Abstract
Background
Leukocyte telomere length (LTL) is a biomarker that is affected by older age, psychosocial stress, and medical comorbidities. Despite the relevance of these factors to obstetric practice, little is known about LTL in pregnancy. Our study explored longitudinal LTL dynamics in pregnant and non-pregnant people.
Objective
This pilot study compares changes in LTL between pregnant and non-pregnant people over time, explores potential correlations between LTL and mental health measures, and investigates associations between short first-trimester LTL and adverse pregnancy outcomes.
Study design
This was a prospective pilot cohort study of nulliparous pregnant and non-pregnant people between ages 18 and 50 who presented for care at a single institution from January to November 2020. Pregnant people were enrolled between 10 and 14 weeks gestation. Participants had two blood samples drawn for LTL; the first on the day of enrollment and the second on postpartum day 1 (pregnant cohort) or 7 months later (non-pregnant cohort). LTL was measured using quantitative PCR. The primary outcome was the difference between pregnant and non-pregnant people in LTL change between the two timepoints (basepair difference per 30-day period). Secondary outcomes included differences in responses to the Patient Health Questionnaire-9 (PHQ-9) and a survey about stress related to COVID-19. Differences in LTL were tested using t-tests and linear regression models, both crude and adjusted for age. A subgroup analysis was conducted within the pregnant cohort to examine whether shorter first-trimester LTL was associated with adverse pregnancy outcomes. We conducted t-tests to compare LTL between people with and without each categorical outcome and computed Pearson correlation coefficients between LTL and continuous outcomes such as gestational age at delivery.
Results
46 pregnant and 30 non-pregnant people were enrolled; 44 pregnant and 18 non-pregnant people completed all LTL assessments. There were no between-group differences in LTL change (−4.2 ± 22.2 bp per 30 days pregnant versus −6.4 ± 11.2 bp per 30 days non-pregnant, adjusted beta 2.1, 95% CI -9.0-13.2, p = 0.60). The prevalence of depression and pandemic-related stress were both low overall. The two groups did not differ in PHQ-9 scores, and no correlations were significant between LTL and PHQ-9 scores. Among the 44 pregnant people, shorter first-trimester LTL was significantly correlated with earlier gestational age at delivery (r = 0.35, p = 0.02).
Conclusion
In this exploratory pilot cohort of reproductive-aged people with low levels of psychological stress, we described baseline changes in LTL over time in pregnant and non-pregnant participants. We found a correlation between shorter first-trimester LTL and earlier gestational age at delivery, which warrants further investigation in a larger cohort.
Keywords: Anxiety, Cellular aging, Coronavirus, Depression, Mental health, Pandemic, Pregnancy, SARS-COV-2, Stress, Telomeres
Highlights
-
•
Shorter leukocyte telomeres associated with earlier gestational age at delivery.
1. Introduction
Short telomere length is a biomarker of emerging interest in perinatal biology due to associations with psychological stress and potential as a transgenerational stress marker (Humphreys et al., 2020; Verner et al., 2021; Epel et al., 2004; Entringer et al., 2018). Despite this potential, very little is known about how pregnancy affects telomere dynamics from which to guide research.
Telomeres are repetitive non-coding deoxyribonucleic acid (DNA) sequences that cap chromosome ends to protect them from shortening over time (Notterman and Schneper, 2020; Vaiserman and Krasnienkov, 2021; Shalev et al., 2013). Although telomeres are present in many cells, most stress-focused research has utilized leukocyte telomere length (LTL) (Demanelis et al., 2020). LTLs normally shorten by 20–30 basepairs (bp) annually in non-pregnant adults (Müezzinler et al., 2013), but accelerated shortening can occur via oxidative stress mechanisms (Fouquerel et al., 2019). Shorter LTLs have been associated with downstream increases in morbidity and mortality in non-pregnant adults, and LTL shortening itself can be accelerated by factors including psychosocial stress and mental health conditions. In fact, maternal psychosocial stress during pregnancy has been linked to shorter LTL in children (Entringer et al., 2013, 2018).
Given these associations, and the fact that pregnancy itself is a state of physical, biological, and emotional stress, it is important to elucidate LTL dynamics in pregnancy before applying this biomarker to perinatal research. It is unknown whether pregnancy affects the natural rate of LTL shortening, and if early pregnancy LTL is associated with adverse outcomes. Since telomere changes over a period as little as one year have been described outside of pregnancy (Puterman et al., 2015; Ridout et al., 2019), and short term telomere changes have also been associated with changes in brain structure (Puhlmann et al., 2019), it is possible that changes in maternal LTL over as short as a single term gestation could occur and be relevant to perinatal research in this area.
Therefore, in this pilot study, we compared LTL change over time in pregnant and non-pregnant individuals, examined correlations of LTL with stress measures, and explored whether maternal LTL in the first trimester was associated with pregnancy complications such as gestational diabetes, hypertensive disorders, or spontaneous preterm birth. We hypothesized that pregnancy would accelerate maternal LTL shortening, that LTL would correlate with psychosocial stress, and that shorter first trimester LTL could have utility as a predictive biomarker for adverse pregnancy outcomes.
