Abstract
Purpose
Many studies report the triple negative breast cancer (TNBC) as the worst subgroup, as such patients do not benefit from anti-hormonal therapy and human epidermal growth factor receptor 2 (HER2) antagonists. While HER2 overexpression was a poor prognostic factor in breast cancer before trastuzumab (Herceptin) was available, TNBC is often reported as the worst BC subgroup since targeted therapy is currently not possible. Since the patience-specific experiences and the current literature did not always align, we aimed to determine the BC subgroup with the shortest survival in our center.
Methods
The records of patients with BC who were admitted to Trakya University Faculty of Medicine Department of Medical and Radiation Oncology between July 1999 and December 2019 were reviewed. Patients were divided into four main groups (Luminal A, Luminal B, TNBC, and HER2-enriched) according to the St Gallen International Consensus Panel and four subgroups in accordance with estrogen receptor, progestin receptor and HER2 positivity. Patient characteristics, treatment characteristics and clinical outcomes of the four main subgroups were evaluated. Survival curves were generated using the Kaplan–Meier method, and the significance of survival differences among the selected variables was compared by using the Log rank test. Factors affecting disease-free survival (DFS) and overall survival (OS) were analyzed by Cox regression analysis.
Results
Statistical analysis was performed on 2017 patients, after excluding patients with phyllodes tumor, carcinoma-in-situ and missing information from a total of 2474 patients with BC. There were 952 (47.1%) patients in the Luminal A group, 236 (34.1%) in the Luminal B group, 236 (11.7%) in the TNBC group and 142 (7.1%) patients in the HER2 enriched group. HER2-enriched patients had the shortest survival (p < 0.001), with 113.70 ± 7.17 months of DFS and 125.45 ± 3.03 months of OS. For patients who received Herceptin, DFS was 101.50 ± 6.4 months and OS was 118.14 ± 6.16. Patients who did not receive Herceptin had 92.79 ± 18 months of DFS and 94.44 ± 15.23 months of OS.
Conclusion
The HER2-enriched subgroup had the worst prognosis despite receiving targeted therapy. While the duration of DFS and OS had no significant difference between TNBC and Luminal A-B subgroups, HER2 enriched subgroup had significantly shorter survival when compared to any other subgroup. HER2-enriched subgroup had a 10-fold greater risk of death compared to the Luminal A subgroup.
Keywords: breast cancer, HER2 enriched subgroup, triple negative breast cancer, subgroup in breast cancer, Luminal-B breast cancer, Luminal-A breast cancer
Introduction
Breast cancer (BC) remains the most common cancer in women, and it is the second leading cause of cancer-related death in women after lung cancer.1 However, because of advances in treatment, long survival is now possible even in patients with metastatic BC, whereas certain groups of patients survive for a very short time despite being diagnosed at an early stage.2 Owing to the discovery of molecular receptors in breast carcinogenesis and pathways responsible for rapid cell proliferation, the differential clinical course of BC gradually becomes clearer.3–6
Immunohistochemical (IHC) staining and in situ fluorescent hybridization (FISH) are currently used methods for identifying tumor subtypes to achieve more accurate treatment and longer survival. In the St. Gallen International Consensus Panel in 2011, four main subtypes have been approved in the classification scheme.4 According to the presence or absence of estrogen receptors (ER), progestin receptors (PR) and human epidermal growth factor receptor 2 (HER2), these molecular subtypes have been defined as Luminal A (ER and PR-positive, HER2-negative, low Ki67), Luminal B (ER and/or PR positive, HER2-positive or high Ki67), HER2-enriched (ER and PR-negative, HER2-positive) and triple-negative (TNBC) (ER, PR, HER2-negative). Each subtype exhibits distinct clinical outcomes and requires different treatment strategies.3–12
In many studies, the TNBC subgroup is stated to have the worst prognosis, as such patients are deprived of antihormonal therapy and trastuzumab (Herceptin) therapy. Additionally, the main systemic treatment is chemotherapy only in most TNBC patients.9–15 HER2 proto-oncogene encodes the transmembrane receptor tyrosine kinase and because of the pathway it activates, the conversion of HER2 to an oncogene increases tumor proliferation and invasion. HER2 amplification may cause more aggressive tumor spread, leading to the development of both local and distant metastases.16,17 HER2 gene is overexpressed in 20–25% patients with BC, which has been associated with poorer survival. Therefore, it is an important prognostic factor for the progression of the disease and lymph node metastasis.18–23
Herceptin is a monoclonal IgG1 class humanized murine antibody, which blocks HER2 overexpression. It was one of the first targeted therapies discovered for HER2 and was revolutionary for this group of patients. However, although Herceptin improves both DFS and OS in early-stage HER2-positive BC, long-term follow-up data show approximately one-quarter of patients still go into relapse.24 This led to the development of new agents such as pertuzumab, a monoclonal antibody that blocks another extracellular subdomain of the HER2 receptor,25,26 conjugate trastuzumab-emtansine (T-DM1),27 and the irreversible pan-HER2 inhibitor neratinib.28 Newer agents can provide double blockage of the HER2 pathway in combination with Herceptin.25–31
Our study aims to determine the worst prognostic subgroup by evaluating Ki67, HER2 overexpression and hormone receptor status, and whether the current classification captures the biodiversity despite Herceptin treatment.
Materials and Method
Following the approval of the Institutional Review Board, records of patients with BC who were admitted to the Radiation and Medical Oncology Department of Trakya University between July 1999 and December 2019 were reviewed. The Human Research Ethical Committee of Trakya University Medical Faculty Hospital approved (TUTF-BAEK 2021/406) the use of these patients’ information for the study. In order to use the relevant information, informed consent forms were obtained from the patients or relatives of the deceased patients from our local ethics committee in accordance with the Declaration of Helsinki.32
Patients were divided into four main groups (Luminal A, Luminal B, TNBC, and HER2-enriched) according to the St Gallen International Consensus Panel and four subgroups according to receptor positivity4 (Table 1). Patient characteristics were age, body mass index (BMI), age at menarche, age at menopause, menstruation status, number of births, family history, breastfeeding, hormone replacement status, histological type, breast localization, tumor quadrant, surgical type, axillary surgery type, tumor size, lymph node metastasis, TNM stage, grade, mitotic index, ER, PR and HER2 positivity, Ki67 level, lymphovascular invasion (LVSI), perineural invasion (PNI), extensive intraductal component (EIC), surgical margin positivity, skin involvement, whether chemotherapy was received, chemotherapy type, whether radiotherapy was received, radiotherapy type, duration of tamoxifen (TAM) use, duration of aromatase inhibitor (AI) use, and duration of luteinizing hormone-releasing hormone (LHRH) use. This study was modeled on the prognostic values of the American Joint Committee for Cancer (AJCC) 8th edition cancer staging system.33
Table 1.
The Subtyping Schemes
| Groups Name | How is the Classification Made? | Group Branches |
|---|---|---|
| Subtyping 1 | Subtype Triple-Negative | Triple-Negative Not-Triple Negative |
| Subtyping 2 | Original Subtype | Triple-Negative Luminal A Luminal B HER2-enriched |
| Subtyping 3 | SubtypeHER2-enriched (received Herceptin) | Triple-Negative Luminal A Luminal B HER2-enriched (received Herceptin) HER2-enriched (did not receive Herceptin) |
| Subtyping 4 | Subtype HER2 positive-negative | Triple-Negative Luminal A Luminal B HER2 positive Luminal B HER2 negative HER2-enriched (received Herceptin) HER2-enriched (did not receive Herceptin) |
| Subtyping 5 | Subtype received Herceptin | Luminal B (received Herceptin) Luminal B (did not receive Herceptin) HER2-enriched (received Herceptin) HER2-enriched (did not receive Herceptin) HER2 negative |
Histopathologic Evaluation
ER and PR positivity assessments were made using Primary Novocastra monoclonal antibodies. ER and PR positivity is determined as ≥1% of tumor cell nuclei being immunoreactive.34
IHC analyses were performed in accordance to DAKO Herceptest scoring. Strong complete staining of the cell membrane in more than 10% of the tumor cells was interpreted as HER2 positivity and was scored 3+. FISH was used to confirm HER2 positivity in weak to moderate staining of the cell membrane in more than 10% of the tumor cells and was scored 2+. Faint, incomplete staining of the cell membrane in more than 10% of the tumor cells was scored 1+ and was interpreted as trace negative. No staining was interpreted as HER2 negative and was scored 0.35,36
Ki67 score was defined as the percentage of stained tumor cell nuclei and was analyzed in paraffin sections by using MIB-1 IHC staining. The stained section was examined using a standard light microscope with a 40x objective and 10 × 10 graticule. At least 1000 stained tumor cell nuclei in ten high-power fields (× 40) was considered evaluable.37
Statistical Analysis
Numerical results are expressed as the mean ± standard deviation, and categorical results are shown as n (%). Survival curves were generated using the Kaplan–Meier method, and the significance of survival differences among the selected variables was compared by using the Log rank test.38 Univariate Cox regression analysis was used to estimate hazard ratios. Then, multivariate Cox regression analysis with the backward elimination method was used to estimate hazard ratios and to identify independent prognostic factors.39 All reported p values are two-sided, and p values below 0.05 were considered significant. Data analysis was performed using SPSS version 20.0 (IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.).
