Abstract
This study compared the meat quality and sensory quality characteristics of broiler pectoralis major (PM) muscle between meat quality classes, including reddish, firm, and non-exudative (RFN) and pale, soft, and exudative (PSE) conditions. Additionally, we also investigated the associations between the meat quality classes and expression levels of cytochrome c, initiator (caspase 9), and effector caspases (caspase 3 and 7) at the early postmortem period. A total of 135 PM muscles from broilers were used, and meat quality classes were determined according to the pH24 h and lightness values and classified into the RFN (N = 81) and PSE (N = 54) conditions. The PSE breasts showed lower muscle pH15 min and pH24 h values compared to the RFN breasts (P < 0.05). A lower lightness value was observed in the RFN group compared to the PSE group (P < 0.001), whereas there were no significant differences in redness and yellowness between the groups (P > 0.05). The PSE group exhibited higher extent of water loss, including drip loss and cooking loss, compared to the RFN group (P < 0.05). For these reasons, samples from the RFN group required less force to breakdown the cooked meat with more moisture in the mouth after chewing compared to samples from the PSE group (P < 0.001); however, flavor intensity did not differ between the groups (P > 0.05). At 15 min postmortem, all apoptosis-related molecules, including cytochrome c, caspase 9, caspase 3, and caspase 7, were present at higher levels in the PSE group than in the RFN group (P < 0.05). These results indicated that higher apoptotic potentials were associated with the development of PSE chicken breasts. Therefore, the variation of meat quality in chicken breast can be explained as being affected by the expression levels of apoptosis-related factors at the early postmortem period.
Key words: cytochrome c, caspases, apoptotic potential, meat quality, chicken breast
INTRODUCTION
One of the important issues in the poultry industry is the occurrence of pale, soft, and exudative (PSE) condition meat, which arises as a result of intensive selection for increased muscling, particularly the pectoralis major (PM) muscle (Owens et al., 2000). This condition occurs in more than 20% of the total production in commercial chicken slaughtering plants in many countries (Grashorn, 2010; Zhu et al., 2012; Karunanayaka et al., 2016). The poultry industry has been working to reduce the incidence of the PSE condition, since it can negatively affect consumer acceptability, meat quality traits, and further processing properties (Kuttappan et al., 2012; Gonzalez, 2020). In general, the development of PSE condition is dependent on the extent of physicochemical changes during the conversion of muscle to meat after bleeding, and the mechanisms responsible for this development are interdependent and extremely complex (Kemp and Parr, 2012). The first phase in this conversion during the postmortem period is the onset of programmed cell death, which is a central homeostatic process. Thus, the biochemical and structural changes caused by the metabolic switch suffered by muscle fibers during apoptosis can influence the meat quality (Lomiwes et al., 2014).
Apoptosis is effectively established by caspases, cysteine aspartate-specific proteases that induce myofibril protein cleavage (Kemp and Parr, 2012). In caspase-mediated apoptosis, it is subdivided into 3 main pathways of activation, including intrinsic, extrinsic, and endoplasmic reticulum-mediated pathways (Kemp and Parr, 2012). The pathway induced by hypoxic/ischemic conditions during postmortem is the intrinsic apoptosis pathway, a form of mitochondrion-centered cell death mediated by the permeabilization of mitochondrial outer membrane (Concannon et al., 2003). Disruption of the mitochondrial membrane leads to the release of cytochrome c, which binds apoptosis protease activating factor 1 in the presence of dATP in the cytoplasm (Concannon et al., 2003). This protein complex, known as the apoptosome, activates caspase 9, triggering a cascade of effector caspases, including caspase 3 and 7 (Concannon et al., 2003). During the postmortem period, these caspase systems are responsible for muscle fiber destruction and affect the meat quality traits of various animals (Garcia-Macia et al., 2014; Guo et al., 2016; Cramer et al., 2018; Lee et al., 2022). Cramer et al. (2018) reported that callipyge lamb meat showed lower expression levels of cytochrome c and caspase 3 compared to normal lamb meat, which may lead to the delayed onset of apoptosis and consequently increased toughness. On the other hand, the occurrence of PSE pork was associated with higher expression levels of caspase enzymes due to their different apoptotic potentials compared to normal quality pork (Xing et al., 2018). However, there is limited information describing whether apoptosis-related factors impact on the meat quality characteristics of broilers, particularly the development of PSE condition. Therefore, this study was designed to investigate the differences in the levels of apoptotic factors, including cytochrome c and caspases, of the PM muscle, and their relationship to meat and sensory quality characteristics in the PSE broilers compared to the normal quality broilers, to understand the association between apoptotic potential and quality variation.