2. Materials and methods
2.1. Study design
This was a prospective pilot cohort study of nulliparous people between ages 18 and 50 years who presented for routine obstetric or gynecologic care at a single institution from January 2020 to November 2020. The study was restricted to nulliparas given prior cross-sectional research demonstrating an association with increased parity and shorter LTL (Pollack et al., 2018). Nulliparity was defined as no prior pregnancy beyond 13 weeks gestation. Pregnant patients were included if they were between 10 weeks 0 days and 14 weeks 0 days by best estimate (The American College of Obstetricians and Gynecologists. Committee Opinion, 2017) with a viable singleton pregnancy. Exclusion criteria were determined based on prior literature (Lin et al., 2016; Valdes et al., 2005; Ma et al., 2011). Eligibility was initially assessed using the electronic medical record by screening all outpatient Obstetrics and Gynecology clinics. Only Gynecology clinic visits for well woman or routine indications were included; people presenting for a procedure were not eligible. All eligible patients who were interested in participating in research were approached during their visit and interviewed to confirm they did not meet exclusion criteria. In both cohorts, patients were excluded if they were current smokers, had a communicable blood-borne disease, were prescribed therapeutic anticoagulation, had a history of solid organ or hematologic malignancy, showed signs of illness or injury, or were planning to relocate. For the pregnant cohort, people were also excluded if they utilized egg donation (Keefe et al., 2007), had a lethal fetal anomaly, or planned pregnancy termination. For the non-pregnant cohort, people were excluded if they were planning pregnancy within the next year. Convenience sampling via the electronic health record was utilized for screening, stratifying by age < or ≥35 years. Age stratification using the cutoff for advanced maternal age was done due to the known contribution of chronological age to telomere shortening (Müezzinler et al., 2013; Blackburn and Epel, 2017).
This study began enrollment immediately before the emergence of the COVID-19 pandemic; thus, enrollment was paused between March and May 2020. It was approved by the Stanford Institutional Review Board (IRB Protocol 44072) and all patients signed informed consent.
2.2. Telomere sample acquisition and processing
The primary outcome was the difference between pregnant and non-pregnant people in the change of LTL over time. All participants had two blood samples drawn; the first was obtained on the day of enrollment (Time 1). For the pregnant cohort, the second draw was done postpartum day 1 (Time 2). For the non-pregnant cohort, the second draw was arranged approximately 7 months after the first (Time 2) to match the timeframe of the pregnant cohort. Due to the unforeseen circumstances that evolved due to the COVID-19 pandemic, which arose after this study began enrollment, additional flexibility was accommodated in the timing of the second draw. To account for differences in the timing of the second draw, the primary outcome was analyzed as the change in LTL per 30 day period between draws.
For all samples, a total of 2–4 cc of whole blood was drawn in EDTA tubes. Within 4 h, samples were aliquoted into 7 tubes (Sardstedt SRS-72-694-006) of 300 μL each and frozen at −80 °C until all samples were ready for simultaneous DNA extraction and analysis. Genomic DNA was extracted using the QIAamp protocol for cultured cells (Qiagen, 2016). LTL was then measured according to previously published protocols (Entringer et al., 2013; Lin et al., 2010; Cawthon, 2002; Wolkowitz et al., 2011; Panelli et al., 2022). As has been previously reported, telomere (“T”) and single copy gene (human beta globin, “S”) lengths were measured via quantitative polymerase chain reaction (qPCR) using a Roche LC480 real-time PCR machine (Roche Diagnostics Corporation, Indianapolis, IN). Samples were run in triplicate on 384-well assay plates in a Roche LightCycler 480. Average concentrations of T and S were used to calculate T/S ratios after Dixon's Q-test, and each sample was measured twice. When one sample's duplicate T/S values differed by greater than 7%, the sample was run a third time and the two closest values were averaged to give the final result. Tubes containing the same reference DNA (human genomic DNA from buffy coat, Sigma-Aldrich, Cat#11691112001) were included in each run to adjust for batch effect. The mean of 8 adjustment factors from 8 DNA samples were applied to convert the raw T/S ratios to adjusted T/S ratios which were converted to bp using the following formula: bp = 3274 + 2413*(T/S) (Entringer et al., 2013; NHANES). This formula was developed in the same lab using the same protocol as the present study, though of note was not validated for this specific cohort; this approach has previously been described.
2.3. Secondary outcomes
Secondary outcomes included differences between pregnant and non-pregnant groups in responses to the Patient Health Questionnaire-9 (PHQ-9) and a survey focusing on the COVID-19 pandemic. The PHQ-9 is a validated screening instrument for depression scored from 0 to 27; a score of ≥10 is considered a positive screen with a sensitivity of 88% and specificity of 85% (Levis et al., 2019). The PHQ-9 was used instead of the Edinburgh Postpartum Depression Scale (EPDS) due to inclusion of non-pregnant people. All participants completed the PHQ-9 twice: once on the day of enrollment and again at the time of the second blood draw.
Given the timing of enrollment (i.e., during the emergence of the pandemic), there were no validated survey tools yet available to assess pandemic-related stress. Thus, we adapted the COVID-19 survey that was administered in this study from a longer survey that had been prepared, piloted, and administered prior to use in the current study (King et al., 2020). The survey included 14 questions assessing whether the patient or their household member contracted COVID-19, and questions exploring objective adversity (e.g. financial impacts) and subjective adversity (e.g. impact of COVID-19 on stress level). Survey responses were measured on Likert scales as well as with multiple selection and free text options.
2.4. Variables
Demographic characteristics were obtained via chart abstraction, including age, body mass index, comorbidities, and social determinants such as race/ethnicity, highest level of education, and insurance type. Race/ethnicity and education level were verbally confirmed by the participants after enrollment. The American College of Obstetricians and Gynecologists has identified high-risk comorbidities that warrant treatment with low-dose aspirin during pregnancy (ACOG. Committee, 2018). Given this is a best-practice guideline, and a widely accepted list of clinically relevant comorbidities, we used this list to compile relevant comorbidities for this study. These included: neurologic disease (epilepsy, multiple sclerosis), active psychiatric disease, chronic hypertension, type 1 or 2 diabetes, asthma, thyroid disease, or autoimmune disorder. Active psychiatric disease was defined as depression receiving medication, anxiety, post-traumatic stress disorder, schizophrenia, or bipolar disorder.
