Abstract
We investigated the roles of fliF, fliS, flhB, fliQ, fliG, and fliI of Helicobacter pylori, predicted by homology to encode structural components of the flagellar basal body and export apparatus. Mutation of these genes resulted in nonmotile, nonflagellate strains. Western blot analysis showed that all the mutants had considerably reduced levels of both flagellin subunits and of FlgE, the flagellar hook protein. RNA slot blot hybridization showed reduced levels of flaA mRNA, indicating that transcription of the major flagellin gene is inhibited in the absence of the early components of the flagellar-assembly pathway. This is the first demonstration of a checkpoint in H. pylori flagellar assembly.
Helicobacter pylori is the causative agent of chronic superficial gastritis and is associated with the development of peptic ulcer disease, gastric carcinoma, and gastric lymphoma (3). Motility is required for colonization by H. pylori and is essential for persistent infection in gnotobiotic piglets and mice (4, 6). The bacterium has a unipolar bundle of flagella and displays active motility in viscous environments inhibitory to the motility of other bacteria (10). The flagellum of H. pylori comprises three structural elements: a basal body, a flexible hook, and a flagellar filament. The filament is composed of two proteins: a minor species, FlaB (57 kDa), and the major species, FlaA (56 kDa) (14). The corresponding genes, flaA and flaB, are not linked on the chromosome, and transcription is controlled by different sigma factors: ς28 and ς54, respectively (16, 23). It has been suggested that H. pylori can alter the mechanical properties of the flagellar filament in response to environmental signals by varying the relative amounts of FlaA and FlaB (24). However, little is known about the regulation of flagellin gene expression or flagellar assembly in this bacterium.
The annotated genome sequences of H. pylori 26695 and J99 contain over 40 genes predicted by homology to be involved in the regulation, secretion, and assembly of the flagellar structure (1, 25). However, compared to that in other bacteria, the organization of these genes is atypical. Whereas other bacterial flagellar genes are usually clustered in well-defined regions, H. pylori flagellar genes are distributed throughout the genome. Further, the genome lacks orthologues of the master regulators FlhC and FlhD and the anti-sigma factor FlgM. It also contains genes coding for additional flagellar proteins, including paralogues of FlaB and FlgE (HP0295 and HP0908, respectively), and two genes which appear to encode polar flagellins (25). These peculiarities suggest that the mechanisms of flagellar assembly and flagellar gene regulation of H. pylori may differ substantially from those of other bacteria.
Putative ς70 promoters have been identified upstream of genes encoding the early components required for flagellar assembly, including those for structural components of the export apparatus, motor, and basal body. An NtrC orthologue, FlgR, was identified as a transcriptional activator of ς54-dependent genes which encode structural components of the basal body-hook complex. The flgR gene is transcribed by ς70-directed RNA polymerase (22). A model for flagellar-gene expression in H. pylori has been proposed in which ς70 directs transcription of genes encoding the early components required for flagellar assembly—the export apparatus, motor, and basal body—and flgR. FlgR in turn activates transcription of ς54-dependent genes encoding the basal body-hook complex and represses flaA transcription (22).
In Salmonella enterica serovar Typhimurium, the first structure in flagellar assembly is the MS ring (FliF). The next structure assembled is the C ring, which contains the switch proteins, FliG, FliM, and FliN. This is followed by rod assembly, for which several proteins, including FliI, FliQ, and FlhB, are required in addition to the rod structural proteins (17, 18). These proteins are believed to be located at the cytoplasmic side of the basal body near the switch and to be components of the flagellum-specific export apparatus (17). FliS, required for efficient elongation of the filament in serovar Typhimurium, is thought to be a cytoplasmic chaperone for flagellin export (18). The genes encoding flagellar components in serovar Typhimurium are expressed in the order in which their products are assembled. This temporal control of gene expression is exerted at the level of transcription by the presence of an anti-sigma factor, FlgM, which must be exported through the central pore of the assembled basal body-hook complex for initiation of flagellin gene transcription (11). Consequently, the absence of any of the structural components of the basal body-hook complex prevents expression of the flagellin gene. However, this is not the case in H. pylori. Mutants defective in FlgE, the hook protein, or other basal body-hook components still express both flagellin subunits, which accumulate intracellularly (19, 22). Further, a mutation in flgR, which encodes the ς54 transcriptional activator, and the resulting defect in expression of basal body and hook genes appear to increase transcription of flaA (22). As H. pylori lacks an FlgM orthologue (25) and the absence of the basal body-hook complex does not appear to prevent flagellin expression, it has been suggested that flagellar biosynthesis in H. pylori is not as highly regulated as in other bacteria.
