Abstract
Obesity is a significant risk factor for the development of knee osteoarthritis (KOA). However, the precise molecular mechanisms linking obesity to OA remain unclear. In the present study, we investigated the effect of short-term high-fat diet (HFD) on the development of OA and the possible role of the adipokine resistin and autophagy-related genes in mediating this effect. Thirty adult male Wistar rats were equally divided into 2 groups: control and obese groups. Body mass index (BMI), levels of lipid profile, glucose, insulin and HOMA-IR index were significantly higher in the obese group compared with control. Our results revealed significantly higher serum and cartilage resistin levels with a significant increase in the mRNA expressions of toll-like receptor 4 (TLR4), matrix metalloproteinase-9 (MMP-9) and interleukin-1β (IL-1β) as well as protein levels of IL-1β, matrix metalloproteinase-13 (MMP-13), ADAMTS 5 (aggrecanase-2) and caspase-3 in the cartilage of obese rats. The HFD induced a significant upregulation of autophagy related 5 (ATG5), beclin-1 and light chain 3 (LC3) mRNA expressions and a significant downregulation of mammalian target of rapamycin (mTOR) expression in cartilage. The protein levels of cartilage ATG5 were also significantly elevated in HFD-fed group. In conclusion, we suggested that increased levels of resistin and expression of autophagy-related genes may contribute in part, to OA development in HFD-fed rats. This provides a novel insight into the early molecular changes in the cartilage associated with obesity.
Subject terms: Physiology, Diseases, Pathogenesis
Introduction
The prevalence of obesity has increased dramatically in recent decades and it is now one of the most serious health problems, with more than 1.9 billion adults are overweight and over 650 million of them are obese1. Obesity is recognized as a major risk factor for several diseases, including osteoarthritis (OA)2. OA is the most common degenerative joint disease, characterized by degradation of articular cartilage, synovial inflammation, and structural changes in bone including the formation of osteophytes and subchondral bone sclerosis3.
Obesity-related OA has been described as a separate “metabolic OA” phenotype, representing approximately 60% of OA population4. Despite the well-established link between obesity and OA, the impact of a short-term HFD on the development of OA and the underlying molecular mechanisms remain unclear.
In recent years, it has been suggested that increased adiposity in metabolic OA is associated with increased concentrations of adipokines that promote a state of chronic, low-grade inflammation contributing to joint damage5. Resistin is a 12 kDa cysteine-rich polypeptide hormone secreted by adipose tissue in macrophages and adipocytes in humans and mice, inducing inflammation and insulin resistance6. The adipokine resistin is highly expressed in serum and synovial joints of patients with OA7. In human articular cartilages, resistin induced the expression of matrix degrading enzymes and proinflammatory cytokines7–9. In addition, resistin has been shown to increase vascular adhesion molecule-1 expression on synovial fibroblasts in OA, whereas the decrease of resistin activity prevented anterior cruciate ligament transection-induced damage to OA rat cartilage10.
Autophagy is a crucial homeostatic mechanism that regulates cell metabolism and removes damaged macromolecules and organelles to maintain cellular equilibrium11. It may be separated into the following phases artificially: autophagosome induction and nucleation, autophagosome elongation, autophagosome maturation and degradation12. Autophagy process is regulated by spatiotemporal coordinated recruitment of autophagy-related proteins (ATG) (the “core machinery”) and accessory proteins13. Numerous recent studies demonstrated the importance of autophagy in normal cartilage maintenance and suggested that dysregulation of autophagy is closely associated with the pathogenesis of OA14,15. Autophagy was shown to be increased in OA-affected cartilage, according to several studies16; however, others found that autophagy was significantly reduced17.
In the present study, we aimed to investigate the effect of short-term high-fat diet on the development of OA in HFD-fed male rats and the possible role of the adipokine resistin and autophagy-related genes in mediating this effect.
Materials and methods
Ethical statement
The current protocol was approved by Alexandria University- Institutional Animal Care and Use Committee (AlexU-IACUC, Approval number: AU 0122232233). All experiments fulfill the guidelines of the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978)18 and the recommendations of Egypt's guide for the care and use of laboratory animals19. The current study adheres to the ARRIVE Guidelines for reporting in vivo experiments20. All efforts were made to curb the distress of rats during the experimental period.
Experimental animals
The present study was conducted on 30 male Wistar rats (aged 2–3 months). Rats were obtained from the animal house of the Medical Research Institute, Alexandria University, Egypt. Animals were kept 5 per cage at 23 °C in a 12 h light/12 h dark cycle under good hygienic conditions and standard humidity with access to food and water.
Experimental design
The animals were classified into two groups according to diet they were receiving: 1. Group I (Control group): 15 male rats that were maintained under normal diet (13.5% kcal fat), 2. Group II (HFD group): 15 male rats that were feeding with high fat diet (60% kcal fat) (D12492; Research Diets, New Brunswick, NJ) for 12 weeks21.
At the end of experiment, final body weight and length were recorded to calculate the body mass index (BMI). Rats were fasted for 12 h and then sacrificed by cervical dislocation under anesthesia (ketamine 100 mg/kg and xylazine 10 mg/kg intraperitoneally)22. Blood samples were collected, centrifuged at 1000×g for 20 min at 4 °C to separate the sera for biochemical analysis.
Cartilage tissues of both groups were gently dissected, cleaned with saline, and divided into two parts. The first part was homogenized in a phosphate buffered saline (PBS) in ratio of 1:9, centrifuged at 4 °C for 10 min at 10,000×g, and the supernatant was collected and stored at − 80 °C for determination of resistin, interleukin-1β (IL-1β), caspase 3, MMP-13, ADAMTS 5 and ATG-5. The second part was used for RNA extraction to analyze gene expression. The third part was used for histopathological analysis.
Biochemical analysis
Serum levels of fasting serum glucose (FBG), triglycerides (TG), total cholesterol (TC), and high density lipoprotein-cholesterol (HDL-C) were assayed using commercial available kits (Bio Med Diagnostic INC, USA). Low density lipoprotein-cholesterol (LDL-C) was estimated according to the Friedewald’s equation: LDL-C (mg/dl) = TC − (HDL-C) − (TAG/5)23.
