Abstract
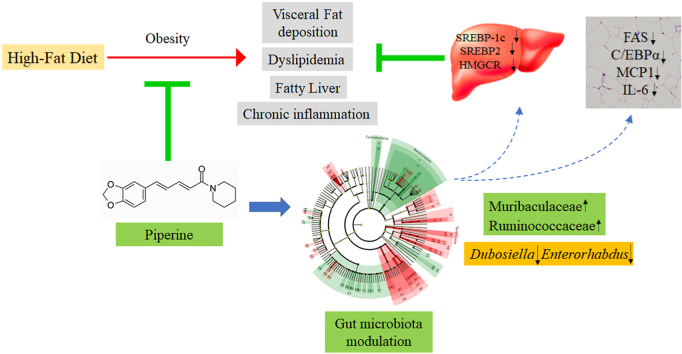
An obese mouse model induced by high-fat diet (HFD) feeding was used to reveal the role of piperine in modulating gut microbiota (GM). Piperine was administrated at 20 and 40 mg/kg body weight every day. As a result, piperine at 40 mg/kg significantly decreased body weight, liver weight, perirenal fat weight, and lowered serum triglycerides, total cholesterol, low-density lipoprotein cholesterol, and glucose levels in HFD-fed mice. Additionally, piperine significantly attenuated fatty liver and modulated hepatic mRNA expressions of SREBP-1c, SREBP2, and HMGCR. In perirenal fat, FAS, C/EBPα, MCP1, and IL-6 expressions were significantly downregulated by piperine. 16S rRNA sequencing revealed that piperine elevated GM diversity. The relative abundance of Muribaculaceae and Ruminococcaceae were significantly elevated, while Dubosiella and Enterorhabdus genera were suppressed by piperine. The Pearson correlation analysis showed that the altered phylotypes were highly correlated with obesity phenotypes. These findings suggest that piperine modulates energy homeostasis and inflammation to alleviate obesity associated with GM regulation.
Keywords: Piperine, Obesity, Fatty liver, Muribaculaceae, Ruminococcaceae
Graphical abstract
Highlights
-
•
Piperine significantly attenuates obesity and elevates gut microbiota diversity.
-
•
Piperine elevates the abundance of Muribaculaceae and Ruminococcaceae families.
-
•
Piperine suppresses Dubosiella and Enterorhabdus genera.
-
•
The piperine-altered phylotypes are highly correlated with obesity phenotypes.
Abbreviations:
- HFD
high-fat diet;
- GM
gut microbiota
- FAS
fatty acid synthase
- SREBP-1c
sterol regulatory element-binding protein 1c
- SREBP-2
sterol regulatory element-binding protein 2
- HMGCR
3-hydroxy-3-methyl-glutaryl-coenzyme A reductase
- CYP7A1
cholesterol 7α-hydroxylase
- LDLR
low-density lipoprotein receptor
- C/EBPα
CCAAT/enhancement-binding protein-α;
- MCP1
monocyte chemoattractant protein-1
- IL-6
interleukin-6
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HE
haematoxylin and eosin
- TG
triacylglycerol
- TC
total cholesterol
- LDL-C
low-density lipoprotein cholesterol
- HDL-C
high-density lipoprotein cholesterol
- AST
aspartate aminotransferase
- ALT
alanine aminotransferase
- PCoA
principal coordinates analysis
- OTUs
operational taxonomic units
- SCFAs
short-chain fatty acids
1. Introduction
Obesity results from complex interactions among inherited, physiological and environmental factors (Jackson et al., 2020). In the past four decades, due to the overconsumption of energy-dense food combined with a sedentary lifestyle, obesity has tripled, leading to an epidemic that affects both children and adults. It is worrisome that 7% of the children and adolescents had obesity in 2016 compared with less than 1% in 1975 (NCD Risk Factor Collaboration, 2017). By 2025, about 1 billion adults will have obesity (Loos and Yeo, 2022). The prevalence raised a growing concern about economic burden worldwide of related chronic diseases, including cardiovascular disease, diabetes, nonalcoholic fatty liver disease, musculoskeletal disorders, and certain cancers (Formica et al., 2020). However, obesity is largely preventable.
Gut microbiota (GM) is an enormous microbial community that plays a vital role in lifestyle-related disorders (Chen et al., 2021). GM is considered an endocrine organ that highly involved in the host regulation of nutrient handling and energy homeostasis, and thus has significant contributions to obesity (Gomes et al., 2018). GM dysbiosis may contribute to the development of metabolic disorders and chronic inflammation present in obesity (Gomes et al., 2018; Li et al., 2017). Therefore, the modulation of GM has been proposed as a potential strategy to prevent obesity and associated metabolic disorders (Cani et al., 2019).
Piperine, one of the most widely used spices, is the main bioactive component in Piper species (Jwa et al., 2012). Previous studies have found the beneficial effects of piperine on metabolic disorders, and multiple mechanisms appear to be involved. It was reported that piperine protected against hepatic steatosis and insulin resistance possibly via suppression of LXRα-mediated lipogenesis (Jwa et al., 2012), and also by activation of adiponectin-AMPK signaling (Choi et al., 2013). The metabolic inflammation was inhibited by piperine, which helped to ameliorate insulin resistance (Liu et al., 2020). Surprisingly, piperine was found to upregulate the metabolic rate of resting muscle to attenuate obesity and diabetes (Nogara et al., 2016). It was recently reported that piperine attenuated liver fibrosis by activating Nrf2 cascade and subsequently suppressing TGF-β1/Smad axis (Shu et al., 2021).
However, whether piperine can improve metabolic disorders by altering GM has not been investigated so far. In this study, for the first time, the role of piperine in GM regulation and the correlation with obesity-induced metabolic dysfunction were revealed using an HFD-fed mice model.
2. Material and methods
2.1. Animal study
Male 5-week-old C57BL/6 mice (20.36 ± 0.83 g, Shanghai Shilaike Laboratory Animal Co, Ltd, Shanghai, China) were randomly divided into four groups (n = 10) and treated daily. The mice in the normal group were fed with a control diet (10% kcal from fat, D12450J, Research Diets, New Brunswick, NJ, USA) and intragastrically (i.g.) treated with 5% lecithin (Macklin Biochemical, Shanghai, China) at 10 mL/kg body weight (Normal). The mice in the model group were fed with an HFD (60% kcal from fat, D12492, Research Diets) and treated with 5% lecithin (Model). The mice in the low-dose piperine (C17H19NO3, purity ≥98%, Macklin Biochemical) group were fed with an HFD and treated with 20 mg/kg piperine suspended in 5% lecithin (PIP 20). The mice in the high-dose piperine group were fed with an HFD and treated with 40 mg/kg piperine suspended in 5% lecithin (PIP 40). The low and high doses were determined according to literature (Aswar et al., 2015; Liu et al., 2020). Food intake was measured daily, and body weight was recorded every other day. The weight gained through the experimental period was divided by cumulative dietary intake to calculate the food efficiency ratio.
At the end of 10 weeks of treatment, the mice were fasted overnight and anaesthetized by inhalation of isoflurane, and the blood was collected via the retro-orbital sinus. In each group, cecal samples (n = 6) were randomly collected and immediately stored in liquid nitrogen for GM analysis. The liver and perirenal fat were isolated by surgery, and quickly weighted. A small portion of the tissue was collected for histopathological evaluation. The remaining tissue was immediately frozen with liquid nitrogen for RNA extraction. All protocols were approved by the Institutional Committee on the Care and Use of Animals of the Third Institute of Oceanography, Ministry of Natural Resources (TIO-IACUC-03-2021-09-01). All animals received humane care in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, USA).