Previous studies have investigated the role of fliI, fliQ, and flhB, which in serovar Typhimurium encode components of the flagellar export apparatus (9, 12). Mutation of any one of these genes in H. pylori results in nonmotile, nonflagellate strains, demonstrating an essential role in flagellar biosynthesis (9, 12). Porwollik and coworkers also constructed fliI and fliQ mutants and confirmed these findings (20). In the study reported here, we have constructed isogenic mutants of fliF, fliG, and fliS. We demonstrate that in addition to fliQ, flhB, and fliI, the genes fliF, fliG, and fliS are required for flagellum synthesis and motility. Further, we show that mutations in any of these genes result in reduced amounts of FlaA and FlaB and the flagellar-hook subunit, FlgE. To determine whether the absence of FlaA was due to transcriptional regulation, we investigated the transcription of flaA in these mutants by slot blot analysis of total RNA.
Bacterial strains and growth conditions.
The wild-type strain was SS1 (8, 15). H. pylori was stored at −80°C in brain heart infusion broth (Oxoid, Basingstoke, United Kingdom) containing 15% glycerol and 10% fetal calf serum (Sigma-Aldrich, Poole, United Kingdom). Strains were grown in brain heart infusion broth containing 10% fetal calf serum or on Helicobacter-selective agar, consisting of blood agar base no. 2 (Oxoid) supplemented with 7% lysed defibrinated horse blood (TCS Microbiology, Botolph Claydon, United Kingdom) and Dent's selective supplement (Oxoid), in a microaerobic atmosphere at 37°C.
Construction of flagellar mutants.
Defined isogenic mutations were constructed in the genes listed in Table 1. Mutants were constructed by inverse PCR mutagenesis and allelic replacement as described previously (5, 9, 27). To minimize the chance of polar effects, plasmid clones in which the kanamycin resistance cassette was inserted in the same orientation as the mutated gene were selected for electroporation, as the cassette lacks transcription terminators (7, 26). Confirmation of double crossovers leading to allelic replacement and elimination of the vector was obtained by gene-specific primer PCR and Southern blotting (data not shown). As the fliI and fliF genes are each transcribed as part of an operon, we performed reverse transcriptase PCR on total RNA from these mutants by using a Superscript cDNA synthesis kit (Life Technologies, Paisley, United Kingdom) to confirm the presence of mRNA specific for the downstream gene in each case (data not shown).
TABLE 1.
Genes mutated, closest orthologues, degree of identity, and orthologue function
| TIGR HP no.a | ASTRA JHP no.b | Orthologue/organism | % Identity/amino acid overlap | Orthologue function |
|---|---|---|---|---|
| 0351 | 0325 | FliF/Salmonella enterica serovar Choleraesuis | 34/524 | Basal body MS-ring protein |
| 0753 | 0690 | FliS/Bacillus subtilis | 32/123 | Chaperone for export? |
| 0770 | 0707 | FlhB/Bacillus subtilis | 39/346 | Flagellar export apparatus? |
| 1419 | 1314 | FliQ/Escherichia coli | 52/84 | Flagellar export apparatus? |
| 0352 | 0326 | FliG/Borrelia burgdorferi | 37/339 | Motor switch protein |
| 1420 | 1315 | FliI/Bacillus subtilis | 48/426 | Flagellar export ATP synthase |
Motility testing.
