Abstract
Background
The English national bowel cancer screening program offering a guaiac fecal occult blood test began in July 2006. In randomized controlled trials of guaiac fecal occult blood test screening, reductions in mortality were accompanied by reductions in advanced stage colorectal cancer (CRC). We aimed to evaluate the effect of participation in the national bowel cancer screening program on stage-specific CRC incidence as a likely precursor of a mortality effect.
Methods
In this population-based case-control study, cases were individuals diagnosed with CRC aged 60-79 years between January 1, 2012, and December 31, 2013. Two controls per case were matched on geographic region, gender, date of birth, and year of first screening invitation. Screening histories were extracted from the screening database. Conditional logistic regression with correction for self-selection bias was used to estimate odds ratios (odds ratios corrected for self-selection bias [cOR]) and 95% confidence intervals (CIs) by Duke stage, sex, and age.
Results
14 636 individuals with CRC and 29 036 without were eligible for analysis. The odds of CRC (any stage) were increased within 30 days of a screening test and decreased thereafter. No reduction in CRC (any stage) among screened individuals compared with those not screened was observed (cOR = 1.00, 95% CI = 0.89 to 1.15). However, screened individuals had lower odds of Duke stage D CRC (cOR = 0.68, 95% CI = 0.50 to 0.93). We estimate 435 fewer Duke D CRC by age 80 years in 100 000 people screened biennially between ages 60 and 74 years compared with an unscreened cohort.
Conclusion
The impact of colorectal screening on advanced CRC incidence suggests that the program will meet its aim of reducing mortality.
In the United Kingdom, approximately 42 000 colorectal cancer (CRC) cases are diagnosed annually (1). Between 2005-2007 and 2015-2017, CRC incidence rates decreased by 4% and death rates by 14% (2).
Randomized controlled trials (RCTs) have demonstrated the efficacy of biennial screening with guaiac fecal occult blood test (gFOBT) for reducing CRC mortality (3). To date, only 1 trial (4) has demonstrated any statistically significant reduction in CRC incidence, and in that trial, a high proportion of subjects in the intervention arms underwent colonoscopy. In all trials, the reduction in mortality was accompanied by a reduction in advanced stage CRC (5-9).
In England, a national bowel cancer screening program (NHSBCSP) offering a gFOBT test was rolled out between 2006 and 2010. Initially, it offered screening to individuals between the ages of 60 and 69 years, but from 2010, it was extended up to age 74 years. People older than 74 years can self-refer (10). Individuals with abnormal test results are offered colonoscopy. In 2013, the NHSBCSP began offering flexible sigmoidoscopy at age 55 years. However, it was never fully rolled out (11), and it was recently discontinued (12). In June 2019, the program changed the test from gFOBT to fecal immunochemical testing.
The NHSBCSP aims to reduce mortality from colorectal cancer by 16% in those invited for screening (13), but it is too early to assess its impact on mortality. The aim of this study is to evaluate the effect of participation in the NHSBCSP on the risk of diagnosis of primary CRC, using changes in advanced stage CRC incidence as early indicators of the likely future impact on mortality.
Methods
Study Population and Data
Cases were individuals with primary CRC (International Classification of Diseases–10 C18, C19 or C20) diagnosed at age 60-79 years between January 1, 2012, and December 31, 2013. Both first and subsequent registrations were included. Controls were individuals with no diagnosis of colorectal cancer prior to the date of diagnosis of their matched case.
Cases were identified by the National Cancer Registration and Analysis Service. The National Health Application and Infrastructure Services system was used to identify 2 matched population controls per case. Controls were individual matched to their case on gender, geography (to 1 of 82 regions of England), date of birth (within 1 month), year of first NHSBCSP invitation (to ensure equal opportunity for screening), and being alive when the case was diagnosed. Individuals who objected to their records being used for research were excluded. Full details have been published previously (14).
Demographics, staging, and cause of death data were retrieved from National Cancer Registration and Analysis Service. Screening histories were extracted from the Bowel Cancer Screening System, which only includes tests taken in response to an invitation for screening. During the period covered by this study, individuals were screened using gFOBT and would not have been offered flexible sigmoidoscopy at age 55 years.
Controls were assigned the date of diagnosis of their matched case as a pseudodiagnosis reference date. Age was the age at diagnosis.
Individuals not invited for screening prior to (pseudo)diagnosis, controls with a prior diagnosis of colorectal cancer, cases diagnosed at younger than 60 years, and death certificate–only cases were excluded from analysis.
Duke staging data were 74.4% complete. To increase the proportion of cases with staging, time between diagnosis and death was considered. Given the poor survival (15) following a diagnosis of Duke D, patients with tumors with missing stage who died within 1 year of diagnosis were classified as suspected Duke D. Those dying between at least 1 year and less than 3 years were classified as Duke B or worse, not otherwise specified (B+ NOS). The remaining tumors with missing stage were checked against tumor-node-metastasis stage data and nodal status data. Any tumor coded as T2-4, N1-3, or M1 and all node-positive tumors were classified as Duke B+ NOS. If no conclusive data were available, tumors were classified as unknown stage. We also carried out 2 sensitivity analyses. First, we derived results including only cases and their matched controls with known stage (ie, assuming stage is missing completely at random). Second, we used inverse probability weighting, estimating probabilities of stage being nonmissing by age, gender, and survival time to deal with missing stage (16).
