Abstract
Class AII and AIII lantibiotics and mersacidin are antibacterial peptides containing unusual residues obtained by posttranslational modifications of prepeptides, presumably catalyzed by LanM. LctM, the LanM for lacticin 481, is essential for the production of this class AII lantibiotic. Using the yeast two-hybrid system, we showed direct contact between the prelacticin 481 and LctM, supporting the proposed LctM function. Sixteen domains are conserved between the 10 known LanM proteins, whereas three additional domains were found only in class AII LanM proteins and in MrsM, the LanM for mersacidin. All the truncated LctM proteins that we tested presented impaired LctA-binding activity.
Bacteriocins are ribosomally synthesized peptides with antibacterial activity. Some bacteriocins from gram-positive bacteria are termed lantibiotics because they present the unique feature of containing unusual residues, leading to intramolecular thioether bridges (20, 21). Lantibiotics are produced as prepeptides encoded by structural genes. The unusual residues are created in the prepeptide C-terminal part (propeptide) by posttranslational modifications, whereas the prepeptide N-terminal leader sequence is cleaved off (4, 20, 21). The unusual residues are mainly the α,β-unsaturated amino acids dehydroalanine (Dha) and dehydrobutyrine (Dhb) and the residues lanthionine (Lan) and 3-methyllanthionine (MeLan) harboring the thioether bonds. Dehydrations of serine and threonine produce Dha and Dhb, respectively, which are targets for nucleophilic addition of the SH group of cysteine residues, yielding Lan and MeLan. The most studied linear (type A) lantibiotics belong to class AI, which includes in particular nisin, subtilin, Pep5, and epidermin (4, 21). Biosynthesis of these lantibiotics requires two modification enzymes, LanB and LanC (Lan refers collectively to homologous proteins of different lantibiotic systems). LanB would be involved in the dehydration process and LanC in thioether bond formation, as shown in the cases of nisin and Pep5, respectively (10, 14). It has been shown in the cases of nisin and subtilin that LanB and LanC form a lantibiotic synthetase complex also including the transmembrane ATP-binding cassette (ABC) transporter LanT and that both LanB and LanC interact directly with the lantibiotic prepeptide (11, 22). In comparison, information related to the biosynthesis of other type A lantibiotics is scarce. Lacticin 481 contains 1 Dhb, 1 MeLan, and 2 Lan residues responsible for a rather globular C terminus, whereas the N-terminal part is unbridged (16, 20, 25). This lantibiotic is representative of several highly similar bacteriocins (streptococcin A-FF22, mutacin II, salivaricin A, variacin, streptococcin A-M49, and butyrivibriocin OR79A) that have been regrouped so far into class AII (9, 13). The gene clusters for lacticin 481, mutacin II, and streptococcin A-FF22 have been reported (3, 13, 18). They are similarly organized, each including the structural gene lanA followed by the genes lanMTFEG (Fig. 1) but no counterpart of lanB or lanC. The six genes of the lacticin 481 operon are sufficient to confer high levels of lantibiotic production to a Lactococcus strain (18). LanT and LanFEG form two ABC transporters, the first one responsible for both the cleavage of the leader peptide and the export of the mature bacteriocin (8), and the second one protecting the strain from its own lantibiotic (18). As LanM proteins show limited similarities with conserved segments of LanC proteins (12, 23), it is assumed that they catalyze the formation of the unusual residues. According to this hypothesis, LanM should be essential for lantibiotic biosynthesis. This was partially verified in the lacticin 481 case, since introduction into Lactococcus lactis IL1403 of lctA and lctM only (lct refers to the lacticin 481 operon genes) resulted in low bacteriocin activity, which was abolished by deleting the 3′ end of lctM (17). The butyrivibriocin OR79A gene cluster has been reported (9) and includes genes similar to lanFEGAM, the lanM (which we name here bviM instead of ORF6) being only partially sequenced. LanM proteins are also encoded by the gene clusters for class AIII lantibiotics, the third subgroup of linear lantibiotics which tentatively includes lactocin S (24) and two-component lantibiotics, the full activity of which requires the synergistic action of two peptides, such as cytolysin, staphylococcin C55, and lacticin 3147 (7, 15, 19). LanM homologues are essential for the production of cytolysin and lactocin S (7, 24). Finally, a LanM counterpart, MrsM, is encoded by the gene cluster for mersacidin (1), which is a lantibiotic more closely related to type B (globular peptides) than to type A lantibiotics (2). In the present study, we showed that LctM is essential for lacticin 481 biosynthesis even when the five other lct genes are expressed, and we used the yeast two-hybrid system to detect and study direct interactions between LctM and the lacticin 481 prepeptide LctA. We also compared the sequences of all known LanM proteins, identifying 19 conserved regions, 3 of which are found only in class AII LanM proteins and in MrsM. Finally, we tested the LctA-binding activity of truncated variants of LctM.