MATERIALS AND METHODS
Animals and Sample Procedure
A total of 135 PM muscles from Ross 308 broiler carcasses (male; 4 wk of age; average live weight 1,587 ± 293 g) were used in the present study. At 15 min postmortem, both the left and right PM muscles were excised during standard slaughtering process at a commercial slaughterhouse in a cold room. The muscle pH was immediately measured from the left side breast. At the same fillets, muscle samples of approximately 20 g were taken for real-time polymerase chain reaction (RT-PCR) analysis, were immediately frozen using liquid nitrogen, and stored at –80°C. The remaining left and entire right fillets were rapidly cooled with ice-cold water to minimize muscle damage and stored at 4°C until further analysis.
After 24 h postmortem, the meat quality traits, including pH24 h, meat color, drip loss, and cooking loss, were measured using the muscle samples from the left side. The quality classes were determined using the values of the pH24 h and lightness (L*) as a measure of the meat color (Carvalho et al., 2014). The reddish, firm, and non-exudative (RFN) quality group (N = 81) had a pH24 h value of ≥5.7 and L* value ranging from 48 to 53, and the PSE quality group (N = 54) had a pH24 h value of under 5.7 and L* value of ≥53. The right PM muscle samples at 24 h postmortem were stored at –20°C for the sensory evaluation.
Meat Quality Characteristics
The muscle pH values (pH15 min and pH24 h) were determined on the surface on PM muscle using a pH instrument equipped with a penetration probe (Testo 206-pH2, Test Inc., Lenzkirch, Germany). Color assessment was performed using a Minolta chromameter (CR-400, Minolta Camera Co., Osaka, Japan) and the color was expressed in terms of CIE values for lightness (L*), redness (a*), and yellowness (b*) (Commission Internationale de l'Eclairage, 1978). For water holding capacity (WHC), percentage of drip loss and cooking loss were measured with reference to Honikel (1998). Drip loss was calculated as the difference in sample weight before and after storage at 4°C for 48 h. To determine cooking loss, muscle samples were weighed before and after cooking, which involved being sealed in a polyethylene bag and heated in a water bath (80°C) until the core temperature reached 71°C. Cooking loss was expressed as a percentage of the initial weight of the muscle sample.
Sensory Quality Characteristics
A total of 135 right breast samples were randomly selected by coding with a 3-digit number and evaluated over 27 sessions, with 5 samples per each session. The panelists training and sensory evaluations were performed at the Kyungpook National University (KNU) according to the guidelines of the American Meat Science Association (2015), and human ethics approval was granted by the Bioethics Committee of KNU (protocol number 2019-0027). Frozen muscle samples were thawed at 4°C for 18 h and then cooked by pan-frying using an induction cooker (CIR-IH300RGL, Cuchen, Cheonan, Korea) until the internal temperature reached 71°C. Cooked samples were cut into 1.3 cm3 cubes and provided to trained panelists. The panelists evaluated the samples for 3 sensory quality attributes, including tenderness, juiciness, and flavor intensity, which were assessed on a 9-point scale.
Quantitative RT-PCR
Total RNA was isolated from the left PM muscles at 15 min postmortem, following the manufacturer's instructions. The quantity of total RNA was assessed by electrophoresis and normalized accordingly. Complementary DNA (cDNA) was synthesized using 1 ng of total RNA. The synthesized cDNA was used for quantitative RT-PCR to measure the expressions of CYCS (cytochrome c; forward 5′-CCC AGT GCC ATA CGG TTG AA-3′ and reverse 5′-CTC ACC CCA AGT GAT ACC TTT GT-3′), CASP9 (caspase 9; forward 5′-AGA TGA AAC TTG CCG ACG TT-3′ and reverse 5′-CTT CAG AAC GGG CGT AAT GT-3′), CASP3 (caspase 3; forward 5′-TGGCCCTCTTGAACTGAAAG-3′ and reverse 5′-TCC ACT GTC TGC TTC AAT ACC-3’), CASP7 (caspase 7; forward 5′-CAT TTA TGG CAC CGA TGG AC-3′ and reverse 5′-CCG GTC CAG AGT CAG TTT GT-3′), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; forward 5′-CGT CCT CTC TGG CAA AGT CC-3′ and reverse 5′-AAG ATA GTG ATG GCG TGC CC-3′). RT-PCR was carried out using SYBR green dye (A25741, Applied Biosystems, Foster City, CA) using an ABI 730 real-time PCR instrument (Applied Biosystems). The comparative 2−ΔΔCt method for relative quantification was used to calculate the relative gene expression. The housekeeping gene was GAPDH which was used to normalize the RT-PCR calculation.
