Abstract
Objective
This study aims to verify the feasibility and efficacy of laparoscopic lower mediastinal lymphadenectomy for Siewert type II/III adenocarcinoma of esophagogastric junction (AEG).
Setting
An exploratory, observational, prospective, cohort study will be carried out under the Idea, Development, Exploration, Assessment and Long-term Follow-up (IDEAL) framework (stage 2b).
Participants
The study will recruit 1,036 patients with cases of locally advanced AEG (Siewert type II/III, clinical stage cT2−4aN0−3M0), and 518 will be assigned to either the laparoscopy group or the open group.
Interventions
Patients will receive lower mediastinal lymphadenectomy along with either total or proximal gastrectomy.
Primary and secondary outcome measures
The primary endpoint is the number of lower mediastinal lymph nodes retrieved, and the secondary endpoints are the surgical safety and prognosis, including intraoperative and postoperative lower-mediastinal-lymphadenectomy-related morbidity and mortality, rate of rehospitalization, R0 resection rate, 3-year local recurrence rate, and 3-year overall survival.
Conclusions
The study will provide data for the guidance and development of surgical treatment strategies for AEG.
Trial registration number
The study has been registered in ClinicalTrials.gov (No. NCT04443478).
Keywords: Esophagogastric junction, laparoscopy, lymph node excision, stomach neoplasms
Introduction
Gastric cancer is the fourth leading cause of tumor death worldwide, according to GLOBOCAN data (1). Meanwhile, the incidence and proportion of adenocarcinoma of the esophagogastric junction (AEG) have been increasing in many regions (2-7). Because of the distinct anatomic features of AEG, a full consensus on its surgical treatment has not yet been reached. According to the Japanese treatment guidelines for gastric cancer (8), lower mediastinal lymphadenectomy is indicated when the AEG tumor invades the esophagus; however, the standard surgical procedures for AEG are ambiguous compared to those of D2 lymphadenectomy.
The JCOG9502 trial demonstrated that the abdominal transhiatal (TH) approach can lead to a long-term survival benefit for Siewert type II/III AEG compared to the left thoracoabdominal approach (9). This result raises the question of whether similar actions can be repeated in the laparoscopy era, given its well-accepted advantages, such as less trauma, enhanced recovery and flexible vision. First, the laparoscopic TH approach showed good technical feasibility and surgical safety based on early experience (10). Then, the author reported that the laparoscopic TH approach could increase the number of dissected lower mediastinal lymph nodes compared to the open approach (2 vs. 1, P=0.002), with comparable postoperative morbidity (11). Regarding long-term survival, a retrospective study revealed that the 5-year overall survival (OS) was significantly higher in the laparoscopy arm than in the open arm (98% vs. 74%). After stratifying patients according to stage, survival benefits were still observed with no significant differences noted (12). A similar study by Huang (13) reported that the 3-year OS was superior in the laparoscopic arm (72.0% vs. 61.5%, P=0.113), and in the Siewert type II subgroup analysis, the 3-year OS (81.3% vs. 66.4%, P=0.011) and 3-year disease-free survival (77.5% vs. 63.8%, P=0.040) were significantly higher in the laparoscopic arm.
As a surgical innovation, laparoscopic lower mediastinal lymphadenectomy displayed good feasibility and safety. This study obtained a technical consensus on the surgical technique and verified the feasibility and efficacy of this technique in a prospective cohort study.
Materials and methods
Study design
The Idea, Development, Exploration, Assessment and Long-term Follow-up (IDEAL) framework is designed for the evaluation of surgical innovation and is established and applied in this study (14). The IDEAL exploration (stage 2b) study is a research phase to obtain a technical consensus on surgical innovation and explore the feasibility of conducting a definitive comparison against the current best treatment.
