Abstract
The Bacillus subtilis mrp (multiple resistance and pH) operon supports Na+ and alkali resistance via an Na+/H+ antiport, as well as cholate efflux and resistance. Among the individual mutants with nonpolar mutations in each of the seven mrp genes, only the mrpF mutant exhibited cholate sensitivity and a cholate efflux defect that were complemented by expression of the deleted gene in trans. Expression of mrpF in the mrp null (VKN1) strain also restored cholate transport and increased Na+ efflux, indicating that MrpF does not require even low levels of other mrp gene expression for its own function. In contrast to MrpF, MrpA function had earlier seemed to depend upon at least modest expression of other mrp genes, i.e., mrpA restored Na+ resistance and efflux to strain VK6 (a polar mrpA mutant which expresses low levels of mrpB to -G) but not to the null strain VKN1. In a wild-type background, each nonpolar mutation in individual mrp genes caused profound Na+ sensitivity at both pH 7.0 and 8.3. The mrpA and mrpD mutants were particularly sensitive to alkaline pH even without added Na+. While transport assays in membrane vesicles from selected strains indicated that MrpA-dependent antiport can occur by a secondary, proton motive force-dependent mechanism, the requirement for multiple mrp gene products suggests that there are features of energization, function, or stabilization that differ from typical secondary membrane transporters. Northern analyses indicated regulatory relationships among mrp genes as well. All the mrp mutants, especially the mrpA, -B, -D, -E, and -G mutants, had elevated levels of mrp RNA relative to the wild type. Expression of an upstream gene, maeN, that encodes an Na+/malate symporter, was coordinately regulated with mrp, although it is not part of the operon.
The mrp operon was first identified in the genome of Bacillus subtilis as a homologue of a locus that had been found to be centrally important to cytoplasmic pH regulation in alkaliphilic Bacillus halodurans C-125 (3, 16). A point mutation in the first gene of the alkaliphile homologue resulted in loss of Na+/H+ antiporter activity (3). Such antiport is widely used by prokaryotes for alkali and Na+ resistance inasmuch as coupled Na+ exclusion and H+ accumulation can be accomplished via electrogenic exchange of cytoplasmic Na+ for a greater number of H+ (14, 20). The complete mrp operon of B. subtilis is predicted to encode seven hydrophobic gene products (9, 12), as is also posited for homologues from diverse organisms, including alkaliphilic Bacillus pseudofirmus OF4 (14, 15), Rhizobium meliloti (21), Staphylococcus aureus (6), and others annotated in genome databases. Apart from the apparent role of the alkaliphile mrp operons in Na+-dependent pH homeostasis, studies with mutants have suggested that the R. meliloti homologue, pha, may encode a K+/H+ antiporter that is required for symbiotic nitrogen fixation (21) and that B. subtilis mrp (called ntr and sha by other investigators [12, 13]) has multiple functions. First, the B. subtilis mrp locus has been shown to play a role in Na+ resistance and in both Na+- and K+-dependent cytoplasmic pH homeostasis (9, 12). This is consistent with one or more mrp genes encoding an Na+(K+)/H+ antiport activity. Recently, Kosono et al. (13) showed that a B. subtilis mrpA (shaA) mutant fails to sporulate normally and suggested that an early step in sporulation is sensitive to the elevated cytoplasmic Na+ concentration that results from mrp mutations. The second B. subtilis mrp activity, in which the mrpF gene has been implicated, encompasses cholate and Na+ efflux activities, which may be mechanistically coupled. Demonstration of cholate efflux activity has thus far been made only in a mutant with a disruption in mrpF that also lowered expression of mrpG (9), but in the current study, separate mutations in mrpF and mrpG have been examined.
Before the discovery of the mrp operon and its homologues, the well-studied examples of Na+/H+ antiporters all involved a single structural gene product (20). Data to date suggest that monovalent cation/H+ antiporter activity requires the first gene of the operon, mrpA, in B. subtilis, but that other genes of the operon are required for some combination of antiporter activity, expression, and assembly (9, 12). That is, MrpA is necessary but not sufficient for Na+/H+ activity. Similarly, Hiramatsu et al. (6) have suggested from studies in which the S. aureus homologue, designated mnh, was expressed in an Na+-sensitive Escherichia coli mutant, that all the genes of the operon may be required for the Na+ resistance conferred in that system. There are recent reports of secondary multidrug transporters with two heterologous protein components (10, 18) but the complexity of the mrp product interactions might be of a much higher order. In addition, the long-recognized sequence similarity of several mrp products to membrane-embedded subunits of energy-coupled NADH dehydrogenase complexes (3, 9) raises the possibility that there is a capacity for electron transport that could provide a primary energy coupling option for mrp functions.
