Abstract
Food allergy affects approximately 2–4% of children and adults. This guideline provides recommendations for managing food allergy from the Global Allergy and Asthma European Network (GA2LEN). A multidisciplinary international Task Force developed the guideline using the Appraisal of Guidelines for Research and Evaluation (AGREE) II framework and the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) approach. We reviewed the latest available evidence as of April 2021 (161 studies) and created recommendations by balancing benefits, harms, feasibility, and patient and clinician experiences. We suggest that people diagnosed with food allergy avoid triggering allergens (low certainty evidence). We suggest that infants with cow's milk allergy who need a breastmilk alternative use either hypoallergenic extensively hydrolyzed cow's milk formula or an amino acid-based formula (moderate certainty). For selected children with peanut allergy, we recommend oral immunotherapy (high certainty), though epicutaneous immunotherapy might be considered depending on individual preferences and availability (moderate certainty). We suggest considering oral immunotherapy for children with persistent severe hen's egg or cow's milk allergy (moderate certainty). There are significant gaps in evidence about safety and effectiveness of the various strategies. Research is needed to determine the best approaches to education, how to predict the risk of severe reactions, whether immunotherapy is cost-effective and whether biological therapies are effective alone or combined with allergen immunotherapy.
Keywords: Food allergy, Food hypersensitivity, Children, Adolescent, Adults
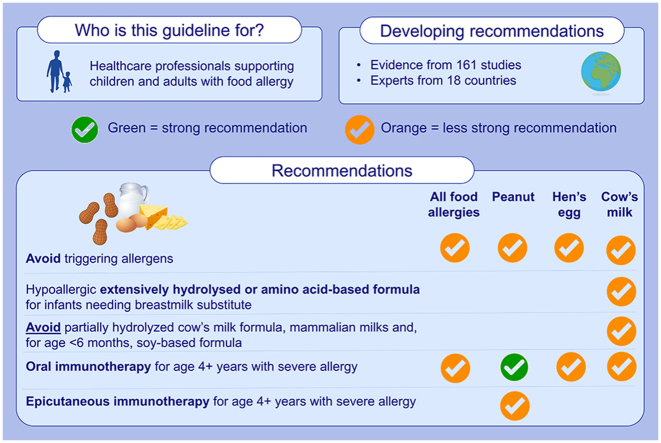
Graphical abstract
Summary of the Ga2len managing food allergy 2022 guideline.
Introduction
Food allergy affects at least 2–4% of children and adults.1 It can have serious consequences, including severe reactions such as anaphylaxis, reduced quality of life, and increased economic burden.2 Affected individuals and their families live with risk and must maintain constant vigilance to prevent exposure to food allergens.3
Food allergy is diagnosed by obtaining a detailed allergy history and tests such as skin prick tests, specific IgE, or molecular allergology (previously called component resolved diagnostics) to detect IgE-sensitization4 (see Box 1 for definitions) and differentiate from other food-related conditions such as lactose intolerance and pharmacologically-mediated reactions such as histamine in tomatoes or amines in cheese. A diagnosis of food allergy may require a food challenge at an experienced center due to the possibility of inducing anaphylaxis.
Box 1. Glossary of terms used in the guideline.
| Age groups | Infants: aged 0–1 year; Children: aged 1–17 years; Adolescents: aged 12–17 years; Adults: aged 18 years or older |
| Certainty of evidence | How confident we are that the available evidence represents the true effect of the intervention. Low certainty means that we are not confident in the findings and further research may make a significant difference. Moderate certainty evidence means that we are confident in the direction of the evidence, but the exact size of the effect may change as further evidence becomes available. High certainty means we are confident in the direction and the size of the effect |
| Food allergy | An adverse reaction to food mediated by an immunologic mechanism, involving specific IgE (IgE-mediated), cell-mediated mechanisms (non-IgE-mediated), or both IgE- and cell-mediated mechanisms (mixed IgE- and non-IgE-mediated) |
| Severe food allergy | Substantial risk of severe reactions and/or substantially impaired quality of life |
| Pollen food allergy syndrome | Oral hypersensitivity symptoms with raw fruit, vegetables, peanut and some tree nuts in people with pollen allergy caused by the cross-reactivity of the foods with pollen allergens |
| Hypoallergenic formula | Hypoallergenicity is nationally regulated in most countries.5,6 There is no unambiguous and generally agreed definition of a hypoallergenic formula. Meanwhile, The American Academy of Pediatrics, the European Society of Pediatric Allergology and Clinical Immunology ESPACI and EAACI defines a hypoallergenic formula one that is tolerated by 90% of individuals with cow's milk allergy.7,8,9 |
| Infant formula | Foodstuffs for use during the first year of life, which satisfy the nutritional requirements of infants until the introduction of appropriate complementary feeding. Follow-on formula is intended for use by infants when appropriate complementary feeding is introduced and constitutes the principal liquid element in a progressively diversified diet |
| Milk | Mammary secretion obtained from milking farmed animals such as cow, goat, sheep and donkey.10 |
| Allergen immunotherapy (AIT) | Repeated allergen administration at regular intervals and increasing dosages to modulate immune response and increase the threshold at which an individual reacts to an allergen |
| Epicutaneous immunotherapy (EPIT) | Form of AIT where the allergen is administered topically on the skin using a specific applicator, such as a patch |
| Oral immunotherapy | Form of AIT where the allergen is ingested as a non-processed food or an oral preparation |
| Subcutaneous immunotherapy (SCIT) | Form of AIT where the allergen is administered as subcutaneous injections |
| Sublingual immunotherapy (SLIT) | Form of AIT where the allergen is administered in liquid form or tablets under the tongue to be absorbed |
| Desensitization | The ability to consume a serving of food containing the trigger allergen during allergen immunotherapy without significant side effects |
| Sustained unresponsiveness | The ability to safely consume a serving of food containing the trigger allergen for a period of time after stopping allergen immunotherapy |
| Tolerance | The ability to consume a serving of food without developing an allergic reaction. |
| Tolerance in the context of immunotherapy | The ability to consume a serving of food containing the trigger allergen indefinitely after allergen immunotherapy has been stopped without significant side effects |
Alt-text: Box 1
Food allergy is best managed by a multidisciplinary team, including clinicians to make a diagnosis and help unravel causes and risks, dietitians to identify unusual allergens and avoid nutrient deficiencies, gastroenterologists where other conditions need to be excluded, and psychologists to support people who are severely affected by anxiety or impacts on quality of life. Allergy nurse specialists and primary care teams have a key role in reassuring those with and without allergy, managing mild cases, making referrals to specialists, and providing ongoing education. Patient-led organizations also have an important role in providing information and support.
Until recently, people with food allergy were just advised to avoid the food and, for some IgE-mediated allergy, to carry adrenaline at all times in case of anaphylaxis. Today there are additional options available. This guideline is designed to support healthcare professionals in managing people diagnosed with food allergy. The guideline sets out the Global Allergy and Asthma European Network's (GA2LEN) recommendations for managing food allergy, based on the latest evidence and expert consensus. Recommendations relate to both IgE and non-IgE mediated food allergy, unless otherwise stated. The European Academy of Allergy and Clinical Immunology (EAACI) is planning to publish complementary guidelines on the diagnosis of food allergy shortly.
Methods
Approach to developing the guideline
This guideline was developed by a multidisciplinary Task Force with representatives from pediatric and adult allergy, dermatology, primary care, dietetics, psychology, education, food science, methodologists, and representatives of patient organizations from 18 countries.
We used the Appraisal of Guidelines for Research and Evaluation (AGREE) II approach11,12 and evaluated evidence and developed recommendations using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) method.13 The work was undertaken between April and December 2021, using monthly online conferences and email discussions.
Guideline focus
The guideline focuses on ways to best manage diagnosed food allergy in infants, children, adolescents, and adults. We mainly focused on IgE-mediated food allergy, although some of the sections are also applicable to non-IgE mediated food allergy. We examined dietary and educational interventions, biological therapies, allergen immunotherapy, and how the risk of severe reactions should influence management plans (see Box 2).
Box 2. Key clinical questions addressed in the guideline.
|
|
|
|
|
Alt-text: Box 2
Reviewing evidence
We undertook 2 systematic reviews and 3 rapid reviews to compile the most robust and up-to-date evidence upon which to base recommendations (Table S1.1). We searched 6–8 bibliographic databases, depending on the topic. In total we screened 23 961 studies and included evidence from 161.
