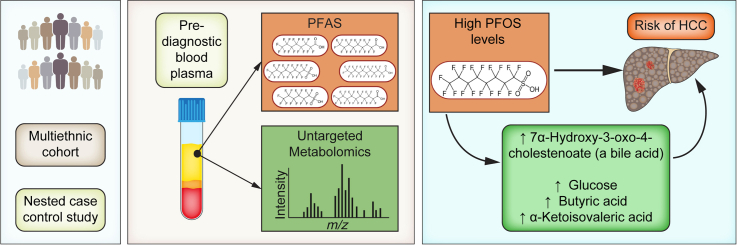
Abstract
Background & Aims
Exposure to poly- and perfluoroalkyl substances (PFAS), a class of persistent organic pollutants, is ubiquitous. Animal studies suggest that PFAS may increase risk of fatty liver and hepatocellular carcinoma (HCC) via impacts on hepatic lipid, amino acid, and glucose metabolism, but human data is lacking. We examined associations between PFAS exposure, altered metabolic pathways, and risk of non-viral HCC.
Methods
In this nested case-control study, pre-diagnostic plasma PFAS and metabolomics were measured in 50 incident HCC cases and 50 individually matched controls from the Multiethnic Cohort (MEC) study. Cases/controls were matched by age, sex, race, and study area. PFAS exposure and risk of HCC were examined using conditional logistic regression. A metabolome-wide association study and pathway enrichment analysis was performed for PFAS exposure and HCC risk, and key metabolites/metabolic pathways were identified using a meet in the middle approach.
Results
High perfluorooctane sulfonic acid (PFOS) levels (90th percentile from NHANES; >55 μg/L) were associated with 4.5-fold increased risk of HCC (odds ratio 4.5, 95% CI 1.2-16.0). Pathway enrichment analysis showed that PFOS exposure was associated with alterations in amino acid and glycan biosynthesis pathways, which were also associated with HCC risk. We identified 4 metabolites linking PFOS exposure with HCC, including glucose, butyric acid (a short-chain fatty acid), α-ketoisovaleric acid (a branched-chain α-keto acid), and 7α-hydroxy-3-oxo-4-cholestenoate (a bile acid), each of which was positively associated with PFOS exposure and risk of HCC.
Conclusion
This proof-of-concept analysis shows that exposure to high PFOS levels was associated with increased risk of non-viral HCC, likely via alterations in glucose, amino acid, and bile acid metabolism. Larger studies are needed to confirm these findings.
Lay summary
Per- and polyfluoroalkyl substances (PFAS), often referred to as “forever chemicals” because they are difficult to break down and stay in the human body for years, are extremely common and can cause liver damage. In a first of its kind study, we found that exposure to high levels of perfluorooctanesulfonic acid, one of the most common PFAS chemicals, was linked to increased risk of hepatocellular carcinoma in humans. Hepatocellular carcinoma is difficult to treat and is one of the most common forms of liver cancer, and these findings may provide new avenues for helping to prevent this disease.
Keywords: Chemical exposure, exposome, perfluorinated alkyl substance, hepatocellular carcinoma, metabolome, bile acid, metabolic pathway
Abbreviations: HCC, hepatocellular carcinoma; HILIC, hydrophilic interaction chromatography; HRMS, high-resolution mass spectrometry; LC, liquid chromatography; MEC, Multiethnic Cohort; MWAS, metabolome-wide association; NAFLD, non-alcoholic fatty liver disease; PFAS, perfluoroalkyl substances; PFDA, perfluorodecanoate; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoate; PFOA, perfluorooctanoate; PFOS, perfluorooctane sulfonate; PFUnDA, perfluoroundecanoic acid; RP, reverse phase; SEER, Surveillance, Epidemiology, and End Results
Graphical abstract
Highlights
-
•
Associations of PFAS and risk of hepatocellular carcinoma were tested in humans.
-
•
PFAS and untargeted metabolomics were assessed in pre-diagnostic samples.
-
•
Exposure to high PFOS levels was linked to increased hepatocellular carcinoma risk.
-
•
The likely mechanisms were via alterations in glucose, amino acid, and bile acid metabolism.
Introduction
Liver cancer was the 6th most common cancer and the 3rd leading cause of cancer death worldwide in 2020.1 In the United States, liver cancer incidence rates have more than tripled since 1980.2 In 2021, liver cancer was the 5th and 7th leading cause of cancer deaths in the US among men and women, respectively.2 Hepatocellular carcinoma (HCC) is the most common form of liver cancer, accounting for 85% of cases.1 With a 5-year survival rate of less than 20%, HCC is one of the most lethal cancers.3 While global vaccination efforts and effective antiviral treatments have helped to decrease the incidence of HCC related to HBV/HCV infection, the incidence of non-alcoholic fatty liver disease (NAFLD)-related HCC is increasing, and NAFLD is projected to become the predominate cause of HCC in many countries by 2030.4 In the USA, the incidence of HCC has decreased since 2011, driven by decreasing HBV and HCV rates.5 However, the incidence of HCC in individuals born after 1985 is beginning to match that of baby boomers, which is likely driven by increasing rates of metabolic syndrome, NAFLD, and non-alcoholic steatohepatitis.5 Mirroring the increasing global rates of obesity, diabetes, and NAFLD, the incidence of HCC is expected to increase further, and the health impact of this increase is compounded by its dismal prognosis.4 Thus, there is an urgent need to identify the risk factors for non-viral-related HCC in order to improve the identification and surveillance of high-risk populations and ultimately reduce the burden of this disease.
Accumulating evidence suggests that poly- and perfluoroalkyl substances (PFAS), a class of pervasive endocrine disruptors, are hepatotoxic.6,7 PFAS are persistent and ubiquitous chemicals which have been widely used in industry and consumer products for more than 60 years.[8], [9], [10], [11] In humans, biological half-lives of long-carbon chain PFAS, such as perfluorooctane sulfonate (PFOS), perfluorooctanoate (PFOA), and perfluorohexane sulfonate (PFHxS), range between 3 and 7 years.12,13 As such, human biomonitoring studies report widespread exposure with detection rates of over 98% in the blood of US adults.[14], [16], [17], [43]
PFAS partition preferentially to the liver, where they have hepatotoxic and metabolism disrupting effects.6,18,19 Rodents exposed to PFAS, even at low levels, developed liver enlargement, hepatocellular hypertrophy, elevated liver enzymes, and hepatic steatosis.[20], [21], [22], [23], [24], [25], [26], [27] In rainbow trout, PFAS increase the incidence of hepatocellular adenomas and tumors.28 PFAS also alter hepatic lipid, amino acid, and carbohydrate metabolism, which is hypothesized to be a mechanism linking PFAS exposure with hepatotoxic endpoints.29
Despite compelling experimental evidence, investigation of hepatotoxic effects in population studies is limited. Several human studies have examined the cross sectional associations of PFAS exposure with liver enzymes, including of alanine aminotransferase (ALT), a marker of liver function.7 For example, in the NHANES study, higher blood PFOS and PFOA concentrations were associated with increased levels of ALT in the general adult population in the USA.30,31 In adults, PFAS have also been linked to alterations in cytokeratin 18, a marker for liver apoptosis.32,33 Recent evidence also suggests that PFAS are associated with increased risk of NAFLD, diagnosed using liver MRI or liver biopsy, in both children and adults.34,35 Using untargeted metabolomics, these studies have also shown that alterations in lipid and amino acid metabolism link PFAS exposure and risk of NAFLD.34,35 However, research examining PFAS exposure and HCC in humans is non-existent.
