Abstract
Background
The effects of sacubitril/valsartan in patients with chronic heart failure with reduced ejection fraction (HFrEF) were recently reported. However, the hemodynamic impact of this well-established treatment in patients with HFrEF has been poorly systematically researched.
Aim
We aimed to investigate the hemodynamic effects of sacubitril/valsartan among patients with HFrEF.
Methods
Between 2016 and 2020, we retrospectively collected data for patients with HFrEF treated at the University Medical Center Mannheim, Germany. Data for 240 patients with HFrEF were available. We systematically analyzed echocardiographic parameters, all-cause hospitalization, and congestion rate.
Results
The left ventricular ejection fraction (LVEF) improved from a median (minimum; maximum) of 28% (3; 65) before initiation of sacubitril/valsartan to a median of 34% (13; 64) at 24-month follow-up (p < 0.001). Systolic pulmonary atrial pressure (PAPsys) decreased from a median of 30 mmHg (13; 115) to 25 mmHg (20; 80) at 24-month follow-up (p = 0.005). The median (minimum; maximum) tricuspid annular plane systolic excursion improved from 17 mm (3; 31) at baseline to 20 mm (9; 30) at 12-month follow-up (p = 0.007). The incidence of severe and moderate mitral, tricuspid, and aortic valvular insufficiency improved after treatment. Hospitalization and congestion rates reduced at 24-month follow-up. The mortality rate in echocardiographic and functional nonresponders was higher than in responders (12.1 vs. 5.2%; p = 0.1 and 11.3 vs. 3.1%; p = 0.01, respectively).
Conclusion
Follow-up 24 months after starting treatment with sacubitril/valsartan revealed sustained improvements in echocardiographic parameters, including LVEF, PAPsys, and cardiac valvular insufficiency. Rates of all-cause hospitalization and congestion had decreased significantly at follow-up. The mortality rate was higher in echocardiographic and functional nonresponders.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40256-022-00525-w.
Key Points
| Beneficial hemodynamic effects of sacubitril/valsartan were sustained over a follow-up of 24 months in patients with heart failure with reduced ejection fraction (HRrEF). |
| Sacubitril/valsartan was effective in reducing hospitalization and congestion rates in patients with HFrEF. |
| Clinical outcomes improved in echocardiographic and functional responders compared with nonresponders. |
Introduction
Treatment with the combined angiotensin receptor neprilysin inhibitor valsartan/sacubitril resulted in a 20% reduced risk of cardiovascular death or hospitalization compared with enalapril in patients with heart failure with reduced ejection fraction (HFrEF) [1]. Tolerability evaluations indicated that patients receiving sacubitril/valsartan also experienced a spectrum of side effects comparable to that experienced by patients receiving enalapril [1].
Left ventricular ejection fraction (LVEF) was reported to improve after treatment with sacubitril/valsartan [2, 3]. In the PARADIGM-HF trial, improvements in LVEF were associated with better outcomes in terms of reducing the risk of death and hospitalizations in patients with HFrEF [4]. In addition, the degree of mitral regurgitation decreased significantly because of decreases in left ventricular end-systolic and end-diastolic volume [5].
Patients with HFrEF develop pulmonary hypertension (PH), which is associated with a poor prognosis [6]. Sacubitril/valsartan has been reported to improve right ventricular remodeling with the recovery of contraction and relaxation related to the reduction of pulmonary pressure [7]. In this context, hemodynamic recovery as an improvement of diastolic and systolic function was recently described in patients with HFrEF receiving sacubitril/valsartan [5, 8].
We investigated the echocardiographic hemodynamic effects of sacubitril/valsartan at 24-month follow-up, systematically analyzing congestion and all-cause hospitalizations before and after treatment. In addition, we investigated clinical outcomes in responders and nonresponders at 24-month follow-up.
Methods
Between 2017 and 2020, we included 240 patients at the University Medical Center Mannheim, University of Heidelberg. These patients had HFrEF diagnosed as per European Society of Cardiology guidelines. We included patients if they had (1) New York Heart Association (NYHA) functional class II or higher heart failure (HF) symptoms despite optimal medical treatment (including angiotensin-converting enzyme inhibitors [ACEIs] or angiotensin II receptor blockers [ARBs], β-blockers, and mineralocorticoid receptor antagonists); (2) LVEF ≤40%; (3) implantation of cardioverter-defibrillator or cardiac resynchronization therapy; and (4) received and tolerated sacubitril/valsartan. Patients initially received 24/26 mg twice daily and increased to 97/103 mg twice daily over 3–6 weeks according to tolerance, during visits to either our HF outpatient clinic or their cardiologist.