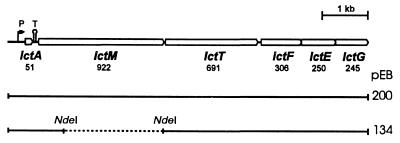
FIG. 1.
Organization of the lacticin 481 operon. The number of codons in each gene is given below its name. P and T indicate the promoter region and a terminator, respectively. pEB200 and pEB134 are fusions of pIL253 and pBluescript containing the inserts shown by the continuous lines. The dashed line corresponds to the deleted NdeI fragment.
LctM is essential for lacticin 481 production.
We previously showed that lctM was necessary to produce lacticin 481 when it was the only gene expressed with lctA (17). To examine whether this is still the case when the remainder of the lacticin 481 operon in addition to lctA is expressed, an in-frame deletion of 77% of lctM was created by removing the 2.1-kb NdeI fragment (Fig. 1) from pEF94, which contains lctAMTFEG in pBluescript KS (18). To allow replication and selection in L. lactis, the resulting plasmid was fused to the vector pIL253, creating pEB134. In L. lactis IL1403, pEB134 failed to induce detectable bacteriocin activity, indicating that culture supernatants contained less than 10 arbitrary units (AU) of lacticin 481 · ml−1. In contrast, a plasmid carrying the complete operon (Fig. 1, pEB200) led to the accumulation of up to 5,000 AU of lacticin 481 · ml−1 in culture supernatants. The lctATFEG genes are thus not sufficient to produce lacticin 481. This could not result from an impaired expression of the lct genes, since pEB134 provided L. lactis IL1403 with the same high level of protection against lacticin 481 as pEB200 (whereas L. lactis IL1403 containing the vector only was inhibited by 10 AU of lacticin 481 · ml−1 [18], 1,280 AU · ml−1 was required when the strain harbored pEB134 or pEB200). These results show that lacticin 481 biosynthesis absolutely requires LctM.
A LctA-LctM interaction is detected by the yeast two-hybrid system.
If the assumption that LctM modifies the propeptide part of LctA is correct, one would expect that the two molecules would make direct physical contact. We examined this hypothesis with the yeast two-hybrid system, which detects even transient protein interactions such as enzyme-substrate interactions (6) and was used to show direct contacts between prelantibiotics and LanB or LanC (11, 22). lctA and lctM were amplified by PCR and cloned into pGBT9 and pGAD424 (Matchmaker GAL4 two-hybrid system; Clontech Laboratories, Palo Alto, Calif.) as indicated in Table 1, in order to produce chimeric proteins including LctA or LctM fused to one of the two separate domains of the yeast transcriptional activator GAL4: the DNA binding domain (BD) and the transcriptional activation domain (AD). Within the yeast strain Saccharomyces cerevisiae SFY526, an interaction between the proteins fused to the GAL4 domains leads to transcriptional activation of the reporter gene lacZ. This activation was examined by using the β-galactosidase colony lift filter assay (yeast protocols handbook, Clontech) on at least 15 yeast colonies resulting from the cotransformation of SFY526 by one pGBT9 and one pGAD424 derivative. The results are shown in Table 2. High β-galactosidase activity was detected in yeasts producing BD::LctA (pHB246) and AD::LctM (pHA689 or pHA888). As yeasts producing BD::LctA and AD or AD::LctM and BD did not contain detectable levels of β-galactosidase, this result indicated an interaction between LctA and LctM. The reciprocal fusions AD::LctA (pHA409) and BD::LctM (pHB685 or pHB887) failed to activate the reporter gene. However, such interactions, which could not be confirmed by the reciprocal fusions, have been reported (6, 26), without invalidating the positive result. When we quantified the enzyme activity from liquid cultures using o-nitrophenyl-β-d-galactopyranoside (ONPG) as the substrate (yeast protocols handbook, Clontech), the activity induced by the BD::LctA-AD::LctM interaction was 9,400-fold higher than the background activity (e.g., BD::LctA-AD) (Table 2). This confirms the interaction between LctA and LctM, which supports the notion that LctM is the enzyme for the posttranslational modifications of prelacticin 481.