Therefore, we performed the first population-based study to examine the association between PFAS exposure and risk of non-viral HCC in a prospective cohort. We hypothesize that PFAS exposure increases risk of HCC via effects on lipid, amino acid, and carbohydrate metabolism. In a well characterized, ethnically diverse cancer cohort, we examined whether pre-diagnostic levels of PFAS were associated with risk of HCC. We also examined the underlying metabolic pathways linking PFAS exposure with risk of HCC using untargeted metabolomics.
Patients and methods
Study population
This study included 50 incident non-viral HCC cases and 50 controls from the Multiethnic Cohort (MEC) study. Cases and controls were matched by age, sex, race/ethnicity, and study area. MEC is a unique, ethnically diverse prospective cohort of >200,000 African Americans, Latinos, Native Hawaiians, Japanese Americans, and Whites followed since the early 1990s in California and Hawaii.36 Incident cancers including HCC over >20-years of follow-up were identified by the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program. Comprehensive epidemiologic risk factor data from questionnaires, health condition data from Medicare claims, and California hospital discharge and mortality information from national mortality databases were also collected. The underlying etiology of HCC was identified using a combination of viral hepatitis testing and Medicare claims and classified into viral (hepatitis B or hepatitis C related), NAFLD, alcohol related, and others as previously described.[37], [38], [39] The breakdown of etiology in HCC cases was 33 (66%) NAFLD, 4 (8%) alcohol related, and 13 (26%) cryptogenic cirrhosis. HCC cases with known viral etiology were excluded from this study. Controls were free from diagnosed liver disease, including metabolic dysfunction-associated fatty liver disease. This study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki, and ethics approval for this study was provided by the Institutional Review Boards at the University of Southern California (HS-17-00714) and the University of Hawaii (CHS 9575).
Plasma PFAS
Plasma PFAS concentrations in fasting pre-diagnostic samples were determined using liquid chromatography with high-resolution mass spectrometry (LC-HRMS). Plasma samples were prepared by adding 2.5 μl of an internal standard solution containing 30 different 13C-labeled PFAS compounds to 40 μl of plasma to obtain final 13C-labeled PFAS concentrations of 5 μg/L. This was followed by adding 80 μl of ice-cold acetonitrile to precipitate proteins. Treated samples were vortex mixed, equilibrated on ice for 30 mins, and then centrifuged for 15 min at 18,000×g, at 4 °C. The resulting supernatant was diluted 2:1 with LC-MS grade water and placed in refrigerated autosamplers. Extracts were analyzed using a Vanquish Binary Pump F Ultra Performance Liquid Chromatography (Thermo Fisher Scientific, Rockford, IL, USA), connected to a Q-Exactive HF-X Orbitrap MS system (Thermo Fisher Scientific, Rockford, IL, USA). PFAS analysis was completed using reverse phase chromatography with negative electrospray ionization. Details on method parameters and operation of the LC-HRMS are provided in the following section.
Following analysis of all study samples, accurate mass m/z peaks corresponding to PFAS and matched 13C internal standard peaks were extracted and integrated using a mass error threshold of 5 ppm in TraceFinder 5.1. PFAS were quantified by comparing the ratio of the analyte peak and corresponding internal standard to a 6-point calibration curve prepared in charcoal stripped plasma. Each batch of samples included replicate analysis of NIST 1957 and 1958, as well as method and instrumental blanks. Analyte recovery for major PFAS exceed 90%, with detection limits in the low pg/ml range and coefficient of variations less than 15%. Method accuracy was evaluated by comparison to NIST, and through participation in the CTQ AMAP Ring Test for Persistent Organic Pollutants in Human Serum. The limit of detection for plasma PFAS were 0.43 μg/L for PFOS, 0.01 μg/L for PFOA, 0.01 μg/L for PFHxS, 0.01 μg/L for perfluorononanoate (PFNA), 0.05 μg/L for perfluoroundecanoic acid (PFUnDA), and 0.01 μg/L for perfluorodecanoate (PFDA).
Untargeted plasma metabolomics
The plasma metabolome was determined in fasting pre-diagnostic samples using LC-HRMS. To maximize detection of endogenous metabolites, we expanded established methods40 to enable analysis using a dual column and dual polarity approach that included analyses with both reverse phase (RP) and hydrophilic interaction chromatography (HILIC). Untargeted analysis was accomplished using a Vanquish Duo liquid chromatography (LC) system (Thermo Fisher Scientific, Rockford, IL, USA) equipped with dual pumps and columns with independent flow paths interfaced to a Q-Exactive HF-X Orbitrap MS system (Thermo Fisher Scientific, Rockford, IL, USA). The dual LC system was configured to enable parallel analytical separation and flushing using columns with the same stationary phase and mobile phases optimized for positive or negative ionization. To enable analysis using all 4 analytical configurations, all study samples were first analyzed using RP, after which the system was switched to HILIC. Details on sample preparation and LC parameters for each mode are provided below.