We gathered data on medical history, NYHA classification, clinical parameters, electrocardiogram, arrhythmias assessed by querying the implantable cardioverter-defibrillator or cardiac resynchronization therapy, and medication at baseline. Patients’ clinical outcomes were assessed by chart review and contact with the outpatient practice at follow-up. Laboratory parameters representing heart or kidney function, electrolytes such as potassium, and glycated hemoglobin were evaluated before initiation of sacubitril/valsartan and at follow-up. The estimated glomerular filtration rate was calculated using the abbreviated Modification of Diet in Renal Disease equation. A doppler transthoracic and standard two-dimensional echocardiogram was used at more timepoints at baseline and after initiation of sacubitril/valsartan at follow-up. All measurements were taken with available echocardiographic instruments (Vivid 9 System, Philips Medical Systems). Echocardiographic parameters such as LVEF, tricuspid annular plane systolic excursion (TAPSE), systolic pulmonary atrial pressure (PAPsys), tricuspid regurgitation jet maximal pressure gradient, tricuspid regurgitation peak systolic velocity, left atrial surface, right atrial surface, E wave/A wave ratio, valvular insufficiency, and the vena cava inferior (VCI) diameter were collected. Serial echocardiographic evaluation was presented by more sonographers at follow-up. In this regard, intra-observer variability due to the nonsignificant differences of ejection fraction measurements was insignificant. Hospitalizations and congestion were also evaluated at follow-up. In addition, this study compared hospitalization and congestion rates before and after initiation of sacubitril/valsartan.
Definitions and Technical Measurements of Echocardiographic Outcome
Ejection fraction (EF) is defined as the volume of blood ejected during left ventricular contraction and is measured using the Simpson biplane method. TAPSE indicates right ventricular longitudinal function and is measured using M-mode echocardiography between the end-diastole and peak systole, with the cursor along the tricuspid lateral annulus in the apical four-chamber view. PAPsys indicates the peak pressure of blood in the right ventricle during systole and is calculated from the tricuspid regurgitation jet maximal pressure gradient and right atrial pressure. Tricuspid regurgitation peak systolic velocity is a quantitative measurement of the peak velocity of blood flow across the tricuspid valve. Left atrial and right atrial size are qualitative descriptions of the size of the atria and are measured in a four-chamber apical view at end-systole. E wave/A wave ratio is determined as the ratio of peak early to peak late transmitral flow velocities reported without units. It is measured to evaluate the left ventricular diastolic function. The VCI measurement is assessed in the subcostal view 1–2 cm from the junction with the right atrium in the long-axis view. VCI values are used to determine systolic pulmonary artery pressure. An echocardiographic response was defined as improved LVEF with an increase of ≥ 5%, and a functional response was defined as an improvement of one class category in NYHA classification [9, 10].
Congestion was defined as the presence of one or more symptoms of fluid overload. The following symptoms are considered: pulmonary rales, third heart sound, jugular venous stasis, hepatomegaly, peripheral edema, high level of N-terminal pro-B-type natriuretic peptide (NT-proBNP), acute depression of heart function diagnosed by echocardiography, and chest radiograph signs of congestion. Hospitalization was defined as any readmission to a hospital.
Statistics
We presented continuous variables with a non-normal distribution as median (minimum; maximum), continuous variables with a normal distribution as mean ± standard deviation, and categorical variables as frequency (%). The Kolmogorov–Smirnov test was used to assess normal distribution. Student’s t test and the Mann–Whitney U test were used to compare continuous variables with normal and non-normal distributions, respectively. Box plot diagrams were presented as medians with 25th and 75th percentiles (boxes) and 5th and 95th percentiles (whiskers). Dots indicated extreme values. The Kruskal–Wallis test with Dunn’s multiple comparisons test was used for multiple comparisons. Hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated in the survival analysis using Cox regression. P values < 0.05 were recognized as statistically significant. Univariate analyses were performed to study the relationship between mortality and other variables, for example, patient characteristics, medical history, heart valve insufficiency, arrhythmias, electronic cardiac disease, and drugs on admission. Predictors of mortality were identified using univariate analysis. Predictors with p < 0.05 were analyzed using Cox multivariate regression. Multivariable Cox regression to investigate predictors of mortality included the following variables: medical history such as type 2 diabetes mellitus, congestion at admission, coronary heart disease, MitraClip, non-sustained ventricular tachycardia, aldosterone antagonist, and insulin. Statistical analysis was performed using SPSS 25.0.
Results
Patient Characteristics
The median (minimum; maximum) age of patients was 66.9 years (32; 89); 55.4% of patients were aged > 65 years. Males predominated (79.6%). Ischemic cardiomyopathy (ICMP) was diagnosed in 51.7% of patients, whereas 51.3% of patients had dilated cardiomyopathy (DCMP). ACEIs were used in 56.5% of patients, whereas 24.3% of patients received ARBs before initiating sacubitril/valsartan. Among cardiovascular risk factors and comorbidities, 36.3% of patients had diabetes mellitus and 72.9% had arterial hypertension. Baseline characteristics are presented in Table 1.
Table 1.
