Abstract
Purpose
Adult T‐cell leukemia/lymphoma (ATLL) is a relatively refractory peripheral T‐cell lymphoma caused by human T‐cell lymphotropic virus type 1 (HTLV‐1). The objective of this study was to investigate the characteristics of long‐term survivors with ATLL.
Methods
We conducted an observational study of 75 aggressive‐type ATLL patients. Flow cytometry was conducted to analyze HTLV‐1 Tax‐specific cytotoxic T‐lymphocytes (CTLs) and T‐cell receptor Vβ gene repertoire.
Results
We first evaluated six long‐term survivors among 37 patients who were newly diagnosed with ATLL and then treated with intensive chemotherapy without mogamulizumab, a monoclonal antibody for C‐C chemokine receptor four antigen. Reversal of the CD4‐to‐CD8 ratio (CD4/CD8) in peripheral mononuclear cells was observed in all six patients. Three of these six patients showed reversed CD4/CD8 immediately after herpes virus infection. Four of these six patients who could be examined demonstrated long‐term maintenance of HTLV‐1 Tax‐specific CTLs. We subsequently identified four long‐term survivors among 38 patients who were newly diagnosed with ATLL and then treated with intensive chemotherapy plus mogamulizumab. All four patients showed reversed CD4/CD8, and three of the four patients contracted herpes virus infection during immunochemotherapy. Six of the total 10 patients were subjected to CTL analyses. Tax‐specific CTLs were observed, and the CTLs that were almost entirely composed of memory CTLs in all patients were recorded. HTLV‐1 provirus was also detected in all six patients.
Conclusions
These data suggest that Tax‐specific memory CTLs probably, together with anticancer agents, eradicate ATLL cells and exhibit long‐term preventive effects from relapse ATLL. Thus, the strong activation of cellular immunity, such as herpes virus infection, seems to be necessary to induce such a potent number of Tax‐specific CTLs.
Keywords: adult T‐cell leukemia/lymphoma, cytotoxic T‐lymphocytes, herpes virus infection, human T‐cell lymphotropic virus type 1, Tax
Long‐term survivors with complete remission of newly diagnosed aggressive‐type adult T‐cell leukemia/lymphoma were investigated clinically and immunologically. HTLV‐1 Tax‐specific memory cytotoxic T lymphocytes were observed in all survivors. Strong activation of cellular immunity, such as herpes virus infection during or immediately after chemotherapy, may be necessary to induce such a potent number of Tax‐specific cytotoxic T lymphocytes.

1. INTRODUCTION
Adult T‐cell leukemia/lymphoma (ATLL) is a CD4‐positive peripheral T‐cell lymphoma and the causative agent of human T‐cell lymphotropic virus type 1 (HTLV‐1). 1 , 2 , 3 , 4 ATLL can be classified into four subtypes: acute, lymphoma, smoldering, and chronic. 5 The prognosis of aggressive‐type ATLL consisting of acute, lymphoma, and chronic type with poor prognostic factors is dismal. 6 Allogeneic hematopoietic stem cell transplantation (allo‐HSCT) can be considered if the patients with aggressive‐type ATLL can achieve complete response (CR) with the first‐line of chemotherapy. 7 HTLV‐1 Tax‐specific cytotoxic T lymphocytes (CTLs) have been reported in some patients with ATLL who were successfully treated with allo‐HSCT. 8 , 9 Mogamulizumab, a monoclonal antibody for C‐C chemokine receptor 4 antigen, and lenalidomide, an immunomodulating agent, have recently been approved for use in the treatment of newly diagnosed and relapsed/refractory aggressive‐type ATLL. 10 , 11 , 12 Patients treated with mogamulizumab often experience mogamulizumab‐induced skin disorders with the infiltration of CD3‐ and CD8‐positive T‐cells. 13 , 14 We recently reported that Tax‐specific CTLs are often observed in newly diagnosed patients with aggressive‐type ATLL and mogamulizumab‐induced skin disorders. Interestingly, a statistically significant overall survival benefit was observed in these patients than in those without mogamulizumab‐induced skin disorders and under the combination therapy of mogamulizumab and intensive chemotherapy such as VCAP‐AMP‐VECP (mLSG15) and etoposide, vincristine, doxorubicin, cyclophosphamide, and prednisolone (EPOCH). 14 However, in a phase‐II study comparing treatment outcome with mogamulizumab plus mLSG15 and with mLSG15 alone, no significant difference was noted in the overall survival and progression‐free survival between the two treatments arms, albeit the combination arm showed a better overall response rate. 11 These results may be attributed to the fact that mogamulizumab was designed to activate natural killer (NK) cells rather than CTLs. 15 These data suggest the importance of antitumor cellular immunity, especially CTLs, in treating aggressive‐type ATLL. It has been reported that dormant tumor lesions in immunocompetent mice treated with methylcholanthrene, a tar component, developed into progressive tumors after the depletion of T lymphocytes. 16 This phenomenon was not observed when the NK cells were depleted. These results indicate the importance of T‐cell immunity as an acquired immune system toward controlling cancer progression. To investigate the clinical and immunological features of long‐term survivors with complete remission of newly diagnosed aggressive‐type ATLL that was treated with intensive chemotherapy with and without mogamulizumab, we analyzed the T lymphocytes of these patients, with a focus on HTLV‐1 Tax‐specific CTLs.
2. PATIENTS AND METHODS
2.1. Patient eligibility
This was a single institution observational study conducted on subjects aged ≥20 years, who were newly diagnosed with aggressive‐type ATLL. All patients included in this study were ineligible for allo‐HSCT and were treated at our institute.
We examined 75 patients, including 37 patients, who received intensive chemotherapy between July 2000 and October 2016, and 38 patients, who received intensive chemotherapy plus mogamulizumab between December 2010 and December 2018.