2.2. Quantitative RT-PCR analysis
Trizol (Biouniquer Technology Co., Ltd, Nanjing, China) was used to extract total RNA from liver or perirenal adipose. A commercial kit (R333-01, Vazyme Biotech, Nanjing, China) was used to convert the RNA from each sample into cDNA. The quantitative RT-PCR was performed on the LightCycler 96 instrument (Roche, Basel, Switzerland) using Taq Pro Universal SYBR qPCR Master Mix (Q712-02, Vazyme Biotech) according to the manufacturer's instructions. The mRNA levels of fatty acid synthase (FAS), sterol regulatory element-binding protein 1c (SREBP-1c), sterol regulatory element-binding protein 2 (SREBP-2), 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase (HMGCR), cholesterol 7α-hydroxylase (CYP7A1), and low-density lipoprotein receptor (LDLR) in the liver were measured. For the perirenal fat, the mRNA expressions of FAS, CCAAT/enhancement-binding protein-α (C/EBPα), monocyte chemoattractant protein-1 (MCP1), F4/80, and interleukin-6 (IL-6) were measured. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the reference gene. The 2−ΔΔCt method was used to analyze the relative changes in gene expression. The primers sequences were shown in Table S1.
2.3. Histological evaluation
The liver and adipose samples were fixed in 10% neutral buffered formalin and subsequently embedded in paraffin. The tissue sections were sliced and stained with haematoxylin and eosin (HE, Leica, Bensheim, Germany) using standard methods. The histological images were taken with Nikon E80i microscopy (Nikon, Tokyo, Japan).
2.4. Biochemical analysis
The serum levels of triacylglycerol (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), aspartate aminotransferase (AST), alanine aminotransferase (ALT), and glucose were measured by an automatic hematology analyzer (BS-240VET, Mindray, Shenzhen, China).
2.5. GM analysis
The 16S rRNA gene sequencing was performed by Gene Denovo Biotechnology Co. Ltd (Guangzhou, China) for identification, classification and quantitation of GM in different treatment groups. The bacterial DNA extraction, target region amplification and sequencing, quality control and clustering, and taxonomy annotation were conducted as previously reported (Zhang et al., 2021).
To analyze the microbiota structure generally, the Chao1 richness index was obtained by the QIIME software package (University of Colorado, Boulder, CO, USA, version 1.9.1), and rank abundance curves were plotted in R project ggplot2 package (version 2.2.1). Principal coordinates analysis (PCoA) was performed to present differences of the gut microbial communities among groups. The stacked bar plot of the community composition on the phylum, family and genus level was visualized using R project ggplot2 package (version 2.2.1). Pearson correlations between the gut microbial composition and host parameters were calculated in R project psych package (version 1.8.4) and the heatmaps were plotted using “pheatmap” package (version 1.0.12) in R project.
2.6. Statistical analysis
Data were presented as mean ± SD. GraphPad Prism 8 (San Diego, CA, USA) was employed to create histograms, and determine the statistical significance between groups by one-way ANOVA with the post-hoc Tukey test. GM analysis was performed using Omicsmart platform (http://www.omicsmart.com). The p values < 0.05 were considered to be statistically significant.
3. Results
3.1. Effect of piperine on obesity-related features in HFD-fed mice
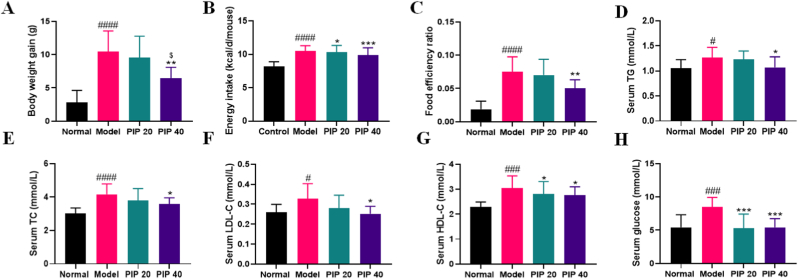
HFD-fed mice gained significantly more body weight than the normal group (Fig. 1A). However, compared with the model group, the piperine-treated groups showed a significant reduction in body weight gain in a dose-dependent manner. Additionally, piperine treatment decreased energy intake and food efficiency ratio dose-dependently (Fig. 1B and C), which explained a less body weight gain.
Fig. 1.
The effect of piperine on the obesity-related features in high-fat diet-fed mice. (A) Body weight gain. (B) Energy intake. (C) Food efficency ratio (the body weight gained divided by cumulative dietary intake). (D) Serum triacylglycerol (TG). (E) Serum total cholesterol (TC). (F) Serum low-density lipoprotein cholesterol (LDL-C). (G) Serum high-density lipoprotein cholesterol (HDL-C). (H) Serum glucose. Mean ± SD (n = 10); #p < 0.05, ###p < 0.001, ####p < 0.0001, Model versus Normal; *p < 0.05, **p < 0.01, ***p < 0.001 versus Model; $ p < 0.05, PIP 40 (piperine at 40 mg/kg) versus PIP 20 (piperine at 20 mg/kg).
Furthermore, the elevated serum TG, TC, LDL-C, HDL-C, and glucose levels by HFD feeding have been lowered to some extent in low- or high-dose (20 or 40 mg/kg) piperine treated groups. Particularly, the serum TG, LDL-C, and glucose of the high-dose group were at the same levels as those in the normal group (Fig. 1D-H).
3.2. Effect of piperine on fatty liver in HFD-fed mice
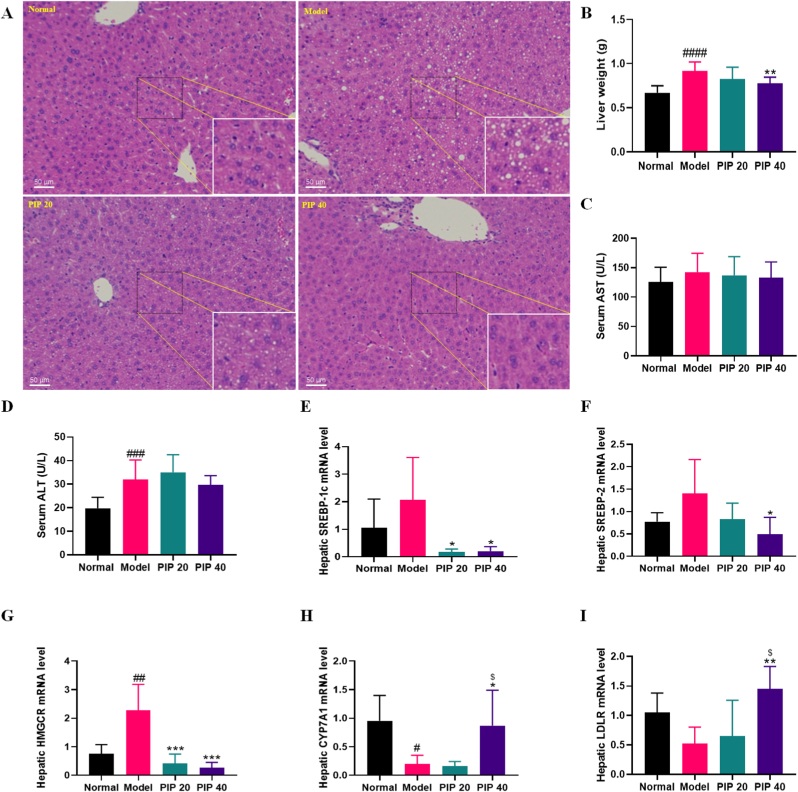
To evaluate the effect of the intervention of piperine on HFD-induced liver injury, the histological change, the serum AST and ALT levels, and the mRNA levels of the hepatic lipid metabolism key regulators, SREBP-1c, SREBP-2, HMGCR, CYP7A1, and LDLR were determined. As shown in Fig. 2A, prominent fat accumulation within hepatocytes was observed in the model group, suggesting the progression of fatty liver induced by long-term HFD feeding. However, piperine dose-dependently alleviated fat deposition in the liver tissue. In addition, the HFD feeding caused a 37% increase of liver weight, which was significantly lowered by high-dose piperine treatment (Fig. 2B). Although serum AST did not show significant differences among different groups, ALT was significantly elevated in the model group, indicating hepatic damage caused by fat deposition (Fig. 2C and D). Compared with the model group, serum ALT was lower in the high-dose piperine group, but the differences were not significant.