Baseline characteristics of patients presenting before sacubitril/valsartan
| Characteristics | Patients before sacubitril/valsartan (n = 240) |
|---|---|
| Demographics | |
| Age, years | 66.9 (32; 89) |
| ≤ 65 | 107/240 (44.6) |
| > 65 | 133/240 (55.4) |
| Male | 191/240 (79.6) |
| BMI | 29.51 ± 6.01 |
| Medical history | 240/240 (100) |
| Smoking | |
| Current | 70/240 (29.2) |
| Ex-smoker | 76/240 (31.6) |
| Lung disease | 48/240 (20) |
| Asthma | 4/48 (8.3) |
| COPD | 44/48 (91.7)) |
| Arterial hypertension | 175/240 (72.9) |
| Type 2 diabetes mellitus | 87/240 (36.3) |
| Positive family history | 59/240 (24.6) |
| History of malignancy | 39/240 (16.3) |
| Myocardial infarction | 98/240 (40.8) |
| STEMI | 71/98 (72.4) |
| NSTEMI | 37/98 (37.8) |
| Coronary heart disease | 158/240 (65.8) |
| Stroke | 30/240 (12.5) |
| Bypass | 36/240 (15) |
| Bleeding | 10/240 (4.2) |
| Heart failure | 240/240 (100) |
| DCMP | 123/240 (51.3) |
| ICMP | 124/240 (51.7) |
| NYHA classification | |
| I | 8/208 (3.8) |
| II | 55/208 (26.4) |
| III | 124/208 (59.6) |
| IV | 21/208 (10.1) |
| Clinical parameter | |
| Systolic BP, mmHg | 121 (80; 190) |
| Diastolic BP, mmHg | 80 (42; 120) |
| HR, bpm | 74 (46; 156) |
| Electrocardiogram | |
| PQ, ms | 170 (96; 396) |
| QTc, ms | 469.50 (207; 696) |
| MitraClip | 14/240 (5.8) |
| Arrhythmias | |
| Atrial fibrillation | 107/240 (44.6) |
| Paroxysmal | 46/107 (43.0) |
| Persistent | 20/107 (18.7) |
| Permanent | 25/107 (23.4) |
| Ventricular fibrillation | 18/47 (38.3) |
| Nonsustained ventricular tachycardia | 22/47 (46.8) |
| Ventricular tachycardia | 18/47 (38.3) |
| Cardiac device | |
| CRT-D | 51/162 (31.5) |
| S-/TV-ICD | 131/162 (80.9) |
| PM | 16/162 (9.9) |
| CCM | 38/162 (23.5) |
| Vagus stimulator | 1/162 (0.6) |
| Medication | |
| β-blocker | 221/239 (92.5) |
| ARB | 58/239 (24.3) |
| Aldosterone antagonist | 155/239 (64.9) |
| ACEI | 135/239 (56.5) |
| Ivabradine | 8/239 (3.3) |
| Diuretics | 189/239 (79.1) |
| Platelet aggregation inhibitors | 116/239 (48.5) |
| Anticoagulation | 113/239 (47.3) |
| Amiodarone | 28/239 (11.7) |
| Sotalol | 1/239 (0.4) |
| Mexiletine | 0/239 (0.0) |
| Statin | 157/239 (65.7) |
| Metformin | 27/231 (11.7) |
| Insulin | 29/231 (12.6) |
| SGLT2 inhibitor | 10/231 (4.3) |
| DPP-4 inhibitor | 17/231 (7.4) |
| GLP-1 agonist | 1/231 (0.4) |
| Sulfonylureas | 3/231 (1.3) |
Data are presented as n (%), median (minimum; maximum) or mean ± standard deviation
ACEI angiotensin-converting enzyme inhibitor, AF atrial fibrillation, ARB angiotensin II receptor blocker, BMI body mass index, BP blood pressure, CCM cardiac contractility modulation, COPD chronic obstructive pulmonary disease, CRT-D cardiac resynchronization therapy with a defibrillator, DCMP dilated cardiomyopathy, DPP-4 dipeptidyl peptidase-4, GLP-1 glucagon-like peptide-1, HR heart rate, ICMP ischemic cardiomyopathy, n number, NSTEMI non-ST-segment elevation myocardial infarction, NYHA New York Heart Association, PM pacemaker, PQ PQ interval, QTc corrected QT interval, SGLT2 sodium-glucose co-transporter-2, S-ICD subcutaneous implantable cardioverter-defibrillator, STEMI ST-segment elevation myocardial infarction, TV-ICD transvenous implantable cardioverter-defibrillator
Hemodynamic Effects of Sacubitril/Valsartan
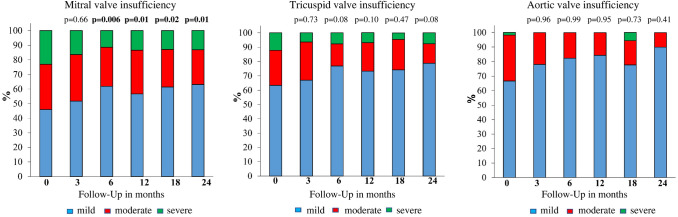
Continuous improvement in LVEF was observed, from a median (minimum; maximum) of 28% (3; 65) at initiation of sacubitril/valsartan to 34% (13; 64) at 24-month follow-up (p < 0.001). The TAPSE improved from an initial median (minimum; maximum) of 17 mm (3; 31) to 18 mm (2.5; 31) at 24-month follow-up (p = 0.47). However, the largest increase of TAPSE was revealed at 12-month follow-up: 20 mm (9; 30); p=0.007. In addition, PAPsys, as an indicator of possible PH, decreased from a median (minimum; maximum) 30 mmHg (13; 115) to 25 mmHg (20; 80) at 24-month follow-up (p = 0.005) (Table 2). Data about the hemodynamic effects at follow-up are available in the appendix in the electronic supplementary material (ESM). Transthoracic echocardiography before initiation of sacubitril/valsartan revealed a higher incidence of severe and moderate valvular insufficiency, whereas numerically more valvular insufficiency was classified as mild after sacubitril/valsartan (Fig. 1). The incidence of mitral, tricuspid, and aortic insufficiency before and after sacubitril/valsartan is presented in Fig. 1.