TABLE 1.
Plasmids used in two-hybrid assays
| Plasmid | Fused genesa | Vector | PCR primersb used or cloning strategy |
|---|---|---|---|
| pHA409 | gal4AD::lctAc | pGAD424 | LCTA1, ATTAAGAATTCATAATGAAAGAACAAAACTCT (EcoRI) |
| LCTT4, ATATTCTGCAGCGATACGTAACTTTTTAT (PstI) | |||
| pHA689 | gal4AD::lctM | pGAD424 | LCTM1, TAATAGAATTCATAGTGAAAAAAAAGACTTAC (EcoRI) |
| LCTM2, TTATACTGCAGATATTAATCAACATATGGC (PstI) | |||
| pHA888 | gal4AD::lctM | pGAD424 | The 2.1-kb NdeI fragment internal to the pHA689 insert was replaced by the corresponding fragment from the cloned lacticin 481 operon |
| pHB246 | gal4BD::lctAc | pGBT9 | LCTA1 and LCTT4 |
| pHB685 | gal4BD::lctM | pGBT9 | LCTM1 and LCTM2 |
| pHB887 | gal4BD::lctM | pGBT9 | The 2.1-kb NdeI fragment internal to the pHB685 insert was replaced by the corresponding fragment from the cloned lacticin 481 operon |
The junctions between the vectors and the 5′ ends of the inserts have been verified by sequencing. The inserts carrying lctA were entirely sequenced.
The names of the primers and their 5′→3′ sequences are given. Only boldfaced bases are complementary to the target sequence. Restriction sites are underlined in the sequences and identified in parentheses.
The insert is a 0.34-kb DNA fragment resulting from the EcoRI-BclI digestion of the larger fragment amplified with LCTA1–LCTT4.
TABLE 2.
Yeast two-hybrid assays of LctA-LctM interaction
| Protein fused to GAL4 AD | Color (β-galactosidase activity)a of colonies with the indicated protein fused to GAL4 BD
|
||
|---|---|---|---|
| LctA | LctM | None | |
| LctA | W | W | W |
| LctM | B (94.0 ± 9.0) | W | W (0.01 ± 0.01) |
| None | W (0.01 ± 0.01) | W | W (0.01 ± 0.01) |
The activation of the lacZ reporter gene was examined by β-galactosidase filter assays (W and B, white and blue colonies, respectively). Values are means ± standard errors from quantitative assays performed in duplicate or triplicate from at least three distinct liquid cultures. They are expressed in Miller units calculated by the equation (OD420 × 1,000)/(OD600 × 0.1 × cell concentration factor × reaction time in minutes), where OD420 and OD600 are optical densities at 420 and 600 nm, respectively.
LanM proteins display conserved domains.