Fig. 2.
Piperine improved fatty liver of high-fat diet-fed mice. (A) Liver sections was stained with HE. (B) Liver weight. (C) AST and (D) ALT in the serum. Hepatic mRNA levels of (E) SREBP-1c, (F) SREBP-2, (G) HMGCR, (H) CYP7A1, and (I) LDLR. Mean ± SD (n = 10). #p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001 Model versus Normal; *p < 0.05, **p < 0.01, ***p < 0.001 versus Model; $ p < 0.05, PIP 40 (piperine at 40 mg/kg) versus PIP 20 (piperine at 20 mg/kg).
Lipid regulators in the liver were largely altered by HFD and piperine treatment. SREBP-1c (p = 0.35) and SREBP-2 (p = 0.19) were upregulated by HFD feeding, but SREBP-1c was dramatically decreased by piperine at 20 or 40 mg/kg, and SREBP-2 was significantly decreased by 40 mg/kg of piperine treatment (Fig. 2E and F). Hepatic HMGCR mRNA level was also highly elevated by HFD, but remarkably downregulated by piperine at both doses (Fig. 2G). The mRNA levels of the cholesterol regulators, CYP7A1 and LDLR were all significantly upregulated by high-dose piperine treatment (Fig. 2H and I). The results showed that piperine attenuated HFD-induced fatty liver in mice, probably by regulating lipid and cholesterol signaling.
3.3. Effect of piperine on perirenal fat in HFD-fed mice
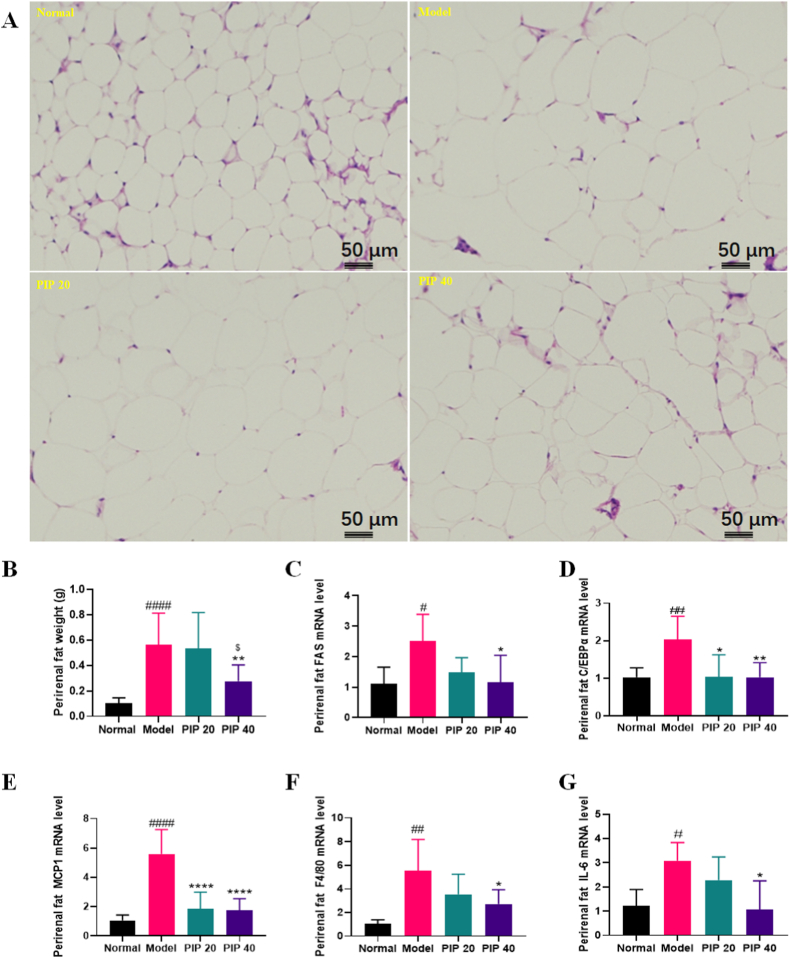
Perirenal adipose tissue was dissected to represent the visceral white adipose tissue. HE staining showed that the cell vacuole was increased by HFD. The enlargement was mitigated by piperine treatment (Fig. 3A). Additionally, the weight of perirenal fat was increased evidently in the model group, but reduced by high-dose piperine treatment (Fig. 3B).
Fig. 3.
Effects of piperine on perirenal fat of high-fat diet-fed mice. (A) Histology examined by HE staining. (B) Perirenal fat weight. mRNA levels of (C) FAS, (D) C/EBPα, (E) MCP1, (F) F4/80, and (G) IL-6. Mean ± SD (n = 10); #p < 0.05, ##p < 0.01, ####p < 0.0001 Model versus Normal; *p < 0.05, **p < 0.01, ****p < 0.0001 versus Model; $ p < 0.05, PIP 40 (piperine at 40 mg/kg) versus PIP 20 (piperine at 20 mg/kg).
To explore the mechanism underlying piperine's effect on the adipose tissue, mRNA expressions of fat deposition regulators FAS and C/EBPα, and inflammation regulators MCP1, F4/80, and IL-6 of perirenal fat were measured. As a result, all of them were significantly upregulated in the model group, and downregulated in the high-dose piperine group. Additionally, C/EBPα and MCP1 were also downregulated in the low-dose group (Fig. 3C–G).
3.4. Effect of piperine on GM profiles in HFD-fed mice
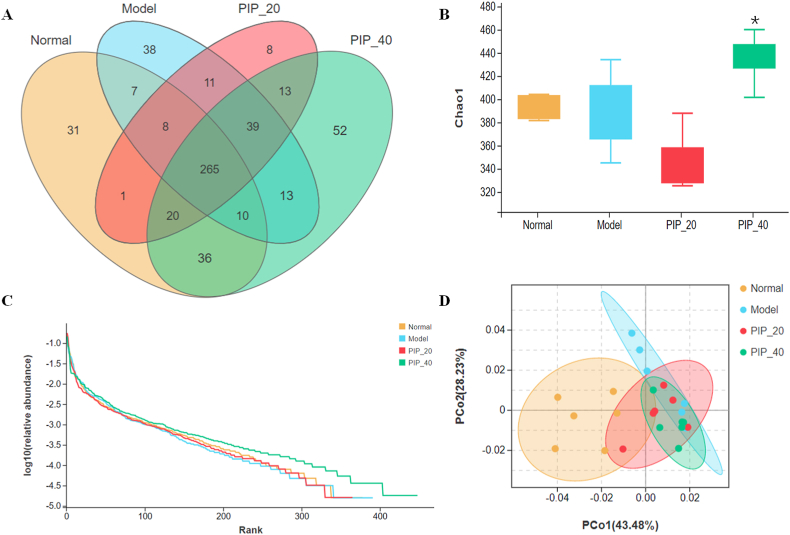
The effects of piperine on GM were investigated by the sequencing of 16S rRNA. According to the Venn diagram, the investigated groups had overlapped and unique operational taxonomic units (OTUs). Notably, high-dose piperine treatment resulted in the most unique OTUs (Fig. 4A). Consistently, the values of Chao1 richness were significantly increased by high-dose piperine treatment compared with the model group (Fig. 4B).