2.5. Subgroup analysis
For pregnant participants, we planned to conduct an exploratory subgroup analysis to examine the relation, if any, between first-trimester LTL and adverse pregnancy outcomes. For this, the exposure was first-trimester maternal LTL (Time 1) and the outcomes were perinatal complications postulated to be inflammatory-mediated (Osborne and Monk, 2013) including gestational diabetes, hypertensive disorders (including gestational hypertension and preeclampsia with and without severe features), gestational age at delivery, and spontaneous preterm birth <37 weeks.
2.6. Statistical analysis
A sample size calculation was conducted using limited available data for serial LTL measurements in non-pregnant adults. For comparability with published literature, we powered our study in bp using a standard conversion from T/S ratio. (NHANES) While this conversion was not developed directly from this cohort, it has been previously extrapolated to other cohorts (Entringer et al., 2013; Pollack et al., 2018). An effect size of 50% difference between groups in the change of LTL over time was selected, as this was deemed to be clinically meaningful (Ridout et al., 2019). To detect a difference of at least 50% in LTL change in pregnancy from a baseline shortening of 25 bp per year in non-pregnant individuals (Müezzinler et al., 2013; Chen et al., 2011), the study would require 42 patients per group (total N = 84), assuming α = 0.05, power = 0.8, a two-sided t-test, and a common pooled standard deviation of 20 bp. This would translate to an additional change of 12.5 basepairs in either direction. Accounting for a 25% loss to follow up in the non-pregnant cohort and 10% in the pregnant cohort, we aimed to recruit 53 non-pregnant and 46 pregnant people.
We restricted the primary analysis to those who had completion of all LTL measurements. We reported demographic characteristics descriptively without statistical comparisons due to small sample size. We evaluated within-person LTL change over time between pregnant and non-pregnant subjects first using paired t-tests, then compared each participant's LTL change using multivariable linear regression adjusting for maternal age given prior research suggesting the role chronologic age plays in telomere shortening (Epel et al., 2004; Blackburn and Epel, 2017; Gotlib et al., 2015). Age adjustment was done despite age-matching due to differential loss to follow-up, as described below. Regression to the mean was assessed, as this has been reported to be a significant biasing effect in other studies examining repeated LTLs within one individual (Barnett et al., 2005; Bateson et al., 2019).
We conducted secondary analyses using psychosocial measures. First, we reported PHQ-9 scores for pregnant and non-pregnant patients using descriptive statistics. Next, the association between PHQ-9 score at Time 1, Time 2, and PHQ score change (independent variables) and LTL change in bp/30 day period (dependent variable) were examined using Spearman correlation coefficients, given the non-normal distribution of PHQ-9 scores.
For the subgroup analysis among pregnant participants, we used two-sample t-tests with unequal variances to compare LTL between participants with and without each adverse pregnancy outcome as LTL were normally distributed. We computed Pearson correlation coefficients between LTL and continuous outcomes such as gestational age at delivery and neonatal birthweight. Statistical significance was set to a two-tailed alpha <0.05. Missing data were not imputed. Statistical analysis was performed using Stata IC 15.1 (StataCorp, College Station, TX).
3. RESULTS
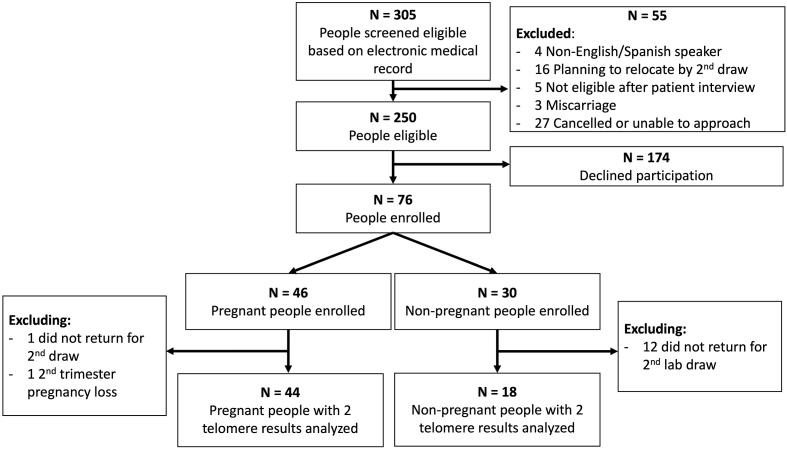
305 patients were potentially eligible based on electronic medical record screening of clinic schedules. Of those approached and interviewed for eligibility, 76 enrolled; 46 were pregnant and 30 were non-pregnant (Fig. 1). Given the exploratory nature of this study, enrollment for the non-pregnant cohort concluded when the pregnant cohort reached the target of 46 participants due to unforeseen enrollment delays and challenges encountered during the pandemic lockdown. Specifically, phlebotomy visits scheduled solely for the purpose of research were not allowed for several months which disproportionately affected the non-pregnant participants, and many non-pregnant individuals had unplanned relocations or changes in medical care during the pandemic. A total of 44 pregnant and 18 non-pregnant people completed all longitudinal biologic assessments and were included in the primary analysis (Fig. 1).
Fig. 1.
Title. Study inclusion flowchart.
The pregnant and non-pregnant cohorts were similar in terms of demographic characteristics (Table 1). Importantly, there was no difference in mean age at enrollment (31.3 ± 3.3 years pregnant versus 31.8 ± 7.1 non-pregnant, p = 0.72).
Table 1.