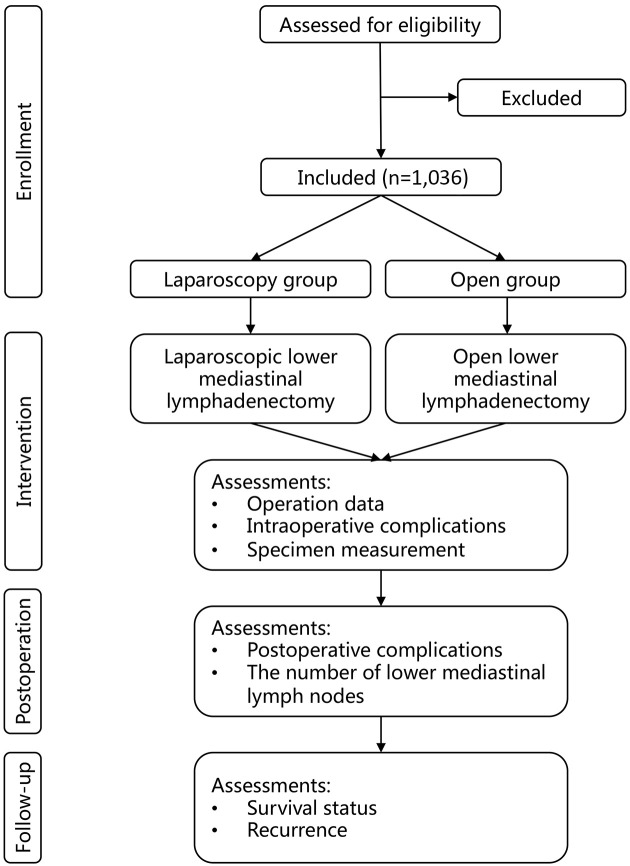
This study is designed under the framework of the IDEAL exploration (stage 2b) study and based on the following facts: 1) the primary surgical technique of laparoscopic lower mediastinal lymphadenectomy has been developed and reported; 2) there is a lack of consensus on the efficacy of the detailed surgical techniques used to treat AEG, such as the surgical boundary, the learning curve, and the quality control; and 3) there is an unknown tendency for surgeons and patients to accept laparoscopic lower mediastinal lymphadenectomy as the most effective form of treatment for AEG and an unknown number of randomized studies on its effectiveness. The trial was registered at ClinicalTrials.gov (NCT04443478. Registered on June 23, 2020). This protocol was developed in accordance with the Standard Protocol Items: Recommendations for Interventional Trials reporting guidelines (15). The description of the protocol is based on the latest version of the study protocol (Version 1.2). Figure 1 shows the study flow chart. Figure 2 shows the schedule of enrollment, intervention and assessment.
Figure 1.
Study design flow chart.
Figure 2.
Schedule of enrollment, intervention and assessments. CT, computed tomography; MRI, magnetic resonance imaging.
Population
Patients will be identified, screened for eligibility and recruited from the participating centers by the clinicians. If they are interested in participating in the study, they will be scheduled for a screening visit. Clinicians will ensure that all the reports related to endoscopy, blood tests and radiology have been conducted to allow for the assessment of participant eligibility for participation. Written informed consent (Appendix 1) will be obtained from a trained member of the research team independent of the referring clinicians.
Eligibility criteria
The eligibility criteria (Table 1) are designed to include patients appropriate for the study protocol. All relevant medical and nonmedical conditions will be taken into consideration by the investigating team to determine whether the protocol is appropriate for a patient to participate.
Table 1. Inclusion and exclusion criteria of this study.
| Inclusion criteria | Exclusion criteria |
| a, AEG is defined as a tumor whose epileft is located between the proximal 5 cm and distal 5 cm of EGJ and whose margin crosses or touches the EGJ; b, According to the 8th AJCC staging system for gastric cancer; c, Hemoglobin ≥80 g/L, neutrophil count ≥1.5×109/L, platelet count ≥75×109/L, alanine transaminase, aspartate transaminase and alkaline phosphatase ≤2.5 ULN, total bilirubin <1.5 ULN, creatinine <1 ULN, and albumin ≥30 g/L; d, At least one node of 3 cm or more in diameter, or at least three consecutive nodes each of diameter 1.5 cm or more, along the coeliac, splenic, common or proper hepatic arteries. ECOG, Eastern Cooperative Oncology Group; KPS, Karnofsky performance score; AEG, adenocarcinoma of esophagogastric junction; ULN, upper limit of normal. | |
| Age >18 years and <80 years | Any neoadjuvant treatments received |
| ECOG≤2 or KPS≥70% | Multiple malignant lesions in the stomach |
| Histology of adenocarcinoma confirmed by biopsy | Suspicious lymph node metastasis in the middle and/or upper mediastinum |
| aTumors meet AEG definition of “Chinses expert consensus on the surgical treatment for AEG (2018 edition)” | Surgical history in the upper abdomen (laparoscopic cholecystectomy excluded) |
| Siewert type II or III | Pregnant or breastfeeding females |
| Length of esophagus involvement ≤2 cm | Uncontrolled epilepsy, central nervous system diseases or mental disorders |
| bClinical stage cT2−4aN0−3M0 | dBulky N2 status |
| cLaboratory tests basically normal | Emergency surgery |
| Signed informed consent | Severe heart diseases |
| History of cerebral infarction or cerebral hemorrhage within 6 months | |
| Organ transplant recipients who need immunosuppressive therapies | |
| Other malignancies diagnosed within 5 years (cured dermoid caner and cervical cancer excluded) | |
Definition of esophagogastric junction (EGJ)
When defining the AEG and determining the Siewert type in the screening visit, the Japanese Classification of Esophageal Cancer (11th edition) will be applied to define the EGJ. The EGJ is defined as the lower margin of palisading small vessels or the oral margin of the longitudinal folds of the greater curvature of the stomach for endoscopic findings, and is defined as the narrowest locus of the lower esophagus for the upper gastrointestinal series, or is defined as the point at which the luminal caliber changes in the area where the tubular esophagus is connected to the vestibule lumen of the stomach for macroscopic definition.