Fig. 4.
Piperine altered gut microbiota profiles in high-fat diet-fed mice. (A) The OTUs distribution among different groups shown as Venn diagram. (B) Chao1 richness of gut microbiota. (C) Species rank abundance curves. (D) PCoA plot of microbial communities based on the weighted UniFrac distances. Mean ± SD (n = 6); *p < 0.05 vesus Model. PIP 20 (piperine at 20 mg/kg). PIP 40 (piperine at 40 mg/kg).
In a rank abundance plot, species richness is indicated by the number of different species, and species evenness is reflected in the slope of the line that best fits the graph (Sharma et al., 2021). Therefore, the rank abundance plot visualizes both the species richness and evenness. As evidently shown in Fig. 4C, the high-dose piperine group had the most diverse GM among the four investigated groups.
For the beta diversity, the PCoA plot showed that the clusters of GM in the model group appeared to be distinct from those in the normal group, whereas the clusters in low- and high-dose piperine groups were between those in the normal and model groups (Fig. 4D).
As a whole, piperine modulated the GM of HFD-fed mice, especially at high-dose, by which the mice had the most diverse GM.
3.5. Effect of piperine on GM composition at different levels in HFD-fed mice
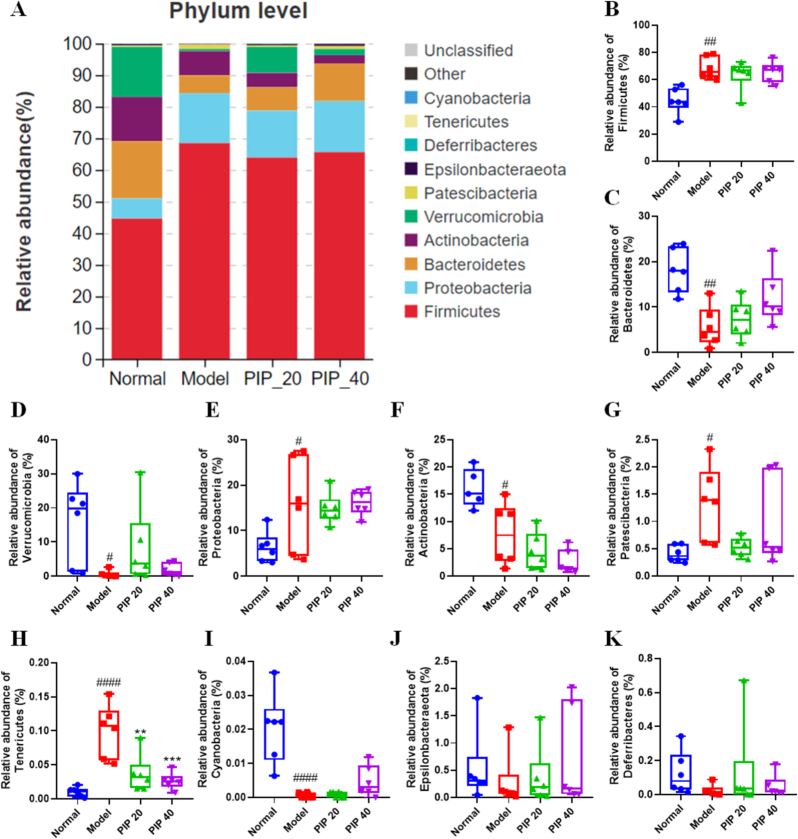
In the present study, GM phylotypes at the phylum, family, and genus levels were analyzed for different groups, to evaluate the effect of piperine on GM composition of mice fed an HFD. As shown in Fig. 5A, GM in the normal group was mainly composed of Firmicutes, Bacteroidetes, Verrucomicrobia, Proteobacteria, and Actinobacteria at the phylum level. All these 5 major phylotypes were evidently altered by HFD. The abundance of Firmicutes and Proteobacteria were significantly elevated, while those of Bacteroidetes, Actinobacteria, and Verrucomicrobia were significantly decreased. Piperine showed no obvious modulatory effect on either of the 5 major phyla (Fig. 5B-F), or the Patescibacteria phylum (Fig. 5G). It is worth noting that the HFD-elevated Tenericutes abundance was decreased by piperine in a dose-dependent manner (Fig. 5H). Cyanobacteria, a very low abundance phylotype, was significantly decreased by HFD (Fig. 5I). Epsilonbacteraeota and Deferribacteres were not significantly altered by either HFD or piperine treatment (Fig. 5J and K).
Fig. 5.
Specific phyla of gut microbiota modulated by piperine intervention in high-fat diet-fed mice. (A) The proportion of gut microbial communities at phylum level. The relative abundance of (B) Firmicutes, (C) Bacteroidetes, (D) Verrucomicrobia, (E) Proteobacteria, (F) Actinobacteria, (G) Patescibacteria, (H) Tenericutes, (I) Cyanobacteria, (J) Epsilonbacteraeota, and (K) Deferribacteres. Mean ± SD (n = 6); #p < 0.05, ##p < 0.01, ####p < 0.0001 Model versus Normal; **p < 0.01, ***p < 0.001 versus Model. PIP 20 (piperine at 20 mg/kg). PIP 40 (piperine at 40 mg/kg).
