/MRP subunit P30) is amplified and measured. All testing was performed using the QX200 ddPCR system (BioRad, Hercules, CA). The target lower limit of input for the RaDaR assay is 2,000 amplifiable copies of cfDNA, and the upper limit is 20,000 copies on the basis of this dPCR measurement.
Multiplex PCR and Sequencing
After primer panel qualification and DNA extraction, RaDaR multiplex PCR was performed on cfDNA from plasma alongside a buffy coat DNA control sample, which was used for identification and removal of germline variants, the removal of variants because of clonal hematopoiesis of indeterminate potential (CHIP) from the analysis, and as a positive amplification control. A negative amplification control was used to ensure against contamination for each panel. In addition, an amplification-positive control was performed for each plate of PCR reactions. After multiplex PCR, reaction-specific index barcode sequences were added to the amplified sequences and then libraries were pooled, combined with a PhiX control, and sequenced using the NovaSeq 6000 system (Illumina Inc).
Processing of Sequencing Data
Sequencing data were analyzed in a multistep process. Briefly, raw Illumina binary base call sequence files were converted to the FASTQ format and demultiplexed using bcl2fastq. FASTQ files were then aligned to the human genome using bwa mem and processed using a pipeline to identify primer pairs and count mutant and reference bases.
Selection of Variants for Tracking
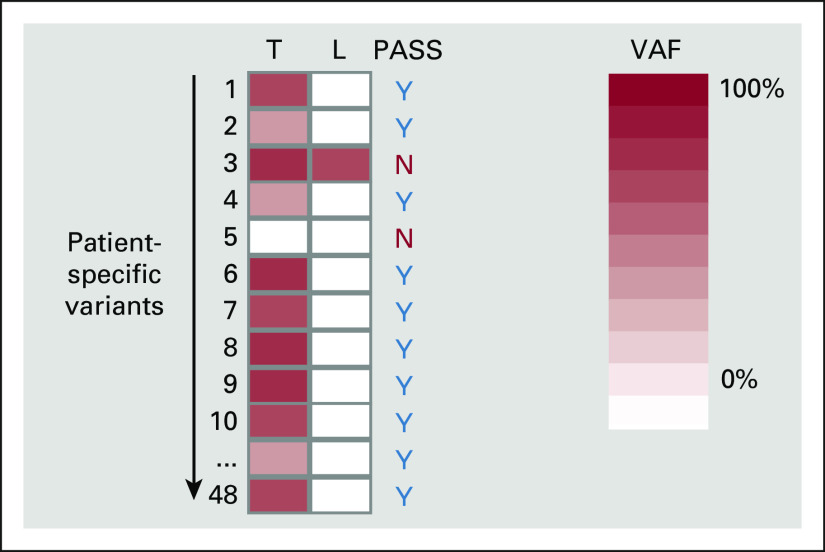
For plasma DNA testing, acceptable variants were required to be present in tumor DNA and absent in leukocyte DNA. Variants present in leukocyte DNA are typically either germline mutations or CHIP and were excluded from further analysis (exemplified by variant 3 in Appendix Fig A2). Variants absent from tumor DNA are either false positives from WES or failure to amplify, sequence, or align the target region (exemplified by variant 5 in Appendix Fig A2). This step both prevents false positives that could be the cause such as through the detection of variants that are in fact CHIP and enables an accurate assessment of each sample limit of detection through knowledge of the number of variants assessed.
Determination of Residual or Recurrent Disease
A statistical model was used to assess the statistical significance of the observed mutant counts for each variant, and the information was integrated over the entire set of filtered personalized variants to obtain evidence of tumor presence or absence at the sample level. This included an assessment of the noise of each individual variant class and the sensitivity and specificity on the basis of the number of variants in the panel. A sample was called as positive for residual disease if its cumulative statistical score was above a preset threshold, as defined during analytical development (as described in Flach et al24). The tumor fraction estimated from this model was then reported (estimated variant allele frequency).
FIG A1.