Plasma samples were analyzed in batches of 70 study samples with 10 pooled quality assurance/quality control samples. Prior to analysis, samples were thawed at 4 °C and plasma (40 μl for RP, and 30 μl for HILIC) was extracted with ice-cold acetonitrile (80 μl for RP, and 90 μl for HILIC) containing 13C-labeled PFAS and internal standards. Treated samples were vortexed for 10 s, equilibrated on ice for 30 min, and then centrifuged for 15 min at 18,000×g, at 4 °C. The supernatant (40 μl for RP, and 30 μl for HILIC) was added to 250 μl LC vials containing water (80 μl for RP) or 1:1 (v/v) water/acetonitrile (90 μl for HILIC analysis) and placed in a refrigerated autosampler. Samples were analyzed with mobile phases optimized for positive or negative ionization. RP analyte separation was accomplished by C18 (TARGA C18 5 μm 50 × 2.1 mm, Higgins Analytical, Inc, Mountain View, CA, USA) for both positive mode and negative modes. Mobile phases for RP included water and acetonitrile containing 0.1% formic acid for positive mode and 10 mM ammonium acetate and 95/5 (v/v) acetonitrile/water (A) for negative mode. For HILIC, positive ESI analysis was completed using a SeQuant ZIC-HILIC column (3.5 μm, 200 A 4.6 × 50 mm; MilliporeSigma, Burlington, MA, USA), and mobile phase including 0.1% formic acid in water and acetonitrile. For negative mode, a Waters XBridge Amide column (3.5 μm 3.0 × 50 mm, Waters Corporation, Milford, MA, USA) was used with mobile phases consisting of 10 mM ammonium acetate in water adjusted to pH 9.60 with ammonium hydroxide and 95/5 (v/v) acetonitrile. Total runtime for each analysis was 7.5 min. Mass spectral data was collected over the scan range 85–1,275 at 120,000 (FWHM) resolution. Spray voltages were maintained at 3.5 kV and 4.0 kV for positive and negative mode, respectively. Sheath and auxiliary gas temperatures were 300 °C and 250 °C, respectively, while sheath and auxiliary gas flow rates were set to 45 and 25 (arbitrary units). To minimize analyte fragmentation at the source, the RF funnel level was set at 35. In addition to full scan data collection, a subset of samples were selected for data-dependent tandem mass spectrometry (MSMS), which collected MSMS spectra for the top 20 most abundant peaks at MS2 resolution of 15,000 using normalized collision energies of 20, 40, and 60. Metabolite peaks were extracted separately for each chromatography and polarity pair using apLCMS and xMSanalyzer,40,41 and metabolite features were uniquely identified based upon detected m/z, retention time and peak intensity. Following data extraction, LC-HRMS features were corrected for batch effects using the ComBat algorithm.42 Features were excluded from the analysis if they were detected in less than 50% of samples or if the quality control samples after batch correction were greater than 0.3, resulting in 4,360 metabolites for analysis.
Statistical analysis
Differences in participant characteristics between cases and controls were tested using t tests for continuous variables and chi-squared tests for categorical variables. Differences in PFAS levels by education status were tested using linear regression, adjusting for case-control status. Associations between PFAS levels and HCC in the matched case-control set were assessed using conditional logistic regression. Adjusted odds ratios (ORs) and 95% CIs were calculated for each case group compared to the reference group. To examine the potential non-linear association between PFAS exposure and risk of HCC, we performed a preliminary analysis modeling PFAS concentrations using smoothing splines within the conditional logistic regression framework. Based upon visual inspection of these models, we observed a potentially non-linear trend between PFOS and risk of HCC. These associations were driven by high levels of PFOS, so for the main analysis, we categorized all PFAS as high vs. low concentration based on the 90th percentile of exposure in NHANES 1999-2000, the earliest date that PFAS monitoring was performed in NHANES.43 In our data, this corresponded to the 85th percentile for PFOS, and so to maintain consistency, the 85th percentile was used to define high vs. low exposure for all other PFAS.
Metabolome-wide association study
To examine the metabolic pathways linking PFAS exposure and risk of HCC, we performed 2 metabolome-wide association (MWAS) studies. For the first analysis, we performed an MWAS examining the associations between PFAS levels and individual metabolites using linear regression. This analysis was performed independently for each PFAS which was found to be significantly associated with risk of HCC. In these models, metabolites were the dependent variable and PFAS concentrations were the independent variable. In order to account for oversampling due to the matching case-control design, models were adjusted for the matching variables, including case/control status, age at blood draw, sex, race/ethnicity, and study area. For the second analysis, we performed an MWAS examining the associations between individual metabolites and HCC status using conditional logistic regression. Prior to analysis, all metabolites were scaled to have a mean of zero and a standard deviation of one.
Metabolite annotation and pathway enrichment analysis
Based on the results from the MWAS analysis, we performed a pathway enrichment analysis using both the mummichog algorithm44 and the gene set enrichment algorithm,45 implemented in version 2 of the peaks to paths module from MetaboAnalyst version 5.0.46 The enrichment analysis was performed independently using the results from both the PFAS to metabolite MWAS as well as the metabolite to HCC MWAS. For this analysis, all LC-MS features from both the positive and negative ion modes were included. The mass tolerance was set to 5.0 ppm, the p value for significant features was set to 0.05, and we included retention time in the analysis to improve metabolite annotation. Pathways which were independently associated with both PFAS exposure and risk of HCC were selected for additional analysis. Metabolites from overlapping significant pathways were identified using a meet in the middle approach.47
Sensitivity analysis
In order to examine the impact of different modeling assumptions on our main findings, we performed the following sensitivity analyses. First, in order to examine the effect of modeling PFAS exposure as a categorical variable, we performed a supplemental analysis looking at the association between PFAS exposure and risk of HCC using conditional logistic regression, and modeling PFAS exposure as a continuous variable scaled to a mean of zero and a standard deviation of one. Second, since conditional logistic regression is prone to bias in small sample sizes,48 we reran our main analysis using ordinary logistic regression, and controlling for the matching variables (age, sex, race/ethnicity, and study area). Third, due to uncertainties about whether obesity and diabetes mellitus may be on the causal pathway linking PFAS exposure with risk of HCC, we performed a supplemental analysis by rerunning the main analysis with either baseline BMI or baseline diabetes mellitus status as covariates.
Results
Characteristics of the study population
Participant characteristics of HCC cases and controls can be found in Table 1. Because of the matching design, age at blood collection, sex, race/ethnicity, and study area were not different between cases and controls. Compared to controls, HCC cases were more likely to be overweight or obese (p = 0.003) and had higher prevalence of diabetes mellitus (p = 0.01). Smoking status and alcohol intake were similar in cases and controls.
Table 1.
Characteristics of HCC cases and controls from the MEC.
| HCC cases (n = 50) | Controls (n = 50) | p value | |
|---|---|---|---|
| Age at blood collection, mean ± SD | 69.7 ± 7.37 | 69.2 ± 7.42 | 0.76 |
| Years between blood collection and diagnosis, Median (range) | 7.2 (0.9, 16.4) | — | — |
| Sex | — | ||
| Male | 62% | 62% | |
| Female | 38% | 38% | |
| Race/ethnicity | — | ||
| White | 18% | 18% | |
| African American | 6% | 6% | |
| Japanese American | 38% | 38% | |
| Latino | 24% | 24% | |
| Native Hawaiian | 14% | 14% | |
| Study area | — | ||
| California | 36% | 36% | |
| Hawaii | 64% | 64% | |
| Education | 0.87 | ||
| Some high school | 18% | 16% | |
| Graduated high school | 20% | 24% | |
| Vocational/some college | 32% | 36% | |
| Graduated college/graduate/professional school | 30% | 24% | |
| BMI (kg/m2) | 0.003 | ||
| <25 | 18% | 38% | |
| 25-30 | 36% | 46% | |
| ≥30 | 46% | 16% | |
| Alcohol intake (g/day) | 0.58 | ||
| 0 | 48% | 58% | |
| <12 | 30% | 26% | |
| ≥12 | 22% | 16% | |
| Smoking status | 0.35 | ||
| Never | 26.0% | 38.0% | |
| Former | 62.0% | 48.0% | |
| Current | 12.0% | 14.0% | |
| Diabetes mellitus | 0.001 | ||
| No | 62% | 92% | |
| Yes | 38% | 8% |
Cases and controls matched on age, sex, race/ethnicity, and study area. Differences in participant characteristics between cases and controls were tested using t tests for continuous variables and chi-squared tests for categorical variables.
