Abstract
BACKGROUND:
Activating point mutations of the RAS gene (NRAS, HRAS, and KRAS) can be seen in benign and malignant thyroid tumors; among these, NRAS mutations are more commonly seen. This study was conducted to evaluate the thyroid risk of malignancy (ROM) associated with RAS mutations in thyroid fine-needle aspiration (FNA) at the authors’ institution.
METHODS:
The authors searched their electronic database system between January 2015 and May 2021 for thyroid FNA cases with any type of RAS mutation. Molecular alterations were identified with the ThyroSeq Genomic Classifier, ThyGeNEXT (thyroid oncogene panel)/ThyraMIR (miRNA classifier), or ThyroSure gene panel.
RESULTS:
A total of 127 cases (age, 51 ± 14 years; 100 females and 27 males) were identified, and 72 had histologic follow-up. The overall ROM associated with RAS mutations (with or without any other molecular alterations) was 29%, whereas the ROM was lower (18%) with RAS mutations only. Isolated NRAS, HRAS, and KRAS mutation–associated ROMs were 15%, 27%, and 14%, respectively. Among these RAS-mutated cases, the cases with a Bethesda category IV cytologic diagnosis had a higher ROM than the cases with a category III diagnosis (38% vs 17%). Twenty-one histologically confirmed malignant cases were mostly classified on cytology as category IV lesions (14 of 34; 41%), and the remainder were either category III (6 of 35; 17%) or V lesions (1 of 1; 100%).
CONCLUSIONS:
This study demonstrated that the overall RAS mutation–associated ROM in thyroid FNA was intermediate (29%), and isolated HRAS mutations appeared to have a higher ROM (27%) than NRAS and KRAS mutations (15% and 14%, respectively).
Keywords: fine-needle aspiration, molecular alterations, RAS mutations, risk of malignancy, thyroid
INTRODUCTION
Fine-needle aspiration (FNA) cytology plays an important role in the accurate and cost-effective evaluation of thyroid nodules.1 The Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) provides a uniform structure for reporting thyroid nodule cytology results with the following 6 diagnostic categories: 1) nondiagnostic, 2) benign, 3) atypia of undetermined significance/follicular lesion of undetermined significance (AUS/FLUS), 4) follicular neoplasm/suspicious for follicular neoplasm (FN), 5) suspicious for malignancy (SM), and 6) malignant.2 The risk of malignancy (ROM) is variable within each TBSRTC diagnosis. The role of molecular testing in thyroid nodules is evolving, and it can be used in combination with cytology as an adjuvant test. These molecular makers are often used in cases with indeterminate cytologic diagnoses (AUS/FLUS, FN, and SM) to guide managament.1 Molecular testing can either be a rule-in test with a high positive predictive value and specificity or a rule-out test with a high negative predictive value and sensitivity.3 Different types of molecular alterations can be seen in thyroid nodules, and certain ones (eg, BRAF V600E) are more frequently associated with malignancy than others.2 RAS gene family (NRAS, HRAS, and KRAS) mutations can be seen in benign and malignant thyroid nodules, with NRAS being the most frequent.4 The ROM associated with RAS mutations is variable and has been reported to be between 31% and 76%.5–7 Each isoform of RAS (NRAS, HRAS, and KRAS) also has a different ROM.6,7 This study was conducted to evaluate our institutional malignancy risk associated with RAS mutation and its isoforms along with cytohistologic correlations.
MATERIAL AND METHODS
Study Characteristics
This study was approved by the Yale University Institutional Review Board. We retrospectively reviewed our institutional electronic database for thyroid FNA cytology cases with any type of RAS mutation between January 1, 2015, and May 31, 2021. Cytologic diagnoses were rendered by board-certified cytopathologists, and the majority of the cases were reviewed at a daily cytopathology consensus conference by 3 or more cytopathologists. We collected demographic details, clinical and imaging features, cytology and subsequent histopathologic details, available ancillary studies, and follow-up. Available histology slides of select cases with molecular alterations were reviewed by 2 pathologists blinded to the histologic diagnosis (S.M.G. and A.J.A.). At our institution, we use the follicular lesion of undetermined significance (FLUS) cytologic diagnostic category for TBSRTC III cases and divide this into the following subcategories: nuclear atypia (equivalent to atypia of undetermined significance), low cellularity with a predominantly microfollicular architecture or Hürthloid features. We also use the follicular neoplasm/Hürthle cell neoplasm (FN/HCN) diagnostic category, which corresponds to TBSRTC category IV (suspicious for follicular neoplasm/follicular neoplasm). For this study, cases with a noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) diagnosis along with adenomas were included in the benign neoplastic category.
Cytologic Features
At our institution, cytopathologists use a standard template, based on TBSRTC guidelines, to report thyroid FNA results, and it documents the cytomorphologic features related to each diagnostic category. The architecture was noted as microfollicular, macrofollicular, clusters/groups, or single cells. Cellularity was categorized semi-quantitatively as low, moderate, or high. Hürthle cell changes were present either focally or in abundance. Any nuclear atypia or changes were documented when observed. Colloid, macrophages, and lymphocytes were also noted when present.
Overall, 127 cases from 127 patients fulfilled our study inclusion criteria. These cytology FNA cases included in-house cases (83%) processed at the Yale Laboratory (Yale–New Haven Hospital) and consult cases (17%). In-house cases had at least 2 FNA passes performed under image guidance by radiologists using 25-gauge needles. Most cases had no onsite evaluation. One smear slide was air-dried and stained with Diff-Quik stain (a modified Romanowsky stain), whereas the other smear slide was fixed in an alcohol solution and later stained with Papanicolaou stain. Needles were rinsed in CytoRich Red solution (Thermo Fisher Scientific) for a ThinPrep liquid-based preparation (Hologic, Marlborough, Massachusetts). Consult cases used different preparations, which included 1 or more of the following: ThinPrep preparations and Diff-Quik–stained, Papanicolaou-stained, or hematoxylin-eosin–stained cell block slides.