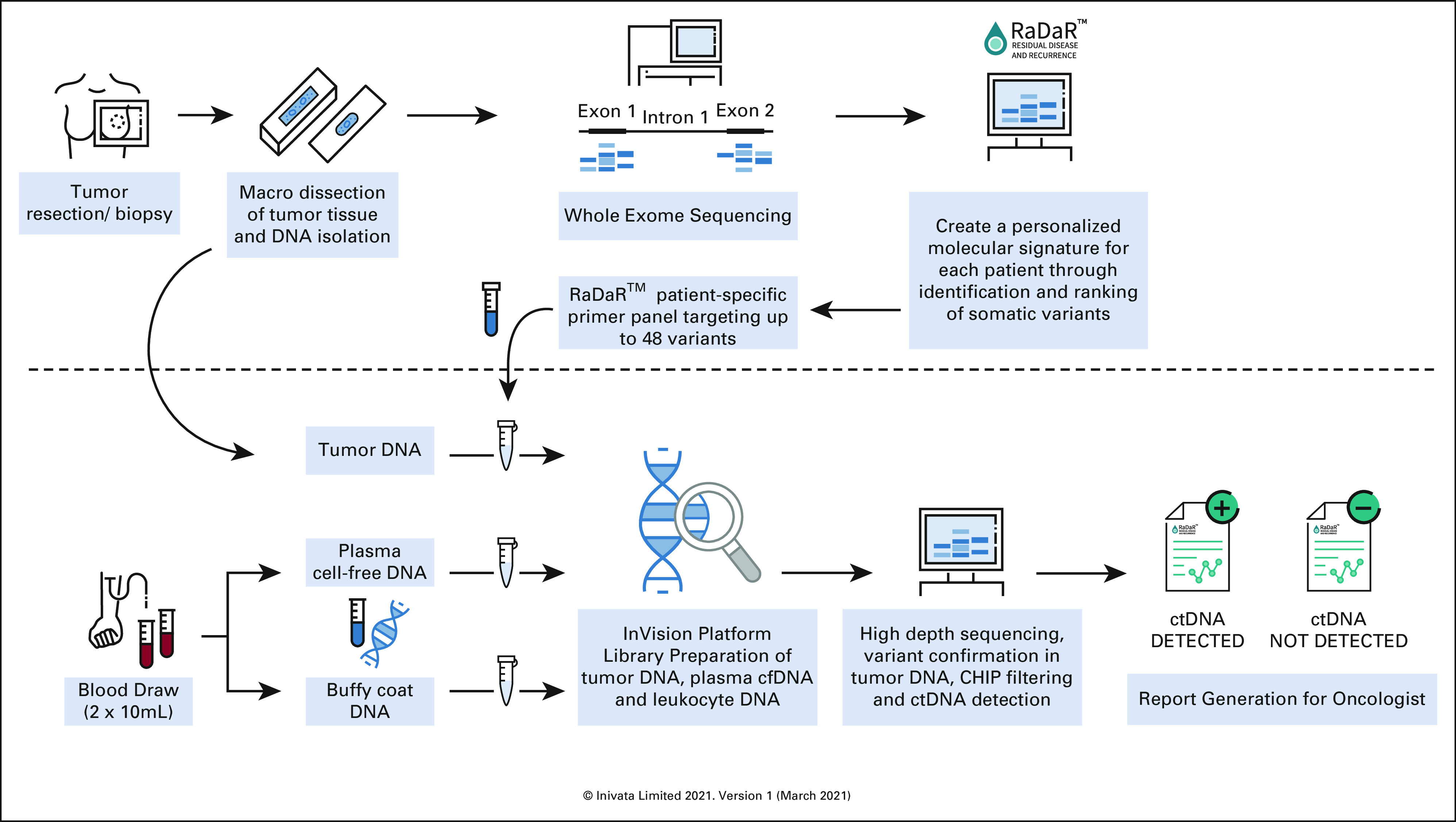
RaDaR assay workflow. Used with permission from Inivata, Inc.38 cfDNA, cell-free DNA; CHIP, clonal hematopoiesis of indeterminate potential; ctDNA, circulating tumor DNA.
FIG A2.
Selection of variants for tracking. Each column represents a different material type from the same patient. Each row represents a different variant. The shade of red represents the VAF. The reference VAF intensity for tumor DNA assessment is shown on the right (range from 0% to 100%). L, lymphocytes; N, no; T, tumor; VAF, variant allele frequency; Y, yes.