HCC, hepatocellular carcinoma; MEC, Multiethnic Cohort.
Geometric mean and selected percentiles of serum concentrations of legacy PFAS (in μg/L) in HCC cases and controls can be found in Table 2. PFOS, PFHxS, PFOS, PFDA, PFNA were detected in all participants. PFUnDA was detected in 29% of all participants. Plasma levels of all PFAS were similar to those reported in NHANES survey years 1999–2000 and 2003–2004.43 PFAS ranged from uncorrelated to highly correlated, with Spearman correlation coefficients ranging from −0.03 to 0.87 (Fig. S1). There were no significant differences in PFAS levels by education status, an indicator of socioeconomic status (p values ranging from 0.26–0.99).
Table 2.
Geometric mean or 75th percentile of serum concentrations of PFAS (in μg/L) in HCC cases and controls.
| PFAS | Controls (n = 50) | Cases (n = 50) |
|---|---|---|
| PFOS, GM (GSD) | 29.2 (1.95) | 29.2 (2.37) |
| PFOA, GM (GSD) | 4.78 (1.89) | 4.21 (2.13) |
| PFHxS, GM (GSD) | 2.07 (2.25) | 1.84 (3.11) |
| PFNA, GM (GSD) | 0.827 (1.85) | 0.844 (2.05) |
| PFDA, GM (GSD) | 0.278 (2.84) | 0.27 (2.97) |
| PFUnDA, 75th percentile | ∗ | 0.89 |
GM, geometric mean; GSD, geometric standard deviation; HCC, hepatocellular carcinoma; PFAS, perfluoroalkyl substances; PFDA, perfluorodecanoate; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoate; PFOA, perfluorooctanoate; PFOS, perfluorooctane sulfonate; PFUnDA, perfluoroundecanoic acid.
Geometric mean not calculated for PFAS with >40% of samples below limit of detection.
Plasma PFAS were associated with risk of HCC
We observed a positive association between pre-diagnostic plasma PFAS concentrations and risk of HCC (Table 3). The strongest association was between PFOS and HCC. Plasma PFOS concentrations >54.9 μg/L were associated with 4.5 times higher odds of HCC (95% CI 1.20–16.00; p = 0.02). This threshold corresponded to the 90th percentile of exposure in NHANES 1999-2000.43 PFUnDA levels greater than 1.22 μg/L were associated with 2.2 times higher odds of HCC, although this association did not reach statistical significance (95% CI 0.92–5.50; p = 0.07).
Table 3.
Odds ratios and 95% CIs evaluating pre-diagnostic serum concentrations of PFAS and risk of HCC in the MEC.
| PFAS | μg/L | Odds ratio (95% CI) | p value |
|---|---|---|---|
| PFOS | >54.9 | 4.50 (1.20, 16.00) | 0.02 |
| PFHxS | >4.3 | 1.10 (0.56, 2.30) | 0.72 |
| PFOA | >8.6 | 1.20 (0.52, 2.80) | 0.67 |
| PFDA | >0.8 | 0.80 (0.31, 2.00) | 0.64 |
| PFNA | >1.5 | 1.20 (0.49, 3.20) | 0.64 |
| PFUdA | >1.2 | 2.20 (0.92, 5.50) | 0.07 |
Effect estimates were calculated using conditional logistic regression to account for the matched case-control study design. HCC, hepatocellular carcinoma; MEC, Multiethnic Cohort; PFAS, perfluoroalkyl substances; PFDA, perfluorodecanoate; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoate; PFOA, perfluorooctanoate; PFOS, perfluorooctane sulfonate; PFUnDA, perfluoroundecanoic acid.
The plasma metabolome was linked to PFAS exposure and to risk of HCC
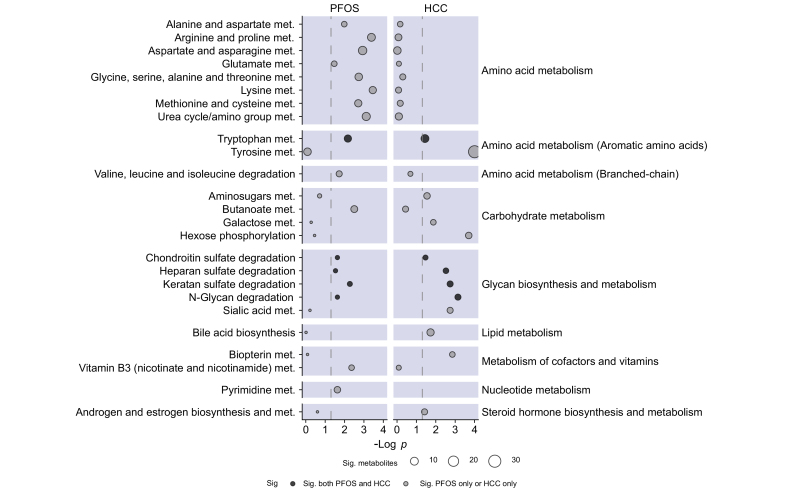
Based on the results of the exposure outcome analysis, we examined the associations between high levels of PFOS and the plasma metabolome using an MWAS. Of the 4,361 metabolomics features included in the MWAS, 433 (9%) were associated with high levels of PFOS exposure at a p <0.05, and 6 metabolites were associated with PFOS exposure at a false discovery rate-corrected p <0.2. Functional pathway analysis of the MWAS results identified significant enrichment of 18 metabolic pathways (Fig. 1). These pathways were primarily related to the metabolism of amino acids, the metabolism of carbohydrates, and glycan biosynthesis and metabolism (Fig. 1).
Fig. 1.
Metabolic pathways associated with exposure to high levels of PFOS (on left) or HCC (on right) in 50 cases and 50 controls from the MEC cohort.
Metabolic pathways are grouped into super pathways as indicated on the right of the plot. Metabolic pathway enrichment was performed using MetaboAnalyst version 5.0. Point size is proportional to the number of significant metabolites associated with each pathway. HCC, hepatocellular carcinoma; MEC, multiethnic cohort; PFOS, perfluorooctane sulfonic acid.