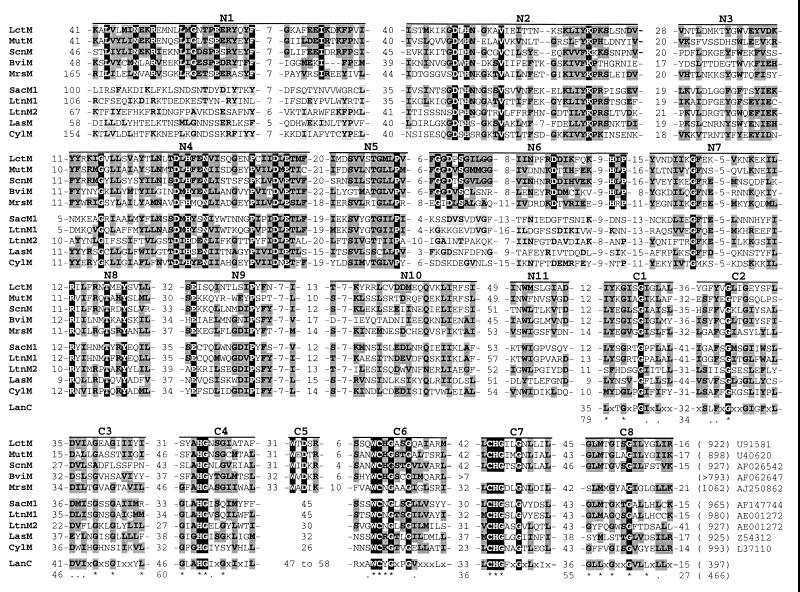
The sequences of LctM, LasM (lactocin S), and CylM (cytolysin) were compared in previous studies, showing that the six (24) or seven (23) domains conserved among LanC proteins are also found in the LanM C-terminal parts and identifying one (23) to four (24) domains shared by their N-terminal parts. Since these works, the sequences of seven other LanM proteins have been reported: MutM (mutacin II), ScnM (streptococcin A-FF22), LtnM1 and LtnM2 (lacticin 3147), SacM1 (staphylococcin C55), BviM (butyrivibriocin OR79A), and MrsM (mersacidin) (1, 5, 9, 13, 15, 27). We compared these sequences and observed that LctM shares 26 to 32% identity with the other class AII LanM proteins (MutM, ScnM, and BviM), 19 to 23% identity with class AIII LanM proteins (SacM1, LtnM1, LtnM2, LasM, and CylM), and 25% identity with MrsM. The conserved amino acids are clustered in distinct domains, and we propose to distinguish 19 of them (Fig. 2 and 3). In the C-terminal regions, domains C1 to C4 and C6 to C8 are conserved within all LanM proteins and correspond to the seven LanC domains (Fig. 2). Furthermore, the H, WC, and CH residues of domains C4, C6, and C7, which could be involved in the enzymatic activity of LanC, disulfide bond formation, or metal-ion binding (12, 23), are common to all LanM proteins. The glycines of the seven domains could be important for the structure or activity of LanC (12, 23), and are also well conserved within LanM proteins. Domain C5 is common to the four class AII LanM proteins but is absent from the class AIII LanM proteins and from LanC. The N-terminal two-thirds of LctM did not show any significant similarity with proteins other than LanM. Within these regions, we propose 11 conserved domains designated N1 to N11 (Fig. 2 and 3). Whereas domains N2 to N5 and N7 to N11 are shared by all LanM proteins, domains N1 and N6 are highly conserved in the four class AII LanM proteins but not in the class AIII LanM proteins. Class AII prepeptides are very closely related in terms of primary sequence and the disposition and identity of the modified residues (9). It is thus not surprising that their putative modification enzymes are more closely related to each other than to class AIII LanM proteins, which process more distantly related prebacteriocins. The conservation or lack of conservation of LanM domains N1, N6, and C5 confirms the proposed classification of lacticin 481, mutacin II, streptococcin A-FF22, and butyrivibriocin OR79A, on the one hand, and of lacticin 3147, staphylococcin C55, cytolysin, and lactocin S, on the other, into two different subgroups. This feature could thus be considered a new criterion of lantibiotic classification. It is not, however, sufficient and should not be considered independently of the characteristics of the lantibiotic and its prepeptide, as shown by the case of mersacidin. Although the latter was not included in class AII or AIII, due to the differences between premersacidin and type A prepeptides (2), its LanM (MrsM) shares all the LanM conserved domains, including the three class AII-specific domains (Fig. 2). The only obvious divergent feature of MrsM compared to the class AII LanM proteins is a longer sequence preceding domain N1 (165 versus 41 to 48 residues). The sequences of other LanM proteins encoded by operons for mersacidin-related lantibiotics will be required in order to draw more-accurate conclusions. Another interpretation would be that mersacidin and class AII lantibiotics are more closely related than previously thought. A similar conclusion was drawn very recently by Altena et al. (1) on the basis of overall gene cluster organization and new comparisons of prepeptide leader sequences. In