Elza C. de Bruin
Employment: AstraZeneca
Stock and Other Ownership Interests: AstraZeneca
Robert McEwen
Employment: AstraZeneca
Stock and Other Ownership Interests: AstraZeneca
Daniel Stetson
Employment: AstraZeneca
Stock and Other Ownership Interests: AstraZeneca
Patents, Royalties, Other Intellectual Property: I have a patent pending for AstraZeneca
Sara M. Tolaney
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Novartis, Pfizer, Merck, Lilly, Nektar, NanoString Technologies, AstraZeneca, Puma Biotechnology, Genentech, Eisai, Sanofi, Bristol Myers Squibb, Paxman, Seattle Genetics, Odonate Therapeutics, G1 Therapeutics, OncoPep, Kyowa Hakko Kirin, Samsung Bioepis, CytomX Therapeutics, Daiichi Sankyo, Athenex, Immunomedics/Gilead, Mersana, Certara Inc, 4D Pharma, Ellipses Pharma, OncoSec, Chugai Pharma, BeyondSpring Pharmaceuticals, OncXerna Therapeutics, Infinity Pharmaceuticals, Zymeworks, Zentalis, Blueprint Medicines, Reveal Genomics
Research Funding: Genentech/Roche (Inst), Merck (Inst), Exelixis (Inst), Pfizer (Inst), Lilly (Inst), Novartis (Inst), Bristol Myers Squibb (Inst), Eisai (Inst), AstraZeneca (Inst), NanoString Technologies (Inst), Cyclacel (Inst), Nektar (Inst), Immunomedics (Inst), Odonate Therapeutics (Inst), Sanofi (Inst), Seattle Genetics (Inst)
Ann H. Partridge
Patents, Royalties, Other Intellectual Property: I receive small royalty payments for co-authoring the breast cancer survivorship section of UpToDate
Open Payments Link: https://openpaymentsdata.cms.gov/physician/835197
Ian E. Krop
Employment: Freeline Therapeutics, PureTech
Leadership: Freeline Therapeutics, PureTech
Stock and Other Ownership Interests: Freeline Therapeutics, PureTech
Honoraria: Genentech/Roche, AstraZeneca
Consulting or Advisory Role: Genentech/Roche, Seattle Genetics, Daiichi Sankyo, Macrogenics, Novartis, Merck, Bristol Myers Squibb, AstraZeneca
Research Funding: Genentech (Inst), Pfizer (Inst), Macrogenics (Inst)
Charlene Knape
Employment: Inivata
Ute Feger
Employment: Inivata
Stock and Other Ownership Interests: Abbott/AbbVie
Giovanni Marsico
Employment: Inivata
Stock and Other Ownership Interests: Inivata
Research Funding: Inivata
Patents, Royalties, Other Intellectual Property: Patents pending for Inivata Ltd core technologies, including the RaDaR assay in this study
Karen Howarth
Employment: Inivata
Stock and Other Ownership Interests: Inivata, NeoGenomics Laboratories
Research Funding: Inivata
Patents, Royalties, Other Intellectual Property: Patent pending
Eric P. Winer
Honoraria: Genentech/Roche, Genomic Health
Consulting or Advisory Role: Leap Therapeutics, Jounce Therapeutics, GlaxoSmithKline, Carrick Therapeutics, Genentech/Roche
Research Funding: Genentech (Inst)
Other Relationship: InfiniteMD
Nancy U. Lin
Stock and Other Ownership Interests: Artera Inc
Consulting or Advisory Role: Seattle Genetics, Puma Biotechnology, Daiichi Sankyo, Denali Therapeutics, AstraZeneca, Prelude Therapeutics, Voyager Therapeutics, Affinia Therapeutics, Pfizer, Olema Pharmaceuticals, Aleta Biotherapeutics, Artera
Research Funding: Genentech (Inst), Pfizer (Inst), Seattle Genetics (Inst), Merck (Inst), Zion (Inst), Olema Pharmaceuticals (Inst)
Patents, Royalties, Other Intellectual Property: Royalties for chapter in UpTtoDate regarding management of breast cancer brain metastases, Royalties, Jones & Bartlett
Heather A. Parsons
Research Funding: Puma Biotechnology (Inst)
No other potential conflicts of interest were reported.
See accompanying editorial on page 2395
PRIOR PRESENTATION
Presented in part at the ASCO Annual Meeting, Chicago, IL, June 3-7, 2022.
SUPPORT
Supported by Komen Scholars grant (SAB190001, E.P.W.); AstraZeneca, and NCI Breast Cancer SPORE at DF/HCC (P50CA168504, N.U.L.); Susan G. Komen Career Catalyst Research Grant, and NCI Mentored Clinical Scientist Research Career Development Award (1K08CA252639, H.A.P.).
