Abdominal contents are primarily fluid in character so that pressure within this compartment follows Pascal’s hydrostatic law. Intra-abdominal pressure (IAP) is the steady state pressure within the abdominal cavity and changes during respiration with an inspiratory increase [1]. Mean perfusion pressure (MPP) is the difference between mean arterial pressure (MAP) and central venous pressure (CVP). Similarly abdominal perfusion pressure (APP) is calculated as the difference between MAP and IAP. MPP has been seen to be associated with progression of organ system injury [2].
Consensus definition
The reference standard for intermittent IAP measurements is via the bladder with a maximal instillation volume of 25 mL of sterile saline. IAP should be expressed in mmHg and measured at end-expiration in the supine position after ensuring abdominal muscle contractions are absent and with the transducer zeroed at the level where the midaxillary line crosses the iliac crest and is approximately 5–7 mmHg in healthy and 10 mmHg in critically ill adults. IAH is defined by a sustained or repeated elevation in IAP ≥ 12 mmHg. ACS is defined as a sustained IAP > 20 mmHg that is associated with new organ dysfunction/failure [3].
Pathophysiology
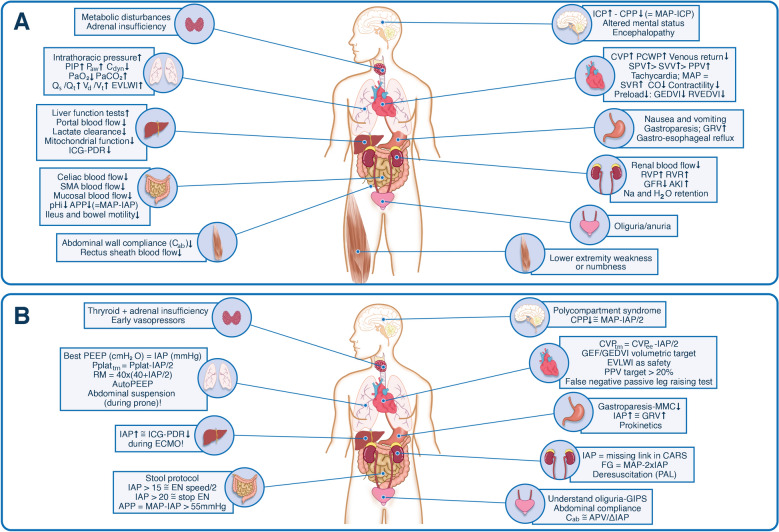
There are four factors affecting IAP: gravity, uniformity of compression, shear stress and deformation, and presence of abdominal muscle contraction [1, 4]. However, in the critically ill patient the abdomen often behaves as a hydraulic system. Raised IAP affects multiple organ systems within and outside the abdominal cavity and understanding the pathophysiology can prevent impending ACS and guide treatment. The kidneys are very vulnerable to raised IAP and some suggest they are the canary in the coalmine for IAH and elevated renal parenchymal and venous pressures are the main causes for AKI. Subsequently, raised IAP decreases chest wall compliance and in combination with the cranial shift of the diaphragm this results in reduced lung volumes causing compression atelectasis, hypoxia and hypercarbia. In addition, the negative effects of increased IAP on the cardiovascular system are explained by significant changes in preload, afterload, and contractility. As a result, reduced cardiac output leads to low blood pressure followed by poor organ perfusion and eventually worsening of IAH. A summary of the effects of raised IAP on other end-organs are shown in Fig. 1 Panel A [3, 5, 6].
Fig. 1.
