Introduction:
Patients with Richter’s Syndrome (RS) treated with chemoimmunotherapy have low complete remission (CR) rates (5-38%). Median survival is <1y. TP53 mutations, which confer chemoresistance, are frequent.(1) Despite an encouraging 65% overall response rate (ORR) with ibrutinib + the PD1 inhibitor nivolumab, median PFS was only 5 months.(2) Novel approaches are required.
Blinatumomab is CD3x19 bi-specific T cell engager (BiTE), approved at 28mcg/d in MRD+ or relapsed/refractory pre-B-ALL.(3, 4) High dose (112mcg/d), blinatumomab is active in relapsed/refractory diffuse large B cell lymphoma (DLBCL).(5)
Methods:
We designed a phase II clinical trial of high-dose blinatumomab in patients with DLBCL sub-type RS. Full eligibility criteria are shown in the supplementary appendix. Blinatumomab was given over 2 courses. C1 consisted of dose-escalation (9mcg/d during week 1, 28mcg/d during week 2) followed by 112mcg/d for 6 weeks. Responding patients received C2 (112mcg/d for 4 weeks) 4 weeks post-C1.
Responses were assigned according to Lugano 2014 criteria.(6) We continously monitored ORR and adverse effects (AEs) using the Bayesian approach of Thall, Simon, Estey as extended by Thall and Sung.(7) The primary end point was ORR following induction.
Flow cytometry data analysis
Blood samples were obtained pre-treatment and on day 1, 7, 14, 28 and 70 from 8 patients (4 non-responders, 3 transient responders, 1 complete responder). Samples were evaluated for expression of CD3, CD4, CD8, CD19, CD45RO, LAG-3, 2B4, PD1, CD160, TIM-3 and TIGIT. Analytic methodology is detailed in the supplementary methods.
Results
Pre-treatment patient characteristics are shown in Supplementary Table 1: all patients had received prior treatment for CLL (median 4, range 1-12) prior therapies; 6 of 9 had received prior therapy for RT (median 2, range 0-9); 8/8 patients with results had complex karyotype, 5/8 had del(17p), 5/7 had TP53 mutation and 5/9 had bulky lymphadenopathy (>5cm). Nine patients were treated before the study was closed for slow accrual.
Efficacy.
Objective reduction in nodal or extra-nodal disease burden was seen in 4/9 patients. ORR was 2/9 (22%): patient #1 achieved partial metabolic remission (PMR) after C1 and complete metabolic remission (CMR) after C2; patient #6 had 42% nodal reduction, with PMR, after C1 but progressive disease (PD) after C2. Best response to therapy is shown in Supplementary Figure 1. Of note, 3 of 5 patients with >10% CLL involvement in bone marrow prior to treatment had reduction in CLL involvement of the marrow to <0.1% at response assessment (#7, #8 and #9), Supplementary Table 2.
All patients who came off study did so for progression or lack of response. Median PFS was 1.9 months and median survival 10.3 months. There were no deaths during treatment.
Tolerability.
Overall, the treatment was well tolerated. No patient stopped treatment due to an AE. The most common therapy-related AE was reversibile neurotoxicity (n=5; grade 1, n=3, grade 2, n=1 and grade 3, n=1). Grade 1-2 cytokine release syndrome (CRS) was seen in 3 patients (grade 1, n=2, grade 2, n=1), with no patient developing grade ≥3 CRS. There were no grade 4 or 5 AEs. Supplementary Table 3 outlines all treatment-emergent AEs. All 9 patients experienced at least one serious adverse event (Supplementary Table 4). Of these, only one case of grade 1 CRS and 1 case of grade 3, reversible neurotoxicity were deemed related to treatment.
Correlative studies
The proportions of B cells and the two T cell subsets varied among patients (Figure 1A). Most patients expressed high levels of multiple immune checkpoints (ICs) on their CD8 and CD4 T cells at baseline and throughout therapy (Figure 1B and 1C). The most consistently highly expressed molecules were TIGIT, PD1 and TIM3; however there was significant inter-patient variability in terms of expression levels, co-expression, specific IC molecules expressed, and the T cell subset that expressed them. The patient who achieved a CMR (#1) expressed much lower levels of PD1 than transient responders and non-responders, and undetectable levels of TIGIT and TIM3.