Statistical Analysis
The meat quality traits and levels of apoptosis-related factors in the meat quality classes were analyzed using a general linear mixed model (GLM; SAS Institute, Cary, NC). To assess the sensory quality characteristics, a GLM was produced, using the pH24 h and L* values as a fixed effects and the trained panelists as a random effect. The probability difference option was set at 5% and used for calculating the significant differences in the least-squares means (LSM) of investigated parameters between the groups. All data were presented as LSM with standard errors.
RESULTS AND DISCUSSION
The breast meat and sensory quality characteristics of the meat quality classes are presented in Table 1. As expected, the RFN group exhibited higher muscle pH15 min (6.38 vs. 6.27, P < 0.05) and pH24 h (6.00 vs. 5.61, P < 0.001) values compared to the PSE group. A higher lightness value was observed in the PSE group than in the RFN group (56.8 vs. 49.6, P < 0.001), although redness and yellowness did not differ between the groups (P > 0.05). Breast muscle samples from the RFN group showed lower drip loss (2.19 vs. 2.66%, P < 0.05) compared to breast samples from the PSE group. Nam et al. (2009) reported that consumers are generally able to distinguish meat in unacceptable condition, especially PSE meat, due to its paler color and large amount of exudated water on the meat surface compared to meat in normal conditions (Livisay et al., 1996; Zequan et al., 2021). The occurrence of PSE meat is accompanied by economic losses for the meat industry as a result of its higher moisture loss and decreased purchases due to it's lower appearance acceptability (Kuttappan et al., 2012). However, when the cooked meat was evaluated by consumer panelists, the results differed among researchers. Livisay et al. (1996) reported a lower palatability score for the PSE compared to the RFN meat. However, Droval et al. (2012) and Nam et al. (2009) confirmed that consumers could not easily distinguish the differences in the organoleptic properties between the PSE and RFN meat. In the current study, the extent of water loss, especially cooking loss, was significantly different between the RFN and PSE groups (11.7 vs. 15.4%, P < 0.001). These differences in the WHC can significantly impact the palatability characteristics of cooked meat between the 2 groups, as cooking loss was negatively correlated with juiciness and tenderness scores. Trained panelists gave lower tenderness (5.29 vs. 5.88, P < 0.001) and juiciness (5.35 vs. 5.74, P < 0.001) scores for cooked samples from the PSE group compared to cooked samples from the RFN group, although there was no significant difference in flavor intensity between the two groups (P > 0.05). Therefore, the PSE chicken breasts tended to have impaired sensory quality compared to the RFN chicken breasts, a situation which may negatively affect the repurchase intentions of consumers for poultry meat.
Table 1.
Comparison of meat quality and sensory quality characteristics of chicken breast between the meat quality classes.
| RFN (N = 81) | PSE (N = 54) | Level of significance | |
|---|---|---|---|
| Meat quality characteristics | |||
| pH15 min | 6.38a (0.03)1 | 6.27b (0.04) | * |
| pH24 h | 6.00a (0.01) | 5.61b (0.02) | *** |
| Lightness (L*) | 49.6b (0.25) | 56.8a (0.30) | *** |
| Redness (a*) | 1.41 (0.10) | 1.22 (0.12) | NS |
| Yellowness (b*) | 7.06 (0.19) | 7.33 (0.23) | NS |
| Drip loss (%) | 2.19b (0.13) | 2.66a (0.17) | * |
| Cooking loss (%) | 11.7b (0.34) | 15.4a (0.39) | *** |
| Sensory quality characteristics2 | |||
| Tenderness | 5.88a (0.09) | 5.29b (0.08) | *** |
| Juiciness | 5.74a (0.08) | 5.35b (0.07) | *** |
| Flavor intensity | 6.29 (0.08) | 6.25 (0.07) | NS |
Abbreviations: RFN, reddish, firm, and non-exudative; PSE, pale, soft, and exudative.