Molecular Studies
At our institution, reflex molecular testing on FNA material was performed at the clinician’s request for cases with an indeterminate cytological diagnosis (mostly AUS/FLUS [Bethesda category III] and suspicious for follicular neoplasm/follicular neoplasm [Bethesda category IV] and infrequently SM [Bethesda category V]). Usually at the time of the procedure, a separate FNA pass was collected for molecular testing in an appropriate medium. Molecular tests were performed with different platforms. The majority of the molecular tests were send-out tests, and different platforms, including the ThyroSeq Genomic Classifier (v2 and v3; ThyroSeq, University of Pittsburgh Medical Center/Sonic Healthcare) and ThyGeNEXT (thyroid oncogene panel)/ThyraMIR (miRNA classifier; Interpace Diagnostics), were used. Selected in-house cases had molecular testing performed at the Yale Laboratory with the ThyroSure gene panel (a Yale-developed next-generation sequencing [NGS]–based gene panel). ThyroSeq v3 evaluated nucleic acids (DNAs/messenger RNAs) of 112 thyroid genes by using NGS.8 ThyroSeq v2 evaluated 14 thyroid tumor genes and 42 gene fusions.9 ThyroSeq results were described as either “negative” or “positive” along with further details of molecular alterations if present. ThyGeNEXT provided DNA and RNA analysis by NGS and included a DNA evaluation of 10 genes and 38 RNA fusion transcripts, whereas ThyraMIR identified the expression of 10 specific microRNAs.10 ThyGeNEXT and ThyraMIR reports showed “presence of mutation” or “no mutation” and “positive” or “negative” results, respectively. The ThyroSure test used DNA and RNA by using NGS to evaluate 78 thyroid cancer–related genes. The ThyroSure gene panel reported results as “negative” or “positive.” If the ThyroSure gene panel result was positive, then a specific alteration or mutation was described in the report.
Statistical Analysis
The statistical analysis was performed with a 2-tailed Fisher exact test in R.11 A P value less than .05 was considered significant.
RESULTS
We identified a total of 127 thyroid FNA cases with any type of RAS mutation from 127 patients (100 females and 27 males with a mean age of 51 years). On cytology, these cases were categorized as follows: nondiagnostic (n = 1), negative (n = 3), FLUS (n = 67), FN (n = 53), and SM (n = 3). Four cases with nondiagnostic and negative cytology diagnoses had prior FNAs from the same side and were FLUS (n = 3) and FN (n = 1), and follow-up FNAs were performed with additional samples for molecular testing. Histologic follow-up was available in 72 cases (Table 1 and Figs. 1–4).
TABLE 1.
Demographic Details
| Variable | Value |
|---|---|
| Age, mean ± SD, y | 51 ± 14 |
| Sex, No. | |
| Female | 100 |
| Male | 27 |
| Site (thyroid gland), No. | |
| Right | 73 |
| Left | 48 |
| Isthmus | 6 |
| Size, mean, cm | 2.3 |
| Cytology diagnosis, No. | 127 |
| ND | 1 |
| NEG | 3 |
| FLUS | 67 |
| FN/HCN | 53 |
| SM | 3 |
| Histology diagnosis, No. | 72 |
| Nonneoplastic | 35 |
| Neoplastic | 16 |
| FA | 7 |
| HCA | 3 |
| NIFTP | 6 |
| Malignant | 21 |
| PTC | 14 |
| FTC | 4 |
| HCC | 2 |
| PDTC | 1 |
Abbreviations: FA, follicular adenoma; FLUS, follicular lesion of undetermined significance; FN/HCN, follicular neoplasm/Hürthle cell neoplasm; FTC, follicular thyroid carcinoma; HCA, Hürthle cell adenoma; HCC, Hürthle cell carcinoma; ND, nondiagnostic; NEG, negative/benign; NIFTP, noninvasive follicular thyroid neoplasm with papillary-like nuclear features; PDTC, poorly differentiated thyroid carcinoma; PTC, papillary thyroid carcinoma; SM, suspicious for malignancy.
Figure 1.
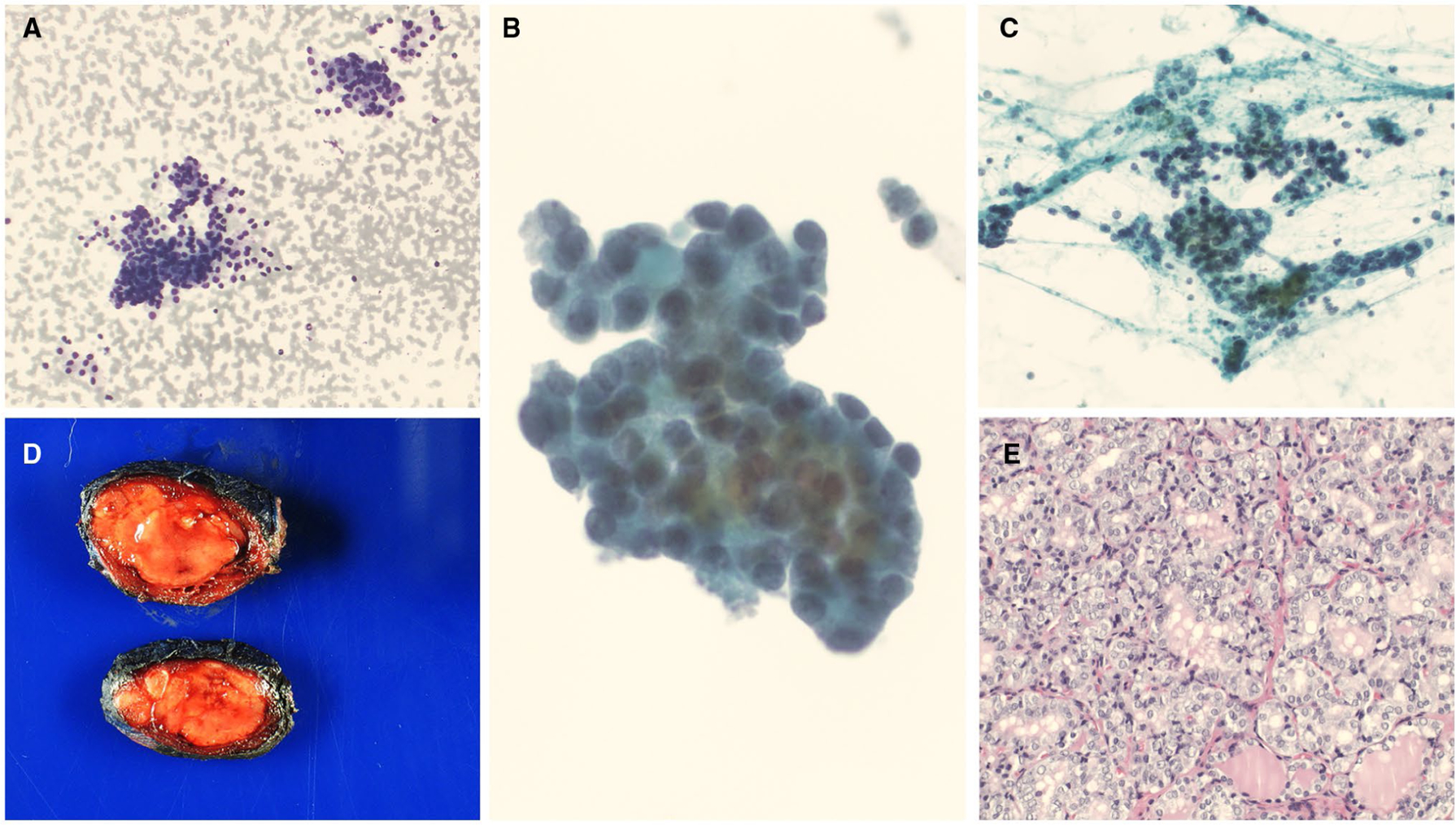
HRAS mutations along with other molecular alterations. (A-C) A cytology evaluation showed clusters and groups of follicular cells in a microfollicular architecture. Some of the follicular cells showed nuclear crowding and slight nuclear enlargement (cytology diagnosis of follicular lesion of undetermined significance with nuclear atypia) ([A] Diff-Quik, ×100; [B] ThinPrep, ×400; [C] Papanicolaou stain, ×200). (D) A macroscopic examination showed a pale tan, somewhat circumscribed nodule (gross image). (E) A histologic evaluation showed papillary thyroid carcinoma, follicular variant (H & E, ×200).