For the main analysis, we considered 1) all stages—includes all eligible cases; 2) Duke stage B or worse—including stage B+ NOS; 3) Duke stage C or worse—cases with stages C1, C2, D, or suspected D; and 4) Duke stage D—including those with suspected Duke D.
Classification of Screening Exposure
Reflecting the maximum time between screening rollout and diagnosis in this study, exposure to screening was assessed during a 7-year look-back window. Screens taken at younger than 60 years were excluded. Tests within 7 days of diagnosis were excluded under the assumption that screening could not lead to diagnosis that quickly.
The effectiveness of gFOBT screening was explored using 2 measures of screening exposure. “Ever screened” was defined as having at least 1 test at age 60 years or older and not within 7 days of diagnosis and/or pseudodiagnosis. “Time since last test” was defined as the time from the most recent test prior to diagnosis/pseudodiagnosis up to the date of the latter.
Statistical Analysis
Conditional logistic regression was used to estimate odds ratios (OR) and 95% confidence intervals (CI). The study was designed to have at least 90% power to detect an odds ratio of 0.80 as statistically significant at 5% level with 2-sided testing (17).
To correct for the fact that individuals who accept the invitation to screening may have a priori better health status compared with individuals who do not (ie, self-selection bias), we used the formula (18)
where cOR is the odds ratio corrected for self-selection bias, is the proportion of individuals participating in screening (assumed 0.6 in this study, to approximate participation [59.9%] in the national program) (19), is the uncorrected odds ratio, and Dr is the risk ratio of CRC in unscreened invited over unscreened not invited. We used data from long-term follow-up of the Nottingham trial (7) to estimate Dr risk ratios by Duke stage by dividing the incidence of cancers diagnosed among individuals offered screening but who chose not to be screened by the corresponding incidence in the control population (Supplementary Table 1, available online).
Time since last test was coded in 2 ways to show the effect of screening over time. We first used overlapping time intervals. From 0 to less than 3 months, intervals were 30 days wide with a 15-day shift from one interval to the next. From 3 months to less than 1 year, intervals were 60 days wide and shifted by 28 days. From 1 to 4 years, intervals were 180 days wide and shifted by 60 days. The lower band of the last interval estimated was 4 years. Overlapping odds ratios and 95% confidence intervals were plotted at the lower band of the time since last test interval. Second, we used time categorized into exclusive intervals: less than 30 days, 30 to less than 60 days, 60 to less than 90 days, 90 to less than 180 days, 180 to less than 365 days, 365 to less than 730 days, 2 years, and 3 or more years. This analysis was corrected for self-selection bias.
We estimated the cumulative risk per 100 000 in a hypothetical cohort of individuals aged 60-79 years in the general population and among individuals screened every 2 years from their 60th birthday until age 74 years, using gender-specific nationally reported rates of CRC (for 2015-2017) in 5-year age groups (20). See the Supplementary Methods (available online).
Ethical approval was given to receive anonymized routinely collected data by the National Research Ethics Service (NRES) Committee London—City & East, reference14/LO/0826 and HRA CAG reference14/CAG/1020.
Results
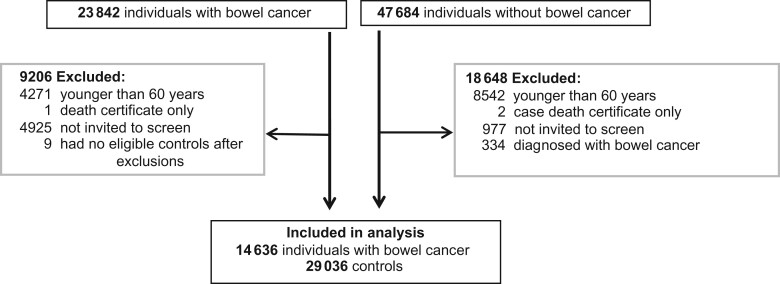
After exclusions, 14 636 individuals with CRC and 29 036 without were eligible for analysis (Figure 1). Most cases excluded were younger than 60 years at diagnosis (n = 4271) or had not been invited for screening prior to diagnosis (n = 4925).
Figure 1.
Flowchart detailing total study population, exclusions, and total included in the analysis.
More than half (57%) of eligible CRC cases were male, and 62% were aged 60-69 years at diagnosis (Table 1). The most common Duke stages were C (25%) and B (23%). Stage was missing for 25% of cases, and other details allowed 1069 (29.2% of those with stage missing) to be classified as Duke stage B+ NOS and 1525 (41.7%) as suspected Duke D.
Table 1.