Results
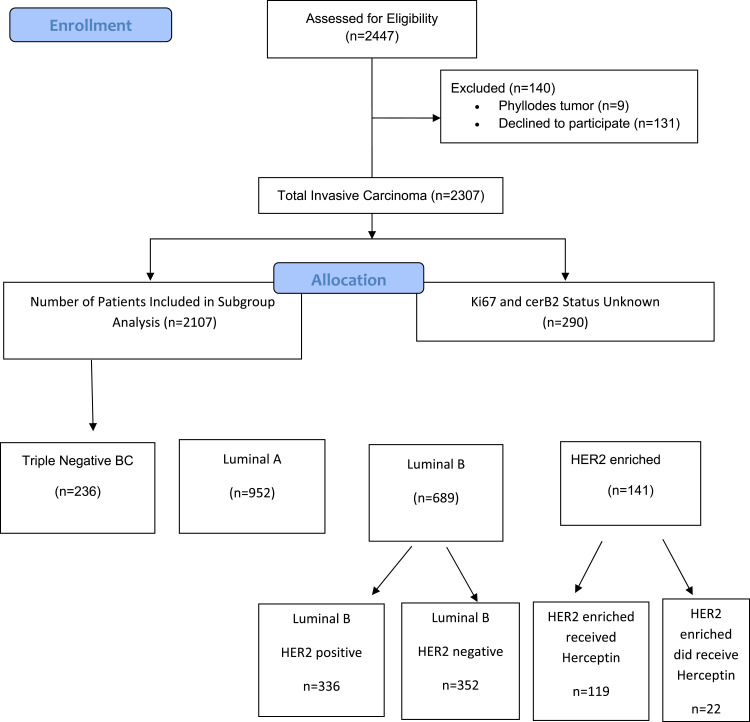
A total of 2474 patients with BC who were treated between July 1999 and December 2019 were evaluated. Patients with missing information were not evaluated. A total of 131 patients with ductal carcinoma-in-situ and lobular carcinoma-in-situ, 9 patients with phyllodes tumors and 244 patients with unobtainable data regarding ER, PR, HER2, and Ki67 were excluded from the analysis. Statistical analysis was performed on 2017 patients with BC (Figure 1). The mean age of the patients was 52.07 years. The mean menopausal age was 48.35 years, and the mean menarche age was 13.15 years. The mean BMI was 29.9. HER2 positivity rate was 23.7%.
Figure 1.
Distribution of BC patients in our series by subtyping.
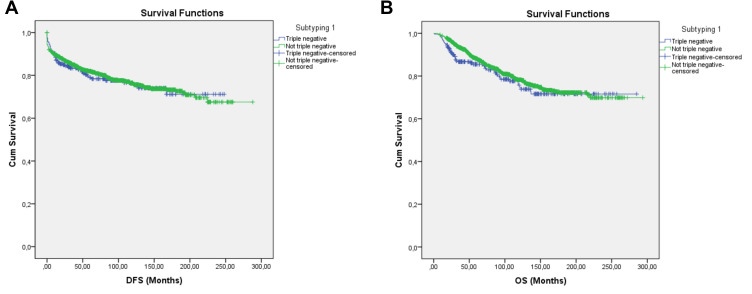
In order to determine the subgroup with the worst prognosis in our series, statistical analyses were performed by dividing the patients using 5 different subtyping schemes (Table 1). In the first subtyping, the patients were divided as TNBC (n = 236) and Not-TNBC (n = 1781). Duration of DFS was 190.37 ± 7.19 months for TNBC and 218.23 ± 3.68 months for Not-TNBC. Duration of OS was 221.68 ± 7.92 months for TNBC, and 231.77 ± 3.29 months for Not-TNBC. Neither DFS (p = 0.739) nor OS (p = 0.252) showed statistical significance between the two groups (Table 2, Figure 2A and B).
Table 2.
Disease-Free Survival, and Overall Survival Times, Comparative Log Rank Test, p-values Obtained Using the Kaplan–Meier Method of Triple-Negative Breast Cancer and Not-Triple-Negative Breast Cancer Subgroups Forming Subtyping 1
| Subtyping 1 | p-value (Log Rank Test) | |||
|---|---|---|---|---|
| Triple-Negative (TNBC) | Not-TNBC | |||
| Disease-free survival | Mean ± SD | 190.3 ± 7.1 | 218.2 ± 3.6 | 0.739 |
| 95% Confidence Interval | 176.2–204.4 | 211.0–225.4 | ||
| Overall survival | Mean ± SD | 221.6 ± 7.9 | 231.7 ± 3.2 | 0.252 |
| 95% Confidence Interval | 206.1–237.2 | 225.3–238.2 | ||
Note: p values are in italic.
Abbreviations: SD, Standard deviation; CI, Confidence Interval.
Figure 2.
Survival curve of DFS (A) and OS (B) for the TNBC and Not-TNBC subgroups producing subtype 1 using the Kaplan–Meier method.
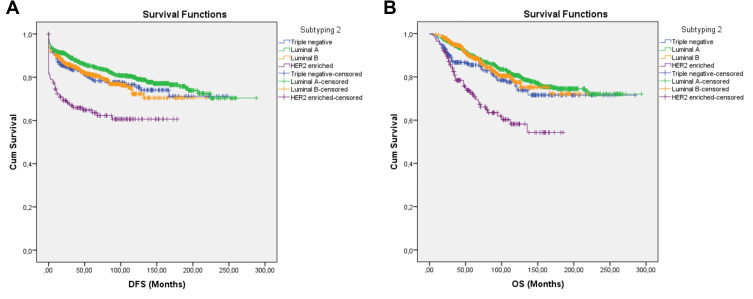
For the second subtyping, the patients were divided into 4 main groups (Table 1). There were 952 (47.1%) patients in the Luminal A group, 236 (34.1%) patients in the Luminal B group, 236 (11.7%) patients in the TNBC group and 142 (7.1%) patients in the HER-2 enriched group. The group with the longest DFS and OS was Luminal A. Patients in the HER-2 enriched had the shortest DFS and OS (Table 3, Figure 3A and B). Duration of DFS was 226.7 ± 4.3 months in the Luminal A group, 168.3 ± 4.3 months in the Luminal B group, 190.3 ± 7.1 months in the TNBC group and 113.7 ± 7.1 months in the HER2 enriched group. Duration of OS was 237.4 ± 3.8 months for the Luminal A group, 180.2 ± 4.0 months for the Luminal B group, 221.6 ± 7.9 months for the TNBC group and 125.4 ± 3.0 months for the HER2 enriched group (Tables 3 and 4).
Table 3.
Disease-Free Survival, and Overall Survival Times, Comparative Log Rank Test, p-values Obtained Using Kaplan–Meier Method of Triple-Negative Breast Cancer, Luminal A, and Luminal B and HER2-Enriched Subgroups Forming Subtyping 2
| Subtyping 2 | Mean ± Std. Error (Months) | 95% Confidence Interval | Triple-Negative | Luminal A | Luminal B | ||
|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||||
| Disease-free survival | Triple-Negative | 190.3 ± 7.1 | 176.2 | 204.4 | |||
| Luminal A | 226.7 ± 4.3 | 218.3 | 235.2 | 0.139 | |||
| Luminal B | 168.3 ± 4.3 | 159.8 | 176.9 | 0.971 | 0.016 | ||
| HER2-enriched | 113.7 ± 7.1 | 99.6 | 127.7 | <0.001 | <0.001 | <0.001 | |
| Overall survival | Triple-Negative | 221.6 ± 7.9 | 206.1 | 237.2 | |||
| Luminal A | 237.4 ± 3.8 | 229.9 | 244.9 | 0.002 | |||
| Luminal B | 180.2 ± 4.0 | 172.3 | 188.2 | 0.160 | 0.450 | ||
| HER2-enriched | 125.4 ± 3.0 | 112.0 | 138.9 | <0.001 | <0.001 | <0.001 | |
Notes: p values are in italic, significant p values are in bold italic.
Figure 3.
Survival curve of DFS (A) and OS (B) for TNBC, Luminal A, Luminal B, and HER2-enriched subgroups producing subtype 2 using the Kaplan–Meier method.
Table 4.
Disease-Free Survival and Overall Survival Times, Comparative Log Rank Test, p-values Obtained Using Kaplan–Meier Method of Triple-Negative Breast Cancer, Luminal A and Luminal B and HER2-Enriched Received Herceptin, HER2-Enriched Did Not Receive Herceptin Subgroups Forming Subtyping 3
| Subtyping 3 | Mean ± Std. Error (Months) | 95% Confidence Interval | Triple Negative | Luminal A | Luminal B | HER2-Enriched Received Herceptin | ||
|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||||
| Disease-free survival | Triple-Negative | 190.37±7.19 | 176.27 | 204.46 | ||||
| Luminal A | 227.02±4.31 | 218.57 | 235.46 | 2085 (0.149) | ||||
| Luminal B | 168.12±4.36 | 159.57 | 176.67 | 0.000 (0.977) | 5.224 (0.022) | |||
| HER2-enriched received Herceptin | 101.50±6.49 | 88.77 | 114.23 | 9.262 (0.002) | 28.443 (<.001) | 13.935 (<.001) | ||
| HER2-enriched did not receive Herceptin | 92.79±18.00 | 57.44 | 128.13 | 10.318 (0.001) | 17.954 (<.001) | 10.409 (0.001) | 1.665 (0.197) | |
| Overall survival | Triple-Negative | 221.68±7.92 | 206.14 | 237.21 | ||||
| Luminal A | 237.44±3.83 | 229.92 | 244.97 | 3.100 (0.078) | ||||
| Luminal B | 180.29±4.04 | 172.37 | 188.22 | 2.122 (0.145) | 0.387 (0.534) | |||
| HER2-enriched received Herceptin | 118.14±6.16 | 106.06 | 130.22 | 4.548 (0.033) | 21.267 (<.001) | 16.439 (<.001) | ||
| HER2-enriched did not receive Herceptin | 94.44±15.23 | 64.58 | 124.30 | 16.092 (<.001) | 32.866 (<.001) | 30.357 (<.001) | 5.602 (0.018) | |
Notes: p values are in italic, significant p values are in bold italic.
When the durations of DFS and OS of each group were individually compared with the HER-2 group, the difference was statistically significant (p < 0.001) (Tables 3 and 4).
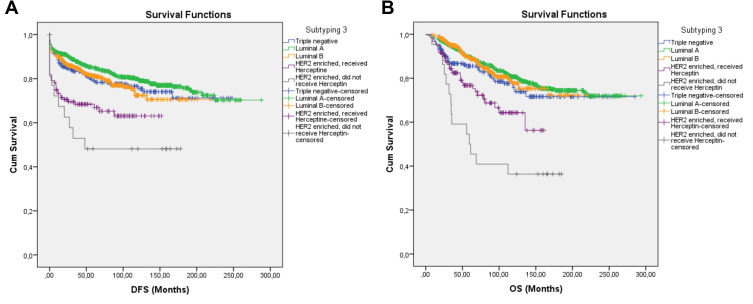
Subgroup with the shortest survival was determined as the HER2 enriched group. Therefore, these patients were further divided as those received Herceptin and those who did not, marking the third subtyping. The HER2 enriched subgroup still had the shortest DFS and OS, despite receiving Herceptin. Herceptin recipients did not have significantly longer DFS. However, Herceptin significantly increased the duration of OS (p = 0.012) (Table 4 Figure 4A and B).