Impact of intra-abdominal pressure on end-organ function. PANEL A Pathophysiologic implications of increased intra-abdominal pressure on end-organ function within and outside the abdominal cavity. PANEL B Clinical implications of increased intra-abdominal pressure at the bedside. Knowing IAP and understanding organ-organ crosstalk is key to adapt organ support therapy. Adapted with permission from Regli et al. according to the Open Access CC BY License 4.0 [6]. AKI acute kidney injury, APP abdominal perfusion pressure = MAP − IAP, ΔIAP respiratory variation in IAP = IAPei − IAPee, APV abdominal pressure variation = ΔIAP/(mean IAP), Cab abdominal wall compliance, Cdyn dynamic respiratory compliance, CARS cardio-abdominal-renal-syndrome, CO cardiac output, CPP cerebral perfusion pressure, CVP central venous pressure, CVPtm transmural central venous pressure, ECMO extracorporeal membrane oxygenation, EN enteral nutrition, EVLWI extravascular lung water index, FG filtration gradient, GEDVI global end-diastolic volume index, GEF global ejection fraction, GFR glomerular filtration rate, GIPS global increased permeability syndrome, GRV gastric residual volume, HR heart rate, IAP intra-abdominal pressure, ICG-PDR indocyanine green plasma disappearance rate, ICP intra-cranial pressure, ITP intra-thoracic pressure, MAP mean arterial pressure, MMC migrating motor complex, PAL combination of PEEP, albumin 20% and LASIX® furosemide, PEEP positive end-expiratory pressure, PIP peak inspiratory pressure, Paw airway pressures, PCWP pulmonary capillary wedge pressure, pHi intra-mucosal gastric pH, PPV pulse pressure variation, Qs/Qt shunt fraction, RM recruitment maneveur, RVEDVI right ventricular end-diastolic volume index, RVP renal venous pressure, RVR renal vascular resistance, SMA superior mesenteric artery, SPV systolic pressure variation, SVR systemic vascular resistance, SVV stroke volume variation, Vd/Vt dead-space ventilation
Intermittent intra-abdominal pressure measurement
Measuring IAP via the bladder is currently the most widely accepted technique due to its simplicity, reliability, user-friendliness, and reproducibility with the added benefits of low cost minimal invasiveness and low complication risks [5]. This technique was originally described by Kron and later modified by Iberti, Cheatham and Malbrain [1] (ESM Fig. 1) to a closed system using a pressure transducer that allowed for multiple IAP measurements [7]. IAP measurements can also be performed via the height of the urine column with a Foley manometer [1] (ESM Fig. 2). Particularly in patients with bladder trauma, neurogenic bladder dysfunction, outflow obstruction, or pelvic hematomas where measurements via the bladder might be unreliable and/or contraindicated, the stomach can then be used as an alternative route (ESM Fig. 3 and 4) [1]. Coca et al. reported data from 205 kidney transplant patients where intermittently measured high IAP was common and associated with delayed graft function, postoperative complications, and absence of graft function recovery [8]. In addition, IAP is a prognostic marker when measured during exploratory laparotomy in surgical patients, with higher numbers associated with mortality, a higher SOFA score and prolonged ileus. Fifty five percent of patients having cardiac surgery were found to have IAH (IAP 12 or more in mmHg) when measured intermittently and a further 24% had higher pressures during the first two hours of ICU stay. Even though routine intermittent IAP measurement suggests that high IAP is uncommon, it should be considered postoperatively in patients with obesity. Other factors associated with development of IAH in cardiac surgery would be prolonged cardiopulmonary bypass or surgical time, and need for vasopressor support.
Continuous intra-abdominal pressure measurement
Intermittent IAP measurements via the bladder in symptomatic patients or those with a high clinical suspicion of developing abdominal compartment syndrome (ACS) every 4 to 6 h is widely accepted as routine practice. Today different techniques are available for continuous intra-abdominal pressure (CIAP) measurement and estimation. CIAP can be measured directly via the peritoneum (this is the technique used during laparoscopy to limit insufflation pressures to 14 mmHg, or chronic ambulatory peritoneal dialysis), however the use of direct intraperitoneal pressure measurement has not been advocated in critically ill patients because its invasiveness, infection risk and complications. The use of balloon-tipped abdominal catheters has also been described [1]. CIAP can also be estimated in critically ill patients via the bladder using a 3-way Foley with continuous irrigation. In the near future another device will become available that can be connected to an existing Foley (Serenno Medical, Yokeneam Illit, Israel). Finally, CIAP can be estimated via a balloon-tipped nasogastric tube (Spiegelberg, Hamburg, Germany) [9]. Recently, the Accuryn Monitoring System (Potrero Medical, Hayward, California, USA) using a special urinary catheter with an extra air-filled balloon at the tip has been FDA cleared for measuring IAP, urinary output (UO), and core body temperature. A significant advantage of this system is near-continuous, high-fidelity and high-resolution IAP monitoring as shown in a recent study in cardiac surgery patients observing persistent increases in IAP that had previously not been shown with traditional spot-check measurements [10]. One more consideration when measuring IAP at high frequency is the signal to noise factor. Applying a 10-min running median and resampling data at longer intervals every 15 min may help mitigate the effect of patient position and factors that induce rapid sudden changes in IAP such as diaphragmatic contraction with a coughing [10].