Descriptive characteristics of individuals eligible for analysis
| Characteristics | Age at diagnosis, ya |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 60-64 |
65-69 |
70-74 |
75-79b |
Total |
||||||
| Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | |
| Total No. | 4263 | 8477 | 4785 | 9504 | 4639 | 9179 | 949 | 1876 | 14636 | 29036 |
| Stage at diagnosis, % | ||||||||||
| Stage A | 15.9 | 14.2 | 14.2 | 12.4 | 14.6 | |||||
| Stage B | 20.9 | 23.7 | 24.1 | 22.9 | 22.9 | |||||
| Stage C | 27.5 | 25.0 | 23.4 | 23.2 | 25.1 | |||||
| Stage D | 13.0 | 13.0 | 11.6 | 10.9 | 12.4 | |||||
| Missing stage, % | ||||||||||
| Suspected Duke Dc | 7.1 | 9.7 | 13.0 | 16.3 | 7.3 | |||||
| Stage B or worse NOSd | 7.0 | 7.1 | 7.2 | 9.7 | 10.4 | |||||
| Stage unknown | 8.6 | 7.3 | 6.5 | 4.6 | 7.3 | |||||
| Gender, % | ||||||||||
| Male | 58.9 | 56.8 | 56.2 | 51.3 | 56.9 | |||||
| Female | 41.1 | 43.2 | 43.8 | 48.7 | 43.1 | |||||
| Screening exposure, % | ||||||||||
| Invited but not screenede | 40.3 | 45.9 | 38.4 | 36.2 | 40.5 | 39.3 | 49.5 | 40.2 | 40.3 | 40.3 |
| Screened | 59.7 | 54.1 | 61.6 | 63.8 | 59.5 | 60.7 | 50.5 | 59.8 | 59.7 | 59.7 |
| Time since last screen, % | ||||||||||
| <1 y | 40.3 | 28.4 | 34.8 | 28.3 | 27.7 | 21.7 | 1.7 | 3.8 | 32.0 | 24.7 |
| >1 y to 2 y | 14.4 | 18.7 | 18.2 | 24.4 | 11.6 | 15.1 | 19.5 | 25.9 | 15.1 | 19.9 |
| >2 y to 3 y | 3.7 | 5.7 | 5.2 | 7.0 | 7.1 | 8.9 | 16.2 | 17.4 | 6.1 | 7.8 |
| >3 y to 4 y | 1.1 | 1.1 | 2.1 | 2.3 | 6.8 | 7.2 | 5.7 | 6.6 | 3.5 | 3.8 |
| >4 y | 0.2 | 0.5 | 1.3 | 1.9 | 6.3 | 7.8 | 7.4 | 6.1 | 3.0 | 3.6 |
Note that prior to excluding cases who were not invited for screening, 47.5% of all cancers were diagnosed at ages 60-69 years. A larger proportion of cases were excluded at ages 70-79 years because they had not been invited prior to diagnosis. B+ NOS = Duke B or worse not otherwise specified.
Three controls opted into screening.
All individuals died within 1 year of diagnosis and hence are suspected to be Duke D.
Survival and other tumor characteristics suggest stage B+ NOS.
Excludes those whose only test was within 7 days of diagnosis or who only had tests at younger than 60 years.
Of the individuals, 60% were screened at least once (Table 1). The mean time since last test was 1.26 (range = 0.02-7.1) years. Individuals invited more than once were more likely to have been screened than were those invited just once (Supplementary Table 2, available online).
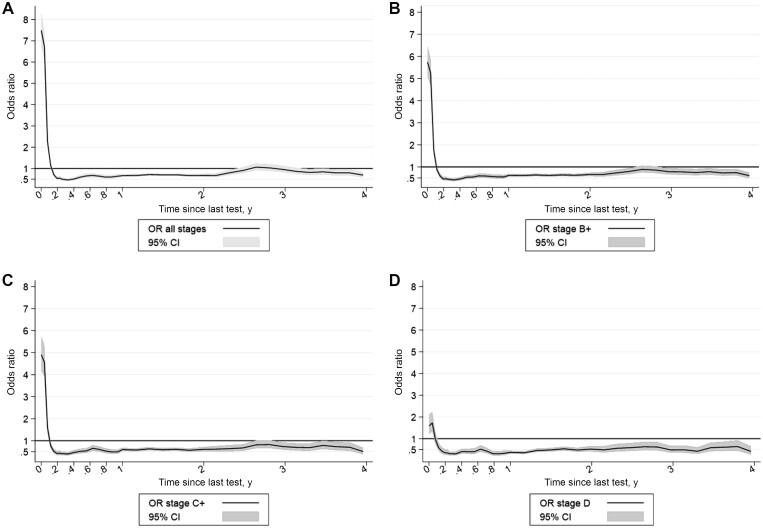
In the main analysis, the odds of being diagnosed with CRC were increased within 60 days of a screening test (Figure 2) and were particularly high within 30 days (cOR all stages = 9.66, 95% CI = 8.17 to 11.43; Supplementary Table 3, available online), suggesting substantial numbers of screen-detected cancers. The magnitude of the effect decreased with increasing stage at diagnosis (Figure 2). After the initial increase in odds ratios, there was a decrease up to 1 year (cOR all stages = 0.53, 95% CI = 0.46 to 0.62) after which the odds ratio began to increase again (Supplementary Table 3, available online).
Figure 2.
Effect of screening on the odds ratio of colorectal cancer by time since last test. A) All stages, B) Duke B or worse, C) Duke C or worse, D) Duke D. CI = confidence interval; OR = odds ratio.