Figure 4.
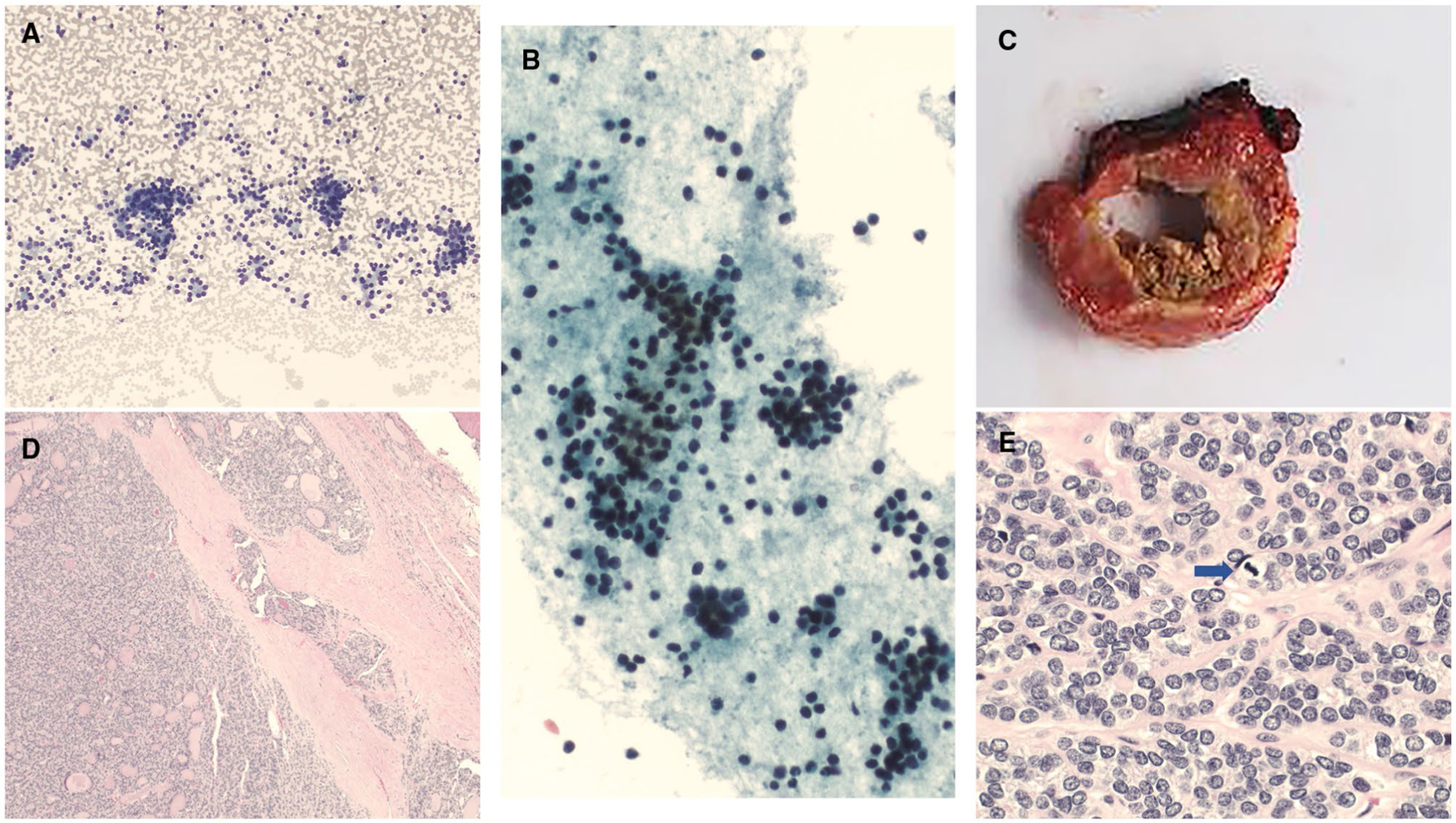
KRAS mutation and MIR (miRNA classifier)-positive. (A,D,E) Cytology preparations showed mostly microfollicular architecture and rare macrofollicles with Hürthloid features (cytology diagnosis of follicular lesion of undetermined significance with Hürthloid features) ([A] Diff-Quik, ×400; [D] Diff-Quik, ×200; [E] ThinPrep, ×400). (B,C) A histologic evaluation showed follicular adenoma ([B] H & E, ×100; [C] H & E, ×400).