Less invasive techniques
Less invasive techniques include the use of a strain gauge, respiratory inductance plethysmography, or abdominal tensiometer, as well as the application of ultrasound-based techniques for IAP monitoring (like tonometry, abdominal wall thickness, Doppler, or laser), bio-electrical impedance analysis, microwave reflectometry, digital image correlation and finally, the use of a wireless motility capsule [11]. Recently, IAP was measured noninvasively in an in vitro model with an advanced abdominal wall phantom using a transient radar method.
Is continuous intra-abdominal pressure measurement the future?
Continuous intra-abdominal pressure has many possibilities: first, an IAP trend allows to assess the effect of treatment, to visualize the interaction between physiologic variables in real time. Second, CIAP allows the user to obtain a continuous APP trend as a potential better resuscitation target and for early identification of impending ACS. Third, CIAP allows automation via computer analysis and calculation of the area under the curve (AUC) and the time above a threshold as in analogy to ICP where the pressure time-burden may be of prognostic value. Fourth, CIAP allows to diagnose poly-compartment syndrome and prediction of acute kidney injury (AKI) and cardio-abdominal-renal syndrome (CARS) earlier than via the intermittent measurement technique. While most prediction models for kidney injury feature urine output in conjunction with other parameters, very few feature IAP, and those that do have used IAP as an intermittent tool in clinically at risk patients. Specifically, the use of CIAP allowed prediction of AKI with a higher precision and recall compared to the use of urinary output alone, and up to 28 h before the event [12]. Fifth, it allows to calculate abdomino-thoracic and thoraco-abdominal pressure transmission which is important to understand organ-organ crosstalk phenomena (ESM Fig. 5) [4, 13–15]. Sixth, it helps us understand problems during ventilation and weaning, for example forced expiration in COPD, ventilator asynchrony, increased transdiaphragmatic pressures and work-of-breathing. Seventh, it might help us to select lung protective ventilation, driving pressure, recruitment maneuver, and optimal PEEP (in cmH2O) setting equal to IAP (in mmHg) [6]. Finally, it provides us with an idea of abdominal wall compliance (Cab) with the respiratory abdominal variation test (RAVT), where the higher abdominal pressure variation (APV) and respiratory variations (ΔIAP) the lower the Cab [4]. Analysis of the raw IAP data signal not only gives us information on absolute values of IAP (with end inspiratory and end expiratory value), but also delta (Δ) IAP (IAPei minus IAPee), APV (ΔIAP/mean IAP), which can be used to assess Cab and splanchnic perfusion. Furthermore, this data can be combined with heart rate, respiratory rate, and relative stroke volume and pulse pressure variation data (ESM Fig. 6, Panels A–D). Medical management options are summarized and listed in ESM Fig. 7.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Conflicts of interest
MLNGM is a Professor of Critical Care Research at the 1st Department of Anaesthesiology and Intensive Therapy, Medical University of Lublin, Poland. He is co-founder, past-President and current Treasurer of WSACS (The Abdominal Compartment Society, http://www.wsacs.org). He is a member of the medical advisory Board of Pulsion Medical Systems (part of Getinge group), Serenno Medical, Potrero Medical, and Baxter, and consults for BBraun, Becton Dickinson, ConvaTec, Spiegelberg, and Holtech Medical. He is the co-founder and President of the International Fluid Academy (IFA). The IFA (http://www.fluidacademy.org) is integrated within the not-for-profit charitable organization iMERiT, International Medical Education and Research Initiative, under Belgian law. AKK is a paid consultant for and chairs the steering committee for the Predict AKI group for Potrero Medical and also consults for Edwards Lifesciences, Philips North America, GE Healthcare, Hill-Rom, and Caretaker Medical. His institution has grant funding from Caretaker Medical for ongoing investigations on portable hemodynamic monitoring. AKK is on the executive advisory board for Medtronic and Retia Medical. AKK receives support from the Wake Forest CTSI via NIH/NCATS KL2 for a trial of continuous portable hemodynamic and saturation monitoring on hospital wards. The other author has no potential conflicts of interest in relation to the contents of this paper. The payment of the Open Access fee was kindly supported by an unrestricted educational grant from Potrero Medical.