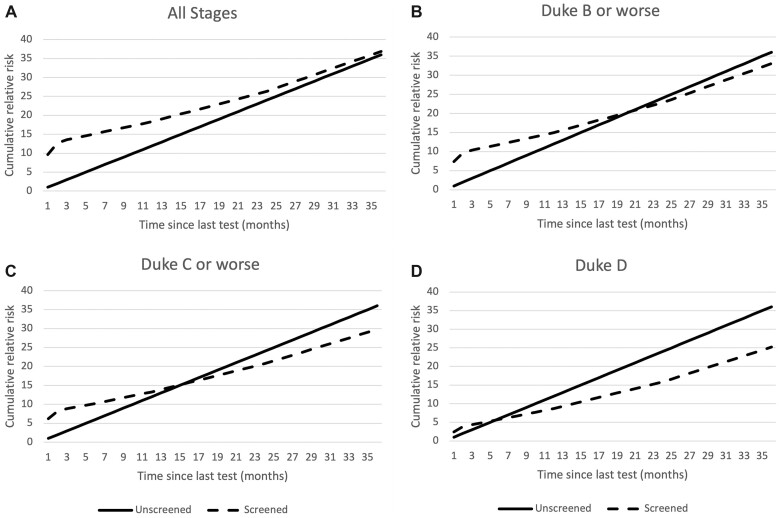
Figure 3 illustrates the cumulative relative risk of CRC over time corrected for self-selection bias. For screened individuals, time zero is the time of the last test. Screened individuals were more likely to have CRC (all stages combined) diagnosed after testing than unscreened individuals, but the risk converges by about 3 years. For stage B or worse, the excess risk disappears by 2 years. For stage C or worse, the cumulative risks cross at about 15 months and are less at 3 years in screened than in unscreened individuals. The excess risk of stage D CRC disappears within 6 months of screening, and the cumulative risk at 3 years is substantially less in screened than in unscreened individuals.
Figure 3.
Cumulative relative risk of colorectal cancer by time since last test and stage in unscreened individuals compared with those screened. Corrected for self-selection bias. A) All stages, B) Duke B or worse, C) Duke C or worse, D) Duke D.
Impact of Ever Having a gFOBT Screen
Overall, no reduction in odds of CRC among screened individuals compared with those who had been invited but not screened was observed (cOR = 1.00, 95% CI = 0.89 to 1.15). However, screened individuals had a 32% lower odds of Duke stage D CRC (cOR = 0.68, 95% CI = 0.50 to 0.93; Table 2). Results for stage B+ and stage C+ were intermediary. Excluding those missing stages yielded higher relative risks associated with screening for all cancers combined but showed a similar impact of screening on stage D CRC among screened individuals (OR = 0.66, 95% CI = 0.58 to 0.74) which became statistically nonsignificant once corrected for self-selection (cOR = 0.92, 95% CI = 0.66 to 1.27; Table 2). Inverse probability weighting generally gave similar results to the main analysis.
Table 2.
Crude and adjusted odds ratios of being diagnosed with colorectal cancer by screening status and Duke stage
| Invitational status—screening status | Main analysisa |
Sensitivity analysis 1–known stage onlyb |
Sensitivity analysis 2– inverse probability weighting |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Controlsc | Cases | OR (95% CI) | cORd (95% CI) | Controlsc | Cases | OR (95% CI) | cORd (95% CI) | Controlsc | Cases | OR (95% CI) | cORd (95% CI) | ||
| All stages, No. | 29 036 | 14636 | 21 779 | 10979 | 29 036 | 14636 | |||||||
| Invited—unscreened | 40.3% | 40.3% | 1.00 (Referent) | 1.00 (Referent) | 40.4% | 38.0% | 1.00 (Referent) | 1.00 (Referent) | 40.3% | 40.3% | 1.00 (Referent) | 1.00 (Referent) | |
| Invited—screened | 59.7% | 59.7% | 1.00 (0.96 to 1.04) | 1.00 (0.89 to 1.15) | 59.6% | 62.0% | 1.11 (1.06 to 1.17) | 1.12 (0.98 to 1.28) | 59.7% | 59.7% | 1.00 (0.96 to 1.04) | 1.00 (0.89 to 1.15) | |
| Stage Duke B or worse, No.e | 22 702 | 11441 | 17 557 | 8847 | 12 356 | 6231 | |||||||
| Invited—unscreened | 40.0% | 44.2% | 1.00 (Referent) | 1.00 (Referent) | 40.3% | 40.7% | 1.00 (Referent) | 1.00 (Referent) | 41.0% | 50.3% | 1.00 (Referent) | 1.00 (Referent) | |
| Invited—screened | 60.0% | 55.8% | 0.83 (0.80 to 0.88) | 0.89 (0.76 to 1.03) | 59.7% | 59.3% | 0.98 (0.93 to 1.04) | 1.05 (0.90 to 1.22) | 59.0% | 49.7% | 0.68 (0.63 to 0.74) | 0.73 (0.62 to 0.85) | |
| Duke C or worse, No. | 13 909 | 7014 | 10 884 | 5489 | 10 023 | 5059 | |||||||
| Invited—unscreened | 40.4% | 47.0% | 1.00 (Referent) | 1.00 (Referent) | 41.0% | 42.2% | 1.00 (Referent) | 1.00 (Referent) | 41.2% | 50.2% | 1.00 (Referent) | 1.00 (Referent) | |