Levels of significance: NS, no significant; * P < 0.05; *** P < 0.001.
Different superscripts in the same row represent significant differences (P < 0.05).
Standard error of least-square means.
Score distribution (1‒9): tenderness, very tough to very tender; juiciness, not juicy to extremely juicy; flavor intensity, very weak to very strong.
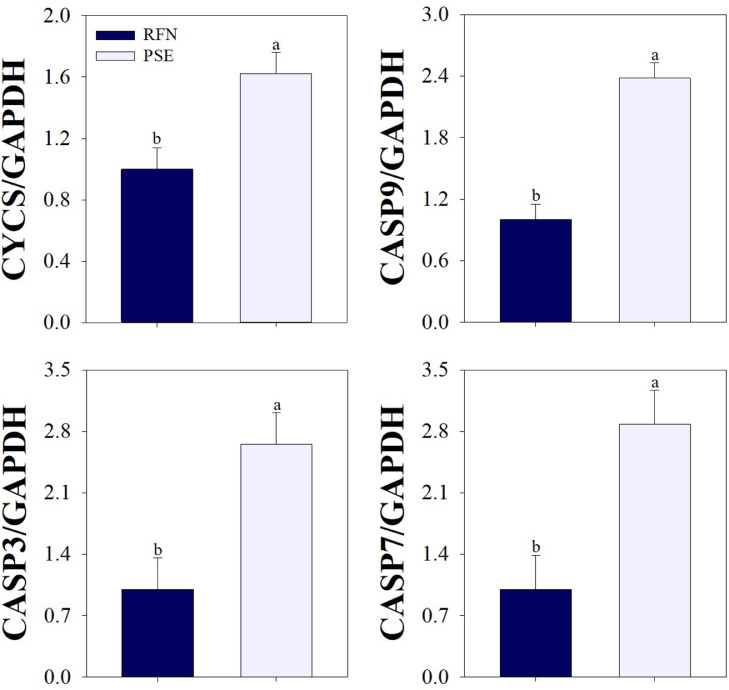
During conversion of muscle to meat after the exsanguination, the muscle metabolism changes. Aerobic metabolism is rapidly stopped and glycolysis becomes the primary form of metabolism as oxygen is depleted, and these conditions cause all muscle fibers to engage in cell death (Choi and Kim, 2009). Meanwhile, adenosine triphosphate (ATP) continues to be synthesized and utilized to sustain cellular homeostasis during postmortem glycolysis (Matarneh et al., 2021). Glycolytic metabolism during the postmortem period is causally linked to the caspase systems, as caspase proteases require ATP for activation in apoptotic muscle cells (Pradelli et al., 2014). Thus, alterations of ATP homeostasis in the postmortem muscle are affected by the extent of glycolysis and caspase activity, and associated with the accumulation of lactate and hydrogen ions, resulting in decreasing muscle pH at the early postmortem (Matarneh et al., 2021). Gorska and Wojtysiak (2018) reported that the caspase 3 activity at 30 min postmortem was significantly higher in the turkey breast muscles with lower muscle pH and higher lightness compared to muscles with higher muscle pH and lower lightness, as caspase 3 activity is accelerated by glycolytic metabolism (Secinaro et al., 2018; Ma et al., 2022). Thus, greater apoptotic and glycolytic potentials may increase the incidence of poultry meat under abnormal condition due to higher proteolytic activity and muscle acidification (Carvalho et al., 2014). However, there have been few studies on the relationship between the levels of other apoptosis-related molecules and chicken meat quality is limited. In the current study, the levels of various apoptosis-related factors were confirmed in chicken PM muscle at the early postmortem, and significant differences were observed between the quality classes (Figure 1). Cytochrome c, a key trigger of the apoptotic pathway, was highly expressed at 15 min postmortem in breast fillets showing PSE condition than in breast fillets showing RFN condition (1.62 vs. 1.00, P < 0.05); as a result, the RFN group had a lower level of initiator caspase 9 compared to the PSE group (1.00 vs. 2.38, P < 0.001). Additionally, muscle samples from the PSE group exhibited a higher level of caspase 3, which is the major caspase contributing to proteolysis in apoptotic cells, compared to those of the RFN group (2.65 vs. 1.00, P < 0.01). Caspase 7, which has a similar specificity profile as caspase 3, was present in lower levels in the RFN group than in the PSE group (1.00 vs. 2.88, P < 0.01). Thus, considering the levels of apoptotic factors in this study, the PSE condition chicken showed greater apoptotic potentials at the early postmortem period compared to the RFN condition chicken.