The standard 0.3% stab agar motility test was used to assess motility (13). The wild-type strain, SS1, formed diffuse colonies with large, swarming halos. In contrast, all mutants formed dense colonies without swarming (data not shown).
Electron microscopy.
Overnight cultures were examined by electron microscopy for the presence of flagellar-organelle structures, as described previously (9). First, 0.5 ml of culture was mixed with 0.5 ml of 2% (vol/vol) glutaraldehyde solution and incubated at room temperature for 10 min. Fixed bacteria were recovered by centrifugation at 3,000 × g for 2 min and were resuspended in 150 μl of water. Whole bacterial cells were negatively stained with 1.5% (wt/vol) potassium phosphotungstate (pH 6.4) and were examined by transmission electron microscopy. Wild-type bacteria had multiple sheathed flagella, in contrast to the mutants, which were all nonflagellate (data not shown). In an H. pylori flhA mutant, short structures (30 to 100 nm long) were occasionally observed and assumed to be flagellar hooks (21). No such structures were apparent in any of the mutants examined in this study.
Western blot analysis.
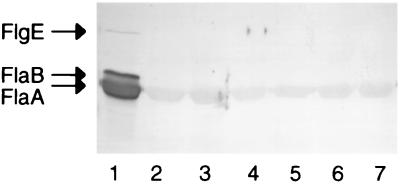
Bacteria were harvested from 10-ml cultures at an optical density at 600 nm of ≈1.0, resuspended in 0.5 ml of phosphate-buffered saline (pH 7.4), and lysed by ultrasound (ultrasonic processor; Jencons Scientific Ltd., Leighton Buzzard, United Kingdom). The soluble fraction was recovered by centrifugation at 10,000 × g for 5 min. A bicinchoninic acid protein assay kit (Pierce, Rockford, Ill.) was used to quantify total protein, and samples containing 20 μg of protein were separated using 12% polyacrylamide gels (Novex Electrophoresis, Frankfurt, Germany). For Western blot analysis, proteins were transferred to nitrocellulose (Hybond C pure; Amersham Pharmacia Biotech, St. Albans, United Kingdom) using a semidry transfer unit (Amersham Pharmacia Biotech). Blots were incubated with mouse antibody F2B9 (12), reactive with FlaA, FlaB, and the hook protein, FlgE, and bound antibodies were detected using polyvalent anti-mouse immunoglobulin-alkaline phosphatase conjugate (Sigma-Aldrich). In the wild-type strain, the two flagellin proteins of 56 kDa (FlaA) and 57 kDa (FlaB) were visible (Fig. 1, lane 1). A 78-kDa band corresponding to the FlgE hook protein was also detected. In contrast, expression of flagellins and the hook protein was greatly reduced in each mutant (Fig. 1, lanes 2 to 7).
FIG. 1.
Western blot of whole-cell lysates of wild-type H. pylori (SS1) (lane 1) and fliI (lane 2), fliG (lane 3), fliQ (lane 4), flhB (lane 5), fliS (lane 6), and fliF (lane 7) mutants.
RNA slot blot hybridizations.