Figure 1.
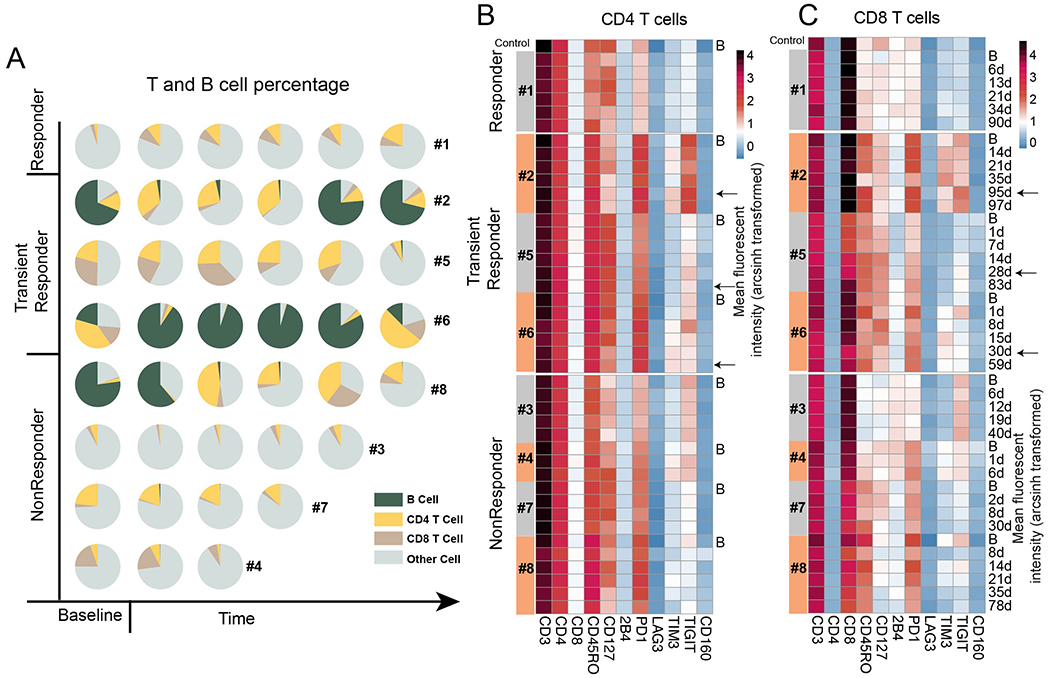
Immune profiling in each patient sample. A. Percentage of B, CD4, CD8 T cells groups in each patient sample. X axis is plotted in time order. Other cell are the cells that were CD3 and CD19 negative. B. Expression level of each immune marker in CD4 T cells for each sample. C. Expression level of each immune marker in CD8 T cells for each sample. B: baseline; Timepoints in days after treatment for each sample are shown next to the last heat map. Color bar indicates the mean arcsinh transformed value of fluorescent intensity.
To further explore the complexity of the CD3 positive cells, the patient samples from all patients at all time points (n=42) were clustered in an unsupervised analysis into different cell populations based on the expression pattern of the 10 markers outlined above (Figure 2A–C). Patients were heterogeneous in the composition of their CD8 and CD4 T subsets (Figure 2D–E). The CMR patient, #1, was enriched for cluster 1 (CD4+ T-cells) and cluster 6 (CD8+ T-cells), both clusters characterized by low or absent expression of checkpoint molecules, including TIGIT, TIM3, LAG3 and PD1.
Figure 2.