their most recent review (20), Sahl and Bierbaum wondered whether the grouping of lacticin 481-related lantibiotics (class AII) into type A (linear) lantibiotics is appropriate, because of their partially globular structure. If one could imagine defining a new lantibiotic type for class AII lantibiotics, type C, intermediate between types A and B (globular), this new type could also include mersacidin and the related actagardin, which have been classified as type B lantibiotics without sharing all their characteristics (2). The question of whether the common features of class AII bacteriocins and mersacidin are sufficient to regroup them in a single type despite their differences remains open. For class AIII lantibiotics, the information related to their structures is too scarce to question their relationship with type A lantibiotics.
FIG. 2.
Conserved domains within LanM and LanC. The domains are designated N1 to N11 and C1 to C8. LanM proteins are divided into two subgroups, one including the four class AII LanM proteins (LctM, MutM, ScnM, and BviM) and MrsM and the other composed of the five class AIII LanM proteins (SacM1, LtnM1, LtnM2, LasM, and CylM). Residues identical either in LanM proteins from the first subgroup (domains N1, N6, and C5) or in all LanM proteins (other domains) are on a solid background, whereas residues similar or identical in at least 60% of the proteins are on a dark shaded background. A light shaded background indicates residues from LanM proteins of the second subgroup that are identical or similar to residues conserved within domains N1 and N6. The numbers of amino acids before, between, and after the sequences represented are given. The total numbers of residues are given in parentheses at the ends of the sequences, followed by the database accession numbers. The consensus sequence for the conserved domains of LanC is given, with x indicating undefined positions. Residues of the LanC consensus that are conserved within LanM are on the corresponding background. The consensus is drawn from the work of Siezen et al. (23) and our own comparison including NisC, SpaC, EpiC, PepC (database accession numbers as in reference 23), MutC, EciC, and Scf1.12 (accession numbers AF154675, Y14023, and AL117322, respectively). A residue was included in the consensus if it or a similar amino acid was found in at least 70% of LanC proteins. Dots and stars indicate residues similar or identical, respectively, in the seven LanC proteins. The smallest and largest numbers of amino acids preceding and following each LanC sequence are given. The total numbers of residues of the smallest and largest LanC proteins are given in parentheses at the end of the sequence.
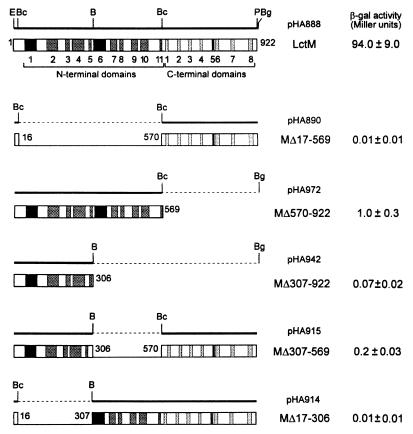
FIG. 3.
LctM and truncated derivatives fused to the GAL4 activator domain. pHA888 is pGAD424 containing the insert (full-length lctM) represented by the heavy top line. The following restriction sites are indicated: B, BamHI; Bc, BclI; Bg, BglII; E, EcoRI; P, PstI. The other plasmids result from deletions in pHA888 created at the indicated restriction sites, with the deleted fragments represented by dashed lines. The encoded proteins are represented by boxes in which the domains conserved between LanM and LanC, among LanM proteins, and between class AII LanM proteins and MrsM are indicated by light shading, dark shading, and black, respectively. The domains are numbered according to Fig. 2. The numbers next to the boxes indicate their last or first residues. The β-galactosidase activities given are means ± standard errors of duplicate or triplicate assays from at least three distinct liquid cultures of yeast cells coexpressing the corresponding AD::LctM fusion and BD::LctA (pHB246).