Interventions
Patients included in the study will undergo radical total or proximal gastrectomy plus lower mediastinal lymphadenectomy. When assigned to the laparoscopy group, both the gastrectomy and the lower mediastinal lymphadenectomy should be performed via laparoscopic approach, and vice versa. The surgical approach will be determined by the investigator in each center before the operation and recorded in electronic case report forms (CRFs). In the laparoscopy group, the conversion to open surgery was defined as the use of a laparotomy wound for any part of the gastrectomy or the lower mediastinal lymphadenectomy. Reasons for conversion will be recorded in CRF. It should be noted that conversion to open surgery is not regarded as a protocol violation, and participants who undergo intraoperative conversion to open surgery will not be excluded from the per-protocol analysis.
For the lower mediastinal lymphadenectomy, the listed anatomic landmarks (Table 2) are chosen as boundaries of the lower mediastinal lymphadenectomy. Any planned thoracotomy for the purpose of lymphadenectomy or reconstruction is prohibited in this study.
Table 2. Boundaries of lower mediastinal lymphadenectomy for this study.
| Side | Boundary |
| Upper (cranial) | Inferior wall of pericardium and pulmonary ligament |
| Lower (caudal) | Diaphragm hiatus |
| Lateral | Mediastinal pleura |
| Front (ventral) | Anterior inferior wall of pericardium and diaphragm |
| Back (dorsal) | Anterior wall of aorta |
Lymph nodes will be retrieved station by station from the fresh specimens according to the Japanese classification of gastric carcinoma (16) by the surgeon or the assistant within 30 min after specimen removal.
Adjuvant chemotherapies are listed as recommended guidelines for locally advanced cancers. The regimens, courses and adverse events will be recorded in the CRF.
Quality control
Photos of surgical fields after lymphadenectomy will be submitted to the electronic database and reviewed by a third-party expert for surgical quality control; these photos will include images of the suprapancreas, hiatus, both sides of the lower mediastinum and surgical incisions. Likewise, photos of the specimens will be submitted and reviewed to verify the tumor size and position data.
Follow-up visits
Patients will either be required to return to the study site for outpatient visits and/or have follow-up phone calls to report survival status and assess for recurrence every 3 months in the 1st and 2nd years postoperatively and every 6 months in the 3rd year postoperatively. Their complete blood counts, comprehensive chemistry profiles and tumor markers will be tested upon each follow-up visit, and contrast computer tomography (abdomen/pelvis) will be performed every 6 months or when clinically indicated postoperatively. To maximize patient retention in the study, investigators will maintain close contact with the participants, and investigators will call the participants if they do not return for follow-up. Participants will be provided with a detailed follow-up timeline upfront.
Outcome measures
Primary endpoint
The primary objective of this study is to explore the effect of laparoscopic lower mediastinal lymphadenectomy for Siewert type II/III AEG compared with open surgery. Thus, the number of lower mediastinal lymph nodes retrieved is set as the primary endpoint (Table 3).