Table 2.
Echocardiographic and laboratory values of patients presenting before and after sacubitril/valsartan (LCZ696)
| Variables | Before LCZ696 | 3 months after LCZ696 | 6 months after LCZ696 | 12 months after LCZ696 | 18 months after LCZ696 | 24 months after LCZ696 | P valuea |
|---|---|---|---|---|---|---|---|
| Echocardiographic values | |||||||
| LVEF, % | 28 (3; 65) | 34 (5; 69) | 33 (15; 60) | 33.5 (13; 60) | 33 (15; 58) | 34 (13; 64) | <0.001 |
| TAPSE, mm | 17 (3; 31) | 18 (8; 38) | 18 (2.3; 32) | 20 (9; 30) | 19 (2.1; 42) | 18 (2.5; 31) | 0.01 |
| PAPsys, mmHg | 30 (13; 115) | 30.5 (15; 68) | 30 (11; 71) | 29 (10; 88) | 25 (12; 73) | 25 (20; 80) | 0.02 |
| TR dPmax, mmHg | 30.5 (13; 101) | 36.5 (14; 68) | 35 (17; 71) | 30 (2; 87) | 30.4 (14; 57) | 29 (25; 75) | 0.69 |
| TR Vmax, m/s | 2.75 (0.7; 4.5) | 3.11 (2.4; 4.13) | 2.8 (0.95; 4.2) | 3.0 (1.8; 5.0) | 2.85 (3; 3) | 2.8 (1.7; 7.0) | 0.81 |
| LA, cm2 | 25 (12; 63) | 25 (14; 48) | 23 (11; 57) | 23 (11; 58) | 24 (11; 43) | 24 (12; 56) | 0.65 |
| RA, cm2 | 19 (7; 44) | 17 (8; 39) | 18.25 (8; 40) | 18.6 (7; 40) | 19 (8.1; 35) | 17 (7; 41) | 0.74 |
| VCI diameter, mm | 20 (3; 40) | 17 (13; 29) | 20 (9; 28) | 17 (7; 40) | 21 (7; 34) | 19 (12; 35) | 0.88 |
| E/A ratio | 1.3 (0.49; 3.8) | 0.87 (0.36; 3.86) | 0.8 (0.3; 3.6) | 0.9 (0.4; 5.8) | 0.9 (0.44; 2.8) | 0.8 (0.4; 2.3) | 0.05 |
| NYHA classification | |||||||
| I | 8/208 (3.8) | 17/185 (9.2) | 21/169 (12.4) | 24/157 (15.3) | 22/130 (16.9) | 25/118 (21.2) | < 0.001 |
| II | 55/208 (26.4) | 68/185 (36.8) | 68/169 (40.2) | 67/157 (42.7) | 60/130 (46.2) | 53/118 (44.9) | < 0.001 |
| III | 124/208 (59.6) | 84/185 (45.4) | 71/169 (42.0) | 61/157 (38.9) | 44/130 (33.8) | 37/118 (31.4) | < 0.001 |
| IV | 21/208 (10.1) | 16/185 (8.6) | 9/169 (5.3) | 5/157 (3.2) | 4/130 (3.1) | 3/118 (2.5) | < 0.001 |
| Laboratory values | |||||||
| Potassium, mmol/l | 4.15 (2.1; 6.5) | 4.23 (2.9; 6.0) | 4.3 (2.7; 4.6) | 4.21 (2.86; 5.7) | 4.30 (2.38; 6.6) | 4.23 (2.8; 5.5) | 0.69 |
| Creatinine, mg/dl | 1.20 (0.34; 6.95) | 1.31 (0.58; 3.37) | 1.28 (0.68; 7.02) | 1.32 (0.65; 4.9) | 1.34 (0.67; 4.40) | 1.25 (0.56; 7.63) | 0.23 |
| GFR, ml/min | 55.0 (10; 128.8) | 51.0 (21; 117) | 50.0 (3; 128.8) | 51.0 (11; 102) | 48.0 (15; 125) | 49.0 (8; 109) | < 0.001 |
| TNI, ng/ml | 0.077 (0.009; 138.690) | 0.021 (0.002; 0.600) | 0.048 (0.013; 0.878) | 0.015 (0.005; 14.945) | 0.019 (0.015; 0.208) | 0.020 (0.006; 0.635) | 0.13 |
| NT-proBNP, pg/ml | 1445.0 (48; 74,676) | 1149.5 (31; 15,505) | 991.5 (78; 26,041) | 770.5 (49; 8598) | 813 (30; 8634) | 569 (13; 4571) | < 0.001 |
| Hb, g/dl | 13.8 (8.2; 17.8) | 13.5 (8.3; 18.3) | 13.6 (9.3; 17.6) | 13.6 (7.1; 17.2) | 13.6 (8.0; 17.2) | 14.0 (8.7; 17.1) | 0.83 |
| HbA1c, % | 6.0 (4.3; 11.5) | 6.4 (5.2; 12.2) | 6.6 (5.3; 11.7) | 6.1 (5.2; 14.4) | 6.3 (5.0; 11.2) | 6.2 (5.2; 9.9) | 0.68 |
Data are presented as n (%) or median (minimum; maximum)
Bold text indicates a statistically significant difference with a p-value less than 0.05
E/A ratio E wave/A wave ratio, GFR glomerular filtration rate, Hb hemoglobin, HbA1c glycated Hb, LA left atrial surface, LCZ696 sacubitril/valsartan, LVEF left ventricular ejection fraction, n number, NT-proBNP N-terminal pro-B-type natriuretic peptide, NYHA New York Heart Association, PAPsys systolic pulmonary arterial pressure, RA right atrial surface, TAPSE tricuspid annular plane systolic excursion, TNI high-sensitivity troponin I, TR dPmax tricuspid regurgitation jet maximal pressure gradient, TR Vmax tricuspid regurgitation peak velocity, VCI vena cava inferior
ap values for the comparison between before and all times after sacubitril/valsartan, p values for pairwise comparisons are presented in the electronic supplementary material
Fig. 1.