2.2. Treatment
Patients were treated with intensive chemotherapy with or without mogamulizumab. The mLSG15, CHOP, and EPOCH therapies were applied as the intensive chemotherapy regimens. 11 , 14 , 17 The mLSG15 regimen consisted of the following three regimens: VCAP (vincristine, 1 mg/m2, maximum 2 mg; cyclophosphamide, 350 mg/m2; doxorubicin, 40 mg/m2; and prednisolone, 40 mg/m2) at day 1; AMP (doxorubicin, 30 mg/m2; ranimustine, 60 mg/m2; and prednisolone, 40 mg/m2) at day 8; and VECP (vindesine, 2.4 mg/m2 at day 15; etoposide, 100 mg/m2 on days 15–17; carboplatin, 250 mg/m2 at day 15; and prednisolone, 40 mg/m2 on days 15–17). The CHOP regimen consisted of doxorubicin (50 mg/m2 at day 1), cyclophosphamide (750 mg/m2 at day 1), vincristine (1.4 mg/m2, maximum 2 mg, at day 1), and prednisolone (40 mg/m2 on days 1–5). The EPOCH regimen consisted of continuous intravenous infusions conducted on days 1–4 with etoposide (50 mg/m2), vincristine (0.4 mg/m2), and doxorubicin (10 mg/m2), with bolus doses of cyclophosphamide (750 mg/m2 at day 6) and oral prednisolone (60 mg/m2 on days 1–6). We recently reported a risk reduction of approximately 30% in patients treated with mogamulizumab + EPOCH compared to those treated with mogamulizumab + mLSG15. 14
2.3. Immunological analysis and HTLV‐1 provirus load
Peripheral blood mononuclear cells (PBMCs) were collected from patients with human leukocyte antigen (HLA)‐A*02:01 and/or HLA‐A*24:02. Tax‐specific CTLs were determined by flow cytometry without any stimulation. HLA‐A*02:01‐restricted Tax tetramer (HTLV‐1 Tax 178–186 Peptide, MBL) and HLA‐A*24‐02‐restricted Tax tetramer (HTLV‐1 Tax 301–309 Peptide, MBL) were used for detecting Tax‐specific CTLs. The analyses were performed by SRL Corporation and at our institution. Tax‐specific CTLs were further analyzed to categorize them into memory, effector, and naïve CTLs by flow cytometry using antibodies for CD27 and CD45RA (Beckman Coulter). Memory CD8‐positive T lymphocytes were defined as the CD8+CD27+CD45RA− population, effector CD8‐positive T lymphocytes as the CD8+CD27−CD45RA+ population, and naïve CD8‐positive T lymphocytes as the CD8+CD27+CD45RA+ population.
Tax‐specific CTLs have been further analyzed for T‐cell receptor (TCR) Vβ gene repertoire by flow cytometry using the Beta Mark TCR Vβ Repertoire Kit (Beckman Coulter) according to the manufacturer's instructions. This kit consisted of Kit‐A for TCR Vβ 5.3, Vβ 7.1, and Vβ 3; Kit‐B for TCR Vβ 9, Vβ 17, and Vβ 16; Kit‐C for TCR Vβ 18, Vβ 5.1, and Vβ 20; Kit‐D for TCR Vβ 13.1, Vβ 13.6, and Vβ 8; Kit‐E for TCR Vβ 5.2, Vβ 2, and Vβ 12; Kit‐F for TCR Vβ 23, Vβ 1, and Vβ 21.3; Kit‐G for TCR Vβ 11, Vβ 22, and Vβ 14; and Kit‐H for TCR Vβ 13.2, Vβ 4, and Vβ 7.2.
The percentages of CD4‐ and CD8‐positive T lymphocytes in the PBMCs were analyzed by flow cytometry at several time points during chemotherapy and the follow‐up period.
To quantify HTLV‐1 provirus load, genomic DNA extracted from the samples were analyzed using real‐time polymerase chain reaction, as previously described. 18 The HTLV‐1 provirus load was determined at the same time when Tax‐specific CTLs were analyzed by SRL Corporation.
Detailed immunological examinations were performed in 10 of the total 75 patients. Written informed consent was obtained from all of the 10 patients for their participation in this study. The study protocol was approved by the Institutional Ethical Review Board and was conducted in accordance with the Declaration of Helsinki and its later amendments. The study design has been illustrated in Figure 1.
FIGURE 1.
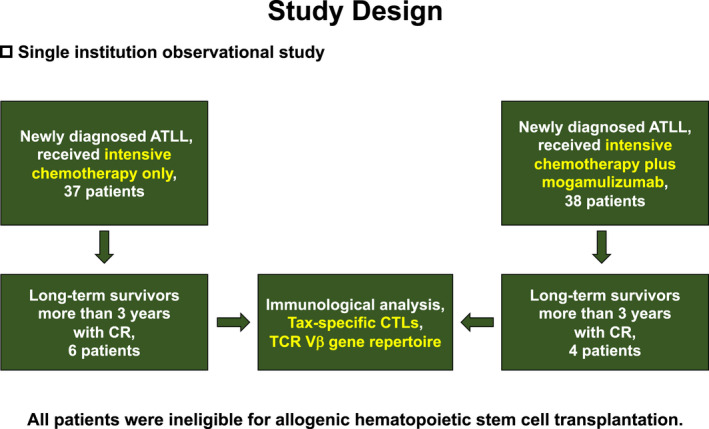
Flow diagram illustrating the study design. ATLL, adult T‐cell leukemia/lymphoma; CR, complete remission; TCR Vβ, T‐cell receptor V beta
3. RESULTS
3.1. Long‐term survivors treated with intensive chemotherapy without mogamulizumab
We first evaluated 37 patients who were newly diagnosed with aggressive‐type ATLL and then treated with intensive chemotherapy alone (Table 1). A total of 34 patients were treated with mLSG15 and three were treated with a CHOP‐like regimen. Of these, six patients achieved long‐term survival with CR. Patients #1, #2, #3, #4, and #6 survived for >10 years, but patient #5 died due to secondary chronic myelomonocytic leukemia without ATLL relapse. The overall survival of patient #5 from the time of initiation of chemotherapy was 71 months.
TABLE 1.
Long‐term survivors with complete remission who were initiated on intensive chemotherapy between July 2000 and October 2016
| Treatment regimen | N |
|---|---|
| VCAP‐AMP‐VECP (mLSG15) | 34 |
| CHOP‐like | 3 |
| Number of patients | Sex | Age at diagnosis | Subtype of ATLL | Survival after starting chemotherapy (months) | CD4/CD8 reversal | Herpes virus infection | HLA |
|---|---|---|---|---|---|---|---|
| 1 | Male | 69 | Lymphoma | >221 | + | + | A*02:01 |
| 2 | Male | 68 | Lymphoma | >211 | + | + | A*02:01 |
| 3 | Female | 62 | Lymphoma | >177 | + | − |
A*02:01 A*24:02 |
| 4 | Female | 61 | Acute | >125 | + | − |
A*02:01 A*24:02 |
| 5 | Female | 71 | Lymphoma | 71 | + | − | N.E. |
| 6 | Male | 60 | Lymphoma | >192 | + | + | N.E. |
Abbreviations: ATLL, adult T‐cell leukemia/lymphoma; HLA, human leukocyte antigen; N.E., not examined.
The reversal of the CD4‐to‐CD8 ratio (CD4/CD8) in PBMCs was noted during chemotherapy for all long‐term survivors. Three of the survivors (i.e., patients #1, #2, and #6) contracted herpes virus infection after obtaining CR during chemotherapy or the completion of chemotherapy. In patient #1, the ratio of CD8‐positive T lymphocytes was higher than that of CD4‐positive T lymphocytes before starting with chemotherapy, but the difference increased after the patient developed herpes zoster (Figure 2A). In patients #2 and #6, the CD4/CD8 reversal was immediately observed after the onset of herpes simplex encephalitis and herpes zoster, respectively (Figure 2B,C).