Table 3. Definition of study endpoints.
| Endpoints | Definitions |
| TH, transhiatal; POD, postoperative day. | |
| Number of lower mediastinal lymph nodes retrieved | Number of lymph nodes retrieved from the surgery, including stations of No.110, 111 and 112 |
| Duration of lower mediastinal lymphadenectomy | Operation time from the dissection of diaphragm hiatus to the completion of esophagus mobilization |
| R0 resection | Operation without any tumor residuals |
| Intraoperative complications related to lower mediastinal lymphadenectomy | Complications occurring in TH procedure, including rupture of mediastinal pleura, pericardium or esophagus, hemorrhage, and deficient anastomosis |
| Postoperative complications related to lower mediastinal lymphadenectomy | Postoperative complications related to TH procedure, including leakage, hemorrhage or stenosis of esophagus-related anastomosis, mediastinitis, hiccup, pulmonary complications, and cardiac complications |
| Postoperative complications | All-cause postoperative complications until POD 90 |
| Postoperative mortality | The all-cause postoperative mortality until POD 90 |
| Reoperation | Any reoperation to manage postoperative complications |
| Rehospitalization | Any rehospitalization to manage the postoperative complications |
| Length of proximal margin | Length from the upper edge of the tumor to the proximal end of the esophagus, which should be measured within 30 min after specimen removal. |
| 3-year local recurrence rate | Percentage of patients with local recurrence in the lower mediastinal area at 3 years measured from the date of operation |
| 3-year overall survival | Percentage of patients who are still alive at 3 years measured from the date of operation |
Secondary endpoints
The secondary objectives of this study are to explore differences in clinical features and prognosis between laparoscopic and open surgery regarding the surgical technique, safety, oncological characteristics and survival. This study will explore the feasibility of conducting a confirmative randomized controlled trial, while collecting preliminary data to provide a computational basis for future research design and reaching agreement on the technical details of the intervention, indications and core outcomes. The intraoperative and postoperative lower-mediastinal-lymphadenectomy-related morbidity and mortality, duration of lower mediastinal lymphadenectomy, rate of rehospitalization, R0 resection rate, length of proximal margin, metastatic rate of lower mediastinal lymph nodes, 3-year local recurrence rate and 3-year OS were set as the secondary endpoints (Table 3).
Adverse events (AEs)
AEs are unfavorable or unintended events that may harm patients during the study, regardless of their relevance to the interventions. AEs will be recorded in details on the CRF and will include the time of occurrence, duration, relevance to the interventions, and whether they led to the discontinuation of treatment. Events that lead to death, permanent or severe disability and significant clinical sequelae are defined as severe AEs (SAEs), which will be reported to the Ethics Committee of Peking University Cancer Hospital within 24 h of initial discovery.
Patient and public involvement
Patients and the public were not involved in the design, conduct, reporting and dissemination plans of this study. Patients will decide which intervention they will receive after being fully and exhaustively informed of the risks and benefits of the two interventions.
Study termination
To minimize the possible SAEs, the study will be terminated when there are more than 10 cases of surgery-related deaths or when there are more than 30 cases of surgery-related postoperative complications, of which the Clavien-Dindo grades (17) are equal to or greater than 4. Additionally, the interim analysis will be carried out 18 months after the study initiation or when half of the patients are included. The study will be terminated under the following circumstances in the interim analysis: the number of enrollments is less than 30%, the number of enrollments in any treatment arm is less than 117, or the proportion of any treatment arm is less than 40%.
Data collection and management
Patients will be asked for permission to obtain their clinical information from their medical records. CRFs will be completed for each patient for each particular visit. Each CRF will be signed by a research investigator to certify that the information on the protocol is valid. All data will be double entered into a secure electronic database in preparation for data analysis. The CRF will not contain identifiable data. Only the authorized investigators will have access to the final dataset. Data monitoring and auditing are conducted by the funding agency annually.
Statistical design and analysis
Sample size consideration
Given the nonrandomized nature of the current study, the sample size needs to be relatively large to provide sufficient statistical power to detect any group differences after adjusting for potential confounders. Propensity score matching will be used for confounding adjustment, and logistic regression will be used to construct the propensity score model. According to the rule of thumb, each candidate parameter in the propensity score model should correspond to at least ten events (i.e., laparoscopic surgery). We identified 46 candidate parameters (Supplementary materials), corresponding to a minimum of 460 patients in the laparoscopy group and the open group with a matching ratio of 1:1. We expect only part of these candidate parameters to be included in the propensity score model, which can help offset the potentially “wasted” sample size during the matching process.