| Invited—screened | 59.6% | 53.0% | 0.76 (0.72 to 0.81) | 0.81 (0.70 to 0.94) | 59.0% | 57.8% | 0.95 (0.89 to 1.02) | 1.01 (0.87 to 1.19) | 58.8% | 49.8% | 0.69 (0.63 to 0.75) | 0.74 (0.62 to 0.87) | |
| Duke D, No. | 6622 | 3340 | 3597 | 1815 | 5448 | 2750 | |||||||
| Invited—unscreened | 40.4% | 57.7% | 1.00 (Referent) | 1.00 (Referent) | 41.8% | 52.0% | 1.00 (Referent) | 1.00 (Referent) | 41.3% | 54.2% | 1.00 (Referent) | 1.00 (Referent) | |
| Invited—screened | 59.6% | 42.3% | 0.49 (0.45 to 0.53) | 0.68 (0.50 to 0.93) | 58.22% | 47.99% | 0.66 (0.58 to 0.74) | 0.92 (0.66 to 1.27) | 58.7% | 45.8% | 0.59 (0.52 to 0.66) | 0.82 (0.59 to 1.13) | |
Individuals with suspected Duke D are assumed to be Duke D. B+ NOS = Duke B or worse not otherwise specified; CI = confidence interval; OR = odds ratio.
This analysis equates to assuming stage is missing completely at random.
Percentages of disease-free controls matched to cases of the relevant stage.
Corrected for self-selection: 1.00 for all stages, 1.04 up to Duke C or worse, and 1.20 for Duke D.
Duke B or worse always includes those with stage recorded as Duke B+ NOS, but Duke C or worse always excludes them.
Results by age and gender revealed 7% lower odds of all stage CRC following screening in females (OR = 0. 93, 95% CI = 0.87 to 0.99), a 9% decrease in those aged 65-69 years (OR = 0.91, 95% CI = 084 to 0.98), and a 33% decrease in those aged 70-79 years (OR = 0.67, 95% CI = 0.57 to 0.79). However, there was an increased risk in males and in those aged 60-64 years following screening (Supplementary Figures 1 and 2, available online). The difference in odds ratio by gender disappeared when results were restricted to Duke stage B+. Point estimates mirrored those of the pooled analysis. Lower odds of CRC were observed at ages 70-79 years compared with ages 60-69 years for all stages except Duke D where no difference was seen.
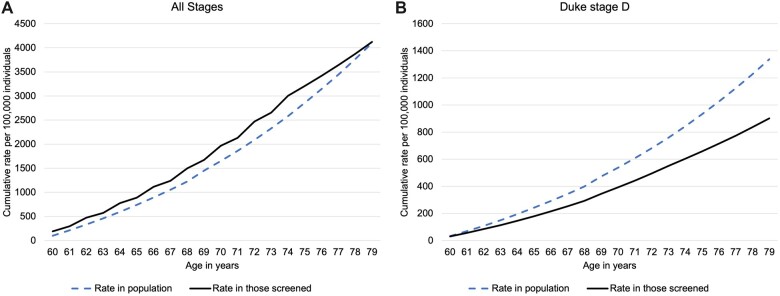
Cumulative risk of CRC from age 60 to 79 years in a hypothetical cohort of individuals screened once every 2 years from age 60 to 74 years compared with general population risk is shown in Figure 4. Screening increases the odds of being diagnosed with CRC during the screening years, but screened individuals have a lower risk of CRC in the years following screening so that by age 79 years, the cumulative rates are almost identical to those in the general population (ie, there is neither overdiagnosis nor prevention of CRC). However, the difference in rates of Duke D CRC between screened individuals and the general population increases with age so that by age 79 years, there were 435 per 100 000 fewer CRC in those screened. The cumulative rate in the general population was 1337 per 100 000.
Figure 4.
Cumulative rate of colorectal cancer per 100 000 in a hypothetical cohort of individuals aged 60 to 79 years among screened individuals and the general population. Corrected for self-selection bias. A) All stages and B) for Duke stage D.
Discussion
Results suggest that screened individuals are 32% less likely to be diagnosed with advanced stage CRC, defined as Duke stage D, compared with individuals invited for screening but who do not attend. Results did not differ by gender. The observed differential in risk with age probably results from a substantially higher harvest of screen-detected cancers on the prevalent screen than on an incident screen.