In the current study, individual in-frame deletions were made in each of the B. subtilis mrp genes for which no such mutations had been made earlier, i.e., mrpB, -C, -D, -E, -F, and -G. For each of those strains, a version was also made in which an active copy of the disrupted gene was returned to the amyE locus of the chromosome under the control of an IPTG (isopropyl-β-d-thiogalactopyranoside)-inducible promoter. Each mrp gene was similarly introduced into the amyE locus of an mrp null mutant, VKN1, of B. subtilis, and into that of a polar mutant, VK6, that lacks mrpA and expresses mrpB to -G at greatly reduced levels. Resistance and transport studies have supported earlier indications that MrpF is the Na+-cholate efflux protein and further show that MrpF activity is independent of the expression of additional mrp genes. By contrast, MrpA function, which is shown to correlate with a protonophore-sensitive Na+ efflux activity, requires all six other mrp genes. In addition, evidence is presented for a complex regulatory relationship between loss of function of particular mrp genes and expression of the polycistronic mrp mRNA.
MATERIALS AND METHODS
Bacterial strains, plasmids, and general growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. The bacteria were routinely grown at 30°C in malate-containing, potassium-replete TKM medium (9). For experiments in which mrp genes were introduced into the amyE locus under control of the pspac promoter, 200 μM IPTG was included in the growth medium.
TABLE 1.
Bacterial strains and plasmids used
| Strain or plasmid | Relevant charcteristic(s) | Source or reference |
|---|---|---|
| E. coli DH5α MCR | F−mcrAΔ1 (mrr-hsd RMS-mcrBC) Φ80dlacZ Δ(lacZYA-argF)U169 deoR recA1 endA1 supE44 λ thi-1 gyr-496 relA1 | Gibco-BRL |
| B. subtilis | ||
| BD99 (wild type) | hisA1 thrS trpC2 | A. Garro |
| VK1A | BD99 ΔmrpA | 9 |
| VK1B | BD99 ΔmrpB | This study |
| VK1C | BD99 ΔmrpC | This study |
| VK1D | BD99 ΔmrpD::Spr | This study |
| VK1E | BD99 ΔmrpE | This study |
| VK1F | BD99 ΔmrpF::Spr | This study |
| VK1G | BD99 ΔmrpG::Spr | This study |
| VKN1 | BD99 ΔmrpA-G::Spr | 9 |
| VK6 | BD99 mrpA::Spr | This study |
| Plasmids | ||
| pGEM11Zf(+) | Cloning vector (Apr) | Promega |
| pDH88 | Cmr vector for cloning B. subtilis chromosomal DNA and integration into the corresponding locus | 5 |
| pDR67 | amyE integration vector with Cmr gene and pspac promoter upstream of multiple cloning site | 8 |
| pDHB1 | pDH88 + ΔmrpB | This study |
| pDHC1 | pDH88 + ΔmrpC | This study |
| pDHE1 | pDH88 + ΔmrpE | This study |
| pDRB1 | pDR67 + mrpB | This study |
| pDRC1 | pDR67 + mrpC | This study |
| pDRD1 | pDR67 + mrpD | This study |
| pDRE1 | pDR67 + mrpE | This study |
| pDRF1 | pDR67 + mrpF | This study |
| pDRG1 | pDR67 + mrpG | This study |
| pGEMD1 | pGEM11Zf(+) + ΔmrpD::Spr | This study |
| pGEMF1 | pGEM11Zf(+) + ΔmrpF::Spr | This study |
| pGEMG1 | pGEM11Zf(+) + ΔmrpG::Spr | This study |
Northern analyses.
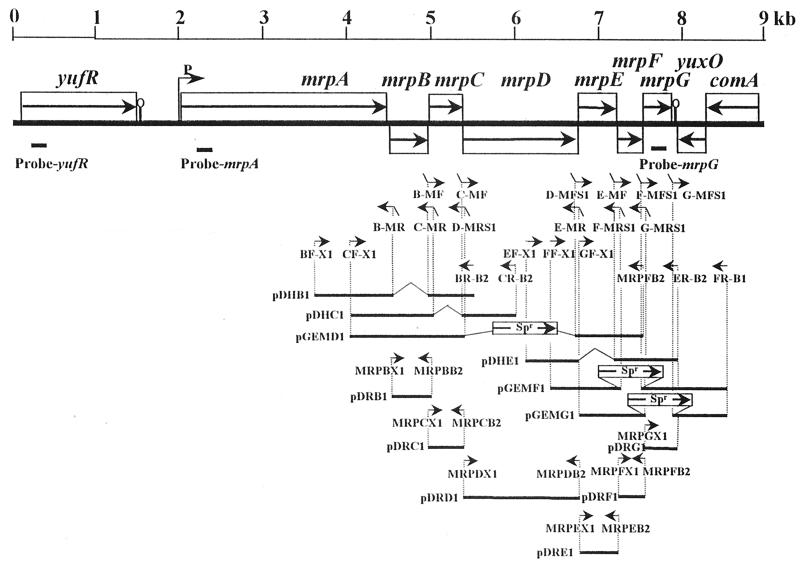
Cells were grown, RNA was prepared, and the Northern procedures were carried out according to methods described by others (2). Three different probes were employed: (i) a probe to a gene upstream of the mrp operon that encodes maeN (yufR) was prepared using primers yufR1 and yufR2; (ii) a probe to part of mrpA was prepared using primers BsmrpA1 and mrpA2T7; and (iii) a probe to part of mrpG was prepared using primers BsmrpG1 and mrpG2T7. For these preparations as well as other PCRs, either Pfx (Life Tech) or Vent (New England Biolabs) DNA polymerase was used. The PCR products were first gel purified and then eluted using a gel extraction kit (Qiagen). The probes were then used as DNA templates for random priming 32P labeling using a random-primed DNA labeling kit (Roche Diagnostics). The locations of the probes are indicated in Fig. 1.
