Abstract
We genetically characterized the Pseudomonas putida mutS gene and found that it encodes a smaller MutS protein than do the genes of other bacteria. This gene is able to function in the mutS mutants of Escherichia coli and Bacillus subtilis. A P. putida mutS mutant has a mutation frequency 1,000-fold greater than that of the wild-type strain.
The MutS protein is part of the MutSLH DNA repair system, which corrects the mismatched DNA produced by DNA replication errors, genetic recombination, and chemical damage to DNA (5). Since MutS proteins are relatively large (∼90 kDa), multifunctional proteins, they probably contain multiple domains. Deletion analysis of the Escherichia coli mutS gene showed that DNA binding takes place in the N-terminal end of MutS and MutS dimerization and MutS-MutL interaction happen in the C-terminal end (9). Functional analysis of Thermus thermophilus MutS showed that it consists of at least three domains, including double-stranded DNA binding, mismatched DNA binding, and APTase (8).
In this study, we identified a mutS gene from Pseudomonas putida which is able to metabolize various aromatic compounds. We showed that this gene encodes a smaller MutS protein than do genes of other bacteria. We used complementation analysis of this gene in E. coli and Bacillus subtilis, as well as disruption of the P. putida mutS gene.
Cloning and sequencing of a P. putida mutS gene.
The amino acid sequences of the E. coli (7), B. subtilis (1), and Azotobacter vinelandii (4) MutS proteins share five highly conserved regions in the C-terminal domain. Based on this data, we designed two oligonucleotide primers [forward primer, 5′-GICATCA(T/C)CCIGTIGTIGA-3′; reverse primer, 5′-TC(A/G)AA(A/G)TA(A/G)TGIGTIG-3′] from two conserved amino acid sequences (GRHPVVE and TLFATHYFELT) and used a PCR with P. putida ATCC 33015 chromosomal DNA as the template. A 450-bp DNA segment was amplified and sequenced, which showed that the DNA encodes three conserved amino acid sequences (IITGPNMGGKSTYMRQ, GRSTFMVEM, and SLVLMDE) in the C-terminal domain of MutS. The amplified DNA was used as a probe to screen a cosmid library of the genomic DNA. All of the DNA was partially digested with Sau3AI and ligated to BamHI-digested cosmid Lorist6 (Nippon Gene). The ligation mixture was packaged through the use of an in vitro packaging module (Amersham Pharmacia Biotech), and about 3,000 recombinant clones of E. coli DH5α were obtained. Southern hybridization screening of 500 clones, in which we used an AlkPhos Direct system for chemiluminescence (Amersham Pharmacia Biotech), yielded three positive colonies. Restriction analysis of these clones showed that all inserts had overlapping regions and each contained a 3.7-kb EcoRI-HindIII fragment which hybridized to the probe (data not shown).
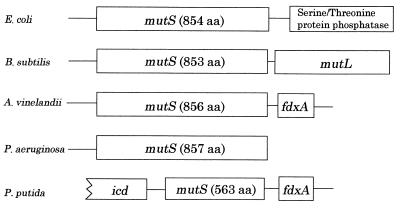
The nucleotide sequence of this fragment was determined, and its genetic organization is shown in Fig. 1. The nucleotide sequence spans 3,726 bp and contains two complete open reading frames of 563 (orf1) and 108 (orf2) codons. For orf1, significant homology to the Pseudomonas aeruginosa (accession no. AF220055), A. vinelandii, E. coli, and B. subtilis proteins MutS (82.1, 81.2, 56.8, and 38.9%, respectively) was found. However, orf1 encoded a smaller protein (about 60 kDa) than that (∼90 kDa) of other bacteria. Therefore, to determine whether this gene is a true mutS gene or not, we performed, using the method described above, genomic Southern hybridization using the orf1 fragment as a probe. Our results detected only one band corresponding to the 3.7-kb fragment in the EcoRI-HindIII-digested chromosomal DNA (data not shown). The amino acid sequence of orf2, which was located in the same direction as the mutS gene, was identical to that of the fdxA (ferredoxin) gene from Pseudomonas ovalis (3) and was very similar in genetic organization to that of A. vinelandii (Fig. 1). Moreover, the N-terminal region (275 amino acids) of the open reading frame, which was located in the direction opposite to the mutS gene, was highly homologous to that of the icd (isocitrate dehydrogenase) gene from A. vinelandii (accession no. D73443). Taken together, the above comparisons suggest that, in spite of an incomplete gene, the small MutS protein of P. putida is likely to be structurally and functionally analogous to that of A. vinelandii.