The odds ratio (Supplementary Table 4, available online) of a Duke D CRC within 30 days (OR range = 1.44-2.21 depending on age) and 30-60 days (OR range = 1.06-1.72) indicate an excess of about 1-2 months’ worth of cancers being diagnosed within 2 months of a screen (ie, if the cOR is 2.21 that corresponds to an extra 2.21-1 months’ worth of cancer). This is consistent with there being a small number of screen-detected Duke D cancers. The increased odds for all stage cancer within 30 days (OR range = 9.14-12.31) and 30-60 days (OR range = 2.25-3.59) indicate an excess of 8-11 months’ worth of cancer in the first month and 1-2.5 in the second month. Hence, overall, about 1 years’ worth of cancer is found by screening. The odds ratios are greater at age 60-62 years, the age of first invitation for screening (an excess of about 14 months of cancer), than at older ages (incident screens) when the excess is similar to the incidence over 10 months.
The results of the sensitivity analysis including only known stage are more conservative than our main analysis (although less so for Duke D in particular). The fact that screen-detected cancers are more likely to have stage recorded (21) confers a conservative bias, increasing the relative risk associated with screening. The results of the sensitivity analysis using inverse probability weighting were similar but not identical to those of our main analysis. The assumption that survival following a diagnosis of CRC would be a good indicator of stage at diagnosis (15) seems reasonable because the stage distribution with this assumption agrees with national reported statistics for England (22).
Individuals who accept the invitation to screening (participants) may have a priori better health compared with individuals who do not (nonparticipants). In the case of CRC screening, gFOBT kit return has been shown to be lower for postcode sectors with poor health (23). Therefore, even without any benefit of screening, those who would participate in screening may be less likely to be diagnosed with CRC and particularly with advanced CRC compared with the general population (ie, self-selection bias) (24). For this reason, we corrected for self-selection bias as described above.
The lower impact of prevalent cancer in Duke D CRC suggests most of these cancers are diagnosed symptomatically (25). This correlates with the observation that self-selection bias is strongest among this subgroup. After 20 years of follow-up in the Nottingham RCT (7), the rate of Duke D CRC among individuals invited for screening but who did not take up the offer was 7.5 per 1000 compared with 6.2 per 1000 among those not invited (unscreened). The differences in diagnosis rates were smaller and statistically non-significant for other stages (Supplementary Table 1, available online). Given that self-selection (not to be screened) is likely to be stronger among individuals at greatest risk of presenting with advanced CRC, our correction factor was greater with increasing stage at diagnosed.
The case-control design has previously been used to great effect in evaluation of the NHS cervical screening program (26). Nevertheless, it is prone to biases (27). The use of centralized national databases to obtain screening history and colorectal cancer diagnoses will have eliminated recall and ascertainment bias. Screening opportunity bias was addressed by giving the controls a pseudodiagnosis date that is the same as that of their matched case and screening history is only considered up to that date (28). However, individuals who were invited more than once were more likely to have been screened, suggesting that some opportunity bias may remain unaccounted for.
When estimating the cumulative rate per 100 000 individuals, it is possible that the absolute risk in the population is inaccurate because it will reflect a mixture of screened and unscreened individuals, and these proportions are changing over time. However, we are confident that the relative risk between the population and those screened is unbiased. Changes in adherence to screening at a population level would have little impact on odds ratios reported here because they report on the effect of being screened.
Traditional case-control evaluation of CRC screening takes individuals who have died from primary cancer as cases, and individuals known to be alive at the time of death of the cases as controls (29,30). One such study of opportunistic gFOBT screening in England (31) found that cases were less likely than controls to have ever been screened, although the effect was not statistically significant (OR = 0.64, 95% CI = 0.34 to 1.15). This study was carried out in the context of high-risk surveillance, prior to the national program.
In 2 case series in the Australian immunochemical testing program, Cole et al. (32) found a similar reduction in Duke D cases, and Ananda et al. (33) found an increase in survival in cancers detected by the program compared with symptomatic cancers. In the first round of the program in England, Ellul et al. (34) found a shift toward more favorable stage compared with cancers diagnosed prior to the program.
Two case-control studies that assess the impact of fecal immunochemical testing screening on CRC incidence in Japan (17,35) indicated reductions in incidence of advanced stage disease or of interval cancers. Neither study corrected for self-selection.
RCTs of gFOBT screening have found differing results with regard to CRC incidence (36,37). The Nottingham trial found a higher yield of Duke A cancers in the intervention arm (9) but no long-term reduction in incidence between groups (7). The trial did find a 13% reduction in CRC mortality. The Minnesota Colon Cancer Control Study found a reduction in incidence (4) and in mortality (6) from CRC after a similar follow-up time as the Nottingham trial. Rehydration of gFOBT in the Minnesota trial led to very high levels of positivity and colonoscopy, which may have contributed to more adenomas being removed in the screening group, explaining the impact on incidence.
The change to immunochemical testing is likely to improve sensitivity to early stage cancer and adenomas compared with gFOBT (3). Therefore, the benefits in terms of prevention of late-stage disease and mortality are likely to be larger in the future.
Surveillance of the impact of bowel screening programs on mortality is possible using intermediate surrogate endpoints. Results suggest no evidence of cancer prevention (or of overdiagnosis) in the NHSBSP. Combination of the 32% reduction in Duke D cancers and the 19% reduction in Duke C or worse with national stage distribution and stage-specific 5-year survival (20) suggests that the program is on course to reduce CRC mortality by 16% in those who participate.