The retrospective study of Sugita et al. (11) found that the number (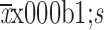 ) of lower mediastinal lymph nodes dissected was 1.85±2.45 and 0.49±0.93 in the laparoscopy and open groups, respectively. Using this information and supposing the number of dissected lower mediastinal lymph nodes follow a Poisson distribution, we estimate that a group size of 460 will produce a 95% confidence interval with a half-width of 12% and 6% for the average number of dissected lower mediastinal lymph nodes in the laparoscopy group and open group, respectively (18). After considering a 10% dropout rate, we determined that 1,036 people will be needed, with 518 patients in each group. This sample size will have 80% of power to detect a minimal difference of 0.17 in the average number of dissected lower mediastinal lymph nodes between the two groups.
) of lower mediastinal lymph nodes dissected was 1.85±2.45 and 0.49±0.93 in the laparoscopy and open groups, respectively. Using this information and supposing the number of dissected lower mediastinal lymph nodes follow a Poisson distribution, we estimate that a group size of 460 will produce a 95% confidence interval with a half-width of 12% and 6% for the average number of dissected lower mediastinal lymph nodes in the laparoscopy group and open group, respectively (18). After considering a 10% dropout rate, we determined that 1,036 people will be needed, with 518 patients in each group. This sample size will have 80% of power to detect a minimal difference of 0.17 in the average number of dissected lower mediastinal lymph nodes between the two groups.
Statistical analysis
Analytic population
The per-protocol set, defined as patients who are enrolled in the study and receive laparoscopic or open lower mediastinal lymphadenectomy without meeting any of the criteria listed in Supplementary materials, will be used as the primary analytic set of all analyses.
Analysis of primary and secondary outcomes
For primary analysis of the number of dissected lower mediastinal lymph nodes, the multivariate between-group comparison will be performed using the method of propensity score matching. The propensity score model that predicts the treatment assignment (i.e., open vs. laparoscopic lymphadenectomy) will be a logistic regression model, and the outcome model that predicts the number of dissected lower mediastinal lymph nodes will be a Poisson regression model. Candidate covariates for adjustment are listed in Supplementary materials and will be selected into the propensity score model by backward stepwise selection. The propensity score matching procedure will be based on the logit of the propensity score, using nearest neighbor matching without replacement and with a caliper of width equal to 0.2 of standard deviation of the logit of the propensity score. The matched dataset will be checked to see if the covariates for adjustment are balanced between the two groups. For analyses of secondary outcomes, appropriate statistical methods will be used. A detailed description of these methods can be found in the Statistical Analysis Plan (Appendix 2).
Sensitivity and subgroup analyses
In the case of missing data due to patient loss to follow-up, worst-best and best-worst case sensitivity analyses will be conducted to check if the impact of missing data is negligible (19). If the sample size permits, we will conduct subgroup analysis of the primary endpoint in different subpopulations according to the Siewert type and length of esophagus invasion: subdivide the population into two groups.
Missing data
A complete case set will be used for primary analyses if one of the criteria listed in Appendix 2 meets. Otherwise, multiple imputations will be used. A more detailed description of these processes can be found in the Statistical Analysis Plan (Appendix 2).
General statistical considerations
All analyses will be performed in standard statistical software. Unless otherwise specified, statistical significance will be declared at a two-sided P<0.05, and 95% confidence intervals will be used. Due to the exploratory nature of the current study, no adjustment for Type I errors will be adopted.
Ethics and dissemination
This study protocol was reviewed and approved by the Ethics Committee of Peking University Cancer Hospital (No. 2020KT71). All study protocols will follow the Helsinki Declaration. Any amendments to the protocol will be reviewed by the Ethics Committee. Each study participant will be made aware of potential risks, benefits, treatment alternatives and costs prior to participating in the study. To document this, study participants (or parents/legally authorized representatives) will sign an informed consent document (Appendix 1). Enrollment will be voluntary, and consent may be withdrawn at any time, without giving reasons and without affecting future medical care. Patient recruitment for this study began in August 2020 and will be completed in July 2026. The findings will be published in peer-reviewed journals and disseminated at national and international meetings.