Figure 4.
Survival curve of DFS (A) and OS (B) for TNBC, Luminal A, Luminal B, and HER2 -enriched subgroups that received Herceptin and the HER2-enriched subgroups that did not receive Herceptin producing subtype 3 using the Kaplan–Meier method.
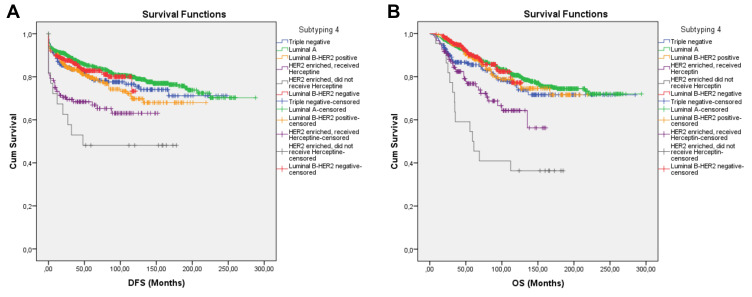
In the fourth subtyping, the Luminal-B subgroup was divided as HER2-positive and HER2-negative (Table 5). Luminal-B patients had longer DFS and OS, however the difference was not statistically significant (Figure 5A and B). Duration of DFS was 163.79 ± 5.78 months in the HER2-positive Luminal B subgroup and 101.23 ± 2.35 months in the HER2-negative Luminal B subgroup (p = 0.239). Duration of OS was 178.95 ± 5.15 months in the HER2-positive Luminal B subgroup and 114.16 ± 2.01 months in the HER2-negative Luminal B subgroup (p = 0.611). HER2 positivity in Luminal B subgroup had no statistical significance for neither DFS nor OS. HER2 enriched subgroup still had the shortest DFS and OS (Table 5, Figure 5A and B). However, receiving Herceptin significantly increased OS in the HER2 enriched group. Regardless of Herceptin use and eligibility, HER2 enriched subgroup had significantly worse DFS and OS than the Luminal B HER2-positive and Luminal B-HER2 negative subgroup (Table 5).
Table 5.
Disease-Free Survival and Overall Survival Times, Comparative Log Rank Test, p-values Obtained Using Kaplan–Meier Method of Triple-Negative Breast Cancer, Luminal A and Luminal B HER2 Positive, Luminal B HER2 Negative and HER2-Enriched Received Herceptin, HER2-Enriched Did Not Receive Herceptin Subgroups Forming Subtyping 4
| Subtyping 4 | Mean ± Std. Error (Months) | 95% Confidence Interval | Triple Negative | Luminal A | Luminal B | Luminal B | HER2-Enriched Received Herceptin | ||
|---|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | HER2 Positive | HER2 Negative | ||||||
| Disease-free survival | Triple-Negative | 190.37±7.19 | 176.27 | 204.46 | |||||
| Luminal A | 227.02±4.31 | 218.57 | 235.46 | 2.085 (0.149) | |||||
|
Luminal B HER2 Positive |
163.79±5.78 | 152.45 | 175.13 | 0.266 (0.606) | 6.980 (0.008) | ||||
|
Luminal B HER2 Negative |
101.23±2.35 | 96.61 | 105.86 | 0.431 (0.512) | 0.941 (0.332) | 1.387 (0.239) | |||
| HER2-enriched received Herceptin | 101.50±6.49 | 88.77 | 114.23 | 9.262 (0.002) | 28,443 (<0.001) | 8.157 (0.004) | 15.406 (<0.001) | ||
| HER2-enriched did not receive Herceptin | 92.79±18.00 | 57.44 | 128.13 | 10,318 (0.001) | 17,954 (<0.001) | 7.883 (0.005) | 13.455 (<0.001) | 1.665 (0.197) | |
| Overall survival | Triple-Negative | 221.68±7.92 | 206.14 | 237.21 | |||||
| Luminal A | 237.44±3.83 | 229.92 | 244.97 | 3.10 (0.078) | |||||
|
Luminal B HER2 Positive |
178.95±5.15 | 168.83 | 189.06 | 1.061 (0.303) | 0.582 (0.446) | ||||
|
Luminal B HER2 Negative |
114.16±2.01 | 110.20 | 118.11 | 2.699 (0.100) | 0.018 (0.892) | 0.259 (0.611) | |||
| HER2-enriched received Herceptin | 118.14±6.16 | 106.06 | 130.22 | 4.548 (0.033) | 21.267 (<0.001) | 11.391 (0.001) | 13.703 (<0.001) | ||
| HER2-enriched did not receive Herceptin | 94.44±15.23 | 64.58 | 124.30 | 16.092 (<0.001) | 32.866 (<0.001) | 25.708 (<0.001) | 30.967 (<0.001) | 5.602 (0.018) | |
Notes: p values are in italic, significant p values are in bold italic.
Figure 5.
Survival curve of DFS (A) and OS (B) for the TNBC, Luminal A, and Luminal B subgroups that received Herceptin, the Luminal B subgroups that did not receive Herceptin, the HER2-enriched subgroup that received Herceptin, and the HER2-enriched subgroup that did not receive Herceptin, producing subtype 4 using the Kaplan–Meier method.
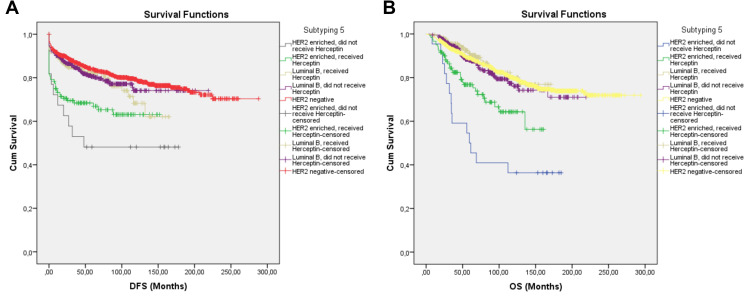
In the fifth subtyping, patients were divided as HER2-negative, Luminal B Herceptin recipients, Luminal B non-Herceptin recipients, HER2 enriched Herceptin recipients, and HER2 enriched non-Herceptin recipients. Length of DFS was 225.237 ± 3.89 months for the HER2-negative group, 126.33 ± 5.10 months for Luminal B Herceptin recipients, 171.69 ± 4.90 months for Luminal B non-Herceptin recipients, 101.50 ± 6.49 months for HER2-enriched Herceptin recipients, and 92.79 ± 18.03 months for HER2-enriched non-Herceptin recipients. Length of OS was 235.49 ± 3.48 months for the HER2-negative group, 148.32 ± 4.17 months for Luminal B Herceptin recipients, 178.20 ± 4.91 months for Luminal B non-Herceptin recipients, 118.14 ± 6.16 months for HER2-enriched Herceptin recipients, and 94.44 ± 15.23 months for HER2-enriched non-Herceptin recipients. HER2-negative subgroup had the best survival. However, this difference was not statistically significant for neither DFS (p = 0.162) nor OS (p = 0.317) from the Luminal-B subgroup regardless of Herceptin use. HER2 enriched subgroup had significantly shorter DFS and OS when compared to the other subgroups (Table 6, Figure 6A and B).
Table 6.
Disease-Free Survival, Overall Survival Times, Comparative Log Rank Test, p-values Obtained Using Kaplan–Meier Method of TNBC, Luminal A, Luminal B Received Herceptin, Luminal B Did Not Receive Herceptin and HER2-Enriched Received Herceptin, HER2-Enriched Did Not Receive Herceptin Subgroups Forming Subtyping 5
| Subtyping 5 | Mean ± Std. Error (Months) | 95% Confidence Interval | HER2 Negative | Luminal B Received Herceptin | Luminal B Did Not Receive Herceptin | HER2-Enriched Received Herceptin | ||
|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||||
| Disease-free survival | HER2 Negative | 225.237±3.89 | 217.60 | 232.86 | ||||
| Luminal B received Herceptin | 126.33±5.10 | 116.32 | 136.34 | 3.006 (0.083) | ||||
| Luminal B did not receive Herceptin | 171.69±4.90 | 162.09 | 181.30 | 1.945 (0.163) | 0.162 (0.688) | |||
| HER2-enriched received Herceptin | 101.50±6.49 | 88.77 | 114.23 | 26.736 (<0.001) | 8.466 (0.004) | 12.783 (<0.001) | ||
| HER2-enriched did not receive Herceptin | 92.79±18,03 | 57.44 | 128.13 | 16.902 (<0.001) | 7.133 (0.008) | 11.736 (0.001) | 1.665 (0.197) | |
| Overall survival | HER2 Negative | 235.49±3.48 | 228.66 | 242.33 | ||||
| Luminal B received Herceptin | 148.32±4.17 | 140.14 | 156.50 | 0.607 (0.436) | ||||
| Luminal B did not receive Herceptin | 178.20±4.91 | 168.56 | 187.84 | 0.386 (0.534) | 0.999 (0.317) | |||
| HER2-enriched received Herceptin | 118.14±6.16 | 106.06 | 130.22 | 18.218 (<0.001) | 14.578 (<0.001) | 11.466 (<0.001) | ||
| HER2-enriched did not receive Herceptin | 94.44±15.23 | 64.58 | 124.30 | 30.150 (<0.001) | 30.660 (<0.001) | 25.569 (<0.001) | 5.602 (0.018) | |
Notes: p values are in italic, significant p values are in bold italic.
Figure 6.