FIG. 1.
Schematic diagram of the yufR (maeN)-mrp region of the B. subtilis chromosome that indicates the probes used for Northern analyses and the primers used in construction of new mrp deletion strains.
Construction of mutant strains.
For each type of mutant, the phenotype of the strain used in the studies was the same as that of several other strains from the construction protocol. Each mutant that was used in the subsequent studies was initially confirmed to have the expected PCR profile and was then directly shown to contain the expected sequence. Sequencing was conducted at the Biotechnology Center at Utah State University (Logan, Utah) with an ABI-100 model 377 sequencer. All in-frame mutations were constructed by gene splicing via overlap extension, as described by others (7). For the deletions of mrpB, mrpC, and mrpE, the approach was precisely as used in earlier construction of the VK1A (mrpA deletion, previously called VK1) strain (9). For deletions in mrpD, mrpF, and mrpG, the earlier approach alone did not yield the desired mutations; therefore, gene splicing via overlap extension was used as the first step, followed by steps that resulted in the introduction of a spectinomycin resistance (Spr) cassette gene, spc (19), into the deleted area.
(i) For construction of in-frame mutant VK1B with a deletion in mrpB, two independent PCRs were performed on wild-type DNA with the sets of primers shown in Fig. 1, BF-XI and B-MR plus BR-B2 and B-MF. The sequences of the primers used in this study will be made available from the authors. BF-X1 has additional nucleotides encoding an XbaI site. BR-B2 has additional nucleotides encoding a BglII site. The two purified PCR products were used as templates for a second PCR with primers BF-X1 and BR-B2. The purified product of this reaction was digested with XbaI and BglII and then cloned into XbaI- and BglII-digested pDH88. The resulting plasmid, pDHB1, was integrated into the mrpB locus in the chromosome by a single crossover using chloramphenicol resistance for selection (5). To prepare strains that had lost the plasmid sequences, leaving a mutated mrpB, cells were grown under nonselective conditions on LBK plates, and the strains thus obtained were confirmed as above.
(ii) For construction of in-frame mutant VK1C with a deletion in mrpC, the strategy (Fig. 1) was the same as for VK1B except that the primer pairs were CF-X1 and C-MR plus CR-B2 and C-MF. The two purified PCR products were used as templates for a second PCR using primers CF-X1 and CR-B2. The product of this reaction was cloned into pDH88 as above, resulting in plasmid pDHC1, which was integrated into the mrpC locus. Strains that had lost the plasmid sequences were isolated and confirmed.
(iii) For construction of in-frame mutant VK1D with a deletion in mrpD, two PCRs were performed on wild-type DNA with primer pairs CF-X1 and D-MRS1 plus MRPFB2 and D-MFS1 (Fig. 1). MRPFB2 has additional nucleotides encoding a BglII site. D-MRS 1 and D-MFS1 have additional nucleotides encoding a SmaI site in the middle of each primer. The two purified PCR products were used as templates for a second PCR with primers CF-X1 and MRPFB2. The purified product of this reaction was digested with XbaI and BglII and then cloned into XbaI- and BamHI-digested pGEM11Zf(+) (Apr; Promega). The recombinant plasmid was digested with SmaI. Just before the SmaI site, the third amino acid from the N terminus of MrpD is encoded, and just after the SmaI site, the third amino acid from the C terminus of MrpD is encoded. A gene encoding Spr was amplified by PCR using primers SpcA and SpcB. The PCR product encoded only the open reading frame region of the spc gene. The spc gene was ligated to this linear plasmid in frame, resulting in a recombinant plasmid encoding a chimeric protein containing a small part of MrpD and the Spcr protein. This chimeric protein conferred spectinomycin resistance. After isolation, the recombinant plasmid, pGEMD1, was digested with XbaI, and the linear plasmid was introduced into wild-type B. subtilis. Mutants with deletions in mrpD were identified by spectinomycin resistance (150 μg/ml) and confirmed.