The serum levels of insulin were assayed using immunoassay kit (EMD Millipore USA). The homeostasis model assessment index for insulin resistance (HOMA-IR) was then calculated using the following formula24:
Determination of resistin, IL-1β, caspase 3, MMP-13, ADAMTS 5 and ATG-5
IL-1β and caspase 3 levels were evaluated in cartilage homogenates using ELISA kits purchased from (MyBioSource, Inc., San Diego, CA, USA and Cusabio Biotech Co., Ltd), respectively according to their manufacturer’s instructions. A competitive ELISA kit was used for the determination of serum and cartilage resistin levels (LifeSpan BioSciences, Inc.). Matrix metalloproteinase-13 (MMP-13) and ADAMTS 5 (aggrecanase-2) were measured in cartilage homogenates using Sandwich ELISA kits purchased from (Life Span Biosciences, Inc and Mybiosource), respectively. ATG-5 protein levels were also measured in cartilage using ELISA kits purchased from (Mybiosource). The total protein concentration was determined using Lowry’s method25.
RNA extraction and real-time RT-PCR
30 mg of cartilage tissues were used for total RNA extraction using the miRNeasy Mini Kit (Qiagen, Germany) according to the manufacturer’s instructions and the concentration and integrity of extracted RNA were checked using nanodrop. The reverse transcription of the extracted RNA was performed using Reverse transcription (RT) was performed by TOPscript RT DryMIX kit (dT18/dN6 plus) (Enzynomics, Korea) according to the manufacturer instructions. The tissues expression of mammalian target of rapamycin (mTOR), beclin-1, light chain 3 (LC3), autophagy related 5 (ATG5), matrix metalloproteinase-9 (MMP-9), toll-like receptor 4 (TLR4) and IL-1β were quantified in the cDNA by CFX Maestro Software (Bio-Rad, USA) using QuantiNova SYBR Green PCR Kit (Qiagen, Germany). Quantitative PCR amplification conditions were adjusted as an initial denaturation at 95 °C for 10 min and then 45 cycles of PCR for amplification as follows: denaturation at 95 °C for 20 s, annealing at 55 °C for 20 s and extension at 70 °C for 15 s. The housekeeping gene 18S rRNA was used as a reference gene for normalization. The primers used for the determination of rat genes are presented in supplementary material (Table S1). The relative change in mRNA expression in samples was estimated using 2−ΔΔCt method.
Cartilage histology
The knee joints of adult rats were fixed in 10% neutral buffered formalin for 48 h. Then placed into hydrochloric acid to decalcify. After decalcification process, the knee joints were cut into approximately 2 equal halves. The joints were processed for paraffin embedding and sectioned at 5 μm for Hematoxylin and Eosin (H&E) stain for histopathological examination.
Statistical analysis
Data were analyzed using SPSS software package version 18.0 (SPSS, Chicago, IL, USA). The data were expressed as mean ± SE and analyzed using Student independent t test to compare between different groups. The p value was assumed to be significant at p < 0.0526. The correlation coefficients (r) between different assayed parameters were evaluated using Pearson correlation coefficient; p < 0.05 was considered as the significance limit for all comparisons.
Results
Effect of HFD feeding on anthropometric and biochemical parameters
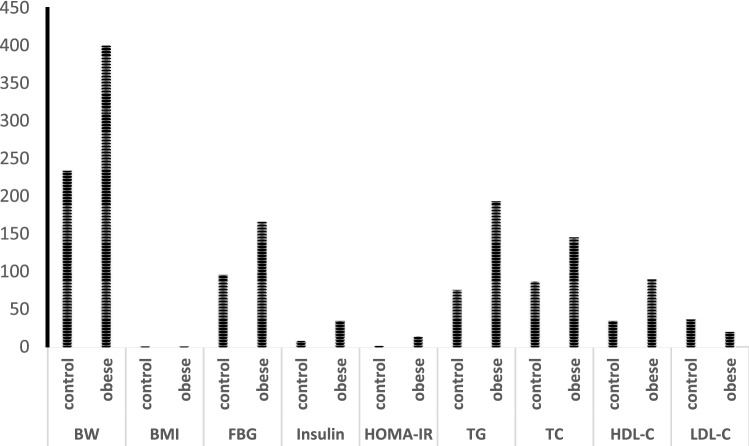
As shown in Fig. 1, our results showed significant elevation in the body weight and body mass index (BMI) in obese rats compared to control by about 71, 61%, respectively. The serum levels of fasting glucose and insulin as well as HOMA-IR index were all significantly increased by about 72, 333, 652%, respectively in the same group compared to control. In addition, HFD feeding induced a significant increase in serum TG, TC and LDL-C levels by about 154, 67 and 148%, respectively compared with normal rats, whereas the serum HDL-C levels were significantly reduced by about—45%.
Figure 1.
Data were illustrated as mean ± SD, *p < 0.05, indicating a statistically significant difference when compared with control group using independent sample t test.
Effect of HFD feeding on resistin levels and TLR4 expression
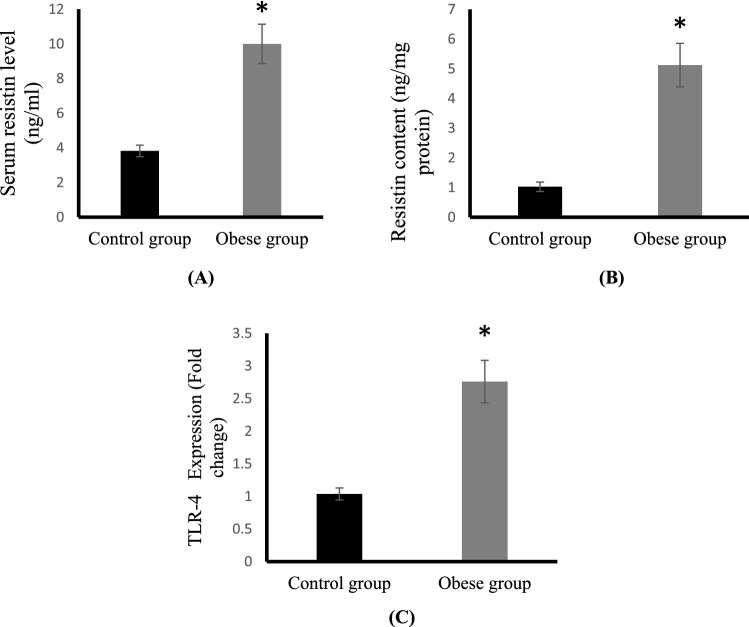
Obese rats showed highly significant increase in the serum level and cartilage tissue content of resistin by about 161, 398%, respectively compared to control rats (Fig. 2A,B). The mRNA expression of TLR4 was significantly upregulated in cartilage of obese group compared to control (Fig. 2C).
Figure 2.
Serum resistin levels (ng/ml) (A), cartilage resistin content (ng/mg protein) (B) and cartilage TLR4 mRNA expression (C) in the control and obese groups.