Molecular testing results showed NRAS mutations in 67 cases (NRAS alone, 39; NRAS with others, 28), HRAS mutations in 32 cases (HRAS alone, 18; HRAS with others, 14); and KRAS mutations in 28 cases (KRAS alone, 17; KRAS with others, 11). Cases with histologic follow-up (n = 72) showed the following molecular alterations: NRAS in 37 cases (NRAS alone, 20; NRAS with others, 17), HRAS in 21 cases (HRAS alone, 11; HRAS with others, 10), and KRAS in 14 cases (KRAS alone, 7; KRAS with others, 7; see Table 2). Twelve cases with NRAS mutations showed malignancy on follow-up histology, whereas the remaining 25 cases were negative (nonneoplastic and benign neoplastic). Six cases with HRAS mutations showed malignant histology on follow-up, and 15 were negative. Most cases with KRAS mutations were negative (n = 11) on histologic follow-up (Table 2). Fifty-five cases with RAS mutations (NRAS, 30; HRAS, 11; KRAS, 14) had no available histologic follow-up.
TABLE 2.
Correlation of Molecular Tests With Histologic Follow-Up
| Histologic Diagnosis | ||||
|---|---|---|---|---|
| Molecular Test | Age, Range, y | Nonneoplastic | Neoplastic | Malignant |
| NRAS (n = 37) | ||||
| NRAS only (n = 20) | 17–79 | n = 12 | n = 5 (NIFTP, 2; FA, 3) | n = 3 (PTC) |
| NRAS and MIR-positive (n = 10) | 33–69 | n = 3 | n = 0 | n = 7 (PTC, 3; FTC, 3; HCC, 1) |
| NRAS and positive gene expression profile (n = 5) | 24–59 | n = 3 | n = 1 (HCA) | n = 1 (HCC) |
| NRAS and TERT promotor gene mutation (n = 1) | 66 | n = 0 | n = 0 | n = 1 (PDTC, PTC) |
| NRAS and EIF1AX mutation (n = 1) | 81 | n = 1 | n = 0 | n = 0 |
| HRAS (n = 21) | ||||
| HRAS only (n = 11) | 47–74 | n = 5 | n = 3 (NIFTP, FA, HCA) | n = 3 (PTC) |
| HRAS and MIR-positive (n = 5) | 23–56 | n = 1 | n = 3 (NIFTP, 2; FA, 1) | n = 1 (PTC) |
| HRAS and positive gene expression profile (n = 3)a | 28–52 | n = 1 | n = 0 | n = 2 (PTC)a |
| HRAS and EIF1AX (n = 2) | 35–54 | n = 2 | n = 0 | n = 0 |
| KRAS (n = 14) | ||||
| KRAS only (n = 7) | 28–71 | n = 5 | n = 1 (NIFTP) | n = 1 (FTC) |
| KRAS and MIR-positive (n = 5) | 28–49 | n = 1 | n = 2 (FA, HCA) | n = 2 (PTC) |
| KRAS and positive gene expression profile (n = 1) | 33 | n = 0 | n = 1 | n = 0 |
| KRAS and PTEN mutation (n = 1) | 61 | n = 1 | n = 0 | n = 0 |
Abbreviations: FA, follicular adenoma; FTC, follicular thyroid carcinoma; HCA, Hürthle cell adenoma; HCC, Hürthle cell carcinoma; MIR, miRNA classifier; NIFTP, noninvasive follicular thyroid neoplasm with papillary-like nuclear features; PDTC, poorly differentiated thyroid carcinoma; PTC, papillary thyroid carcinoma.
Two had copy number alterations.
Sixty-six samples were tested by ThyroSeq (ThyroSeq v3, 51; ThyroSeq v2, 15), 52 were tested by ThyGeNEXT/ThyraMIR (miRNA classifier), and 9 were tested by ThyroSure. Histologic follow-up was available for 35 of the 66 cases with ThyroSeq results, and 7 showed malignancy. The ROMs with ThyroSeq molecular testing were as follows: overall, 20%; RAS alone (any type), 17%; and RAS with other molecular alterations, 25%. Thirty-one cases with ThyGeNEXT/ThyraMIR testing had histologic follow-up available, and 12 of those cases were malignant. The overall ROM was 39%, the ROM for RAS alone (any type) was 18%, and the ROM for RAS with other alterations (all had RAS mutations and miRNA-positive results) was 50%. Only 9 cases had ThyroSure testing, and 6 of those, including 2 malignant cases, had histologic follow-up. The overall ROM was 33%, and the ROM for RAS alone was 25% (Table 3).
TABLE 3.
Comparison of Different Molecular Platforms
| ThyroSeq (n = 66) | ThyGenX/ThyraMIR (n = 52) | ThyroSure (n = 9) | ||||
|---|---|---|---|---|---|---|
| RAS Alone | RAS With Others | RAS Alone | RAS With Others | RAS Alone | RAS With Others | |
| Histology, No. | n = 35 | n = 31 | n = 6 | |||
| Malignant | 4 | 3 | 2 | 10 | 1 | 1 |
| Benign | 19 | 9 | 9 | 10 | 3 | 1 |
| ROM (RAS alone), % | 17 | 18 | 25 | |||
| ROM (RAS with others), % | 25 | 50 | —a | |||
| ROM (overall), % | 20 | 39 | 33 | |||
Abbreviation: ROM, risk of malignancy.
The number was too low for an appropriate calculation.
Twenty-one cases with any RAS mutation showed malignancy on histologic follow-up, with papillary thyroid carcinoma (PTC) being the most frequent malignancy (n = 14); it was followed by follicular thyroid carcinoma (FTC; n = 4), Hürthle cell carcinoma (n = 2), and then poorly differentiated thyroid carcinoma (n = 1; Figs. 1–3). Fourteen of the 21 malignant cases showed RAS mutations along with other alterations, and the remaining 7 cases showed RAS mutations only (Table 4). Cytologically, the malignant cases were classified with the following TBSRTC categories: FN/HCN (IV), 14 (67%); FLUS (III), 6 (28%); and SM (V), 1 (5%). Four of the 6 cases with an FLUS diagnosis were characterized as FLUS with nuclear atypia, and 2 were characterized as FLUS with low cellularity with a microfollicular architecture. The overall ROM associated with RAS mutations (with or without any other molecular alterations) was 29%. The ROMs associated with NRAS, HRAS, and KRAS mutations alone and along with any other molecular alterations were 15%, 27%, and 14% and 53%, 30%, and 29%, respectively (Table 5).