Figure 1.
Quantitative RT-PCR for expression of cytochrome c (CYCS), caspase 9 (CASP9), caspase 3 (CASP3), and caspase 7 (CASP7) in the broiler pectoralis major muscle for the quality classes. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used to control for normalization. Bars indicate the standard error of the mean. a-bDifferent letters denote significant differences (P < 0.05). Abbreviations: RFN, reddish, firm, and non-exudative; PSE, pale, soft, and exudative.
Taken together, these results indicate that the variation of meat quality in chicken PM muscles is associated with the rate of caspase-mediated apoptosis and glycolysis, which in turn are mediated by the levels of apoptosis-related factors at the early postmortem, and higher apoptotic potentials may lead to the PSE condition chicken breasts.
ACKNOWLEDGEMENTS
This research was supported by the National Research Foundation of Korea (NRF-2020R1A2C1010756).
DISCLOSURES
The authors did not provide a conflict of interest statement.
REFERENCES
- American Meat Science Association . 2nd ed. American Meat Science Association; Champaign, IL: 2015. Research Guideline for Cookery, Sensory Evaluation, and Instrumental Tenderness Measurements of Fresh Meat. [Google Scholar]
- Carvalho H., Soares A.L., Honorato D.C.B., Guarnieri P.D., Pedrao M.R., Paiao F.G., Oba A., Ida E.I., Shimokomaki M. The incidence of pale, soft, and exudative (PSE) turkey meat at a Brazilian commercial plant and the functional properties in its meat product. LWT. 2014;59:883–888. [Google Scholar]
- Choi Y.M., Kim B.C. Muscle fiber characteristics, myofibrillar protein isoforms, and meat quality. Livest. Sci. 2009;122:105–118. [Google Scholar]
- Commission Internationale de l'Eclairage. 1978. Recommentations on uniform color spaces, color differences equations, psychometric colour terms. CIE Publication (15 (E-1.3.3) 1971/(TO-1.3) (Suppl. 15). Bureau Central del la CIE, Paris, France.
- Concannon G.G., Gorman A.M., Samali A. On the role of Hsp27 in regulating apoptosis. Apoptosis. 2003;8:61–70. doi: 10.1023/a:1021601103096. [DOI] [PubMed] [Google Scholar]
- Cramer T., Penick M.L., Waddell J.N., Bidwell C.A., Kim Y.H.B. A new insight into meat toughness of callipyge lamb loins - the relevance of anti-apoptotic systems to decreased proteolysis. Meat Sci. 2018;140:66–71. doi: 10.1016/j.meatsci.2018.03.002. [DOI] [PubMed] [Google Scholar]
- Droval A.A., Benassi V.T., Rossa A., Prudencio S.H., Paiao F.G., Shimokomaki M. Consumer attitudes and preferences regarding pale, soft, and exudative broiler breast meat. J. Appl. Poult. Res. 2012;21:502–507. [Google Scholar]
- Garcia-Macia M., Sierra V., Palanca A., Vega-Naredo I., De Gonzalo-Calvo D., Rodriguez-Gonalez S., Olivan M., Coto-Montes A. Autophagy during beef aging. Autophagy. 2014;10:137–143. doi: 10.4161/auto.26659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez J.M. Poultry and pork muscle defects and meat quality – consequences, causes, and management. J. Anim. Sci. 2020;98:skaa63. doi: 10.1093/jas/skaa263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorska M., Wojtysiak D. Comparison of plasma corticosterone concentration, muscle fibre diameter, and apoptotic markers between normal and pale, soft, exudative (PSE) turkey breast muscles. Med. Weter. 2018;74:387–391. [Google Scholar]
- Grashorn M.A. Research into poultry meat quality. Br. Poult. Sci. 2010;51:60–67. doi: 10.1080/00071668.2010.506761. [DOI] [PubMed] [Google Scholar]
- Guo B., Zhang W., Tume R.K., Hudson N.J., Huang F., Yin Y., Zhou G. Disorder of endoplasmic reticulum calcium channel components is associated with the increased apoptotic potential in pale, soft, exudative pork. Meat Sci. 2016;115:34–40. doi: 10.1016/j.meatsci.2016.01.003. [DOI] [PubMed] [Google Scholar]
