PURPOSE
Antitumor activity in preclinical models and a phase I study of patients with dedifferentiated liposarcoma (DD-LPS) was observed with selinexor. We evaluated the clinical benefit of selinexor in patients with previously treated DD-LPS whose sarcoma progressed on approved agents.
METHODS
SEAL was a phase II-III, multicenter, randomized, double-blind, placebo-controlled study. Patients age 12 years or older with advanced DD-LPS who had received two-five lines of therapy were randomly assigned (2:1) to selinexor (60 mg) or placebo twice weekly in 6-week cycles (crossover permitted). The primary end point was progression-free survival (PFS). Patients who received at least one dose of study treatment were included for safety analysis (ClinicalTrials.gov identifier: NCT02606461).
RESULTS
Two hundred eighty-five patients were enrolled (selinexor, n = 188; placebo, n = 97). PFS was significantly longer with selinexor versus placebo: hazard ratio (HR) 0.70 (95% CI, 0.52 to 0.95; one-sided P = .011; medians 2.8 v 2.1 months), as was time to next treatment: HR 0.50 (95% CI, 0.37 to 0.66; one-sided P < .0001; medians 5.8 v 3.2 months). With crossover, no difference was observed in overall survival. The most common treatment-emergent adverse events of any grade versus grade 3 or 4 with selinexor were nausea (151 [80.7%] v 11 [5.9]), decreased appetite (113 [60.4%] v 14 [7.5%]), and fatigue (96 [51.3%] v 12 [6.4%]). Four (2.1%) and three (3.1%) patients died in the selinexor and placebo arms, respectively. Exploratory RNA sequencing analysis identified that the absence of CALB1 expression was associated with longer PFS with selinexor compared with placebo (median 6.9 v 2.2 months; HR, 0.19; P = .001).
CONCLUSION
Patients with advanced, refractory DD-LPS showed improved PFS and time to next treatment with selinexor compared with placebo. Supportive care and dose reductions mitigated side effects of selinexor. Prospective validation of CALB1 expression as a predictive biomarker for selinexor in DD-LPS is warranted.
INTRODUCTION
Liposarcoma is one of the most common soft tissue sarcoma type in adults, representing 24% and 45% of extremity and retroperitoneal soft tissue sarcomas, respectively.1 Liposarcomas are classified into five distinct types with dedifferentiated liposarcoma (DD-LPS) being the most common subtype.2 While surgery is the primary therapy for localized liposarcomas, approximately 40% of patients eventually die from advanced unresectable or metastatic disease, emphasizing the need for effective new drugs.2 Standard palliative chemotherapy for advanced disease includes single agent or combinations of parenteral chemotherapies including doxorubicin and ifosfamide, or eribulin or trabectedin, which are indicated for treatment of liposarcoma. Gemcitabine and docetaxel may be used but have mainly been evaluated in earlier lines of primarily well-differentiated LPS.3 First-line treatment of DD-LPS with doxorubicin monotherapy has shown an overall response rate (ORR) between 0% and 8% with a median progression-free survival (PFS) ranging from 1.5 to 4 months.3-6 Anthracycline-containing regimens as a front-line treatment for retroperitoneal well-differentiated/DD-LPS have resulted in an ORR of 26% with a median PFS and overall survival (OS) of 4 and 25 months, respectively.3 Second or subsequent lines of therapy with US Food and Drug Administration–approved agents eribulin and trabectedin result in a median PFS of approximately 2 months.7,8
CONTEXT
Key Objective
Current treatment options of advanced dedifferentiated liposarcoma (DD-LPS) are limited in number and efficacy. The SEAL trial evaluated monotherapy with selinexor, a selective inhibitor of nuclear export, and, to our knowledge, is the first and largest study conducted exclusively on patients with DD-LPS whose sarcoma progressed on approved agents with no further approved therapeutic options.
Knowledge Generated
Single-agent oral selinexor provided prolonged progression-free survival, time to next treatment, and reduced pain in patients with previously treated DD-LPS. Exploratory molecular biomarker data revealed CALB1 expression to be associated with selinexor resistance resulting in a potential application for future patient stratification.
Relevance
Our results provide the rationale to further investigate selinexor in patients with advanced or metastatic DD-LPS and as a basis to further assess selinexor treatment in patients with DD-LPS with CALB1 expression.
MDM2 and CDK4 amplifications are hallmarks of DD-LPS.9,10 MDM2 is an E3-ligase which tags the tumor suppressor protein p53 (and other proteins) with ubiquitin for proteasome-mediated degradation, primarily in the cytoplasm of the cell. A majority of DD-LPS tumor cells that overexpress MDM2 carry wild-type p53, consistent with the notion that p53 is inactivated in these tumors through MDM2-facilitated degradation.11 Nuclear export of p53 is mediated by exportin-1 (XPO1) alone, but it is greatly facilitated by MDM2-mediated ubiquitination of p53.12 CDK4 expression is associated with poor survival in patients with DDLPS. The tumor suppressor protein p21 (CIP1/WAF1) is a negative regulator of CDK4, and nuclear p21 attenuates CDK4 activity. XPO1 facilitates the nuclear export and consequent functional inactivation of p21, thus potentiating the effect of CDK4. Overexpression of XPO1 has been reported in sarcomas,13 while MDM2 and CDK4 amplifications and expression are associated with poor survival in patients with DD-LPS.9,10
Selinexor is a potent, oral, selective inhibitor of nuclear export compound that specifically blocks XPO1 by covalently and reversibly binding to cysteine-528, an essential residue for XPO1 cargo binding.14-16 Blockade of XPO1 leads to nuclear retention and functional activation of multiple tumor suppressor proteins. Treatment with selinexor has demonstrated increased p53 nuclear accumulation and retention followed by reactivation of its tumor suppressor activity, even in the presence of MDM2 overexpression.13,17 When selinexor was administered both in vitro and in vivo, there were increased levels of nuclear p21 and downregulation of CDK4-mediated oncogenic pathways.13,17,18 Furthermore, in cancer, nuclear factor kappa B (NF-κB) activates pro-oncogenic, chemotherapy resistance and inflammatory gene transcription activity. This activity is inhibited endogenously by several proteins, the most potent of which is IκB (inhibitor of NF-κB). IκB is exported from the nucleus—and its NF-κB inhibiting activity blocked, solely by XPO1. Therefore, inhibition of XPO1 by selinexor prevents IκB transport to the cytoplasm and, instead, leads to its accumulation in the nucleus and potent inhibition of NF-κB pro-oncogenic activity.19
Currently approved for use in patients with multiple myeloma20,21 and diffuse large B-cell lymphoma,14 selinexor has demonstrated antitumor activity in in vitro and in vivo models of DD-LPS, inducing apoptosis in multiple liposarcoma cell lines, including those with MDM2 and CDK4 amplification17 and downregulating CDK4-mediated oncogenic pathways through increased nuclear retention of p21, a negative regulator of CDK4 in vitro.17 In murine xenograft models of human liposarcoma, selinexor inhibited tumor growth and reduced levels of XPO1,22 increased nuclear retention of p53,23 and inhibited NF-κB.19
In a phase I clinical trial of selinexor in advanced sarcomas, patients with DD-LPS had prolonged stable disease.18 We therefore conducted a phase II-III randomized trial to evaluate the activity of selinexor in patients with advanced or metastatic DD-LPS who had two-five prior lines of systemic therapy and report here the phase III results.
METHODS
Study Design and Participants
The SEAL trial was a phase II-III, multicenter, randomized, double-blind study of selinexor versus placebo in patients with advanced unresectable DD-LPS including 70 sites in 10 countries. Patients were randomly assigned in a 2:1 ratio. The Protocol (online only) was approved by institutional review boards at individual enrolling institutions and performed in accordance with the International Conference on Harmonization Good Clinical Practice Guidelines and the Declaration of Helsinki. Disease response was assessed by an Independent Review Committee.
Eligible patients age 12 years or older had histologically confirmed DD-LPS with measurable disease per RECIST v1.1 as assessed by an independent review committee, had shown radiologic evidence of disease progression, and had received two-five prior systemic therapies. An Eastern Cooperative Oncology Group performance status of ≤ 1, creatinine clearance > 30 mL/min, and adequate laboratory hematopoietic and hepatic function were required. Patients with other subtypes of liposarcoma or with known central nervous system metastases were excluded. A full list of inclusion or exclusion criteria is provided in the Data Supplement (online only). All patients provided written informed consent.
Procedures
Selinexor (60 mg) or matching placebo was administered twice weekly in 6-week cycles. Random assignment was stratified on the basis of (1) prior eribulin use (prior eribulin v no prior eribulin), (2) prior trabectedin use (prior trabectedin v no prior trabectedin), and (3) the number of prior systemic therapies excluding eribulin and trabectedin (≤ 2 v ≥ 3). Stratification by prior eribulin or trabectedin was implemented as these are the most recently approved agents for the treatment of LPS. With a 2:1 random assignment, a block size of six was used. Supportive care measures included a 5-hydroxytryptamine-3 antagonist (eg, ondansetron), olanzapine and, if needed, low-dose glucocorticoids. Treatment was administered until disease progression, discontinuation, or unacceptable side effects. If radiographic progression was confirmed by central independent radiology review in the placebo arm, eligible patients were allowed to cross over to selinexor. Patients on selinexor with confirmed progression were permitted to continue selinexor if their treating physician considered them to be benefiting from the therapy.
Outcomes
The primary end point was PFS, defined as the time from date of random assignment until the first date of progression confirmed by central radiographic review, on the basis of RECIST v1.1, or death due to any cause. Secondary end points were OS (additional details in the Data Supplement), OS among patients who did not cross over, time to progression on study treatment, ORR, duration of response, time to next treatment, and health-related quality of life (HR-QoL). Exploratory end points included tumor biomarker analysis in tumor tissue. Adverse events (AEs) were graded according to National Cancer Institute, Division of Cancer Treatment and Diagnosis Common Terminology Criteria for Adverse Events Grading Scale, version 4.03.24
Exploratory Molecular Correlative Studies
Per preplanned analysis, RNA sequencing was performed on formalin-fixed paraffin-embedded resected tumors or tumor biopsies of patients who were treated on study for at least one complete cycle. Details of the patient samples analyzed are included in the Data Supplement.
Statistical Analysis
The sample size was designed to have 90% power to detect a hazard ratio (HR) of 0.6 between selinexor and placebo for the primary efficacy end point of PFS, using a one-sided test with a nominal level of 0.025. The intent-to-treat population was used for efficacy analysis and consisted of all patients randomly assigned to study treatment. The safety population included patients who received at least one dose of blinded study treatment. For categorical variables, summary tabulations of the number and percentage of patients within each category were used (with a category for missing data) of the parameter, as well as two-sided 95% CIs. For continuous variables, summary statistics included the number of patients, mean, median, standard deviation, minimum, and maximum. For time-to-event variables, the Kaplan-Meier method was used for descriptive summaries. SAS version 9.4 was used for the analysis.