Funding
This research was funded by the National Institute for Health Research (NIHR) Policy Research Programme, conducted through the Policy Research Unit in Cancer Awareness, Screening, and Early Diagnosis, PR-PRU-1217-21601. AC and PS are supported by a Cancer Research UK programme grant (grant number C8162/A16892 to PS).
Notes
Role of the funders: The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Disclosures: The authors have no disclosures.
Author contributions: SD: conceptualization, funding acquisition, supervision, writing—review & editing. AC: formal analysis, writing—original draft. DP: data curation, project administration, writing—review & editing. NM: conceptualization, methodology, writing—review & editing. PS: methodology, formal analysis, writing—review & editing.
Acknowledgements: Data for this study is based on information collected and quality assured by the Public Health England (PHE) National Cancer Registration and Analysis Service. Access to the data was facilitated by the PHE Office for Data Release. This work uses data provided by patients and collected by the NHS as part of their care and support.
Disclaimers: The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Supplementary Material
Contributor Information
Alejandra Castanon, Cancer Prevention Group, Faculty of Life Sciences & Medicine, School of Cancer & Pharmaceutical Sciences, King’s College London, London, UK.
Dharmishta Parmar, Centre for Prevention, Detection and Diagnosis,Wolfson Institute of Population Health, Queen Mary University of London, London, UK.
Nathalie J Massat, Centre for Prevention, Detection and Diagnosis,Wolfson Institute of Population Health, Queen Mary University of London, London, UK.
Peter Sasieni, Cancer Prevention Group, Faculty of Life Sciences & Medicine, School of Cancer & Pharmaceutical Sciences, King’s College London, London, UK.
Stephen W Duffy, Centre for Prevention, Detection and Diagnosis,Wolfson Institute of Population Health, Queen Mary University of London, London, UK.
Data Availability
The data underlying this article were provided by NHS Digital under the Data Sharing Agreement reference ODR1516_019. Source data included in this study is available by application through NHS Digital’s Data Access Request Service (https://digital.nhs.uk/services/data-access-request-service-dars). Available data is included in this manuscript.
References
- 1. Cancer Research UK. Cancer incidence for common cancers. https://www.cancerresearchuk.org/health-professional/cancer-statistics/incidence/common-cancers-compared#heading-Two. Accessed June 1, 2022
- 2. Cancer Research UK. Bowel cancer statistics. https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/bowel-cancer#heading-Zero. Accessed June 1, 2022.
- 3. Massat NJ, Moss SM, Halloran SP, et al. Screening and primary prevention of colorectal cancer: a review of sex-specific and site-specific differences. J Med Screen. 2013;20(3):125-148. [DOI] [PubMed] [Google Scholar]
- 4. Mandel JS, Church TR, Bond JH, et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med. 2000;343(22):1603-1607. [DOI] [PubMed] [Google Scholar]
- 5. Kronborg O, Fenger C, Olsen J, et al. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996;348(9040):1467-1471. [DOI] [PubMed] [Google Scholar]
- 6. Mandel JS, Church TR, Ederer F, et al. Colorectal cancer mortality: effectiveness of biennial screening for fecal occult blood. J Natl Cancer Inst. 1999;91(5):434-437. [DOI] [PubMed] [Google Scholar]
- 7. Scholefield JH, Moss SM, Mangham CM, et al. Nottingham trial of faecal occult blood testing for colorectal cancer: a 20-year follow-up. Gut. 2012;61(7):1036-1040. [DOI] [PubMed] [Google Scholar]
- 8. Lindholm E, Brevinge H, Haglind E.. Survival benefit in a randomized clinical trial of faecal occult blood screening for colorectal cancer. Br J Surg. 2008;95(8):1029-1036. [DOI] [PubMed] [Google Scholar]
- 9. Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348(9040):1472-1477. [DOI] [PubMed] [Google Scholar]
- 10. Morris EJ, Whitehouse LE, Farrell T, et al. A retrospective observational study examining the characteristics and outcomes of tumours diagnosed within and without of the English NHS Bowel Cancer Screening Programme. Br J Cancer. 2012;107(5):757-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Richards M. Report of the independent review of adult screening programmes in England; 2019. https://www.england.nhs.uk/wp-content/uploads/2019/02/report-of-the-independent-review-of-adult-screening-programme-in-england.pdf. Accessed June 1, 2022.
- 12. UK National Screening Committee. Consultation on the permanent discontinuation of Bowelscope in the English Bowel Screening Programme, 2020. https://legacyscreening.phe.org.uk/policydb_download.php?doc=1312. Accessed February 2, 2022
- 13. Logan RF, Patnick J, Nickerson C, et al. ; for the English Bowel Cancer Screening Evaluation Committee. Outcomes of the Bowel Cancer Screening Programme (BCSP) in England after the first 1 million tests. Gut. 2012;61(10):1439-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Massat NJ, Sasieni PD, Parmar D, et al. An ongoing case-control study to evaluate the NHS Bowel Cancer Screening Programme. BMC Cancer. 2014;14:945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Office for National Statistics. Cancer survival by stage at diagnosis for England (experimental statistics): adults diagnosed 2012, 2013 and 2014 and followed up to 2015. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/cancersurvivalbystageatdiagnosisforenglandexperimentalstatistics/adultsdiagnosed20122013and2014andfollowedupto2015. Accessed June 1 2022.