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Malbrain ML. Different techniques to measure intra-abdominal pressure (IAP): time for a critical re-appraisal. Intensive Care Med. 2004;30(3):357–371. doi: 10.1007/s00134-003-2107-2. [DOI] [PubMed] [Google Scholar]
- 2.Ostermann M, Hall A, Crichton S. Low mean perfusion pressure is a risk factor for progression of acute kidney injury in critically ill patients—a retrospective analysis. BMC Nephrol. 2017;18(1):151. doi: 10.1186/s12882-017-0568-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirkpatrick AW, Roberts DJ, De Waele J, Jaeschke R, Malbrain ML, De Keulenaer B, et al. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med. 2013;39(7):1190–1206. doi: 10.1007/s00134-013-2906-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malbrain M, Peeters Y, Wise R. The neglected role of abdominal compliance in organ-organ interactions. Crit Care. 2016;20:67. doi: 10.1186/s13054-016-1220-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sugrue M, De Waele JJ, De Keulenaer BL, Roberts DJ, Malbrain ML. A user's guide to intra-abdominal pressure measurement. Anaesthesiol Intensive Ther. 2015;47(3):241–251. doi: 10.5603/AIT.a2015.0025. [DOI] [PubMed] [Google Scholar]
- 6.Regli A, Pelosi P, Malbrain ML. Ventilation in patients with intra-abdominal hypertension: what every critical care physician needs to know. Ann Intensive Care. 2019;9(1):52. doi: 10.1186/s13613-019-0522-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Waele JJ, De Laet I, Malbrain ML. Rational intraabdominal pressure monitoring: how to do it? Acta Clin Belg. 2007;62(Suppl 1):16–25. doi: 10.1179/acb.2007.62.s1.004. [DOI] [PubMed] [Google Scholar]
- 8.Coca A, Arias-Cabrales C, Perez-Saez MJ, Fidalgo V, Gonzalez P, Acosta-Ochoa I, et al. Impact of intra-abdominal pressure on early kidney transplant outcomes. Sci Rep. 2022;12(1):2257. doi: 10.1038/s41598-022-06268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balogh Z, De Waele JJ, Malbrain ML. Continuous intra-abdominal pressure monitoring. Acta Clin Belg. 2007;62(Suppl 1):26–32. doi: 10.1179/acb.2007.62.s1.005. [DOI] [PubMed] [Google Scholar]
- 10.Khanna A, Minear S, Kurz A, Moll V, Stanton K, et al. Intra-abdominal hypertension in cardiac surgery patients: a multicenter observational sub-study of the Accuryn registry. J Clin Monit Comput. 2022 doi: 10.1007/s10877-022-00878-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tayebi S, Gutierrez A, Mohout I, Smets E, Wise R, Stiens J, et al. A concise overview of non-invasive intra-abdominal pressure measurement techniques: from bench to bedside. J Clin Monit Comput. 2021;35(1):51–70. doi: 10.1007/s10877-020-00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prabhakar A, Stanton K, Burnett D, Egan K, Keeling B, Moll V. 1130: combining urine output and intra-abdominal pressures predict acute kidney injury early. Crit Care Med. 2021;49(1):567. doi: 10.1097/01.ccm.0000730408.55242.7c. [DOI] [Google Scholar]
- 13.Malbrain ML, Roberts DJ, Sugrue M, De Keulenaer BL, Ivatury R, Pelosi P, et al. The polycompartment syndrome: a concise state-of-the-art review. Anaesthesiol Intensive Ther. 2014;46(5):433–450. doi: 10.5603/AIT.2014.0064. [DOI] [PubMed] [Google Scholar]
- 14.Malbrain ML, Wilmer A. The polycompartment syndrome: towards an understanding of the interactions between different compartments! Intensive Care Med. 2007;33(11):1869–1872. doi: 10.1007/s00134-007-0843-4. [DOI] [PubMed] [Google Scholar]
- 15.Malbrain MLNG, De Laet I, De Waele J. The Polycompartment syndrome: what’s all the fuss about? In: Vincent JL, editor. Yearbook of intensive care and emergency medicine. Berlin: Springer; 2010. pp. 465–484. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.