M.L.-S. and E.C.D.B. contributed equally. K.S. and R.M. contributed equally. N.U.L. and H.A.P. contributed equally.
DATA SHARING STATEMENT
Deidentified data are provided in the Supplemental Tables. Sequencing data will be uploaded to dbGaP.
AUTHOR CONTRIBUTIONS
Conception and design: Elza C. de Bruin, Sara M. Tolaney, Ian E. Krop, Karen Howarth, Eric P. Winer, Nancy U. Lin, Heather A. Parsons
Financial support: Eric P. Winer, Nancy U. Lin, Heather A. Parsons
Administrative support: Katheryn Santos, Melissa E. Hughes, Ute Feger, Nancy U. Lin, Heather A. Parsons
Provision of study materials or patients: Melissa E. Hughes, Nancy U. Lin, Heather A. Parsons
Collection and assembly of data: Marla Lipsyc-Sharf, Katheryn Santos, Ashka Patel, Gregory J. Kirkner, Melissa E. Hughes, Sara M. Tolaney, Ute Feger, Giovanni Marsico, Nancy U. Lin, Heather A. Parsons
Data analysis and interpretation: Marla Lipsyc-Sharf, Elza C. de Bruin, Robert McEwen, Daniel Stetson, Melissa E. Hughes, Sara M. Tolaney, Ann H. Partridge, Charlene Knape, Giovanni Marsico, Karen Howarth, Nancy U. Lin, Heather A. Parsons
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Circulating Tumor DNA and Late Recurrence in High-Risk Hormone Receptor–Positive, Human Epidermal Growth Factor Receptor 2-–Negative Breast Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Elza C. de Bruin
Employment: AstraZeneca
Stock and Other Ownership Interests: AstraZeneca
Robert McEwen
Employment: AstraZeneca
Stock and Other Ownership Interests: AstraZeneca
Daniel Stetson
Employment: AstraZeneca
Stock and Other Ownership Interests: AstraZeneca
Patents, Royalties, Other Intellectual Property: I have a patent pending for AstraZeneca
Sara M. Tolaney
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Novartis, Pfizer, Merck, Lilly, Nektar, NanoString Technologies, AstraZeneca, Puma Biotechnology, Genentech, Eisai, Sanofi, Bristol Myers Squibb, Paxman, Seattle Genetics, Odonate Therapeutics, G1 Therapeutics, OncoPep, Kyowa Hakko Kirin, Samsung Bioepis, CytomX Therapeutics, Daiichi Sankyo, Athenex, Immunomedics/Gilead, Mersana, Certara Inc, 4D Pharma, Ellipses Pharma, OncoSec, Chugai Pharma, BeyondSpring Pharmaceuticals, OncXerna Therapeutics, Infinity Pharmaceuticals, Zymeworks, Zentalis, Blueprint Medicines, Reveal Genomics
Research Funding: Genentech/Roche (Inst), Merck (Inst), Exelixis (Inst), Pfizer (Inst), Lilly (Inst), Novartis (Inst), Bristol Myers Squibb (Inst), Eisai (Inst), AstraZeneca (Inst), NanoString Technologies (Inst), Cyclacel (Inst), Nektar (Inst), Immunomedics (Inst), Odonate Therapeutics (Inst), Sanofi (Inst), Seattle Genetics (Inst)
Ann H. Partridge
Patents, Royalties, Other Intellectual Property: I receive small royalty payments for co-authoring the breast cancer survivorship section of UpToDate
Open Payments Link: https://openpaymentsdata.cms.gov/physician/835197
Ian E. Krop
Employment: Freeline Therapeutics, PureTech
Leadership: Freeline Therapeutics, PureTech
Stock and Other Ownership Interests: Freeline Therapeutics, PureTech
Honoraria: Genentech/Roche, AstraZeneca
Consulting or Advisory Role: Genentech/Roche, Seattle Genetics, Daiichi Sankyo, Macrogenics, Novartis, Merck, Bristol Myers Squibb, AstraZeneca
Research Funding: Genentech (Inst), Pfizer (Inst), Macrogenics (Inst)
Charlene Knape
Employment: Inivata
Ute Feger
Employment: Inivata
Stock and Other Ownership Interests: Abbott/AbbVie
Giovanni Marsico
Employment: Inivata
Stock and Other Ownership Interests: Inivata
Research Funding: Inivata
Patents, Royalties, Other Intellectual Property: Patents pending for Inivata Ltd core technologies, including the RaDaR assay in this study
Karen Howarth
Employment: Inivata
Stock and Other Ownership Interests: Inivata, NeoGenomics Laboratories
Research Funding: Inivata
Patents, Royalties, Other Intellectual Property: Patent pending
Eric P. Winer
Honoraria: Genentech/Roche, Genomic Health
Consulting or Advisory Role: Leap Therapeutics, Jounce Therapeutics, GlaxoSmithKline, Carrick Therapeutics, Genentech/Roche
Research Funding: Genentech (Inst)
Other Relationship: InfiniteMD
Nancy U. Lin
Stock and Other Ownership Interests: Artera Inc