FIG. 1.
Genetic organization of the mutS gene loci of various microorganisms. aa, amino acid residues.
Complementation analysis of a P. putida mutS gene.
To examine the possibility that P. putida MutS could complement the mutS mutants of other bacteria, we constructed two expression plasmids capable of replicating in E. coli and B. subtilis. First, we constructed the expression vector pKK223-3M, which modified the multicloning sites of pKK223-3 (Amersham Pharmacia Biotech) by ligating HindIII multicloning sites of pHSG299 (Takara Shuzo) and an EcoRI into them. This created the XbaI site. A 1,692-bp fragment of the P. putida mutS coding region was amplified by PCR on chromosomal DNA. The forward primer had an EcoRI site (5′-GGGAATTCATGGGATACCAGAAAATC-3′; the underlined bases correspond to the mutS sequence), and the reverse primer had an XbaI site (5′-GGTCTAGATTATAACAGGTTCTTTAG-3′). The fragment was cloned into the EcoRI and XbaI sites of pKK223-3M. The resulting plasmid, designated pEPPS, was transformed into the BMH71-18 mutS mutant strain of E. coli (NIG Collection). We also constructed plasmid pEEES by inserting a 2,562-bp fragment of the E. coli mutS coding region into the EcoRI and XbaI sites of pKK223-3M, and this was transformed into the BMH71-18 mutS strain and used as a control. For B. subtilis, a 1,972-bp BamHI- and HindIII fragment containing the tac promoter and the mutS coding sequences of pEPPS was cloned into the BamHI and HindIII sites of B. subtilis plasmid pHY300PLK (Takara Shuzo) and the resulting plasmid, designated pBPPS, was transformed into the 168trp-S B. subtilis mutS strain (M. Sasaki and Y. Kurusu, unpublished data).
To investigate whether P. putida MutS could complement the mutS mutants of both bacteria, we compared the spontaneous mutation rates of these transformants. Through the use of previously described procedures for E. coli and B. subtilis (1, 2), we measured their frequencies of mutation to rifampin resistance. As shown in Table 1, a P. putida mutS gene was partially complemented in an E. coli mutS mutant and was completely complemented in a B. subtilis mutS mutant. The expression of the P. putida mutS gene in both mutS mutants was relatively low, since a typical MutS protein could not be detected by sodium dodecyl sulfate-polyacrylamide gel electrophoresis in either cell (data not shown). These results suggested that the small MutS protein of P. putida could function in both bacteria.
TABLE 1.
Spontaneous mutation frequencies of various strains
| Strain | Avg frequency of Rifr cells ± SDa | Relative mutation frequencyb |
|---|---|---|
| E. coli | ||
| BMH71-18 mutS (MutS−) | (3.5 ± 0.4) × 10−6 | 220 |
| BMH71-18 mutS(pEEES) | (7.8 ± 1.5) × 10−8 | 1 |
| BMH71-18 mutS(pEPPS) | (7.2 ± 1.3) × 10−7 | 10 |
| B. subtilis | ||
| 168trp (MutS+) | (8.7 ± 1.8) × 10−8 | 1 |
| 168trp-S (MutS−) | (2.9 ± 0.9) × 10−7 | 30 |
| 168trp-S(pBPPS) | (4.5 ± 1.6) × 10−8 | 2 |
| P. putida | ||
| 33015 (MutS+) | (1.3 ± 0.4) × 10−9 | 1 |
| 33015-S (MutS−) | (1.5 ± 0.5) × 10−6 | 1,000 |
| 33015-S(pPPPS) | (1.4 ± 0.5) × 10−9 | 1 |
All strains grown to the stationary phase in Luria-Bertani medium were inoculated into this medium at approximately 50 cells/ml and grown for 25 generations. Aliquots of the cultures were then diluted and spread on Luria-Bertani plates containing rifampin for the selection of spontaneous mutants. Rifampin was used at the following concentrations: for E. coli, 100 μg/ml; for B. subtilis, 30 μg/ml; for P. putida, 50 μg/ml. Cell concentration was determined after the growth of 25 generations. To calculate the standard deviation, all of the experiments were repeated at least five times. Frequencies were calculated from both the total number of cells and the number of Rifr cells.