Discussion
Compared with perigastric lymphadenectomy (D2 resection), there have been few studies concerning the clinical value of lower mediastinal lymphadenectomy for AEG. According to previous studies, the metastatic rate of lower mediastinal lymph nodes was 8%−18.1% in Siewert type II AEG and 5%−6% in Siewert type III AEG (20-24). A nationwide retrospective study (7) revealed that the metastatic rate of No. 110 station was 5.1% and that of No. 111 station was less than 5% in esophagus-predominant EGJ cancers, and the rates of both stations were less than 5% in stomach-predominant EGJ cancers. Furthermore, the therapeutic index at stations No. 110, 111 and 112 was 1.9, 1.1 and 0, respectively, which was relatively low compared to 13.4 and 9.2 at stations No. 3 and 1. The nationwide study concluded that the benefit of mediastinal node dissection remained an issue to be addressed in managing patients with esophagus-predominant EGJ cancers. Another prospective study (25), which included 363 cases from 42 centers, showed that the metastatic rates of No. 110, 111, and 112 were 9.3%, 3.4%, and 2.0%, respectively. The rates were positively correlated with the length of esophagus involvement. When the length was longer than 2 cm, the metastatic rate of station No. 110 could exceed 10%, and when it was longer than 4 cm, the metastatic rate of station No. 111 could also exceed 10%; thus, the authors recommended a strategy to manage lower mediastinal lymphadenectomy based on esophageal involvement.
The surgical techniques of lower mediastinal lymphadenectomy were rarely explicated and illustrated in previous studies, which led to the poor standardization and repeatability of this procedure. Similar to the perigastric lymphadenectomy, we attempted to define the boundaries of the lower mediastinal lymph nodes. Based on the experiences at Gastrointestinal Cancer Center, Peking University Cancer Hospital & Institute, the following boundaries were recommended in this study: upper (cranial) is the inferior wall of the pericardium and the pulmonary ligament, lower (caudal) is the diaphragm hiatus, front (ventral) is the anterior inferior wall of the pericardium and the diaphragm, back (dorsal) is the anterior wall of the aorta, and lateral is the mediastinal pleura (Table 2).
The CLASS-10 study is aimed at verifying the feasibility and exploring the efficacy of laparoscopic lower mediastinal lymphadenectomy for Siewert type II/III AEG under the IDEAL framework. There are some potential limitations to this study. First, as a nonrandomized cohort study, the between-group heterogeneous baseline characteristics may lead to different outcomes between the two arms; thus, multivariate analyses and the propensity score model will be used to handle this confounding issue. Second, as a surgical innovation, laparoscopic lower mediastinal lymphadenectomy lacks safety data based on large-scale samples, and the termination criterion for this study is relatively restrictive, which might cause premature termination. Third, the definition of AEG is relatively strict in this study, which requires the tumor to cross or touch the EGJ. Consequently, this could lead to slow participant accrual.
Table S1. Candidate variables for propensity score model.
| Candidate variables | Categories | No. of parameters |
| Pre-treatment variables that may be associated with treatment preference and/or primary outcomes | ||
| Range of gastrectomy | Total gastrectomy, proximal gastrectomy | 1 |
| No. of comorbidities | Including a quadratic term for non-linearity | 2 |
| Lung function | Abnormal, normal | 1 |
| Tumor size via preoperative assessment, long diameter | Including a quadratic term for non-linearity | 2 |
| Tumor size via preoperative assessment, short diameter | Including a quadratic term for non-linearity | 2 |
| Age (year) | <45, 45−55, 56−65, 66−75, >75 | 4 |
| cT stage | 2, 3, 4a | 2 |
| cN stage | 0, 1, 2, 3 | 3 |
| Body mass index | Underweight, normal, overweight, obese | 3 |
| Preoperative hemoglobin | Including a quadratic term for non-linearity | 2 |
| No. of lung comorbidities | Including a quadratic term for non-linearity | 2 |
| History of smoking and smoking cessation | Non-quitted, quitted, non-smokers | 2 |
| Length of esophageal invasion | Including a quadratic term for non-linearity | 2 |
| Timing of surgery | Morning, afternoon, evening | 2 |
| History of cardiac diseases | Yes, no | 1 |
| Siewert type | I, II | 1 |
| CEA | Including a quadratic term for non-linearity | 2 |
| CA199 | Including a quadratic term for non-linearity | 2 |
| CA125 | Including a quadratic term for non-linearity | 2 |
| Geographical location | East and central west, Southwest and northwest, North and northeast | 2 |
| Hospital type | Cancer specialized, general | 1 |
| Additional pre-treatment variables that may be associated with primary outcomes alone | ||
| Differentiation | Well, moderate, poor | 2 |
| Lauren type | Diffused, mixed, intestinal | 2 |
| Gender | Male, female | 1 |
Table S2. Potential confounding covariates.