FIGURE 2.
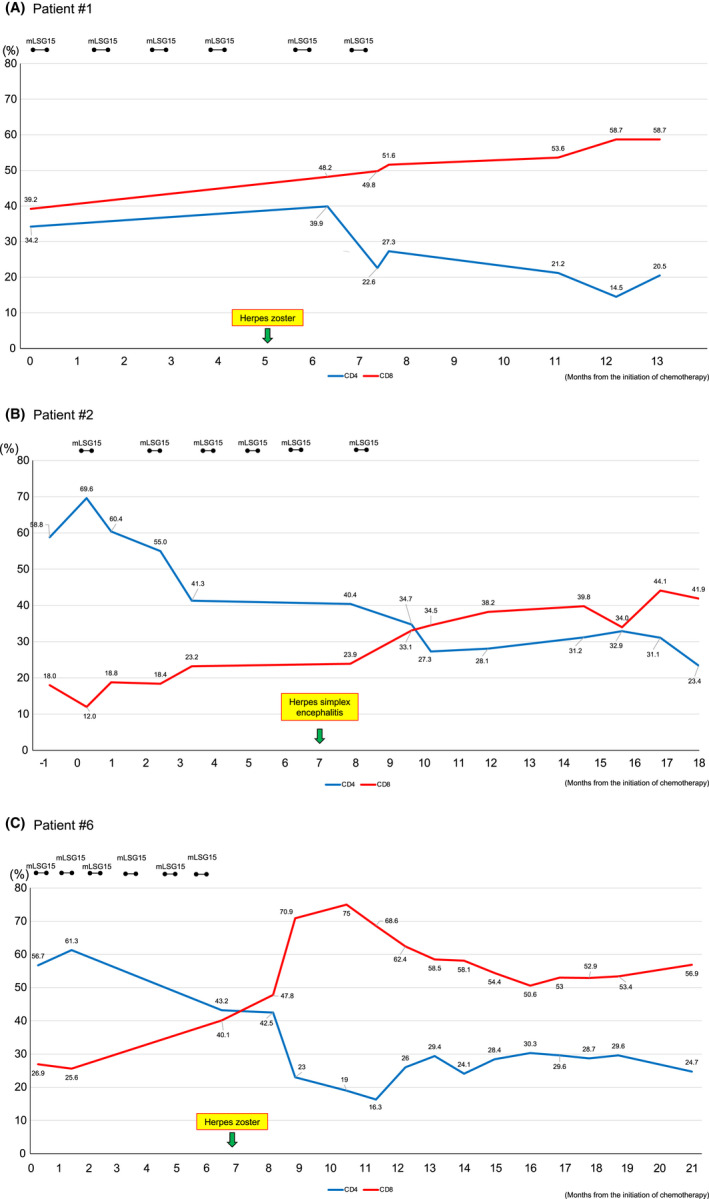
Changes in the levels of CD4‐ and CD8‐positive T‐lymphocytes in three aggressive‐type ATLL patients treated with intensive chemotherapy alone. (A) Patient #1; (B); Patient #2; and (C) Patient #6. The percentages of CD4‐positive (blue lines) and CD8‐positive (red lines) T‐lymphocytes are indicated. Black bars indicate VCAP‐AMP‐VECP (mLSG15) chemotherapy. The occurrences of herpes virus infection are indicated with arrows
Patients #1 and #2 had HLA‐A*02:01, whereas patients #3 and #4 both showed HLA‐A*02:01 and HLA‐A*24:02. These four patients were subjected to Tax‐specific CTL analysis twice after the completion of the chemotherapy regimen (Table 2). Tax‐specific CTLs were detected in all patients. The representative flow cytometry data are presented in Figure 3. Although these analyses were performed without any stimulation, the percentage of Tax‐specific CTLs in the lymphocytes was extremely high in all four patients (0.05%–0.73%), except for HLA‐A*24:02‐restricted CTLs in patient #3 (0.01%). Although all four patients maintained CR at each examination time, HTLV‐1 provirus remained detectable in small quantities (7.1–61.9 copies/1000 PBMCs) (Table 2).
TABLE 2.
Tax‐specific CTLs and HTLV‐1 provirus load in the peripheral blood samples
| Number of patients | Date of examination from starting chemotherapy (months) | HLA | Tax‐specific CTLs/lymphocyte (%) | HTLV‐1 provirus DNA (copies/1000PBMCs) |
|---|---|---|---|---|
| 1 | 133 | A*02:01 | 0.05 | 35.4 |
| 155 | 0.05 | 61.9 | ||
| 2 | 118 | A*02:01 | 0.23 | 24.4 |
| 129 | 0.41 | 14.7 | ||
| 3 | 84 | A*02:01 | 0.26 | 7.1 |
| A*24:02 | 0.01 | |||
| 104 | A*02:01 | 0.29 | 13.3 | |
| A*24:02 | 0.01 | |||
| 4 | 31 | A*02:01 | N.E. | 26.4 |
| A*24:02 | 0.64 | |||
| 53 | A*02:01 | 0.33 | 27.9 | |
| A*24:02 | 0.73 |
Abbreviations: CTLs, cytotoxic T lymphocytes; HLA, human leukocyte antigen; HTLV‐1, human T lymphotropic virus type 1; N.E., not examined; PBMCs, peripheral blood mononuclear cells.
FIGURE 3.
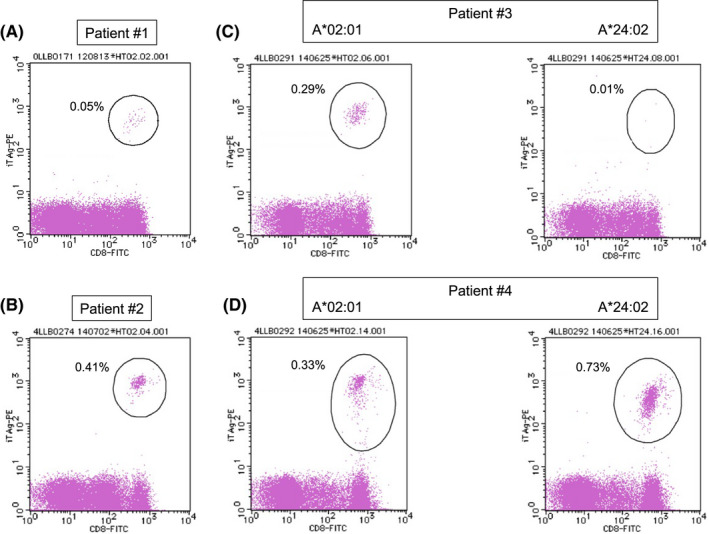
HTLV‐1 Tax‐specific CTLs in four patients with aggressive‐type ATLL treated with intensive chemotherapy alone. (A) Patient #1; (B) Patient #2. (C) Patient #3; and (D) Patient #4. The percentages of Tax‐specific CTLs in lymphocytes are indicated. The dots surrounded by the circles indicate Tax‐specific CTLs
3.2. Long‐term survivors treated with intensive chemotherapy with mogamulizumab
We next investigated 38 patients who were newly diagnosed with aggressive‐type ATLL and then treated with intensive chemotherapy with mogamulizumab (Table 3). Of these, 22 patients were treated with mogamulizumab plus EPOCH, 14 with mogamulizumab plus mLSG15, and two with mogamulizumab plus a CHOP‐like regimen. Of these 22 patients, four achieved long‐term survival with CR. CD4/CD8 reversal was observed after the first cycle of immunochemotherapy in each patient, mainly due to the effect of mogamulizumab. Three of the four patients contracted herpes virus infection (herpes zoster for patient #7 and herpes simplex for patients #8 and #9) during immunochemotherapy. Patient #7 showed both HLA‐A*02:01 and HLA‐A*24:02. Patient #8 showed HLA‐A*24:02, patient #9 showed HLA‐A*02:01, and patient #10 showed neither.