For HCC, 499 (11%) of LC-MS features were associated with HCC at a p <0.05, and 109 LC-MS features were associated with HCC at a false discovery rate-corrected p <0.2. Functional pathway analysis of the MWAS results identified significant enrichment of 13 metabolic pathways (Fig. 1). These pathways were primarily related to carbohydrate metabolism, glycan biosynthesis and metabolism, and aromatic amino acid metabolism (Fig. 1).
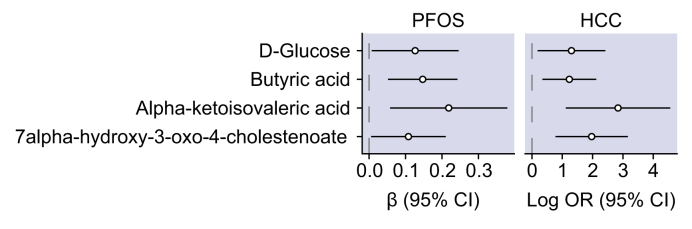
Five metabolic pathways were enriched for both PFOS and HCC. These included tryptophan metabolism, keratin sulfate, heparin sulfate, chondroitin sulfate, and N-glycan degradation. Additionally, 4 metabolites were positively associated with both PFOS exposure and risk of HCC (Fig. 2). These included glucose, butyric acid (a short-chain fatty acid), α-ketoisovaleric acid (a branched-chain α-keto acid), and 7α-hydroxy-3-oxo-4-cholestenoate (a bile acid).
Fig. 2.
Effect estimates for metabolites associated with high levels of PFOS and risk of HCC in 50 HCC cases and 50 controls from the MEC.
For PFOS exposure, effect estimates were calculated using linear regression adjusting for age, sex, race/ethnicity, and study site, and indicate the mean difference and 95% CI in the log2-transformed metabolite intensity between high (≥85th percentile) vs. low levels of PFOS exposure. Effect estimates for HCC were calculated using conditional logistic regression, and indicate the OR and 95% CI for the risk of HCC per doubling of pre-diagnostic metabolite levels. HCC, hepatocellular carcinoma; MEC, multiethnic cohort; OR, odds ratio; PFOS, perfluorooctane sulfonic acid.
Sensitivity analysis
When modeling PFAS exposure as continuous, we observed a similar positive association between PFOS and risk of HCC, although this association did not reach statistical significance (OR 1.2; 95% CI 0.91–1.60; p = 0.18; Table S1). When analyzing our results using ordinary logistic regression and controlling for matching variables, we observed a similar positive association between high levels of PFOS and HCC (OR 4.40; 95% CI 1.20–20.00; p = 0.03; Table S2). When rerunning the main analysis and accounting for baseline BMI, we observed a similar positive association between PFOS and risk of HCC, although this association did not reach statistical significance (OR 2.90; 95% CI 0.78–10.00; p = 0.11; Table S3). When rerunning the main analysis and accounting for baseline diabetes mellitus, we observed a similar positive association between PFOS and risk of HCC (OR 5.70; 95% CI 1.10–30.00; p = 0.04; Table S4).
Discussion
To our knowledge, this is the first prospective study to examine the association between PFAS exposure and risk of HCC. We found that exposure to high levels of PFOS was associated with increased risk of non-viral HCC. Using untargeted metabolomics, we identified several metabolites positively associated with PFOS exposure and risk of HCC. Our findings suggest that PFAS exposure may increase risk of HCC via alterations in glucose metabolism, bile acid metabolism, and metabolism of branched-chain amino acids.
Research examining the associations between PFAS exposure and liver cancer is limited. One existing study has examined the prospective association between PFOA and PFOS concentrations with incident cancer, including liver cancer, in the general Danish population between 1993-2006.49 Although this study reported null associations between PFAS levels and risk of liver cancer, a major limitation of this study was that liver cancer was not split by cancer type, and etiology of liver cancer was not available. Between 2004-2006, HCC only accounted for 43% of liver cancer diagnoses in Denmark50; it was not until after 2007 that the incidence of HCC dramatically increased, which paralleled increases in obesity, diabetes, and NAFLD.51 Therefore, non-viral HCC cases were likely a small portion of the total liver cancer cases, which may explain the null findings reported in this study.
Studies examining associations of PFAS exposure with risk of other cancers, such as kidney cancer, in the general population have found similar associations to those reported here. For example, in the only existing nested case-control study examining the prospective association between PFAS levels and risk of renal cell carcinoma, PFOS levels >50 μg/L were associated with a more than 2-fold increased risk of developing renal cell carcinoma (OR 2.51; 95% CI 1.28–4.92), and similar associations were reported for PFOA and PFHxS.52 These findings are notable due to the similarity in PFOS concentrations associated with risk of HCC in our study. Other epidemiological studies examining associations of PFAS and cancers have occurred in highly exposed cohorts such as the C8 project,53 which can limit the generalizability of findings to different populations. However, participants in the MEC had PFAS exposure levels similar to the general population. MEC participants were recruited in the early 2000s, and the geometric mean of PFOS in our study (29.2 μg/L) is close to the geometric mean for PFOS in the 1999-2000 NHANES survey (30.4 μg/L, 95% CI 27.1–33.9).
PFAS exposure has been linked to insulin dysregulation and type 2 diabetes,54 which is an emerging risk factor for HCC.38,55 In the current study, using untargeted metabolomics we found that PFAS exposure was associated with higher fasting glucose levels, and that higher glucose levels were associated with increased risk of HCC. Increased fasting glucose is a hallmark of type 2 diabetes, suggesting that the association between PFAS exposure and risk of HCC may be partially due to effects of PFAS on glucose and/or insulin dysregulation.
In addition to glucose, we identified 3 additional metabolites linking PFAS exposure and HCC. The compound most strongly associated with both PFOS exposure and with risk of HCC was α-ketoisovaleric acid, a branched-chain ketoacid and a byproduct in the catabolism of branched-chain amino acids.56 Recent evidence in rats suggests that branched-chain ketoacids are associated with alterations in insulin secretion, hepatic lipid metabolism, and hepatic steatosis.57 Previous studies have also shown that alterations in branched-chain amino acid metabolism may link early life PFAS exposure with risk of liver injury in children.58 The second compound which was associated with PFOS and HCC was 7α-hydroxy-3-oxo-4-cholestenoate, a bile acid synthesized in the liver. Bile acids are steroid acids with important roles in signaling energy availability and nutrient status.59 As such, they play important roles in metabolic diseases including obesity, diabetes, and non-alcoholic steatohepatitis.59 Recently, increased levels of primary bile acids have been linked to risk of HCC in humans.60 Animal and human studies also suggests that PFAS impact bile acid metabolism, increasing risk of liver injury.34,61 The third compound associated with both PFOS and HCC was butyric acid, a short-chain fatty acid which is primarily produced via intestinal fermentation. Short-chain fatty acids play an important role in energy sensing and metabolic regulation in a variety of tissues,62 and have been implicated in NAFLD.63 While previous research has identified the importance of branched-chain ketoacids, bile acids, and short-chain fatty acids in the etiology of metabolic disorders and liver disease, our results provide the first human evidence that PFAS-associated alterations in these compounds may increase the risk of HCC.