Figure 3.
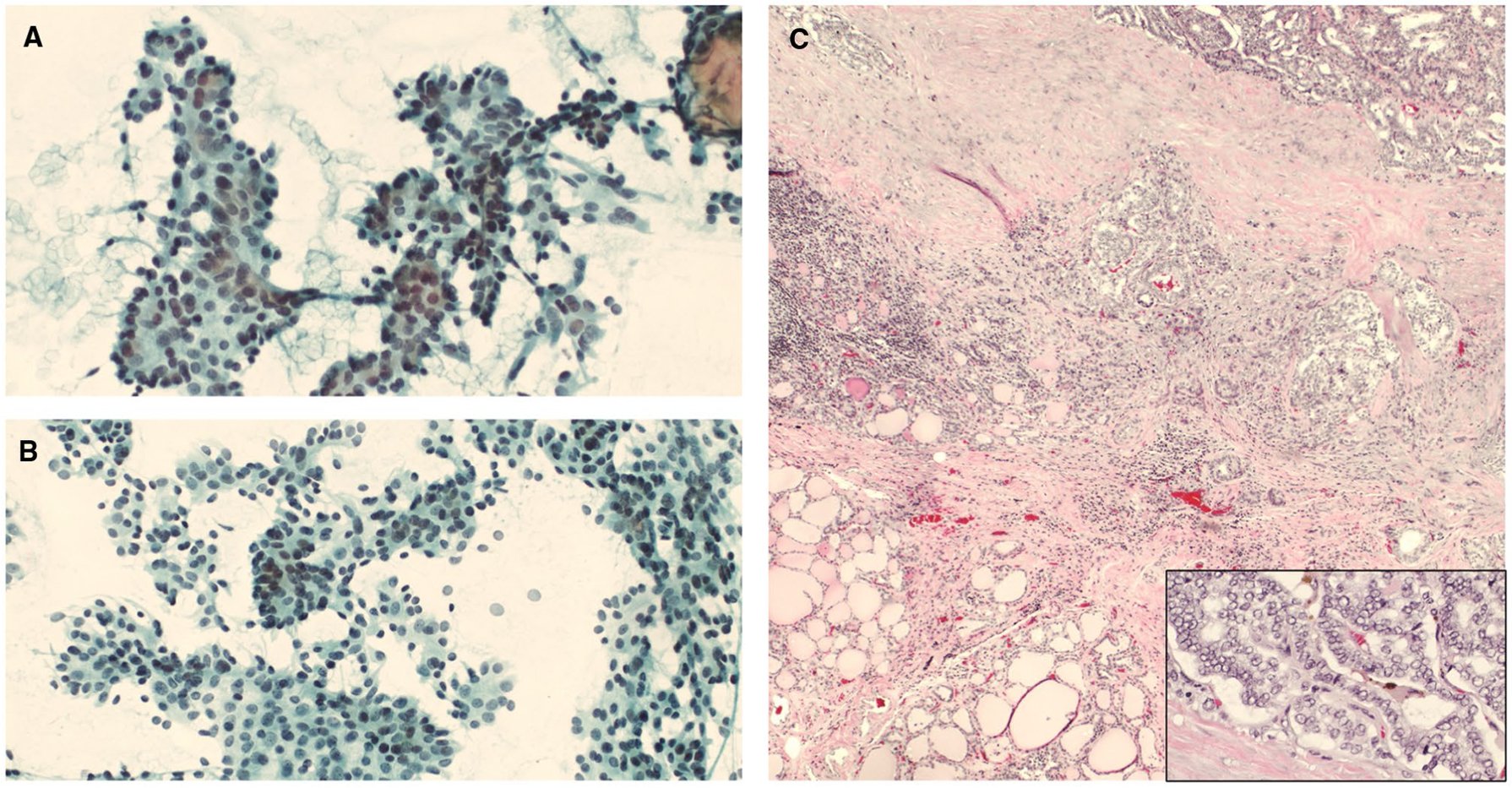
KRAS mutation and MIR (miRNA classifier)-positive. (A,B) Cytology smears showed increased cellularity of follicular cells arranged in groups with nuclear crowding and overlapping, some loosely cohesive clusters, and microfollicles (cytology diagnosis of follicular neoplasm) (Papanicolaou stains, ×200). (C) A histologic evaluation showed PTC with foci of follicular architecture (H & E, ×40), and high power showed PTC nuclear features (H & E, ×400). PTC indicates papillary thyroid carcinoma.
TABLE 4.
Correlation of Malignant Cases With RAS Mutations
| Histologic Diagnosis | Age, Range, y | Sex, No. | Procedure, No. | Tumor Site, No. | Tumor Size, Range, cm | LVI, No. | Molecular Alteration, No. |
|---|---|---|---|---|---|---|---|
| PTC (n = 14) | |||||||
| FV (n = 10) | 23–69 | F = 6 | T = 4 | RS = 7 | 1.7–6.3 | Focal = 3 | HRAS alone = 3 |
| M = 4 | L = 6 | LS = 3 | Ext = 1 | HRAS with others = 3 | |||
| NRAS alone = 1 | |||||||
| NRAS with others = 2 | |||||||
| KRAS with others = 1 | |||||||
| Other (n = 4)a | 34–60 | F = 4 | T = 2 | RS = 4 | 0.8–3.4 | — | NRAS alone = 2 |
| L = 2 | NRAS and MIR = 1 | ||||||
| KRAS and MIR = 1 | |||||||
| FTC (n = 4) | 33–71 | F = 4 | T = 3 | RS = 3 | 2–7.9 | Focal = 1 | KRAS alone = 1 |
| L = 1 | LS = 1 | Ext = 1 | NRAS and MIR = 3 | ||||
| HCC (n = 2) | 53–58 | F = 2 | T = 2 | RS = 2 | 0.8–1.5 | Focal = 1 | NRAS and MIR = 1 |
| NRAS and GEF = 1 | |||||||
| PDTC (n = 1) | 66 | F | T | LS | 4.8 | Ext | NRAS and TERT |
Abbreviations: Ext, extensive; F, female; FTC, follicular thyroid carcinoma; FV, follicular variant; GEF, gene expression profile; HCC, Hürthle cell carcinoma; L, lobectomy; LS, left side; LVI, lymphovascular invasion; M, male; MIR, miRNA classifier; PDTC, poorly differentiated thyroid carcinoma; PTC, papillary thyroid carcinoma; RS, right side; T, total thyroidectomy.