Effect of HFD feeding on osteoarthritis markers
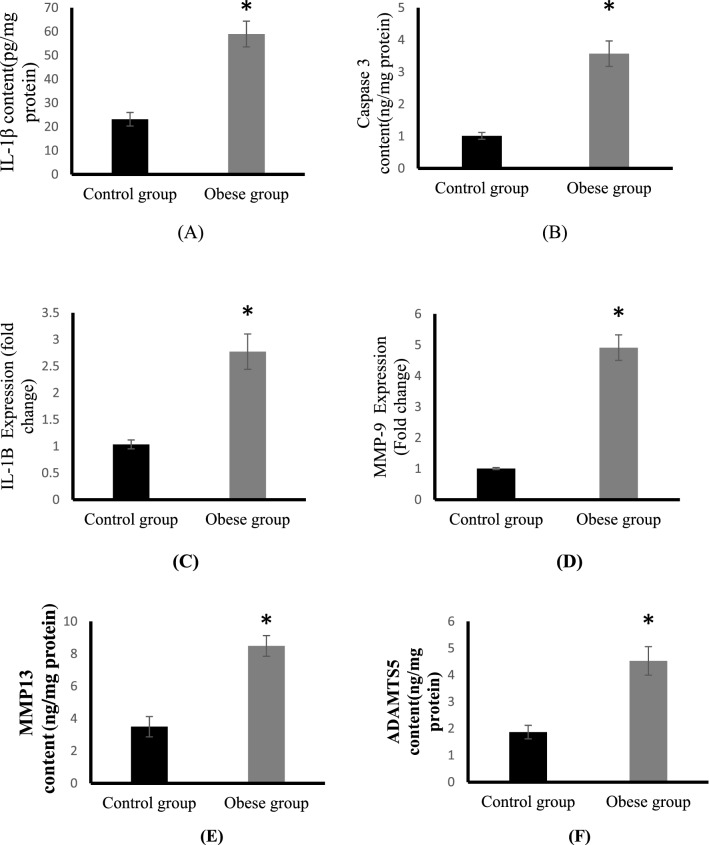
As shown in Fig. 3, all osteoarthritis parameters including IL-1β, caspase 3 and MMP-9 were significantly increased in the obese group compared to control. Obese rats showed highly significant increase in cartilage contents of IL-1β and caspase 3 by about 154, 252%, respectively compared to control rats (Fig. 3A,B). The Cartilage tissue expression (fold change) of IL-1β and MMP-9 are shown in (Fig. 3C,D), respectively. Our results revealed that HFD promoted a significant upregulation of IL-1β expression by threefold in obese group compared to control, whereas MMP-9 expression was significantly increased fivefold in the same group than control group. Also, HFD caused a significant increase in MMP13 and ADAMTS5 protein levels in cartilage (Fig. 3E,F).
Figure 3.
Cartilage mRNA expressions of IL-1β (pg/mg protein) (A) and caspase 3 (ng/mg protein) (B) IL-1β expression (C) and MMP-9 expression (D), MMP13 (ng/mg protein) (E) and ADAMTS5 (ng/mg protein) (F) in the control and obese groups.
Effect of HFD feeding on autophagy markers
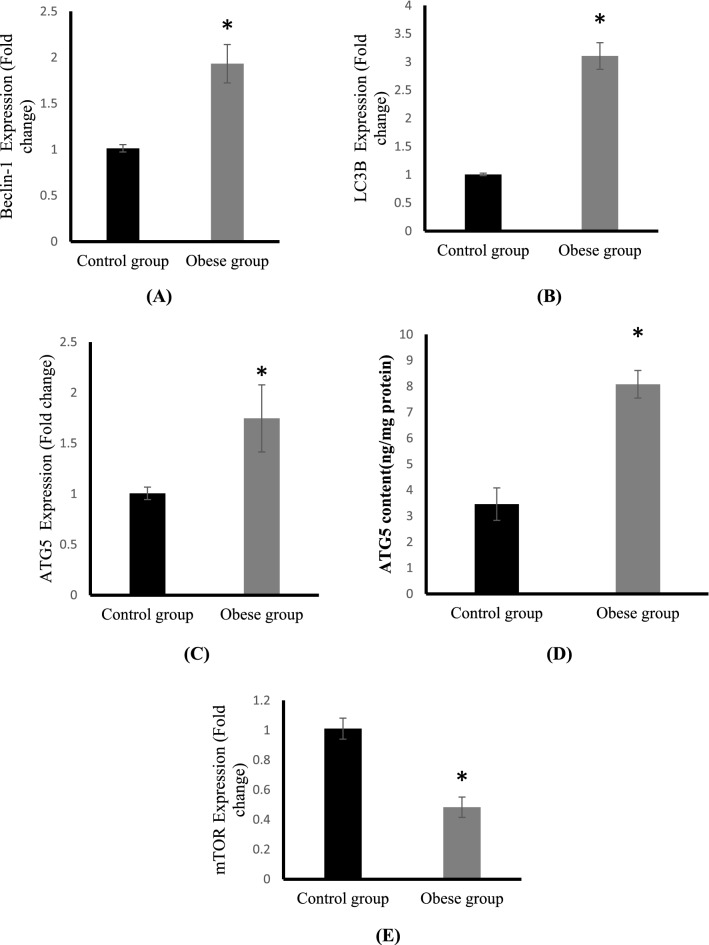
The expression of autophagy genes including Beclin-1, LC3B and ATG5 and the negative regulator of autophagy, mTOR in cartilage tissue are shown in Fig. 4. HFD feeding induced a significant upregulation of mRNA expressions of beclin-1 and LC3B in cartilage tissues by two fold as compared to control (Fig. 4A,B). A significant increase in ATG5 mRNA expression to one and half fold was also detected in the cartilage tissues of obese rats compared with cartilage tissues of control rats (Fig. 4C). In addition, a significant increase in ATG-5 protein levels were detected in the cartilage of HFD-fed rats (Fig. 4D).
Figure 4.
Cartilage mRNA expressions of Beclin-1 (A), LC3B (B), ATG5 (C), ATG5 protein levels (D) and mTOR expression (E) in the control and obese groups.
As shown in Fig. 4E, compared with control group, the mRNA expression of mTOR in articular cartilage was significantly downregulated and reduced to half in the obese group.