Truncated LctM showed impaired interactions with LctA.
In order to examine if the interaction detected between LctA and LctM could be assigned to a particular region of LctM, we constructed several derivatives of pHA888, each containing a deletion (in frame when internal to lctM) allowing the expression of a truncated version of LctM fused to the GAL4 activation domain (Fig. 3). None of these fusions induced β-galactosidase activities higher than 0.01 ± 0.01 Miller units when coexpressed with the GAL4 BD alone. The β-galactosidase activities assayed from yeast cells coexpressing one of these proteins and BD::LctA are shown in Fig. 3. We first looked for the interactions between LctA and either the N- or the C-terminal region of LctM. Whereas the C-terminal region (MΔ17-569) failed to interact with LctA, the N-terminal region (MΔ570-922) retained a significant LctA-binding ability, inducing a β-galactosidase activity 100-fold higher than the background. This activity was, however, about 100-fold lower than that obtained with the complete LctM, suggesting that the C-terminal region somehow participates in the LctA-binding activity. We further subdivided the N-terminal region in two parts comprising domains N1 to N5 (up to residue 306) and domains N6 (without its first 3 residues, FGG) to N11 (residues 307 to 569). The deletion of the second part reduced the β-galactosidase activity either 14-fold (MΔ307-922 versus MΔ570-922) or 470-fold (MΔ307-569 versus LctM). The absence of residues 17 to 306 (M13017-306) abolished the interaction with LctA, but these residues by themselves (MΔ307-922) were not sufficient to account for the affinity of LctM for LctA. We thus could not assign the LctA-binding activity of LctM to a particular region of the latter. It is likely that this activity requires several residues scattered within LctM. Since LctM is a much larger protein than LctA (922 versus 51 amino acids), we could speculate that the spatial distribution of these residues in the native LctM forms a LctA-binding pocket. Truncating any portion of the protein could therefore alter such a LctA-binding site not only by removing one or several residues involved in the direct contact with LctA but also by changing their appropriate spacing or by preventing correct folding of the protein. To gather data on the involved residues, one would need to analyze the LctA-binding activity of LctM harboring point mutations. The residues that we identified here as identical either in all LanM proteins (18 and 13 residues in the N- and C-terminal domains, respectively) or in domains N1, N6, and C5 of class AII LanM proteins and MrsM (14 and 2 residues in the N- and C-terminal domains, respectively) would probably be interesting mutagenesis targets.
Acknowledgments
P.U. was the recipient of a doctoral fellowship from the Région Bretagne.