(iv) For construction of in-frame mutant VK1E with a deletion in mrpE, the strategy was the same as for VK1B (Fig. 1) except that the primer pairs were EF-X1 and E-MR plus ER-B2 and E-MF. The two purified PCR products were used as templates for a second PCR with primers EF-X1 and ER-B2. The purified PCR product was cloned into pDH88, resulting in plasmid pDHE1, which was integrated into the mrpE locus. Clones that had lost the plasmid sequence were isolated and confirmed.
(v) For construction of in-frame mutant VK1F with a deletion in mrpF, two PCR were performed on wild-type DNA with the sets of primers shown in Fig. 1, FF-X1 and F-MRS1 plus FR-B1 and F-MFS1 (Table 2). FF-X1 has additional nucleotides encoding an XbaI site. FR-B1 has additional nucleotides encoding a BamHI site. F-MRS1 and F-MFS1 have additional nucleotides encoding a SmaI site in the middle of each primer. The two purified PCR products were used as templates for a second PCR with primers FF-X1 and FR-B1. The purified product of this reaction was digested with XbaI and BamHI and then cloned into XbaI- and BamHI-digested pGEM11Zf(+). The recombinant plasmid was digested with SmaI. Just before the SmaI site, the first amino acid from the N terminus of MrpF is encoded, and just after the SmaI site, the sixth animo acid from the C terminus of MrpF is encoded. A recombinant plasmid, pGEMF1, containing a very short part of mrpF and an Spr gene was constructed as for VK1D. The plasmid was introduced into B. subtilis, and deletion of mrpF was confirmed.
TABLE 2.
Na+ resistance of B. subtilis strains with mutations in selected mrp genes
| Strain | MICa(M) of Na+ at:
|
|||
|---|---|---|---|---|
| pH 7.0
|
pH 8.3
|
|||
| Na+ concn (M) | A600 | Na+ concn (M) | A600 | |
| Wild type | 1.30 ± 0.05 | 0.996 | 0.71 ± 0.03 | 0.646 |
| VKN1 | 0.09 ± 0.03 | 0.984 | 0.024 ± 0.006 | 0.694 |
| VK1A | 0.15 ± 0.02 | 0.912 | 0.03 ± 0.004 | 0.301 |
| VK1B | 0.12 ± 0.02 | 0.946 | 0.025 ± 0.005 | 0.763 |
| VK1C | 0.21 ± 0.02 | 0.896 | 0.057 ± 0.005 | 0.733 |
| VK1D | 0.22 ± 0.01 | 0.832 | 0.015 ± 0.004 | 0.141 |
| VK1E | 0.11 ± 0.02 | 0.951 | 0.023 ± 0.005 | 0.637 |
| VK1F | 0.19 ± 0.02 | 0.836 | 0.052 ± 0.007 | 0.684 |
| VK1G | 0.22 ± 0.02 | 0.864 | 0.073 ± 0.008 | 0.648 |
| VK1A (mrpA) | 1.15 ± 0.05 | 0.991 | 0.45 ± 0.02 | 0.598 |
| VK1B (mrpB) | 1.11 ± 0.03 | 0.947 | 0.51 ± 0.02 | 0.604 |
| VK1C (mrpC) | 1.30 ± 0.04 | 0.925 | 0.73 ± 0.04 | 0.675 |
| VK1D (mrpD) | 0.90 ± 0.03 | 0.954 | 0.47 ± 0.03 | 0.497 |
| VK1E (mrpE) | 1.32 ± 0.04 | 0.961 | 0.62 ± 0.04 | 0.668 |
| VK1F (mrpF) | 1.35 ± 0.05 | 0.924 | 0.72 ± 0.04 | 0.675 |
| VK1G (mrpG) | 1.22 ± 0.04 | 0.978 | 0.71 ± 0.03 | 0.661 |
Minimal Na+ concentration at which no growth was observed after 15 h. The values are means of at least eight separate determinations ± standard deviations. A600 values are after 15 h.
(vi) For construction of in-frame mutant VK1G with a deletion in mrpG, the strategy was as for VK1F. Two independent PCRs were performed on wild-type DNA using primer pairs GF-X1 and G-MRS1 plus FR-B1 and G-MFS1. GF-X1 has additional nucleotides encoding an XbaI site; FR-B1 has a BamHI site; and G-MRS1 and G-MFS1 have a SmaI site in the middle of each primer. The two purified PCR products were used as templates for a second PCR with primers GF-X1 and FR-B1. The PCR product was cloned into pGEM11Zf(+) as for VK1F. The recombinant plasmid was digested with SmaI. Just before the SmaI site, the sixth amino acid from the N terminus of MrpG is encoded, and just after the SmaI site, the stop codon of MrpG is encoded. A recombinant plasmid containing a small part of mrpG and a spectinomycin resistance gene was introduced into B. subtilis, and deletion of mrpG was confirmed.