Role of Funding Source
The funder of the trial was involved in trial design, data collection, data analysis, data interpretation, and writing of the report. All authors had full access to all the data and had final responsibility for the decision to submit for publication.
RESULTS
Patient Characteristics
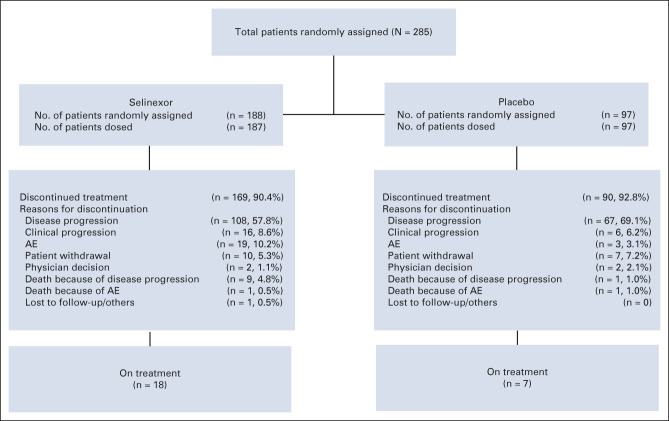
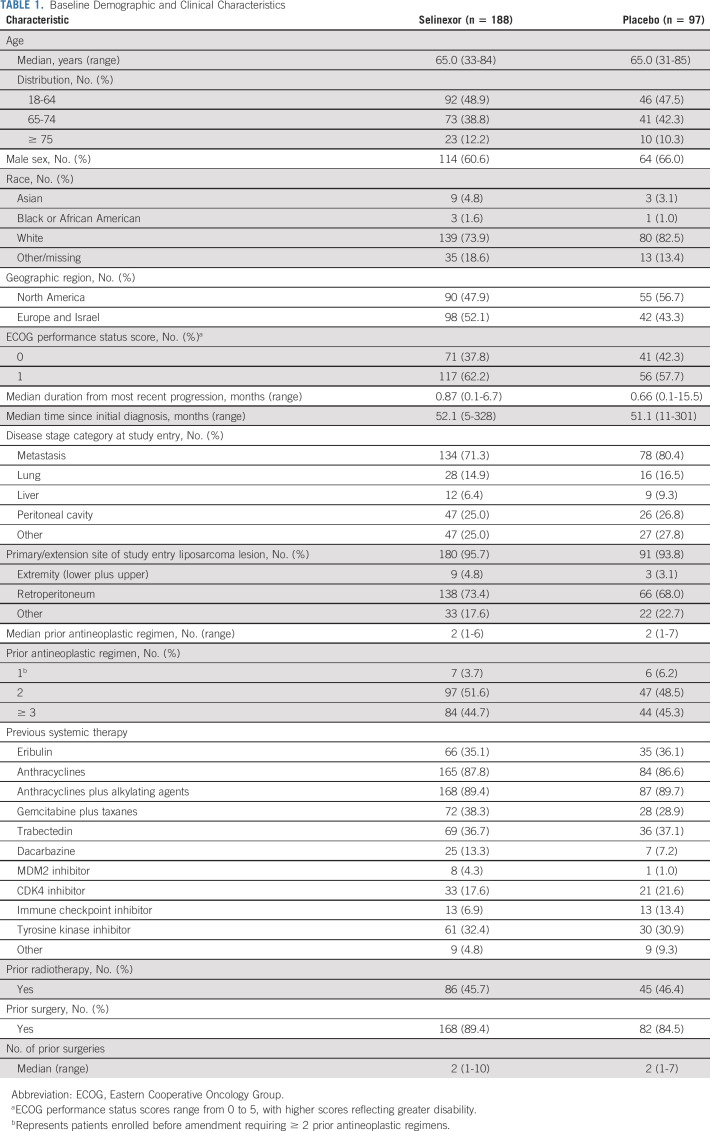
A total of 285 patients were enrolled in the phase III component of the trial with 188 and 97 patients randomly assigned to selinexor or placebo, respectively (Fig 1). Both arms were balanced with a median age of 65 years (interquartile range, 56.0-71.0). The most common primary site of disease at study entry was retroperitoneal (73.4% selinexor; 68.0% placebo). The majority of patients had metastatic disease at study entry (71.3% selinexor; 80.4% placebo) and had prior treatment with doxorubicin, gemcitabine, eribulin, or trabectedin (Table 1).
FIG 1.
CONSORT diagram. AE, adverse event.
TABLE 1.
Baseline Demographic and Clinical Characteristics
Efficacy
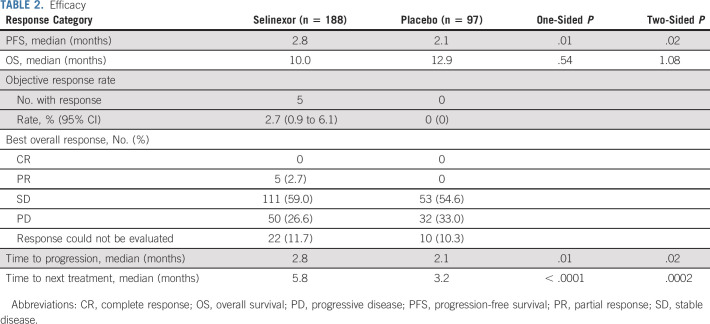
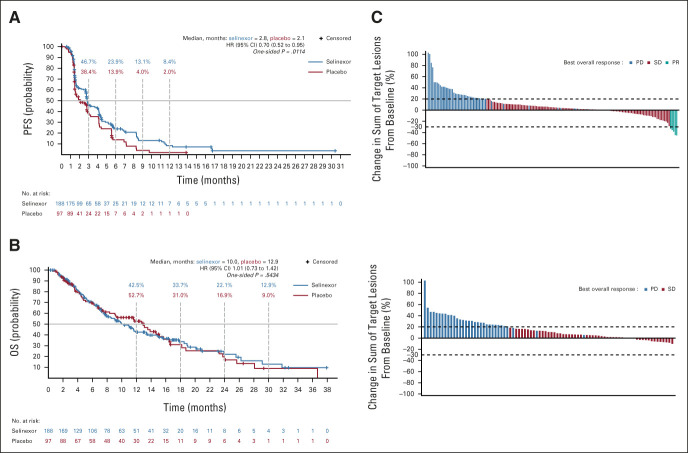
Selinexor was associated with a 30% improvement in PFS as compared with placebo (HR, 0.70; 95% CI, 0.52 to 0.95; one-sided P = .01; two-sided P = .02; median PFS 2.8 months and 2.1 months, respectively; Table 2). At 12 weeks or longer, the PFS was significantly longer with selinexor (46.8%, n = 88; 95% CI, 39.6 to 55.1) when compared with placebo (34.0%, n = 33; 95% CI, 29.1 to 50.7; one-sided P = .02; two-sided P = .04). The 6- and 12-month PFS rates for selinexor and placebo were 23.9% (95% CI, 17.7 to 32.4) v 13.9% (95% CI, 29.1 to 50.7) and 8.4% (95% CI, 4.3 to 16.2) v 2.0% (95% CI, 0.3 to 13.4), respectively (Fig 2A). Of the 135 (71.8%) and 74 (76.3%) PFS events in the selinexor and placebo arms, respectively, there were 10 (5.3%) deaths with selinexor and five (5.2%) with placebo. The ORR on the basis of RECIST v1.1 per independent central radiologic review was 2.7% (five patients) with selinexor, while no responses were observed with placebo. Three patients from the selinexor arm continued into selinexor open-label after radiographic progressive disease because of continued clinical benefit. The median duration of response was 7.4 months (95% CI, not reached to not reached) with selinexor. The time to next treatment was also significantly longer with selinexor versus placebo (HR, 0.49 [95% CI, 0.37 to 0.66], one-sided P < .0001; two-sided P = .0002; medians 5.8 months v 3.2 months). Of note, the number of patients receiving subsequent therapies was similar in each arm (Data Supplement). Following independent radiographic confirmation of progression, 58.8% patients on placebo arm crossed over to receive open-label selinexor. At a median follow-up of 14.6 months (interquartile range, 8.2-23.5), there was no difference in OS for selinexor and placebo (HR, 1.02; 95% CI, 0.73 to 1.42, one-sided P = .54; two-sided P = 1.08; median 10.0 months v 12.9 months; Fig 2B). OS among patients who did not cross over showed a HR of 0.69 (95% CI, 0.43 to 1.11; one-sided P = .06; two-sided P = .12; median 10.0 months with selinexor v 9.1 months with placebo).
TABLE 2.
Efficacy
FIG 2.
Efficacy by treatment arm. (A) Median progression-free survival. Kaplan-Meier curves by treatment arm. (B) Median OS. Kaplan-Meier curves by treatment arm. (C) Best overall response waterfall plots during blinded treatment: selinexor (upper panel) and placebo (lower panel). HR, hazard ratio; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response; SD, stable disease.
Safety
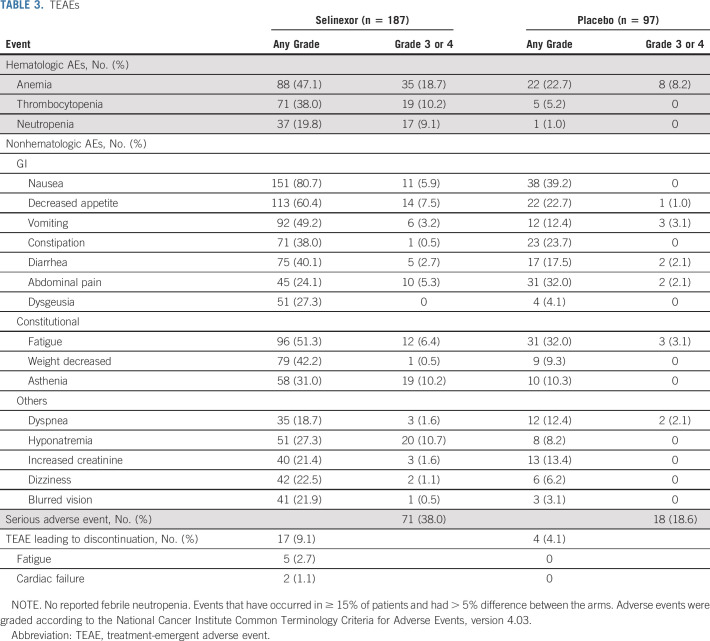
Discontinuation of study treatment because of treatment-emergent adverse events (TEAEs) occurred in 10.2% of patients on selinexor and 3.1% of patients on placebo. Overall, the most frequently reported TEAEs were nausea (80.7% v 39.2%), decreased appetite (60.4% v 22.7%), fatigue (51.3% v 32.0%), and weight loss (42.2% v 9.3%), the majority of which were grade 1 or 2 and reversible. Most common grade 3/4 AEs were anemia (18.7% v 8.2%), hyponatremia (nearly all asymptomatic, 10.7% v 0%), asthenia (10.2% v 0%), and thrombocytopenia (10.2% v 0%) with selinexor and placebo, respectively (Table 3). Two of 71 (2.8%) patients in the selinexor arm with thrombocytopenia had grade 3 bleeding: duodenal invasion by the tumor in one patient and bleeding from an anastomotic ulcer in the other. Of note, there were no reports of febrile neutropenia. Unique grade 3/4 AEs with selinexor included increased creatinine (1.6%), blurred vision (0.5%), and dizziness (1.1%; Table 3).