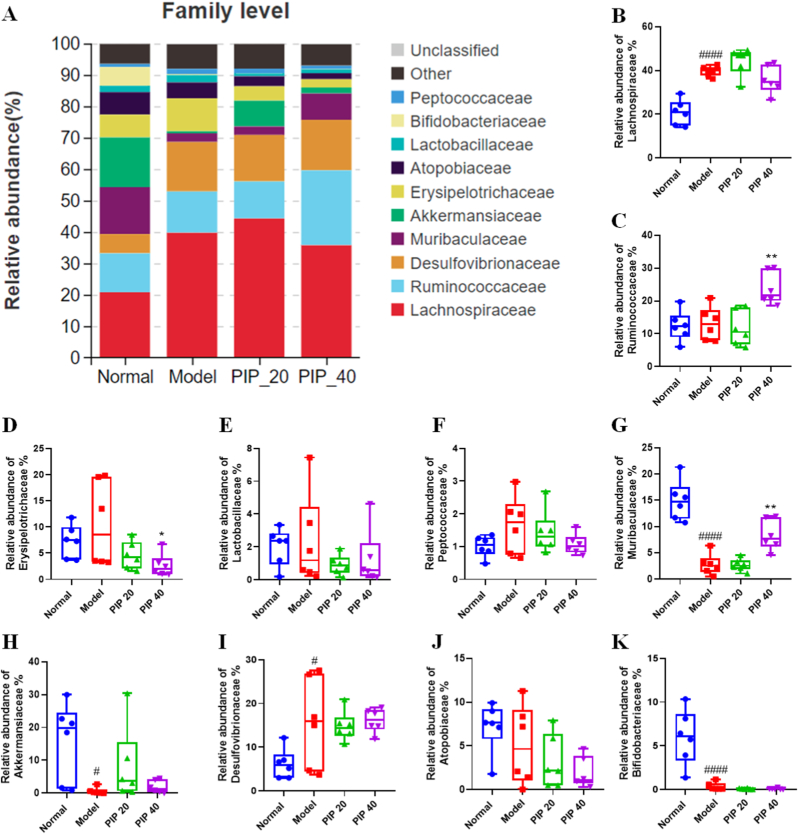
It seems that HFD feeding had a great impact on the abundance of all the major phyla, but piperine had little influence. However, at the family level, the effects of piperine surfaced (Fig. 6A). Fig. 6B-F showed the abundance of the Firmicutes members in each group. The Lachnospiraceae family was elevated about 2-fold by HFD feeding, and low- or high-dose piperine did not reverse the elevation (Fig. 6B). The abundance of the Ruminococcaceae family showed no differences among normal, model, and low-dose piperine groups, but was significantly elevated in the high-dose group (Fig. 6C). Conversely, the Erysipelotrichaceae family was decreased by high-dose piperine (Fig. 6D). The abundance of Lactobacillaceae family and Peptococcaceae family appeared to be elevated by HFD feeding in some individual mice, and the average abundance of both families were lower in piperine treatment groups compared with the model group. However, the differences were not significant (Fig. 6E and F).The Bacteroidetes member, Muribaculaceae accounted for 14.92 ± 3.79% of total bacteria in the normal group, but was decreased to 2.78 ± 1.99% by HFD feeding. However, high-dose piperine restored the abundance to 8.38 ± 2.93% (Fig. 6G). The growth of the Verrucomicrobia member, the Akkermansiaceae family was fully inhibited in the HFD-fed mice, but piperine administration restored it in some individual animals (Fig. 6H). Piperine did not show the opposite effect to HFD on the families Desulfovibrionaceae, Atopobiaceae, and Bifidobacteriaceae (Fig. 6I-K).
Fig. 6.
Specific families of gut microbiota modulated by piperine intervention in high-fat diet-fed mice. (A) The proportion of gut microbial communities at the family level. The relative abundance of (B) Lachnospiraceae, (C) Ruminococcaceae, (D) Erysipelotrichaceae, (E) Lactobacillaceae, and (F) Peptococcaceae belonging to the Firmicutes phylum, (G) Muribaculaceae belonging to the Bacteroidetes phylum, (H) Akkermansiaceae belonging to the Verrucomicrobia phylum, (I) Desulfovibrionaceae belonging to the Proteobacteria phylum, and (J) Atopobiaceae and (K) Bifidobacteriaceae belonging to the Actinobacteria phylum. Mean ± SD (n = 6); #p < 0.05, ####p < 0.0001 Model versus Normal; *p < 0.05, **p < 0.01 versus Model. PIP 20 (piperine at 20 mg/kg). PIP 40 (piperine at 40 mg/kg).
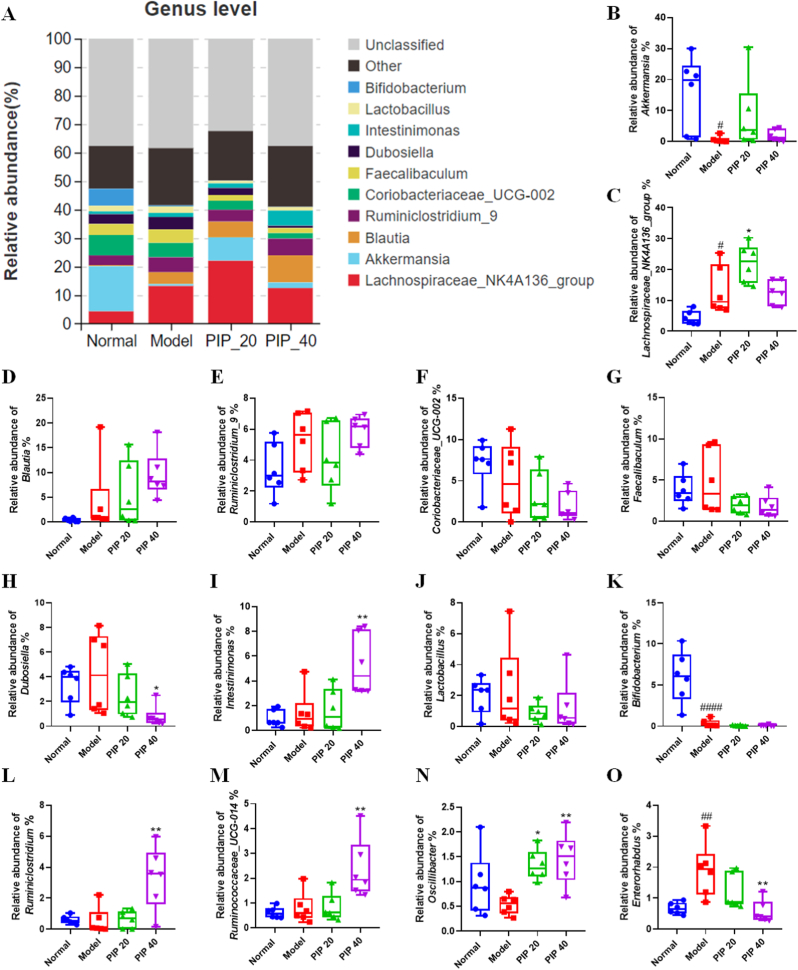
At the genus level, Akkermansia was the most dominant in the normal group, while Lachnospiraceae_NK4A136_group was the most abundant in all the other three groups (Fig. 7A), indicating that HFD could strongly inhibit Akkermansia (Fig. 7B), but stimulate Lachnospiraceae_NK4A136_group (Fig. 7C). Among the top 10 genera (shown in Fig. 7B-K), high-dose piperine significantly decreased the abundance of the Erysipelotrichaceae family member Dubosiella (Fig. 7H), but elevated the abundance of Intestinimonas (Fig. 7I), which belongs to the Ruminococcaceae family. We also found that high-dose piperine highly elevated the abundance of the other 3 genera that belong to the Ruminococcaceae family, including Ruminiclostridium, Ruminococcaceae_UCG-014, and Oscillibacter (Fig. 7L–N). The Eggerthellaceae family member, Enterorhabdus was elevated in HFD-fed mice, but reduced by high-dose piperine treatment (Fig. 7O).
Fig. 7.
Specific genera of gut microbiota modulated by piperine intervention in high-fat diet-fed mice. (A) The proportion of gut microbial communities at the genus level. The relative abundance of (B) Akkermansia, (C) Lachnospiraceae_NK4A136_group, (D) Blautia, (E) Ruminiclostridum_9, (F) Coriobacteriaceae_UCG-002, (G) Faecalibaculum, (H) Dubosiella, (I) Intestinimonas, (J) Lactobacillus, (K) Bifidobacterium, (L) Ruminiclostridium, (M) Ruminococcaceae_UCG-014, (N) Oscillibacter, and (O) Enterorhabdus. Mean ± SD (n = 6); #p < 0.05, ##p < 0.01, ####p < 0.0001 Model versus Normal; *p < 0.05, **p < 0.01 versus Model. PIP 20 (piperine at 20 mg/kg). PIP 40 (piperine at 40 mg/kg).