Echocardiographic outcome of patients after sacubitril/valsartan presented with significantly lower moderate or severe valvular insufficiency at follow-up; p values for the comparison between follow-up time and month 0
Laboratory Parameters
The glomerular filtration rate decreased from a median (minimum; maximum) 55 ml/min (10; 128.8) before treatment to 49 ml/min (8; 109) after sacubitril/valsartan at 24-month follow-up (p = 0.98). The largest decrease in glomerular filtration rate was observed at 18-month follow-up: median (minimum; maximum) 48 ml/min (15; 125); p < 0.001. However, NT-proBNP values reduced significantly from a median (minimum; maximum) of 1445 pg/ml (48; 74,676) before sacubitril/valsartan to 569 pg/ml (13; 4571) at 24-month follow-up (p < 0.001) (Table 2). Data on NT-proBNP levels at follow-up are available in the ESM.
Clinical Outcomes in Responders vs. Nonresponders
Echocardiographic and functional nonresponse occurred more in patients with ICMP than in those with DCMP (echocardiographic nonresponse 63.8 vs. 41.4%; functional nonresponse 57.7 vs. 43.3%). NT-ProBNP was significantly reduced in echocardiographic responders compared with nonresponders at 24-month follow-up (median [minimum; maximum] 452 pg/ml [13; 4203] vs. 1097 pg/ml [159; 4571]; p = 0.003). The mortality rate in echocardiographic and functional nonresponders was higher than in responders (12.1 vs. 5.2%; p = 0.1 and 11.3 vs. 3.1%; p = 0.01, respectively). The rate of congestion was significantly higher in echocardiographic nonresponders than in responders (20 vs. 4.3%; p = 0.05). Table 3 presents the differences between echocardiographic and functional responders compared with nonresponders.
Table 3.
Clinical characteristics and outcomes in responders vs. nonresponders at 24-month follow-up
| Variables | Echocardiographic responder | Echocardiographic nonresponder | p valuea | Functional responder | Functional nonresponder | p valuea |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age, years | 64.5 (33; 88) | 67.5 (33; 88) | 0.43 | 65 (35; 85) | 66 (33; 88) | 0.66 |
| Male | 89/116 (76.7) | 52/58 (89.7) | 0.04 | 76/97 (78.4) | 80/97 (82.5) | 0.47 |
| BMI | 28.98 ± 5.56 | 29.68 ± 5.52 | 0.44 | 29.43 ± 5.62 | 28.41 ± 6.27 | 1.00 |
| Medical history | ||||||
| DCMP | 66/116 (56.9) | 24/58 (41.4) | 0.05 | 56/97 (57.7) | 42/97 (43.3) | 0.04 |
| ICMP | 50/116 (43.1) | 37/58 (63.8) | 0.01 | 44/97 (45.4) | 56/97 (57.7) | 0.09 |
| Laboratory values | ||||||
| NT-proBNP pg/ml | 452 (13; 4203) | 1097 (159; 4571) | 0.003 | 544.5 (13; 4571) | 457 (152; 4331) | 0.75 |
| NYHA classification | ||||||
| III | 19/69 (27.5) | 13/35 (37.1) | 0.08 | 10/64 (15.6) | 25/44 (56.8) | <0.001 |
| IV | 1/69 (1.4) | 2/35 (5.7) | 0.08 | 0/64 (0.0) | 3/44 (6.8) | <0.001 |
| Echocardiographic values | ||||||
| LVEF, % | 38 (15; 64) | 22.5 (13; 55) | < 0.001 | 33 (15; 60) | 37 (13; 64) | 0.11 |
| TAPSE, mm | 19.5 (2.5; 31) | 17 (11; 27) | 0.01 | 19 (11; 31) | 18 (2.5; 31) | 0.34 |
| Clinical outcomes | ||||||
| Mortality | 6/116 (5.2) | 7/58 (12.1) | 0.1 | 3/97 (3.1) | 11/97 (11.3) | 0.01 |
| Congestion | 5/70 (4.3) | 7/35 (20.0) | 0.05 | 6/65 (9.2) | 5/45 (11.1) | 0.75 |
| Hospitalization | 40/74 (54.1) | 21/38 (55.3) | 0.97 | 38/67 (56.7) | 23/52 (44.2) | 0.18 |
Data are presented as n (%), median (minimum; maximum), or mean ± standard deviation unless otherwise indicated
Bold text indicates a statistically significant difference with a p-value less than 0.05
BMI body mass index, DCMP dilated cardiomyopathy, ICMP ischemic cardiomyopathy, LVEF left ventricular ejection fraction, NT-proBNP N-terminal pro-B-type natriuretic peptide, NYHA New York Heart Association, TAPSE tricuspid annular plane systolic excursion
ap values for the comparison between responders and nonresponders
Predictors of Mortality
Cox multivariate analysis for mortality determined congestion at admission as an independent predictor for mortality (HR 5.57; 95% CI 1.45–21.48; p = 0.01), Table 4.