TABLE 3.
TEAEs
Serious TEAEs were reported in 38.0% patients with selinexor and 18.6% patients with placebo, of which GI disorders were the most frequent in both arms: selinexor (11.8%) and placebo (6.2%; Data Supplement). The most common TEAEs leading to discontinuation with selinexor were fatigue (2.7%) and cardiac failure (1.1%). With placebo, the most frequent TEAEs leading to discontinuation were sepsis (2.1%), abdominal pain (1.0%), and decreased appetite (1.0%). Compared with placebo, patients treated with selinexor had a higher rate of AEs leading to dose reduction (35.8% v 3.1%) and interruption (63.1% v 16.5%). TEAEs leading to death were similar for selinexor (2.1%) and placebo (3.1%).
Exploratory Molecular Correlative Studies
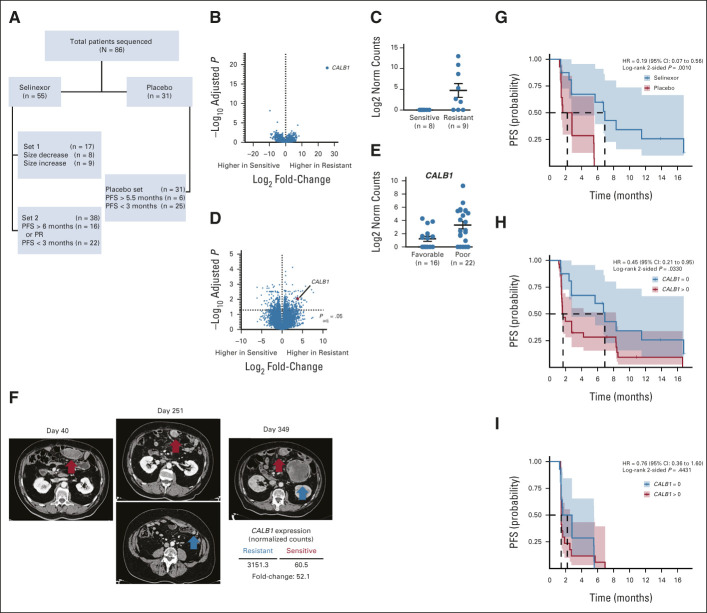
To identify potential genetic markers of response to selinexor, we performed RNA sequencing of pretreatment tumor samples from two nonoverlapping sets of 55 patients treated with at least one full cycle of selinexor. Set 1 included patients for whom a radiographically measured target lesion was recently biopsied and available for sequencing. Biopsies from lesions that decreased in size were defined as selinexor sensitive (–6% to –73%, n = 8), and those from lesions that increased in size were defined as resistant (+10% to +93%, n = 9; Data Supplement). Set 2 included patients who were stratified on the basis of PFS and RECIST response criteria. Patients who experienced progressive disease within 3 months of starting selinexor were defined as poor benefit (n = 22), and patients who were progression-free for at least 6 months or achieved a partial response were defined as favorable benefit (n = 16; Fig 3A; Data Supplement). Differential expression analysis of sensitive and resistant tumors in set 1 (target size change comparison) revealed that expression of CALB1 (calbindin 1) was strongly associated with resistance to selinexor (multiple test correction adjusted P value [Padj] = 7.5 × 10−20), and sensitive tumors lacked expression of CALB1 (Figs 3B and 3C; Data Supplement). In set 2 (short v long PFS), CALB1 was among the top differentially expressed genes and its expression was significantly higher in tumors from patients with short (< 3 months) PFS (P = 2.54 × 10−05; Padj = .0091; Figs 3D and 3E; Data Supplement). Finally, paired post-treatment tumor tissues from a patient who initially responded to selinexor and then developed a resistant metastasis were available, and RNA sequencing showed that the selinexor-resistant tumor had 52-fold higher expression of CALB1 compared with the sensitive tumor (Fig 3F).
FIG 3.
CALB1 expression is associated with selinexor resistance. (A) Flow diagram of patient samples that were sequenced. (B) Volcano plot showing the significance (y-axis) and fold-change (x-axis) of all genes compared between sensitive and resistant tumors in the set 1 comparison using RECIST tumor size change. (C) Expression of CALB1 in the set 1 tumor samples. (D) Significance and fold-change of all genes in the set 2 comparison of patients with favorable and poor PFS. (E) Expression of CALB1 in set 2. (F) CT scans from a patient who had a mesentery lesion that steadily reduced in size over 4 months after starting selinexor treatment on SEAL and then remained stable. A scan on day 251 revealed a small nodule that rapidly grew while the patient continued selinexor treatment, until both tumors were resected on day 349. (G) PFS of phase III selinexor and placebo arm patients restricted to those patients without detectable CALB1. (H and I) PFS of phase III (H) selinexor or (I) placebo arm patients stratified by CALB1 expression. Shaded areas represent 95% CIs. HR, hazard ratio; PFS, progression-free survival; PR, partial response.
In an exploratory analysis of patients whose tumors had no detectable CALB1 expression (n = 30), those randomly assigned to selinexor (n = 16) had significantly improved PFS compared with placebo (6.9 v 2.2 months; HR = 0.19 [0.07 to 0.56], P = .001; Fig 3G; Data Supplement). In addition, among patients on the selinexor arm, those with no tumor CALB1 expression (n = 16/45) had improved PFS compared with those whose tumors expressed CALB1 (n = 29/45; 6.9 v 1.7 months; HR = 0.45 [0.21 to 0.95], P = .03; Fig 3H). There was no association between PFS and CALB1 expression among patients on the placebo arm (P = .44; Fig 3I; Data Supplement) or in an analysis of the DD-LPS cohort of The Cancer Genome Research Atlas (TCGA) database (Data Supplement).25
DISCUSSION
To our knowledge, SEAL is the first and largest global phase II-III trial focused exclusively on patients with relapsed and refractory DD-LPS who had received all agents of known clinical benefit. Despite the rarity of this tumor type (approximately 2,200 in the United States annually26), feasibility of randomized studies in rare cancers was enabled by international cooperation.
In patients with advanced, refractory DD-LPS, oral, twice-weekly selinexor showed a 30% improvement in PFS. In the context of the HR of 0.7, the modest improvement (2.8 months) in the median PFS over placebo (2.1 months) is consistent with a benefit in a subset of the population. Along these lines, at 12 weeks or longer, the PFS was significantly longer with selinexor compared with placebo: 46.8% v 34.0% (one-sided P = .02). In this context, in the first-line setting, the median PFS for single-agent doxorubicin was 1.5 months while combinations with ifosfamide ranged from 2 to 4 months.3-6 In the third-line setting, patients with DD-LPS randomly assigned to trabectedin also showed modest improvement in the median PFS of 2.2 months (v 1.9 months for dacarbazine) and did not show an improvement in OS (12.4 months v 12.9 months for dacarbazine; HR 0.87).7 Eribulin showed no improvement in median PFS (2.0 v 2.1 months for dacarbazine); however, in a small subset analysis of patients with DD-LPS, eribulin (n = 31) demonstrated a significant improvement in OS (HR, 0.42, 18.0 v 8.1 months) over dacarbazine (n = 34).8 Single-arm, phase II studies of CDK4/6 inhibitors palbociclib and abemaciclib have demonstrated median PFS ranging from 18 to 30 weeks with most patients treated in the first-line (37%-50%) or second-line setting.27-29 In contrast, 51.6% and 44.7% of patients in the SEAL study had failed two and three prior lines of therapies, respectively.
With crossover to selinexor allowed on confirmation of progression for patients on placebo, the SEAL trial was designed to assess noninferiority for OS as key secondary end point; overall, there was no OS difference between the groups (HR of 1.0). In addition, as compared with the patients on placebo who did not cross over, those randomly assigned to selinexor showed a trend toward improved OS (P = .06). Additionally, a significant improvement of time to next treatment was observed with selinexor (HR, 0.50). In patients with refractory DD-LPS whose disease has progressed on two to five prior lines of therapy, a median PFS of 2.8 months with single-agent oral selinexor, while modest, is in line with currently used cytotoxic chemotherapies.7,8 The ORR for selinexor in DD-LPS was low at 2.7%, which is similar to monotherapy with doxorubicin monotherapy, eribulin or trabectedin,4,6-8 and CDK4/627,29 or investigational MDM2 inhibitors30 that range from 0% to 8%.
Regarding HR-QoL, as recently published, a total of 255 patients completed baseline assessments, including 168 and 88 in the selinexor and placebo arms, respectively.31 At baseline, pain scores were significantly higher in the selinexor group. By day 169, patients treated with selinexor had significant reductions in pain compared with placebo.
AEs with selinexor were mostly grade 1 and grade 2, GI and/or constitutional and required prophylactic antiemetics, supportive care, and dose modifications and/or reductions; the vast majority were reversible and tolerable. Nausea, dysgeusia, decreased appetite, vomiting, fatigue, and weight loss were notable (all grades). These symptoms were also reported, albeit at lower frequencies, in the placebo arm and reflect, at least in part, the natural history of DD-LPS. Grade 3/4 anemia, thrombocytopenia, and neutropenia occurred in 18.7%, 10.2%, and 9.1%, respectively. Notably, no febrile neutropenia, mucositis, transaminitis, or alopecia was observed.
Twice-weekly oral selinexor provides the convenience of oral (including at home) administration and absence of neuropathy and transaminase elevations, which occurred in 19% and 45% with eribulin and trabectedin, respectively. However, there is a need to counsel and actively manage GI and constitutional AEs with supportive care, and dose modifications are important. Antiemetic regimens used with selinexor include 5-hydroxytryptamine-3 inhibitors, olanzapine and, if needed, low-dose steroids. This was reflected in patients on selinexor who reported worse HR-QoL (except for pain levels) primarily during the first 43 days. With time, the differences between selinexor and placebo dissipated, and this presumably reflects the investigators' increased experience at addressing TEAEs. Finally, patients on the selinexor arm had significant improvements in pain symptoms, a critical issue for patients with advanced DD-LPS.32
The study demonstrated an overall improvement in PFS of 30%, while median PFS improvement was modest. Thus, a subset of patients had a significant and durable benefit with selinexor. To better delineate which patients were more likely to benefit from selinexor, we conducted exploratory molecular biomarker studies using a representative subset of patients (Data Supplement). These analyses revealed that expression of CALB1, a calcium binding protein, was strongly associated with resistance to selinexor. We confirmed that CALB1 is not a prognostic marker in DD-LPS, as there was no association between CALB1 expression and outcomes in the placebo arm or the TCGA data. CALB1 was an unexpected discovery as it is highly expressed in the central nervous system and kidneys, where it acts as a buffer and calcium sensor.33 Although most human adult tissues do not express CALB1, its ectopic expression has been observed in several cancer types.34,35 Recent functional studies have demonstrated a novel oncogenic activity of CALB1 where it binds MDM2 to enhance MDM2-mediated suppression of p53 signaling.34 This is especially important in the context of DD-LPS, as MDM2 overexpression, leading to p53 degradation, is a hallmark of this disease. Treatment with selinexor forces nuclear retention and functional activation of p53, even in the presence of high MDM2 levels. Thus, we speculate that CALB1 expression could prevent selinexor from overcoming MDM2-mediated suppression of p53; laboratory work investigating this is ongoing.