Overall, piperine effectively modulated GM composition in HFD-fed mice, which was especially obvious at the high dose.
3.6. The correlation of piperine-altered GM phylotypes with host parameters
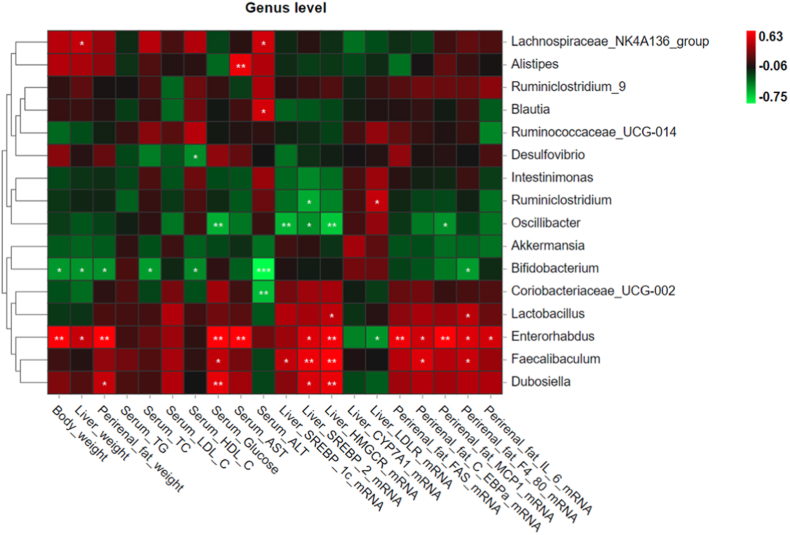
The potential association between GM and host parameters was measured by the Pearson correlation analysis. At the genus level, GM is associated with HFD-related indexes including body weight, organ weight, serum indicators, and gene expressions of hepatic or perirenal fat regulators or biomarkers (Fig. 8). It is worth noting that several Ruminococcaceae family members, like Intestinimonas, Ruminiclostridium, Ruminococcaceae_UCG-014 and Oscillibacter showed a negative correlation with body weight, liver weight, and fat weight and the other obesity-related features, while these bacteria exhibited a positive correlation with regulators that promote lipid metabolism, like hepatic CYP7A1 and LDLR mRNA levels. Since the abundance of these bacteria were significantly elevated by high-dose piperine treatment (Fig. 7I, 7L-7N), it was proposed that the mitigating effect of piperine on HFD-induced obestiy was associated with the alteration.
Fig. 8.
Pearson correlations between the gut bacterial genera and obesity-related indexes of the host. Green or red color means negative or positive correlation. The asterisks indicate significant correlations between variables. *p < 0.05, **p < 0.01, ***p < 0.001. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Meanwhile, the Erysipelotrichaceae members Faecalibaculum and Dubosiella, the Lactobacillaceae member Lactobacillus, and the Eggerthellaceae member Enterorhabdus were positively correlated with obesity-related parameters, and negatively correlated with hepatic CYP7A1 and LDLR expressions, indicating that they might be significant genera for the development of HFD-induced obesity. Since piperine downregulated Dubosiella (Fig. 7H) and Enterorhabdus (Fig. 7O) abundance, it can be inferred that piperine exhibited its anti-obesity effect associated with Dubosiella and Enterorhabdus suppression.
4. Discussion
Besides body weight gain, obesity is associated with local fat accumulation, insulin resistance, chronic inflammation, and progressive alteration in lipid and glucose metabolism. In this study, 10-week HFD feeding effectively resulted in obesity, evidenced by remarkable body weight gain, abnormal serum indicators, fatty liver, and visceral fat deposition, which largely imitated human obesity.
Piperine lowered body weight gain with a decreased energy intake and a much lower food efficiency compared with the model group. Additionally, piperine treatment resulted in obvious improvement of the elevated serum indicators in HFD-fed mice. Furthermore, the serum TG, LDL-C, and glucose were lowered to similar levels to those in the normal mice.
The classical role of HDL-C is to deliver cholesterol to the liver to excrete, so it has been regarded as “good cholesterol” for decades. However, this function depends more on the number of HDL particles, as well as the protein and lipid composition, than on the HDL-C concentration (März et al., 2017). Therefore, the HDL-C concentration is not always inversely associated with heart disease. Recently, a U-shape relationship between HDL-C and cardiovascular disease was found, which means an extremely high HDL-C is also associated with elevated cardiovascular risk (Feng et al., 2020). In the current study, HFD-feeding highly increased serum HDL-C, but piperine significantly lowered it at both low and high doses (Fig. 1G). According to the recent literature, the lowering of HDL-C level of HFD-fed mice by piperine might be beneficial.
The HFD-induced fatty liver was ameliorated by piperine dose-dependently. Moreover, key regulators of hepatic lipid metabolism were investigated. As a result, SREBP-1c and SREBP-2 were dramatically lowered by piperine. Evidently, the highly increased hepatic HMGCR mRNA level in HFD-fed mice was remarkably decreased by the piperine treatment. CYP7A1 encodes the rate-limiting enzyme that catalyze the conversion of cholesterol to bile acids. Hepatic CYP7A1 expression was decreased by HFD feeding and elevated by high-dose piperine treatment. Thus, piperine might reduce serum cholesterol level by promoting the synthesis of bile acids. The hepatic mRNA level of LDLR, which helps to bind serum low-density lipoprotein, was also significantly upregulated by high-dose piperine, contributing to a lowered serum LDL-C level.
Many enzymes are involved in the complex process of lipid metabolism. FAS catalyzes the synthesis of saturated fatty acids and subsequently increases the TG levels in the tissue. CEBP/α is the master transcription factor that regulates preadipocytes differentiation. In our study, HFD feeding elevated FAS and C/EBPα expression in the perirenal adipose tissue, while they were downregulated by 40 mg/kg piperine. Thus, piperine might downregulate fat synthesis and deposition in visceral adipose tissue.
Obesity is often accompanied by low-grade metabolic inflammation. In obesity, M1-like macrophages are recruited into visceral adipose tissue and secrete a great amount of pro-inflammatory cytokines (Liu et al., 2020). MCP-1 is a key chemokine involved in the migration and infiltration of monocytes and macrophages (Deshmane et al., 2009). F4/80 is the major marker of macrophages (dos Anjos Cassado, 2017). IL-6 is a cytokine that plays multiple roles in immune responses and inflammation (Tanaka et al., 2014). To investigate the intervention of piperine on the inflammation of adipose tissue, the mRNA levels of MCP1, F4/80, and IL-6 were measured. The mRNA levels of MCP1, F4/80, and IL-6 were vigorously increased by HFD feeding, and greatly lowered by piperine, especially at 40 mg/kg. Our data suggested that piperine might attenuate the inflammatory state in the visceral adipose tissue, and may be used to lower obesity-relevant metabolic inflammation.
Taken together, piperine effectively ameliorated obesity induced by HFD feeding in mice.
GM is considered to play a critical role in the development of obesity. Interstingly, we found that GM was modulated by piperine. Comparing GM composition among groups at different levels could provide key information on the effects of piperine.
At the phylum level, HFD exhibited an extensive effect on different phyla, and piperine treatment did not alter the major phyla with the exception of Tenericutes. Tenricutes displayed a high level of correlation with obesity phenotypes (Fig. S1). Tenericutes is represented by a single class, Mollicutes, which has been shown to be increased in the HFD-fed mice and can cause ulcerative colitis in humans (Allen et al., 2015; Wirostko et al., 1990). Although Tenericutes had a very low abundance, the role of Tenericutes related to the beneficial effects of piperine on obesity should be pursued in future studies.