Allergen immunotherapy and biologicals are relatively new treatments for food allergy, so we prioritized these topics for the most in-depth exploration of evidence. Independent methodologists worked with clinicians and patient representatives to undertake full systematic reviews about these topics. The methods and findings have been published.14,15 The reviews included randomized controlled trials published until April 30, 2021 for immunotherapy and until September 30, 2021 for biologicals. We assessed the risk of bias and certainty of evidence and constructed summary of findings tables to inform recommendations.16
We conducted rapid reviews about risk management, dietary interventions, and education for people affected by food allergy. We knew that the evidence-base about these topics was suboptimal, so we included non-randomized trials with a simultaneous comparison group as well as randomized controlled trials. We also included observational studies about identifying risks. We undertook a systematic search for studies published up until April 30, 2021 and assessed the risk of bias and the certainty of evidence.
Supplement 1 contains fuller details about the methods.
Identifying recommendations and gaps
We considered the strength and consistency of the evidence when formulating evidence-based recommendations.13 Informed by GRADE, we assessed the certainty of evidence about each management approach as high, moderate, low or very low. We considered the importance of the issue, desirable and undesirable effects, certainty of evidence, patient preferences, resources required, accessibility, and feasibility.
Task Force working groups reviewed the evidence in detail and proposed recommendations which were then explained and voted on by the full Task Force. All recommendations were agreed by consensus. At least 80% of those voting needed to agree in order for a recommendation to be included. We prepared tables summarizing the reasons for each recommendation (Supplements 2–6). We also identified and prioritized gaps where evidence is lacking.
Table 1 describes the conventions we used to describe the strength of recommendations and how this relates to policy and practice.
Table 1.
Wording conventions used in recommendations in this guideline.
| Strength and direction | Wording | What does this mean? |
|---|---|---|
| Strong recommendation for an intervention | “The GA2LEN Task Force recommends …” |
|
| Conditional recommendation for an intervention | “The GA2LEN Task Force suggests …” |
|
| Conditional recommendation against an intervention | “The GA2LEN Task Force suggests against …” |
|
| Strong recommendation against an intervention | “The GA2LEN Task Force recommends against …” |
|
| No recommendation | “The GA2LEN Task Force makes no recommendation for or against using …” |
|
We also provided good practice statements setting out points for healthcare professionals to consider in areas where there was insufficient evidence to make a formal recommendation.
Peer review and public comment
A draft was peer reviewed by invited experts from a range of organizations, countries, and professional backgrounds. The draft was also publicly available on the GA2LEN website for a two-week period in January/February 2022 to gain feedback from stakeholders. Final revisions were made, incorporating feedback received.
Editorial independence and managing conflicts
Task Force members volunteered their time. GA2LEN contributed resources for administration and sourcing evidence. The funder did not have any influence on the content or decision to publish.
Task Force members declared interests at the start and end of the process. Anyone with a direct financial conflict of interest, apart from consultancy, was not involved in decisions or voting about relevant recommendations. Methodologists who had no conflict of interests independently compiled evidence about the effectiveness of immunotherapy and biologicals and reviewed the strength of evidence for recommendations about other topics.
Updating the guidelines
The Task Force plans to update this guideline in 2027 unless there are important advances before then.
Guideline recommendations
Table 2 sets out our evidence-based recommendations. This section provides brief justifications of our recommendations. Online supplements 2–6 contain fuller details, including a summary of relevant evidence and our confidence in it.
Table 2.
Guideline recommendations.
| Recommendation | Certainty of evidence |
|---|---|
| Dietary interventions | |
| The GA2LEN Task Force suggests that people with a documented food allergy avoid the offending food unless their individual circumstances and risks allow for some consumption, as advised by their healthcare professional. We suggest that most breastfeeding mothers whose infants have a food allergy do not need to avoid the offending food themselves, though in rare cases this might be considered. | Low |
| The GA2LEN Task Force suggests that most infants (aged 0–1 years) diagnosed with cow's milk allergy who need a breastmilk alternative use a documented hypoallergenic extensively hydrolyzed cow's milk formula, or an amino-acid based formula if better tolerated or more appropriate. We suggest against partially hydrolyzed cow's milk formula, mammalian milks and, also for infants under 6 months, against soy-based formula. | Moderate |
| Allergen immunotherapy | |
| The GA2LEN Task Force recommends offering peanut oral immunotherapy under specialist supervision with standardized evidence-based protocols using peanut products (or licensed pharmaceutical products, where appropriate), to selected children (aged 4+ years) with clinically diagnosed, severe, IgE-mediated, peanut allergy to increase the amount of peanut tolerated while on therapy. | High |
| The GA2LEN Task Force suggests offering peanut epicutaneous immunotherapy under specialist supervision using licensed pharmaceutical products if they become available to selected children aged 4–11 years with clinically diagnosed, severe, IgE-mediated, peanut allergy to increase the amount of peanut tolerated while on therapy. | Moderate |
| The GA2LEN Task Force suggests offering oral immunotherapy under specialist supervision with standardized evidence-based protocols using food products to selected children (aged 4+ years) with clinically diagnosed persistent severe IgE-mediated hen's egg or cow's milk allergy to increase the amount of allergen tolerated while on therapy. | Moderate |
Note: The certainty of evidence refers to how confident we are that the available evidence represents the true effect of the intervention. See Box 1 for definitions. Further information including rationale and practical consideration is available in the text.
We found insufficient evidence to make recommendations about some strategies (Table 3), though we were able to suggest good practice statements based on expert opinion from the multidisciplinary guideline group to help support the allergy consultation in some areas (Box 3).
Table 3.
Areas where guideline makes no recommendation for or against.
| Topic | Certainty of evidence |
|---|---|
| Dietary interventions | |
| The GA2LEN Task Force makes no recommendation for or against any prebiotics, probiotics or synbiotics that have been evaluated so far for managing food allergy, whether used as a supplement or added to infant formula. | Very low |
| The GA2LEN Task Force makes no recommendation for or against hydrolyzed plant-based formulas including rice hydrolysates that have been evaluated so far for managing food allergy in infancy. | Very low |
| Allergen immunotherapy | |
|
Very low |
| Biological therapies | |
| The GA2LEN Task Force makes no recommendation for or against offering etokimab for treating food allergy. | Very low |
| The GA2LEN Task Force makes no recommendation for or against offering omalizumab for treating food allergy, alone or in combination with immunotherapy. | Very low |
Note: The certainty of evidence refers to how confident we are that the available evidence represents the true effect of the intervention. See Box 1 for definitions.
Box 3. Good practice statements.
|
|
|
|
Note: The certainty of evidence for all good practice statements was very low.
Alt-text: Box 3
Dietary interventions
The GA2LEN Task Force suggests that people with a documented food allergy avoid the offending food unless their individual circumstances and risks allow for some consumption, as advised by their healthcare professional. We suggest that most breastfeeding mothers whose infants have a food allergy do not need to avoid the offending food themselves, though in rare cases this might be considered.
Reason for recommendation
Our rapid review identified 4 studies about eliminating food allergens from the diet (n = 389, 2 RCTs) (Tables S2.1-2.7).17, 18, 19, 20 We concluded that avoiding food allergens is likely to reduce allergic reactions and symptoms (see Supplement 2). However, there is potential for suboptimal nutrition and growth, so it is important that only documented food allergens are avoided. There are circumstances where people may not have to completely avoid their food allergen(s). For example, some people with allergies to milk or egg may tolerate baked milk or baked egg21,22 and individuals with pollen food allergy syndrome may be able to tolerate cooked fruits and vegetables.23
It is important for breastfeeding mothers to have an adequate diet. Infants with IgE-mediated allergy are rarely so sensitive that they react to the very low levels of food allergens in breastmilk. The harm associated with avoiding foods during breastfeeding may be greater than any benefits for managing food allergy in infants.
Strength of recommendation
This guideline supports avoiding the triggering food allergen; however, despite avoidance being the main management approach, this is not the strongest recommendation possible as it has only been assessed in a small number of studies with heterogeneous approaches, giving a low certainty of evidence. There may also be some instances where individuals can be less restrictive in their diet.
Practical implications
Healthcare professionals should aim to accurately identify a person's food allergies. This will allow people with food allergy to avoid only their specific allergens and prevent an overly restrictive diet. Dieticians can help ensure the diet continues to meet nutritional needs.