Consulting or Advisory Role: Seattle Genetics, Puma Biotechnology, Daiichi Sankyo, Denali Therapeutics, AstraZeneca, Prelude Therapeutics, Voyager Therapeutics, Affinia Therapeutics, Pfizer, Olema Pharmaceuticals, Aleta Biotherapeutics, Artera
Research Funding: Genentech (Inst), Pfizer (Inst), Seattle Genetics (Inst), Merck (Inst), Zion (Inst), Olema Pharmaceuticals (Inst)
Patents, Royalties, Other Intellectual Property: Royalties for chapter in UpTtoDate regarding management of breast cancer brain metastases, Royalties, Jones & Bartlett
Heather A. Parsons
Research Funding: Puma Biotechnology (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.American Institute for Cancer Research : Worldwide cancer data. https://www.wcrf.org/dietandcancer/worldwide-cancer-data/
- 2.Parise CA, Bauer KR, Brown MM, et al. : Breast cancer subtypes as defined by the estrogen receptor (ER), progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2) among women with invasive breast cancer in California, 1999-2004. Breast J 15:593-602, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Pan H, Gray R, Braybrooke J, et al. : 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med 377:1836-1846, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pedersen RN, Esen BÖ, Mellemkjær L, et al. : The incidence of breast cancer recurrence 10-32 years after primary diagnosis. J Natl Cancer Inst 114:391-399, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leone JP, Vallejo CT, Hassett MJ, et al. : Factors associated with late risks of breast cancer-specific mortality in the SEER registry. Breast Cancer Res Treat 189:203-212, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pedersen RN, Mellemkjær L, Ejlertsen B, et al. : Mortality after late breast cancer recurrence in Denmark. J Clin Oncol 40:1450-1463, 2022 [DOI] [PubMed] [Google Scholar]
- 7.American Cancer Society : Breast Cancer Facts and Figures 2019-2020. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts-and-figures-2019-2020.pdf [Google Scholar]
- 8.Mariotto AB, Etzioni R, Hurlbert M, et al. : Estimation of the number of women living with metastatic breast cancer in the United States. Cancer Epidemiol Biomarkers Prev 26:809-815, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dowling RJO, Sparano JA, Goodwin PJ, et al. : Toronto workshop on late recurrence in estrogen receptor-positive breast cancer: Part 2: Approaches to predict and identify late recurrence, research directions. JNCI Cancer Spectr 3:pkz049, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peto R, Davies C, Godwin J, et al. : Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 379:432-444, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies C, Pan H, Godwin J, et al. : Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 381:805-816, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Comprehensive Cancer Network : Breast Cancer (version 2.2022) https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
- 13.Parsons HA, Rhoades J, Reed SC, et al. : Sensitive detection of minimal residual disease in patients treated for early-stage breast cancer. Clin Cancer Res 26:2556-2564, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coombes RC, Page K, Salari R, et al. : Personalized detection of circulating tumor DNA antedates breast cancer metastatic recurrence. Clin Cancer Res 25:4255-4263, 2019 [DOI] [PubMed] [Google Scholar]
- 15.Magbanua MJM, Swigart LB, Wu HT, et al. : Circulating tumor DNA in neoadjuvant-treated breast cancer reflects response and survival. Ann Oncol 32:229-239, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Murillas I, Chopra N, Comino-Méndez I, et al. : Assessment of molecular relapse detection in early-stage breast cancer. JAMA Oncol 5:1473-1478, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Murillas I, Schiavon G, Weigelt B, et al. : Mutation tracking in circulating tumor DNA predicts relapse in early breast cancer. Sci Transl Med 7:302ra133, 2015 [DOI] [PubMed] [Google Scholar]
- 18.McDonald BR, Contente-Cuomo T, Sammut SJ, et al. : Personalized circulating tumor DNA analysis to detect residual disease after neoadjuvant therapy in breast cancer. Sci Transl Med 11:eaax7392, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olsson E, Winter C, George A, et al. : Serial monitoring of circulating tumor DNA in patients with primary breast cancer for detection of occult metastatic disease. EMBO Mol Med 7:1034-1047, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burstein HJ, Lacchetti C, Anderson H, et al. : Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: ASCO clinical practice guideline focused update. J Clin Oncol 37:423-438, 2019 [DOI] [PubMed] [Google Scholar]