REFERENCES
- 1.Altena K, Guder A, Cramer C, Bierbaum G. Biosynthesis of the lantibiotic mersacidin: organization of a type B lantibiotic gene cluster. Appl Environ Microbiol. 2000;66:2565–2571. doi: 10.1128/aem.66.6.2565-2571.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bierbaum G, Brötz H, Koller K-P, Sahl H-G. Cloning, sequencing and production of the lantibiotic mersacidin. FEMS Microbiol Lett. 1995;127:121–126. doi: 10.1111/j.1574-6968.1995.tb07460.x. [DOI] [PubMed] [Google Scholar]
- 3.Chen P, Qi F, Novak J, Caufield P W. The specific genes for lantibiotic mutacin II biosynthesis in Streptococcus mutans T8 are clustered and can be transferred en bloc. Appl Environ Microbiol. 1999;65:1356–1360. doi: 10.1128/aem.65.3.1356-1360.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Vos W M, Kuipers O P, van der Meer J R, Siezen R J. Maturation pathway of nisin and other lantibiotics: post-translationally modified antimicrobial peptides exported by Gram-positive bacteria. Mol Microbiol. 1995;17:427–437. doi: 10.1111/j.1365-2958.1995.mmi_17030427.x. [DOI] [PubMed] [Google Scholar]
- 5.Dougherty B A, Hill C, Weidman J F, Richardson D R, Venter J C, Ross R P. Sequence and analysis of the 60 kb conjugative, bacteriocin-producing plasmid pMRC01 from Lactococcus lactis DPC3147. Mol Microbiol. 1998;29:1029–1038. doi: 10.1046/j.1365-2958.1998.00988.x. [DOI] [PubMed] [Google Scholar]
- 6.Fields S, Sternglanz R. The two-hybrid system: an assay for protein-protein interactions. Trends Genet. 1994;10:286–291. doi: 10.1016/0168-9525(90)90012-u. [DOI] [PubMed] [Google Scholar]
- 7.Gilmore M S, Segarra R A, Booth M C, Bogie C P, Hall L R, Clewell D B. Genetic structure of the Enterococcus faecalis plasmid pAD1-encoded cytolytic toxin system and its relationship to lantibiotic determinants. J Bacteriol. 1994;176:7335–7344. doi: 10.1128/jb.176.23.7335-7344.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Håvarstein L S, Diep D B, Nes I F. A family of bacteriocin ABC transporters carry out proteolytic processing of their substrates concomitant with export. Mol Microbiol. 1995;16:229–240. doi: 10.1111/j.1365-2958.1995.tb02295.x. [DOI] [PubMed] [Google Scholar]
- 9.Kalmokoff M L, Lu D, Whitford M F, Teather R M. Evidence for production of a new lantibiotic (butyrivibriocin OR79A) by the ruminal anaerobe Butyrivibrio fibrisolvens OR79: characterization of the structural gene encoding butyrivibriocin OR79A. Appl Environ Microbiol. 1999;65:2128–2135. doi: 10.1128/aem.65.5.2128-2135.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karakas Sen A, Narbad A, Horn N, Dodd H M, Parr A J, Colquhoun I, Gasson M J. Post-translational modification of nisin. The involvement of NisB in the dehydration process. Eur J Biochem. 1999;261:524–532. doi: 10.1046/j.1432-1327.1999.00303.x. [DOI] [PubMed] [Google Scholar]
- 11.Kiesau P, Eikmanns U, Gutowski-Eckel Z, Weber S, Hammelmann M, Entian K-D. Evidence for a multimeric subtilin synthetase complex. J Bacteriol. 1997;179:1475–1481. doi: 10.1128/jb.179.5.1475-1481.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kupke T, Götz F. Expression, purification, and characterization of EpiC, an enzyme involved in the biosynthesis of the lantibiotic epidermin, and sequence analysis of Staphylococcus epidermidis epiC mutants. J Bacteriol. 1996;178:1335–1340. doi: 10.1128/jb.178.5.1335-1340.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McLaughlin R E, Ferretti J J, Hynes W L. Nucleotide sequence of the streptococcin A-FF22 lantibiotic regulon: model for production of the lantibiotic SA-FF22 by strains of Streptococcus pyogenes. FEMS Microbiol Lett. 1999;175:171–177. doi: 10.1111/j.1574-6968.1999.tb13616.x. [DOI] [PubMed] [Google Scholar]