Professionals should assess whether individuals can be less restrictive with their diet, by trying cooked or baked foodstuffs, and by regularly re-evaluating the diagnosis to make sure that people have not grown out of their allergy; this is common with milk and egg allergies in early childhood where 6–12 monthly reassessments are desirable. Any reintroduction or less restrictive diets should always be individually determined with a healthcare professional.
Breastfeeding mothers should only consider an avoidance diet to manage food allergies affecting their infant if advised on an individual basis by a healthcare professional.
The GA2LEN Task Force suggests that most infants (aged 0–1 years) diagnosed with cow's milk allergy who need a breastmilk alternative use a documented hypoallergenic extensively hydrolyzed cow's milk formula, or an amino-acid based formula if better tolerated or more appropriate. We suggest against using partially hydrolyzed cow's milk formula, mammalian milks and also, for infants under 6 months, against soy-based formula.
Reason for recommendation
Our rapid review identified 12 studies about extensively hydrolyzed cow's milk based infant formula (n = 743, 10 RCTs) and 8 studies about amino-acid based infant formula for infants with cow's milk allergy (n = 483, 7 RCTs) (Supplement 3). We concluded that the benefits outweigh potential harms for extensively hydrolyzed cow's milk formula and amino acid-based formula. The cost of amino acid-based formula may not make it the first choice.
We suggest against partially hydrolyzed cow's milk formula and mammalian milks due to the potential for allergic reactions. We also suggest against soy-based formula for those under six months due to risk of allergic reactions and the unknown effect of their potential phytate, aluminum, and phytoestrogen content on the infant.24
There was not enough evidence to draw conclusions about hydrolyzed rice formula or other hydrolyzed alternatives. There are very few studies on milk from other mammals, such as goat and donkey milk.25 There is a high degree of cross-reactivity between goat/sheep and cow's milk. Some studies found that using goat's milk in children with cow's milk allergy resulted in allergic reactions, including anaphylaxis, in a large proportion. See online supplement for more details, including comments on nutritional adequacy (Table S2.2).
Strength of recommendation
This is not the strongest recommendation possible because the certainty of evidence is moderate-to-low due to the small number of studies about each formula and heterogeneity of reported outcomes.
Practical implications
Breastfeeding is preferable for infants with cow's milk allergy. Where this is not possible, professionals should assist families to identify the best alternative for individual infants. Most tolerate an extensively hydrolyzed formula but some may continue to have significant symptoms or poor growth. Amino-acid formula may be helpful as an alternative when excluding multiple foods in those with severe complex gastrointestinal food allergies, eosinophilic esophagitis, faltering growth or symptoms while exclusively breastfeeding. Formula should have documented hypoallergenicity7,8 and be nutritionally sufficient (confirmed with dietician input). Due to a high degree of cross-reactivity with cow's milk proteins and therefore risk of allergic reactions, other mammalian milks, especially goat's/sheep's milk, are not recommended. After one year of age, consideration can be given to adding plant-based drink (supplemented by micronutrients) to the child's diet, depending on the child's growth and overall nutrition. Further practical advice can be found in online supplement (Table S2.2).
Allergen immunotherapy
The immunological pathways underlying IgE-mediated food allergy can potentially be targeted with allergen immunotherapy. This involves carefully-controlled exposure using increasing doses of food allergens, which can modify the immune response and increase the threshold at which they react.26 Immunotherapy may be administered via the oral, epicutaneous, sublingual or subcutaneous routes.
The GA2LEN Task Force recommends offering peanut oral immunotherapy under specialist supervision with standardized evidence-based protocols using peanut products (or licensed pharmaceutical products, where appropriate), to selected children (aged 4+ years) with clinically diagnosed, severe, IgE-mediated, peanut allergy to increase the amount of peanut tolerated while on therapy.
The GA2LEN Task Force suggests offering peanut epicutaneous immunotherapy under specialist supervision using licensed pharmaceutical products if they become available to selected children aged 4–11 years with clinically diagnosed, severe, IgE-mediated, peanut allergy to increase the amount of peanut tolerated while on therapy.
The GA2LEN Task Force suggests offering oral allergen immunotherapy under specialist supervision with standardized evidence-based protocols using food products (or licensed pharmaceutical products, where appropriate), to selected children (aged 4+ years) with clinically diagnosed persistent severe IgE-mediated hen's egg or cow's milk allergy to increase the amount of allergen tolerated while on therapy.
Reason for recommendations
Supplement 3 (Tables S3.1-3.8) contains the evidence and rationale for these recommendations.
Our recommendations about oral immunotherapy (OIT) focus on children with severe, IgE-mediated allergy given the potential time and emotional and physical burden of this therapy, the risk of rare severe reactions and the cost. In this context, we defined severe food allergy as having a substantial risk of severe reactions and/or substantially impaired quality of life.
Our systematic review and meta-analysis found that OIT in children aged 4–17 years probably results in a large increase in threshold for reaction to peanut whilst on therapy. It probably also increases the threshold for hen's egg and cow's milk.14 Sustained unresponsiveness is not achieved in many individuals.14 Severe allergic reactions occurred but were rare (Supplement 3, Tables S3.1, S3.3). Box 4 lists which children to consider for this therapy.
Box 4. Indications and contraindications for allergen immunotherapy for food allergy.
Indication:
All the following need to be in place:
-
•
History of IgE-mediated systemic allergic reactions after ingestion and/or positive oral food challenge (especially where allergy may be transient)9,31
- •
-
•
Primary food allergy, as opposed to pollen food allergy syndrome due to cross-reactivity
-
•
Persistent food allergy with low likelihood of spontaneous resolution
-
•
Affected people and care givers (where relevant) have a full understanding of effectiveness, side effects, logistics and the potentially life-long duration of the therapy32,33
-
•
Affected people and their care givers should be motivated, adherent and capable of administering emergency treatment (including intramuscular adrenaline) in the case of adverse effects34
-
•
Previous severe reactions to the food35 or impaired quality of life due to burden of food allergy36,37
-
•
Willingness of all stakeholders to incorporate the food into diet38,39
-
•
Stability of living and family situation
Absolute.
-
•
Inadequate adherence to therapy and/or safety recommendations
-
•
Uncontrolled or severe asthma42
-
•
Active malignant neoplasia(s)
-
•
Active systemic autoimmune disorders
-
•
Systemic immunosuppression therapy
-
•
Untreated/uncontrolled active eosinophilic esophagitis and other eosinophilic gastrointestinal disorders
-
•
Initiation during pregnancy
Relative.
-
•
Severe systemic conditions such as cardiovascular diseases
-
•
Systemic autoimmune disorders in remission or organ specific (i.e. thyroiditis)
-
•
Uncontrolled active atopic dermatitis/eczema
-
•
Uncontrolled chronic urticaria
-
•
Therapy with beta-blockers or ACE inhibitors
-
•
Systemic mastocytosis
-
•
Concurrent up-dosing with other immunotherapy
-
•
Chronic gastrointestinal symptoms without a clear diagnosis
-
•
Unable to consume study product (e.g. vomiting, taste problems, allergy to vehicle)
-
•
Psychological problems, suspicion/confirmation of eating disorders
Appropriate staffing, environment and approach: 43
-
•
Personnel trained and experienced in the use of immunotherapy for food allergy, including a medical doctor and nurse experienced in the diagnosis of food allergy and in recognition and treatment of allergic reactions, including anaphylaxis
-
•
Provision to provide appropriate intervention and observation dependent on the severity of any allergic reaction (may involve transfer to another facility)34
-
•
Emergency equipment and medications to manage medical emergencies including severe anaphylaxis and rapid access intensive care if needed
-
•
Standardized, evidence-based protocol; licensed pharmaceutical product where available
Alt-text: Box 4
We focus on children aged 4+ years as this is where most evidence exists. Randomized placebo-controlled trial evidence of the efficacy and safety of peanut OIT for the induction of sustained unresponsiveness in children below 4 years of age has recently been published,27 after our review of the evidence. There are similar data from a recent real-world study.28 However, we make recommendations for allergen immunotherapy from 4 years of age based on the effectiveness evidence, the potential to outgrow the allergy, the logistics and potential harms. Clinicians may consider other age groups depending on individual circumstances.
OIT may be useful for selected adults with IgE-mediated food allergy where potential benefits outweigh risks, but there was no or minimal evidence to support making a recommendation about this.