This study has several strengths. The identification of HCC was based on linkages with SEER-based cancer registries, which are over 95% complete.64 We included pre-diagnostic levels of PFAS and metabolomics, which decreases the possibility of reverse causality explaining the observed association between PFAS exposure and HCC. Additionally, the use of fasting plasma samples for determination of the untargeted metabolome removes the potential for confounding due to recent dietary intake, especially for metabolites related to glucose or lipid metabolism. The nested case-control study design allowed us to efficiently examine associations of PFAS exposure with HCC. We were able to individually match cases and controls on age, sex, race/ethnicity, and geographical region, removing potential confounding due to these known risk factors for HCC.37 Despite these strengths, there are some limitations worth noting. First, the sample size of 50 cases and 50 controls is a potential limitation. Because of the limited sample size, we were not able to examine effect modification by known risk factors such as race, sex, BMI, or diabetes status. Second, PFAS exposure can change across time, and we were only able to measure PFAS at a single timepoint. However, the long biological half-life of PFAS, and especially PFOS, means that risk of exposure misclassification is low.
This is the first prospective study to demonstrate that PFAS exposure was associated with risk of HCC. Findings from this proof-of-concept study suggest that PFAS exposure may play an important role in the pathology of HCC. These associations may be driven by alterations in branched-chain keto acids and bile acids. These findings may provide a new insight into the mechanisms of environmental-associated liver disease; however, larger studies are warranted to confirm these findings.
Financial support
Funding for this study was provided by the Southern California Environmental Health Science Center supported by the National Institutes of Health (P30ES007048). Additional funding from NIH supported Dr. Chatzi (R01ES030691, R01ES029944, R01ES030364, R21ES029681, R21ES028903), Dr. Goodrich (T32-ES013678), Dr. Conti (P01CA196569, P30ES007048, R01ES030691, R01ES030364, R21ES029681, R01ES029944) and Dr. Setiawan (R01CA228589, R01CA209798, R01MD015971, R01CA227133).
Authors’ contributions
Concept and design: Goodrich, Walker, McConnell, Conti, Chatzi, Setiawan. Acquisition, analysis, or interpretation of data: Goodrich, Walker, Lin, Wang, Lim, McConnell, Conti, Chatzi, Setiawan. Drafting of the manuscript: Goodrich, Walker, Lim, Conti, Chatzi, Setiawan. Critical revision of the manuscript for important intellectual content: Goodrich, Walker, Lin, Wang, Lim, McConnell, Conti, Chatzi, Setiawan. Administrative, technical, or material support: Walker, Lin, Lim.
Data availability statement
The data shown in this article are available from the corresponding authors upon a reasonable request.
Conflict of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2022.100550.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Society AC . 2021. Cancer facts & figures 2021. [Google Scholar]
- 3.Brar G., Greten T.F., Graubard B.I., McNeel T.S., Petrick J.L., McGlynn K.A., et al. Hepatocellular carcinoma survival by etiology: a SEER-medicare database analysis. Hepatol Commun. 2020;4:1541–1551. doi: 10.1002/hep4.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ioannou G.N. Epidemiology and risk-stratification of NAFLD-associated HCC. J Hepatol. 2021;75:1476–1484. doi: 10.1016/j.jhep.2021.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Han J., Wang B., Liu W., Wang S., Chen R., Chen M., et al. Declining disease burden of HCC in the United States, 1992-2017: a population-based analysis. Hepatology. 2022 doi: 10.1002/hep.32355. [DOI] [PubMed] [Google Scholar]
- 6.Wahlang B., Jin J., Beier J.I., Hardesty J.E., Daly E.F., Schnegelberger R.D., et al. Mechanisms of environmental contributions to fatty liver disease. Curr Environ Health Rep. 2019;6:80–94. doi: 10.1007/s40572-019-00232-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costello E., Rock S., Stratakis N., Eckel S.P., Walker D.I., Valvi D., et al. Exposure to per- and polyfluoroalkyl substances and markers of liver injury: a systematic review and meta-analysis. Environ Health Perspect. 2022;130 doi: 10.1289/EHP10092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindstrom A.B., Strynar M.J., Libelo E.L. Polyfluorinated compounds: past, present, and future. Environ Sci Technol. 2011;45:7954–7961. doi: 10.1021/es2011622. [DOI] [PubMed] [Google Scholar]
- 9.Sunderland E.M., Hu X.C., Dassuncao C., Tokranov A.K., Wagner C.C., Allen J.G. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J Expo Sci Environ Epidemiol. 2019;29:131–147. doi: 10.1038/s41370-018-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cousins I.T., DeWitt J.C., Gluge J., Goldenman G., Herzke D., Lohmann R., et al. The high persistence of PFAS is sufficient for their management as a chemical class. Environ Sci Process Impacts. 2020;22:2307–2312. doi: 10.1039/d0em00355g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taxvig C., Rosenmai A.K., Vinggaard A.M. Polyfluorinated alkyl phosphate ester surfactants - current knowledge and knowledge gaps. Basic Clin Pharmacol Toxicol. 2014;115:41–44. doi: 10.1111/bcpt.12208. [DOI] [PubMed] [Google Scholar]
- 12.Li Y., Fletcher T., Mucs D., Scott K., Lindh C.H., Tallving P., et al. Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occup Environ Med. 2018;75:46–51. doi: 10.1136/oemed-2017-104651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olsen G.W., Burris J.M., Ehresman D.J., Froehlich J.W., Seacat A.M., Butenhoff J.L., et al. Half-life of serum elimination of perfluorooctanesulfonate,perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect. 2007;115:1298–1305. doi: 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kannan K., Corsolini S., Falandysz J., Fillmann G., Kumar K.S., Loganathan B.G., et al. Perfluorooctanesulfonate and related fluorochemicals in human blood from several countries. Environ Sci Technol. 2004;38:4489–4495. doi: 10.1021/es0493446. [DOI] [PubMed] [Google Scholar]