Includes cases with mixed features (follicular and solid features; n = 1), classic variant (n = 1), and papillary thyroid microcarcinoma (n = 2).
TABLE 5.
Correlation of Molecular Alterations Between Cases With Malignant and Benign Histology
| Molecular Alteration | Malignant Histology, No. | Benign Histology, No. | Risk of Malignancy, % | OR | P |
|---|---|---|---|---|---|
| NRAS alone | 3 | 17 | 15 | 0.166 | .0322 |
| NRAS with others | 9 | 8 | 53 | ||
| HRAS alone | 3 | 8 | 27 | 0.88 | .63 |
| HRAS with others | 3 | 7 | 30 | ||
| KRAS alone | 1 | 6 | 14 | 0.44 | .5 |
| KRAS with others | 2 | 5 | 29 | ||
| RAS mutation (overall) | 21 | 51 | 29 | ||
| RAS mutation (alone) | 7 | 31 | 18 | 0.33 | .031 |
| RAS with others | 14 | 20 | 41 |
Abbreviation: OR, odds ratio.
We retrospectively reviewed the slides of the available and selected histologic cases. Most cases showed previous FNA changes; some cases without FNA changes were submitted entirely, or a lesional nodule/capsule was submitted completely, for microscopic evaluation.
The cytologic diagnoses of the cases with histologic follow-up were as follows: nondiagnostic, 1; negative, 1; FLUS, 35; FN, 34; and SM, 1. The overall ROM associated with an FLUS diagnosis with an RAS mutation was 17% (RAS alone, 17%; RAS with other molecular alterations, 17%). Fourteen cases with an FN cytology diagnosis were malignant on histologic follow-up. The overall ROM in RAS-mutated FN cases was 38% (RAS alone, 15%; RAS with other alterations, 57%; Table 6).
TABLE 6.
Cases With Histologic Follow-Up and Correlation With Indeterminate Cytology
| Variable | FN (n = 34) | FLUS (n = 35) | OR | P |
|---|---|---|---|---|
| Age, range, y | 17–79 | 20–74 | — | — |
| Sex, No. | — | — | ||
| Female | 30 | 26 | ||
| Male | 4 | 9 | ||
| Molecular alterations, No. | ||||
| RAS alone | 13 | 23 | 0.33 | .02 |
| RAS with others | 21 | 12 | ||
| Procedure, No. | ||||
| Lobectomy | 15 | 22 | 0.47 | .09 |
| Total | 19 | 13 | ||
| Histology, No. | ||||
| Nonneoplastic | 14 | 19 | 0.3 | .025a |
| Neoplastic | 6 | 10 | ||
| Malignant | 14 | 6 | ||
| Molecular alteration and histology correlation, No. | ||||
| RAS alone + negative histology | 11 | 19 | 1.15 | .72 |
| RAS alone + positive histology | 2 | 4 | ||
| RAS and others + negative histology | 9 | 10 | 0.159 | .03 |
| RAS and others + positive histology | 12 | 2 | ||
| Risk of malignancy, % | ||||
| RAS alone | 15 | 17 | 0.27 | .003 |
| RAS with others | 57 | 17 | ||
| RAS (overall) | 38 | 17 |
Abbreviations: FLUS, follicular lesion of undetermined significance; FN, follicular neoplasm; OR, odds ratio.
Nonneoplastic + neoplastic versus malignant = P value.
DISCUSSION
The occurrence of RAS gene mutations in thyroid nodules can be seen in benign and malignant processes. These mutations possibly suggest a clonal process and are mostly seen in follicular-patterned lesions.12 In a study of 63 RAS-positive cases, Gupta et al12 identified 11 histologically confirmed benign cases (7 follicular adenomas and 4 hyperplastic nodules). However, upon the microdis-section of representative hyperplastic nodule cases, they identified the homogeneous presence of an RAS mutation and suggested a clonal neoplastic process despite the lack of typical neoplastic histology. On the contrary, limited data are available for comparisons of the molecular behavior of thyroid nodules with benign cytology.13 In this study, on histologic examination, 16 cases were classified as neoplastic, and 35 were classified as nonneoplastic. The majority of the nonneoplastic cases (>50%) were characterized as adenomatoid nodules.
The overall ROM associated with RAS mutations is variable. A study of 1172 thyroid FNAs reported more RAS-like mutations in TBSRTC III and IV (82% and 73%, respectively) in comparison with BRAF-like mutations (18% and 27%, respectively). In their study, the ROM associated with RAS-like mutations was 42.6%.14 Others have demonstrated more RAS mutations in TBSRTC IV (51%) versus TBSRTC III (35%). On histologic follow-up, 83% were malignant, and 17% were benign. Among the malignant PTC cases, follicular variant was the most frequent diagnosis (46 cases, including 31 encapsulated noninvasive cases), and it was followed by FTC (4 cases). Interestingly, they found 1 case each of medullary thyroid carcinoma (HRAS) and anaplastic thyroid cancer.12 Valderrabano et al5 studied 182 patients with indeterminate thyroid cytology results (III, IV, and V) and identified 21 RAS-mutated cases, with NRAS being the most frequent mutation (n = 14). They observed a 25% ROM associated with RAS mutations. In this study, the ROM associated with RAS mutations was 29%. Further evaluation showed that isolated RAS mutations had a lower percentage of malignancy than RAS mutations with other molecular alterations (18% vs 41%; P = .031).
In a systemic review, Goldner et al7 calculated the positive predictive value of RAS for malignancy. They noted for RAS a positive predictive value of 66% and for its isoforms the following: HRAS, 63%; NRAS, 38%; and KRAS, 25%. Others have noted a 76% ROM with RAS mutations in cases with an indeterminate cytology diagnosis (TBSRTC III, IV, and V); among these, HRAS was more frequently associated with malignancy (92%), and it was followed by NRAS (74%) and then KRAS (61%).6 We evaluated the ROM for RAS mutations alone and found that HRAS had a higher ROM than NRAS or KRAS (27% vs 15% and 14%, respectively). However, when an RAS mutation was present along with other molecular alterations, NRAS mutations had a higher ROM than HRAS and KRAS mutations (53% vs 30% and 29%, respectively). Our data showed that the malignancy rate with RAS mutations (overall) in TBSRTC IV was higher in comparison with TBSRTC III (38% vs 17%). These findings are comparable but are at the higher end of TBSRTC ROMs for categories III and IV (6%−18% and 10%−40%).2 However, the ROM was significantly higher in TBSRTC IV versus III when an RAS mutation was noted along other molecular alterations (57% vs 17%; P = .003).