Epicutaneous immunotherapy in children aged 4–11 years probably results in an increase in the threshold at which they react to peanut whilst on therapy. This intervention is not currently available or licensed, but the task force felt it was important to highlight the positive evidence in trials to date. If it becomes available, professionals and families need to make a shared decision about whether OIT or epicutaneous immunotherapy is best for an individual based on relative effectiveness, safety, and logistics.
There was insufficient evidence to make recommendations about other applications of immunotherapy by route or for different types of foods. There was also insufficient evidence to make a recommendation about adding omalizumab to immunotherapy.
Strength of recommendations
We make a strong recommendation in favor of peanut OIT given the high certainty about the evidence regarding desensitization. The number needed to treat to achieve 1 person tolerating 300 mg or 1000 mg of peanut protein while on therapy was 2 people with food allergy.14
Our recommendations about OIT for hen's egg and cow's milk allergy and for epicutaneous immunotherapy for peanut are positive, but not the strongest possible because we had moderate certainty in the evidence, there are likely variations in individual preferences and we considered the potential burden and cost of treatment.
Practical implications
Allergen immunotherapy should only be used when an individual has proven IgE-mediated, primary food allergy. Given the complexity of allergen immunotherapy and its potential side-effects, clinical staff should be trained and experienced in its use and have the facilities available to deal with any side-effects (Box 4). Treatment should be under the supervision of a specialist with the requisite competencies in food allergy immunotherapy.
Only standardized protocols with evidence of effectiveness and safety should be used, under specialist supervision. If food products are used, care should be taken that doses are appropriate and consistent in terms of their allergen content, biological potency and lack of contaminants. Given these considerations, clinicians and patients may prefer to use licensed medicinal products prepared under Good Manufacturing Practices for pharmaceutical products. Affordability, quality of an alternative, risk-benefit, patient preference and local context should also be taken into consideration.
Clinicians should discuss the potential benefits and harms to help families choose whether immunotherapy is right for them and whether they are capable of adhering to therapy and managing any side effects. Some people may prefer to avoid the offending allergen instead. Careful selection is needed to avoid unnecessary treatment as many children outgrow hen's egg or cow's milk allergies by school age.29,30
Biological therapies
The immunological pathways underlying IgE-mediated food allergy are a potential target for biological therapies. This approach has the potential to target multiple different food allergies and coexisting allergic diseases. Etokimab and omalizumab are monoclonal antibodies targeting IL-33 and IgE respectively. There are published data on their effectiveness for treating food allergy. Other biologics, such as dupilumab (anti-IL-4Rα) are currently being assessed in phase 2–3 studies.
The GA2LEN Task Force makes no recommendation for or against offering omalizumab or etokimab for treating food allergy.
Reasons for recommendations
Our systematic review found insufficient evidence to make recommendations about biological monotherapy for people with food allergy.15 The certainty of evidence was very low. One randomized controlled trial of omalizumab and another of etokimab found trends towards higher threshold for reaction in adults with peanut allergy, but the studies were small, heterogeneous and too few to draw conclusions.44,45 (Supplement 4, Tables S4.1-4.7)
Practical implications
There is some promising early data from non-controlled studies, which were outside the scope of our review.46 Confirmatory evidence from large, adequately powered trials is required before biologicals can be recommended for general use in food allergy.
Omalizumab is already an established treatment for severe allergic asthma and so may be the preferred biological for asthma in people with coexisting food allergy. Clinicians may also consider testing the therapy in selected people, such as those with recurrent episodes of anaphylaxis despite allergen avoidance.
Educating individuals and families
It is good practice to offer structured education to people with food allergy and their family about managing food allergy routinely and in an emergency, tailored to their age group and individual needs.
Reason for good practice statement
People with food allergy and their care givers need knowledge and skills to recognize reactions of differing severities and how to manage them.47 Our rapid review identified 6 randomized controlled trials assessing educational strategies. We concluded that there is not yet enough evidence to recommend one form of education over others (Supplement 5).
Structured group or individual education sessions focused on self-management48,49 and behavioral change,50 referral to patient support groups and digital technologies are all options. Whatever approach is used, education should be tailored to each person's experience of allergy, their risk of reactions and their personal and social circumstances. There is a potential to increase anxiety if information is not provided with appropriate support or phrased in a sensitive and contextually appropriate manner.51 There is also a potential for over-confidence, leading to a risk of inappropriate exposure to food allergens.
In children and adults with other conditions, education that incorporates psychological, motivational or behavioral change concepts has reduced anxiety and improved people's confidence to self-manage long-term conditions.52 This may be useful in food allergy. Motivational and behavioral change principles can be used by a wide range of professionals, with minimal training.53
Further details can be found in Tables S5.1-5.5.
Identifying and managing risk
Our rapid review found that there was insufficient evidence to make recommendations about how to accurately identify individuals most at risk of severe reactions and which interventions might reduce risk (Supplement 6, Tables S6.1-6.7). We instead suggest good practice for clinicians to consider.
Adolescents and young adults with food allergy are at increased risk of severe reactions, so it is good practice to put into place effective risk management and transition strategies.
Reason for good practice statement
Our rapid review found that adolescents and young adults with food allergy are at increased risk of severe reactions compared to those of other ages with food allergy. This may be compounded by inadequate transitioning, as this age group starts to take responsibility for their own health. It is good practice to support transitioning from around age 11 years with approaches to improve confidence in self-management.54 However, the extent to which such interventions reduce the risk of severe reactions has not been adequately studied.
It is good practice to optimize asthma control in people with food allergy as this reduces morbidity and mortality due to asthma. It might reduce the risk of severe food-induced allergic reactions, though the evidence about this is unclear.
Reason for good practice statement
There are limited and contradictory data about whether a diagnosis of asthma and/or poor asthma control is a risk factor for severe food allergy reactions. Achieving good asthma control in people with food allergy will reduce morbidity and mortality due to asthma. This has the potential to reduce the risk of severe food-induced allergic reactions, but the relationship is not straightforward or certain.
It is good practice for clinicians to consider the severity of previous symptoms and the likely triggering dose when evaluating the risk of anaphylaxis. Allergen-specific IgE alone is not useful in predicting risk of anaphylaxis.
Reason for good practice statement
Allergy history can help clinicians personalize management. History alone is not a good predictor of future severe anaphylaxis because severity depends on a range of factors. Absence of prior anaphylaxis does not exclude future risk of anaphylaxis.55 If people has a history of reactions to only large doses, but no or minimal symptoms to smaller doses, they may not need to strive for complete avoidance.
There is not a simple relationship between triggering dose and reaction severity, so people who react to smaller doses of a food allergen are not necessarily at risk of severe reactions. Additionally, severity depends on the presence or absence of cofactors (eg, exercise, concurrent viral infections, use of non-steroidal anti-inflammatory drugs, sleep deprivation, and alcohol). It is important that people with food allergy are aware of these cofactors.
Most individuals with food allergy will experience oral symptoms to smaller amounts of allergen; the occurrence of oral symptoms alone to low doses should not be assumed to imply pollen food allergy syndrome.
In general, the level of allergen-specific IgE does not predict risk of anaphylaxis. For some foods, molecular allergology may be useful in predicting higher or lower risk of anaphylaxis. Some studies report that in people with tree nut allergy, IgE against 2S albumins is associated with increased risk of anaphylaxis. For peanut, IgE-monosensitization to Ara h 8 is associated with a lower risk and implies pollen food allergy syndrome. Sensitization to lipid transfer proteins may imply higher risk of anaphylaxis in some regions.
Discussion
Summary
This guideline provides evidence-based recommendations and good practice statements to help healthcare professionals, patients and families to manage food allergy (Table 2, Box 3). Our recommendations are different from previous guidelines because the latest robust evidence supports more proactive management strategies such as the use of some types of allergen immunotherapy for selected children with IgE-mediated food allergy. This is a substantial change to past guidance which recommended only dietary avoidance due to lack of evidence on other strategies.9 Table 4 lists practical considerations when implementing the recommendations; the local context may mean that some may need to be modified or will not be applicable.
Table 4.