In conclusion, to our knowledge, the SEAL trial was the first and largest study conducted exclusively on patients with heavily pretreated DD-LPS. The results of the SEAL study showed that the novel mechanism of action provided by single-agent oral selinexor conferred a 30% improvement in PFS with an important minority of patients deriving longer-term benefits as demonstrated by clinical improvements in 3-, 6-, and 12-month PFS. The most common AEs were typically low grade and reversible and could be mitigated with proactive supportive care. Further investigation is warranted for selinexor as a treatment for patients with DD-LPS with low or high CALB1 expression. Oral selinexor may represent a therapeutic option for patients with DD-LPS who have exhausted treatments of known clinical benefit.
Mrinal M. Gounder
Honoraria: Medscape, More Health, Physicians Education Resource, touchIME
Consulting or Advisory Role: Athenex, Ayala, Bayer, Boehringer Ingelheim, Daiichi, Epizyme, Karyopharm, Rain, Springworks, Tracon, TYME
Research Funding: National Cancer Institute, National Institutes of Health (P30CA008748)—core grant (CCSG shared resources and core facility for MSKCC)
Royalties: Wolters Kluwer, patents with MSKCC (GODDESS PRO), uncompensated research with Foundation Medicine
Other Relationship: Guidepoint, GLG, Third Bridge, Flatiron Health
Albiruni Abdul Razak
Consulting or Advisory Role: Merck, Adaptimmune, Bayer
Research Funding: Deciphera, Karyopharm Therapeutics, Pfizer, Roche/Genentech, Bristol Myers Squibb, MedImmune, Amgen, GlaxoSmithKline, Blueprint Medicines, Merck, AbbVie, Adaptimmune, Iterion Therapeutics
Neeta Somaiah
Consulting or Advisory Role: Bayer, Blueprint Medicines, Deciphera, Immune Design
Research Funding: AstraZeneca, Deciphera, Karyopharm, GSK, Daichii, Ascentage
Sant Chawla
Consulting or Advisory Role: Amgen, CytRx Corporation, GlaxoSmithKline, Ignyta, Immune Design, Janssen, Karyopharm Therapeutics, Roche, SARC: Sarcoma Alliance for Research though Collaboration, Threshold Pharmaceuticals, TRACON Pharma
Speakers' Bureau: Amgen, CytRx Corporation, GlaxoSmithKline, Ignyta, Immune Design, Janssen, Karyopharm Therapeutics, Roche, SARC: Sarcoma Alliance for Research though Collaboration, Threshold Pharmaceuticals, TRACON Pharma
Research Funding: Amgen, CytRx Corporation, GlaxoSmithKline, Ignyta, Immune Design, Janssen, Karyopharm Therapeutics, Roche, SARC: Sarcoma Alliance for Research though Collaboration, Threshold Pharmaceuticals, TRACON Pharma
Other Relationship: Amgen, CytRx Corporation, GlaxoSmithKline, Ignyta, Immune Design, Janssen, Karyopharm Therapeutics, Roche, SARC: Sarcoma Alliance for Research though Collaboration, Threshold Pharmaceuticals, TRACON Pharma
Javier Martin-Broto
Leadership: Chairman of Spanish Sarcoma Group (GEIS) 2010–October 2018, GEIS Leadership Role. Vice-chair 2018-ongoing, CTOS Leadership Role. Board of directors 2015-2017, ESMO Leadership Role. Chair sarcoma faculty member 2018-2020, SELNET Leadership Role. Sarcoma European & Latin American Network
Consulting or Advisory Role: PharmaMar, Eli-Lilly, Bayer, Eisai, Roche, Daichii, Eli-Lilly, PharmaMar, Roche
Speakers' Bureau: PharmaMar, Eli-Lilly, Bayer, Eisai, Roche, Daichii
Research Funding: Lilly, PharmaMar, GSK, Eisai, Novartis, IMMIX Biopharma, Eisai, Daiichi Sankyo, Karyopharm, Celgene, Pfizer, BMS, Blueprint, Deciphera, Nektar, Forma, Amgen, Lixte
Giovanni Grignani
Honoraria: Bayer, Novartis, Lilly, Pfizer, Merck Serono, EISAI, PharmaMar, GlaxoSmithKline
Consulting or Advisory Role: EISAI, PharmaMar, Bayer, Merck, GlaxoSmithKline
Speakers’ Bureau: GlaxoSmithKline
Research Funding: PharmaMar (Inst)
Travel, Accommodations, Expenses: PharmaMar, Tesaro
Scott M. Schuetze
Research Funding: Adaptimmune, Amgen, Blueprint Medicines, GlaxoSmithKline, Karyopharm Therapeutics
Other Relationship: Blueprint Medicines
Bruno Vincenzi
Consulting or Advisory Role: Lilly, GlaxoSmithKline, Abbott
Speakers’ Bureau: PharmaMar
Research Funding: BD Bard
Andrew J. Wagner
Honoraria: Deciphera
Consulting or Advisory Role: Lilly, Daiichi Sankyo, Deciphera, Mundipharma, Cogent Biosciences, Epizyme, Boehringer Ingelheim, AADi
Research Funding: Lilly (Inst), Plexxikon (Inst), Daiichi Sankyo (Inst), Karyopharm Therapeutics (Inst), Deciphera (Inst), Foghorn Therapeutics (Inst), AADi (Inst), Rain Therapeutics (Inst)
Bartosz Chmielowski
Consulting or Advisory Role: Iovance Biotherapeutics, IDEAYA Biosciences, Sanofi, OncoSec, Genentech, Nektar, Novartis
Research Funding: Bristol Myers Squibb (Inst), Macrogenics (Inst), Array BioPharma (Inst), Daiichi Sankyo (Inst), Merck (Inst), Karyopharm Therapeutics (Inst), Infinity Pharmaceuticals (Inst), Rgenix (Inst), Biothera (Inst), Advenchen Laboratories (Inst), Idera (Inst), Neon Therapeutics (Inst), Xencor (Inst), Compugen (Inst), Iovance Biotherapeutics (Inst), PACT Pharma (Inst), RAPT Therapeutics (Inst), Immunocore (Inst), Lilly (Inst), IDEAYA Biosciences (Inst), Tolero Pharmaceuticals (Inst), Ascentage Pharma (Inst), Novartis (Inst), Atreca (Inst), Replimune (Inst), InstilBio (Inst), InstilBio (Inst)
Robin L. Jones
Consulting Fees: Adaptimmune, Athenex, Bayer, Boehringer Ingelheim, Blueprint, Clinigen, Eisai, Epizyme, Daichii, Deciphera, Immunedesign, Lilly, Merck, Pharmamar, Springworks, Tracon, UptoDate
Richard F. Riedel
Employment: Limbguard
Stock and Other Ownership Interests: Limbguard
Consulting or Advisory Role: Bayer, Blueprint, Daiichi Sankyo, Deciphera, Ignyta, Lilly, Loxo, NanoCarrier, Springworks
Research Funding: AADi, Arog, Bayer, Blueprint, Daiichi Sankyo, Deciphera, GSK, Ignyta, Immune Design, Karyopharm Therapeutics, Lilly, NanoCarrier, Oncternal, Plexxikon, Roche/Genentech, Springworks, TRACON Pharma
Patents, Royalties, Other Intellectual Property: PandoNet—Limbguard
Other Relationship: Daiichi Sankyo, Ignyta, NanoCarrier
Silvia Stacchiotti
Consulting or Advisory Role: Bavarian Nordic, Bayer, Daiichi Sankyo, Deciphera, Epizyme, Immune Design, Lilly, MaxiVax, PharmaMar
Research Funding: Advenchen Laboratories, Amgen, Bayer, Blueprint Medicines, Daiichi Sankyo, Epizyme, Karyopharm, Lilly, Novartis, Pfizer, PharmaMar
Other Relationship: Lilly, Takeda, PharmaMar
Elizabeth T. Loggers
Research Funding: Epizyme (Inst), Karyopharm Therapeutics (Inst), SpringWorks Therapeutics (Inst)
Kristen Ganjoo
Consulting or Advisory Role: Daiichi Sankyo, Foundation Medicine
Axel Le Cesne
Honoraria: Bayer, PharmaMar, Deciphera
Antoine Italiano
Honoraria: Bayer, Daiichi Sankyo, Lilly, Epizyme, Novartis, Roche, IPSEN
Consulting or Advisory Role: Roche, Daiichi Sankyo, Immune Design, Epizyme, Bayer, Lilly
Research Funding: Roche, Bayer, AstraZeneca/MedImmune, PharmaMar, MSD Oncology, Merck Serono
Patents, Royalties, Other Intellectual Property: BMS
Xavier Garcia del Muro
Consulting or Advisory Role: Bristol-Myers Squibb, EusaPharma, Ipsen, Lilly, Pfizer, PharmaMar, Roche
Speakers' Bureau: Astellas Pharma, Bristol-Myers Squibb, Ipsen, Pfizer, PharmaMar
Research Funding: AstraZeneca
Other Relationship: Bristol-Myers Squibb, Pfizer, Roche
Melissa Burgess
Consulting or Advisory Role: EMD Serono
Research Funding: Merck & Co
Other Relationship: SpringWorks Therapeutics
Uncompensated Relationships: TRACON Pharmaceuticals
Sophie Piperno-Neumann
Consulting or Advisory Role: Immunocore, Atlanthera
Christopher Ryan
Consulting or Advisory Role: AstraZeneca, AVEO, Bristol Myers Squibb, Daiichi Sankyo, Exelixis, Partner Therapeutics, Synox
Research Funding: Bristol Myers Squibb (Inst), Daiichi Sankyo (Inst), Exelixis (Inst), Genentech (Inst), GlaxoSmithKline/Novartis (Inst), Karyopharm Therapeutics (Inst), Merck (Inst), Pfizer (Inst), Xynomic Pharma (Inst), Nektar (Inst), Leducq (Inst)
Charles Forscher
Speakers’ Bureau: Deciphera
Anthony Elias
Stock and Other Ownership Interests: AbbVie, Merck, Gilead Sciences, Allergan, Pfizer, Abbott Laboratories, Amgen, Bristol Myers Squibb, United Health Group, Align Oncology, Illumina, Exact Sciences, Lilly, Agilent, Cigna, Alexion Pharmaceuticals, Biogenerix
Research Funding: Astellas Pharma (Inst), Genentech (Inst), Deciphera (Inst), Xencor (Inst), Infinity Pharmaceuticals (Inst), Karyopharm Therapeutics (Inst), TopAlliance BioSciences Inc (Inst), Orinove (Inst), BioAtla (Inst)
Uncompensated Relationships: Seiyax
Tony Philip
Consulting or Advisory Role: Daiichi Sankyo, Deciphera
Thierry Alcindor
Consulting or Advisory Role: Amgen, Bayer, Bristol-Myers Squibb, Eisai, Lilly, Merck, Novartis, Canada Pharmaceuticals, Pfizer/EMD Serono, Roche Canada, Taiho Pharmaceutical
Bernd Kasper
Honoraria: Bayer, GlaxoSmithKline, Pharmamar-zeltia