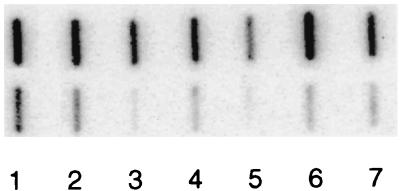
Total RNA was isolated from 10-ml cultures at an optical density at 600 nm of ≈1.0 using the RNeasy protocol (Qiagen, Crawley, United Kingdom), and genomic DNA was eliminated using DNase I (Promega, Southampton, United Kingdom). RNA for hybridization was applied to Hybond N (Amersham Pharmacia Biotech) using a slot blot filtration manifold (Hoefer) as previously described (2). DNA probes were labeled with [α-32P]dCTP (3,000 Ci mmol−1) using a Rediprime kit (Amersham Pharmacia Biotech) and were hybridized with the blots at 42°C for 16 h in buffer containing formamide (AMS Biotechnology Ltd., Witney, United Kingdom). The flaA and 16S rRNA probes were generated by PCR using primers (flaA, 5′-ATGGCTTTTCAGGTCAATAC-3′ and 5′-AGTTAAAAGCCTTAAGATAT-3′; 16S rRNA, 5′-ACGCCGCGTGGAGGATG-3′ and 5′-GCTCCCCACGCTTTCGC-3′) based on DNA sequences obtained from the website of The Institute for Genomic Research (http://www.tigr.org/tdb/CMR/ghp/htmls/SplashPage.html). Membranes were washed with 0.1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) 0.1% sodium dodecyl sulfate at 55°C and were analyzed using a Storm 840 scanner (Molecular Dynamics, St. Albans, United Kingdom). Hybridizations were quantified using ImageQuant, version 5.0, software (Molecular Dynamics), and the flaA hybridization intensities were adjusted using the 16S rRNA hybridization data to allow for minor differences in the amounts of wild-type and mutant RNA. Hybridizations were repeated at least three times using a minimum of two independent RNA preparations, and the median hybridization intensities are reported in Table 2. A typical hybridized blot is shown in Fig. 2. The results show that the levels of flaA mRNA are considerably reduced in all mutants compared to the wild-type strain.
TABLE 2.
Intensities of hybridization of a flaA probe expressed as a percentage of wild-type intensity
| Strain | Median % intensity of hybridization (range)a |
|---|---|
| SS1 (wild type) | 100 |
| fliS mutant | 57 (42–71) |
| fliF mutant | 23 (11–38) |
| fliI mutant | 26 (18–56) |
| fliG mutant | 18 (13–36) |
| fliQ mutant | 28 (25–46) |
| flhB mutant | 42 (7–49) |
Hybridizations were repeated at least three times using a minimum of two independent RNA preparations.
FIG. 2.
Slot blot hybridization of total RNAs with probes specific for either 16S rRNA (top), to determine that equal amounts of RNA were loaded, or flaA mRNA (bottom). Tests were performed on H. pylori SS1 (wild-type strain) (lane 1) and fliS (lane 2), fliF (lane 3), fliI (lane 4), fliG (lane 5), fliQ (lane 6), and flhB (lane 7) mutants. Hybridization was detected by phosphorimaging with an exposure of 72 h.
Our studies have shown that a feedback mechanism, which prevents expression of flagellin subunits when a fully functional flagellum cannot be assembled, occurs at the earliest stage in flagellar assembly. The absence of either FliF or FliG, structural components of the MS ring and switch complex, respectively, prevents the expression of FlaA, FlaB, and FlgE. In the case of FlaA expression, control is exerted at the level of transcription. This study has also shown that the absence of FliS, FlhB, FliQ, or FliI—components of the flagellum-specific export apparatus—also reduces flaA transcription. To our knowledge, this is the first direct demonstration that flaA transcription in H. pylori is inhibited in the absence of the early components of the flagellar-assembly pathway. The mechanism by which this control is exerted is unknown. However, the demonstration that a mutation in flgR, the gene encoding the NtrC orthologue, results in enhanced transcription of flaA (22) suggests that this transcription factor may be responsible. Further studies are required to fully elucidate the regulatory control of flagellar biosynthesis in H. pylori.
Acknowledgments
We gratefully acknowledge Angela Whiley and Lynne Batty for technical assistance, Richard Ferrero for strain SS1, and Chrystala Constantinidou and Charles Penn for the monoclonal antibody F2B9. We are also indebted to Graham McPhail for electron microscopy.
This work was supported by the Medical Research Council, United Kingdom.