TABLE 3.
Long‐term survivors with complete remission who were initiated on mogamulizumab plus intensive chemotherapy between December 2010 and December 2018
| Treatment regimen | N |
|---|---|
| Mogamulizumab plus EPOCH | 22 |
| Mogamulizumab plus VCAP‐AMP‐VECP (mLSG15) | 14 |
| Mogamulizumab plus CHOP‐like | 2 |
| Number of patients | Sex | Age at diagnosis | Subtype of ATLL | Survival after starting chemotherapy (months) | CD4/CD8 reversal | Herpes virus infection | HLA |
|---|---|---|---|---|---|---|---|
| 7 | Male | 78 | Acute | >47 | + | + |
A*02:01 A*24:02 |
| 8 | Female | 62 | Acute | >41 | + | + | A*24:02 |
| 9 | Female | 69 | Acute | >37 | + | − | A*02:01 |
| 10 | Female | 80 | Lymphoma | >55 | + | + |
A*26:03 A*31:01 |
Abbreviations: ATLL, adult T‐cell leukemia/lymphoma; HLA, human leukocyte antigen.
3.3. HTLV‐1 Tax‐specific CTLs and TCR repertoire analysis
We performed a combined analysis of Tax‐specific CTLs and TCR Vβ gene repertoire to investigate the subdivision of memory, effector, and naïve CTLs as well as the clonality of the CTLs in patients #2, #3, #4, #7, #8, and #9 (Table 4, Figure 4). The analyses were performed after the initiation of the first antitumor treatment, and CR was maintained at the time of the analyses in all six patients (Table 4). Patients #2, #7, and #8 contracted herpes virus infection during the antitumor treatment. Tax‐specific CTLs were detected in all six patients at high percentages (0.09%–1.40%). The majority of these CTLs consisted of memory CTLs. In the HLA‐A*24:02‐restricted CTLs in patient #4, 60% were composed of memory CTLs, but, in all other cases, memory CTLs accounted for >80% (83.0%–99.8%).
TABLE 4.
HTLV‐1 Tax‐specific CTLs, HTLV‐1 provirus DNA, and TCR repertoire analyses in the peripheral blood samples
| Number of patients | HLA | Tax‐specific CTLs/lymphocyte (%) | Tax‐specific CTL subtype | TCR V beta usage major clones | HTLV‐1 provirus load (copies/1000PBMCs) | Date of examination from starting therapy (months) | ||
|---|---|---|---|---|---|---|---|---|
| Memory (%) | Effector (%) | Naïve (%) | ||||||
| 2 a | A*02:01 | 0.266 | 96.2 | 3.8 | 0.0 |
V beta 14: 60.4% V beta 12: 26.2% V beta 16: 14.0% |
50.2 | 211 |
| 3 | A*02:01 | 0.153 | 83.0 | 5.7 | 9.4 |
V beta 22: 25.0% V beta 2: 15.2% |
12.3 | 177 |
| A*24:02 | 0.011 | N.E. | Not examined | |||||
| 4 | A*02:01 | 0.308 | 95.7 | 1.1 | 3.2 | Not determined | 66.6 | 125 |
| A*24:02 | 0.882 | 61.2 | 0.8 | 38.1 | Not determined | |||
| 7 a | A*02:01 | 0.065 | 96.3 | 0.0 | 3.7 |
V beta 14: 43.6% V beta 3: 13.9% V beta 20: 13.6% V beta 8: 13.0% V beta 21.3: 10.5% |
15.3 | 45 |
| A*24:02 | 0.878 | 99.8 | 0.1 | 0.1 | Not determined | |||
| 8 a | A*24:02 | 0.09 | 90.3 | 0.0 | 9.7 |
V beta 3: 61.1% V beta 12: 18.2% |
137.3 | 39 |
| 9 | A*02:01 | 1.40 | 91.7 | 1.7 | 6.5 | Not determined | 22.2 | 35 |
Abbreviations: CTLs, cytotoxic T lymphocytes; HLA, human leukocyte antigen; HTLV‐1, human T lymphotropic virus type 1; not determined, the usage of TCR V beta genes >10% was not detected in this study; TCR, T‐cell receptor.
Patients who experienced herpes virus infection during chemotherapy.
FIGURE 4.
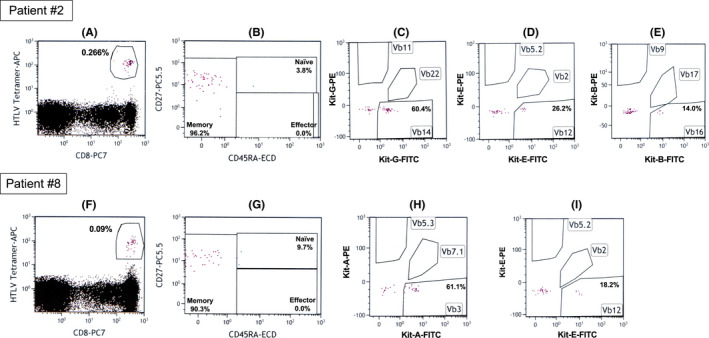
Representative data of HTLV‐1 Tax‐specific CTLs and T‐cell receptor repertoire analysis. The upper panels from (A–E) show the data on Patient #2, whereas the lower panels from (F–I) show the data on Patient #8. CD8‐positive and HTLV‐1 Tax‐specific tetramer‐positive T‐lymphocytes surrounded by the circles indicate HTLV‐1 Tax‐specific CTLs in panels (A and F). HTLV‐1 Tax‐specific memory, effector, and naïve CTLs are shown in panels (B and G). Selected T‐cell receptor Vβ gene repertoire in HTLV‐1 Tax‐specific CTLs are shown in panels (C, D, E, H, and I). Peripheral blood mononuclear cells were directly analyzed by flow cytometry without any stimulation using the HTLV‐1 Tax‐specific tetramers (BML) and the Beta Mark TCR Vβ repertoire kit (Beckman Coulter) as specified by the manufacturer
The Beta Mark TCR Vβ Repertoire Kit used for TCR Vβ gene repertoire analysis of Tax‐specific CTLs could be used to examine 24 TCR Vβ genes simultaneously. The usage of the TCR Vβ genes at >10% is listed in Table 4. Clonal expansion of Tax‐specific CTLs was detected in four of the six patients. In particular, >60% usage of the TCR Vβ genes was observed in patients #2 and #8. In patients #4 and #9, no clonality of TCR Vβ gene usage was detected. The representative flow cytometry data are presented in Figure 4. The percentages of Tax‐specific CTLs in lymphocytes, as indicated by circles for patients #2 and #8, were 0.266% and 0.09%, respectively (Figure 4A,F). In both the patients, >90% of Tax‐specific CTLs consisted of the memory subtype (Figure 4B,G). Selected data of the TCR Vβ repertoire analysis of the memory Tax‐specific CTLs are presented in Figure 4C–E,H,I. HLA‐A*02:01‐restricted memory Tax‐specific CTLs using TCR Vβ 14 was a major clone in patient #2, whereas HLA‐A*24:02‐restricted memory Tax‐specific CTLs using TCR Vβ 3 was a major clone in patient #8.