Considerations for implementing guideline recommendations.
| Topic | Barriers to implementation | Facilitators to implementation | Audit criteria | Resource implications |
|---|---|---|---|---|
| Elimination diet for children and adults with any food allergy |
|
|
|
|
| Elimination diet in breastfeeding mothers whose infant has a food allergy |
|
|
|
|
|
Extensively hydrolyzed cow's milk or amino acid based infant formula in infants with cow's milk allergy |
|
|
|
|
| Topic |
Barriers to implementation |
Facilitators to implementation |
Audit criteria |
Resource implications |
| Avoidance of partially hydrolyzed cow's milk based formula in infants with cow's milk allergy |
|
|
|
|
| Avoidance of soy-protein based formula in infants with cow's milk allergy under 6 months |
|
|
|
|
| Oral immunotherapy for peanut, hen's egg or cow's milk allergy in children |
|
|
|
|
| Epicutaneous immunotherapy for peanut allergy |
|
|
|
|
Strengths and limitations
A strength of this guideline is that it is based on rigorous up-to-date systematically collated evidence using the GRADE approach. The reviews were led by independent methodologists with no conflicts of interest. Recommendations are based on randomized controlled trial data and controlled clinical trials to provide the highest quality available evidence. The evidence was interpreted and applied to real world settings by an international, multidisciplinary guideline group containing a mix of patient representatives, clinicians, and other stakeholders.
The key limitation is gaps in the existing evidence base, which made it difficult to develop recommendations on some topics. Table 5 lists key gaps and priorities for future research.
Table 5.
Gaps in the evidence for managing food allergy.
| Gaps | Suggestion to address | Priority |
|---|---|---|
| Dietary interventions | ||
| Long-term effect of dietary avoidance on nutrition and quality of life | High quality prospective, multi-site studies focusing on nutrition, growth and quality of life | Medium |
| Impact of a nutrition consultation by a dietitian on reducing accidental exposures, supporting growth and maintaining nutritional status, including support for breastfeeding | Food allergy part of core dietetic training curriculumAccess to dietetic support for every specialist food allergy service could be a requirement for national/international accreditationAuditing practice to assess outcome | Medium |
| Knowledge of the role of nutrition in supporting tolerance development | Training of dietitians/nutritionist to be able to provide information on tolerance development such as oral immunotherapy protocols, intake of foods with altered/reduced allergenicity and modulation of the microbiome and immune system as information becomes available | Medium |
| Indications for the use of different types of infant formula | Large cohort studies of children with cow's milk allergy comparing the cost-effectiveness of types of formulas at different ages and different clinical symptoms | Medium |
| The optimal dietary regimen for non-IgE mediated food allergy | High quality prospective trials of infants and young children with documented non-IgE mediated food allergy | Medium |
| Most useful parameters in evaluating the need for total exclusion of the culprit food or a ‘partial’ diet allowing consumption of ‘may contain’, small amounts or modified food allergens (e.g. baked milk and egg) | Re-evaluating data from existing studies | Medium |
| New diagnostic approaches to delayed-type food allergies to guide dietary interventions beyond the empirical approach | Basic science studies to develop candidate diagnostic tests | Medium |
| Effect of supplementation with different probiotic strains or prebiotics for management of food allergy | High quality prospective trials of infants and young children with documented food allergy | Low |
| Immunotherapy | ||
| Long term benefits and harms of immunotherapy including sustained unresponsiveness, including the impact of oral immunotherapy on health-related quality of life, and its cost effectiveness |
Large randomized controlled trials powered to detect moderate differences in health-related quality of life and utility, and including cost information Trials with long-term follow up |
High |
| Gaps |
Suggestion to address |
Priority |
| Predictors of response to immunotherapy, including effect of using modified food allergens (e.g. baked milk and egg) to improve and accelerate tolerance in IgE and non-IgE mediated food allergy/use of raw or cooked egg in oral immunotherapy | Studies to assess the ability for different factors and biomarkers to predict good response to therapy in different age groups | High |
| Effect of co-administration of biological therapy on the efficacy and safety of immunotherapy for food allergy | Large randomized controlled trials looking at optimal duration and dose and efficacy after stopping biologicals | High |
| Standardized definitions and measurement approach to adverse events and efficacy outcomes | Qualitative studies, surveys and cost-effectiveness studies to identify most relevant performance indicators. | High |
| Biological therapy | ||
| Most suitable candidates for biological therapy for food allergy | Analysis of existing observational data and new controlled trials | High |
| Specific and sensitive biomarkers to predict the response to biological therapy for food allergy | Analysis of existing observational data and new controlled trials | High |
| Education | ||
| Most effective approaches for delivering education, including digital technologies | Needs assessmentCoproduction with stakeholdersLarge multicenter study looking at learning and skill acquisition and psychological impact with long term follow up to address de-skilling | Medium |
| Effectiveness of educational programs, support and tools offered by patient organizations | Research collaborations with patient organizations to validate impactful interventions and share best practices | Medium |
| Best interval between retraining for people with food allergy and care givers | Longitudinal studies | Medium |
| Best approach to utilize psychological support for individuals with food allergy | RCT to evaluate the impact of psychological intervention and identify which individuals have the most to benefit | Medium |
| Risk prediction and management | ||
| Factors which might predict severity | Analysis of prospective data relating to reactions collected systematically Case-control studies evaluating risk factors for life-threatening reactions | High |
| Impact of risk mitigation strategies on outcomes | Large randomized control trials to specifically evaluate interventions designed to reduce risk of accidental reactions and their severity | Medium |
Priority allocated according to voting of all guideline group.
Research gaps
Our ability to manage people with food allergy is currently limited. We lack ways to accurately predict who is most at risk of severe reactions and need to improve how we individualize avoidance advice to minimize the negative impact on quality of life. Education for people with food allergy and their families is fundamental to help people keep themselves safe. We therefore urgently need randomized trials to understand the best and most cost-effective approaches to education, support and shared decision-making processes.56
Similarly, for immunotherapy, there is sparse evidence about patient preferences, impacts on quality of life and cost-effectiveness. We are also lacking adequate and robust studies into the effectiveness of biological therapies alone and with immunotherapy. Immunotherapy and biological therapies have the potential to revolutionize the life of people with food allergy and should be a focus of further research. Having standardized definitions and measurement approaches for adverse events, severe reactions and tolerance levels would significantly aid comparability and allow for more targeted support and guidance.
Conclusions
This is an exciting time in the field of food allergy, with fundamental changes now possible to support growing numbers of affected individuals. Applying the recommendations in this guideline, alongside shared decision-making processes with patients and families, may help to reduce the substantial and growing burden of food allergy in Europe and around the world. Introducing new ways of managing food allergy requires system-wide changes and collaboration between allergy specialists, community healthcare professionals, psychologists, patient organizations, patients, and their families.
Funding
GA2LEN.
Availability of data and materials:
No new data generated as part of this manuscript.
Author contributions
All authors conceptualized the work, contributed to discussions about evidence, voted on recommendations, critically reviewed the manuscript and approved the guideline for submission. In addition, DdS and GR drafted and edited the manuscript, which all authors input into. AM, GR, MW, and SH chaired the guidelines Task Force. EK and GR coordinated a working group focused on immunotherapy, AM and SA coordinated a group focused on biological therapies. ADG and PS coordinated a group examining educational initiatives. SH and BN led a group reviewing dietary interventions. PJT and SA led a group focused on risk identification and management.
Ethics approval
Not applicable.
Authors’ consent for publication
All the authors approved the final manuscript and decision to publish.
Declaration of competing interest
Alessandro Fiocchi: personal fees: Ferrero; grant: Danone, HIPP.
Amena Warner: none declared; organization received funding to support projects from Aimmune, DBV, Nutricia, Abbott Nutrition, Reckitt Benckiser, Novartis, MEDA and ThermoFisher.
Angel Sanchez, none declared.
Anna Nowak-Wegrzyn: personal fees: Nestle, Novartis, Sanofil.
Antonella Cianferoni: personal Fees: AstraZeneca, DBV.
Antonella Muraro: personal fees: Aimmune, DVB, Mylan, ALK, Nestle, Novartis, Nutricia Research. Grant: Aimmune, Sanofi.
Antoine Deschildre: personal fees from Novartis, ALK, GSK, Sanofi, Aimmune Therapeutics, DBV Technologies, Nestlé Health Science, Boehringer Ingelheim, Stallergenes Greer, DBV Technologies, Nutricia. Grant from Fondation du Souffle, Conseil Régional Hauts-de-France Research Program 2014–2018.
Audrey DunnGalvin: grant: DBV, WAO; personal fees: Aimmune.
Barbara Ballmer-Weber: personal fees: Novartis, Thermofisher, ALK, Allergopharma, Stallergenes, Menarini, Sanofi, Aimmune.
Berber Vlieg-Boerstra: personal fees: Marfo Food group, Nestle, Nutricia. Grant Nutricia.