Consulting or Advisory Role: Ayala Pharmaceuticals, Bayer, Blueprint Medicines, GlaxoSmithKline, SpringWorks Therapeutics
Peter Reichardt
Consulting or Advisory Role: Bayer, Clinigen, BMS, Roche, MSD, Deciphera, Novartis, Pfizer, PharmaMar, Lilly, Amgen
Jean-Yves Blay
Honoraria: Novartis, GSK, Bayer, Roche, Deciphera, Ignyta, BMS, MSD, Pharmamar, Karyopharm
Research Support: Novartis, GSK, Bayer, Roche, Deciphera, Ignyta, BMS, MSD, Pharmamar, Karyopharm
Christine Chevreau
Consulting or Advisory Role: Bristol Myers Squibb, Ipsen, Pfizer, EISAI, GlaxoSmithKline
Travel, Accommodations, Expenses: Ipsen
Claudia Maria Valverde Morales
Consulting or Advisory Role: Lilly, PharmaMar, Pfizer, Eisai, Bayer, Mundipharma, GlaxoSmithKline
Research Funding: Lilly (Inst), Novartis (Inst), Pfizer (Inst), PharmaMar (Inst), Karyopharm Therapeutics (Inst), Incyte (Inst), Adaptimmune (Inst), GlaxoSmithKline (Inst)
Travel, Accommodations, Expenses: PharmaMar, Lilly, Novartis, Pfizer, Bayer, Rovi
Gary K. Schwartz
Stock and Other Ownership Interests: Pfizer
Consulting or Advisory Role: Bionaut Labs, Ellipses Pharma, Gencirq, Epizyme, Array BioPharma, Apexigen, Oncogenuity, OnCusp, Concarlo, Shanghai Pharma, Astex Pharmaceuticals, January Therapeutics, Sellas Life Sciences, Purtech
Research Funding: Astex Pharmaceuticals, Incyte (Inst), Calithera Biosciences (Inst), Lilly (Inst), Daiichi Sankyo (Inst), Fortress Biotech (Inst), Karyopharm Therapeutics (Inst), Oxford BioTherapeutics (Inst), Astex Pharmaceuticals (Inst), TopAlliance BioSciences Inc (Inst), Adaptimmune (Inst), Clovis Oncology (Inst), SpringWorks Therapeutics (Inst), TRACON Pharma (Inst)
Patents, Royalties, Other Intellectual Property: Companion diagnostics for CD4 inhibitors (Inst), patent granted to develop a new technology called PNAs for cancer therapy
Travel, Accommodations, Expenses: Array BioPharma, Epizyme
James L. Chen
Consulting or Advisory Role: Syapse, Tempus
Speakers’ Bureau: Foundation Medicine
Research Funding: Eisai
Patents, Royalties, Other Intellectual Property: MatchTX
Hari Deshpande
Honoraria: Daiichi Sankyo, Deciphera, Blueprint Medicines, Exelixis
Consulting or Advisory Role: Daiichi Sankyo, Deciphera, Blueprint Medicines, Exelixis
Research Funding: Deciphera, SpringWorks Therapeutics, Eisai
Travel, Accommodations, Expenses: Deciphera, Daiichi Sankyo
Open Payments Link: https://openpaymentsdata.cms.gov/physician/156300
Elizabeth J. Davis
Honoraria: MJH Life Sciences
Consulting or Advisory Role: Deciphera
Speakers’ Bureau: Physicans’ Education Resource
Research Funding: Incyte (Inst), Five Prime Therapeutics (Inst), Genentech (Inst), Karyopharm Therapeutics (Inst), Bristol Myers Squibb (Inst), Actuate Therapeutics (Inst), TopAlliance BioSciences Inc (Inst)
Florence Duffaud
Consulting or Advisory Role: Bayer Health, BluPrint Oncology, GlaxoSmithKline
Travel, Accommodations, Expenses: PharmaMar, Leo Pharma
Antonio Casado Herráez
Consulting or Advisory Role: Roche, PharmaMar, EISAI, Merck Sharp & Dohme
Research Funding: Pharmamar (Inst)
Travel, Accommodations, Expenses: Pharmamar, Roche, Lilly Spain
Other Relationship: Lilly (Inst)
Mikael Eriksson
Consultant or Advisory Role: Blueprint Medicines, Clinigen, Bayer
Other Relationship: Trial physician in the Scandinavian Sarcoma Group that receives trial support from Novartis
Christian Meyer
Consulting or Advisory Role: Deciphera, Intellisphere, AADi
Speakers’ Bureau: Novartis
Other Relationship: UpToDate
Margaret von Mehren
Honoraria: Deciphera, NCCN, Blueprint
Consulting or Advisory Role: Deciphera, NCCN, Blueprint
Research Funding: Novartis
Travel, Accommodations, Expenses: Deciphera, NCCN, Blueprint
Other Relationship: Arog, GenMab, ASCO, Gradalis
Brian A. Van Tine
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Leadership: Polaris
Honoraria: Bionest Partner, Horizon CME, Research to Practice
Consulting or Advisory Role: EMD Serono, Novartis, Epizyme, Daiihi Sankyo, Pfizer, Adaptimmune, Bayer, GlaxoSmithKline, Lilly, Cytokinetics, Apexigen, Deciphera Pharmaceuticals, Immune Design, ADRx, Ayala Pharmaceuticals, Intellisphere
Speakers’ Bureau: Novartis, Lilly, Adaptimmune, GlaxoSmithKline
Research Funding: Pfizer, Merck, TRACON Pharma, GlaxoSmithKline
Patents, Royalties, Other Intellectual Property: Patent on the use of ME1 as a biomarker, patent on ALEXT3102, Accuronix Therapeutics–Licensing agreement. Sigma-2 Receptor Ligands and Therapeutic uses therefor (006766), Modular Platform for Targeted Therapeutic Delivery (006755), Sigma-2 Receptor Ligand Drug Conjugates as Antitumor Compounds, Methods of synthesis and Uses Thereof (014229)
Expert Testimony: Health Advances
Travel, Accommodations, Expenses: Adaptimmune, Advenchen Laboratories, GlaxoSmithKline, Lilly
Aviad Zick
Research Funding: Merck (Inst), Roche Molecular Diagnostics (Inst), Karyopharm Therapeutics (Inst)
Alexander Lee
Consulting or Advisory Role: Astex Pharmaceuticals, AstraZeneca/MedImmune
Patents, Royalties, Other Intellectual Property: Material and methods for stratifying and treating cancers
Anna Estival Gonzalez
Honoraria: Roche, MSD Oncology, AstraZeneca Spain, Bayer, PharmaMar, Takeda
Travel, Accommodations, Expenses: Lilly, Roche, Roche, PharmaMar, Bristol Myers Squibb, Bayer, MSD, Pfizer, AstraZeneca Spain, Takeda
Mark A. Dickson
Employment: Memorial Sloan Kettering Cancer Center
Research Funding: Karyopharm Inc, AADI, Eli-Lilly, National Institutes of Health/National Cancer Institute Cancer Center Support Grant (P30CA008748)
Dayana Michel
Employment: Karyopharm Therapeutics
Travel, Accommodations, Expenses: Karyopharm Therapeutics
Changting Meng
Employment: Karyopharm Therapeutics
Stock and Other Ownership Interests: Karyopharm Therapeutics
Travel, Accommodations, Expenses: Karyopharm Therapeutics
Jianjun Liu
Employment: Karyopharm Therapeutics
Stock and Other Ownership Interests: Karyopharm Therapeutics
Osnat Ben-Shahar
Employment: Karyopharm Therapeutics
Stock and Other Ownership Interests: Karyopharm Therapeutics
Dane R. Van Domelen
Employment: Karyopharm Therapeutics
Stock and Other Ownership Interests: Karyopharm Therapeutics
Christopher J. Walker
Employment: Karyopharm Therapeutics
Stock and Other Ownership Interests: Karyopharm Therapeutics
Patents, Royalties, Other Intellectual Property: Pending patents for biomarkers related to selinexor efficacy
Travel, Accommodations, Expenses: Karyopharm Therapeutics
Hua Chang
Employment: Karyopharm Therapeutics
Stock and Other Ownership Interests: Karyopharm Therapeutics
Travel, Accommodations, Expenses: Karyopharm Therapeutics
Yosef Landesman
Employment: Karyopharm Therapeutics
Stock and Other Ownership Interests: Karyopharm Therapeutics
Jatin J. Shah
Employment: Karyopharm Therapeutics
Stock and Other Ownership Interests: Karyopharm Therapeutics
Sharon Shacham
Employment: Karyopharm Therapeutics
Stock and Other Ownership Interests: Karyopharm Therapeutics
Patents, Royalties, Other Intellectual Property: 8999996, 9079865, 9714226, PCT/US12/048319, and I574957 on hydrazide-containing nuclear transport modulators and uses. Pending patents: PCT/US12/048319, 499/2012, PI20102724, and 2012000928 on hydrazide-containing nuclear transport modulators and uses
Michael G. Kauffman
Employment: Karyopharm Therapeutics
Stock: Karyopharm Therapeutics
Steven Attia
Research Funding: AB Science, Adaptimmune, Advenchen Laboratories, Bavarian Nordic, Bayer, Blueprint Medicines, BTG, CBA Pharma, CytRx Corporation, Daiichi Sankyo, Deciphera, Desmoid Tumor Research Foundation, Epizyme, FORMA Therapeutics, Genmab, GlaxoSmithKline, Gradalis, Immune Design, Incyte, Karyopharm Therapeutics, Lilly, Merck, Novartis, Philogen, PTC Therapeutics, Takeda, TRACON Pharma
Other Relationship: Immune Design
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the Connective Tissue Oncology Society Virtual Annual Meeting, November 18-21, 2020.
SUPPORT
Supported by research funding from Karyopharm Therapeutics, Inc. JetPub Scientific Communications LLC, supported by Karyopharm Therapeutics, Inc, assisted in the preparation of this manuscript in accordance with Good Publication Practice (GPP3) guidelines.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
Karyopharm Therapeutics agrees to share individual participant data that underlie the results reported in this article (after deidentification), including the study protocol and statistical analysis plan. Data availability will begin 9 months after publication and will be available 36 months after publication. To gain access, data requestors should submit a proposal to medicalinformation@karyopharm.com. Proposals will be reviewed by an independent review committee identified for this purpose.