4. DISCUSSION
The main target tissues of ATLL are lymph nodes and the peripheral blood, but the skin and muscular tissues are also occasionally targeted. 19 HTLV‐1 infects CD4‐positive T cells and causes peripheral T‐cell lymphoma. However, an occasional shift can occur in cell‐surface markers from CD4 to CD8 during the disease course in patients with ATLL. 20 Thus, ATLL is not a straightforward disease, and its treatment is fraught with several challenges.
The various physiological activities of the Tax and HBZ proteins possibly contribute to the therapeutic difficulty of ATLL. 21 , 22 On the contrary, the gene sequence of HTLV‐1 is different from that of humans and hence, its gene product could possibly be recognized as a tumor antigen by host‐immunocompetent cells. 23 However, several reports have demonstrated that ATLL cells have a high incidence of genetic and epigenetic alterations, which induces several ATLL cells to either not express Tax at all or express it at extremely low levels in the body. 24 , 25 , 26 , 27 , 28 , 29 , 30 On the other hand, the detection of the tax mRNA expression in ATLL cells and Tax‐specific CTLs have been reported in ATLL patients, which suggests that the Tax expression in ATLL patients cannot be ignored. 8 , 9 , 14 , 31 Therefore, the clinical and immunological features, including Tax‐specific CTLs, in the long‐term survivors with CR who were affected by ATLL and treated with chemotherapy with or without mogamulizumab should be investigated.
In chemotherapy treatment conducted without mogamulizumab, we found that CD4/CD8 reversal was observed in all long‐term survivors with ATLL (Table 1). We speculated that this phenomenon could demonstrate the importance of CTLs in the treatment of ATLL. However, the modalities for examining Tax‐specific CTLs in routine medical practice were not available until 2012. Tax‐specific CTLs were examined once in 2012 and then once in 2014 in four long‐term survivors with ATLL. Although HTLV‐1 provirus DNA was detected in all these four patients, Tax‐specific CTLs were maintained in all cases (Table 2, Figure 3). Considering that T lymphocytes can maintain cancer dormancy in carcinogen‐induced cancer‐bearing mice, 16 the identification of HTLV‐1 in long‐term survivors may indicate that some ATLL cells remain dormant for a long time. It is therefore possible that Tax‐specific CTLs may have worked together with anticancer drugs during the chemotherapy treatment period and may have played a role in preventing the regrowth of these dormant ATLL cells after the chemotherapy period in the long‐term survivors. Notably, the CD4/CD8 reversal was observed immediately after herpes virus infection in three patients (Table 1, Figure 2). Moreover, past studies have reported that herpes virus infection and varicella‐zoster virus reactivation can elicit a rapid and potent immune response and thereafter the activation of CTLs. 32 , 33 , 34 In this process, antigen‐presenting cells (APCs) also get activated, which in turn may enhance the ability to present Tax peptides to CD8‐positive T cells. 8 , 9 Although patient #4 was not infected with a herpes virus, the patient was treated with EPOCH therapy with PEGylated interferon α2b drug after the relapse of ATLL. In general, achieving CR and long‐term survival is extremely difficult in relapsed cases of ATLL. However, patient #4 survived for >10 years with Tax‐specific CTLs. 35 Furthermore, it has been reported that the cross‐presentation of tumor antigens by dendritic cells activated by type‐I interferon is essential for the induction of antitumor immunity. 36 , 37 Moreover, a past study reported that interferon α, a type‐I interferon, induces the upregulation of major histocompatibility complex class‐1 molecule on tumor cells as well as activates CTLs, which in turn facilitates the recognition of tumor cells by CTLs. 38 Therefore, the application of interferon α may have contributed to long‐term survival for patient #4 by activating APCs and Tax‐specific CTLs. As a future challenge, incorporating interferon α into the treatment of ATLL to enhance host cellular immunity might be considered. In general, the identification of tumor‐specific CTLs is not simple. For example, PBMCs are cultured for approximately 14 days with any stimulation by tumor antigen peptides and interleukin 2, and then assessed. 39 However, it remains intriguing that the Tax‐specific CTLs identified in this study were examined without any stimulation, and their values were extremely high (Table 2, Figure 3). Based on these data, we speculated that the upregulation of cellular immunity caused by fighting against herpes virus infection or interferon α treatment may have upregulated cellular immunity against Tax, which could have ultimately led to long‐term survival in these patients. In fact, the treatment of ATLL with the combination of zidovudine and interferon α with or without arsenic have been reported. 40
In chemotherapy treatment conducted with mogamulizumab, CD4/CD8 reversal was noted in most patients in the early stage of the immunochemotherapy. This may be because almost all CD4‐positive cells express the CCR4 antigen. However, CD4/CD8 reversal does not always lead to long‐term survival. For instance, in a recent report, we demonstrated that Tax‐specific CTLs were often observed in ATLL patients with mogamulizumab‐induced skin disorders, and such patients obtained statistically longer survival than those without mogamulizumab‐induced skin disorders. 14 The phase‐II study compared mLSG15 plus mogamulizumab combination therapy with mLSG15 monotherapy in newly diagnosed aggressive ATLL patients who did not show any survival benefit, although the CR rates were higher in the combination arm. 11 These results suggest that CD4/CD8 reversal induced by mogamulizumab treatment alone may be insufficient to control ATLL and that the upregulation of Tax‐specific CTLs is essential for long‐term survival, as in the case of ATLL patients with mogamulizumab‐induced skin disorders. This result may be partially attributed to the fact that mogamulizumab was designed to primarily activated NK cells, and not CTLs. 15 All four long‐term survivors who underwent intensive chemotherapy with mogamulizumab demonstrated CD4/CD8 reversal. Furthermore, three of the four patients got infected with herpes virus infection during the immunochemotherapy period (Table 3). Recently, we encountered an interesting case report related to patient #7. Patient #7 developed anaplastic lymphoma kinase‐negative anaplastic large cell lymphoma (ALCL) after achieving CR with mogamulizumab plus EPOCH therapy for aggressive ATLL. We found that the patient suffered from herpes zoster during the immunochemotherapy, after which upregulation of Tax‐specific CTLs was noted. Fortunately, the patient achieved CR for ALCL through treatment with romidepsin. The Tax‐specific CTLs were maintained at high percentages during the romidepsin therapy and until the last follow‐up without any relapse of ATLL and ALCL in this patient. 