Demographics among pregnant and non-pregnant participants, N = 62.
| Characteristic | Non-pregnanta N = 18 |
Pregnant N = 44 |
|---|---|---|
| Mean maternal age at enrollment (years) | 31.8 ± 7.1 | 31.3 ± 3.3 |
| Mean BMI at enrollment (kg/m2) | 24.8 ± 4.0 | 22.8 ± 3.8 |
| Race | ||
| Asian or Pacific Islander | 7 (38.9) | 18 (40.9) |
| Black | 0 | 0 |
| White | 11 (61.1) | 21 (47.7) |
| Other or unknown | 0 | 5 ( ) |
| Hispanic ethnicity | 0 | 5 (11.4) |
| Highest level of education | ||
| Completed high school | 0 | 3 (6.8) |
| Completed college | 6 (33.3) | 9 (20.5) |
| Pursuing or completed higher degree | 10 (55.6) | 30 (68.2) |
| Unknown or missing | 2 (11.1) | 2 (4.6) |
| Comorbidity at time of enrollment (any) | 6 (33.3) | 24 (54.6) |
| Neurologic (e.g. epilepsy, multiple sclerosis) | 0 | 1 (2.3) |
| Psychiatric (e.g. depression, anxiety) | 1 (5.6) | 9 (20.5) |
| Chronic hypertension | 1 (5.6) | 0 |
| Asthma | 0 | 4 (9.1) |
| Thyroid (hypo or hyper) | 0 | 6 (13.6) |
| Type 1 or 2 Diabetes | 0 | 1 (2.3) |
| Autoimmune (e.g. lupus) | 0 | 1 (2.3) |
| Other | 4 (22.2) | 12 (27.3) |
| Spontaneous conception | – | 37 (84.1) |
| Enrolled prior to COVID-19 lockdown (March 17, 2020) | 8 (44.4) | 24 (54.6) |
| Median PHQ-9 score at enrollment (Time 1) | 1 (0,3) | 2 (1,4) |
| Median PHQ-9 score Time 2 | 1 (0,2) | 2 (1,4) |
| PHQ-9 score increase between two timepointsb | 6 (35.3) | 14 (38.9) |
Data shown as number (column percent) for categorical variables and mean ± standard deviation for continuous.
Denominator is N = 53 people with PHQ-9 scores from both Time 1 and Time 2.
For our primary outcome, we did not identify a significant difference in LTL change between groups over the course of the study. The non-pregnant cohort had LTL shortening of −6.4 ± 11.2 bp per 30 day period whereas the pregnant cohort had shortening of −4.2 ± 22.2 bp per 30 day period (adjusted beta coefficient 2.1, 95% CI -9.0 - 13.2, Table 2). The mean duration between Time 1 and Time 2 was significantly longer for the non-pregnant cohort (242.0 ± 50.7 non-pregnant versus 195.6 ± 11.4 days pregnant, p = 0.001). Of note, in contrast to the power calculation utilizing 20 bp for expected standard deviation, the overall sample (N = 62) LTL difference over time was −35.7 ± 130.7 bp. Regression to the mean was not observed (Supplemental Fig. 1) Supplemental Table 1 shows results in T/S ratio format.
Table 2.
Leukocyte telomere length over time compared between pregnant and non-pregnant people with serial leukocyte telomere length (LTL) measurements in basepairs (bp), N = 62.
| Non-pregnant Mean ± SD (Referent) N = 18 |
Pregnant Mean ± SD N = 44 |
P-valuea | Crude beta, (95% CI)b | Adjusted beta, (95% CI)c | |
|---|---|---|---|---|---|
| LTL change rate (bp per 30 days) | −6.4 ± 11.2 | −4.2 ± 22.2 | 0.60 | 2.3 (−8.8, 13.3) | 2.1 (−9.0, 13.2) |
| Mean days between samples | 242.0 ± 50.7 | 195.6 ± 11.4 | 0.001 | – | – |
Maternal leukocyte telomere length was normally distributed in both groups, so two-sample t-test was used for all comparisons.
Crude linear regression with pregnant versus non-pregnant as independent variable and telomere length, difference, or change rate as dependent variables.
Adjusted for age at enrollment.
There were no differences between groups in PHQ-9 scores (Table 1). One third of all participants had an increase in their PHQ-9 score over the study period. There were no associations between LTL and PHQ-9 score at Time 1 (r = 0.01, p = 0.97), Time 2 (r = 0.11, p = 0.43), nor between LTL change and PHQ-9 score difference (r = −0.12, p = 0.38). On the COVID-19 survey, the majority of respondents reported moderate or significant worsening of stress related to the pandemic (61.8% pregnant versus 71.4% non-pregnant, Supplemental Table 2).
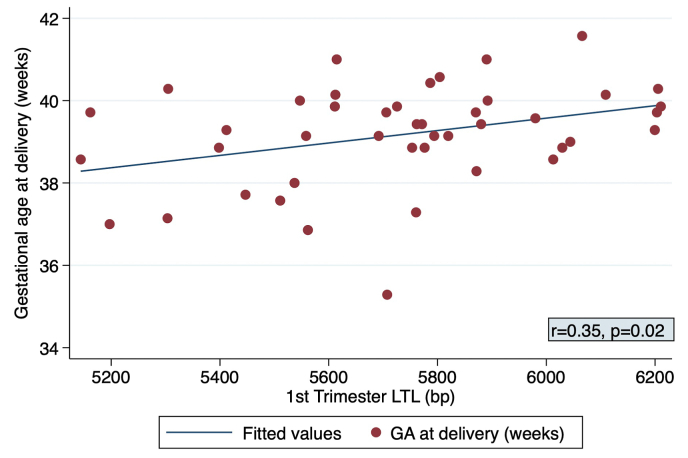
In the subgroup analysis of the 44 pregnant people, shorter first-trimester maternal LTL was significantly correlated with earlier gestational age at delivery (r = 0.35, p = 0.02, Fig. 2). This association persisted in linear regression modeling after adjusting for maternal age, using LTL in 200 bp increments (beta 0.30, 95% CI 0.04–0.56, p = 0.03). Additionally, Table 3 demonstrates that there were non-significant crude trends for shorter first-trimester LTL in people who developed hypertensive disorders (5,648.9 ± 382.8 bp with versus 5,748.9 ± 250.3 bp without, p = 0.47) and spontaneous preterm birth (5,488.8 ± 262.8 bp preterm versus 5,750.7 ± 279.5 bp term, p = 0.22).