Integration of various mrp genes into the amyE locus of particular mutant strains was performed as described elsewhere using plasmid pDR67 (8). For construction of a plasmid carrying the intact mrpB gene, PCR was performed on wild-type DNA using primers MRPBX1 and MRPBB2 (Fig. 1). For mrpC, the primers were MRPCX1 and MRPCB2. For mrpD, the primers were MRPDX1 and MRPDB2. For mrpE, the primers were MRPEX1 and MRPEB2. For mrpF, the primers were MRPFX1 and MRPFB2. For mrpG, the primers were MRPGX1 and ER-B2. Each amplified fragment was cloned into XbaI- and BglII-digested pDR67, yielding pDRB1, pDRC1, pDRD1, pDRE1, pDRF1, and pDRG1, respectively. Each plasmid was linearized with NruI and used to transform particular mutants to a chloramphenicol-resistant, amylase-negative phenotype. The plasmids used in this study are listed together with the bacterial strains in Table 1. All were confirmed to have the correct sequences.
Determination of MICs of Na+ and inhibition profile for cholate.
The MIC of Na+ was determined in TKM medium at pH 7.0 or 8.3 exactly as described elsewhere (9). Sensitivity to cholate was also assessed as previously described (9).
Transport assays.
Measurements of cholate efflux were conducted on whole cells that were preloaded with cholate and then assayed using a filtration assay as described in connection with earlier studies of the mrp operon (9). Measurements of 22Na+ efflux were conducted in both whole cells and right-side-out membrane vesicles. The whole-cell 22Na+ efflux assays conducted in connection with MrpF function were carried out precisely as described earlier (9). For the assays in membrane vesicles, right-side-out vesicles were prepared by a modification of the method of Kaback (11). Protoplasts were prepared from logarithmic-phase cells by incubating at 37°C in 100 mM potassium phosphate (pH 7.5)–20% sucrose–300 μg of lysozyme per ml until the cells had rounded up. The protoplasts were shocked in 50 mM potassium phosphate (pH 7.5) plus 1 mM MgSO4 by diluting 100-fold and passing through a syringe with a 19G1/2 needle. Vesicles were passively loaded with 5 mM 22NaCl (10 μCi/ml) for 18 h at 4°C. For assays of Na+ efflux energized by the electron transport chain, vesicles (100 μg of protein/ml) were incubated at 10°C. No further additions were made, or 10 mM potassium ascorbate–0.1 mM phenazine methosulfate (PMS) was added. The protonophore carbonyl cyanide p-chlorophenylhydrazone (CCCP) was added to some reactions, as indicated. Samples were taken at various times and filtered, and radioactivity was measured by liquid scintillation counting.
In some assays Na+ efflux was driven by a potassium diffusion potential. The membrane vesicles loaded with 5 mM 22Na+ in buffer containing 100 mM potassium phosphate (pH 7.5) were treated with 10 μM valinomycin and diluted to various extents into 50 mM Tris-HCl (pH 7.5) in order to generate potassium diffusion potentials of different magnitudes. Samples were taken at 5 s after dilution, and radioactivity was counted as above. Protein was determined by the method of Lowry et al. (17), using egg white lysozyme as the standard.
RESULTS
Northern analyses of the mutant strains.
Northern analyses were conducted on each of the newly constructed strains, the VK1A strain constructed earlier (9), and the wild type. Three different probes were used. One was to the upstream maeN (yufR) gene, which has been shown to encode an Na+/malate symporter (Y. Wei, unpublished data). This gene is transcribed in the same direction as the mrp genes, and the possibility that it is cotranscribed or coregulated with them was of interest. The other two probes were to the mrp genes at the ends of the known operon, mrpA and mrpG. These probes were used to determine whether the new in-frame deletion mutations were in fact nonpolar, and whether all of the new mutants exhibited increased mrp RNA abundance. The mrpA deletion in strain VK1A had earlier been shown to be nonpolar and to result in much higher levels of mrp RNA than the wild type (9). As shown in Fig. 2, all the new inframe deletion mutations (mrp BCDEF) are nonpolar inasmuch as the cells express mrpG at wild-type levels of RNA abundance or greater. In Fig. 2, an arrow indicates the location of the wild-type mrp band. This band could only be visualized more distinctly upon longer exposures, which made it too difficult to discern any detail in most of the other lanes. The sizes of the mrp bands in the mutants were as expected from the size of the deletion combined, in three of them, with the introduction of a cassette. As had been observed for mrp RNA in VK1A (9) (which is shown again in Fig. 2 together with the new mutants), the new mutants with deletions in mrpB, mrpE, mrpD, and mrpG all exhibited a significant increase in mrp RNA, as did the retested mrpA mutant. Two of the new mutants with deletions in mrpC or mrpF did not exhibit an elevation of mrp RNA comparable to that seen in the other strains, although there was still an increase relative to the wild-type strain. In the RNA preparations from some of the mutants, there were also bands that reacted with mrp gene probes that were smaller than the major, expected band and may represent degradation products.