In this study, we noted an interesting finding: most RAS-mutated cases were classified as either FLUS (n = 67; 53%) or FN/HCN (n = 53; 42%). RAS mutations, with or without other alterations, were present in PTC, FTC, Hürthle cell carcinoma, and poorly differentiated thyroid carcinoma, and the majority of PTCs showed a predominance of a follicular pattern. This finding brings up an important point: most RAS-mutated lesions are follicular-patterned lesions and can pose diagnostic challenges on cytologic material. Similar findings have been reported by other authors who evaluated 68 RAS-mutated FNA samples; 63 of those samples (including AUS/FLUS [n = 22] and FN [n = 32]) had an indeterminate cytology diagnosis.12
RAS mutations can be seen in a variety of neoplastic entities.15 Paulson et al16 studied 27 RAS-mutated thyroid tumors and noted that 16 of the 27 tumors (59%) were NIFTP, with NRAS being the most frequent mutation (8 of 16); it was followed by HRAS (7 of 16). In this study, we identified 6 cases of NIFTP; 3 had NRAS mutations alone, and 3 had HRAS mutations (including 2 ThyraMIR-positive cases). In a study of 162 TBSRTC category III cases, the authors identified 6 RAS-mutated cases with histologic follow-up, and 2 of those were malignant.17 Yoon et al18 evaluated 31 RAS-mutated thyroid nodules. Seven of these (22.6%) were malignant, whereas 24 (77.4%) were benign on histologic follow-up; this suggested a limited role for RAS in determining malignancy. In a study of 911 patients, Rossi et al19 identified 31 with only an RAS mutation, and 11 of those had histologic follow-up. Two of the 11 cases showed malignancy, whereas 6 were follicular adenomas, and 3 were nonneoplastic. The majority of our RAS-mutated cases with histologic follow-up were not malignant (51 of 72 [71%]: 28 lobectomies and 23 total thyroidectomies), and 35 of those cases were nonneoplastic. Twenty-one RAS-mutated cases were malignant, and the majority underwent a total thyroidectomy (12 of 21; 57%). Nine patients (43%) had an initial lobectomy, and 5 underwent a completion thyroidectomy.
There are a few limitations pertinent to this study, including the retrospective review of the data. In addition, the molecular testing was not homogeneous because the tests were performed with different platforms according to the preference of the referring clinician. Although the number of patients in our study was not very large and many lacked histologic follow-up (but there was still a reasonable number of cases), we successfully demonstrated our institutional ROM associated with RAS mutations. The selection of only RAS-mutated thyroid FNA cases raises the possibility of a selection bias; however, the purpose of our study was to determine the ROM specifically associated with RAS mutations. The majority of our cytology cases (97%) were indeterminate (FLUS, FN, or suspicious), and this limited the evaluation of the RAS mutation prevalence in benign/negative cytology FNA cases.
We conclude that RAS mutations can be seen in both benign and malignant thyroid conditions. RAS-mutated thyroid FNA cases had a higher overall ROM in TBSRTC category IV versus category III (38 vs 17%), but it was comparable to the ROM reported for TBSRTC IV and III without any mutations.2 However, for RAS-mutated cases with additional molecular alterations, this ROM was significantly higher in category IV versus category III (57% vs 17%; P < .05).
Figure 2.
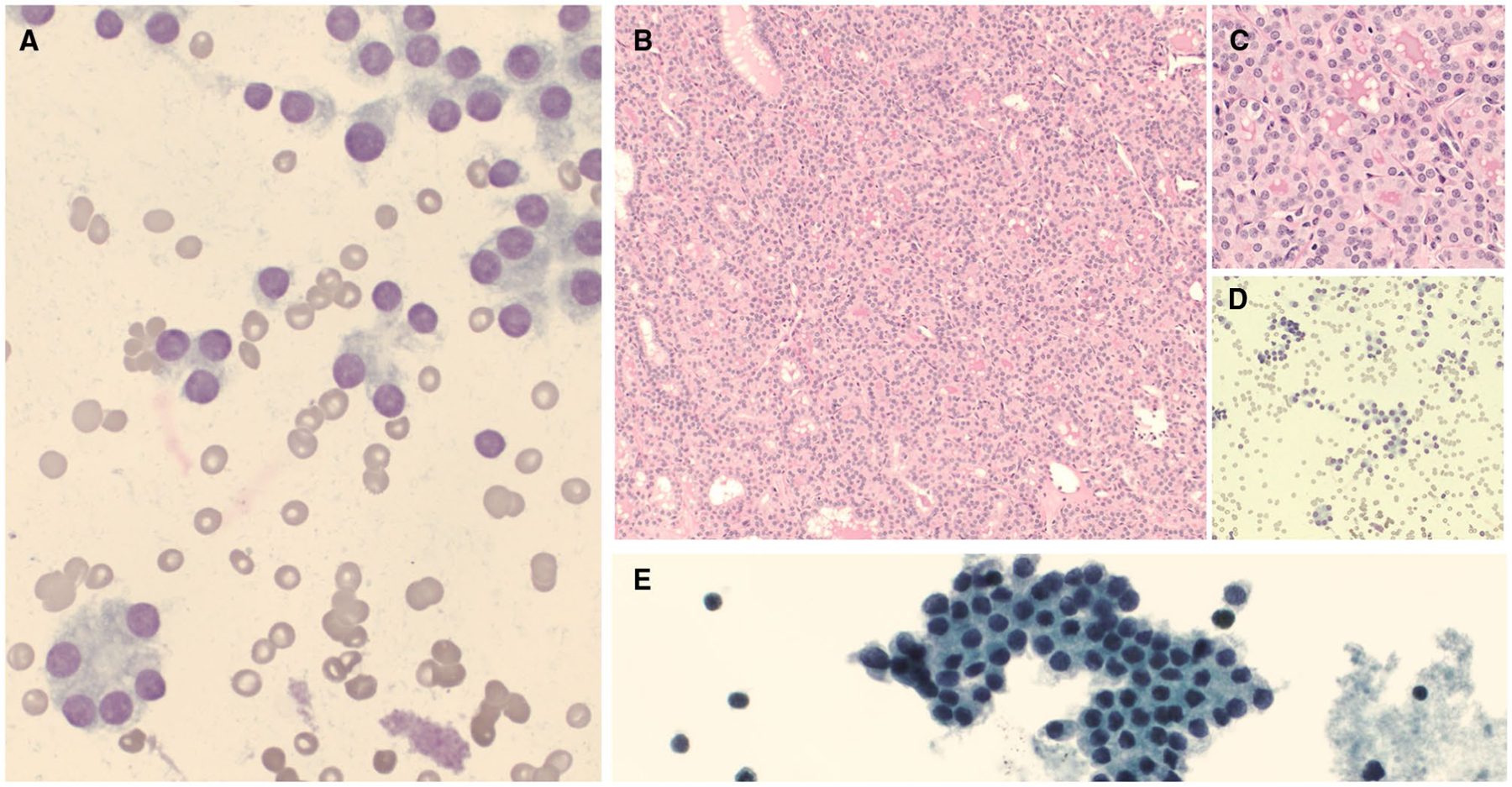
NRAS and TERT mutation. (A,B) A cytologic preparation showed cellular smears with a microfollicular architecture (cytology diagnosis of follicular neoplasm) ([A] Diff-Quik, ×100; [B] Papanicolaou stain, ×200). (C) A macroscopic examination showed a brown-yellow nodule with a fleshy cut surface (gross image). (D) A microscopic examination showed a poorly differentiated carcinoma (H & E stain, ×40). (E) Further review showed scattered mitosis (blue arrow; H & E stain, ×400).
FUNDING SUPPORT
James Garritano is supported by the National Institutes of Health (F30HG011193 and Medical Scientist Training Program Training Grant T32GM007205). The other authors made no disclosures.