Fig. 2.
Title. Correlation between first trimester leukocyte telomere length and gestational age at delivery among pregnant participants, N = 44.
Table 3.
Categorical maternal and obstetric outcomes by first trimester maternal leukocyte telomere length (LTL) in pregnant cohort, N = 44.
| Outcome | Outcome present Total N = 44 N (%) |
First trimester LTL by presence or absence of each outcome Mean ± SD |
P-valuea | |
|---|---|---|---|---|
| Absent | Present | |||
| Gestational diabetes (any) | 6 (13.6) | 5,730.2 ± 288.8 | 5,749.3 ± 270.8 | 0.88 |
| Hypertensive disorder of pregnancy | 9 (20.5) | 5,748.9 ± 250.3 | 5,648.9 ± 382.8 | 0.47 |
| Gestational hypertension | 4 (9.1) | 5,724.0 ± 286.4 | 5,820.7 ± 271.3 | 0.54 |
| Preeclampsia with or without severe features | 5 (11.4) | 5,711.4 ± 429.7 | 5,761.2.4 ± 253.4 | 0.27 |
| Spontaneous preterm birth <37 weeks | 3 (6.5) | 5,750.7 ± 279.5 | 5,488.8 ± 262.8 | 0.22 |
Data for maternal first trimester LTL were normally distributed so student's t-test was used.
4. Discussion
4.1. Principal findings
In this exploratory, prospective, longitudinal pilot study, we describe LTL dynamics in pregnant and non-pregnant individuals with a low prevalence of psychosocial stress. We did not find any differences between pregnant and non-pregnant groups in LTL change per 30 day period. It should be noted that we were inadequately powered to answer this question, as we were unable to attain target follow-up in the non-pregnant cohort. Similarly, we did not detect any associations between LTL and psychosocial factors, including depressive symptoms and COVID-specific stress. In the pregnant cohort, shorter first trimester maternal LTL may be associated with earlier gestational age at delivery, a finding that warrants further evaluation in a larger sample. These pilot data can be used to more appropriately power future studies and inform ongoing research investigating LTL as a predictive biomarker in pregnancy.
4.2. Results in the context of available literature
Although limited by small sample size in our non-pregnant cohort, we did not identify any trends towards group differences in LTL change per 30 day period. First, it is noteworthy that our non-pregnant cohort had a rate of decrease that would correspond to 72 basepairs over a year, which is greater than the 20–30 basepairs per year that has been reported in the literature (Demanelis et al., 2020; Müezzinler et al., 2013). We interpret this with caution, given that extrapolating the monthly rate to a 12-month period may introduce bias; however, it is still notable that this group, which was followed for an average of 242 days, had double the change in basepairs compared to what has been previously reported. While it is possible that pandemic-related stressors contributed to this, our cohort overall had a low level of pandemic-related stress. It is possible that this result is related to our small sample size or to the fact that this is a reproductive-aged cohort, whereas most studies on LTL dynamics include a wider age range because telomere shortening may slow with older compared to younger age (Vaiserman and Krasnienkov, 2021).
While other data are lacking regarding the effect of pregnancy on LTL to contextualize our results, one cross-sectional study reported shorter LTL measurements in parous compared to nulliparous people which might suggest that pregnancy is associated with shorter LTL (Pollack et al., 2018). However, the timeline between LTL sampling and prior deliveries in that study was unclear due to its cross-sectional design; as such, it is difficult to elucidate whether pregnancy or the postpartum state was contributing to shorter LTL. Longitudinal follow up of our cohort may provide further insight into this question.
There is biologic plausibility for why maternal LTL might be protected from stressors and not change dramatically over time. This is particularly important given some studies have demonstrated it is possible to identify differences in telomere shortening rates in as little as 1 year outside of pregnancy, advocating against the notion that a term gestation would be too short a timeframe to see major LTL changes (Puterman et al., 2015; Ridout et al., 2019). It is possible that the immunotolerant state of pregnancy (Oreshkova et al., 2012) might create a resilient environment that protects LTL from degradation that would normally occur via stress-related pathways (Fouquerel et al., 2019; Von Zglinicki, 2002). In fact, one study from Rwanda which might provide support for this hypothesis did not find correlations between short LTL and pro-inflammatory biomarkers such as CRP in pregnancy (Nsereko et al., 2020). Replicating our results in a larger study and determining how LTL fits into the larger biochemical mileu of pregnancy (Aghaeepour et al., 2017) is are important next steps to aid in interpreting our results.
While few investigators have examined the effects of pregnancy on maternal LTL, even fewer researchers have assessed the predictive utility of LTL for perinatal outcomes (Panelli and Bianco, 2021). What has been studied more extensively is the role of telomeres in the placenta, amnion, and cord blood. Interestingly, these telomeres may be involved in the processes controlling the onset of parturition (Polettini and da Silva, 2020). Mouse models have shown that the proportion of short telomeres in placental and fetal membrane tissues increases prior to the onset of labor (Phillippe et al., 2019). It is not known whether placental or fetal membrane telomeres are correlated with maternal LTL. If they are related, this could provide an explanation for why shorter maternal LTL is associated with earlier delivery.