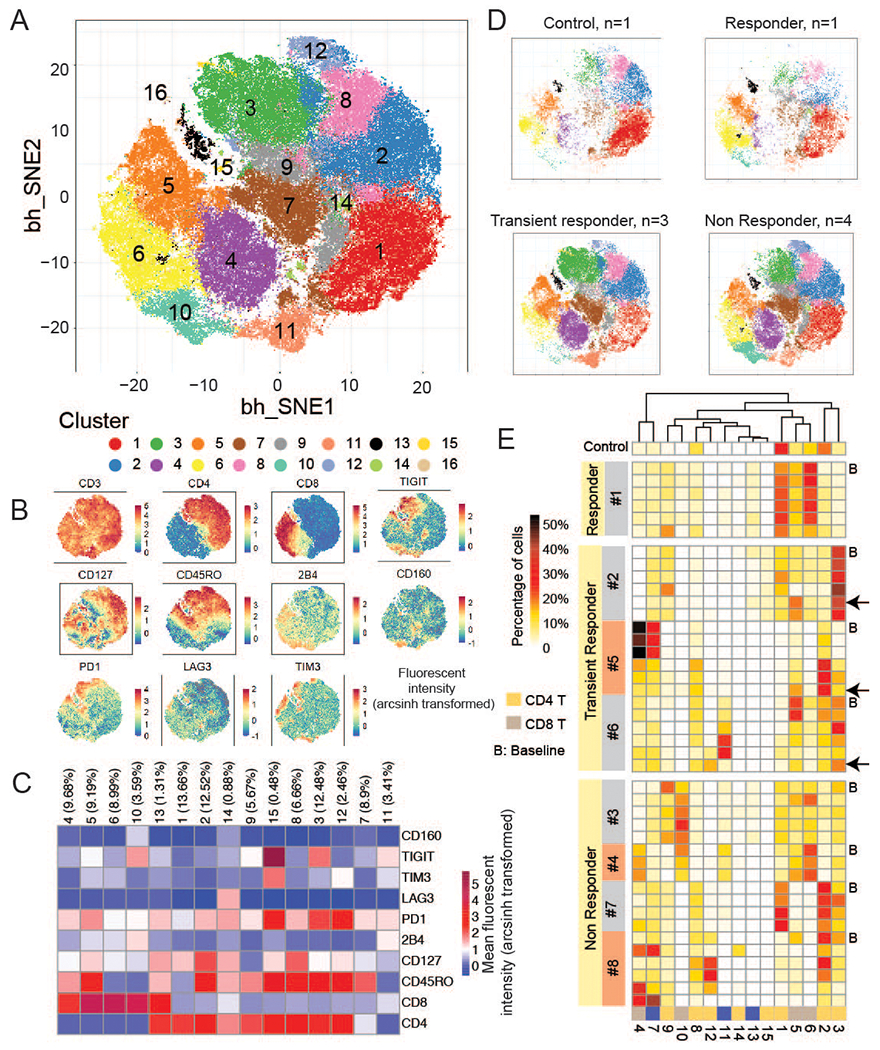
Clustering of cell populations via viSNE analysis on immune markers in all 42 patient samples and a control. A.ViSNE map for PhenoGraph clusters using immune markers in all samples (8 patients and 1 control). B. ViSNE map showing the expression levels of each marker. Color bar indicates the arcsinh transformed value of fluorescent intensity. C. Heatmap of mean immune cell marker expression in each cluster. The number in the brackets represents the size of cluster in percentage. D. ViSNE map separated to control, responder, transient responder, non responder groups. n denotes the number of control individuals or patients. E. Proportions of T cell subpopulations in all 8 patients at different time points and a control sample. B: baseline. Black arrow indicates that samples are collected after disease relapse in transiently responding patients.