Bertine Flokstra: employment: General Practitioners Research Institute (GPRI); GPRI has conducted investigator- and sponsor-initiated research funded by non-commercial organizations, academic institutes, and pharmaceutical companies (including AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Mundipharma, Novartis, and Teva).
Bright Nwaru: personal fees: AstraZeneca, DBV Technologies.
Carla Jones: none declared; organization received funding to support projects from Aimmune, DBV, Nutricia, Abbott Nutrition, Reckitt Benckiser, Novartis, MEDA and ThermoFisher.
Carina Venter: grant: Reckitt, personal fees from Reckitt, Danone, Abbott, Nestle Nutrition Institute, Sifter.
Caroline Nilsson: grant: Aimmune, Thermofisher.
Carsten Bindslev-Jensen: personal fees: Allakos, CEV SA; grant: Novartis.
Céline Demoulin: grants: ALK-Abelló, Mylan-Viatris, Stallergenes Greer, DBV, Bioprojet, Aimmune.
Clare Mills: grants: Food Standards Agency, YU Research and Innovation, European Food Safety Authority, Reacta Biotech Ltd; consulting: Reacta Biotech Ltd; attending meeting: Romer Laboratories UK Ltd; patent: oral food challenges; leadership: Reacta Biotech Ltd, Food Standards Agency advisory committee; stock: Reacta Biotech Ltd.
Debra de Silva: none declared.
Ekaterina Khaleva: none declared.
Elizabeth Angier: grant: Natasha Allergy Research Foundation; expert testimony UK Medicines and Healthcare Regulatory Authority; leadership BSACI, EAACI, WONCA.
Gary Wong: none declared.
Giovanni Pajno: none declared.
Graham Roberts: grant: UK Food Standards Agency, Natasha Allergy Research Foundation; consulting: DBV; leadership: BSACI.
Hania Szajewska: clinical investigator, advisory board member, consultant, and/or speaker: Danone/Nutricia, Nestlé/Nestlé Nutrition Institute, and Mead Johnson.
Hasan Arshad: grant: Food Standard Agency, Natasha Allergy Research Foundation.
Hugh A Sampson: personal fees: DBV Technologies, N-fold, Siolta Therapeutics; grants: NIAID, NIH.
Jennifer Gerdts: none declared.
Josefine Gradman: none declared.
Kate Grimshaw: personal fees: Abbott, Danone, Nestle.
Kirsten Beyer: personal fees: Aimmune, Bencard, Danone, DBV, HIPP, Hycor, Infectopharm, Jenapharma, Mylan/Meda, Nestle, Novartis, Nutricia Research, Thermofisher; grants: Aimmune, Danone/Nutricia/Milipa, DBV, Hipp, Hycor, Infectopharm.
Lars Poulsen: none declared.
Margitta Worm: personal fees: Abbvie, Aimmune, ALK, DBV, Eli Lilly, Mylan, Novartis, Pfizer, Sanofi, Thermofisher; grants: Mylan and Novartis.
Marcia Podestà: none declared.
Mary-Jane Marchisotto, none declared.
Mika Makela: none declared.
Montserrat Alvaro Lozano; personal fees: from Aimmune, DBV technologies, ALK, LETI Pharma, Merck, Allergy Therapeutics, Stallergenes, Diater, Novartis, Uriach, FAES Pharma, Nestle, Mead-Johnson and Sanofi Genzyme.
Montserrat Fernandez Rivas: personal fees: Aimmune Therapeutics, ALK, Allergy Therapeutics, DBV, Diater, GSK, HAL Allergy, Novartis, Medscape, Reacta Healthcare, Thermofisher Scientific, SPRIM.
Motohiro Ebisawa: personal fees: Mylan.
Nicolette W. de Jong: personal fees: Stallargenes Greer; grants: Friesland Campina.
Pablo Rodríguez del Río; personal fees: Aimmune.
Paul J Turner: personal fees: from Aimmune Therapeutics, DBV Technologies, Allergenis, UK Food Standards Agency; grants: National Institute for Health Research (NIHR)/Imperial Biomedical Research Centre, UK Medical Research Council, UK Food Standards Agency, End Allergies Together, Jon Moulton Charity Trust.
Peter Smith: personal fees Viatris, GSK, AZ, Novartis. Grant GSK, Sanofi.
Philippe Begin: personal fees: ALK, Aralez, Astra-Zeneca, Bausch health, DBV, Food Allergy Canada, Novartis, Pfizer, Sanofi, grants: Canadian Allergy and Immunology Foundation, Canadian Institutes of Health Research, DBV, Fonds de Recherches du Québec, Ministère de l’économie et de l'innovation du Québec, Novartis, Ontario Research Funds, Regeneron, Sanofi.
Rosan Meyer: personal fees: Abbott, Danone, Nestle, Mead Johnson.
Richard Loh: none declared.
Robert Wood: research support: NIH, Aimmune, DBV, Genentech, Novartis, Regeneron, Siolta.
Ronald van Ree: consulting: HAL Allergy BV, Citeq BV, Angany Inc, Reacta Healthcare Ltd, Mission MightMe, AB Enzyme GmBH; lectures: HAL Allergy, ThermoFisher Scientific, ALK; meeting attendance: HAL Allergy BV, ThermoFisher Scientific; leadership: HESI Protein Allergenicity Toxicity Bioinformatics Committee, Peer-review Panel COMPARE allergen database; Stock: Angany Inc.
Rosan Meyer: consulting: Abbott, Nestle; speaker: Nutricia, Danone, Nestle, Mead Johnson, Abbott.
Sabine Schnadt: personal fees: Aimmune.
Stefania Arasi: none declared.
Susanne Halken: speaker and chair fees: Purino Nestlé, Abigo Pharma A/S and GSK.
Susanne Lau: personal fees: DBV, Sanofi-Aventis, Leti, Allergopharma, ALK, Nutricia.
Torsten Zuberbier: personal fees: Abbvie, ALK, Almirall, Bayer, Bencard, Berlin Chemie, Faes, HAL, Leti, L'Oreal, Meda, Menarini, Merck, Novartis, Pfizer, Sanofil, Stallergenes, Takeda, UCB; grant: Henkel.
Ulugbek Nurmatov: none declared.
Supporting information legend
Managing Food Allergy: GA2LEN Guideline 2022 online supplementary marterials covering methods used to compile evidence; justification for each recommendation; studies screened, included and excluded; details of studies included; summary of risk of bias; and reasons why studies screened as full text were excluded.
Acknowledgements
The Global Allergy and Asthma European Network (GA2LEN) provided unrestricted funding to support the completion of the guideline. The funder did not input into the clinical questions, the recommendations or the decision to publish. Task force members volunteered their time. We would like to thank Ingrid van Hofman for her support for the development of the guideline, Dr Alessia Baseggio Conrado for supporting the risk section, Lynn Regent for her work on the education section, the Expert Reviewers (Priya Bansal, Roberto Berni–Canani, Katharina Blumchen, Andreas Bonertz, Melisande Bourgoin-Heck, Ozlem Ceylon, Amandine Divaret-Chauveau, David Fleischer, Maximiliano Gomez, Marion Groetch, Domingo Barber Hernandez, Betina Hjorth, Lydia Collins Hussey, André C. Knulst, Agnes Leung, Douglas Mack, Vera Mahler, Francesca Mori, Leyla Namazova-Baranova, Kati Palosuo, Claudio Alberto Salvador Parisi, Antonio Carlos Pastorino, Odilija Rudzeviciene, Maria Said, Piotr Sawiec, Scott Sicherer, Sakura Sato, Svitlana Zubchenko) and everyone who provided feedback during the public review process. Finally, we would like to dedicate this guideline to Giovanni Pajno who sadly passed always after the document was completed.
Footnotes
Full list of author information is available at the end of the article
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2022.100687.
Contributor Information
Antonella Muraro, Email: muraro@centroallergiealimentari.eu.
Graham Roberts, Email: g.c.roberts@soton.ac.uk.