41 These results suggest that mogamulizumab alone is insufficient to induce adequate Tax‐specific CTLs production. Herpes virus infection has been reported to activate innate and adaptive immune responses, which include the release of inflammatory cytokines and chemokines. 42 The activation and proliferation of CD8‐positive T cells during viral infection have also been reported. 43 , 44 An event that elicits a strong immune response in patients, such as herpes virus infection, is believed to be necessary to induce adequate Tax‐specific CTLs. The phenomenon of bacterial infection leading to cancer regression was known empirically in the 18th century. Dr. William Coley conducted a trial in which cancer patients were injected with a mixture of bacteria including Streptococcus pyogenes and Serratia marcescens for therapeutic purposes, which led to some effective case developments. 45 In addition, Dr. Hilton Levy and colleagues tested the use of viral products as interferon inducers in various carcinomas as anticancer immunotherapy. Of the 25 subjects tested, two (one with adult acute leukemia and another one with pediatric acute lymphoblastic leukemia) were reported to have achieved therapeutic outcomes. 46 These results suggest that bacterial and viral infections can activate the immunity of cancer patients, and that the induced cytokines, such as tumor necrosis factor and interferon α can lead to a therapeutic effect. 47 , 48 In the present study, 10 of the 75 patients with aggressive ATLL were long‐term survivors, of which six were infected with herpes virus during or immediately after (immuno) chemotherapy. This is the first report on herpes virus infection that possibly activated the patient's immune system, thereby enhancing the efficacy of treatment for aggressive ATLL.
Finally, we further investigated the nature of the Tax‐specific CTLs in the six long‐term survivors with HLA‐A*02:01 and/or HLA‐A*24:02 (Table 4, Figure 4). The majority of Tax‐specific CTLs were memory phenotype, and the clonal expansion was observed in patients #2, #3, #7, and #8 by TCR Vβ repertoire analyses. However, the TCR Vβ repertoire analyses could not determine the clonal expansion of HLA‐A*02:01‐restricted and HLA‐A*24:02‐restricted Tax‐specific CTLs in patient #4, HLA‐A*24:02‐restricted Tax‐specific CTLs in patient #7, and HLA‐A*02:01‐restricted Tax‐specific CTLs in patient #9, suggesting that the clones had expanded by using other TCR Vβ genes that could not be identified with the kit used in the present study. The combination of these Tax tetramer assays with TCR Vβ repertoire assays in the present study is the first of its kind and has never been reported earlier. This novel combination of assays revealed that most of the Tax‐specific CTLs maintained in aggressive ATLL patients with long‐term survival and CR on intensive chemotherapy with or without mogamulizumab treatment were memory CTLs. HTLV‐1 provirus DNA was detected even in patients #7, #8, and #9 who were treated with intensive chemotherapy plus mogamulizumab. Therefore, chemotherapy with or without mogamulizumab and Tax‐specific CTLs could bring about CR and long‐term survival in aggressive ATLL, albeit it could not eradicate HTLV‐1 completely. Thus, we suggest that Tax‐specific CTLs can form memory CTLs, which in turn can be maintained to prevent the relapse of ATLL.
In summary, we investigated the clinical and immunological characteristics of long‐term survivors of aggressive ATLL who were treated with intensive chemotherapy alone or in combination with mogamulizumab. Out of the 75 patients, 10 were long‐term survivors, six of whom contracted herpes virus infection during or immediately after the chemotherapy. All survivors exhibited and maintained Tax‐specific CTLs for a long time, which were mostly composed of memory CTLs. Furthermore, residual HTLV‐1 provirus DNA was reported in all survivors despite long‐term CR. These results suggest that Tax‐specific CTLs, together with anticancer agents, eradicated ATLL cells during chemotherapy and, subsequently, became memory CTLs to function in the prevention of the relapse of ATLL. The strong activation of cellular immunity during herpes virus infection or via interferon administration may hence be necessary to induce such a sufficiently potent number of Tax‐specific CTLs. In addition, it is clear that mogamulizumab alone is insufficient to effectively induce such Tax‐specific CTLs. This study has some limitations, including it being a single‐center observational study and the small number of long‐term survivors assessed. Furthermore, the patients did not receive the same intensive chemotherapy. Nevertheless, the findings on Tax‐specific memory CTLs and herpes virus infection are intriguing and not reported earlier. Although this study was a retrospective observational study, future prospective studies are warranted to explore the possibility that herpes virus infection upregulating Tax‐specific CTLs may contribute to long‐term survival following (immuno) chemotherapy for ATLL. We believe that the development of a method to effectively activate the cellular immunity against ATLL cells will be necessary for the treatment of ATLL, in addition to immunochemotherapy.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Tatsuro Jo designed the work; collected, analyzed, and interpreted data; and drafted the manuscript. Kazuhiro Noguchi, Takahiro Sakai, Sadaharu Irie, Masatoshi Matsuo, Jun Taguchi, Kuniko Abe, and Kazuto Shigematsu collected and analyzed data. Ritsuko Kubota‐Koketsu interpreted the data and drafted the manuscript. All authors have read and approved the final version of this manuscript.
PATIENT CONSENT STATEMENT
Written informed consent was obtained from all patients who were examined by immunological analyses and HTLV‐1 provirus load.
PERMISSION TO REPRODUCE MATERIAL FROM OTHER SOURCES
No material from other sources was used in the drafting of this manuscript.
CLINICAL TRIAL REGISTRATION
Not applicable.
ETHICS STATEMENT
The study was approved by the Institutional Ethical Review Board (Approval Number: R3‐668) and was conducted in accordance with the Declaration of Helsinki and its later amendments.
ACKNOWLEDGMENTS
The authors would like to thank Crimson Interactive Private Limited for English language editing.