| Candidate variables | Categories |
| Patient baseline characteristics and conditions | |
| Age (year) | <45, 45−55, 56−65, 66−75, >75 |
| Body mass index | Underweight, normal, overweight, obese |
| No. of comorbidities | − |
| No. of lung comorbidities | − |
| Lung function | Abnormal, normal |
| History of smoking and smoking cessation | Non-quitted, quitted, non-smokers |
| History of cardiac diseases | Yes, no |
| Preoperative hemoglobin | − |
| Preoperative tumor characteristics | |
| Tumor size, long diameter | − |
| Tumor size, short diameter | − |
| Length of esophageal invasion | − |
| cT stage | 2, 3, 4a |
| cN stage | 0, 1, 2, 3 |
| Siewert type | I, II |
| CEA | − |
| CA199 | − |
| CA125 | − |
| Surgical characteristics | |
| Range of gastrectomy | Total gastrectomy, proximal gastrectomy |
| Timing of surgery | Morning, afternoon, evening |
| Research site characteristics | |
| Geographical location | East and central west, Southwest and northwest, North and northeast |
| Hospital type | Cancer specialized, general |
Supplementary materials
Participants meeting one of the following criteria will be excluded from the per-protocol set:
a) Laparoscopic exploration confirmed peritoneal or distant metastasis;
b) Failure to dissect the tumor; or R1/R2 resection confirmed;
c) Planned thoracotomy to dissect lower mediastinal lymph nodes and/or digestive tract reconstruction (not including unplanned thoracotomy due to intraoperative bleeding, intraoperative injury, etc., which will be recorded as intraoperative complications);
d) Para-aortic lymphadenectomy (No.16 group);
e) An unknown number of lower mediastinal lymph nodes;
f) The participant or participant’s legal representative requests to withdraw from the study;
g) Participants who are considered unfit to continue their studies as a result of adverse events.
Complete case set will be used for primary analyses if one of the following criteria meets:
a) It is relatively sure that data are missing completely at random. This will be judged by Little’s test plus subjective knowledge that missing completely at random is plausible under the circumstance;
b) Only the dependent variable (aka outcome variables) has missing values;
c) The proportions of missing data are below 5% (as a rule of thumb), and the potential impact of the missing data is negligible;
d) The proportions of missing data are above 40% (as a rule of thumb);
e) Missing not at random is plausible.
Acknowledgements
The authors would like to thank the Chinese Laparoscopic Gastrointestinal Surgery Study (CLASS) Group for organizing this series of studies and all the support from the participating centers involved in this study. The study is supported by the Chinese Medical Foundation (No. 2020064).
Acknowledgments
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Contributor Information
Ziyu Li, Email: ziyu_li@hsc.pku.edu.cn.
Jiafu Ji, Email: jijiafu@hsc.pku.edu.cn.
References
- 1.Sung H, Ferlay J, Siegel RL, et al Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Liu K, Yang K, Zhang W, et al Changes of esophagogastric junctional adenocarcinoma and gastroesophageal reflux disease among surgical patients during 1988-2012: A single-institution, high-volume experience in China. Ann Surg. 2016;263:88–95. doi: 10.1097/SLA.0000000000001148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kusano C, Gotoda T, Khor CJ, et al Changing trends in the proportion of adenocarcinoma of the esophagogastric junction in a large tertiary referral center in Japan. J Gastroenterol Hepatol. 2008;23:1662–5. doi: 10.1111/j.1440-1746.2008.05572.x. [DOI] [PubMed] [Google Scholar]
- 4.Buas MF, Vaughan TL Epidemiology and risk factors for gastroesophageal junction tumors: understanding the rising incidence of this disease. Semin Radiat Oncol. 2013;23:3–9. doi: 10.1016/j.semradonc.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wijnhoven BPL, Louwman MWJ, Tilanus HW, et al Increased incidence of adenocarcinomas at the gastro-oesophageal junction in Dutch males since the 1990s. Eur J Gastroenterol Hepatol. 2002;14:115–22. doi: 10.1097/00042737-200202000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Wayman J, Forman D, Griffin SM Monitoring the changing pattern of esophago-gastric cancer: data from a UK regional cancer registry. Cancer Causes Control. 2001;12:943–9. doi: 10.1023/a:1013756531219. [DOI] [PubMed] [Google Scholar]
- 7.Yamashita H, Seto Y, Sano T, et al. Results of a nation-wide retrospective study of lymphadenectomy for esophagogastric junction carcinoma. Gastric Cancer 2017;20(Suppl 1):69-83.