Finally, despite prior research reporting associations of increased stress and depression with shorter LTL (Humphreys et al., 2020; Epel et al., 2004), we did not identify similar associations in this cohort. Whether this is related to our sample size, low baseline prevalence of mental health disorders, lack of direct impact of COVID-19 on participants, or the presence of a more nuanced underlying relation remains to be seen.
4.3. Clinical implications of results
Mental health has deteriorated for many people over the course of the pandemic (Ettman et al., 2020; Ayaz et al., 2020; Luo et al., 2020). In our study, we saw increases in PHQ-9 scores in both pregnant and non-pregnant cohorts, and the majority of participants reported worsening of stress due to COVID-19. Notably, this was in a cohort with an overall low background prevalence of depression or pandemic-related stress. These results highlight the importance of mental health screening not only in the postpartum period, as is commonly done, but also earlier in pregnancy, as others have advocated (Bunevicius et al., 2009; Murray and Cox, 1990). For gynecologic patients, our results may facilitate consideration of routine mental health screening for well woman visits.
4.4. Implications for future research
The association we identified between shorter first trimester LTL and earlier gestational age at delivery raises questions that warrant further investigation. Whether this association translates to an increased risk of spontaneous preterm birth with shorter first trimester LTL remains unanswered, given that the majority of our patients delivered at term. Although we did identify shorter mean first trimester LTL among those with spontaneous preterm birth, we were not specifically powered for this. A future case-control study may be appropriate to address this question.
Further, our sample size was originally computed based on sparse available data for what normal LTL changes might be in reproductive-aged people. We did not conduct a post-hoc power calculation given caveats to this approach (Goodman and Berlin, 1994). Although the variability in LTL in our control group (84 basepairs) was greater than what we had originally estimated (20 basepairs), and the emergence of the COVID-19 pandemic affected our loss to follow up rate, our pilot data can be used in determining power for future studies examining maternal LTL. For example, using our data, replicating the power analysis using our crude shortening of 55 bp with standard deviation of 84 in the referent group, same effect size, alpha, and beta, N = 153 would actually be needed in each group (total N = 306).
4.5. Strengths and limitations
Our results must be interpreted within the context of our limitations. Given the impact of the pandemic on enrollment and retainment, we did not attain our target sample size in the control group, which decreased our statistical power. Therefore, our negative results may be due to Type 2 error. The pandemic also affected the mean duration between LTL samples; the non-pregnant group had a significantly longer interval between samples due to research limitations in 2020, which may have resulted in a greater crude LTL difference. To account for this, bp rate change per 30 day period was added as a primary outcome. Despite survey responses showing worsening COVID-related stress in our participants, no participants reported that they had been diagnosed with COVID-19, few reported psychosocial or financial impacts of the pandemic, and the overall prevalence of depression based on PHQ-9 scores was low. This may limit the external generalizability of our findings, although it provides internal validity given the core study question of how pregnancy itself affects LTL dynamics. Replicating our study in other populations that are affected more directly by the pandemic and its sequelae, or for whom stronger relations between stress and short telomeres are seen (Pantesco et al., 2018), are important directions for future research.
Despite these limitations, our study has a number of strengths. We provide granular pilot data on the relations between medical and psychiatric comorbidities and LTL in reproductive-aged people, which is an important contribution to the literature. In addition, the deterioration in mental health demonstrated by our repeated assessments highlights the importance of psychiatric screening for both obstetric and gynecologic patients. Further, no bias from regression to the mean on our longitudinal LTL measurements was noted. Lastly, despite limitations of external generalizability, our internal validity aids in interpreting LTL differences, as these have been shown to vary across different populations (Pantesco et al., 2018; Geronimus et al., 2015; Diez Roux et al., 2009; Zhu et al., 2011).
5. Conclusions
In conclusion, in this prospective pilot cohort, we did not identify significant differences between pregnant and non-pregnant people in LTL change over the course of a term gestation. Further, no relationship was seen between LTL and PHQ-9 scores. Notably, among pregnant people, shorter first trimester LTL was associated with earlier gestational age at delivery. In addition to providing baseline pilot information about the effect of pregnancy on LTL dynamics, our findings emphasize the need for replication in a larger study as well as continued research on LTL as a predictive biomarker in pregnancy.
Funding source
This study was funded by a Clinician Educator grant from the Maternal and Child Health Research Institute at Stanford University, for which Dr. Katherine Bianco was Principal Investigator. Dr. Danielle Panelli's time is partially funded by the Women's Reproductive Health Research National Institutes of Health K-12 program at Stanford University.
Declaration of competing interest
The authors report no conflict of interest.