GA2LEN Food Allergy Guideline Group:
Priya Bansal, Roberto Berni–Canani, Katharina Blumchen, Andreas Bonertz, Melisande Bourgoin-Heck, Ozlem Ceylon, Amandine Divaret-Chauveau, David Fleischer, Maximiliano Gomez, Marion Groetch, Domingo Barber Hernandez, Betina Hjorth, Lydia Collins Hussey, André C. Knulst, Agnes Leung, Douglas Mack, Vera Mahler, Francesca Mori, Leyla Namazova-Baranova, Kati Palosuo, Claudio Alberto Salvador Parisi, Antonio Carlos Pastorino, Odilija Rudzeviciene, Maria Said, Piotr Sawiec, Scott Sicherer, Sakura Sato, and Svitlana Zubchenko
Appendix ASupplementary data
The following is the Supplementary data to this article:
Multimedia component 1
References
- 1.Sicherer S.H., Sampson H.A. Food allergy: a review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J Allergy Clin Immunol. 2018;141(1):41–58. doi: 10.1016/j.jaci.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Bilaver L.A., Chadha A.S., Doshi P., O'Dwyer L., Gupta R.S. Economic burden of food allergy: a systematic review. Ann Allergy Asthma Immunol. 2019;122(4):373–380. doi: 10.1016/j.anai.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 3.Stensgaard A., Bindslev-Jensen C., Nielsen D., Munch M., DunnGalvin A. Quality of life in childhood, adolescence and adult food allergy: patient and parent perspectives. Clin Exp Allergy. 2017;47(4):530–539. doi: 10.1111/cea.12849. [DOI] [PubMed] [Google Scholar]
- 4.Roberts G., Ollert M., Aalberse R., et al. A new framework for the interpretation of IgE sensitization tests. Allergy. 2016;71(11):1540–1551. doi: 10.1111/all.12939. [DOI] [PubMed] [Google Scholar]
- 5.Authority EFS . 2021. Scientific and Technical Guidance for the Preparation and Presentation of an Application for Authorisation of an Infant And/or Follow-On Formula Manufactured from Protein Hydrolysates 2017.https://www.efsa.europa.eu/en/efsajournal/pub/4779 [cited 2021. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Food and Drug administration (FDA) 2020. Infant Formula Guidance Documents & Regulatory Information 2014.https://www.fda.gov/food/guidance-documents-regulatory-information-topic-food-and-dietary-supplements/infant-formula-guidance-documents-regulatory-information [cited 2020. Available from: [Google Scholar]
- 7.American Academy of Pediatrics Committee on Nutrition. Hypoallergenic infant formulas. Pediatrics. 2000;106(2 Pt 1):346–349. [PubMed] [Google Scholar]
- 8.Muraro A., Dreborg S., Halken S., et al. Dietary prevention of allergic diseases in infants and small children: part I: immunologic background and criteria for hypoallergenicity. Pediatr Allergy Immunol. 2004;15:103–111. doi: 10.1046/j.1399-3038.2003.00129.x. [DOI] [PubMed] [Google Scholar]
- 9.Muraro A., Werfel T., Hoffmann-Sommergruber K., et al. EAACI food allergy and anaphylaxis guidelines: diagnosis and management of food allergy. Allergy. 2014;69(8):1008–1025. doi: 10.1111/all.12429. [DOI] [PubMed] [Google Scholar]
- 10.European Union. Provision of Information on Substances or Products Causing Allergies or Intolerances as Listed in Annex II to Regulation (EU) No 1169/2011 of the European Parliament and of the Council on the Provision of Food Information to Consumers (2017/C 428/01). 13th July 2017. (last accessed 14th March 2022).
- 11.AGREE Collaboration Development and validation of an international appraisal instrument for assessing the quality of clinical practice guidelines: the AGREE project. Qual Saf Health Care. 2003;12(1):18–23. doi: 10.1136/qhc.12.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brouwers M.C., Kho M.E., Browman G.P., et al. Agree II: advancing guideline development, reporting and evaluation in health care. CMAJ (Can Med Assoc J) 2010;182(18):E839–E842. doi: 10.1503/cmaj.090449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andrews J., Guyatt G., Oxman A.D., et al. GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol. 2013;66(7):719–725. doi: 10.1016/j.jclinepi.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 14.de Silva D., Rodríguez del Río P., de Jong N.W., et al. Allergen immunotherapy and/or biologicals for IgE-mediated food allergy: systematic review and meta-analysis. Allergy. 2022 doi: 10.1111/all.15211. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Silva D., Singh C., Arasi S., et al. Systematic review of monotherapy with biologicals for children and adults with IgE-mediated food allergy. Clin Transl Allergy. 2020 doi: 10.1002/clt2.12123. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y., Akl E.A., Schunemann H.J. Using systematic reviews in guideline development: the GRADE approach. Res Synth Methods. 2019;10(3):312–329. doi: 10.1002/jrsm.1313. [DOI] [PubMed] [Google Scholar]
- 17.Caffarelli C., Plebani A., Poiesi C., Petroccione T., Spattini A., Cavagni G. Determination of allergenicity to three cow's milk hydrolysates and an amino acid-derived formula in children with cow's milk allergy. Clin Exp Allergy. 2002;32:74–79. doi: 10.1046/j.0022-0477.2001.01262.x. [DOI] [PubMed] [Google Scholar]
- 18.Dupont C., Hol J., Nieuwenhuis E.E. Cow's milk allergy modified by elimination and lactobacilli study group. an extensively hydrolyzed casein-based formula for infants with cow's milk protein allergy: tolerance/hypo-allergenicity and growth catch-up. Br J Nutr. 2015;113(7):1102–1112. doi: 10.1017/S000711451500015X. [DOI] [PubMed] [Google Scholar]
- 19.Niggemann B., Binder C., Dupont C., Hadji S., Arvola T., Isolauri E. Prospective, controlled, multi-center study on the effect of an amino-acid-based formula in infants with cow's milk allergy/intolerance and atopic dermatitis. Pediatr Allergy Immunol. 2001;12(2):78–82. doi: 10.1034/j.1399-3038.2001.012002078.x. [DOI] [PubMed] [Google Scholar]
- 20.Viljanen M., Kuitunen M., Haahtela T., Juntunen-Backman K., Korpela R., Savilahti E. Probiotic effects on faecal inflammatory markers and on faecal IgA in food allergic atopic eczema/dermatitis syndrome infants. Pediatr Allergy Immunol. 2005;16(1):65–71. doi: 10.1111/j.1399-3038.2005.00224.x. [DOI] [PubMed] [Google Scholar]
- 21.Lambert R., Grimshaw K.E.C., Ellis B., Jaitly J., Roberts G. Evidence that eating baked egg or milk influences egg or milk allergy resolution: a systematic review. Clin Exp Allergy. 2017;47(6):829–837. doi: 10.1111/cea.12940. [DOI] [PubMed] [Google Scholar]
- 22.Esmaeilzadeh H., Alyasin S., Haghighat M., Nabavizadeh H., Esmaeilzadeh E., Mosavat F. The effect of baked milk on accelerating unheated cow's milk tolerance: a control randomized clinical trial. PAI. 2018;29(7):747–753. doi: 10.1111/pai.12958. [DOI] [PubMed] [Google Scholar]
- 23.Bohle B., Zwölfer B., Heratizadeh A., et al. Cooking birch pollen–related food: divergent consequences for IgE-and T cell–mediated reactivity in vitro and in vivo. J Allergy Clin Immunol. 2006;118(1):242–249. doi: 10.1016/j.jaci.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 24.ESPGHAN Committee on Nutrition. Agostoni C., Axelsson I., Goulet O., et al. Soy protein infant formulae and follow-on formulae: a commentary by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2006;42(4):352–561. doi: 10.1097/01.mpg.0000189358.38427.cd. [DOI] [PubMed] [Google Scholar]
- 25.Vita D., Passalacqua G., Di Pasquale G., et al. Ass's milk in children with atopic dermatitis and cow's milk allergy: crossover comparison with goat's milk. Pediatr Allergy Immunol. 2007;18(7):594–598. doi: 10.1111/j.1399-3038.2007.00567.x. [DOI] [PubMed] [Google Scholar]
- 26.Barshow S.M., Kulis M.D., Burks A.W., Kim E.H. Mechanisms of oral immunotherapy. Clin Exp Allergy. 2021;51(4):527–535. doi: 10.1111/cea.13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones S.M., Kim E.H., Nadeau K.C., et al. Efficacy and safety of oral immunotherapy in children aged 1–3 years with peanut allergy (the Immune Tolerance Network IMPACT trial): a randomised placebo-controlled study. Lancet. 2022;399(10322):359–371. doi: 10.1016/S0140-6736(21)02390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soller L., Abrams E.M., Carr S., et al. First real-world effectiveness analysis of preschool peanut oral immunotherapy. J Allergy Clin Immunol Pract. 2021;9:1349–1356. doi: 10.1016/j.jaip.2020.10.045. [DOI] [PubMed] [Google Scholar]