AUTHOR CONTRIBUTIONS
Conception and design: Mrinal M. Gounder, Neeta Somaiah, Sant Chawla, Robin L. Jones, Silvia Stacchiotti, Gary K. Schwartz, Antonio Casado Herráez, Roberto Diaz Beveridge, Mark A. Dickson, Dayana Michel, Lingling Li, Sharon Shacham, Michael G. Kauffman, Steven Attia
Provision of study materials or patients: Mrinal M. Gounder, Albiruni Abdul Razak, Neeta Somaiah, Javier Martin-Broto, Scott M. Schuetze, Bruno Vincenzi, Andrew J. Wagner, Bartosz Chmielowski, Robin L. Jones, Richard F. Riedel, Silvia Stacchiotti, Elizabeth T. Loggers, Kristen N. Ganjoo, Antoine Italiano, Christopher Ryan, Nicolas Penel, Scott Okuno, Anthony Elias, Tony Philip, Bernd Kasper, Peter Reichardt, Lore Lapeire, Jean-Yves Blay, Christine Chevreau, Claudia Maria Valverde Morales, Gary K. Schwartz, James L. Chen, Elizabeth J. Davis, Garth Nicholas, Stefan Gröschel, Helen Hatcher, Antonio Casado Herráez, Christian Meyer, Margaret von Mehren, Katharina Götze, Filomena Mazzeo, Alexander Lee, Mark A. Dickson, Hua Chang
Collection and assembly of data: Mrinal M. Gounder, Albiruni Abdul Razak, Neeta Somaiah, Sant Chawla, Javier Martin-Broto, Giovanni Grignani, Scott M. Schuetze, Bruno Vincenzi, Andrew J. Wagner, Bartosz Chmielowski, Robin L. Jones, Richard F. Riedel, Silvia Stacchiotti, Elizabeth T. Loggers, Kristen N. Ganjoo, Antoine Italiano, Xavier Garcia del Muro, Melissa Burgess, Sophie Piperno-Neumann, Christopher Ryan, Mary F. Mulcahy, Charles Forscher, Nicolas Penel, Scott Okuno, Lee Hartner, Tony Philip, Bernd Kasper, Peter Reichardt, Lore Lapeire, Jean-Yves Blay, Christine Chevreau, Claudia Maria Valverde Morales, Gary K. Schwartz, James L. Chen, Elizabeth J. Davis, Garth Nicholas, Stefan Gröschel, Helen Hatcher, Antonio Casado Herráez, Roberto Diaz Beveridge, Giuseppe Badalamenti, Mikael Eriksson, Margaret von Mehren, Brian A. Van Tine, Katharina Götze, Alexander Yakobson, Aviad Zick, Alexander Lee, Anna Estival Gonzalez, Andrea Napolitano, Mark A. Dickson, Dayana Michel, Lingling Li, Hua Chang
Data analysis and interpretation: Mrinal M. Gounder, Albiruni Abdul Razak, Sant Chawla, Giovanni Grignani, Scott M. Schuetze, Bruno Vincenzi, Andrew J. Wagner, Bartosz Chmielowski, Robin L. Jones, Silvia Stacchiotti, Elizabeth T. Loggers, Kristen N. Ganjoo, Axel Le Cesne, Antoine Italiano, Melissa Burgess, Scott Okuno, Anthony Elias, Tony Philip, Thierry Alcindor, Bernd Kasper, Peter Reichardt, Claudia Maria Valverde Morales, Gary K. Schwartz, James L. Chen, Hari Deshpande, Elizabeth J. Davis, Garth Nicholas, Florence Duffaud, Antonio Casado Herráez, Roberto Diaz Beveridge, Christian Meyer, Filomena Mazzeo, Alexander Lee, Mark A. Dickson, Dayana Michel, Lingling Li, Jianjun Liu, Osnat Ben-Shahar, Dane R. Van Domelen, Christopher J. Walker, Hua Chang, Yosef Landesman, Jatin J. Shah, Sharon Shacham, Michael G. Kauffman, Steven Attia
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Selinexor in Advanced, Metastatic Dedifferentiated Liposarcoma: A Multinational, Randomized, Double-Blind, Placebo-Controlled Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Mrinal M. Gounder
Honoraria: Medscape, More Health, Physicians Education Resource, touchIME
Consulting or Advisory Role: Athenex, Ayala, Bayer, Boehringer Ingelheim, Daiichi, Epizyme, Karyopharm, Rain, Springworks, Tracon, TYME
Research Funding: National Cancer Institute, National Institutes of Health (P30CA008748)—core grant (CCSG shared resources and core facility for MSKCC)
Royalties: Wolters Kluwer, patents with MSKCC (GODDESS PRO), uncompensated research with Foundation Medicine
Other Relationship: Guidepoint, GLG, Third Bridge, Flatiron Health
Albiruni Abdul Razak
Consulting or Advisory Role: Merck, Adaptimmune, Bayer
Research Funding: Deciphera, Karyopharm Therapeutics, Pfizer, Roche/Genentech, Bristol Myers Squibb, MedImmune, Amgen, GlaxoSmithKline, Blueprint Medicines, Merck, AbbVie, Adaptimmune, Iterion Therapeutics
Neeta Somaiah
Consulting or Advisory Role: Bayer, Blueprint Medicines, Deciphera, Immune Design
Research Funding: AstraZeneca, Deciphera, Karyopharm, GSK, Daichii, Ascentage
Sant Chawla
Consulting or Advisory Role: Amgen, CytRx Corporation, GlaxoSmithKline, Ignyta, Immune Design, Janssen, Karyopharm Therapeutics, Roche, SARC: Sarcoma Alliance for Research though Collaboration, Threshold Pharmaceuticals, TRACON Pharma
Speakers' Bureau: Amgen, CytRx Corporation, GlaxoSmithKline, Ignyta, Immune Design, Janssen, Karyopharm Therapeutics, Roche, SARC: Sarcoma Alliance for Research though Collaboration, Threshold Pharmaceuticals, TRACON Pharma
Research Funding: Amgen, CytRx Corporation, GlaxoSmithKline, Ignyta, Immune Design, Janssen, Karyopharm Therapeutics, Roche, SARC: Sarcoma Alliance for Research though Collaboration, Threshold Pharmaceuticals, TRACON Pharma
Other Relationship: Amgen, CytRx Corporation, GlaxoSmithKline, Ignyta, Immune Design, Janssen, Karyopharm Therapeutics, Roche, SARC: Sarcoma Alliance for Research though Collaboration, Threshold Pharmaceuticals, TRACON Pharma
Javier Martin-Broto
Leadership: Chairman of Spanish Sarcoma Group (GEIS) 2010–October 2018, GEIS Leadership Role. Vice-chair 2018-ongoing, CTOS Leadership Role. Board of directors 2015-2017, ESMO Leadership Role. Chair sarcoma faculty member 2018-2020, SELNET Leadership Role. Sarcoma European & Latin American Network
Consulting or Advisory Role: PharmaMar, Eli-Lilly, Bayer, Eisai, Roche, Daichii, Eli-Lilly, PharmaMar, Roche
Speakers' Bureau: PharmaMar, Eli-Lilly, Bayer, Eisai, Roche, Daichii
Research Funding: Lilly, PharmaMar, GSK, Eisai, Novartis, IMMIX Biopharma, Eisai, Daiichi Sankyo, Karyopharm, Celgene, Pfizer, BMS, Blueprint, Deciphera, Nektar, Forma, Amgen, Lixte
Giovanni Grignani
Honoraria: Bayer, Novartis, Lilly, Pfizer, Merck Serono, EISAI, PharmaMar, GlaxoSmithKline
Consulting or Advisory Role: EISAI, PharmaMar, Bayer, Merck, GlaxoSmithKline
Speakers’ Bureau: GlaxoSmithKline
Research Funding: PharmaMar (Inst)
Travel, Accommodations, Expenses: PharmaMar, Tesaro
Scott M. Schuetze
Research Funding: Adaptimmune, Amgen, Blueprint Medicines, GlaxoSmithKline, Karyopharm Therapeutics
Other Relationship: Blueprint Medicines
Bruno Vincenzi
Consulting or Advisory Role: Lilly, GlaxoSmithKline, Abbott
Speakers’ Bureau: PharmaMar
Research Funding: BD Bard
Andrew J. Wagner
Honoraria: Deciphera
Consulting or Advisory Role: Lilly, Daiichi Sankyo, Deciphera, Mundipharma, Cogent Biosciences, Epizyme, Boehringer Ingelheim, AADi
Research Funding: Lilly (Inst), Plexxikon (Inst), Daiichi Sankyo (Inst), Karyopharm Therapeutics (Inst), Deciphera (Inst), Foghorn Therapeutics (Inst), AADi (Inst), Rain Therapeutics (Inst)
Bartosz Chmielowski
Consulting or Advisory Role: Iovance Biotherapeutics, IDEAYA Biosciences, Sanofi, OncoSec, Genentech, Nektar, Novartis
Research Funding: Bristol Myers Squibb (Inst), Macrogenics (Inst), Array BioPharma (Inst), Daiichi Sankyo (Inst), Merck (Inst), Karyopharm Therapeutics (Inst), Infinity Pharmaceuticals (Inst), Rgenix (Inst), Biothera (Inst), Advenchen Laboratories (Inst), Idera (Inst), Neon Therapeutics (Inst), Xencor (Inst), Compugen (Inst), Iovance Biotherapeutics (Inst), PACT Pharma (Inst), RAPT Therapeutics (Inst), Immunocore (Inst), Lilly (Inst), IDEAYA Biosciences (Inst), Tolero Pharmaceuticals (Inst), Ascentage Pharma (Inst), Novartis (Inst), Atreca (Inst), Replimune (Inst), InstilBio (Inst), InstilBio (Inst)
Robin L. Jones
Consulting Fees: Adaptimmune, Athenex, Bayer, Boehringer Ingelheim, Blueprint, Clinigen, Eisai, Epizyme, Daichii, Deciphera, Immunedesign, Lilly, Merck, Pharmamar, Springworks, Tracon, UptoDate
Richard F. Riedel
Employment: Limbguard
Stock and Other Ownership Interests: Limbguard
Consulting or Advisory Role: Bayer, Blueprint, Daiichi Sankyo, Deciphera, Ignyta, Lilly, Loxo, NanoCarrier, Springworks
Research Funding: AADi, Arog, Bayer, Blueprint, Daiichi Sankyo, Deciphera, GSK, Ignyta, Immune Design, Karyopharm Therapeutics, Lilly, NanoCarrier, Oncternal, Plexxikon, Roche/Genentech, Springworks, TRACON Pharma
Patents, Royalties, Other Intellectual Property: PandoNet—Limbguard
Other Relationship: Daiichi Sankyo, Ignyta, NanoCarrier
Silvia Stacchiotti
Consulting or Advisory Role: Bavarian Nordic, Bayer, Daiichi Sankyo, Deciphera, Epizyme, Immune Design, Lilly, MaxiVax, PharmaMar
Research Funding: Advenchen Laboratories, Amgen, Bayer, Blueprint Medicines, Daiichi Sankyo, Epizyme, Karyopharm, Lilly, Novartis, Pfizer, PharmaMar
Other Relationship: Lilly, Takeda, PharmaMar
Elizabeth T. Loggers
Research Funding: Epizyme (Inst), Karyopharm Therapeutics (Inst), SpringWorks Therapeutics (Inst)
Kristen Ganjoo
Consulting or Advisory Role: Daiichi Sankyo, Foundation Medicine
Axel Le Cesne
Honoraria: Bayer, PharmaMar, Deciphera
Antoine Italiano
Honoraria: Bayer, Daiichi Sankyo, Lilly, Epizyme, Novartis, Roche, IPSEN
Consulting or Advisory Role: Roche, Daiichi Sankyo, Immune Design, Epizyme, Bayer, Lilly
Research Funding: Roche, Bayer, AstraZeneca/MedImmune, PharmaMar, MSD Oncology, Merck Serono
Patents, Royalties, Other Intellectual Property: BMS
Xavier Garcia del Muro
Consulting or Advisory Role: Bristol-Myers Squibb, EusaPharma, Ipsen, Lilly, Pfizer, PharmaMar, Roche
Speakers' Bureau: Astellas Pharma, Bristol-Myers Squibb, Ipsen, Pfizer, PharmaMar
Research Funding: AstraZeneca
Other Relationship: Bristol-Myers Squibb, Pfizer, Roche