Table 4.
Predictors for mortality in echocardiographic and/or functional responder
| Variable | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR | p value | HR | 95% CI | p value | |
| Demographics | |||||
| Age > 65 years | 1.89 | 0.18 | |||
| BMI ≥ 30 | 0.64 | 0.37 | |||
| Medical history | |||||
| T2DM | 2.80 | 0.02 | 2.17 | 0.59–7.92 | 0.24 |
| Congestion at admission | 8.79 | < 0.001 | 5.57 | 1.45–21.48 | 0.01 |
| Coronary heart disease | 4.63 | 0.04 | 3.70 | 0.47–29.44 | 0.22 |
| MitraClip | 5.20 | < 0.001 | 1.39 | 0.39–4.97 | 0.61 |
| Arrhythmia before sacubitril/valsartan | |||||
| Nonsustained ventricular tachycardia | 3.42 | 0.02 | 1.66 | 0.46–6.02 | 0.44 |
| Drugs on admission | |||||
| Aldosterone antagonist | 0.24 | 0.002 | 0.41 | 0.13–1.26 | 0.12 |
| Insulin | 3.67 | 0.01 | 1.15 | 0.34–3.87 | 0.82 |
Bold text indicates a statistically significant difference with a p-value less than 0.05
R2 value 0.21
BMI body mass index, CI confidence interval, HR hazard ratio, T2DM type 2 diabetes mellitus
Clinical Outcomes in All Patients at Follow-Up
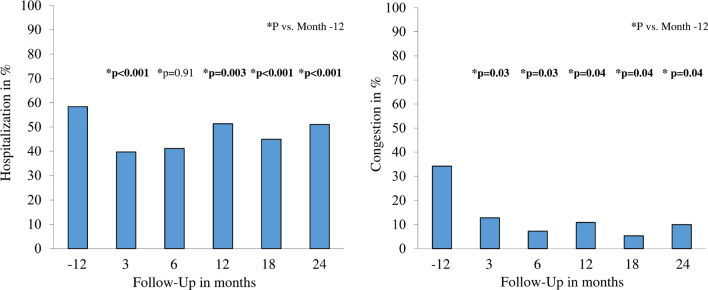
The hospitalization rate was 58.3% at 12 months before treatment and 39.7% at 3 months, 41.1% at 6 months, 51.3% at 12 months, 45% at 18 months, and 51.1% at 24 months after treatment (p < 0.001). Congestion also decreased from 34.2% at treatment initiation to 10% at 24-month follow-up (p = 0.04) (Fig. 2).
Fig. 2.
The rate of hospitalization and congestion of patients before and after sacubitril/valsartan; data in percentage values; p values for the comparison between follow-up time and month 0
Discussion
Our study presents the hemodynamic effects observed over a 24-month follow-up after initiation of sacubitril/valsartan in a retrospective analysis. The main findings are that (1) LVEF, TAPSE, and PAPsys improved at follow-up, (2) the incidence of severe and moderate mitral, tricuspid, and aortic insufficiency reduced after treatment with sacubitril/valsartan, (3) hospitalization and congestion rates were significantly lower after than before sacubitril/valsartan.
Hemodynamic Effects
Consistent with our data, Martens et al. [5] reported that improvements in LVEF after sacubitril/valsartan were probably due to reverse remodeling. TAPSE has also been observed to increase from 18.5 to 19.7 mm at 6-month follow-up after initiation of sacubitril/valsartan [11]. This result is comparable to our result, where TAPSE increased from 17 to 18 mm at 6-month follow-up. Furthermore, PAPsys has been observed to reduce from 31 to 25 mmHg at 6-month follow-up [11]. Our data showed a decrease of PAPsys from 30 to 25 mmHg at 24-month follow-up. In our cohort, PAPsys at 6-month follow-up was equivalent to that at baseline before initiation of sacubitril/valsartan. Regarding heart valve insufficiency, our data illustrated an improvement in grade of mitral, tricuspid, and aortic valve insufficiency after sacubitril/valsartan at follow-up. Kang et al. [12] reported a reduction of effective mitral regurgitant orifice area in patients treated with sacubitril/valsartan as compared with the valsartan group. In this regard, we revealed a decrease of severe and moderate mitral insufficiency at follow-up (23 and 31.1% before vs. 13.2 and 23.7% after sacubitril/valsartan). Treatment with combined sacubitril/valsartan may improve the reverse remodeling that is associated with a reduction of left ventricular volume and sphericity and may improve left ventricular function, which decreases the severity of mitral insufficiency [13]. Our study also found a reduction in the percentage of severe and moderate tricuspid and aortic insufficiency at 24-month follow-up (tricuspid 12.2 and 24.4% before vs. 7.6 and 13.6% after treatment; aortic 1.8 and 31.6% before vs. 0 and 10% after treatment). In 205 patients with HFrEF, tricuspid velocity reduced from 2.8 ± 0.55 to 2.64 ± 0.59 m/s at 6-month follow-up after sacubitril/valsartan [8]. Data about the systematic evaluation of tricuspid and aortic insufficiency remain limited.