REFERENCES
- 1.Alm R A, Ling L S L, Moir D T, King B L, Brown E D, Doig P C, Smith D R, Noonan B, Guild B C, deJonge B L, Carmel G, Tummino P J, Caruso A, Uria-Nickelsen M, Mills D M, Ives C, Gibson R, Merberg D, Mills S D, Jiang Q, Taylor D E, Vovis G F, Trust T J. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature. 1999;397:176–180. doi: 10.1038/16495. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Greene Publishing and Wiley Interscience; 1993. [Google Scholar]
- 3.Blaser M J. Ecology of Helicobacter pylori in the human stomach. J Clin Investig. 1997;100:759–762. doi: 10.1172/JCI119588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danon S J, Eaton K A. Isogenic flagella mutants of Helicobacter pylori: candidates for an attenuated vaccine? Gut. 1998;43(S2):A38. [Google Scholar]
- 5.Dorrell N, Gyselman V G, Foynes S, Li S R, Wren B W. Improved efficiency of inverse PCR mutagenesis (IPCRM) BioTechniques. 1996;21:604–608. doi: 10.2144/96214bm07. [DOI] [PubMed] [Google Scholar]
- 6.Eaton K A, Suerbaum S, Josenhans C, Krakowka S. Colonization of gnotobiotic piglets by Helicobacter pylori deficient in two flagellin genes. Infect Immun. 1996;64:2445–2448. doi: 10.1128/iai.64.7.2445-2448.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrero R L, Cussac V, Courcoux P, Labigne A. Construction of isogenic urease-negative mutants of Helicobacter pylori by allelic exchange. J Bacteriol. 1992;174:4212–4217. doi: 10.1128/jb.174.13.4212-4217.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrero R L, Thiberge J M, Huerre M, Labigne A. Immune responses of specific-pathogen-free mice to chronic Helicobacter pylori (strain SS1) infection. Infect Immun. 1998;66:1349–1355. doi: 10.1128/iai.66.4.1349-1355.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foynes S, Dorrell N, Ward S J, Zhang Z W, McColm A A, Farthing M J G, Wren B W. Functional analysis of the roles of FliQ and FlhB in flagellar expression in Helicobacter pylori. FEMS Microbiol Lett. 1999;174:33–39. doi: 10.1111/j.1574-6968.1999.tb13546.x. [DOI] [PubMed] [Google Scholar]
- 10.Hazell S L, Lee A, Brady L, Hennessy W. Campylobacter pyloridis and gastritis: association with intercellular spaces and adaptation to an environment of mucus as important factors in colonization of the gastric epithelium. J Infect Dis. 1986;153:658–663. doi: 10.1093/infdis/153.4.658. [DOI] [PubMed] [Google Scholar]
- 11.Hughes K T, Gillen K L, Semon M J, Karlinsey J E. Sensing structural intermediates in bacterial flagellar assembly by export of a negative regulator. Science. 1993;262:1277–1280. doi: 10.1126/science.8235660. [DOI] [PubMed] [Google Scholar]
- 12.Jenks P J, Foynes S, Ward S J, Constantinidou C, Penn C W, Wren B W. A flagellar-specific ATPase (FliI) is necessary for flagellar export in Helicobacter pylori. FEMS Microbiol Lett. 1997;152:205–211. doi: 10.1111/j.1574-6968.1997.tb10429.x. [DOI] [PubMed] [Google Scholar]
- 13.Josenhans C, Labigne A, Suerbaum S. Comparative ultrastructural and functional studies of Helicobacter pylori and Helicobacter mustelae flagellin mutants: both flagellin subunits, FlaA and FlaB, are necessary for full motility in Helicobacter species. J Bacteriol. 1995;177:3010–3020. doi: 10.1128/jb.177.11.3010-3020.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kostrzynska M, Betts J D, Austin J W, Trust T J. Identification, characterization, and spatial localization of two flagellin species in Helicobacter pylori flagella. J Bacteriol. 1991;173:937–946. doi: 10.1128/jb.173.3.937-946.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee A, O'Rourke J, DeUngria M C, Robertson B, Daskalopoulos G, Dixon M F. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology. 1997;112:1386–1397. doi: 10.1016/s0016-5085(97)70155-0. [DOI] [PubMed] [Google Scholar]