Survival curves of DFS (A) and OS (B) for the HER2-negative, Luminal B subgroup receiving Herceptin, the Luminal B subgroup that did not receive Herceptin, the HER2-enriched subgroup that received Herceptin, and the HER2-enriched subgroup that did not receive Herceptin, producing subtype 5 using the Kaplan–Meier method.
HER2 enriched subgroup had the shortest DFS and OS regardless of Herceptin use (Table 7). Herceptin recipients and non-recipients in the HER2 enriched group were individually compared to all other subgroups in the 2nd–5th subtyping schemes (Table 7). There was no significant difference between the lengths of DFS of Herceptin recipients and non-recipients in the HER2 enriched group (p = 0.179). However, all other pairwise comparisons were either significant or close to significance. The group showing the greatest difference in DFS and OS from the HER2 enriched group was Luminal A group (p < 0.001).
Table 7.
Comparison of HER2-Enriched Subgroup with Other Subgroups
| Disease-Free Survival | Overall survival | |||||
|---|---|---|---|---|---|---|
| Pearson Chi-Square | Asymptotic Significance (2-Sided) p-value |
Pearson Chi-Square | Asymptotic Significance (2-Sided) p-value | |||
| Subtyping 1 | TNBC vs Not-TNBC | 0.207 | 0.649 | 1.375 | 0.252 | |
| Subtyping 2 | HER2-enriched vs Luminal A | 39.820 | <0.001 | 39.518 | <0.001 | |
| HER2-enriched vs Luminal B | 19.845 | <0.001 | 29.819 | <0.001 | ||
| HER2-enriched vs TNBC | 12.876 | <0.001 | 9.715 | 0.002 | ||
| Subtyping 3 | Received Herceptin | HER2-enriched vs Luminal A | 26.563 | <0.001 | 17.540 | <0.001 |
| HER2-enriched vs Luminal B | 12.484 | <0.001 | 12.787 | <0.001 | ||
| HER2-enriched vs TNBC | 8.652 | 0.003 | 3.437 | 0.064 | ||
| Did not receive Herceptin | HER2-enriched vs Luminal A | 17.954 | <0.001 | 32.866 | <0.001 | |
| HER2-enriched vs Luminal B | 10.271 | 0.001 | 29.516 | <0.001 | ||
| HER2-enriched vs TNBC | 10.391 | 0.001 | 16.155 | <0.001 | ||
| Subtyping 4 | Received Herceptin | HER2-enriched vs Luminal A | 24.648 | <0.001 | 17.784 | <0.001 |
| HER2-enriched vs Luminal B HER2 positive | 9.208 | 0.002 | 10.882 | 0.001 | ||
| HER2-enriched vs Luminal B HER2 negative | 9.410 | 0.002 | 9.072 | 0.003 | ||
| HER2-enriched vs TNBC | 7.911 | 0.005 | 3.544 | 0.060 | ||
| HER2-enriched vs HER2-enriched did not receive Herceptin | 1.805 | 0.179 | 6.231 | 0.003 | ||
| Did not receive Herceptin | HER2-enriched vs Luminal A | 17.954 | <0.001 | 32.866 | <0.001 | |
| HER2-enriched vs Luminal B HER2 positive | 9.068 | 0.003 | 26.983 | <0.001 | ||
| HER2-enriched vs Luminal B HER2 negative | 13.214 | <0.001 | 27.485 | <0.001 | ||
| HER2-enriched vs TNBC | 10.391 | 0.001 | 16.155 | <0.001 | ||
| Subtyping 5 | Received Herceptin | HER2-enriched vs HER2 negative | 22.721 | <0.001 | 14.808 | <0.001 |
| HER2-enriched vs Luminal B received Herceptin | 6.715 | 0.010 | 12.398 | <0.001 | ||
| HER2-enriched vs Luminal B did not receive Herceptin | 10.613 | 0.001 | 9.278 | 0.002 | ||
| Did not receive Herceptin | HER2-enriched vs HER2 negative | 16.761 | <0.001 | 29.920 | <0.001 | |
| HER2-enriched vs Luminal B received Herceptin | 6.904 | 0.009 | 30.298 | <0.001 | ||
| HER2-enriched vs Luminal B did not receive Herceptin | 11.633 | 0.001 | 25.437 | <0.001 | ||
| HER2-enriched received Herceptin vs HER2-enriched did not receive Herceptin | 1.805 | 0.179 | 6.333 | 0.012 | ||
Notes: p values are in italic, significant p values are in bold italic.
Risk factors affecting DFS and OS were calculated in accordance with the patient characteristics, treatment regiments, and the different subgroups using Cox regression test (Tables 8 and 9). In the univariate analysis, age (<35 years), early menarche, being in the postmenopausal period, advanced T and N stages, no breast and/or axillary node surgery, high tumor grade, high mitotic index, skin infiltration, multifocal tumors, ER and PR negativity, HER2 positivity, EIC positivity, LVI positivity, Ki67 ≥15, metastasis (M), no chemotherapy and radiotherapy, use of tamoxifen (TAM) or aromatase inhibitor (AI) less than 5 years, use of LHRH less than 2 years, and having HER2-enriched BC were determined to be negative factors for DFS. Absence of axillary surgery, advanced T and N stages, not receiving radiotherapy, using TAM less than 5 years, and using LHRH for less than 2 years were significant risk factors for DFS in the multivariate analysis (Table 8).
Table 8.
Univariable and Multivariable Analysis of Breast Cancer Survival Using Cox’s Proportional Hazards Model Within Disease-Free Survival
| Patients Descriptions | Events/Total (%) | Univariate Analysis | p | Multivariate Analysis | p |
|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | Hazard Ratio (95% CI) | ||||
| BMI | |||||
| < 25 | 73/373 (19.5) | 1.059 (0.823–1.364) | 0.656 | ||
| ≥ 25 | 342/1644 (20.8) | ||||
| Menopause Age (mean) | |||||
| Events 48.36 years | 260/2017 (12.8) | 1.010 (0.984–1.037) | 0.470 | ||
| None Events 48.30 years | |||||
| Menstruation Age (mean) | |||||
| Events 13.04 years | 415/2017 (20.5) | 0.915 (0.850–0.986) | 0.019 | 0.959 (0.851–1.080) | 0.486 |
| None Events 13.17 years | |||||
| Menstruation situation | |||||
| Premenopause | 149/787 (18.9) | 1.221 (0.998–1.495) | 0.052 | 1.053 (0.746–1.487) | 0.769 |
| Postmenopause | 260/1217 (21.3) | ||||
| Number of births | |||||
| No birth | 38/162 (23.4) | 1 (Reference) | 0.435 | ||
| 1–2 birth | 255/1320 (19.3) | 0.834 (0.593–1.173) | 0.296 | ||
| 3 and more | 115/520 (22.1) | 0.928 (0.643–1.339) | 0.690 | ||
| Family History | |||||
| Positive | 115/632 (18.1) | ||||
| Negative | 300/1385 (21.6) | 0.850 (0.685–1.054) | 0.138 | ||
| Breast-feeding | |||||
| Positive | 229/1192 (19.2) | ||||
| Negative | 186/825 (22.5) | 0.869 (0.716–1.055) | 0.156 | ||
| Breast site | |||||
| Left | 205/1013 (20.2) | 1 (Reference) | |||
| Right | 186/935 (19.8) | 0.000 (0.000–6.51) | 0.939 | ||
| Bilateral | 24/69 (34.7) | <0.001 (0.000–2.31) | 0.935 | ||
| Location | |||||
| Unilateral | 391/1948 (20) | 1 (Reference) | |||
| Metacron | 17/46 (36.9) | 1.590 (0.978–2.586) | 0.061 | ||
| Sencron | 7/23 (30.4) | 1.770 (0.838–3.739) | 0.135 | ||
| Tumor Quadrant | |||||
| Inner | 80/402 (19.9) | 1 (Reference) | 1 (Reference) | ||
| Outer | 234/1205 (19.4) | 0.994 (0.771–1.281) | 0.962 | 0.924 (0.697–1.225) | 0.583 |
| Periareolar | 54/259 (20.8) | 1.081 (0.766–1.527) | 0.657 | 0.713 (0.479–1.062) | 0.096 |
| Multifokal | 47/150 (31.3) | 1.819 (1.269–2.608) | 0.001 | 0.721 (0.466–1.117) | 0.143 |
| Histopathologic Type | |||||
| IDC | 344/1652 (20.8) | 1 (Reference) | 0.646 | ||
| ILC | 26/122 (21.3) | 0.954 (0.640–1.422) | 0.817 | ||