Correlation results
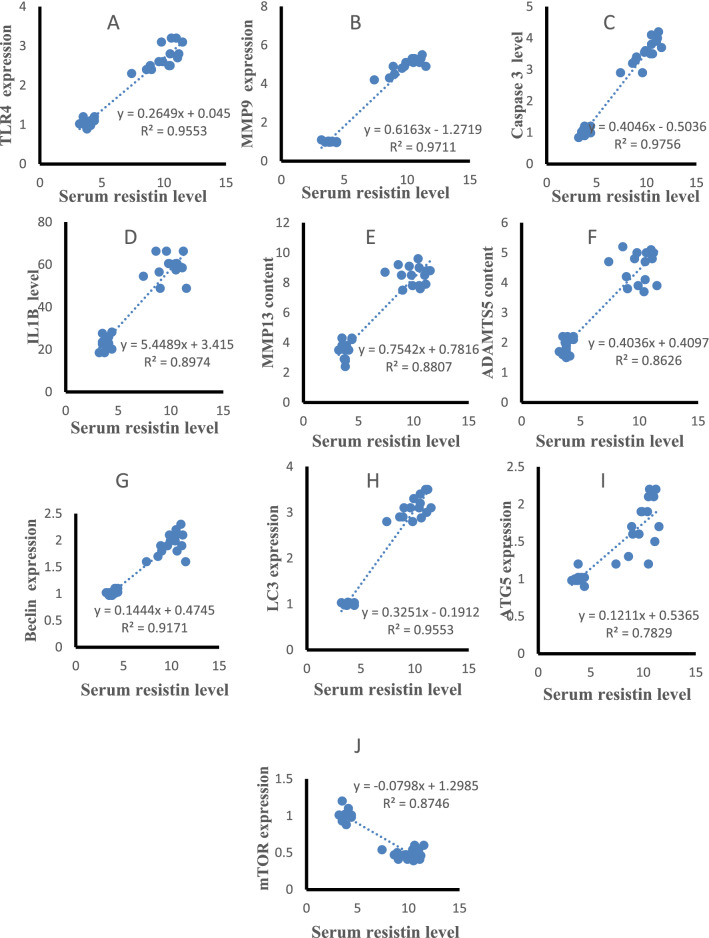
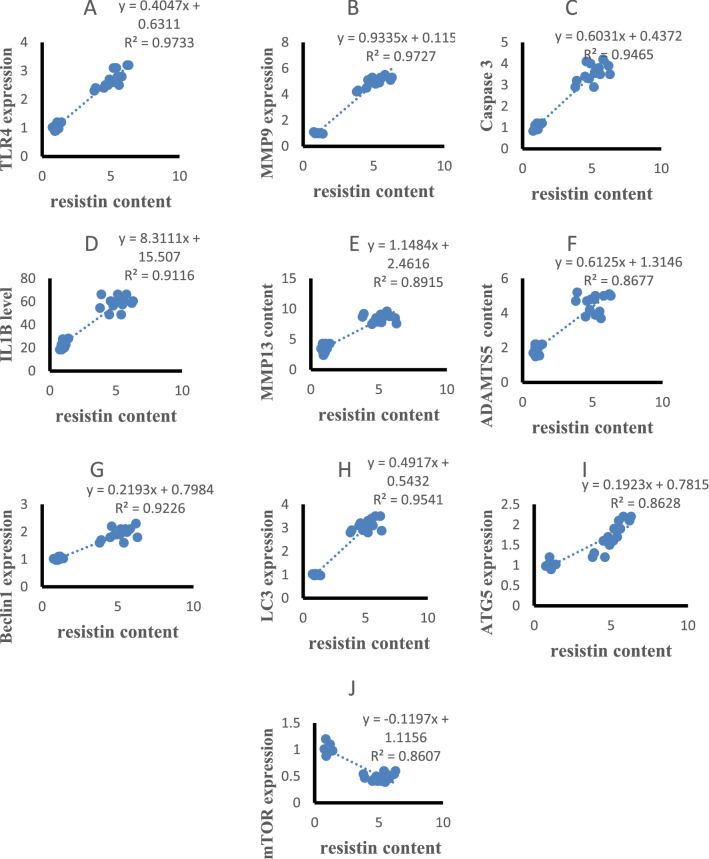
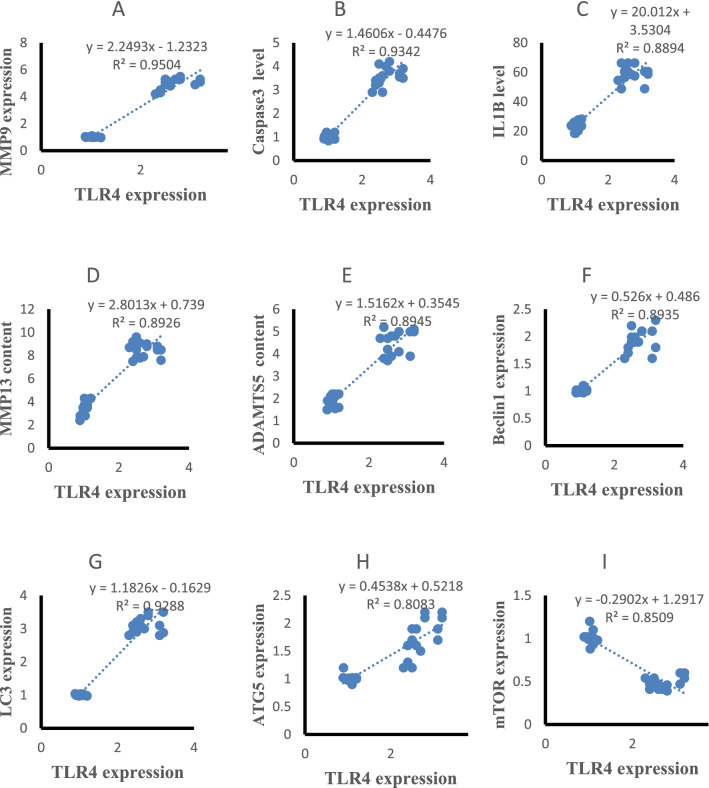
The statistical analysis using Pearson correlation revealed significant positive correlations of serum resistin with TLR4 and MMP9 expression (r = 0.947, p < 0.001, r = 0.951, p < 0.001, Fig. 5A,B), caspase 3, IL-1β, MMP-13 and ADAMTS5 content (r = 0.915, p < 0.001, r = 0.955, p < 0.001, r = 0.951, p < 0.001, r = 0.944, p < 0.001, Fig. 5C–F), Beclin-1, LC3B, ATG5 expression (r = 0.938, p < 0.001, r = 0.948, p < 0.001, r = 0.840, p < 0.001 Fig. 5G–I) and negatively correlated with mTOR expression(r = − 0.924, p < 0.001, Fig. 5J) in all studied groups. Cartilage resistin content was positively correlated with TLR4 and MMP9 expression (r = 0.934, p < 0.001, r = 0.955, p < 0.001, Fig. 6A,B), caspase 3, IL-1β, MMP-13 and ADAMTS5 content (r = 0.955, p < 0.001, r = 0.910, p < 0.001, r = 0.962, p < 0.001, r = 0.952, p < 0.001, Fig. 6C–F), Beclin-1, LC3B, ATG5 expression (r = 0.918, p < 0.001, r = 0.949, p < 0.001, r = 0.815, p < 0.001 Fig. 6G–I) and negatively correlated with mTOR expression (r = − 0.944, p < 0.001, Fig. 6J) in all studied groups. TLR4 expression was positively correlated with MMP9 expression (r = 0.963, p < 0.001, Fig. 7A), caspase 3, IL-1β, MMP-13 and ADAMTS5 content (r = 0.946, p < 0.001, r = 0.927, p < 0.001, r = 0.967, p < 0.001, r = 0.916, p < 0.001, Fig. 7B–E), Beclin-1, LC3B, ATG5 expression (r = 0.894, p < 0.001, r = 0.962, p < 0.001, r = 0.883, p < 0.001 Fig. 7F–H) and negatively correlated with mTOR expression (r = − 0.947, p < 0.001, Fig. 7I) in all studied groups.