Jo T, Noguchi K, Sakai T, et al. HTLV‐1 Tax‐specific memory cytotoxic T lymphocytes in long‐term survivors of aggressive‐type adult T‐cell leukemia/lymphoma. Cancer Med. 2022;11(17):3238–3250. doi: 10.1002/cam4.4689
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Uchiyama T, Yodoi J, Sagawa K, Takatsuki K, Uchino H. Adult T‐cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977;50(3):481‐492. [PubMed] [Google Scholar]
- 2. Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T‐cell lymphoma. Proc Natl Acad Sci USA. 1980;77(12):7415‐7419. doi: 10.1073/pnas.77.12.7415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Miyoshi I, Kubonishi I, Yoshimoto S, et al. Type C virus particles in a cord T‐cell line derived by co‐cultivating normal human cord leukocytes and human leukaemic T‐cells. Nature. 1981;294(5843):770‐771. doi: 10.1038/294770a0 [DOI] [PubMed] [Google Scholar]
- 4. Yoshida M, Seiki M, Yamaguchi K, Takatsuki K. Monoclonal integration of human T‐cell leukemia provirus in all primary tumors of adult T‐cell leukemia suggests causative role of human T‐cell leukemia virus in the disease. Proc Natl Acad Sci USA. 1984;81(8):2534‐2537. doi: 10.1073/pnas.81.8.2534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T‐cell leukemia‐lymphoma. A report from the lymphoma study group (1984‐87). Br J Haematol. 1991;79(3):428‐437. doi: 10.1111/j.1365-2141.1991.tb08051.x [DOI] [PubMed] [Google Scholar]
- 6. Ishitsuka K, Tamura K. Human T‐cell leukaemia virus type I and adult T‐cell leukaemia‐lymphoma. Lancet Oncol. 2014;15(11):e517‐e526. doi: 10.1016/S1470-2045(14)70202-5 [DOI] [PubMed] [Google Scholar]
- 7. Bazarbachi A, Suarez F, Fields P, Hemine O. How I treat adult T‐cell leukemia/lymphoma. Blood. 2011;118(7):1736‐1745. doi: 10.1182/blood-2011-03-345702 [DOI] [PubMed] [Google Scholar]
- 8. Harashima N, Kurihara K, Utsunomiya A, et al. Graft‐versus‐tax response in adult T‐cell leukemia patients after hematopoietic stem cell transplantation. Cancer Res. 2004;64(1):391‐399. doi: 10.1158/0008-5472.can-03-1452 [DOI] [PubMed] [Google Scholar]
- 9. Harashima N, Tanosaki R, Shimizu Y, et al. Identification of two new HLA‐A*1101‐restricted tax epitopes recognized by cytotoxic T lymphocytes in an adult T‐cell leukemia patient after hematopoietic stem cell transplantation. J Virol. 2005;79(15):10088‐10092. doi: 10.1128/JVI.79.15.10088-10092.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ishida T, Joh T, Uike N, et al. Defucosylated anti‐CCR4 monoclonal antibody (KW‐0761) for relapsed adult T‐cell leukemia‐lymphoma: a multicenter phase II study. J Clin Oncol. 2012;30(8):837‐842. doi: 10.1200/JCO.2011.37.3472 [DOI] [PubMed] [Google Scholar]
- 11. Ishida T, Jo T, Takemoto S, et al. Dose‐intensified chemotherapy alone or in combination with mogamulizumab in newly diagnosed aggressive adult T‐cell leukaemia‐lymphoma: a randomized phase II study. Br J Haematol. 2015;169(5):672‐682. doi: 10.1111/bjh.13338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ishida T, Fujiwara H, Nosaka K, et al. Multicenter phase II study of lenalidomide in relapsed or recurrent adult T‐cell leukemia/lymphoma: ATLL‐002. J Clin Oncol. 2016;34(34):4086‐4093. doi: 10.1200/JCO.2016.67.7732 [DOI] [PubMed] [Google Scholar]
- 13. Yonekura K, Kanzaki T, Gunshin K, et al. Effect of anti‐CCR4 monoclonal antibody (mogamulizumab) on adult T‐cell leukemia‐lymphoma: cutaneous adverse reactions may predict the prognosis. J Dermatol. 2014;41(3):239‐244. doi: 10.1111/1346-8138.12419 [DOI] [PubMed] [Google Scholar]
- 14. Jo T, Matsuzaka K, Shioya H, et al. Mogamulizumab plus EPOCH therapy for patients with newly diagnosed aggressive adult T‐cell leukemia/lymphoma. Anticancer Res. 2020;40(9):5237‐5243. doi: 10.21873/anticanres.14527 [DOI] [PubMed] [Google Scholar]
- 15. Ishii T, Ishida T, Uysunomiya A, et al. Defucosylated humanized anti‐CCR4 monoclonal antibody KW‐0761 as a novel immunotherapeutic agent for adult T‐cell leukemia/lymphoma. Clin Cancer Res. 2010;16(5):1520‐1531. doi: 10.1158/1078-0432.CCR-09-2697 [DOI] [PubMed] [Google Scholar]
- 16. Koebel CM, Vermi W, Swann JB, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450(7171):903‐907. doi: 10.1038/nature06309 [DOI] [PubMed] [Google Scholar]
- 17. Tsukasaki K, Utsunomiya A, Fukuda H, et al. VCAP‐AMP‐VECP compared with biweekly CHOP for adult T‐cell leukemia‐lymphoma: Japan Clinical Oncology Group Study JCOG9801. J Clin Oncol. 2007;25(34):5458‐5464. doi: 10.1200/JCO.2007.11.9958 [DOI] [PubMed] [Google Scholar]
- 18. Yasunaga J, Sakai T, Nosaka K, et al. Impaired production of naïve T lymphocytes in human T‐cell leukemia virus type I‐infected indivisuals: its implications in the immunodeficient state. Blood. 2001;97(10):3177‐3183. doi: 10.1182/blood.v97.10.3177 [DOI] [PubMed] [Google Scholar]
- 19. Jo T, Shigematsu K. Extensive and destructive invasion of adult T‐cell leukemia/lymphoma cells into systemic muscular tissues. Blood. 2014;124(10):1690. doi: 10.1182/blood-2014-03-561001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Joh T, Yamada Y, Seto M, Kamihira S, Tomonaga M. Expression of CD8β and alteration of cell surface phenotype in adult T‐cell leukaemia cells. Br J Haematol. 1997;98(1):151‐156. doi: 10.1046/j.1365-2141.1997.1853002.x [DOI] [PubMed] [Google Scholar]
- 21. Kataoka K, Nagata Y, Kitanaka A, et al. Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat Genet. 2015;47(11):1304‐1315. doi: 10.1038/ng.3415 [DOI] [PubMed] [Google Scholar]
- 22. Satou Y, Yasunaga J, Yoshida M, Matsuoka M. HTLV‐I basic leucine zipper factor gene mRNA supports proliferation of adult T cell leukemia cells. Proc Natl Acad Sci USA. 2006;103(3):720‐725. doi: 10.1073/pnas.0507631103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Suzuki S, Masaki A, Ishida T, et al. Tax is a potential molecular target for immunotherapy of adult T‐cell leukemia/lymphoma. Cancer Sci. 2012;103(10):1764‐1773. doi: 10.1111/j.1349-7006.2012.02371.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yasunaga J, Matsuoka M. Leukaemogenic mechanism of human T‐cell leukaemia virus type I. Rev Med Virol. 2007;17(5):301‐311. doi: 10.1002/rmv.548 [DOI] [PubMed] [Google Scholar]