- 29.Schoemaker A.A., Sprikkelman A.B., Grimshaw K.E., et al. Incidence and natural history of challenge-proven cow's milk allergy in European children–EuroPrevall birth cohort. Allergy. 2015;70:963–972. doi: 10.1111/all.12630. [DOI] [PubMed] [Google Scholar]
- 30.Xepapadaki P., Fiocchi A., Grabenhenrich L., et al. Incidence and natural history of hen's egg allergy in the first 2 years of life—the EuroPrevall birth cohort study. Allergy. 2016;71:350–357. doi: 10.1111/all.12801. [DOI] [PubMed] [Google Scholar]
- 31.Muraro A., Agache I., Clark A., et al. EAACI food allergy and anaphylaxis guidelines: managing patients with food allergy in the community. Allergy. 2014;69(8):1046–1057. doi: 10.1111/all.12441. [DOI] [PubMed] [Google Scholar]
- 32.Chinthrajah R.S., Cao S., Dunham T., et al. Oral immunotherapy for peanut allergy: the pro argument. World Allergy Organization Journal. 2020;13(8) doi: 10.1016/j.waojou.2020.100455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leonard S.A., Laubach S., Wang J. Integrating oral immunotherapy into clinical practice. J Allergy Clin Immunol. 2021;147(1):1–3. doi: 10.1016/j.jaci.2020.11.011. [DOI] [PubMed] [Google Scholar]
- 34.Muraro A., Worm M., Alviani C., et al. EAACI guideline: anaphylaxis (2021 update) Allergy. 2022;77:357–377. doi: 10.1111/all.15032. [DOI] [PubMed] [Google Scholar]
- 35.Greenhawt M., Marsh R., Gilbert H., Sicherer S., DunnGalvin A., Matlock D. Understanding caregiver goals, benefits, and acceptable risks of peanut allergy therapies. Ann Allergy Asthma Immunol. 2018;121(5):575–579. doi: 10.1016/j.anai.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 36.Hourihane J.O., Beyer K., Abbas A., et al. Efficacy and safety of oral immunotherapy with AR101 in European children with a peanut allergy (ARTEMIS): a multicentre, double-blind, randomised, placebo-controlled phase 3 trial. Lancet Child Adolesc Health. 2020;4(10):728–739. doi: 10.1016/S2352-4642(20)30234-0. [DOI] [PubMed] [Google Scholar]
- 37.Itoh-Nagato N., Inoue Y., Nagao M., Fujisawa T., Shimojo N., Iwata T. Desensitization to a whole egg by rush oral immunotherapy improves the quality of life of guardians: a multicenter, randomized, parallel-group, delayed-start design study. Allergol Int. 2018;67:209–216. doi: 10.1016/j.alit.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 38.van Erp F.C., Boot J., Knulst A.C., Pasmans S.G., van der Ent C.K., Meijer Y. Reintroduction failure after negative peanut challenges in children. Pediatr Allergy Immunol. 2014;25(6):580–585. doi: 10.1111/pai.12266. [DOI] [PubMed] [Google Scholar]
- 39.van Der Valk J.P., Van Wijk R.G., Vergouwe Y., De Jong N.W. Failure of introduction of food allergens after negative oral food challenge tests in children. Eur J Pediatr. 2015;174(8):1093–1099. doi: 10.1007/s00431-015-2504-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bégin P., Chan E.S., Kim H., et al. CSACI guidelines for the ethical, evidence-based and patient-oriented clinical practice of oral immunotherapy in IgE-mediated food allergy. Allergy Asthma Clin Immunol. 2020;16(1):1–45. doi: 10.1186/s13223-020-0413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pajno G.B., Fernandez-Rivas M., Arasi S., et al. EAACI Guidelines on allergen immunotherapy: IgE-mediated food allergy. Allergy. 2018;73(4):799–815. doi: 10.1111/all.13319. [DOI] [PubMed] [Google Scholar]
- 42.Chung K.F., Wenzel S.E., Brozek J.L., et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–373. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 43.Rodríguez del Río P., Alvarez-Perea A., Blumchen K., et al. Food immunotherapy practice: nation differences across Europe, the FIND project. Allergy. 2021 doi: 10.1111/all.15016. in press. [DOI] [PubMed] [Google Scholar]
- 44.Sampson H.A., Leung D.Y., Burks A.W., et al. A phase II, randomized, double-blind, parallel-group, placebo-controlled oral food challenge trial of Xolair (omalizumab) in peanut allergy. J Allergy Clin Immunol. 2011;127(5):1309–1310. doi: 10.1016/j.jaci.2011.01.051. [DOI] [PubMed] [Google Scholar]
- 45.Chinthrajah S., Cao S., Liu C., et al. Phase 2a randomized, placebo-controlled study of anti–IL-33 in peanut allergy. JCI insight. 2019;4(22) doi: 10.1172/jci.insight.131347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fiocchi A., Artesani M.C., Riccardi C., et al. Impact of omalizumab on food allergy in patients treated for asthma: a real-life study. J Allergy Clin Immunol Pract. 2019;7(6):1901–1909. doi: 10.1016/j.jaip.2019.01.023. e5. [DOI] [PubMed] [Google Scholar]
- 47.Kastner M., Harada L., Waserman S. Gaps in anaphylaxis management at the level of physicians, patients, and the community: a systematic review of the literature. Allergy. 2010;65(4):435–444. doi: 10.1111/j.1398-9995.2009.02294.x. [DOI] [PubMed] [Google Scholar]
- 48.Brockow K., Schallmayer S., Beyer K., et al. Effects of a structured educational intervention on knowledge and emergency management in patients at risk for anaphylaxis. Allergy. 2015;70(2):227–235. doi: 10.1111/all.12548. [DOI] [PubMed] [Google Scholar]
- 49.Shemesh E., D'Urso C., Knight C., et al. Food-allergic adolescents at risk for anaphylaxis: a randomized controlled study of supervised injection to improve comfort with epinephrine self-injection. J Allergy Clin Immunol Pract. 2017;5(2):391–397. doi: 10.1016/j.jaip.2016.12.016. e4. [DOI] [PubMed] [Google Scholar]
- 50.Boyle R.J., Umasunthar T., Smith J.G., et al. A brief psychological intervention for mothers of children with food allergy can change risk perception and reduce anxiety: outcomes of a randomized controlled trial. Clin Exp Allergy. 2017;47(10):1309–1317. doi: 10.1111/cea.12981. [DOI] [PubMed] [Google Scholar]
- 51.Howe L.C., Leibowitz K.A., Perry M.A., et al. Changing patient mindsets about non-life-threatening symptoms during oral immunotherapy: a randomized clinical trial. J Allergy Clin Immunol Pract. 2019;7(5):1550–1559. doi: 10.1016/j.jaip.2019.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Viana L.V., Gomes M.B., Zajdenverg L., Pavin E.J., Azevedo M.J., Brazilian Type 1 Diabetes Study Group Interventions to improve patients' compliance with therapies aimed at lowering glycated hemoglobin (HbA1c) in type 1 diabetes: systematic review and meta-analyses of randomized controlled clinical trials of psychological, telecare, and educational interventions. Trials. 2016;17:94. doi: 10.1186/s13063-016-1207-6. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mahoney B., Walklet E., Bradley E., O'Hickey S. Improving adrenaline autoinjector adherence: a psychologically informed training for healthcare professionals. Immun Inflamm Dis. 2019;7(3):214–228. doi: 10.1002/iid3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roberts G., Vazquez-Ortiz M., Knibb R., et al. EAACI Guidelines on the effective transition of adolescents and young adults with allergy and asthma. Allergy. 2020;75(11):2734–2752. doi: 10.1111/all.14459. [DOI] [PubMed] [Google Scholar]
- 55.Turner P.J., Baumert J.L., Beyer K., et al. Can we identify patients at risk of life-threatening allergic reactions to food? Allergy. 2016;71(9):1241–1255. doi: 10.1111/all.12924. [DOI] [PubMed] [Google Scholar]
- 56.Blaiss M.S., Steven G.C., Bender B., Bukstein D.A., Meltzer E.O., Winders T. Shared decision making for the allergist. Ann Allergy Asthma Immunol. 2019;122:463–470. doi: 10.1016/j.anai.2018.08.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multimedia component 1
Data Availability Statement
No new data generated as part of this manuscript.