Relative frequencies were obtained by comparison with BMH71-18 mutS(pEEES) as 1 for E. coli, 168trp (MutS+) as 1 for B. subtilis, and 33015 (MutS+) as 1 for P. putida.
Disruption of the P. putida mutS gene.
To confirm that the incomplete mutS gene is a mutator gene in P. putida, we constructed a mutS mutant of P. putida that could not synthesize MutS and compared its spontaneous mutation rate to that of the wild-type strain. We obtained a 1,277-bp internal fragment of the mutS gene by digesting plasmid pEPPS with SacI and EcoT14I and then treating it with T4 DNA polymerase. By inserting this fragment into the HincII site of E. coli plasmid pHSG299, which contained the kanamycin resistance (Kmr) gene as the selectable marker, we constructed an integrative plasmid. This plasmid was integrated into the chromosomal wild-type mutS locus by homologous recombination, which resulted in the plasmid separating two partial-deletion-containing copies of mutS. These disruptants were analyzed by Southern hybridization with a suitable probe (data not shown), and one disruptant, designated 33015-S, was used to measure the spontaneous mutation frequency. As shown in Table 1, strain 33015-S had a mutation frequency 1,000-fold greater than that of the wild-type strain. To confirm that the P. putida mutS gene could complement the mutS mutant of P. putida, we constructed plasmid pPPPS by inserting a 1,972-bp BamHI- HindIII fragment, containing the tac promoter and the mutS coding sequences of pEPPS, into the BamHI and HindIII sites of P. putida plasmid pSUP104 (6) and transformed them into strain 33015-S. As shown in Table 1, 33015-S carrying pPPPS had a frequency of spontaneous mutation similar to that of wild-type strain 33015. These results indicate that the mutS gene is a mutator gene in P. putida.
Nucleotide sequence accession number.
The nucleotide sequence described here has been deposited in the DDBJ/GenBank/EMBL database under accession no. AB039965.
REFERENCES
- 1.Ginetti F, Perego M, Albertini A M, Galizzi A. Bacillus subtilis mutS mutL operon: identification, nucleotide sequence and mutagenesis. Microbiology. 1996;142:2021–2029. doi: 10.1099/13500872-142-8-2021. [DOI] [PubMed] [Google Scholar]
- 2.Glickman B W, Radman M. Escherichia coli mutator mutants deficient in methylation-instructed DNA mismatch correction. Proc Natl Acad Sci USA. 1980;77:1063–1067. doi: 10.1073/pnas.77.2.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hase T, Wakabayashi S, Matsubara H, Ohmori D, Suzuki K. Pseudomonas ovalis ferredoxin: similarity to Azotobacter and Chromatium ferredoxins. FEBS Lett. 1978;91:315–319. doi: 10.1016/0014-5793(78)81200-9. [DOI] [PubMed] [Google Scholar]
- 4.Le O, Shen B, Iismaa S E, Burgess B K. Azotobacter vinelandii mutS: nucleotide sequence and mutant analysis. J Bacteriol. 1993;175:7707–7710. doi: 10.1128/jb.175.23.7707-7710.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Modrich P, Lahue R. Mismatch repair in replication fidelity, genetic and cancer biology. Annu Rev Biochem. 1996;65:101–133. doi: 10.1146/annurev.bi.65.070196.000533. [DOI] [PubMed] [Google Scholar]
- 6.Priefer U B, Simon R, Fuhler A. Extension of the host range of Escherichia coli vectors by incorporation of RSF1010 replication and mobilization functions. J Bacteriol. 1985;163:324–330. doi: 10.1128/jb.163.1.324-330.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schlesog V, Boeck A. The Escherichia coli fdv gene probably encodes MutS and is located at minute 58.8 adjacent to the hyp-hyc gene cluster. J Bacteriol. 1991;173:7414–7415. doi: 10.1128/jb.173.23.7414-7415.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tachiki H, Kato R, Masui R, Hasegawa K, Itakura H, Fukuyama K, Kuramitsu S. Domain organization and functional analysis of Thermus thermophilus MutS protein. Nucleic Acids Res. 1998;26:4153–4159. doi: 10.1093/nar/26.18.4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu, Te-Hui, Marinus M G. Deletion analysis of the mutS gene in Escherichia coli. J Biol Chem. 1999;274:5948–5952. doi: 10.1074/jbc.274.9.5948. [DOI] [PubMed] [Google Scholar]