Laboratory Parameters
Our study presents a reduction in NT-proBNP from a median (minimum; maximum) of 1445 pg/ml (48; 74,676) before sacubitril/valsartan to 569 pg/ml (13; 4571) at 24-month follow-up. The PIONEER-HF trial also revealed a decrease in NT-proBNP in patients with de novo and chronic HF after sacubitril/valsartan [14].
Clinical Outcomes in Responders vs. Nonresponders
In our study, nonresponse to treatment occurred more in patients with ICMP than in those with DCMP. This showed the impact of HF etiology in the treatment of patients with HFrEF. In addition, mortality and congestion rates after initiation of sacubitril/valsartan were higher in echocardiographic and functional nonresponders than in responders. Data in this regard are limited.
Hospitalization and Congestion
We observed that all-cause hospitalization and congestion rates reduced significantly at follow-up after initiation of sacubitril/valsartan. All-cause hospitalization was 58.3% before sacubitril/valsartan at 12 months and 51.1% at 24-month follow-up after treatment. ACEIs or ARBs were used in 80.8% of all patients before sacubitril/valsartan. However, the largest decrease in hospitalization rate was 39.7% at 3-month follow-up, maybe because of superior medication adherence at the beginning of the treatment. Medication adherence was reported to be superior at the beginning of treatment compared with later. In this context, patient education about self-management minimized the risk of medication nonadherence, which was associated with a reduction in all-cause hospital readmissions and improved quality of life [15–17]. The PARADIGM-HF trial indicated that sacubitril/valsartan was superior to enalapril in terms of HF-related hospitalization rates (12.8 vs. 15.6%) [1]. In a systematic review, all-cause hospitalizations decreased after sacubitril/valsartan compared with standard of care [18]. In our study, the incidence of congestion was 34.2% at 12 months before starting the treatment and 10% at 24-month follow-up after initiation of sacubitril/valsartan. The rate of congestion was lower in patients with HFrEF treated with sacubitril/valsartan than in those receiving enalapril [18]. Consistent with our data, congestion reduced from the beginning compared with at 19-month follow-up in the sacubitril/valsartan group [18].
Limitations
This study has several limitations. It was a monocenter study, had a limited follow-up time, and had a limited number of patients. Therefore, caution is required when interpreting data. The NYHA class was evaluated without using a qualitative evaluation questionnaire. Diet and exercise habits were not systematically analyzed. Cardiac magnet resonance tomography was not used to evaluate the left ventricular and right ventricular volumes and LVEF. Some echocardiographic parameters useful for the evaluation of reverse remodeling, such as left ventricular mass index, left atrial volume index, and strain analysis, were not presented. Interobserver variability regarding echocardiographic parameters cannot be excluded.
Conclusions
This study confirmed the hemodynamic effects of sacubitril/valsartan on left and right heart function, indicating improvements in valvular insufficiency, LVEF, and TAPSE and reduced PH. All-cause hospitalization and congestion rates reduced after initiation of sacubitril/valsartan compared with before treatment with ACEIs and ARBs. Mortality and congestion rates were lower in echocardiographic and functional responders than in nonresponders. Sacubitril/valsartan is a milestone in the treatment of HFrEF to improve hemodynamics and reduce the need for early interventions for heart valve disease.
Supplementary Information
Below is the link to the electronic supplementary material.
Author Contributions
MA and IA made substantial contributions to the study concept and design. Data were collected by JD, CK, and CP. MA and JD analyzed all data. SL approved the statistical analysis. IE, MB, AA, and IA supervised the project and reviewed the manuscript. MA, AM, and IA prepared the manuscript. All authors approved the final version
Funding
Open Access funding enabled and organized by Projekt DEAL.
Declarations
Funding
No external funding was used in the preparation of this manuscript.
Conflict of interest
Mohammad Abumayyaleh, Jonathan Demmer, Ibrahim El-Battrawy, Carina Krack, Christina Pilsinger, Michael Behnes, Assem Aweimer, Andreas Mügge, Siegfried Lang, and Ibrahim Akin have no potential conflicts of interest that might be relevant to the contents of this manuscript.
Ethics approval
This study was executed in compliance with the Declaration of Helsinki regarding investigations in human subjects, and the study protocol was approved by the Ethics Committee of the Medical Faculty Mannheim Heidelberg University.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The authors are willing to share anonymized primary data upon reasonable research request.