Figure 5.
Correlations of serum resistin levels with other studied parameters in control and HFD groups.
Figure 6.
Correlations of cartilage resistin levels with other studied parameters in control and HFD groups.
Figure 7.
Correlations of cartilage TLR4 expression with other studied parameters in control and HFD groups.
Histopathological results
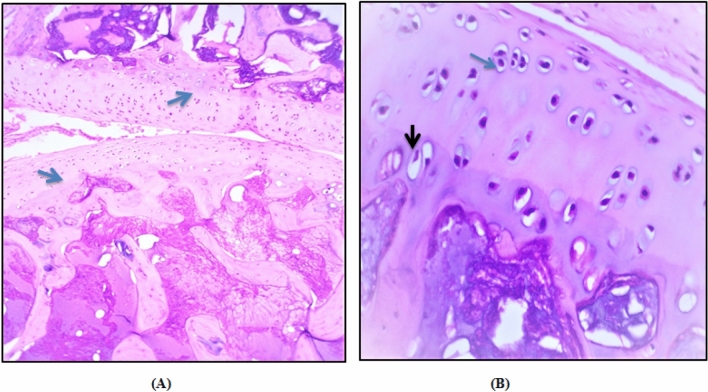
In the control group, H&E staining of cartilage showed a preserved morphological structure with no signs of cartilage degradation. The surface of knee cartilage appeared white, shiny, and firm. It has a smooth surface without fissures. Further, Non-calcified and calcified portions of articular cartilage were separated by a basophilic tidemark (Fig. 8A). The chondrocytes were lying centrally within its lacunae (Fig. 8B). Moreover, they showed finely granular cytoplasm and centrally located nuclei. The most hypertrophic chondrocytes was observed to locate in the calcified cartilage zone.
Figure 8.
Photomicrographs illustrating the normal microscopic features of sections of articular cartilage of the adult rat. (A) Articular cartilage of proximal portion of the tibia the distal portion of the femur is separated into calcified and noncalcified region by a basophilic tidemark (blue arrow). Magnification × 10. (B) Chondrocytes lying centrally within its lacunae with centrally located nuclei (blue arrow) and hypertrophic chondrocytes (black arrow) are located in the calcified cartilage near the subchondral bone. Magnification × 40.
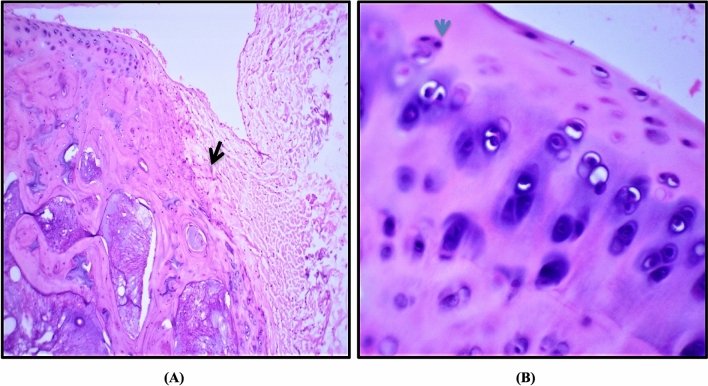
Knee cartilage of the HFD group showed dull and irregular surface with deep surface cleft. Moreover, disappearance of cells from tangential zone was observed with enlargement and disorganization of the chondrocytes in other zones which are not arranged in columns. This was accompanied by changes in the cartilaginous matrix including fibrillation at the articular surface (Fig. 9A). Also, the subchondral bone showed Fibrillation and the basophilic tidemark is no longer intact. A significant chondrocyte loss but with collagen retention was also observed (Fig. 9B). These structural alterations led to reduction of cartilage thickness of the superficial and middle zones.
Figure 9.
Photomicrographs illustrating the lesions resulting from HFD. (A) Fibrillation and fissuring of articular cartilage progressed to erosion of non-calcified cartilage was shown (black arrow) and the tidemark is no longer intact. Magnification × 10. (B) Most of chondrocytes were highly destructed with no clear lacunae (blue arrow) and pyknotic nuclei was also observed. Magnification × 40.
Discussion
Obesity is considered one of the well-recognized and modifiable risk factors of knee osteoarthritis (KOA)27. Previous studies have demonstrated that obesity causes a state of low-grade chronic inflammation that consists of inflammatory cytokines and abnormal metabolites, leading to cartilage matrix impairment, synovitis and subchondral bone remodeling28,29. In the present study, the protocol implemented to generate obesity was a 12-week high fat diet, which led to a significant increase in the total body mass and as expected was associated with dyslipidemia, hyperglycemia and insulin resistance. Our findings showed that short-term HFD feeding for 12 weeks induced a significant increase in mRNA expressions of matrix metalloproteinase-9 (MMP-9) and interleukin-1β (IL-1β) as well as the protein levels of IL-1β, matrix metalloproteinase-13 (MMP-13), ADAMTS 5 (aggrecanase-2) and caspase-3 in the cartilage tissue, leading to articular cartilage degeneration. This was confirmed by histopathological investigation of articular cartilage of HFD-fed rats. In the present study, H&E staining showed clear characteristic features of OA in HFD group as compared to the control group. The number of chondrocytes was markedly decreased, which could be explained by chondrocyte death due to necrosis or apoptosis as pyknotic nuclei were observed. Also, fissures gradually extended through the full thickness of the cartilage and into subchondral bone led to complete loss of cartilage. This was concordant with the previous works in knee joints used different model systems. Sun et al.30 found that obesogenic diets consisting of high-fat, high-carbohydrate promoted systemic and local synovial inflammation and contributed to the development of OA. In another study by Collins et al.31, a high-fat/high-sucrose (HFS) diet caused OA-like joint degeneration in the shoulders, knees, and hips in a rat model of OA. In a mouse model, it was also discovered that a 16-week high-fat diet introduction was linked to alterations in knee cartilage mechanical characteristics, which are thought to be an early predictor of OA development32.