Footnotes
CONFLICT OF INTEREST DISCLOSURE
The authors made no disclosures.
This study was presented as a poster presentation at the 2021 Annual Meeting of the United States and Canadian Academy of Pathology.
REFERENCES
- 1.Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cibas ES, Ali SZ. The 2017 Bethesda System for Reporting Thyroid Cytopathology. Thyroid. 2017;27:1341–1346. [DOI] [PubMed] [Google Scholar]
- 3.Roth MY, Witt RL, Steward DL. Molecular testing for thyroid nodules: review and current state. Cancer. 2018;124:888–898. [DOI] [PubMed] [Google Scholar]
- 4.Hernandez-Prera JC, Valderrabano P, Creed JH, et al. Molecular determinants of thyroid nodules with indeterminate cytology and RAS mutations. Thyroid. 2021;31:36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valderrabano P, Khazai L, Leon ME, et al. Evaluation of ThyroSeq v2 performance in thyroid nodules with indeterminate cytology. Endocr Relat Cancer. 2017;24:127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel SG, Carty SE, McCoy KL, et al. Preoperative detection of RAS mutation may guide extent of thyroidectomy. Surgery. 2017;161:168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldner WS, Angell TE, McAdoo SL, et al. Molecular variants and their risks for malignancy in cytologically indeterminate thyroid nodules. Thyroid. 2019;29:1594–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nikiforova MN, Mercurio S, Wald AI, et al. Analytical performance of the ThyroSeq v3 genomic classifier for cancer diagnosis in thyroid nodules. Cancer. 2018;124:1682–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nikiforov YE, Carty SE, Chiosea SI, et al. Impact of the multi-gene ThyroSeq next-generation sequencing assay on cancer diagnosis in thyroid nodules with atypia of undetermined significance/follicular lesion of undetermined significance cytology. Thyroid. 2015;25:1217–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciarletto AM, Narick C, Malchoff CD, et al. Analytical and clinical validation of pairwise microRNA expression analysis to identify medullary thyroid cancer in thyroid fine-needle aspiration samples. Cancer Cytopathol. 2021;129:239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2013:201. [Google Scholar]
- 12.Gupta N, Dasyam AK, Carty SE, et al. RAS mutations in thyroid FNA specimens are highly predictive of predominantly low-risk follicular-pattern cancers. J Clin Endocrinol Metab. 2013;98:E914–E922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marotta V, Bifulco M, Vitale M. Significance of RAS mutations in thyroid benign nodules and non-medullary thyroid cancer. Cancers (Basel). 2021;13:3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellevicine C, Migliatico I, Sgariglia R, et al. Evaluation of BRAF, RAS, RET/PTC, and PAX8/PPARg alterations in different Bethesda diagnostic categories: a multicentric prospective study on the validity of the 7-gene panel test in 1172 thyroid FNAs deriving from different hospitals in South Italy. Cancer Cytopathol. 2020;128:107–118. [DOI] [PubMed] [Google Scholar]
- 15.Nikiforov YE. Molecular diagnostics of thyroid tumors. Arch Pathol Lab Med. 2011;135:569–577. [DOI] [PubMed] [Google Scholar]
- 16.Paulson VA, Shivdasani P, Angell TE, et al. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features accounts for more than half of “carcinomas” harboring RAS mutations. Thyroid. 2017;27:506–511. [DOI] [PubMed] [Google Scholar]
- 17.Bellevicine C, Sgariglia R, Migliatico I, et al. Different qualifiers of AUS/FLUS thyroid FNA have distinct BRAF, RAS, RET/PTC, and PAX8/PPARg alterations. Cancer Cytopathol. 2018;126:317–325. [DOI] [PubMed] [Google Scholar]
- 18.Yoon JH, Kwon HJ, Lee HS, Kim EK, Moon HJ, Kwak JY. RAS mutations in AUS/FLUS cytology: does it have an additional role in BRAFV600E mutation–negative nodules? Medicine (Baltimore). 2015;94:e1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rossi M, Buratto M, Tagliati F, et al. Relevance of BRAF(V600E) mutation testing versus RAS point mutations and RET/PTC rear-rangements evaluation in the diagnosis of thyroid cancer. Thyroid. 2015;25:221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]