Code availability
Not applicable.
References
- 1.McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 2.Januzzi JL, Jr, Prescott MF, Butler J, et al. Association of change in n-terminal pro-b-type natriuretic peptide following initiation of sacubitril-valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction. JAMA. 2019;332:1–11. doi: 10.1001/jama.2019.12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Almufleh A, Marbach J, Chih S, et al. Ejection fraction improvement and reverse remodeling achieved with sacubitril/valsartan in heart failure with reduced ejection fraction patients. Am J Cardiovasc Dis. 2017;7(6):108–113. [PMC free article] [PubMed] [Google Scholar]
- 4.Solomon SD, Claggett B, Desai AS, et al. Influence of ejection fraction on outcomes and efficacy of sacubitril/valsartan (lcz696) in heart failure with reduced ejection fraction: The prospective comparison of arni with acei to determine impact on global mortality and morbidity in heart failure (paradigm-hf) trial. Circ Heart Fail. 2016;9(3):e002744. doi: 10.1161/CIRCHEARTFAILURE.115.002744. [DOI] [PubMed] [Google Scholar]
- 5.Martens P, Belien H, Dupont M, Vandervoort P, Mullens W. The reverse remodeling response to sacubitril/valsartan therapy in heart failure with reduced ejection fraction. Cardiovasc Ther. 2018;36(4):e12435. doi: 10.1111/1755-5922.12435. [DOI] [PubMed] [Google Scholar]
- 6.Zern EK, Cheng S, Wolfson AM, et al. Angiotensin receptor-neprilysin inhibitor therapy reverses pulmonary hypertension in end-stage heart failure patients awaiting transplantation. Circ Heart Fail. 2020;13(2):e006696. doi: 10.1161/CIRCHEARTFAILURE.119.006696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharifi Kia D, Benza E, Bachman TN, Tushak C, Kim K, Simon MA. Angiotensin receptor-neprilysin inhibition attenuates right ventricular remodeling in pulmonary hypertension. J Am Heart Assoc. 2020;9(13):e015708. doi: 10.1161/JAHA.119.015708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romano G, Vitale G, Ajello L, et al. The effects of sacubitril/valsartan on clinical, biochemical and echocardiographic parameters in patients with heart failure with reduced ejection fraction: the "hemodynamic recovery". J Clin Med. 2019;8(12):2165. doi: 10.3390/jcm8122165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakai T, Ikeya Y, Kogawa R, et al. What are the expectations for cardiac resynchronization therapy? A validation of two response definitions. J Clin Med. 2021;10(3):514. doi: 10.3390/jcm10030514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daubert MA, Massaro J, Liao L, et al. High-risk percutaneous coronary intervention is associated with reverse left ventricular remodeling and improved outcomes in patients with coronary artery disease and reduced ejection fraction. Am Heart J. 2015;170(3):550–558. doi: 10.1016/j.ahj.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 11.Yenercag M, Arslan U, Dereli S, Coksevim M, Dogdus M, Kaya A. Effects of angiotensin receptor neprilysin inhibition on pulmonary arterial stiffness in heart failure with reduced ejection fraction. Int J Cardiovasc Imaging. 2021;37(1):165–173. doi: 10.1007/s10554-020-01973-8. [DOI] [PubMed] [Google Scholar]
- 12.Kang DH, Park SJ, Shin SH, et al. Angiotensin receptor neprilysin inhibitor for functional mitral regurgitation. Circulation. 2019;139(11):1354–65. [DOI] [PubMed]
- 13.Mullens W, Martens P. Sacubitril/valsartan to reduce secondary mitral regurgitation. Circulation. 2019;139(11):1366–1370. doi: 10.1161/CIRCULATIONAHA.118.038135. [DOI] [PubMed] [Google Scholar]
- 14.Ambrosy AP, Braunwald E, Morrow DA, et al. Angiotensin receptor-neprilysin inhibition based on history of heart failure and use of renin-angiotensin system antagonists. J Am Coll Cardiol. 2020;76(9):1034–1048. doi: 10.1016/j.jacc.2020.06.073. [DOI] [PubMed] [Google Scholar]
- 15.Ditewig JB, Blok H, Havers J, van Veenendaal H. Effectiveness of self-management interventions on mortality, hospital readmissions, chronic heart failure hospitalization rate and quality of life in patients with chronic heart failure: a systematic review. Patient Educ Couns. 2010;78(3):297–315. doi: 10.1016/j.pec.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 16.Glader EL, Sjolander M, Eriksson M, Lundberg M. Persistent use of secondary preventive drugs declines rapidly during the first 2 years after stroke. Stroke. 2010;41(2):397–401. doi: 10.1161/STROKEAHA.109.566950. [DOI] [PubMed] [Google Scholar]
- 17.Brown MT, Bussell JK. Medication adherence: who cares? Mayo Clin Proc. 2011;86(4):304–314. doi: 10.4065/mcp.2010.0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Proudfoot C, Studer R, Rajput T, et al. Real-world effectiveness and safety of sacubitril/valsartan in heart failure: a systematic review. Int J Cardiol. 2021;331:164. doi: 10.1016/j.ijcard.2021.01.061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.