- Honikel K.O. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 1998;49:447–457. doi: 10.1016/s0309-1740(98)00034-5. [DOI] [PubMed] [Google Scholar]
- Karunanayaka D.S., Jayasena D.D., Jo C. Prevalence of pale, soft, and exudative (PSE) condition in chicken meat used for commercial meat processing and its effect on roasted chicken breast. J. Anim. Sci. Technol. 2016;58:27. doi: 10.1186/s40781-016-0110-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp C.M., Parr T. Advances in apoptotic mediated proteolysis in meat tenderization. Meat Sci. 2012;92:252–259. doi: 10.1016/j.meatsci.2012.03.013. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Lee Y.S., Erf G.F., Meullenet J.F.C., McKee S.R., Owens C.M. Consumer acceptance of visual appearance of broiler breast meat with varying degrees of white striping. Poult. Sci. 2012;91:1240–1247. doi: 10.3382/ps.2011-01947. [DOI] [PubMed] [Google Scholar]
- Lee B., Kim J.Y., Choi Y.M. Associations of apoptotic and anti-apoptotic factors with beef quality, histochemical characteristics, and palatability of Hanwoo longissimus thoracis muscle. Animals. 2022;12:467. doi: 10.3390/ani12040467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livisay S.A., Xiong Y.L., Moody W.G. Proteolytic activity and calcium effect in dark-firm-dry and pale-soft-exudative meat. LWT. 1996;29:123–128. [Google Scholar]
- Lomiwes D., Farouk M.M., Wiklund E., Young O.A. Small heat shock proteins and their role in meat tenderness: a review. Meat Sci. 2014;96:26–40. doi: 10.1016/j.meatsci.2013.06.008. [DOI] [PubMed] [Google Scholar]
- Ma J., Yu Q., Han L. The effect of postmortem pH decline rate on caspase-3 activation and tenderness of bovine skeletal muscle during aging. J. Food Biochem. 2022;00:e14215. doi: 10.1111/jfbc.14215. [DOI] [PubMed] [Google Scholar]
- Matarneh S.K., Silva S.L., Gerrard D.E. New insights in muscle biology that alter meat quality. Annu. Rev. Anim. Biosci. 2021;9:355–377. doi: 10.1146/annurev-animal-021419-083902. [DOI] [PubMed] [Google Scholar]
- Nam Y.J., Choi Y.M., Jeong D.W., Kim B.C. A comparison of postmortem meat quality and consumer sensory characteristic evaluations, according to porcine quality classification. Food Sci. Biotechnol. 2009;18:307–311. [Google Scholar]
- Owens C.M., Hirschler E.M., Martine-Dawson R., Sams A.R. The characterization and incidence of pale, soft, exudative turkey meat in a commercial plant. Poult. Sci. 2000;79:553–558. doi: 10.1093/ps/79.4.553. [DOI] [PubMed] [Google Scholar]
- Pradelli L.A., Villa E., Zunino B., Marchetti S., Ricci J.E. Glucose metabolism is inhibited by caspases upon the induction of apoptosis. Cell Death Dis. 2014;5:e1406. doi: 10.1038/cddis.2014.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secinaro M.A., Fortner K.A., Dienz O., Logan A., Murphy M.P., Anathy V., Boyson J.E., Budd R.C. Glycolysis promotes caspase-3 activation in lipid rafts in T cells. Cell Death Dis. 2018;9:62. doi: 10.1038/s41419-017-0099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing T., Gao F., Tume R.K., Zhou G., Xu X. Stress effects on meat quality: a mechanistic perspective. Compr. Rev. Food Sci. Saf. 2018;18:380–401. doi: 10.1111/1541-4337.12417. [DOI] [PubMed] [Google Scholar]
- Zequan X., Yonggang S., Guangjuan L., Shijun X., Li Z., Mingrui Z., Yanli X., Zirong W. Proteomics analysis as an approach to understand the formation of pale, soft, and exudative (PSE) pork. Meat Sci. 2021;177 doi: 10.1016/j.meatsci.2020.108353. [DOI] [PubMed] [Google Scholar]
- Zhu X., Xu X., Min H., Zhou G. Occurrence and characterization of pale, soft, exudative-like broiler muscle commercially produced in China. J. Integr. Agric. 2012;11:1384–1390. [Google Scholar]