Melissa Burgess
Consulting or Advisory Role: EMD Serono
Research Funding: Merck & Co
Other Relationship: SpringWorks Therapeutics
Uncompensated Relationships: TRACON Pharmaceuticals
Sophie Piperno-Neumann
Consulting or Advisory Role: Immunocore, Atlanthera
Christopher Ryan
Consulting or Advisory Role: AstraZeneca, AVEO, Bristol Myers Squibb, Daiichi Sankyo, Exelixis, Partner Therapeutics, Synox
Research Funding: Bristol Myers Squibb (Inst), Daiichi Sankyo (Inst), Exelixis (Inst), Genentech (Inst), GlaxoSmithKline/Novartis (Inst), Karyopharm Therapeutics (Inst), Merck (Inst), Pfizer (Inst), Xynomic Pharma (Inst), Nektar (Inst), Leducq (Inst)
Charles Forscher
Speakers’ Bureau: Deciphera
Anthony Elias
Stock and Other Ownership Interests: AbbVie, Merck, Gilead Sciences, Allergan, Pfizer, Abbott Laboratories, Amgen, Bristol Myers Squibb, United Health Group, Align Oncology, Illumina, Exact Sciences, Lilly, Agilent, Cigna, Alexion Pharmaceuticals, Biogenerix
Research Funding: Astellas Pharma (Inst), Genentech (Inst), Deciphera (Inst), Xencor (Inst), Infinity Pharmaceuticals (Inst), Karyopharm Therapeutics (Inst), TopAlliance BioSciences Inc (Inst), Orinove (Inst), BioAtla (Inst)
Uncompensated Relationships: Seiyax
Tony Philip
Consulting or Advisory Role: Daiichi Sankyo, Deciphera
Thierry Alcindor
Consulting or Advisory Role: Amgen, Bayer, Bristol-Myers Squibb, Eisai, Lilly, Merck, Novartis, Canada Pharmaceuticals, Pfizer/EMD Serono, Roche Canada, Taiho Pharmaceutical
Bernd Kasper
Honoraria: Bayer, GlaxoSmithKline, Pharmamar-zeltia
Consulting or Advisory Role: Ayala Pharmaceuticals, Bayer, Blueprint Medicines, GlaxoSmithKline, SpringWorks Therapeutics
Peter Reichardt
Consulting or Advisory Role: Bayer, Clinigen, BMS, Roche, MSD, Deciphera, Novartis, Pfizer, PharmaMar, Lilly, Amgen
Jean-Yves Blay
Honoraria: Novartis, GSK, Bayer, Roche, Deciphera, Ignyta, BMS, MSD, Pharmamar, Karyopharm
Research Support: Novartis, GSK, Bayer, Roche, Deciphera, Ignyta, BMS, MSD, Pharmamar, Karyopharm
Christine Chevreau
Consulting or Advisory Role: Bristol Myers Squibb, Ipsen, Pfizer, EISAI, GlaxoSmithKline
Travel, Accommodations, Expenses: Ipsen
Claudia Maria Valverde Morales
Consulting or Advisory Role: Lilly, PharmaMar, Pfizer, Eisai, Bayer, Mundipharma, GlaxoSmithKline
Research Funding: Lilly (Inst), Novartis (Inst), Pfizer (Inst), PharmaMar (Inst), Karyopharm Therapeutics (Inst), Incyte (Inst), Adaptimmune (Inst), GlaxoSmithKline (Inst)
Travel, Accommodations, Expenses: PharmaMar, Lilly, Novartis, Pfizer, Bayer, Rovi
Gary K. Schwartz
Stock and Other Ownership Interests: Pfizer
Consulting or Advisory Role: Bionaut Labs, Ellipses Pharma, Gencirq, Epizyme, Array BioPharma, Apexigen, Oncogenuity, OnCusp, Concarlo, Shanghai Pharma, Astex Pharmaceuticals, January Therapeutics, Sellas Life Sciences, Purtech
Research Funding: Astex Pharmaceuticals, Incyte (Inst), Calithera Biosciences (Inst), Lilly (Inst), Daiichi Sankyo (Inst), Fortress Biotech (Inst), Karyopharm Therapeutics (Inst), Oxford BioTherapeutics (Inst), Astex Pharmaceuticals (Inst), TopAlliance BioSciences Inc (Inst), Adaptimmune (Inst), Clovis Oncology (Inst), SpringWorks Therapeutics (Inst), TRACON Pharma (Inst)
Patents, Royalties, Other Intellectual Property: Companion diagnostics for CD4 inhibitors (Inst), patent granted to develop a new technology called PNAs for cancer therapy
Travel, Accommodations, Expenses: Array BioPharma, Epizyme
James L. Chen
Consulting or Advisory Role: Syapse, Tempus
Speakers’ Bureau: Foundation Medicine
Research Funding: Eisai
Patents, Royalties, Other Intellectual Property: MatchTX
Hari Deshpande
Honoraria: Daiichi Sankyo, Deciphera, Blueprint Medicines, Exelixis
Consulting or Advisory Role: Daiichi Sankyo, Deciphera, Blueprint Medicines, Exelixis
Research Funding: Deciphera, SpringWorks Therapeutics, Eisai
Travel, Accommodations, Expenses: Deciphera, Daiichi Sankyo
Open Payments Link: https://openpaymentsdata.cms.gov/physician/156300
Elizabeth J. Davis
Honoraria: MJH Life Sciences
Consulting or Advisory Role: Deciphera
Speakers’ Bureau: Physicans’ Education Resource
Research Funding: Incyte (Inst), Five Prime Therapeutics (Inst), Genentech (Inst), Karyopharm Therapeutics (Inst), Bristol Myers Squibb (Inst), Actuate Therapeutics (Inst), TopAlliance BioSciences Inc (Inst)
Florence Duffaud
Consulting or Advisory Role: Bayer Health, BluPrint Oncology, GlaxoSmithKline
Travel, Accommodations, Expenses: PharmaMar, Leo Pharma
Antonio Casado Herráez
Consulting or Advisory Role: Roche, PharmaMar, EISAI, Merck Sharp & Dohme
Research Funding: Pharmamar (Inst)
Travel, Accommodations, Expenses: Pharmamar, Roche, Lilly Spain
Other Relationship: Lilly (Inst)
Mikael Eriksson
Consultant or Advisory Role: Blueprint Medicines, Clinigen, Bayer
Other Relationship: Trial physician in the Scandinavian Sarcoma Group that receives trial support from Novartis
Christian Meyer
Consulting or Advisory Role: Deciphera, Intellisphere, AADi
Speakers’ Bureau: Novartis
Other Relationship: UpToDate
Margaret von Mehren
Honoraria: Deciphera, NCCN, Blueprint
Consulting or Advisory Role: Deciphera, NCCN, Blueprint
Research Funding: Novartis
Travel, Accommodations, Expenses: Deciphera, NCCN, Blueprint
Other Relationship: Arog, GenMab, ASCO, Gradalis
Brian A. Van Tine
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Leadership: Polaris
Honoraria: Bionest Partner, Horizon CME, Research to Practice
Consulting or Advisory Role: EMD Serono, Novartis, Epizyme, Daiihi Sankyo, Pfizer, Adaptimmune, Bayer, GlaxoSmithKline, Lilly, Cytokinetics, Apexigen, Deciphera Pharmaceuticals, Immune Design, ADRx, Ayala Pharmaceuticals, Intellisphere
Speakers’ Bureau: Novartis, Lilly, Adaptimmune, GlaxoSmithKline
Research Funding: Pfizer, Merck, TRACON Pharma, GlaxoSmithKline
Patents, Royalties, Other Intellectual Property: Patent on the use of ME1 as a biomarker, patent on ALEXT3102, Accuronix Therapeutics–Licensing agreement. Sigma-2 Receptor Ligands and Therapeutic uses therefor (006766), Modular Platform for Targeted Therapeutic Delivery (006755), Sigma-2 Receptor Ligand Drug Conjugates as Antitumor Compounds, Methods of synthesis and Uses Thereof (014229)
Expert Testimony: Health Advances
Travel, Accommodations, Expenses: Adaptimmune, Advenchen Laboratories, GlaxoSmithKline, Lilly
Aviad Zick
Research Funding: Merck (Inst), Roche Molecular Diagnostics (Inst), Karyopharm Therapeutics (Inst)
Alexander Lee
Consulting or Advisory Role: Astex Pharmaceuticals, AstraZeneca/MedImmune
Patents, Royalties, Other Intellectual Property: Material and methods for stratifying and treating cancers
Anna Estival Gonzalez
Honoraria: Roche, MSD Oncology, AstraZeneca Spain, Bayer, PharmaMar, Takeda
Travel, Accommodations, Expenses: Lilly, Roche, Roche, PharmaMar, Bristol Myers Squibb, Bayer, MSD, Pfizer, AstraZeneca Spain, Takeda
Mark A. Dickson
Employment: Memorial Sloan Kettering Cancer Center
Research Funding: Karyopharm Inc, AADI, Eli-Lilly, National Institutes of Health/National Cancer Institute Cancer Center Support Grant (P30CA008748)
Dayana Michel
Employment: Karyopharm Therapeutics
Travel, Accommodations, Expenses: Karyopharm Therapeutics
Changting Meng
Employment: Karyopharm Therapeutics
Stock and Other Ownership Interests: Karyopharm Therapeutics
Travel, Accommodations, Expenses: Karyopharm Therapeutics
Jianjun Liu
Employment: Karyopharm Therapeutics
Stock and Other Ownership Interests: Karyopharm Therapeutics
Osnat Ben-Shahar
Employment: Karyopharm Therapeutics
Stock and Other Ownership Interests: Karyopharm Therapeutics
Dane R. Van Domelen
Employment: Karyopharm Therapeutics
Stock and Other Ownership Interests: Karyopharm Therapeutics
Christopher J. Walker
Employment: Karyopharm Therapeutics
Stock and Other Ownership Interests: Karyopharm Therapeutics
Patents, Royalties, Other Intellectual Property: Pending patents for biomarkers related to selinexor efficacy
Travel, Accommodations, Expenses: Karyopharm Therapeutics
Hua Chang
Employment: Karyopharm Therapeutics
Stock and Other Ownership Interests: Karyopharm Therapeutics
Travel, Accommodations, Expenses: Karyopharm Therapeutics
Yosef Landesman
Employment: Karyopharm Therapeutics
Stock and Other Ownership Interests: Karyopharm Therapeutics
Jatin J. Shah
Employment: Karyopharm Therapeutics
Stock and Other Ownership Interests: Karyopharm Therapeutics
Sharon Shacham
Employment: Karyopharm Therapeutics
Stock and Other Ownership Interests: Karyopharm Therapeutics
Patents, Royalties, Other Intellectual Property: 8999996, 9079865, 9714226, PCT/US12/048319, and I574957 on hydrazide-containing nuclear transport modulators and uses. Pending patents: PCT/US12/048319, 499/2012, PI20102724, and 2012000928 on hydrazide-containing nuclear transport modulators and uses
Michael G. Kauffman
Employment: Karyopharm Therapeutics
Stock: Karyopharm Therapeutics
Steven Attia
Research Funding: AB Science, Adaptimmune, Advenchen Laboratories, Bavarian Nordic, Bayer, Blueprint Medicines, BTG, CBA Pharma, CytRx Corporation, Daiichi Sankyo, Deciphera, Desmoid Tumor Research Foundation, Epizyme, FORMA Therapeutics, Genmab, GlaxoSmithKline, Gradalis, Immune Design, Incyte, Karyopharm Therapeutics, Lilly, Merck, Novartis, Philogen, PTC Therapeutics, Takeda, TRACON Pharma
Other Relationship: Immune Design
No other potential conflicts of interest were reported.