- 16.Leying H, Suerbaum S, Geis G, Haas R. Cloning and genetic characterization of a Helicobacter pylori flagellin gene. Mol Microbiol. 1992;6:2863–2874. doi: 10.1111/j.1365-2958.1992.tb01466.x. [DOI] [PubMed] [Google Scholar]
- 17.Macnab R M. Flagella and motility. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C.: ASM Press; 1996. pp. 123–145. [Google Scholar]
- 18.Minamino T, Macnab R M. Components of the Salmonella flagellar export apparatus and classification of export substrates. J Bacteriol. 1999;181:1388–1394. doi: 10.1128/jb.181.5.1388-1394.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Toole P W, Kostrzynska M, Trust T J. Nonmotile mutants of Helicobacter pylori and Helicobacter mustelae defective in flagellar hook production. Mol Microbiol. 1994;14:691–703. doi: 10.1111/j.1365-2958.1994.tb01307.x. [DOI] [PubMed] [Google Scholar]
- 20.Porwollik S, Noonan B, O'Toole P W. Molecular characterization of a flagellar export locus of Helicobacter pylori. Infect Immun. 1999;67:2060–2070. doi: 10.1128/iai.67.5.2060-2070.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmitz A, Josenhans C, Suerbaum S. Cloning and characterization of the Helicobacter pylori flbA gene, which codes for a membrane protein involved in coordinated expression of flagellar genes. J Bacteriol. 1997;179:987–997. doi: 10.1128/jb.179.4.987-997.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spohn G, Scarlato V. Motility of Helicobacter pylori is coordinately regulated by the transcriptional activator FlgR, an NtrC homolog. J Bacteriol. 1999;181:593–599. doi: 10.1128/jb.181.2.593-599.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suerbaum S, Josenhans C, Labigne A. Cloning and genetic characterization of the Helicobacter pylori and Helicobacter mustelae flaB flagellin genes and construction of H. pylori flaA- and flaB-negative mutants by electroporation-mediated allelic exchange. J Bacteriol. 1993;175:3278–3288. doi: 10.1128/jb.175.11.3278-3288.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suerbaum S. The complex flagella of gastric Helicobacter species. Trends Microbiol. 1995;3:168–170. doi: 10.1016/s0966-842x(00)88913-1. [DOI] [PubMed] [Google Scholar]
- 25.Tomb J F, White O, Kerlavage A R, Clayton R A, Sutton G G, Fleischmann R D, Ketchum K A, Klenk H P, Gill S, Dougherty B A, Nelson K, Quackenbush J, Zhou L X, Kirkness E F, Peterson S, Loftus B, Richardson D, Dodson R, Khalak H G, Glodek A, McKenney K, Fitzgerald L M, Lee N, Adams M D, Hickey E K, Berg D E, Gocayne J D, Utterback T R, Peterson J D, Kelley J M, Cotton M D, Weidman J M, Fujii C, Bowman C, Watthey L, Wallin E, Hayes W S, Borodovsky M, Karp P D, Smith H O, Fraser C M, Venter J C. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 26.Trieu-Cuot P, Gerbaud G, Lambert T, Courvalin P. In vivo transfer of genetic information between gram-positive and gram-negative bacteria. EMBO J. 1985;4:3583–3587. doi: 10.1002/j.1460-2075.1985.tb04120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wren B W, Henderson J, Ketley J M. A PCR-based strategy for the rapid construction of defined bacterial deletion mutants. BioTechniques. 1994;16:994–996. [PubMed] [Google Scholar]