- 25. Takeda S, Maeda M, Morikawa S, et al. Genetic and epigenetic inactivation of tax gene in adult T‐cell leukemia cells. Int J Cancer. 2004;109(4):559‐567. doi: 10.1002/ijc.20007 [DOI] [PubMed] [Google Scholar]
- 26. Furukawa Y, Kubota R, Tara M, Izumo S, Osame M. Existence of escape mutant in HTLV‐I tax during the development of adult T‐cell leukemia. Blood. 2001;97(4):987‐993. doi: 10.1182/blood.v97.4.987 [DOI] [PubMed] [Google Scholar]
- 27. Tamiya S, Matsuoka M, Etoh K, et al. Two types of defective human T‐lymphotropic virus type I provirus in adult T‐cell leukemia. Blood. 1996;88(8):3065‐3073. doi: 10.1182/blood.V88.8.3065.bloodjournal8883065 [DOI] [PubMed] [Google Scholar]
- 28. Miyazaki M, Yasunaga J, Taniguchi Y, Tamiya S, Nakahata T, Matsuoka M. Preferential selection of human T‐cell leukemia virus type 1 provirus lacking the 5′ long terminal repeat during oncogenesis. J Virol. 2007;81(11):5714‐5723. doi: 10.1128/JVI.02511-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Koiwa T, Hamano‐Usami A, Ishida T, et al. 5′‐long terminal repeat‐selective CpG methylation of latent human T‐cell leukemia virus type 1 provirus in vitro and in vivo. J Virol. 2002;76(18):9389‐9397. doi: 10.1128/jvi.76.18.9389-9397.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Taniguchi Y, Nosaka K, Yasunaga J, et al. Silencing of human T‐cell leukemia virus type I gene transcription by epigenetic mechanisms. Retrovirology. 2005;2:64. doi: 10.1186/1742-4690-2-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kinoshita T, Shimoyama M, Tobinai K, et al. Detection of mRNA for the tax/rex1 gene of human T‐cell leukemia virus type 1 in fresh peripheral blood mononuclear cells of adult T‐cell leukemia patients and viral carriers by using the polymerase chain reaction. Proc Natl Acad Sci USA. 1989;86(14):5620‐5624. doi: 10.1073/pnas.86.14.5620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mueller SN, Jones CM, Chen W, et al. The early expression of glycoprotein B from herpes simplex virus can be detected by antigen‐specific CD8+ T cells. J Virol. 2003;77(4):2445‐2451. doi: 10.1128/jvi.77.4.2445-2451.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Steain M, Gowrishankar K, Rodriguez M, Slobedman B, Abendroth A. Upregulation of CXCL10 in human dorsal root ganglia during experimental and natural varicella‐zoster virus infection. J Virol. 2011;85(1):626‐631. doi: 10.1128/JVI.01816-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weinberg A, Levin MJ. VZV T cell‐mediated immunity. Curr Top Microbiol Immunol. 2010;342:341‐357. doi: 10.1007/82_2010_31 [DOI] [PubMed] [Google Scholar]
- 35. Jo T, Kaneko Y, Oishi T, et al. Elevation of memory cytotoxic T lymphocytes, including human T Lymphotropic virus type 1 tax‐specific and hepatitis virus type C‐specific cytotoxic T lymphocytes, in a patient with adult T‐cell leukemia/lymphoma and hepatocellular carcinoma. Case Rep Oncol. 2020;13(2):802‐806. doi: 10.1159/000508092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Diamond MS, Kinder M, Matsushita H, et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 2011;208(10):1989‐2003. doi: 10.1084/jem.20101158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fuertes MB, Kacha AK, Kline J, et al. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J Exp Med. 2011;208(10):2005‐2016. doi: 10.1093/jnci/djq009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mocellin S, Pasquali S, Rossi CR, Nitti D. Interferon alpha adjuvant therapy in patients with high‐risk melanoma: a systematic review and meta‐analysis. J Natl Cancer Inst. 2010;102(7):493‐501. doi: 10.1093/jnci/djq009 [DOI] [PubMed] [Google Scholar]
- 39. Yoshikawa T, Nakatsugawa M, Suzuki S, et al. HLA‐A2‐restricted dlypican‐3 peptide‐specific CTL clone induced by peptide vaccine show high avidity and antigen‐specific killing activity against tumor cells. Cancer Sci. 2011;102(5):918‐925. doi: 10.1111/j.1349-7006.2011.01896.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nasr R, Marcais A, Hermine O, Bazarbachi A. Overview of targeted therpies for adult T‐cell leukemia/lymphoma. Methods Mol Biol. 2017;1582:197‐216. doi: 10.1007/978-1-4939-6872-5_15 [DOI] [PubMed] [Google Scholar]
- 41. Jo T, Sakai T, Matsuzaka K, et al. Successful treatment of a patient with brentuximab vedotin‐refractory ALK‐negative anaplastic large cell lymphoma with romidepsin. Case Rep Oncol. 2020;13(3):1402‐1409. doi: 10.1159/000511111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang L, Wang R, Xu C, Zhou H. Pathogenesis of herpes stromal keratitis: immune inflammatory response mediated by inflammatory regulators. Front Immunol. 2020;11:766. doi: 10.3389/fimmu.2020.00766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Callan MF, Tan L, Annels N, et al. Direct visualization of antigen‐specific CD8+ T cells during the primary immune response to Epstein‐Barr virus in vivo. J Exp Med. 1998;187(9):1395‐1402. doi: 10.1084/jem.187.9.1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Murali‐Krishna K, Altman JD, Suresh M, et al. Counting antigen‐specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8(2):177‐187. doi: 10.1016/s1074-7613(00)80470-7 [DOI] [PubMed] [Google Scholar]
- 45. Coley WB. Contribution to the knowledge of sarcoma. Ann Surg. 1891;14(3):199‐220. doi: 10.1097/00000658-189112000-00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Levine AS, Sivulich M, Wiernik PH, Levy HB. Initial clinical trials in cancer patients of polyriboinosinic‐polyribocytidylic acid stabilized with poly‐L‐lysine, in carboxymethylcellulose [poly(ICLC)], a highly effective interferon inducer. Cancer Res. 1979;39(5):1645‐1650. [PubMed] [Google Scholar]
- 47. Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9(5):361‐371. doi: 10.1038/nrc2628 [DOI] [PubMed] [Google Scholar]
- 48. Galluzzi L, Vacchelli E, Eggermont A, et al. Trial watch: experimental toll‐like receptor agonists for cancer therapy. Oncoimmunology. 2021;1(5):699‐716. doi: 10.4161/onci.20696 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