- 21.van Hellemond IEG, Geurts SME, Tjan-Heijnen VCG: Current status of extended adjuvant endocrine therapy in early stage breast cancer. Curr Treat Options Oncol 19:26, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Etienne G, Guilhot J, Rea D, et al. : Long-Term follow-up of the French stop imatinib (STIM1) study in patients with chronic myeloid leukemia. J Clin Oncol 35:298-305, 2017 [DOI] [PubMed] [Google Scholar]
- 23.Lin NU, Vanderplas A, Hughes ME, et al. : Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer 118:5463-5472, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flach S, Howarth K, Hackinger S, et al. : Liquid BIOpsy for MiNimal RESidual DiSease Detection in Head and Neck Squamous Cell Carcinoma (LIONESS)-a personalised circulating tumour DNA analysis in head and neck squamous cell carcinoma. Br J Cancer 126:1186-1195, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gale D, Heider K, Ruiz-Valdepenas A, et al. : Residual ctDNA after treatment predicts early relapse in patients with early-stage non-small cell lung cancer. Ann Oncol 33:500-510, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plagnol V, Woodhouse S, Howarth K, et al. : Analytical validation of a next generation sequencing liquid biopsy assay for high sensitivity broad molecular profiling. PLoS One 13:e0193802, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.BCBIo-Nextgen v1.2.3. 10.5281/zenodo.3743344 [DOI] [Google Scholar]
- 28.Lai Z, Markovets A, Ahdesmaki M, et al. : VarDict: A novel and versatile variant caller for next-generation sequencing in cancer research. Nucleic Acids Res 44:e108, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.R Core Team : R: A Language and Environment for Statistical Computing. Vienna, Austria, R Foundation for Statistical Computing, 2020. https://www.R-project.org/ [Google Scholar]
- 30.Gu Z, Eils R, Schlesner M: Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 32:2847-2849, 2016 [DOI] [PubMed] [Google Scholar]
- 31.Razavi P, Chang MT, Xu G, et al. : The genomic landscape of endocrine-resistant advanced breast cancers. Cancer Cell 34:427-438.e6, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dowsett M, Sestak I, Regan MM, et al. : Integration of clinical variables for the prediction of late distant recurrence in patients with estrogen receptor–positive breast cancer treated with 5 years of endocrine therapy: CTS5. J Clin Oncol 36:1941-1948, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turner N, Swift C, Jenkins B, et al. : Primary results of the cTRAK TN trial: A clinical trial utilising ctDNA mutation tracking to detect minimal residual disease and trigger intervention in patients with moderate and high risk early stage triple negative breast cancer. Presented at 2021 San Antonio Breast Cancer Symposium (virtual), December 7-10, 2021 (abstr GS3-06) [DOI] [PubMed]
- 34.National Cancer Institute, Breast Cancer Steering Committee . https://www.cancer.gov/about-nci/organization/ccct/steering-committees/nctn/breast-cancer
- 35.Cescon DW, Kalinsky K, Parsons HA, et al. : Therapeutic targeting of minimal residual disease to prevent late recurrence in hormone-receptor positive breast cancer: Challenges and new approaches. Front Oncol 11:667397, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Afifi AM, Saad AM, Al-Husseini MJ, et al. : Causes of death after breast cancer diagnosis: A US population-based analysis. Cancer 126:1559-1567, 2020 [DOI] [PubMed] [Google Scholar]
- 37.Lansdorp-Vogelaar I, Gulati R, Mariotto AB, et al. : Personalizing age of cancer screening cessation based on comorbid conditions: Model estimates of harms and benefits. Ann Intern Med 161:104-112, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inivata. https://www.inivata.com/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Deidentified data are provided in the Supplemental Tables. Sequencing data will be uploaded to dbGaP.