Acknowledgements
The authors would like to acknowledge Dr. Jue Lin for her assistance with sample analysis.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2022.100506.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- ACOG. Committee Opinion 743: low-dose aspirin use during pregnancy. Obstet. Gynecol. 2018;132(1):44–52. doi: 10.1097/AOG.0000000000002708. [DOI] [PubMed] [Google Scholar]
- Aghaeepour N., Ganio E.A., Mcilwain D., et al. An immune clock of human pregnancy. Sci. Immunol. 2017;2(15):1–12. doi: 10.1126/sciimmunol.aan2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayaz R., Hocaoǧlu M., Günay T., Yardlmcl O.D., Turgut A., Karateke A. Anxiety and depression symptoms in the same pregnant women before and during the COVID-19 pandemic. J. Perinat. Med. 2020;48(9):965–970. doi: 10.1515/jpm-2020-0380. [DOI] [PubMed] [Google Scholar]
- Barnett A.G., van der Pols J.C., Dobson A.J. Regression to the mean: what it is and how to deal with it. Int. J. Epidemiol. 2005;34(1):215–220. doi: 10.1093/ije/dyh299. [DOI] [PubMed] [Google Scholar]
- Bateson M., Eisenberg D.T.A., Nettle D. Controlling for baseline telomere length biases estimates of the rate of telomere attrition. R. Soc. Open Sci. 2019;6(10) doi: 10.1098/rsos.190937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn E.H., Epel E.S. First Edit. Grand Central Publishing; 2017. The Telomere Effect: A Revolutionary Approach to Living Younger, Healthier, Longer. [Google Scholar]
- Bunevicius A., Kusminskas L., Pop V.J., Pedersen C.A., Bunevicius R. Screening for antenatal depression with the Edinburgh depression scale. J. Psychosom. Obstet. Gynecol. 2009;30(4):238–243. doi: 10.3109/01674820903230708. [DOI] [PubMed] [Google Scholar]
- Cawthon R.M. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30(10):e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Kimura M., Kim S., et al. Longitudinal versus cross-sectional evaluations of leukocyte telomere length dynamics: age-dependent telomere shortening is the rule. J. Geronol. Ser A Biol. Sci. Med. Sci. 2011;66A(3):312–319. doi: 10.1093/gerona/glq223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demanelis K., Jasmine F., Chen L.S., et al. Determinants of telomere length across human tissues. Science. 2020;369(6509) doi: 10.1126/SCIENCE.AAZ6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez Roux A.V., Ranjit N., Jenny N.S., et al. Race/ethnicity and telomere length in the multi-ethnic study of atherosclerosis. Aging Cell. 2009;8(3):251–257. doi: 10.1111/j.1474-9726.2009.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S., Epel E.S., Lin J., et al. Maternal psychosocial stress during pregnancy is associated with newborn leukocyte telomere length. Am. J. Obstet. Gynecol. 2013;208(2):134.e1–134.e7. doi: 10.1016/j.ajog.2012.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S., de Punder K., Buss C., Wadhwa P.D. The fetal programming of telomere biology hypothesis: an update. Philos. Trans. R. Soc. B Biol. Sci. 2018;373(1741):1–15. doi: 10.1098/rstb.2017.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel E.S., Blackburn E.H., Lin J., et al. Accelerated telomere shortening in response to life stress. Proc. Natl. Acad. Sci. U.S.A. 2004;101(49):17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettman C.K., Abdalla S.M., Cohen G.H., Sampson L., Vivier P.M., Galea S. Prevalence of depression symptoms in US adults before and during the COVID-19 pandemic. JAMA Netw. Open. 2020;3(9) doi: 10.1001/jamanetworkopen.2020.19686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouquerel E., Barnes R.P., Uttam S., Watkins S.C., Bruchez M.P., Opresko P.L. Targeted and persistent 8-oxoguanine base damage at telomeres promotes telomere loss and crisis. Mol. Cell. 2019;75(1):117–130.e6. doi: 10.1016/j.molcel.2019.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus A.T., Pearson J.A., Linnenbringer E., et al. Race-ethnicity, poverty, urban stressors, and telomere length in a detroit community-based sample. J. Health Soc. Behav. 2015;56(2):199–244. doi: 10.1177/0022146515582100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman S.N., Berlin J.A. The use of predicted confidence intervals when planning experiments and the misuse of power when interpreting results. Ann. Intern. Med. 1994;121(3):200–206. doi: 10.7326/0003-4819-121-3-199408010-00008. [DOI] [PubMed] [Google Scholar]
- Gotlib I.H., Lemoult J., Colich N.L., et al. Telomere length and cortisol reactivity in children of depressed mothers. Mol. Psychiatr. 2015;20(5):615–620. doi: 10.1038/mp.2014.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys K.L., Sisk L.M., Manczak E.M., Lin J., Gotlib I.H. Depressive symptoms predict change in telomere length and mitochondrial DNA copy number across adolescence. J. Am. Acad. Child Adolesc. Psychiatry. 2020;59(12):1364–1370.e2. doi: 10.1016/j.jaac.2019.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe D.L., Liu L., Marquard K. Telomeres and aging-related meiotic dysfunction in women. Cell. Mol. Life Sci. 2007;64(2):139–143. doi: 10.1007/s00018-006-6466-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King L.S., Feddoes D.E., Kirshenbaum J.S., Humphreys K.L., Gotlib I.H. 2020. Pregnancy during the Pandemic: the Impact of COVID-19-Related Stress on Risk for Prenatal Depression. PsyArXiv. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levis B., Benedetti A., Thombs B.D. Accuracy of Patient Health Questionnaire-9 (PHQ-9) for screening to detect major depression: individual participant data meta-analysis. BMJ. 2019;365 doi: 10.1136/bmj.l1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J., Epel E., Cheon J., et al. Analyses and comparisons of telomerase activity and telomere length in human T and B cells: insights for epidemiology of telomere maintenance. J. Immunol. Methods. 2010;352(1–2):71–80. doi: 10.1016/j.jim.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J., Cheon J., Brown R., et al. Systematic and cell type-specific telomere length changes in subsets of lymphocytes. J. Immunol. Res. 2016;2016 doi: 10.1155/2016/5371050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M., Guo L., Yu M., Wang H. The psychological and mental impact of coronavirus disease 2019 (COVID-19) on medical staff and general public – a systematic review and meta-analysis. Psychiatr. Res. 2020;291(April) doi: 10.1016/j.psychres.2020.113190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H., Zhou Z., Wei S., et al. Shortened Telomere length is associated with increased risk of cancer: a meta-analysis. PLoS One. 2011;6(6):1–9. doi: 10.1371/journal.pone.0020466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müezzinler A., Zaineddin A.K., Brenner H. A systematic review of leukocyte telomere length and age in adults. Ageing Res. Rev. 2013;12(2):509–519. doi: 10.1016/j.arr.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Murray D., Cox J.L. Screening for depression during pregnancy with the Edinburgh depression scale (EPDS) J. Reprod. Infant Psychol. 1990;8(2):99–107. doi: 10.1080/02646839008403615. [DOI] [Google Scholar]
- NHANES. Telomere Mean and Standard Deviation (Surplus) Data Documentation, Codebook, and Frequencies.