At the family level, Muribaculaceae (previously known as S24-7), a dominant Bacteroidetes phylum member, was remarkably decreased by HFD, but largely restored by piperine treatment (Fig. 6G). Muribaculaceae is found almost exclusively in the guts of homeothermic animals, including human being (Ormerod et al., 2016). Studies have suggested that Muribaculaceae is capable of producing short-chain fatty acids (SCFAs) and succinate (Ormerod et al., 2016; Van den Abbeele et al., 2022), all of which may play a beneficial role in the prevention and treatment of obesity and its comorbidities (Canfora et al., 2019). Since few species in the Muribaculaceae are classified, the correlation analysis at the genus level could not be done. However, at the family level, Muribaculaceae was significantly negatively correlated with body weight, liver weight, perirenal fat weight, serum ALT, and mRNA level of MCP1 and F4/80 in the perirenal fat (Fig. S2), suggesting that it might play a vital role in prevention of obesity by piperine.
The abundance of the Ruminococcaceae family members, Intestinimonas, Ruminiclostridium, Ruminococcaceae_UCG-014 and Oscillibacter were found to be increased by piperine. Coincidentally, all of the 4 Ruminococcaceae members were negatively correlated with obesity phenotypes (Fig. 8). This finding was in agreement with previous results claiming that Ruminococcaceae might play an important role in preventing metabolic syndrome (Feng et al., 2021; Jiang et al., 2015). Ruminococcaceae is common in mammalian intestines, and has the ability to degrade cellulose and hemicellulose to produce SCFAs, which are absorbed in the gut and used as energy by the host. In addition, SCFAs have been demonstrated to play a protective role against obesity (Kim et al., 2019).
Therefore, the beneficial effects of piperine on the maintenance of normal body weight and serum markers in the high-dose group might be partly due to the elevation of Muribaculaceae and Ruminococcaceae abundance to produce SCFAs and succinate.
Dubosiella genus (Firmicutes phylum) is from the Erysipelotrichidae family with one known species (Dubosiella newyorkensis), found in murine intestinal microbiota (Cox et al., 2017). Dubosiella was reported to be increased by HFD (Bai et al., 2019). Furthermore, it is positively correlated with body weight gain but negatively correlated with fecal SCFAs, indicating a potential role in the development of obesity-related GM dysbiosis (Qiu et al., 2021). Enterorhabdus (Actinobacteria phylum) was first isolated from the inflamed ileal samples of TNFdeltaARE mice (Clavel et al., 2009), and was found to be related to inflammation and obesity (Li et al., 2019; Zhuang et al., 2020). In this study, the abundance of Dubosiella and Enterorhabdus was significantly decreased by piperine at 40 mg/kg, and both were positively correlated with obesity-related features (Fig. 8). Therefore, the anti-obesity effect of piperine might be associated with the inhibition of the growth of Dubosiella and Enterorhabdus.
5. Conclusion
Piperine significantly mitigated HFD-induced obesity in mice, evidenced by the lowered body weight gain, ameliorated serum indicators, the attenuation of fatty liver, and the decrease of visceral fat deposition. At the same time, piperine elevated GM diversity and altered GM composition. The abundance of Muribaculaceae and Ruminococcaceae families were elevated, and the Dubosiella and Enterorhabdus genera were suppressed. Intriguingly, piperine-altered phylotypes were highly correlated with the phenotypes of obesity. Therefore, we concluded that piperine's anti-obesity effect might partly attributed to GM regulation. Our findings reveal a new mechanism that may underlie the anti-obesity action of piperine.
CRediT authorship contribution statement
Jianlin He: Conceptualization, Methodology, Formal analysis, Funding acquisition, Writing – review & editing. Qingqing Le: Resources, Methodology, Visualization, Data curation. Yufeng Wei: Conceptualization, Writing – review & editing. Longhe Yang: Resources, Methodology, Data curation. Bing Cai: Conceptualization, Resources, Project administration. Yuansen Liu: Resources, Methodology, Data curation. Bihong Hong: Conceptualization, Resources, Project administration, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We appreciate the financial support of the Scientific Research Foundation of Third Institute of Oceanography, Ministry of Natural Resources, China (No.2018020).
Handling Editor: Dr. Yeonhwa Park
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crfs.2022.08.018.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Allen J.M., Berg Miller M.E., Pence B.D., Whitlock K., Nehra V., Gaskins H.R., White B.A., Fryer J.D., Woods J.A. Voluntary and forced exercise differentially alters the gut microbiome in C57BL/6J mice. J. Appl. Physiol. 2015;118:1059–1066. doi: 10.1152/japplphysiol.01077.2014. [DOI] [PubMed] [Google Scholar]
- Aswar U., Shintre S., Chepurwar S., Aswar M. Antiallergic effect of piperine on ovalbumin-induced allergic rhinitis in mice. Pharm. Biol. 2015;53:1358–1366. doi: 10.3109/13880209.2014.982299. [DOI] [PubMed] [Google Scholar]
- Bai Y.F., Wang S.W., Wang X.X., Weng Y.Y., Fan X.Y., Sheng H., Zhu X.T., Lou L.J., Zhang F. The flavonoid-rich Quzhou Fructus Aurantii extract modulates gut microbiota and prevents obesity in high-fat diet-fed mice. Nutr. Diabetes. 2019;9:30. doi: 10.1038/s41387-019-0097-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfora E.E., Meex R.C., Venema K., Blaak E.E. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 2019;15:261–273. doi: 10.1038/s41574-019-0156-z. [DOI] [PubMed] [Google Scholar]
- Cani P.D., Van Hul M., Lefort C., Depommier C., Rastelli M., Everard A. Microbial regulation of organismal energy homeostasis. Nat. Metab. 2019;1:34–46. doi: 10.1038/s42255-018-0017-4. [DOI] [PubMed] [Google Scholar]
- Chen Y., Zhou J., Wang L. Role and mechanism of gut microbiota in human disease. Front. Cell. Infect. Microbiol. 2021;11 doi: 10.3389/fcimb.2021.625913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S., Choi Y., Choi Y., Kim S., Jang J., Park T. Piperine reverses high fat diet-induced hepatic steatosis and insulin resistance in mice. Food Chem. 2013;141:3627–3635. doi: 10.1016/j.foodchem.2013.06.028. [DOI] [PubMed] [Google Scholar]
- Clavel T., Charrier C., Braune A., Wenning M., Blaut M., Haller D. Isolation of bacteria from the ileal mucosa of TNFdeltaARE mice and description of Enterorhabdus mucosicola gen. nov., sp. nov. Int. J. Syst. Evol. Microbiol. 2009;59:1805–1812. doi: 10.1099/ijs.0.003087-0. [DOI] [PubMed] [Google Scholar]