FIG. 2.
Northern analyses of mutants with individual deletions in each of the seven B. subtilis mrp genes. RNA was prepared from wild-type B. subtilis and probed with the DNA probes corresponding to parts of the first and last mrp genes and to part of the upstream maeN (yufR) gene. The locations of the probes are shown in Fig. 1, and the procedures are described in Materials and Methods. The strain is indicated above each lane, e.g., Wt, wild type; A, VK1A; etc. The probe used for the particular blot is indicated below the panel.
A distinct band corresponding to the molecular size of MaeN alone was observed with the maeN probe. This indicates that maeN is expressed on a transcript that probably encodes no other genes and is not included on the large mrp transcript under the conditions used here. Interestingly, the level of maeN RNA, which was higher in the wild type than the level of mrp RNA, exhibited a pattern of increase among the mrp mutants that was similar to that for mrp RNA. maeN RNA was elevated over its wild-type level in all the mrp mutants except the mrpA mutant. That mutant, VK1A, showed less maeN RNA than the wild type. Among the other mrp mutants, there again appeared to be a smaller increase in the strains with deletions in mrpC or mrpF relative to the other, maeN-overexpressing mutants.
MrpF-dependent cholate resistance, cholate efflux, and Na+ efflux.
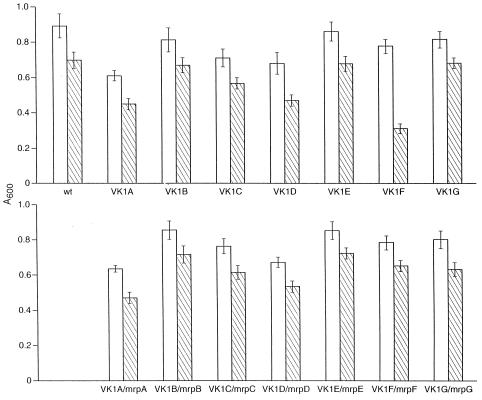
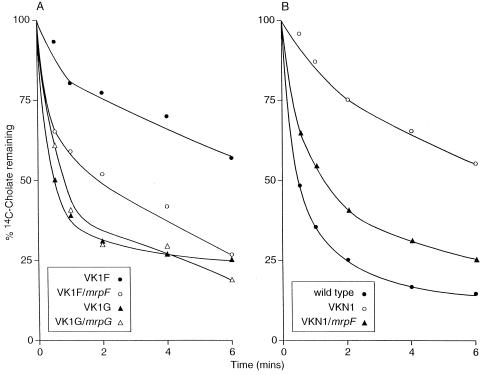
Cholate resistance was examined in our initial studies of the B. subtilis mrp operon because the BLAST analysis (1) of the deduced mrpF product revealed sequence similarities to Na+-coupled bile acid transporters. The difference in cholate resistance found between the wild type and mrpF (polar) mutant, VK15, was significant and reproducible but not large enough to be captured as a difference in MIC (9). As shown in Fig. 3 for each of the single mrp gene mutants and their complemented versions, a large increase in sensitivity to cholate was found only in the new mrpF (nonpolar) mutant VK1F, and only in that mutant was the restoration of an active gene in the amyE locus accompanied by significantly greater resistance to cholate. To correlate the resistance with cholate transport capacity, the efflux of cholate from preloaded cells of the wild type was compared to efflux from the mrpF and mrpG mutants, VK1F and VK1G, respectively, and the versions of each in which the affected gene was expressed from the amyE locus. As shown in Fig. 4A, efflux of cholate was not defective in the mrpG mutant strain but was significantly reduced in the mrpF mutant strain VK1F. Expression in trans of the mrpF gene in VK1F increased the cholate efflux activity of the mutant almost to the level in VK1G (Fig. 4A) or the wild-type strain (Fig. 4B). Earlier studies indicated that expression of mrpF in the mrp null strain of B. subtilis did not restore cholate resistance to that strain (9). However, the results here led us to reexamine the possibility that MrpF can function independently of any other mrp genes in experiments in which transport of cholate itself was assayed. Efflux and resistance might not be observed in parallel, for example, if cholate reentry was pronounced relative to the capacity and rate of efflux. As shown in Fig. 4B, expression of mrpF under control of an IPTG-inducible promoter in the amyE locus of mrp null strain VKN1 resulted in a dramatic increase in cholate efflux. Expression of mrpF in strain VKN1 also resulted in an increase in Na+ efflux (Fig. 5), although, as noted below, this increase too was not sufficient to be reflected in an increase in Na+ resistance.
FIG. 3.