- 16. Seaman SR, White IR.. Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res. 2013;22(3):278-295. [DOI] [PubMed] [Google Scholar]
- 17. Nakajima M, Saito H, Soma Y, et al. Prevention of advanced colorectal cancer by screening using the immunochemical faecal occult blood test: a case-control study. Br J Cancer. 2003;89(1):23-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Duffy S, Cuzick J, Tabar L, et al. Correcting for non-compliance bias in case-control studies to evaluate cancer screening programmes. Appl Statist. 2002;51(2):235-243. [Google Scholar]
- 19. Public Health England. NHS Screening Programmes in England. 1 April 2016 to 31 March 2017; November 2017. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/661677/NHS_Screening_Programmes_in_England_2016_to_2017_web_version_final.pdf. Accessed June 1, 2022.
- 20. Cancer Research UK. Bowel cancer incidence by age 2015-2017. https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/bowel-cancer/incidence#heading-One. Accessed June 1, 2022.
- 21. Spolverato G, Capelli G, Battagello J, et al. More favorable short and long-term outcomes for screen-detected colorectal cancer patients. Front Oncol. 2021;11:620644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cancer Research UK. Bowel cancer incidence by stage at diagnosis. https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/bowel-cancer/incidence#heading-Three. Accessed June 1, 2022.
- 23. von Wagner C, Good A, Wright D, et al. Inequalities in colorectal cancer screening participation in the first round of the national screening programme in England. Br J Cancer. 2009;101(suppl 2):S60-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Puliti D, Miccinesi G, Paci E.. Overdiagnosis in breast cancer: design and methods of estimation in observational studies. Prev Med. 2011;53(3):131-133. [DOI] [PubMed] [Google Scholar]
- 25. Koo MM, Swann R, McPhail S, et al. Presenting symptoms of cancer and stage at diagnosis: evidence from a cross-sectional, population-based study. Lancet Oncol. 2020;21(1):73-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sasieni P, Castanon A, Cuzick J.. Effectiveness of cervical screening with age: population based case-control study of prospectively recorded data. BMJ. 2009;339:b2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Walter SD. Mammographic screening: case-control studies. Ann Oncol. 2003;14(8):1190-1192. [DOI] [PubMed] [Google Scholar]
- 28. Connor RJ, Boer R, Prorok PC, et al. Investigation of design and bias issues in case-control studies of cancer screening using microsimulation. Am J Epidemiol. 2000;151(10):991-998. [DOI] [PubMed] [Google Scholar]
- 29. Wahrendorf J, Robra BP, Wiebelt H, et al. Effectiveness of colorectal cancer screening: results from a population-based case-control evaluation in Saarland, Germany. Eur J Cancer Prev. 1993;2(3):221-227. [PubMed] [Google Scholar]
- 30. Bertario L, Russo A, Crosignani P, et al. Reducing colorectal cancer mortality by repeated faecal occult blood test: a nested case-control study. Eur J Cancer. 1999;35(6):973-977. [DOI] [PubMed] [Google Scholar]
- 31. Lamah M, Norris J, Caffarey SM, et al. Effect of faecal occult blood testing on colorectal cancer mortality in the surveillance of subjects at moderate risk of colorectal neoplasia: a case-control study. Int J Colorectal Dis. 2001;16(5):313-317. [DOI] [PubMed] [Google Scholar]
- 32. Cole SR, Tucker GR, Osborne JM, et al. Shift to earlier stage at diagnosis as a consequence of the National Bowel Cancer Screening Program. Med J Aust. 2013;198(6):327-330. [DOI] [PubMed] [Google Scholar]
- 33. Ananda S, Wong H, Faragher I, et al. Survival impact of the Australian National Bowel Cancer Screening Programme. Intern Med J. 2016;46(2):166-171. [DOI] [PubMed] [Google Scholar]
- 34. Ellul P, Fogden E, Simpson CL, et al. Downstaging of colorectal cancer by the National Bowel Cancer Screening programme in England: first round data from the first centre. Colorectal Dis. 2010;12(5):420-422. [DOI] [PubMed] [Google Scholar]
- 35. Nishida H, Urano S.. Effectiveness of repeated screening using the fecal occult blood test and its impact on reducing false-negative cancer cases. Eur J Cancer Prev. 2011;20(3):184-189. [DOI] [PubMed] [Google Scholar]
- 36. Lauby-Secretan B, Vilahur N, Bianchini F, et al. ; for the International Agency for Research on Cancer Handbook Working Group. The IARC perspective on colorectal cancer screening. N Engl J Med. 2018;378(18):1734-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. International Agency for Research on Cancer Handbook Working Group. Colorectal Cancer Screening. IARC Handbooks of Cancer Prevention. Volume 17. International Agency for Research on Cancer: Lyon, France; 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article were provided by NHS Digital under the Data Sharing Agreement reference ODR1516_019. Source data included in this study is available by application through NHS Digital’s Data Access Request Service (https://digital.nhs.uk/services/data-access-request-service-dars). Available data is included in this manuscript.