Regardless of the fact that there is a well-established link between obesity and OA, the underlying mechanisms are still poorly understood. In recent years, the involvement of adipokines in the development of OA pathogenesis has gained increasing attention, and it is suggested to be crucial mediators linking obesity to chronic, low-grade inflammation and joint damage33.
Resistin, also known as ADSF (adipose tissue-specific secretory factor) or FIZZ3, (in inflammatory zone 3) is a novel adipokine that has been suggested to play an important role in obesity, insulin resistance and inflammation34. It is a cysteine-rich polypeptide secreted from adipocytes in rodents and humans; however, macrophages are the major source of resistin in humans34. Resistin has recently been revealed to have a role in the pathophysiology and development of OA6. Our findings demonstrated that HFD-induced obese rats had considerably greater serum and cartilage resistin levels than control rats.
Several research have looked at resistin's function in the pathophysiology of OA. Clinical investigations have found a substantial positive relationship between OA severity and levels of resistin in human OA serum, synovial fluid, and cartilage tissues, which is consistent with the current findings35. In patients with knee osteoarthritis, the serum levels of resistin were related to cartilage defects and bone marrow lesions36.
According to a recent research, it was shown that the levels of resistin in OA patients were higher than in healthy controls by Alissa et al.27, and they were positively linked with inflammatory markers. In articular cartilages from humans, the production of matrix degrading enzymes and inflammatory cytokines has been found to be increased by resistin8,9. Resistin has also been shown to rise systemically and locally following joint damage, and to have a direct influence on cartilage matrix turnover and cytokine production in vitro37. Inflammatory cytokines are known to trigger apoptosis in chondrocytes by releasing cytochrome c from mitochondria and activating the caspase gene38.
Resistin's inflammatory effects in OA have been documented7,10. In human chondrocytes, Zhao et al.7 suggested that resistin stimulates expression of proinflammatory cytokines and matrix-degrading enzymes via activation of p38 mitogen-activated protein kinase (p38-MAPK) and nuclear factor-κB (NF-κB) signaling pathways. Furthermore, Chen et al.10 demonstrated that resistin increases the expression of vascular adhesion molecule-1 (VCAM-1) on human synovial fibroblasts in OA by inhibiting miR 381 synthesis. Upregulation of VCAM-1 facilitates the adhesion of monocytes to synovial fibroblasts, leading to increased synovial inflammation.
In rats and humans, resistin contains a variety of functional receptors, and these receptors play a significant role in resistin-induced inflammation6. Toll-like receptor 4 (TLR4) is linked to metabolic inflammation and insulin resistance in obese people39,40 and OA patients41. Activation of TLR4 induces NF-κB signaling and proinflammatory cytokine production, which are both upregulated in OA joint tissues42.
Several studies reported that TLR4 acts as a functional receptor directly binding resistin and is involved in the proinflammatory effect of resistin in different tissues39. Li et al.43 found that resistin binds to TLR4 and causes macrophage infiltration by increasing CCL4 expression in the nucleus pulposus cells of the human intervertebral disc. TLR4-mediated activation of swine alveolar macrophages by resistin was shown to promote the inflammatory cytokines production44. Moreover, Miao et al.45 demonstrated that resistin suppressed autophagy in mice hypothalamus through TLR4, which subsequently led to increased inflammation. In the present study, we demonstrated that TLR4 expression is increased in cartilage tissue of the obese group compared with control. It was positively associated with resistin levels, matrix metalloproteinase-9 (MMP-9) and TLR4 expressions and protein levels of IL-1β, caspase-3, MMP-13 and ADAMTS5 content. This finding indicated that TLR4 could be a putative receptor of resistin in the articular cartilage. However, more future investigations are needed to determine whether resistin directly binds to TLR-4 in the cartilage and to uncover the roles of resistin-TLR4 pathway and other resistin receptors such as decorin, receptor tyrosine kinase-like orphan receptor 1 (ROR1), and adenylyl cyclase-associated protein 1 (CAP1) in the pathophysiology and progression of OA.
Autophagy is a lysosomal degradation pathway that is necessary for regulation of energy and nutrition as well as maintenance of energy metabolism in the body12,46. The autophagy-related proteins (ATG) (the core machinery) and accessory proteins are recruited in a spatially coordinated manner13. Among ATG proteins, autophagy related 5 (ATG5), beclin-1 and light chain 3 (LC3) are regarded the most commonly targeted genes and widely used markers for monitoring autophagy activity and flux47,48. Autophagy process is also controlled by the mammalian target of rapamycin (mTOR), a serine/threonine protein kinase that is a major negative regulator of autophagy49.
Recently, deregulation of autophagy has been implicated in the pathogenesis and progression of both obesity50 and OA14,15, but the results regarding changes in autophagy are contradictory. The current research revealed significant upregulation of ATG5, Beclin-1 and LC3 mRNA expressions and downregulation of mTOR mRNA expression as well as increased ATG5 protein levels in the cartilage of obese group, which is consistent with Shin et al.14, study on monosodium iodoacetate (MIA)-induced rodent model of OA. We suggested that the upregulation of autophagy in this context may be a part of a cellular defense mechanism under obesity-associated stresses.
Our results are in contrast with other reports that showed reduced autophagy level in OA cartilage15 and alleviated OA severity by autophagy activation51. With the progression of OA, mTOR was shown to rise, resulting in the repression of autophagy in articular cartilage and the encouragement of cartilage degradation52.
It has been shown that autophagy is upregulated in chondrocytes and cartilage during the early degenerative phase of OA to regulate changes in OA-like gene expression by managing oxidative stress and apoptosis. Thus, autophagy is an adaptive, protective response in cartilage, whereas reduced autophagy leads to cell death as cartilage degrades53.
In Yao et al., study54, HFD feeding over 28 weeks generated a reduction in beclin1 and LC3B protein expression and an increase in the expression of p-mTOR in articular cartilage, resulting in OA-like lesions, which contradicts our results. This might be linked to the length of time on HFD. Thus, we suggested that the duration of HFD consumption is one of the most important aspects affecting autophagic response in the cartilage.