- 8.Japanese Gastric Cancer Association Japanese gastric cancer treatment guidelines 2018 (5th edition) Gastric Cancer. 2021;24:1–21. doi: 10.1007/s10120-020-01042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurokawa Y, Sasako M, Sano T, et al Ten-year follow-up results of a randomized clinical trial comparing left thoracoabdominal and abdominal transhiatal approaches to total gastrectomy for adenocarcinoma of the oesophagogastric junction or gastric cardia. Br J Surg. 2015;102:341–8. doi: 10.1002/bjs.9764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kinoshita T, Gotohda N, Kato Y, et al Laparoscopic transhiatal resection for Siewert type II adenocarcinoma of the esophagogastric junction: operative technique and initial results. Surg Laparosc Endosc Percutan Tech. 2012;22:e199–203. doi: 10.1097/SLE.0b013e31825a72e2. [DOI] [PubMed] [Google Scholar]
- 11.Sugita S, Kinoshita T, Kaito A, et al Short-term outcomes after laparoscopic versus open transhiatal resection of Siewert type II adenocarcinoma of the esophagogastric junction. Surg Endosc. 2018;32:383–90. doi: 10.1007/s00464-017-5687-6. [DOI] [PubMed] [Google Scholar]
- 12.Sugita S, Kinoshita T, Kuwata T, et al Long-term oncological outcomes of laparoscopic versus open transhiatal resection for patients with Siewert type II adenocarcinoma of the esophagogastric junction. Surg Endosc. 2021;35:340–8. doi: 10.1007/s00464-020-07406-w. [DOI] [PubMed] [Google Scholar]
- 13.Huang CM, Lv CB, Lin JX, et al Laparoscopic-assisted versus open total gastrectomy for Siewert type II and III esophagogastric junction carcinoma: a propensity score-matched case-control study. Surg Endosc. 2017;31:3495–503. doi: 10.1007/s00464-016-5375-y. [DOI] [PubMed] [Google Scholar]
- 14.Ergina PL, Barkun JS, McCulloch P, et al IDEAL framework for surgical innovation 2: observational studies in the exploration and assessment stages. BMJ. 2013;346:f3011. doi: 10.1136/bmj.f3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan AW, Tetzlaff JM, Gøtzsche PC, et al SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586. doi: 10.1136/bmj.e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Japanese Gastric Cancer Association Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–12. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
- 17.Dindo D, Demartines N, Clavien PA Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chow SC, Shao J, Wang H, et al. Sample Size Calculations in Clinical Research: Third Edition. Boca Raton: Chapman and Hall/CRC, 2017.
- 19.Jakobsen JC, Gluud C, Wetterslev J, et al When and how should multiple imputation be used for handling missing data in randomised clinical trials - a practical guide with flowcharts. BMC Med Res Methodol. 2017;17:162. doi: 10.1186/s12874-017-0442-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rüdiger Siewert J, Feith M, Werner M, et al Adenocarcinoma of the esophagogastric junction: results of surgical therapy based on anatomical/topographic classification in 1,002 consecutive patients. Ann Surg. 2000;232:353–61. doi: 10.1097/00000658-200009000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feith M, Stein HJ, Siewert JR Adenocarcinoma of the esophagogastric junction: surgical therapy based on 1602 consecutive resected patients. Surg Oncol Clin N Am. 2006;15:751–64. doi: 10.1016/j.soc.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 22.Koyanagi K, Kato F, Kanamori J, et al Clinical significance of esophageal invasion length for the prediction of mediastinal lymph node metastasis in Siewert type II adenocarcinoma: A retrospective single-institution study. Ann Gastroenterol Surg. 2018;2:187–96. doi: 10.1002/ags3.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hosoda K, Yamashita K, Katada N, et al Impact of lower mediastinal lymphadenectomy for the treatment of esophagogastric junction carcinoma. Anticancer Res. 2015;35:445–56. [PubMed] [Google Scholar]
- 24.Yoshikawa T, Takeuchi H, Hasegawa S, et al Theoretical therapeutic impact of lymph node dissection on adenocarcinoma and squamous cell carcinoma of the esophagogastric junction. Gastric Cancer. 2016;19:143–9. doi: 10.1007/s10120-014-0439-y. [DOI] [PubMed] [Google Scholar]
- 25.Kurokawa Y, Takeuchi H, Doki Y, et al Mapping of lymph node metastasis from esophagogastric junction tumors: A prospective nationwide multicenter study. Ann Surg. 2021;274:120–7. doi: 10.1097/SLA.0000000000003499. [DOI] [PubMed] [Google Scholar]