| Other | 45/243 (18.5) | 0.864 (0.633–1.179) | 0.356 | ||
| Surgical Type | |||||
| BCS | 122/1016 (12) | 1 (Reference) | 1 (Reference) | ||
| MRM | 226/930 (24.3) | 1.978 (1.586–2.466) | <0.001 | 0.834 (0.634–1.090) | 0.184 |
| No surgery | 67/71 (94.3) | 27.941 (20.296–38.465) | <0.001 | 1.444 (0.676–3.087) | 0.343 |
| Axillary Surgery Type | |||||
| SLND | 37/451 (8.2) | 1 (Reference) | 1 (Reference) | ||
| AD | 304/1477 (20.5) | 2.210 (1.569–3.111) | <0.001 | 0.645 (0.427–0.975) | 0.037 |
| No axillary surgery | 74/89 (83.1) | 21.759 (14.573–32.487) | <0.001 | 1.056 (0.455–2.448) | 0.900 |
| Stage | |||||
| I | 23/415 (5.54) | 1 (Reference) | <0.001 | ||
| II | 96/881 (10.8) | 1.960 (1.244–3.090) | 0.004 | ||
| III | 158/583 (27.1) | 5.447 (3.517–8.436) | <0.001 | ||
| IV | 138/138 (100) | 95.570 (60.478–151.023) | <0.001 | ||
| T stage | |||||
| T1 | 67/670 (10) | 1 (Reference) | 1 (Reference) | ||
| T2 | 220/1048 (20.9) | 2.207 (1.679–2.902) | <0.001 | 1.426 (1.037–1.962) | 0.029 |
| T3 | 38/155 (24.5) | 2.438 (1.637–3.631) | <0.001 | 1.579 (1.012–2.464) | 0.044 |
| T4 | 89/143 (62.2) | 11.090 (8.050–15.279) | <0.001 | 1.794 (1.053–3.057) | 0.032 |
| Positive Axillary Node Count | |||||
| 0 | 78/861 (9.06) | 1 (Reference) | 1 (Reference) | ||
| 1–3 | 80/531 (15.07) | 1.668 (1.221–2.278) | 0.001 | 0.811 (0.561–1.171) | 0.263 |
| 4–9 | 145/402 (36.07) | 5.000 (3.795–6.587) | <0.001 | 1.338 (0.928–1.929) | 0.119 |
| ≥10 | 111/222 (50) | 7.384 (5.524–9.870) | <0.001 | 1.644 (1.116–2.423) | 0.012 |
| Metastasis site | |||||
| None | 25/1627 (1.53) | 1 (Reference) | 1 (Reference) | ||
| Bone | 142/142 (100) | 156.760 (102.099–240.686) | <0.001 | 158.568 (100.278.250.742) | <0.001 |
| Lung | 25/25 (100) | 139.613 (79.878–244.018) | <0.001 | 131.993 (72.208–241.278) | <0.001 |
| Liver | 15/15 (100) | 171.002 (89.530–326.613) | <0.001 | 133.403 (64.540–275.738) | <0.001 |
| Brain | 21/21 (100) | 173.699 (96.477–312.733) | <0.001 | 129.981 (68.258–247.517) | <0.001 |
| Multiple organs | 185/185 (100) | 164.232 (107.535–250.822) | <0.001 | 126.654 (79.530–201.699) | <0.001 |
| Skin infiltration | |||||
| Positive | 80/152 (52.6) | 4.664 (3.642–5.974) | <0.001 | 1.249 (0.783–1.991) | 0.351 |
| Negative | 335/1865 (18) | ||||
| Surgical margin | |||||
| Positive | 69/367 (18.8) | 1.004 (0.775–1.301) | 0.975 | ||
| Negative | 346/1650 (21) | ||||
| Grade | |||||
| 1 | 23/304 (7.5) | 1 (Reference) | 1 (Reference) | ||
| 2 | 155/987 (15.7) | 2.290 (1.478–3.550) | <0.001 | 0.712 (0.424–1.195) | 0.198 |
| 3 | 237/726 (32.6) | 5.417 (3.528–8.317) | <0.001 | 1.017 (0.595–1.739) | 0.950 |
| Mitotic index | |||||
| 1 | 97/775 (12.5) | 1 (Reference) | 1 (Reference) | ||
| 2 | 98/637 (15.3) | 1.546 (1.164–2.053) | 0.003 | 1.033 (0.734–1.455) | 0.851 |
| 3 | 218/591 (36.8) | 4.303 (3.369–5.497) | <0.001 | 0.996 (0.726–1.367) | 0.982 |
| ER | |||||
| Positive | 300/1598 (18.7) | 0.648 (0.523–0.804) | <0.001 | 0.838 (0.403–1.350) | 0.324 |
| Negative | 115/419 (27.4) | ||||
| PR | |||||
| Positive | 240/1337 (18) | 0.640 (0.526–0.777) | <0.001 | 1.029 (0.769–1.376) | 0.847 |
| Negative | 175/680 (25.7) | ||||
| Ki67 | |||||
| <15 | 204/1130 (18) | 1.758 (1.443–2.143) | <0.001 | 1.062 (0.811–1.389) | 0.662 |
| ≥15 | 210/885 (23.7) | ||||
| HER2 | |||||
| Positive | 125/478 (26.1) | 1.646 (1.333–2.032) | <0.001 | 1.077 (0.730–1.590) | 0.708 |
| Negative | 290/1539 (18.8) | ||||
| EIC | |||||
| Positive | 96/334 (28.7) | 1.646 (1.310–2.069) | <0.001 | 1.175 (0.895–1.542) | 0.247 |
| Negative | 319/1683 (18.9) | ||||
| LVI | |||||
| Positive | 211/954 (22.1) | 1.190 (0.981–1.443) | 0.077 | 1.077 (0.832–1.394) | 0.574 |
| Negative | 204/1063 (19.1) | ||||
| PNI | |||||
| Positive | 103/437 (23.5) | 1.145 (0.917–1.431) | 0.233 | ||
| Negative | 312/1580 (19.7) | ||||
| Chemotherapy | |||||
| None | 40/324 (12.3) | 1 (Reference) | 1 (Reference) | ||
| Neoadjuvant | 78/235 (33.1) | 3.202 (2.186–4.690) | <0.001 | 1.137 (0.699–1.851) | 0.604 |
| Adjuvant | 297/1458 (20.3) | 1.553 (1.116–2.161) | <0.001 | 0.840 (0.563–1.252) | 0.301 |
| Chemotherapy Protocol | |||||
| None | 40/324 (12.3) | 1 (Reference) | |||
| FAC | 54/166 (32.5) | 1.858 (1.255–2.750) | 0.002 | ||
| AC+TXT | 34/273 (12.4) | 1.429 (0.569–3.592) | 0.447 | ||
| Other | 279/1235 (22.5) | 1.694 (1.247–2.301) | 0.001 | ||
| Radiotherapy | |||||
| Positive | 295/1757 (16.7) | 0.302 (0.244–0.374) | <0.001 | 0.470 (0.352–0.626) | <0.001 |
| Negative | 120/260 (46.1) | ||||
| Radiotherapy Type | |||||
| None | 120/260 (46.1) | 1 (Reference) | |||
| Breast alone | 44/587 (7.5) | 0.127 (0.090–0.179) | <0.001 | ||
| Locoregional | 51/1170 (4.3) | 0.389 (0.312–0.484) | <0.001 | ||
| TAM period | |||||
| No TAM | 1 (Reference) | 1 (Reference) | |||
| TAM ≤5 years | 130/652 (19.9) | 0.769 (0.623–0.949) | 0.014 | 1.022 (0.733–1.425) | 0.896 |
| TAM >5 years | 9/107 (8.4) | 0.283 (0.146–0.551) | <0.001 | 0.425 (0.196–0.922) | 0.030 |
| AI period | |||||
| No AI | 1 (Reference) | 1 (Reference) | |||
| AI ≤5 years | 191/937 (20.3) | 0.812 (0.664–0.992) | 0.042 | 0.861 (0.643–1.154) | 0.317 |
| AI >5 years | 32/265 (12) | 0.404 (0.278–0.589) | <0.001 | 0.817 (0.505–1.319) | 0.408 |
| LHRH period | |||||
| None LHRH | 1 (Reference) | 1 (Reference) | |||
| ≤2 years | 8/35 (22.8) | 1.242 (0.616–2.504) | 0.544 | 2.426 (1.057–5.568) | 0.037 |
| >2 years | 57/343 (16.6) | 0.754 (0.570–0.998) | 0.048 | 1.225 (0.798–1.880) | 0.353 |
| Subtyping 2 | |||||
| HER2-enriched | 51/142 (35.9) | 1 (Reference) | 1 (Reference) | ||
| TNBC | 51/236 (21.6) | 0.479 (0.325–0.707) | <0.001 | 0.794 (0.438–1.438) | 0.447 |
| Luminal A | 182/952 (19.1) | 0.380 (0.278–0.520) | <0.001 | 0.891 (0.336–2.362) | 0.817 |
| Luminal B | 131/688 (19.0) | 0.488 (0.353–0.675) | <0.001 | 1.157 (0.543–2.463) | 0.706 |
| HER2-enriched received Herceptin | 40/120 (33.3) | ||||
| HER2-enriched did not receive Herceptin | 11/22 (50) | 1.515 (0.777–2.954) | 0.223 |
Notes: p values are in italic, significant p values are in bold italic.
Abbreviations: BMI, Body Mass Index; IDC, Invasive Ductal Carcinoma; ILC, Invasive Lobular Carcinoma; ER, Estrogen Receptor; PR, Progesterone Receptor; HER-2, Human Epidermal Growth Factor Receptor 2; TNM, Tumor-Node-Metastasis staging system based on the system of the American Joint Committee on Cancer; SLND, Sentinel Lymph Node Dissection; AD, Axillary Dissection; EIC, Extensive Intraductal Component; LVI, Lymphovascular Invasion; PNI, Perineural Invasion; TAM, Tamoxifen; AI, Aromatase Inhibitor; LHRH, Luteinizing Hormone-Releasing Hormone; FAC, Fluorouracil; Adriamycin (Doxorubicin) Cyclophosphamide; AC+TXT, Adriamycin (Doxorubicin), Cyclophosphamide + Taxotere.
Table 9.