- Cox L.M., Sohn J., Tyrrell K.L., Citron D.M., Lawson P.A., Patel N.B., Iizumi T., Perez-Perez G.I., Goldstein E.J., Blaser M.J. Description of two novel members of the family Erysipelotrichaceae: Ileibacterium valens gen. nov., sp. nov. and Dubosiella newyorkensis, gen. nov., sp. nov., from the murine intestine, and emendation to the description of Faecalibacterium rodentium. Int. J. Syst. Evol. Microbiol. 2017;67:1247. doi: 10.1099/ijsem.0.001793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmane S.L., Kremlev S., Amini S., Sawaya B.E. Monocyte chemoattractant protein-1 (MCP-1): an overview. J. Interferon Cytokine Res. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Anjos Cassado A. vol. 62. Springer; Cham: 2017. pp. 161–179. (F4/80 as a Major Macrophage Marker: the Case of the Peritoneum and Spleen. Macrophages). [DOI] [PubMed] [Google Scholar]
- Feng L., Zhou J., Zhang L., Liu P., Wan X. Gut microbiota-mediated improvement of metabolic disorders by Qingzhuan tea in high fat diet-fed mice. J. Funct.Foods. 2021;78 doi: 10.1016/j.jff.2021.104366. [DOI] [Google Scholar]
- Feng M., Darabi M., Tubeuf E., Canicio A., Lhomme M., Frisdal E., Lanfranchi-Lebreton S., Matheron L., Rached F., Ponnaiah M. Free cholesterol transfer to high-density lipoprotein (HDL) upon triglyceride lipolysis underlies the U-shape relationship between HDL-cholesterol and cardiovascular disease. Eur. J. Prev. Cardiol. 2020;27:1606–1616. doi: 10.1177/2047487319894114. [DOI] [PubMed] [Google Scholar]
- Formica V., Morelli C., Riondino S., Renzi N., Tesauro M. Obesity and common pathways of cancer and cardiovascular disease. Endocrinol. Metab. Sci. 2020;1 doi: 10.1016/j.endmts.2020.100065. [DOI] [Google Scholar]
- Gomes A.C., Hoffmann C., Mota J.F. The human gut microbiota: metabolism and perspective in obesity. Gut Microb. 2018;9:308–325. doi: 10.1080/19490976.2018.1465157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S.E., Llewellyn C.H., Smith L. vol. 8. SAGE Open Med; 2020. (The Obesity Epidemic – Nature via Nurture: A Narrative Review of High-Income Countries). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W., Wu N., Wang X., Chi Y., Zhang Y., Qiu X., Hu Y., Li J., Liu Y. Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease. Sci. Rep. 2015;5:8096. doi: 10.1038/srep08096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jwa H., Choi Y., Park U.-H., Um S.-J., Yoon S.K., Park T. Piperine, an LXRα antagonist, protects against hepatic steatosis and improves insulin signaling in mice fed a high-fat diet. Biochem. Pharmacol. 2012;84:1501–1510. doi: 10.1016/j.bcp.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Kim K.N., Yao Y., Ju S.Y. Short chain fatty acids and fecal microbiota abundance in humans with obesity: a systematic review and meta-analysis. Nutrients. 2019;11:2512. doi: 10.3390/nu11102512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Li J., Mao G., Yan L., Hu Y., Ye X., Tian D., Linhardt R.J., Chen S. Effect of the sulfation pattern of sea cucumber-derived fucoidan oligosaccharides on modulating metabolic syndromes and gut microbiota dysbiosis caused by HFD in mice. J. Funct.Foods. 2019;55:193–210. doi: 10.1016/j.jff.2019.02.001. [DOI] [Google Scholar]
- Li X., Watanabe K., Kimura I. Gut microbiota dysbiosis drives and implies novel therapeutic strategies for diabetes mellitus and related metabolic diseases. Front. Immunol. 2017;8:1882. doi: 10.3389/fimmu.2017.01882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Yuan Y., Zhou J., Hu R., Ji L., Jiang G. Piperine ameliorates insulin resistance via inhibiting metabolic inflammation in monosodium glutamate-treated obese mice. BMC Endocr. Disord. 2020;20:1–15. doi: 10.1186/s12902-020-00617-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loos R.J.F., Yeo G.S.H. The genetics of obesity: from discovery to biology. Nat. Rev. Genet. 2022;23:120–133. doi: 10.1038/s41576-021-00414-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- März W., Kleber M.E., Scharnagl H., Speer T., Zewinger S., Ritsch A., Parhofer K.G., von Eckardstein A., Landmesser U., Laufs U. HDL cholesterol: reappraisal of its clinical relevance. Clin. Res. Cardiol. 2017;106:663–675. doi: 10.1007/s00392-017-1106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCD Risk Factor Collaboration Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 2017;390:2627–2642. doi: 10.1016/S0140-6736(17)32129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogara L., Naber N., Pate E., Canton M., Reggiani C., Cooke R. Piperine's mitigation of obesity and diabetes can be explained by its up-regulation of the metabolic rate of resting muscle. Proc. Natl. Acad. Sci. U.S.A. 2016;113:13009–13014. doi: 10.1073/pnas.1607536113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormerod K.L., Wood D.L., Lachner N., Gellatly S.L., Daly J.N., Parsons J.D., Dal'Molin C.G., Palfreyman R.W., Nielsen L.K., Cooper M.A. Genomic characterization of the uncultured Bacteroidales family S24-7 inhabiting the guts of homeothermic animals. Microbiome. 2016;4:1–17. doi: 10.1186/s40168-016-0181-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X., Macchietto M.G., Liu X., Lu Y., Ma Y., Guo H., Saqui-Salces M., Bernlohr D.A., Chen C., Shen S. Identification of gut microbiota and microbial metabolites regulated by an antimicrobial peptide lipocalin 2 in high fat diet-induced obesity. Int. J. Obes. 2021;45:143–154. doi: 10.1038/s41366-020-00712-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R., Kumar A., Singh N., Sharma K. 16S rRNA gene profiling of rhizospheric microbial community of Eichhornia crassipes. Mol. Biol. Rep. 2021;48:4055–4064. doi: 10.1007/s11033-021-06413-x. [DOI] [PubMed] [Google Scholar]
- Shu G., Yusuf A., Dai C., Sun H., Deng X. Piperine inhibits AML-12 hepatocyte EMT and LX-2 HSC activation and alleviates mouse liver fibrosis provoked by CCl4: roles in the activation of the Nrf2 cascade and subsequent suppression of the TGF-beta1/Smad axis. Food Funct. 2021;12:11686–11703. doi: 10.1039/D1FO02657G. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Narazaki M., Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harbor Perspect. Biol. 2014;6 doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Abbeele P.V.d., Ghyselinck J., Marzorati M., Koch A.-M., Lambert W., Michiels J., Chalvon-Demersay T. The effect of amino acids on production of SCFA and bCFA by members of the porcine colonic microbiota. Microogranisms. 2022;10:762. doi: 10.3390/microorganisms10040762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirostko E., Johnson L., Wirostko B. Ulcerative colitis associated chronic uveitis. Parasitization of intraocular leucocytes by mollicute-like organisms. J. Submicr. Cytol. Pathol. 1990;22:231–239. [PubMed] [Google Scholar]
- Zhang Y., Zhao N., Yang L., Hong Z., Cai B., Le Q., Yang T., Shi L., He J., Cui C.B. Insoluble dietary fiber derived from brown seaweed Laminaria japonica ameliorate obesity-related features via modulating gut microbiota dysbiosis in high-fat diet-fed mice. Food Funct. 2021;12:587–601. doi: 10.1039/d0fo02380a. [DOI] [PubMed] [Google Scholar]
- Zhuang P., Zhang Y., Shou Q., Li H., Zhu Y.e., He L., Chen J., Jiao J. Eicosapentaenoic and docosahexaenoic acids differentially alter gut microbiome and reverse high‐fat diet–induced insulin resistance. Mol. Nutr. Food Res. 2020;64 doi: 10.1002/mnfr.201900946. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.