T-cells from transient responders and non-responders, on the other hand, were dominated by clusters that expressed multiple different checkpoints. For patients 2 and 6, the major T cell subsets were in cluster 3, CD45RO+CD4+ T-cells characterized by co-expression of PD1 and TIGIT, and cluster 5, CD45 RO+CD8+ T cells expressing PD-1. The smaller CD4+CD45RO+ T-cell cluster 15, which co-expressed high PD1, TIM3 and TIGIT, was mainly detected in patient #2. Among non-responders, while T-cells in patient #3 had comparably low PD1 expression (Figure 1C), the TIGIT level was much higher and accordingly, this patient was enriched for cluster 10 (high TIGIT+ CD8+ T-cells) during treatment (Figure 2E). Patient #4 had a large cluster of high PD1-expressing CD8+CD45RO+ T-cells (cluster 4) and TIGIT+CD8+ T-cells (cluster 10), which dominated over a small population of cluster 6, CD8+ T cells with low checkpoint expression. Similarly, although cluster 1, another cluster with low inhibitory markers, was present in patient #7, a non-responder, the T-cell repertoire of this patient was dominated by cluster 2, a PD1 high, CD127 high memory CD4 T-cell cluster.
Four patients had re-biopsy at disease progression. All retained CD19 expression.
Treatment after progression.
Patterns of disease progression demonstrated significant heterogeneity One patient (#6) had a complete nodal response, but progressed with bone marrow involvement and pancytopenia.
Post-progression salvage treatment was also heterogenous (supplementary table 2). Of interest given our immune checkpoint expression data, 4 patients received therapy with ibrutinib + nivolumab immediately prior to or after treatment with blinatumomab: patient #2 had a transient response to blinatumomab, with clearance of circulating disease, before relapse with widespread bony lesions. She subsequently received ibrutinib + nivolumab and achieved an MRD-negative CR lasting 13 months before progressing with CNS disease. This patient had moderate levels of PD1 on her CD8+ T cells at baseline, which remained elevated during treatment with blinatumomab. Patient #6 had high levels of PD1 on both CD4 and CD8 T cells and achieved durable partial response with ibrutinib + nivolumab as next therapy. Finally, patient #1 received ibrutinib + nivolumab, without response, and started blinatumomab 10 weeks later. He had multi-focal, bulky, extra-nodal disease and achieved CR with blinatumomab therapy that lasted approximately 1 year from completion of C2. He had very low levels of PD1 expression on both CD4 and CD8 T cells. Patient #9 had also received ibrutinib + nivolumab immediately prior to blinatumomab (last dose 3 weeks prior to blinatumomab), but did not respond to blinatumomab. Samples for correlative analysis were not available for this patient.
Discussion.
Blinatumomab showed activity in high genomic risk and heavily pre-treated patients with RS and in the co-occurring CLL, with a low-incidence of treatment-related AEs. However, most responses were incomplete and transient.
In DLBCL treated with CD19-directed CAR-T, loss of CD19 expression, T cell exhaustion and IC expression, especially PD1,(8) have been implicated in treatment failure.(9) CD19 loss was not seen at progressionin our study. Most patients showed an exhausted T cell phenotype, expressing multiple ICs, particularly PD1, TIGIT and TIM3. Patient #1, who achieved durable CR, had the lowest levels of IC expression, especially PD1 (and had not responded to ibrutinib + nivolumab). Taken together, this suggests that IC expression on T cells may play a role in resistance to blinatumomab. Novel, long-active bi-specific antibodies, such as glofitamab(10) and epcoritamab(11) have achieved durable remissions in multiply-refractory patients with DLBCL. A multi-center study of epcoritamab (NCT04623541) includes a large cohort of patients with RS. CAR-T have also been successfully utilized in RS(12, 13). Understanding mechanisms of treatment failure, such as the high level expression of IC molecules, which could be targeted by clinically available inhibitory antibodies, is critical to improving upon results of treatment with these agents.
Several phase 2 studies have demonstrated activity of PD1 inhibitors in RS. TIGIT antibodies and TIM3 antibodies are in various stages of development. Our data provide a rationale for combining bi-specific antibodies plus IC blockade in RS for two reasons: first, ICs could target a mechanism of tumor escape from killing by blinatumomab; second, PD1 inhibitors have no activity in CLL,(14) but blinatumomab achieved potent debulking of concurrent CLL in the bone marrow in the majority of patients in this study, suggesting that addition of blinatumomab or other bi-specific antibodies could be a useful complementary approach to PD1 inhibition, to enable clearance of all compartments. There is, however, considerable inter-patient immunologic heterogeneity and multiple ICs are expressed by most patients. In the future, therefore, individualized strategies for targeting single or multiple ICs may be necessary to fully unlock the potential of bispecifc antibodies in RS.