In recent years, the relationship between resistin/TLR4 signaling and autophagy has been studied. Miao et al.45 showed that resistin/TLR4 is regarded a regulatory pathway of neuronal autophagy, but the role of resistin/TLR4 in cartilage autophagy was not yet investigated and remains unknown. Our findings revealed that serum and cartilage resistin levels as well as TLR4 expression were positively associated with cartilage expressions of ATG5, Beclin-1 and LC3 expressions and negatively correlated with mTOR, suggesting that resistin and its receptor, TLR4 may have a role in the activation of autophagy and subsequent cartilage damage. However, such an assumption will require further studies to be established and to determine if there is a direct link between resistin/TLR4 signaling and autophagy in chondrocytes.
In conclusion, this study indicated that short-term HFD increases resistin levels and upregulates autophagy-related genes in the cartilage, which may contribute to OA development. This finding provides a novel insight into the underlying mechanisms linking obesity to OA pathogenesis and opens the door for future work investigating the role of resistin/TLR4/autophagy pathway.
Supplementary Information
Author contributions
H.M.A. suggested the research topic, participated in the experimental design, performed the biochemical analysis and ELISA techniques, wrote the paper, and revised the paper. S.A.S. performed molecular experiments, carried out the statistical analysis of data and helped in the writing of the paper, and revised of the paper. M.S. performed histopathological analysis. All authors have read and approved the final paper.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Data availability
The datasets used and/or analysed are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-19481-1.
References
- 1.World Health Organization. Obesity and overweight. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (2020).
- 2.Raud B, et al. Level of obesity is directly associated with the clinical and functional consequences of knee osteoarthritis. Sci. Rep. 2020;10(1):3601. doi: 10.1038/s41598-020-60587-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumavat R, et al. Biomarkers of joint damage in osteoarthritis: Current status and future directions. Mediat. Inflamm. 2021;2021:5574582. doi: 10.1155/2021/5574582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rios JL, et al. Protective effect of prebiotic and exercise intervention on knee health in a rat model of diet-induced obesity. Sci. Rep. 2019;9(1):3893. doi: 10.1038/s41598-019-40601-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tu C, He J, Wu B, Wang W, Li Z. An extensive review regarding the adipokines in the pathogenesis and progression of osteoarthritis. Cytokine. 2019;113:1–12. doi: 10.1016/j.cyto.2018.06.019. [DOI] [PubMed] [Google Scholar]
- 6.Zhao CW, et al. An update on the emerging role of resistin on the pathogenesis of osteoarthritis. Mediat. Inflamm. 2019;2019:1532164. doi: 10.1155/2019/1532164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao CW, et al. Resistin induces chemokine and matrix metalloproteinase production via CAP1 receptor and activation of p38-MAPK and NF-κB signalling pathways in human chondrocytes. Clin. Exp. Rheumatol. 2021 doi: 10.21203/rs.2.24441/v1. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Z, et al. Resistin induces expression of proinflammatory cytokines and chemokines in human articular chondrocytes via transcription and messenger RNA stabilization. Arthritis Rheum. 2010;62(7):1993–2003. doi: 10.1002/art.27473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Z, et al. Resistin stimulates expression of chemokine genes in chondrocytes via combinatorial regulation of C/EBPβ and NF-κB. Int. J. Mol. Sci. 2014;15(10):17242–17255. doi: 10.3390/ijms151017242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen WC, et al. Resistin enhances VCAM-1 expression and monocyte adhesion in human osteoarthritis synovial fibroblasts by inhibiting MiR-381 expression through the PKC, p38, and JNK signaling pathways. Cells. 2020;9(6):1369. doi: 10.3390/cells9061369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang C, et al. Metformin mitigates cartilage degradation by activating AMPK/SIRT1-mediated autophagy in a mouse osteoarthritis model. Front. Pharmacol. 2020;11:1114. doi: 10.3389/fphar.2020.01114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duan R, Xie H, Liu ZZ. The role of autophagy in osteoarthritis. Front. Cell Dev. Biol. 2020;8:608388. doi: 10.3389/fcell.2020.608388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakatogawa H. Mechanisms governing autophagosome biogenesis. Nat. Rev. Mol. Cell Biol. 2020;21:439–458. doi: 10.1038/s41580-020-0241-0. [DOI] [PubMed] [Google Scholar]
- 14.Shin HJ, et al. Pink1-mediated chondrocytic mitophagy contributes to cartilage degeneration in osteoarthritis. J. Clin. Med. 2019;8(11):1849. doi: 10.3390/jcm8111849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng L, et al. Circulating microRNA let-7e is decreased in knee osteoarthritis, accompanied by elevated apoptosis and reduced autophagy. Int. J. Mol. Med. 2020;45(5):1464–1476. doi: 10.3892/ijmm.2020.4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Almonte-Becerril M, et al. Cell death of chondrocytes is a combination between apoptosis and autophagy during the pathogenesis of osteoarthritis within an experimental model. Apoptosis. 2010;15(5):631–638. doi: 10.1007/s10495-010-0458-z. [DOI] [PubMed] [Google Scholar]
- 17.Caramés B, Taniguchi N, Otsuki S, Blanco FJ, Lotz M. Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheum. 2010;62(3):791–801. doi: 10.1002/art.27305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Care IoLARCo, Animals UoL, Resources NIoHDoR. Guide for the Care and Use of Laboratory Animals (National Academies, 1985).
- 19.Fahmy SR, Gaafar K. Establishing the first institutional animal care and use committee in Egypt. Philos. Ethics Humanit. Med. 2016;11:2. doi: 10.1186/s13010-016-0035-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGrath JC, Drummond GB, McLachlan EM, Kilkenny C, Wainwright CL. Guidelines for reporting experiments involving animals: The ARRIVE guidelines. Br. J. Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griffin TM, Huebner JL, Kraus VB, Yan Z, Guilak F. Induction of osteoarthritis and metabolic inflammation by a very high-fat diet in mice: Effects of short-term exercise. Arthritis Rheum. 2012;64:443–453. doi: 10.1002/art.33332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The University of Iowa. Office of animal resources, corp-author. Institutional animal care and use committee. IACUC Guidelines: Anesthesia. https://animal.research.uiowa.edu/iacuc-guidelines-anesthesia [26 Dec 2018].