- 14.Meyer C, Bierbaum G, Heidrich C, Reis M, Süling J, Iglesias-Wind M I, Kempter C, Molitor E, Sahl H-G. Nucleotide sequence of the lantibiotic Pep5 biosynthetic gene cluster and functional analysis of PepP and PepC. Evidence for a role of PepC in thioether formation. Eur J Biochem. 1995;232:478–489. doi: 10.1111/j.1432-1033.1995.tb20834.x. [DOI] [PubMed] [Google Scholar]
- 15.Navaratna M A D B, Sahl H-G, Tagg J R. Identification of genes encoding two-component lantibiotic production in Staphylococcus aureus C55 and other phage group II S. aureus strains and demonstration of an association with the exfoliative toxin B gene. Infect Immun. 1999;67:4268–4271. doi: 10.1128/iai.67.8.4268-4271.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piard J-C, Kuipers O P, Rollema H S, Desmazeaud M J, de Vos W M. Structure, organization, and expression of the lct gene for lacticin 481, a novel lantibiotic produced by Lactococcus lactis. J Biol Chem. 1993;268:16361–16368. [PubMed] [Google Scholar]
- 17.Rincé A, Dufour A, Le Pogam S, Thuault D, Bourgeois C M, Le Pennec J-P. Cloning, expression, and nucleotide sequence of genes involved in production of lactococcin DR, a bacteriocin from Lactococcus lactis subsp. lactis. Appl Environ Microbiol. 1994;60:1652–1657. doi: 10.1128/aem.60.5.1652-1657.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rincé A, Dufour A, Uguen P, Le Pennec J-P, Haras D. Characterization of the lacticin 481 operon: the Lactococcus lactis genes lctF, lctE, and lctG encode a putative ABC transporter involved in bacteriocin immunity. Appl Environ Microbiol. 1997;63:4252–4260. doi: 10.1128/aem.63.11.4252-4260.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryan M P, Jack R W, Josten M, Sahl H-G, Jung G, Ross R P, Hill C. Extensive post-translational modification, including serine to d-alanine conversion, in the two-component lantibiotic, lacticin 3147. J Biol Chem. 1999;274:37544–37550. doi: 10.1074/jbc.274.53.37544. [DOI] [PubMed] [Google Scholar]
- 20.Sahl H-G, Bierbaum G. Lantibiotics: biosynthesis and biological activities of uniquely modified peptides from Gram-positive bacteria. Annu Rev Microbiol. 1998;52:41–79. doi: 10.1146/annurev.micro.52.1.41. [DOI] [PubMed] [Google Scholar]
- 21.Sahl H-G, Jack R W, Bierbaum G. Biosynthesis and biological activities of lantibiotics with unique post-translational modifications. Eur J Biochem. 1995;230:827–853. doi: 10.1111/j.1432-1033.1995.tb20627.x. [DOI] [PubMed] [Google Scholar]
- 22.Siegers K, Heinzmann S, Entian K-D. Biosynthesis of lantibiotic nisin. Posttranslational modification of its prepeptide occurs at a multimeric membrane-associated lanthionine synthetase complex. J Biol Chem. 1996;271:12294–12301. doi: 10.1074/jbc.271.21.12294. [DOI] [PubMed] [Google Scholar]
- 23.Siezen R J, Kuipers O P, de Vos W M. Comparison of lantibiotic gene clusters and encoded proteins. Antonie Leeuwenhoek. 1996;69:171–184. doi: 10.1007/BF00399422. [DOI] [PubMed] [Google Scholar]
- 24.Skaugen M, Abildgaard C I, Nes I F. Organization and expression of a gene cluster involved in the biosynthesis of the lantibiotic lactocin S. Mol Gen Genet. 1997;253:674–686. doi: 10.1007/s004380050371. [DOI] [PubMed] [Google Scholar]
- 25.van den Hooven H W, Lagerwerf F M, Heerma W, Haverkamp J, Piard J-C, Hilbers C W, Siezen R J, Kuipers O P, Rollema H S. The structure of the lantibiotic lacticin 481 produced by Lactococcus lactis: location of the thioether bridges. FEBS Lett. 1996;391:317–322. doi: 10.1016/0014-5793(96)00771-5. [DOI] [PubMed] [Google Scholar]
- 26.Voelker U, Voelker A, Haldenwang W G. The yeast two-hybrid system detects interactions between Bacillus subtilis ςB regulators. J Bacteriol. 1996;178:7020–7023. doi: 10.1128/jb.178.23.7020-7023.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woodruff W A, Novak J, Caufield P W. Sequence analysis of mutA and mutM genes involved in the biosynthesis of the lantibiotic mutacin II in Streptococcus mutans. Gene. 1998;206:37–43. doi: 10.1016/s0378-1119(97)00578-7. [DOI] [PubMed] [Google Scholar]