- 16.Hu X.C., Andrews D.Q., Lindstrom A.B., Bruton T.A., Schaider L.A., Grandjean P., et al. Detection of poly- and perfluoroalkyl substances (PFASs) in U.S. Drinking water linked to industrial sites, military fire training areas, and wastewater treatment plants. Environ Sci Technol Lett. 2016;3:344–350. doi: 10.1021/acs.estlett.6b00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ingelido A.M., Abballe A., Gemma S., Dellatte E., Iacovella N., De Angelis G., et al. Biomonitoring of perfluorinated compounds in adults exposed to contaminated drinking water in the Veneto Region, Italy. Environ Int. 2018;110:149–159. doi: 10.1016/j.envint.2017.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Mamsen L.S., Bjorvang R.D., Mucs D., Vinnars M.T., Papadogiannakis N., Lindh C.H., et al. Concentrations of perfluoroalkyl substances (PFASs) in human embryonic and fetal organs from first, second, and third trimester pregnancies. Environ Int. 2019;124:482–492. doi: 10.1016/j.envint.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 19.Perez F., Nadal M., Navarro-Ortega A., Fabrega F., Domingo J.L., Barcelo D., et al. Accumulation of perfluoroalkyl substances in human tissues. Environ Int. 2013;59:354–362. doi: 10.1016/j.envint.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Das K.P., Wood C.R., Lin M.T., Starkov A.A., Lau C., Wallace K.B., et al. Perfluoroalkyl acids-induced liver steatosis: effects on genes controlling lipid homeostasis. Toxicology. 2017;378:37–52. doi: 10.1016/j.tox.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hui Z., Li R., Chen L. The impact of exposure to environmental contaminant on hepatocellular lipid metabolism. Gene. 2017;622:67–71. doi: 10.1016/j.gene.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 22.Rebholz S.L., Jones T., Herrick R.L., Xie C., Calafat A.M., Pinney S.M., et al. Hypercholesterolemia with consumption of PFOA-laced Western diets is dependent on strain and sex of mice. Toxicol Rep. 2016;3:46–54. doi: 10.1016/j.toxrep.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Son H.Y., Kim S.H., Shin H.I., Bae H.I., Yang J.H. Perfluorooctanoic acid-induced hepatic toxicity following 21-day oral exposure in mice. Arch Toxicol. 2008;82:239–246. doi: 10.1007/s00204-007-0246-x. [DOI] [PubMed] [Google Scholar]
- 24.Tan X., Xie G., Sun X., Li Q., Zhong W., Qiao P., et al. High fat diet feeding exaggerates perfluorooctanoic acid-induced liver injury in mice via modulating multiple metabolic pathways. PLoS One. 2013;8 doi: 10.1371/journal.pone.0061409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang L., Wang Y., Liang Y., Li J., Liu Y., Zhang J., et al. Specific accumulation of lipid droplets in hepatocyte nuclei of PFOA-exposed BALB/c mice. Sci Rep. 2013;3:2174. doi: 10.1038/srep02174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu X., Liang M., Yang Z., Su M., Yang B. Effect of acute exposure to PFOA on mouse liver cells in vivo and in vitro. Environ Sci Pollut Res Int. 2017;24:24201–24206. doi: 10.1007/s11356-017-0072-5. [DOI] [PubMed] [Google Scholar]
- 27.Wu X., Xie G., Xu X., Wu W., Yang B. Adverse bioeffect of perfluorooctanoic acid on liver metabolic function in mice. Environ Sci Pollut Res Int. 2018;25:4787–4793. doi: 10.1007/s11356-017-0872-7. [DOI] [PubMed] [Google Scholar]
- 28.Butenhoff J.L., Chang S.C., Olsen G.W., Thomford P.J. Chronic dietary toxicity and carcinogenicity study with potassium perfluorooctanesulfonate in Sprague Dawley rats. Toxicology. 2012;293:1–15. doi: 10.1016/j.tox.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Jacobsen A.V., Norden M., Engwall M., Scherbak N. Effects of perfluorooctane sulfonate on genes controlling hepatic fatty acid metabolism in livers of chicken embryos. Environ Sci Pollut Res Int. 2018;25:23074–23081. doi: 10.1007/s11356-018-2358-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gleason J.A., Post G.B., Fagliano J.A. Associations of perfluorinated chemical serum concentrations and biomarkers of liver function and uric acid in the US population (NHANES), 2007-2010. Environ Res. 2015;136:8–14. doi: 10.1016/j.envres.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Lin C.Y., Lin L.Y., Chiang C.K., Wang W.J., Su Y.N., Hung K.Y., et al. Investigation of the associations between low-dose serum perfluorinated chemicals and liver enzymes in US adults. Am J Gastroenterol. 2010;105:1354–1363. doi: 10.1038/ajg.2009.707. [DOI] [PubMed] [Google Scholar]
- 32.Bassler J., Ducatman A., Elliott M., Wen S., Wahlang B., Barnett J., et al. Environmental perfluoroalkyl acid exposures are associated with liver disease characterized by apoptosis and altered serum adipocytokines. Environ Pollut. 2019;247:1055–1063. doi: 10.1016/j.envpol.2019.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salihovic S., Stubleski J., Karrman A., Larsson A., Fall T., Lind L., et al. Changes in markers of liver function in relation to changes in perfluoroalkyl substances - a longitudinal study. Environ Int. 2018;117:196–203. doi: 10.1016/j.envint.2018.04.052. [DOI] [PubMed] [Google Scholar]
- 34.Sen P., Qadri S., Luukkonen P.K., Ragnarsdottir O., McGlinchey A., Jantti S., et al. Exposure to environmental contaminants is associated with altered hepatic lipid metabolism in non-alcoholic fatty liver disease. J Hepatol. 2022;76:283–293. doi: 10.1016/j.jhep.2021.09.039. [DOI] [PubMed] [Google Scholar]
- 35.Jin R., McConnell R., Catherine C., Xu S., Walker D.I., Stratakis N., et al. Perfluoroalkyl substances and severity of nonalcoholic fatty liver in children: an untargeted metabolomics approach. Environ Int. 2020;134 doi: 10.1016/j.envint.2019.105220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kolonel L.N., Henderson B.E., Hankin J.H., Nomura A.M., Wilkens L.R., Pike M.C., et al. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol. 2000;151:346–357. doi: 10.1093/oxfordjournals.aje.a010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barzi A., Zhou K., Wang S., Dodge J.L., El-Khoueiry A., Setiawan V.W. Etiology and outcomes of hepatocellular carcinoma in an ethnically diverse population: the multiethnic cohort. Cancers (Basel) 2021;13 doi: 10.3390/cancers13143476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Setiawan V.W., Hernandez B.Y., Lu S.C., Stram D.O., Wilkens L.R., Le Marchand L., et al. Diabetes and racial/ethnic differences in hepatocellular carcinoma risk: the multiethnic cohort. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noureddin M., Zelber-Sagi S., Wilkens L.R., Porcel J., Boushey C.J., Le Marchand L., et al. Diet associations with nonalcoholic fatty liver disease in an ethnically diverse population: the multiethnic cohort. Hepatology. 2019 doi: 10.1002/hep.30967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu K.H., Nellis M., Uppal K., Ma C., Tran V., Liang Y., et al. Reference standardization for quantification and harmonization of large-scale metabolomics. Anal Chem. 2020;92:8836–8844. doi: 10.1021/acs.analchem.0c00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Q., Walker D., Uppal K., Liu Z., Ma C., Tran V., et al. Addressing the batch effect issue for LC/MS metabolomics data in data preprocessing. Sci Rep. 2020;10 doi: 10.1038/s41598-020-70850-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson W.E., Li C., Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8:118–127. doi: 10.1093/biostatistics/kxj037. [DOI] [PubMed] [Google Scholar]