Sensitivity of wild type (wt) and mrp mutant strains of B. subtilis to growth inhibition by cholate. Cells were grown in TKM medium (pH 7.0) in the presence (hatched bars) or absence (open bars) of 0.08% (wt/vol) cholate. After 6 h of incubation with shaking at 30°C, the A600 was determined. The results represent the mean of at least eight determinations, and standard deviations are shown as error bars.
FIG. 4.
Cholate efflux from whole cells of wild-type and selected mrp mutant strains of B. subtilis. The cells were starved and loaded with 20 μM [14C]cholate. Efflux was initiated by diluting 100-fold into buffer containing 10 mM glucose. Samples were taken at various times, filtered, and washed. The radioactivity was determined by liquid scintillation counting. (A) mrpF mutant VKIF and mrpG mutant VK1G, both without (solid symbols) and with (open symbols) the deleted gene expressed from an IPTG-inducible promoter in the amyE locus. (B) Wild-type strain, the mrp null mutant VKN1, and VKN1 expressing mrpF under control of an IPTG-inducible promoter in the amyE locus.
FIG. 5.
22Na+ efflux from wild-type (wt) B. subtilis, the mrp null strain VKN1, and VKN1 expressing mrpF in trans. The cells were washed, energy depleted, and loaded with 5 mM 22NaCl as described previously (9). Efflux was initiated by diluting the cell suspension 100-fold into buffer containing 5 mM NaCl with no further additions (○) or in the presence of 10 mM glucose (●). Samples were taken at various times, filtered, and washed. The radioactivity was determined by liquid scintillation counting.
Na+ and alkali- sensitivity of the mrp mutants.
The MIC of Na+ was determined in the wild type (BD99), in the mrp null strain (VKN1), in each of the single mrp gene deletion strains, and in each of the deletion strains with the gene restored in the amyE locus. The determinations were made at pH 7.0 and at pH 8.3 as described in Materials and Methods. The two pHs were examined because of the role of the Na+/H+ antiport in both Na+ and alkali resistance and because Na+ cytotoxicity is elevated at alkaline pH (14, 20). A record was kept of the A600 after 15 h of growth in the absence of added Na+ at each pH (i.e., the same time at which growth was recorded for Na+-containing cultures) as an indicator of cell yield of each mutant at the two pHs. As shown in Table 2, all of the mrp mutants were from 6 to >10 times more sensitive to Na+ than the wild-type strain at pH 7.0, and there were no major differences in the absorbances reached by the different mutants and the wild type in the absence of added Na+. Restoration of the mutated gene to each of the single deletion mutants resulted in complementation in trans that resulted in essentially wild-type MICs for each of the mutants. At pH 8.3, the patterns were more complex. First, the mrpA and mrpD mutants, VK1A and VK1D, respectively, exhibited poor growth at the elevated pH in the absence of added Na+. This deficit was especially pronounced with VK1D, in which reintroduction of the gene in the amyE locus did not completely restore wild-type levels of growth. Second, all of the mutants exhibited at least 10-fold-greater sensitivity to Na+ than the wild type at pH 8.3, but the sensitivity of the mrpA, mrpB, mrpD, and mrpE mutants (VK1A, VK1B, VK1D, and VK1E, respectively) was reproducibly greater than that of the other three strains, with VK1D being the most Na+ sensitive of all the mutants. The most sensitive strains were also those that were not completely complemented up to wild-type levels upon restoration of the affected gene in trans.
Effects of expression of individual mrp genes in trans on the Na+ resistance of VK6 and VKN1 mutant strains.
Unlike cholate resistance, which depended upon the status of a single mrp gene, wild-type levels of Na+ resistance clearly depended upon the status of multiple mrp genes. Previous work had shown that expression of mrpA from an IPTG-inducible promoter in the amyE locus of mutant strain VK6 led to a significant increase in the Na+ resistance as well as the 22Na+ efflux activity of that strain. VK6 has a disrupted mrpA and reduced expression of mrpB through -G (9). No such increase in either resistance or efflux was observed upon expression of mrpA in the mrp null strain VKN1. We sought to assess whether MrpA is the likeliest candidate for the Na+-translocating protein in Mrp-dependent Na+/H+ antiport, albeit dependent in some manner on all the other mrp gene products as well. Each mrp gene was individually expressed in VK6 (VK6/mrpA through VK6/mrpG) and VKN1, and the MIC of Na+ was determined pH 7.0 and 8.3. As expected from earlier assays (9), expression of mrpA and mrpF increased the resistance of strain VK6 to Na+. Only mrpB expression among all the remaining mrp genes caused a significant increase in resistance and that was restricted to pH 7.0 (Table 3). In the mrp null strain VKN1, no single mrp gene caused a significant, reproducible increase in Na+ resistance (data not shown).
TABLE 3.