Univariable and Multivariable Analysis of Breast Cancer Survival Using Cox’s Proportional Hazards Model Within Overall Survival
| Patients Descriptions | Events/Total (%) | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | p | Hazard Ratio (95% CI) | p | ||
| Age group | |||||
| <35 years | 21/84 (25) | 1 (Reference) | 1 (Reference) | ||
| 35–50 years | 101/756 (13.3) | 0.476 (0.297–0.762) | 0.002 | 0.598 (0.354–1.012) | 0.055 |
| > 50 years | 241/1177 (20.4) | 0.873 (0.559–1.363) | 0.550 | 1.033 (0.569–1.876) | 0.914 |
| BMI | |||||
| <25 | 72/373 (19.3) | 0.919 (0.710–1.190) | 0.524 | ||
| ≥25 | 291/1644 (17.7) | ||||
| Menopause Age (mean) | |||||
| Alive 48.35 years | 1.003 (0.977–1.030) | 0.832 | |||
| Death 48.14 years | 244/2017 (12.1) | ||||
| Menstruation Age (mean) | |||||
| Alive 13.15 years | 363/2017 (18) | 0.939 (0.870–1.015) | 0.112 | ||
| Death 13.11 years | |||||
| Menstruation situation | |||||
| Premenopause | 114/787 (14.5) | ||||
| Postmenopause | 244/1217 (20) | 1.582 (1.266–1.976) | <0.001 | 0.665 (0.679–1.403) | 0.966 |
| Number of births | |||||
| No birth | 28/162 (17.3) | 1 (Reference) | |||
| 1–2 birth | 209/1320 (15.8) | 0.937 (0.631–1.389) | 0.745 | ||
| 3 and more | 120/520 (23.1) | 1.298 (0.860–1.958) | 0.214 | ||
| Family History | |||||
| Positive | 87/632 (13.8) | 0.709 (0.557–0.902) | 0.005 | 0.902 (0.696–1.168) | 0.434 |
| Negative | 276/1385 (20) | ||||
| Breast-feeding | |||||
| Positive | 224/1192 (18.8) | 1.156 (0.935–1.430) | 0.180 | ||
| Negative | 139/825 (16.8) | ||||
| Breast site | |||||
| Left | 185/1013 (18.3) | 1 (Reference) | 0.995 | ||
| Right | 178/935 (19) | 0.975 (0.794–1.198) | 0.811 | ||
| Bilateral | 14/69 (20.3) | ||||
| Location | |||||
| Unilateral | 363/1948 (18.6) | 1 (Reference) | 0.484 | ||
| Metacron | 9/46 (19.6) | 0.778 (0.401–1.509) | 0.458 | ||
| Sencron | 5/23 (21.7) | 1.524 (0.630–3.687) | 0.350 | ||
| Tumor Quadrant | |||||
| Inner | 73/402 (18.1) | 1 (Reference) | 1 (Reference) | ||
| Outer | 210/1205 (17.4) | 1.002 (0.768–1.308) | 0.987 | 0.990 (0.734–1.335) | 0.948 |
| Periareolar | 42/259 (16.2) | 0.951 (0.651–1.391) | 0.797 | 0.884 (0.577–1.353) | 0.569 |
| Multifocal | 38/150 (25.3) | 1.659 (1.121–2.456) | 0.011 | 0.713 (0.448–1.136) | 0.155 |
| Histopathologic Type | |||||
| Invasive ductal carcinoma | 293/1652 (17.7) | 1 (Reference) | 0.752 | ||
| Invasive lobular carcinoma | 22/122 (18) | 0.937 (0.607–1.445) | 0.768 | ||
| Other | 48/243 (19.7) | 1.109 (0.817–1.504) | 0.508 | ||
| Surgical Type | |||||
| BCS | 93/1016 (9.15) | 1 (Reference) | 1 (Reference) | ||
| MRM | 221/930 (23.8) | 2.344 (1.839–2.987) | <0.001 | 1.204 (0.873–1.599) | 0.279 |
| No surgery | 49/71 (69) | 19.760 (13.887–28.117) | <0.001 | 1.154 (0.561–2.374) | 0.697 |
| Axillary surgery | |||||
| SLND | 21/451 (4.7) | 1 (Reference) | 1 (Reference) | ||
| Axillary dissection | 286/1477 (19.4) | 3.040 (1.950–4.741) | <0.001 | 1.466 (0.896–2.400) | 0.128 |
| No axillary surgery | 56/89 (62.9) | 22.238 (13.458–36.747) | <0.001 | 3.251 (1.451–7.283) | 0.004 |
| Stage | |||||
| I | 22/415 (5.3) | 1 (Reference) | |||
| II | 108/881 (12.3) | 2.199 (1.390–3.477) | 0.001 | ||
| III | 150/583 (25.7) | 5.085 (3.250–7.954) | <0.001 | ||
| IV | 83/138 (60.1) | 26.548 (16.530–42.638) | <0.001 | ||
| T Stage | |||||
| T1 | 57/670 (8.5) | 1 (Reference) | 1 (Reference) | ||
| T2 | 183/1048 (17.5) | 2.110 (1.567–2.841) | <0.001 | 1.719 (1.227–2.410) | 0.002 |
| T3 | 37/155 (23.9) | 2.571 (1.699–3.889) | <0.001 | 1.749 (1.099–2.786) | 0.018 |
| T4 | 85/143 59.4 () | 12.764 (9.091–17.920) | <0.001 | 1.843 (1.081–3.143) | 0.025 |
| Infiltrated Axillary Node Count | |||||
| 0 | 88/861 (10.2) | 1 (Reference) | 1 (Reference) | ||
| 1–3 | 69/531 (13) | 1.214 (0.886–1.664) | 0.228 | 0.897 (0.624–1.289) | 0.556 |
| 4–9 | 122/402 (30.3) | 3.710 (2.819–4.882) | <0.001 | 1.390 (0.957–2.018) | 0.084 |
| ≥10 | 83/222 (37.4) | 4.563 (3.379–6.161) | <0.001 | 1.099 (0.726–1.662) | 0.657 |
| Metastasis site | |||||
| None | 117/1627 (7.19) | 1 (Reference) | 1 (Reference) | ||
| Bone | 73/142 (51.4) | 8.934 (6.667–11.972) | <0.001 | 5.123 (3.696–7.100) | <0.001 |
| Lung | 15/25 (60) | 11.240 (6.562–19.252) | <0.001 | 4.350 (2.361–8.015) | <0.001 |
| Liver | 10/15 (66.6) | 14.344 (7.513–27.385) | <0.001 | 10.520 (5.270–20.999) | <0.001 |
| Brain | 20/21 (95.2) | 23.899 (14.826–38.522) | <0.001 | 7.798 (4.372–13.909) | <0.001 |
| Multiple organs | 126/185 (68.1) | 15.101 (11.720–19.458) | <0.001 | 5.059 (3.710–6.899) | <0.001 |
| Skin infiltration | |||||
| Positive | 83/152 (54.6) | 6.585 (5.127–8.459) | <0.001 | 2.093 (1.359–3.223) | 0.001 |
| Negative | 280/1865 (15) | ||||
| Surgical margins | |||||
| Positive | 73/367 (19.9) | 1.427 (1.103–1.846) | 0.007 | 1.236 (0.922–1.656) | 0.156 |
| Negative | 290/1650 (17.6) | ||||
| Grade | |||||
| 1 | 28/304 (9.2) | 1 (Reference) | 1 (Reference) | ||
| 2 | 148/987 (15) | 1.805 (1.205–2.704) | 0.004 | 0.656 (0.413–1.042) | 0.074 |
| 3 | 187/726 (25.8) | 3.484 (2.341–5.185) | <0.001 | 0.535 (0.330–0.870) | 0.012 |
| Mitotic index | |||||
| 1 | 47/775 (6) | 1 (Reference) | 1 (Reference) | ||
| 2 | 57/637 (8.9) | 2.157 (1.462–3.182) | <0.001 | 1.819 (1.182–2.799) | 0.006 |
| 3 | 256/591 (43.3) | 12.288 (8.955–16,860) | <0.001 | 5.904 (4.086–8.532) | <0.001 |
| ER | |||||
| Positive | 254/1598 (15.9) | 0.578 (0.462–0.723) | <0.001 | 0.758 (0.410–1.404) | 0.379 |
| Negative | 109/419 (26) | ||||
| PR | |||||
| Positive | 213/1337 (15.9) | 0.641 (0.520–0.790) | <0.001 | 0.990 (0.711–1.378) | 0.950 |
| Negative | 150/680 (22) | ||||
| Ki67 | |||||
| <15 | 183/1130 (16.2) | 2.025 (1.636–2.507) | <0.001 | 2.627 (1.478–4.670) | 0.001 |
| ≥15 | 179/885 (20.2) | ||||
| HER2 | |||||
| Positive | 98/478 (20.5) | 1.500 (1.188–1.894) | 0.001 | 1.154 (0.729–1.827) | 0.541 |
| Negative | 265/1539 (17.2) | ||||
| EIC | |||||
| Positive | 90/334 (27) | 1.815 (1.430–2.304) | <0.001 | 1.193 (0.879–1.621) | 0.258 |
| Negative | 273/1683 (16.2) | ||||
| LVI | |||||
| Positive | 187/954 (19.6) | 1.242 (1.011–1.527) | 0.039 | 1.099 (0.844–1.431) | 0.484 |
| Negative | 176/1063 (16.5) | ||||
| PNI | |||||
| Positive | 97/437 (22.1) | 1.215 (0.963–1.533) | 0.101 | ||
| Negative | 266/1580 (16.8) | ||||
| Chemotherapy | |||||
| None | 42/324 (13) | 1 (Reference) | 1 (Reference) | ||
| Neoadjuvant | 57/235 (24.3) | 0.816 (0.483–1.379) | 0.447 | 0.774 (0.458–1.309) | 0.340 |
| Adjuvant | 264/1458 (57.6) | 0.648 (0.437–0.959) | 0.03 | 0.628 (0.424–0.930) | 0.02 |
| Chemotherapy Protocol | |||||
| None | 42/324 (13) | 1 (Reference) | |||
| FAC | 55/166 (33.1) | 1.483 (1.004–2.192) | 0.048 | ||
| AC+TXT | 44/273 (16.1) | 2.269 (1.113–4.627) | 0.024 | ||
| Other | 216/1235 (17.4) | 1.230 (0.900–1.682) | 0.194 | ||
| Radiotherapy | |||||
| Positive | 276/1757 (15.7) | 0.427 (0.335–0.543) | <0.001 | 0.885 (0.637–1.230) | 0.467 |
| Negative | 87/260 (33.4) | ||||
| Radiotherapy Type | |||||
| No | 87/1757 (15.7) | 1 (Reference) | |||
| Breast alone | 48/587 (8.2) | 0.220 (0.155–0.313) | <0.001 | ||
| Locoregional | 228/1170 (19.5) | 0.524 (0.409–0.672) | <0.001 | ||
| TAM period | |||||
| No TAM | 262/1258 (20.8) | 1 (Reference) | 1 (Reference) | ||
| TAM ≤5 years | 96/652 (14.7) | 0.539 (0.426–0.683) | <0.001 | 0.540 (0.376–775) | 0.001 |
| TAM >5 years | 5/107 (4.6) | 0.146 (0.060–0.354) | <0.001 | 0.141 (0.075–0.367) | <0.001 |
| AI period | |||||
| No AI | 169/815 (20.7) | 1 (Reference) | 1 (Reference) | ||
| AI ≤5 years | 178/937 (19) | 0.828 (0.671–1.022) | 0.079 | 0.612 (0.442–0.848) | 0.003 |
| AI >5 years | 16/265 (6) | 0.193 (0.116–0.323) | <0.001 | 0.140 (0.092–0.259) | <0.001 |
| LHRH period | |||||
| No LHRH | 324/1634 (19.8) | 1 (Reference) | 1 (Reference) | ||
| ≤2 years | 7/35 (20) | 1.121 (0.530–2.370) | 0.765 | 1.402 (0.587–3.345) | 0.447 |
| >2 years | 31/343 (9) | 0.430 (0.298–0.622) | <0.001 | 1.004 (0.613–1.644) | 0.987 |
| Subtyping2 | |||||
| HER2-enriched | 45/142 (32) | 1 (Reference) | 1 (Reference) | ||
| TNBC | 49/236 (20.7) | 0.493 (0.330–0.737) | 0.001 | 0.900 (0.471–1.722) | 0.751 |
| Luminal A | 178/952 (18.7) | 0.368 (0.266–0.510) | <0.001 | 10.551 (2.956–37.668) | <0.001 |
| Luminal B | 91/688 (13.2) | 0.391 (0.275–0.557) | <0.001 | 1.268 (0.584–2.755) | 0.548 |
| HER2-enriched received Herceptin | 33/120 (27.5) | 2.109 (1.121–3.965) | 0.021 | ||
| HER2-enriched did not receive Herceptin | 14/22 (63.6) | ||||
Notes: p values are in italic, significant p values are in bold italic.