- 23.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18(6):499–502. doi: 10.1093/clinchem/18.6.499. [DOI] [PubMed] [Google Scholar]
- 24.Caumo A, Perseghin G, Brunani A, Luzi L. New insights on the simultaneous assessment of insulin sensitivity and β-cell function with the HOMA2 method. Diabetes Care. 2006;29(12):2733–2734. doi: 10.2337/dc06-0070. [DOI] [PubMed] [Google Scholar]
- 25.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 26.Hagen S. SPSS in practice. Nurse Res. 2002;10(2):86–87. doi: 10.7748/nr.10.2.86.s14. [DOI] [PubMed] [Google Scholar]
- 27.Alissa EM, Alzughaibi LS, Marzouki ZM. Relationship between serum resistin, body fat and inflammatory markers in females with clinical knee osteoarthritis. Knee. 2020;27:45–50. doi: 10.1016/j.knee.2019.12.009. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Hunter D, Xu J, Ding C. Metabolic triggered inflammation in osteoarthritis. Osteoarthr. Cartil. 2015;23:22–30. doi: 10.1016/j.joca.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Scanzello CR. Role of low-grade inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2017;29(1):79–85. doi: 10.1097/BOR.0000000000000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun AR, et al. Obesity-associated metabolic syndrome spontaneously induces infiltration of pro-inflammatory macrophage in synovium and promotes osteoarthritis. PLoS One. 2017;12:e0183693. doi: 10.1371/journal.pone.0183693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Collins KH, Hart DA, Seerattan RA, Reimer RA, Herzog W. High-fat/high-sucrose diet-induced obesity results in joint-specific development of osteoarthritis-like degeneration in a rat model. Bone Joint Res. 2018;7(4):274–281. doi: 10.1302/2046-3758.74.BJR-2017-0201.R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collins AT, et al. Obesity alters the collagen organization and mechanical properties of murine cartilage. Sci. Rep. 2021;11:1626. doi: 10.1038/s41598-020-80599-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lambova SN, et al. Serum leptin and resistin levels in knee osteoarthritis-clinical and radiologic links: Towards precise definition of metabolic type knee osteoarthritis. Biomedicines. 2021;9(8):1019. doi: 10.3390/biomedicines9081019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su KZ, et al. Relation of circulating resistin to insulin resistance in type 2 diabetes and obesity: A systematic review and meta-analysis. Front. Physiol. 2019;10:1399. doi: 10.3389/fphys.2019.01399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koskinen A, Vuolteenaho K, Moilanen T, Moilanen E. Resistin as a factor in osteoarthritis: Synovial fluid resistin concentrations correlate positively with interleukin 6 and matrix metalloproteinases mmp-1 and mmp-3. Scand. J. Rheumatol. 2014;43:249–253. doi: 10.3109/03009742.2013.853096. [DOI] [PubMed] [Google Scholar]
- 36.Wang K, et al. Serum levels of resistin and interleukin-17 are associated with increased cartilage defects and bone marrow lesions in patients with knee osteoarthritis. Mod. Rheumatol. 2016;27(2):339–344. doi: 10.1080/14397595.2016.1205777. [DOI] [PubMed] [Google Scholar]
- 37.Lee JH, et al. Resistin is elevated following traumatic joint injury and causes matrix degradation and release of inflammatory cytokines from articular cartilage in vitro. Osteoarthr. Cartil. 2009;17:613–620. doi: 10.1016/j.joca.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 38.Dondelinger Y, Darding M, Bertrand MJ, Walczak H. Poly-ubiquitination in TNFR1-mediated necroptosis. Cell Mol. Life Sci. 2016;73:2165–2176. doi: 10.1007/s00018-016-2191-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fresno M, Alvarez R, Cuesta N. Toll-like receptors, inflammation, metabolism and obesity. Arch. Physiol. Biochem. 2011;117:151–164. doi: 10.3109/13813455.2011.562514. [DOI] [PubMed] [Google Scholar]
- 40.Benomar Y, Taouis M. Molecular mechanisms underlying obesity-induced hypothalamic inflammation and insulin resistance: Pivotal role of resistin/TLR4 pathways. Front. Endocrinol. (Lausanne) 2019;10:140. doi: 10.3389/fendo.2019.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gomez R, Villalvilla A, Largo R, Gualillo O, Herrero-Beaumont G. TLR4 signalling in osteoarthritis—Finding targets for candidate DMOADs. Nat. Rev. Rheumatol. 2015;11:159–170. doi: 10.1038/nrrheum.2014.209. [DOI] [PubMed] [Google Scholar]
- 42.Liu-Bryan R, Terkeltaub R. Emerging regulators of the inflammatory process in osteoarthritis. Nat. Rev. Rheumatol. 2015;11:35–44. doi: 10.1038/nrrheum.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Z, et al. Resistin promotes CCL4 expression through toll-like receptor-4 and activation of the p38-MAPK and NF-κB signaling pathways: Implications for intervertebral disc de-generation. Osteoarthr. Cartil. 2017;25(2):341–350. doi: 10.1016/j.joca.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 44.Li B, Fang J, Zuo Z, et al. Activation of the porcine alveolar macrophages via toll-like receptor 4/NF-κB mediated pathway provides a mechanism of resistin leading to inflammation. Cytokine. 2018;110:357–366. doi: 10.1016/j.cyto.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 45.Miao J, et al. Resistin inhibits neuronal autophagy through Toll-like receptor 4. J. Endocrinol. 2018;238:77–89. doi: 10.1530/JOE-18-0096. [DOI] [PubMed] [Google Scholar]
- 46.Yu L, Chen Y, Tooze SA. Autophagy pathway: Cellular and molecular mechanisms. Autophagy. 2018;14:207–215. doi: 10.1080/15548627.2017.1378838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klionsky DJ, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–175. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ye X, Zhou XJ, Zhang H. Exploring the role of autophagy-related gene 5 (ATG5) yields important insights into autophagy in autoimmune/autoinflammatory diseases. Front. Immunol. 2018;9:2334. doi: 10.3389/fimmu.2018.02334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu Z, et al. Balancing mTOR signaling and autophagy in the treatment of Parkinson's disease. Int. J. Mol. Sci. 2019;20(3):728. doi: 10.3390/ijms20030728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ju L, et al. Obesity-associated inflammation triggers an autophagy-lysosomal response in adipocytes and causes degradation of perilipin 1. Cell Death Dis. 2019;10:121. doi: 10.1038/s41419-019-1393-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caramés B, et al. Autophagy activation by rapamycin reduces severity of experimental osteoarthritis. Ann. Rheum. Dis. 2012;71(4):575–581. doi: 10.1136/annrheumdis-2011-200557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vasheghani F, et al. PPARg deficiency results in severe, accelerated osteoarthritis associated with aberrant mTOR signalling in the articular cartilage. Ann. Rheum. Dis. 2015;74:569–578. doi: 10.1136/annrheumdis-2014-205743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li YS, et al. Autophagy in osteoarthritis. Joint Bone Spine. 2016;83:143–148. doi: 10.1016/j.jbspin.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 54.Yao J, et al. Curcumin-alleviated osteoarthritic progression in rats fed a high-fat diet by inhibiting apoptosis and activating autophagy via modulation of MicroRNA-34a. J. Inflamm. Res. 2021;14:2317–2331. doi: 10.2147/JIR.S312139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed are available from the corresponding author on reasonable request.