- 43.Centers for Disease Control and Prevention . Centers for Disease Control and Prevention; 2019. Fourth National Report on human exposure to environmental chemicals, updated tables, January 2019. [Google Scholar]
- 44.Li S., Park Y., Duraisingham S., Strobel F.H., Khan N., Soltow Q.A., et al. Predicting network activity from high throughput metabolomics. PLOS Comput Biol. 2013;9 doi: 10.1371/journal.pcbi.1003123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., Gillette M.A., et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pang Z., Chong J., Zhou G., de Lima Morais D.A., Chang L., Barrette M., et al. MetaboAnalyst 5.0: narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021;49:W388–W396. doi: 10.1093/nar/gkab382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chadeau-Hyam M., Athersuch T.J., Keun H.C., De Iorio M., Ebbels T.M., Jenab M., et al. Meeting-in-the-middle using metabolic profiling - a strategy for the identification of intermediate biomarkers in cohort studies. Biomarkers. 2011;16:83–88. doi: 10.3109/1354750X.2010.533285. [DOI] [PubMed] [Google Scholar]
- 48.Greenland S. Small-sample bias and corrections for conditional maximum-likelihood odds-ratio estimators. Biostatistics. 2000;1:113–122. doi: 10.1093/biostatistics/1.1.113. [DOI] [PubMed] [Google Scholar]
- 49.Eriksen K.T., Sorensen M., McLaughlin J.K., Lipworth L., Tjonneland A., Overvad K., et al. Perfluorooctanoate and perfluorooctanesulfonate plasma levels and risk of cancer in the general Danish population. J Natl Cancer Inst. 2009;101:605–609. doi: 10.1093/jnci/djp041. [DOI] [PubMed] [Google Scholar]
- 50.Montomoli J., Erichsen R., Norgaard M., Hoyer M., Hansen J.B., Jacobsen J.B. Survival of patients with primary liver cancer in central and northern Denmark, 1998-2009. Clin Epidemiol. 2011;3(Suppl 1):3–10. doi: 10.2147/CLEP.S20623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jepsen P., Andersen M.W., Villadsen G.E., Ott P., Vilstrup H. Time-trends in incidence and prognosis of hepatocellular carcinoma in Denmark: a nationwide register-based cohort study. Liver Int. 2017;37:871–878. doi: 10.1111/liv.13340. [DOI] [PubMed] [Google Scholar]
- 52.Shearer J.J., Callahan C.L., Calafat A.M., Huang W.Y., Jones R.R., Sabbisetti V.S., et al. Serum concentrations of per- and polyfluoroalkyl substances and risk of renal cell carcinoma. J Natl Cancer Inst. 2021;113:580–587. doi: 10.1093/jnci/djaa143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bartell S.M., Vieira V.M. Critical review on PFOA, kidney cancer, and testicular cancer. J Air Waste Manag Assoc. 2021;71:663–679. doi: 10.1080/10962247.2021.1909668. [DOI] [PubMed] [Google Scholar]
- 54.Fenton S.E., Ducatman A., Boobis A., DeWitt J.C., Lau C., Ng C., et al. Per- and polyfluoroalkyl substance toxicity and human health review: current state of knowledge and strategies for informing future research. Environ Toxicol Chem. 2020 doi: 10.1002/etc.4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pinyopornpanish K., Khoudari G., Saleh M.A., Angkurawaranon C., Pinyopornpanish K., Mansoor E., et al. Hepatocellular carcinoma in nonalcoholic fatty liver disease with or without cirrhosis: a population-based study. BMC Gastroenterol. 2021;21:394. doi: 10.1186/s12876-021-01978-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burrage L.C., Nagamani S.C., Campeau P.M., Lee B.H. Branched-chain amino acid metabolism: from rare Mendelian diseases to more common disorders. Hum Mol Genet. 2014;23:R1–R8. doi: 10.1093/hmg/ddu123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.White P.J., McGarrah R.W., Grimsrud P.A., Tso S.C., Yang W.H., Haldeman J.M., et al. The BCKDH kinase and phosphatase integrate BCAA and lipid metabolism via regulation of ATP-citrate lyase. Cell Metab. 2018;27:1281–1293 e1287. doi: 10.1016/j.cmet.2018.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stratakis N., Conti D.V., Jin R., Margetaki K., Valvi D., Siskos A.P., et al. Prenatal exposure to perfluoroalkyl substances associated with increased susceptibility to liver injury in children. Hepatology. 2020 doi: 10.1002/hep.31483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Perino A., Schoonjans K. Metabolic Messengers: bile acids. Nat Metab. 2022 doi: 10.1038/s42255-022-00559-z. [DOI] [PubMed] [Google Scholar]
- 60.Thomas C.E., Luu H.N., Wang R., Xie G., Adams-Haduch J., Jin A., et al. Association between pre-diagnostic serum bile acids and hepatocellular carcinoma: the Singapore Chinese health study. Cancers (Basel) 2021;13 doi: 10.3390/cancers13112648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roth K., Yang Z., Agarwal M., Liu W., Peng Z., Long Z., et al. Exposure to a mixture of legacy, alternative, and replacement per- and polyfluoroalkyl substances (PFAS) results in sex-dependent modulation of cholesterol metabolism and liver injury. Environ Int. 2021;157 doi: 10.1016/j.envint.2021.106843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mishra S.P., Karunakar P., Taraphder S., Yadav H. Free fatty acid receptors 2 and 3 as microbial metabolite sensors to shape host health: pharmacophysiological view. Biomedicines. 2020;8 doi: 10.3390/biomedicines8060154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dai X., Hou H., Zhang W., Liu T., Li Y., Wang S., et al. Microbial metabolites: critical regulators in NAFLD. Front Microbiol. 2020;11 doi: 10.3389/fmicb.2020.567654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.National Institutes of Health. National Cancer Institute SEER Training Modules. [cited; Available from: training.seer.cancer.gov/followup/intro/requirements.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data shown in this article are available from the corresponding authors upon a reasonable request.