Abbreviations: BMI, body mass index; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; ER, estrogen receptor; PR, progesterone receptor; HER-2, human epidermal growth factor receptor 2; TNM, tumor-node-metastasis staging system based on the system of the American Joint Committee on Cancer; SLND, Sentinel Lymph Node Dissection; AD, Axillary Dissection; EIC, Extensive Intraductal Component; LVI, Lymphovascular Invasion; PNI, Perineural Invasion; TAM, Tamoxifen; AI, Aromatase Inhibitor; LHRH, Luteinizing Hormone-Releasing Hormone; FAC, Fluorouracil, Adriamycin (Doxorubicin) Cyclophosphamide; AC+TXT, Adriamycin (Doxorubicin), Cyclophosphamide + Taxotere.
In the univariate analysis, negative factors for OS were age (<35 years), being in the postmenopausal period, advanced T and N stages, no breast and/or axillary node surgery, high tumor grade, high mitotic index, skin infiltration, multifocal tumors, ER and PR negativity, HER2 positivity, metastases, EIC positivity, LVI positivity, Ki67 ≥15, positive surgical margin, no chemotherapy and radiotherapy, using TAM or AI less than 5 years, using LHRH less than 2 years, and having HER2 enriched BC. In the multivariate analysis, age (<35 years), no axillary surgery, Ki67 ≥15, high tumor grade, high mitotic index, skin infiltration, advanced T and N stages, metastases, no treatment with chemotherapy, using TAM or AI less than 5 years, and having HER2-enriched BC were the negative factors for OS (Table 9).
Having HER2 enriched BC was a significant risk factor for DFS in the univariate analysis. It was a significant risk factor in both univariate and multivariate analyses for OS. Being in the HER2 enriched subgroup increased the risk of death by 10.551 (2956–37,668) compared to the Luminal-A group (p< 0.001).
Discussion
The molecular subgroup classification of BC is a reliable guide for clinicians in using the most accurate treatment options and the best follow-up strategy. Appropriate treatment for BC patients can be provided based on the biological characteristics of the tumor. The need to determine the subgroup with the worst prognosis emerged from our patient-specific experiences, which were not always in line with the current literature.40–44
Although HER2-specific antagonists have revolutionized the treatment of HER2-overexpressing BC and a better clinical outcome for the HER2-enriched subgroup is now possible, it was still identified as the subgroup with the lowest DFS and OS in our study. In the HER2 enriched subgroup, Herceptin decreased the risk of death 2.109 times compared to the patients who could not receive Herceptin (p=0.021). Additionally, Luminal-A subgroup had a 10.551 times (p = <0.001) lower risk of death compared to the HER2 enriched subgroup. While the duration of DFS and OS had no significant difference between TNBC and Luminal A-B subgroups, HER2 enriched subgroup had significantly shorter survival when compared to any other subgroup.
Foulkes et al9 report TNBC as a biologically aggressive subgroup in which certain patients benefit more from chemotherapy than others, and targeted therapy is currently not possible. Despite limited treatment options for TNBC, this subgroup was reported to have better survival than the HER2 overexpressing BC patients who could not receive trastuzumab.9 In 1987, long before trastuzumab was in use, Slamon et al23 reported that patients with HER2 overexpressing BC had significantly shorter OS and relapse times.
ER activates the HER2 receptor signaling pathway,17,20–23 making trastuzumab (Herceptin) more effective since it also enables the use of anti-estrogen drugs such as TAM and AI.7,19,45,46 In the HER2-enriched subgroup, ER and PR are negative and only HER2 is overexpressed. Therefore, the efficacy of treatment is dependent on Herceptin. Single-drug dependency may have caused the HER2-enriched group to have a worse prognosis. Although HER2 overexpression is positive in the Luminal B subgroup as well, it has better survival than the HER2 enriched subgroup. Luminal B subgroup also benefits from anti-hormonal treatment, which may be the reason for longer survival. Although Herceptin improves both DFS and OS in early-stage HER2-positive BC, nearly a quarter of patients were reported to develop recurrence in long-term follow-up.24
Clinical trials show that newly discovered HER2 antagonists contribute to better clinical outcome and longer survival for HER2 positive BC patients. One such agent is pertuzumab, a humanized recombinant monoclonal antibody that prevents the heterodimerization of HER2 to HER3 by interfering with ligand-dependent HER3 and inhibiting the signaling pathway. In the prospective, randomized CLEOPATRA study, OS was significantly and clinically improved by pertuzumab, trastuzumab, and docetaxel for HER2 positive metastatic BC patients.29 Although dual HER2 inhibition with pertuzumab and trastuzumab did not significantly improve OS compared to placebo in the 6-year follow-up in early stage BC, DFS was longer especially in patients with positive lymph nodes.25,26
In the KATHERINE trial, an antibody–drug conjugate of trastuzumab T-DM1 and the maytansine derivative, microtubule inhibitor cytotoxic agent emtansine (DM1) was tested in metastatic BC patients who received chemotherapy and targeted therapy for HER2-positive BC. Compared to those received trastuzumab alone, patients who received a dual combination of HER2 antagonists had longer DFS, especially in the hormone receptor-negative subgroup.27 In another Phase 2 prospective study, trastuzumab and the irreversible pan-HER2 inhibitor neratinib were tested. Pathological complete response rate in patients who received trastuzumab plus neratinib was higher than in those who received a single drug.28 It is clear that further studies are necessary for the patients in the HER2 enriched subgroup that do not benefit from anti-hormonal treatment, and new agents may be particularly promising for this subgroup.
Although not receiving trastuzumab is a poor prognostic factor for the HER2 enriched subgroup, it would not be appropriate to decide on the local treatment regiment solely on a molecular basis.42 However, mastectomy may be preferred for the selected HER2-enriched BC patients instead of breast-conserving surgery because of the multicentric and multifocal localization of tumors in addition to the higher probability of lymph node involvement. In another subgroup analysis, the HER2-enriched subgroup showed higher rates of local recurrence than the TNBC subtype, in addition to being associated with higher possibility of lymph node metastases.43 Another study on Spanish women reported that HER2-enriched, TNBC and unclassified subgroups had a higher risk of death than the Luminal subgroups.44
Consistent with the literature, Cox regression analysis showed that Ki67 score greater than 15 negatively affects OS.32,47,48 Additionally, multivariate analysis showed that hazard ratio was 2.627 (1.478–4.670) (p = 0.001). Another remarkable finding in our study was that TAM use longer than 5 years reduces relapse and mortality risk, and AI use longer than 5 years reduces the risk of death.49–51
As drug trials for personalized treatment options are being conducted, classification guidelines based on the distinct biological, clinical and molecular characteristics of BC subtypes will continue to be one of the main tools for planning patient-specific treatment. Subtyping also captures most of the biodiversity in BC. However, treatment regiments may be altered in order to fit the individual needs of each BC patient.
One possible limitation of this study is that it reflects the retrospective data of a single center. Our study is one of the first studies expressing HER2 as the worst BC subgroup despite targeted therapy. However, prospective studies in multiple centers testing the next generation of well-designed targeted therapies may be necessary.
Conclusion
Our study shows that the HER2-enriched subgroup has the worst prognosis despite receiving targeted therapy. The misconception about the extent of issues that targeted therapy can resolve may cloud the clinicians’ judgment regarding which patients will have worse prognosis. Therefore, patients in the HER2-enriched subgroup need to be followed carefully, and new treatment options should be tested.