REFERENCES
- 1.Crago AM, Singer S: Clinical and molecular approaches to well differentiated and dedifferentiated liposarcoma. Curr Opin Oncol 23:373-378, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas DM, Conyers R, Young S: Liposarcoma: Molecular genetics and therapeutics. Sarcoma 2011:483154, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Livingston JA, Bugano D, Barbo A, et al. : Role of chemotherapy in dedifferentiated liposarcoma of the retroperitoneum: Defining the benefit and challenges of the standard. Sci Rep 7:1-8, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Italiano A, Toulmonde M, Cioffi A, et al. : Advanced well-differentiated/dedifferentiated liposarcomas: Role of chemotherapy and survival. Ann Oncol 23:1601-1607, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Jones RL, Fisher C, Al-Muderis O, et al. : Differential sensitivity of liposarcoma subtypes to chemotherapy. Eur J Cancer 41:2853-2860, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Stacchiotti S, Van der Graaf W, Doms H, et al. : 1629MO—First-line chemotherapy (CT) in advanced well-differentiated/dedifferentiated liposarcoma (WD/DD LPS): An EORTC Soft Tissue and Bone Sarcoma Group (STBSG) retrospective analysis. Ann Oncol 31:S978, 2020 [Google Scholar]
- 7.Demetri GD, Von Mehren M, Jones RL, et al. : Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: Results of a phase III randomized multicenter clinical trial. J Clin Oncol 34:786-793, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demetri GD, Schöffski P, Grignani G, et al. : Activity of eribulin in patients with advanced liposarcoma demonstrated in a subgroup analysis from a randomized phase III study of eribulin versus dacarbazine. J Clin Oncol 35:3433-3439, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Ricciotti RW, Baraff AJ, Jour G, et al. : High amplification levels of MDM2 and CDK4 correlate with poor outcome in patients with dedifferentiated liposarcoma: A cytogenomic microarray analysis of 47 cases. Cancer Genet 218–219:69-80, 2017 [DOI] [PubMed] [Google Scholar]
- 10.Bill KLJ, Seligson ND, Hays JL, et al. : Degree of MDM2 amplification affects clinical outcomes in dedifferentiated liposarcoma. Oncologist 24:989-996, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singer S, Socci ND, Ambrosini G, et al. : Gene expression profiling of liposarcoma identifies distinct biological types/subtypes and potential therapeutic targets in well-differentiated and dedifferentiated liposarcoma. Cancer Res 67:6626-6636, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Nie L, Sasaki M, Maki CG: Regulation of p53 nuclear export through sequential changes in conformation and ubiquitination. J Biol Chem 282:14616-14625, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Garg M, Kanojia D, Mayakonda A, et al. : Molecular mechanism and therapeutic implications of selinexor (KPT-330) in liposarcoma. Oncotarget 8:7521-7532, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalakonda N, Maerevoet M, Cavallo F, et al. : Selinexor in patients with relapsed or refractory diffuse large B-cell lymphoma (SADAL): A single-arm, multinational, multicentre, open-label, phase 2 trial. Lancet Haematol 7:e511-e522, 2020 [DOI] [PubMed] [Google Scholar]
- 15.Ben-Barouch S, Kuruvilla J: Selinexor (KTP-330)—A selective inhibitor of nuclear export (SINE): Anti-tumor activity in diffuse large B-cell lymphoma (DLBCL). Expert Opin Investig Drugs 29:15-21, 2020 [DOI] [PubMed] [Google Scholar]
- 16.Abdul Razak AR, Mau-Soerensen M, Gabrail NY, et al. : First-in-class, first-in-human phase I study of selinexor, a selective inhibitor of nuclear export, in patients with advanced solid tumors. J Clin Oncol 34:4142-4150, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakayama R, Zhang YX, Czaplinski JT, et al. : Preclinical activity of selinexor, an inhibitor of XPO1, in sarcoma. Oncotarget 7:16581-16592, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gounder MM, Zer A, Tap WD, et al. : Phase IB study of selinexor, a first-in-class inhibitor of nuclear export, in patients with advanced refractory bone or soft tissue sarcoma. J Clin Oncol 34:3166-3174, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nair JS, Musi E, Schwartz GK: Selinexor (KPT-330) induces tumor suppression through nuclear sequestration of IκB and downregulation of survivin. Clin Cancer Res 23:4301-4311, 2017 [DOI] [PubMed] [Google Scholar]
- 20.Grosicki S, Simonova M, Spicka I, et al. : Once-per-week selinexor, bortezomib, and dexamethasone versus twice-per-week bortezomib and dexamethasone in patients with multiple myeloma (BOSTON): A randomised, open-label, phase 3 trial. Lancet 396:1563-1573, 2020 [DOI] [PubMed] [Google Scholar]
- 21.Chari A, Vogl DT, Gavriatopoulou M, et al. : Oral selinexor–dexamethasone for triple-class refractory multiple myeloma. N Engl J Med 381:727-738, 2019 [DOI] [PubMed] [Google Scholar]
- 22.Nair JS, Tap W, Vasudeva SD, et al. : Abstract 5210: KPT-330, a selective small molecule inhibitor of nuclear export, is active in bone and soft tissue sarcoma. Cancer Research. American Association for Cancer Research (AACR), 2013, pp 5210
- 23.Crochiere ML, Kashyap T, Klebanov B, et al. : Abstract 3810: Selinexor (KPT-330), a novel selective inhibitor of nuclear export (SINE), shows single agent efficacy against alveolar soft part sarcoma (ASPS) in vivo. Cancer Research. American Association for Cancer Research (AACR), 2014, pp 3810
- 24.US Department of Health and Human Services: National Institutes of Health and National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). Version 4.3, 2010 . https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf [Google Scholar]
- 25.Cancer Genome Atlas Research Network : Comprehensive and integrated genomic characterization of adult soft tissue sarcomas. Cell 171:950-965, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bock S, Hoffmann DG, Jiang Y, et al. : Increasing incidence of liposarcoma: A population-based study of national surveillance databases, 2001–2016. Int J Environ Res Public Health 17:2710, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dickson MA, Schwartz GK, Keohan ML, et al. : Phase 2 trial of the CDK4 inhibitor palbociclib (PD0332991) at 125 mg dose in well-differentiated or dedifferentiated liposarcoma. JAMA Oncol 2:937, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dickson MA, Tap WD, Keohan ML, et al. : Phase II trial of the CDK4 inhibitor PD0332991 in patients with advanced CDK4-amplified well-differentiated or dedifferentiated liposarcoma. J Clin Oncol 31:2024-2028, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dickson MA, Koff A, D’Angelo SP, et al. : Phase 2 study of the CDK4 inhibitor abemaciclib in dedifferentiated liposarcoma. J Clin Oncol 37, 2019. (suppl; abstr 11004) [Google Scholar]
- 30.Gounder MM, Bauer TM, Schwartz GK, et al. : Milademetan, an oral MDM2 inhibitor, in well-differentiated/dedifferentiated liposarcoma: Results from a phase 1 study in patients with solid tumors or lymphomas. Eur J Cancer 138:S3-S4, 2020 [Google Scholar]
- 31.Gounder M, Abdul Razak AR, Gilligan AM, et al. : Health-related quality of life and pain with selinexor in patients with advanced dedifferentiated liposarcoma. Futur Oncol 17:2923-2939, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDonough J, Eliott J, Neuhaus S, et al. : Health-related quality of life, psychosocial functioning, and unmet health needs in patients with sarcoma: A systematic review. Psychooncology 28:653-664, 2019 [DOI] [PubMed] [Google Scholar]
- 33.Rhoten WB, Bruns ME, Christakos S: Presence and localization of two vitamin D-dependent calcium binding proteins in kidneys of higher vertebrates. Endocrinology 117:674-683, 1985 [DOI] [PubMed] [Google Scholar]
- 34.Cao LQ, Wang YN, Liang M, et al. : CALB1 enhances the interaction between p53 and MDM2, and inhibits the senescence of ovarian cancer cells. Mol Med Rep 19:5097-5104, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pelc K, Vincent S, Ruchoux MM, et al. : Calbindin-D28k: A marker of recurrence for medulloblastomas. Cancer 95:410-419, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Karyopharm Therapeutics agrees to share individual participant data that underlie the results reported in this article (after deidentification), including the study protocol and statistical analysis plan. Data availability will begin 9 months after publication and will be available 36 months after publication. To gain access, data requestors should submit a proposal to medicalinformation@karyopharm.com. Proposals will be reviewed by an independent review committee identified for this purpose.