- Notterman D.A., Schneper L. Telomere time-why we should treat biological age cautiously. JAMA Netw. Open. 2020;3(5) doi: 10.1001/jamanetworkopen.2020.4352. [DOI] [PubMed] [Google Scholar]
- Nsereko E., Uwase A., Muvunyi C.M., et al. Association between micronutrients and maternal leukocyte telomere length in early pregnancy in Rwanda. BMC Pregnancy Childbirth. 2020;20(1):1–13. doi: 10.1186/s12884-020-03330-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oreshkova T., Dimitrov R., Mourdjeva M. A cross-talk of decidual stromal cells, trophoblast, and immune cells: a prerequisite for the success of pregnancy. Am. J. Reprod. Immunol. 2012;68(5):366–373. doi: 10.1111/j.1600-0897.2012.01165.x. [DOI] [PubMed] [Google Scholar]
- Osborne L.M., Monk C. Perinatal depression-The fourth inflammatory morbidity of pregnancy? Theory and literature review. Psychoneuroendocrinology. 2013;38(10):1929–1952. doi: 10.1016/j.psyneuen.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panelli D.M., Bianco K. Cellular aging and telomere dynamics in pregnancy. Curr. Opin. Obstet. Gynecol. 2021 doi: 10.1097/GCO.0000000000000765. Epub ahead. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panelli D.M., Leonard S.A., Wong R.J., et al. Leukocyte telomere dynamics across gestation in uncomplicated pregnancies and associations with stress. BMC Pregnancy Childbirth. 2022;22(381):1–12. doi: 10.1186/s12884-022-04693-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantesco E.J., Leibel D.K., Ashe J.J., et al. Multiple forms of discrimination, social status, and telomere length: interactions within race. Psychoneuroendocrinology. 2018;98(August):119–126. doi: 10.1016/j.psyneuen.2018.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillippe M., Sawyer M.R., Edelson P.K. The telomere gestational clock: increasing short telomeres at term in the mouse. Am. J. Obstet. Gynecol. 2019;220(5) doi: 10.1016/j.ajog.2019.01.218. 496.e1-496496.e8. [DOI] [PubMed] [Google Scholar]
- Polettini J., da Silva M.G. Telomere-related disorders in fetal membranes associated with birth and adverse pregnancy outcomes. Front. Physiol. 2020;11(October) doi: 10.3389/fphys.2020.561771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack A.Z., Rivers K., Ahrens K.A. Parity associated with telomere length among US reproductive age women. Obstet. Gynecol. Surv. 2018;73(6):357–358. doi: 10.1093/humrep/dey024. [DOI] [PubMed] [Google Scholar]
- Puhlmann L.M.C., Valk S.L., Engert V., et al. Association of short-term change in leukocyte telomere length with cortical thickness and outcomes of mental training among healthy adults A randomized clinical trial. JAMA Netw. Open. 2019;2(9):1–16. doi: 10.1001/jamanetworkopen.2019.9687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puterman E., Lin J., Krauss J., Blackburn E.H., Epel E.S. Determinants of telomere attrition over 1 year in healthy older women: stress and health behaviors matter. Mol. Psychiatr. 2015;20(4):529–535. doi: 10.1038/mp.2014.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiagen Qiagen. Sample & assay technologies. Qiagen. 2016. http://www.qiagen.com/knowledge-and-support/resource-center/resource-download.aspx?id=67893a91-946f-49b5-8033-394fa5d752ea&lang=en 5, 1-71.
- Ridout K.K., Ridout S.J., Guille C., Mata D.A., Akil H., Sen S. Physician-training stress and accelerated cellular aging. Biol. Psychiatr. 2019;86(9):725–730. doi: 10.1016/j.biopsych.2019.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev I., Entringer S., Wadhwa P.D., et al. Stress and telomere biology: a lifespan perspective. Psychoneuroendocrinology. 2013;38(9):1835–1842. doi: 10.1016/j.psyneuen.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The American College of Obstetricians and Gynecologists. Committee Opinion Methods for estimating the due date. Obstet. Gynecol. 2017;700:1–5. doi: 10.1097/AOG.0000000000002042. [DOI] [Google Scholar]
- Vaiserman A., Krasnienkov D. Telomere length as a marker of biological age: state-of-the-art, open issues, and future perspectives. Front. Genet. 2021;11(January) doi: 10.3389/fgene.2020.630186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes A.M., Andrew T., Gardner J.P., et al. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366(9486):662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- Verner G., Epel E., Lahti-Pulkkinen M., et al. Maternal psychological resilience during pregnancy and new born telomere length: a prospective study. Am. J. Psychiatr. 2021;178(2):183–192. doi: 10.1176/appi.ajp.2020.19101003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem. Sci. 2002;27(7):339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- Wolkowitz O.M., Mellon S.H., Epel E.S., et al. Leukocyte telomere length in major depression: correlations with chronicity, inflammation and oxidative stress - preliminary findings. PLoS One. 2011;6(3):1–10. doi: 10.1371/journal.pone.0017837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H., Wang X., Gutin B., et al. Leukocyte telomere length in healthy Caucasian and African-American adolescents: relationships with race, sex, adiposity, adipokines, and physical activity. J. Pediatr. 2011;158(2):215–220. doi: 10.1016/j.jpeds.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.