Supplementary Material
Acknowledgements
This was supported, in part, by M.D. Anderson Cancer Center Support Grant P30 CA016672. The study drug and funding for the study was provided by Amgen. Amgen had no role in the conduct or analysis of the study or the writing of the paper.
Footnotes
Conflict of interest disclosure: P.A.T. consulted for Amgen, Genentech and AbbVie. The remainder of the authors declare no relevant conflicts of interest.
Deidentified data will be shared with other researchers upon reasonable request to the corresponding author (pathompson2@mdanderson.org). The sharing will require a detailed proposal to the study investigators, and a data transfer agreement must be signed.
References
- 1.Rossi D, Spina V, Gaidano G. Biology and treatment of Richter syndrome. Blood. 2018;131(25):2761–72. [DOI] [PubMed] [Google Scholar]
- 2.Younes A, Brody J, Carpio C, Lopez-Guillermo A, Ben-Yehuda D, Ferhanoglu B, et al. Safety and activity of ibrutinib in combination with nivolumab in patients with relapsed non-Hodgkin lymphoma or chronic lymphocytic leukaemia: a phase 1/2a study. Lancet Haematol. 2019;6(2):e67–e78. [DOI] [PubMed] [Google Scholar]
- 3.Gökbuget N, Dombret H, Bonifacio M, Reichle A, Graux C, Faul C, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood. 2018;131(14):1522–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topp MS, Gokbuget N, Stein AS, Zugmaier G, O’Brien S, Bargou RC, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16(1):57–66. [DOI] [PubMed] [Google Scholar]
- 5.Viardot A, Goebeler M-E, Hess G, Neumann S, Pfreundschuh M, Adrian N, et al. Phase 2 study of bispecific T-cell engager (BiTE®) antibody blinatumomab in relapsed/refractory diffuse large B cell lymphoma. Blood. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thall PF, Sung HG. Some extensions and applications of a Bayesian strategy for monitoring multiple outcomes in clinical trials. Stat Med. 1998;17(14):1563–80. [DOI] [PubMed] [Google Scholar]
- 8.Fraietta JA, Lacey SF, Orlando EJ, Pruteanu-Malinici I, Gohil M, Lundh S, et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med. 2018;24(5):563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah NN, Fry TJ. Mechanisms of resistance to CAR T cell therapy. Nature reviews Clinical oncology. 2019;16(6):372–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hutchings M, Morschhauser F, Iacoboni G, Carlo-Stella C, Offner FC, Sureda A, et al. Glofitamab, a Novel, Bivalent CD20-Targeting T-Cell–Engaging Bispecific Antibody, Induces Durable Complete Remissions in Relapsed or Refractory B-Cell Lymphoma: A Phase I Trial. Journal of Clinical Oncology. 2021;39(18):1959–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hutchings M, Mous R, Clausen MR, Johnson P, Linton KM, Chamuleau MED, et al. Dose escalation of subcutaneous epcoritamab in patients with relapsed or refractory B-cell non-Hodgkin lymphoma: an open-label, phase 1/2 study. Lancet. 2021;398(10306):1157–69. [DOI] [PubMed] [Google Scholar]
- 12.Ying Z, Huang XF, Xiang X, Liu Y, Kang X, Song Y, et al. A safe and potent anti-CD19 CAR T cell therapy. Nature medicine. 2019;25(6):947–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kittai AS, Bond DA, William B, Saad A, Penza S, Efebera Y, et al. Clinical activity of axicabtagene ciloleucel in adult patients with Richter syndrome. Blood Advances. 2020;4(19):4648–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding W, LaPlant BR, Call TG, Parikh SA, Leis JF, He R, et al. Pembrolizumab in patients with CLL and Richter transformation or with relapsed CLL. Blood. 2017;129(26):3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