Disclosure
The authors declare that they have no conflicts of interest.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi: 10.3322/caac.21708 [DOI] [PubMed] [Google Scholar]
- 2.Mareel M, Constantino S. Ecosystems of invasion and metastasis in mammary morphogenesis and cancer. Int J Dev Biol. 2011;55(7–9):671–684. doi: 10.1387/ijdb.113386mm [DOI] [PubMed] [Google Scholar]
- 3.Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. doi: 10.1038/35021093 [DOI] [PubMed] [Google Scholar]
- 4.Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22(8):1736–1747. doi: 10.1093/annonc/mdr304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. doi: 10.1038/nature11412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burstein HJ, Curigliano G, Thürlimann B, et al. Customizing local and systemic therapies for women with early breast cancer: the St. Gallen International Consensus Guidelines for treatment of early breast cancer 2021. Ann Oncol. 2021;32(10):1216–1235. doi: 10.1016/j.annonc.2021.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciriello G, Sinha R, Hoadley KA, et al. The molecular diversity of Luminal A breast tumors. Breast Cancer Res Treat. 2013;141(3):409–420. doi: 10.1007/s10549-013-2699-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24(9):2206–2223. doi: 10.1093/annonc/mdt303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363(20):1938–1948. doi: 10.1056/NEJMra1001389 [DOI] [PubMed] [Google Scholar]
- 10.Ma D, Jiang YZ, Xiao Y, et al. Integrated molecular profiling of young and elderly patients with triple-negative breast cancer indicates different biological bases and clinical management strategies. Cancer. 2020;126(14):3209–3218. doi: 10.1002/cncr.32922 [DOI] [PubMed] [Google Scholar]
- 11.Prat A, Pineda E, Adamo B, et al. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast. 2015;24(Suppl 2):S26–S35. doi: 10.1016/j.breast.2015.07.008 [DOI] [PubMed] [Google Scholar]
- 12.Abubakar M, Figueroa J, Ali HR, et al. Combined quantitative measures of ER, PR, HER2, and KI67 provide more prognostic information than categorical combinations in luminal breast cancer. Mod Pathol. 2019;32:1244–1256. doi: 10.1038/s41379-019-0270-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brewster AM, Chavez-MacGregor M, Brown P. Epidemiology, biology, and treatment of triple-negative breast cancer in women of African ancestry. Lancet Oncol. 2014;15(13):e625–e634. doi: 10.1016/S1470-2045(14)70364-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schettini F, Giuliano M, De Placido S, Arpino G. Nab-paclitaxel for the treatment of triple-negative breast cancer: rationale, clinical data and future perspectives. Cancer Treat Rev. 2016;50:129–141. doi: 10.1016/j.ctrv.2016.09.004 [DOI] [PubMed] [Google Scholar]
- 15.Dey N, Barwick BG, Moreno CS, et al. Wnt signaling in triple negative breast cancer is associated with metastasis. BMC Cancer. 2013;13:537. doi: 10.1186/1471-2407-13-537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J, Xu B. Targeted therapeutic options and future perspectives for HER2-positive breast cancer. Sig Transduct Target Ther. 2019;34. doi: 10.1038/s41392-019-0069-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turke AB, Song Y, Costa C, et al. MEK inhibition leads to PI3K/AKT activation by relieving a negative feedback on ERBB receptors. Cancer Res. 2012;72(13):3228–3237. doi: 10.1158/0008-5472.CAN-11-3747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ban M, Petrić Miše B, Vrdoljak E. Early HER2-positive breast cancer: current treatment and novel approaches. Breast Care. 2020;15(6):560–569. doi: 10.1159/000511883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loi S, Dafni U, Karlis D, et al. Effects of estrogen receptor and human epidermal growth factor receptor-2 levels on the efficacy of trastuzumab: a secondary analysis of the HERA trial. JAMA Oncol. 2016;(8):1040–1047. PMID: 27100299. doi: 10.1001/jamaoncol.2016.0339 [DOI] [PubMed] [Google Scholar]
- 20.Seshadri R, Firgaira FA, Horsfall DJ, McCaul K, Setlur V, Kitchen P. Clinical significance of HER-2/neu oncogene amplification in primary breast cancer. The South Australian Breast Cancer Study Group. J Clin Oncol. 1993;11(10):1936–1942. doi: 10.1200/JCO.1993.11.10.1936 [DOI] [PubMed] [Google Scholar]
- 21.Harari D, Yarden Y. Molecular mechanisms underlying ErbB2/HER2 action in breast cancer. Oncogene. 2000;19(53):6102–6114. doi: 10.1038/sj.onc.1203973 [DOI] [PubMed] [Google Scholar]
- 22.Iqbal N, Iqbal N. Human epidermal growth factor receptor 2 (HER2) in cancers: overexpression and therapeutic implications. Mol Biol Int. 2014;2014:852748. doi: 10.1155/2014/852748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–182. doi: 10.1126/science.3798106 [DOI] [PubMed] [Google Scholar]
- 24.Cameron D, Piccart-Gebhart MJ, Gelber RD, et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017;389(10075):1195–1205. doi: 10.1016/S0140-6736(16)32616-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Minckwitz G, Procter M, de Azambuja E, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med. 2017;377(2):122–131. doi: 10.1056/NEJMoa1703643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piccart M, Procter M, Fumagalli D, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer in the APHINITY trial: 6 years’ follow-up. J Clin Oncol. 2021;39(13):1448–1457. doi: 10.1200/JCO.20.01204 [DOI] [PubMed] [Google Scholar]
- 27.von Minckwitz G, Huang C-S, Mano MS, et al. Trastuzumab Emtansine for residual invasive HER2-positive breast cancer. N Eng J Med. 2019;380(7):617–628. doi: 10.1056/nejmoa1814017 [DOI] [PubMed] [Google Scholar]
- 28.Jacobs SA, Robidoux A, Abraham J, et al. NSABP FB-7: a Phase II randomized neoadjuvant trial with paclitaxel + trastuzumab and/or neratinib followed by chemotherapy and postoperative trastuzumab in HER2+ breast cancer. Breast Cancer Res. 2019;21(1):133. doi: 10.1158/1538-7445.SABCS15-PD5-04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swain SM, Kim SB, Cortés J, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, Phase 3 study. Lancet Oncol. 2013;14(6):461–471. doi: 10.1016/S1470-2045(13)70130-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harbeck N, Gluz O, Christgen M, et al. De-escalation strategies in human epidermal growth factor receptor 2 (HER2)-positive early Breast Cancer (BC): final analysis of the West German Study Group adjuvant dynamic marker-adjusted personalized therapy trial optimizing risk assessment and therapy response prediction in early BC HER2- and hormone receptor-positive Phase II randomized trial-efficacy, safety, and predictive markers for 12 weeks of neoadjuvant trastuzumab emtansine with or without Endocrine Therapy (ET) versus trastuzumab plus ET. J Clin Oncol. 2017;35(26):3046–3054. doi: 10.1200/JCO.2016.71.9815 [DOI] [PubMed] [Google Scholar]
- 31.Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(1):25–32. doi: 10.1016/S1470-2045(11)70336-9 [DOI] [PubMed] [Google Scholar]
- 32.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 33.Lee SB, Sohn G, Kim J, et al. A retrospective prognostic evaluation analysis using the 8th edition of the American Joint Committee on Cancer staging system for breast cancer. Breast Cancer Res Treat. 2018;169(2):257–266. doi: 10.1007/s10549-018-4682-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28(16):2784–2795. doi: 10.1200/JCO.2009.25.6529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolff AC, Hammond ME, Allison KH, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline focused update. Arch Pathol Lab Med. 2018;142(11):1364–1382. doi: 10.5858/arpa.2018-0902-SA [DOI] [PubMed] [Google Scholar]
- 36.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007;131(1):18–43. doi: 10.5858/2007-131-18-ASOCCO [DOI] [PubMed] [Google Scholar]
- 37.Chen R, Ye Y, Yang C, et al. Assessment of the predictive role of pretreatment Ki-67 and Ki-67 changes in breast cancer patients receiving neoadjuvant chemotherapy according to the molecular classification: a retrospective study of 1010 patients. Breast Cancer Res Treat. 2018;170(1):35–43. doi: 10.1007/s10549-018-4730-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bewick V, Cheek L, Ball J. Statistics review 12: survival analysis. Crit Care. 2004;8(5):389–394. doi: 10.1186/cc2955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bradburn MJ, Clark TG, Love SB, Altman DG. Survival analysis part II: multivariate data analysis–an introduction to concepts and methods. Br J Cancer. 2003;89(3):431–436. doi: 10.1038/sj.bjc.6601119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sayed S, Fan S, Moloo Z, et al. Breast cancer risk factors in relation to molecular subtypes in breast cancer patients from Kenya. Breast Cancer Res. 2021;23(1):68. doi: 10.1186/s13058-021-01446-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martínez ME, Cruz GI, Brewster AM, Bondy ML, Thompson PA. What can we learn about disease etiology from case-case analyses? Lessons from breast cancer. Cancer Epidemiol Biomarkers Prev. 2010;19(11):2710–2714. doi: 10.1158/1055-9965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wiechmann L, Sampson M, Stempel M, et al. Presenting features of breast cancer differ by molecular subtype. Ann Surg Oncol. 2009;16(10):2705–2710. doi: 10.1245/s10434-009-0606-2 [DOI] [PubMed] [Google Scholar]
- 43.Park S, Koo JS, Kim MS, et al. Characteristics and outcomes according to molecular subtypes of breast cancer as classified by a panel of four biomarkers using immunohistochemistry. Breast. 2012;21(1):50–57. doi: 10.1016/j.breast.2011.07.008 [DOI] [PubMed] [Google Scholar]
- 44.Puig-Vives M, Sánchez MJ, Sánchez-Cantalejo J, et al. Distribution and prognosis of molecular breast cancer subtypes defined by immunohistochemical biomarkers in a Spanish population-based study. Gynecol Oncol. 2013;130(3):609–614. doi: 10.1016/j.ygyno.2013.05.039 [DOI] [PubMed] [Google Scholar]
- 45.Shou J, Massarweh S, Osborne CK, et al. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst. 2004;96(12):926–935. doi: 10.1093/jnci/djh166 [DOI] [PubMed] [Google Scholar]
- 46.Cheang MC, Chia SK, Voduc D, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101(10):736–750. doi: 10.1093/jnci/djp082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol. 2010;11(2):174–183. doi: 10.1016/S1470-2045(09)70262-1 [DOI] [PubMed] [Google Scholar]
- 48.Bustreo S, Osella-Abate S, Cassoni P, et al. Optimal Ki67 cut-off for luminal breast cancer prognostic evaluation: a large case series study with a long-term follow-up. Breast Cancer Res Treat. 2016;157(2):363–371. doi: 10.1007/s10549-016-3817-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burstein HJ, Lacchetti C, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: ASCO clinical practice guideline focused update. J Clin Oncol. 2019;37(5):423–438. doi: 10.1200/JCO.18.01160 [DOI] [PubMed] [Google Scholar]
- 50.Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381(9869):805–816. doi: 10.1016/S0140-6736(12)61963-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goss PE, Ingle JN, Martino S, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003;349(19):1793–1802. doi: 10.1056/NEJMoa032312 [DOI] [PubMed] [Google Scholar]