Na+ resistance of the polar mrpA mutant of B. subtilis strain VK6 upon expression of individual mrpA genes
| Strain | MICa (M) of Na+ at:
|
|
|---|---|---|
| pH 7.0 | pH 8.3 | |
| Wild type | 1.30 ± 0.05 | 0.71 ± 0.03 |
| VK6 | 0.29 ± 0.03 | 0.025 ± 0.01 |
| VK6/mrpA | 0.62 ± 0.04 | 0.15 ± 0.02 |
| VK6/mrpB | 0.36 ± 0.02 | 0.04 ± 0.01 |
| VK6/mrpC | 0.19 ± 0.02 | 0.031 ± 0.01 |
| VK6/mrpD | 0.18 ± 0.02 | 0.025 ± 0.01 |
| VK6/mrpE | 0.23 ± 0.02 | 0.028 ± 0.01 |
| VK6/mrpF | 0.46 ± 0.02 | 0.16 ± 0.01 |
| VK6/mrpG | 0.22 ± 0.02 | 0.045 ± 0.01 |
Minimal Na+ concentration at which no growth was observed after 15 h. The values are means of at least eight separate determinations ± standard deviations.
Energy-dependent efflux of 22Na+ from right-side-out membrane vesicles of B. subtilis wild-type and selected mrp mutant strains.
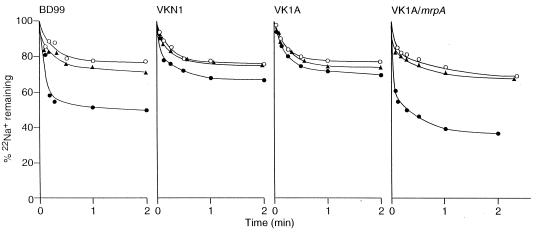
Prior studies of mrp-dependent Na+ fluxes had been conducted on respiring whole cells, in which significant cyanide- and protonophore-dependent inhibition was observed, especially for efflux from cells of mutant VK1A, in which mrpA expression was induced in trans (9). Reproducible data showing that an imposed diffusion potential could also energize such efflux had not been obtained in the whole-cell system. In order to further explore an MrpA-dependent capacity for secondary Na+/H+ antiport, right-side-out membrane vesicles of the wild-type and selected mutant strains were assayed for MrpA- and energy-dependent 22Na+ efflux. Ascorbate-PMS was found to support significant enhancement of the rate of 22Na+ efflux from vesicles prepared from the wild type and from VK1A/mrpA but only slight enhancement of efflux from the control preparations from the null VKN1 or VK1A strains (Fig. 6). The protonophore CCCP abolished the ascorbate-PMS–dependent efflux. The four preparations were then examined at a range of imposed valinomycin-mediated K+ diffusion potentials, with points taken during the initial efflux period under each condition. The percent 22Na+ remaining at 5 s is plotted in Fig. 7 for each preparation as a function of the magnitude of the imposed diffusion potential. The difference among the preparations correlated well, especially at the lowest diffusion potential established, −60 mV, with the ascorbate-PMS–dependent pattern. That is, Na+ efflux from the wild type and VK1A/mrpA was significantly greater than that from VKN1 or VK1A. At higher imposed potentials, efflux via some other transporter may be a more dominant contributor to the observed pattern.
FIG. 6.
Ascorbate-PMS–dependent 22Na+ efflux from right-side-out membrane vesicles of wild-type B. subtilis (BD99), mrp null mutant VKN1, mrpA mutant VK1A, and VK1A to which mrpA is restored in the amyE locus. The vesicles were passively loaded overnight at 4°C with 5 mM 22NaCl in 100 mM potassium phosphate (pH 7.5) plus 5 mM MgSO4. For assays of Na+ efflux, vesicles (100 μg of protein/ml) were incubated at 10°C. No further additions were made (○), or 10 mM potassium ascorbate plus 0.1 mM PMS was added in the absence (●) or presence (▴) of 10 μM CCCP. Samples were taken at various times and rapidly filtered. The radioactivity on the filters was measured by liquid scintillation counting.
FIG. 7.
Efflux of 22Na+ from right-side-out membrane vesicles of wild-type B. subtilis (BD99) and strains VKN1, VK1A, and VK1A/mrpA as a function of the magnitude of an imposed potassium diffusion potential. Membrane vesicles were loaded with 22Na+ as described in the legend to Fig. 6 in buffer containing 100 mM potassium phosphate and 10 μM valinomycin. These vesicles were diluted to different extents into 50 mM Tris-HCl (pH 7.5), in order to generate diffusion potentials of different magnitudes (indicated on the bottom of the figure). Samples were taken at 5 s after dilution and rapidly filtered. The radioactivity was determined by scintillation counting.
DISCUSSION
The mrp operon and its homologues are widely distributed among diverse prokaryotes and function in multiple processes involving ion-coupled transport reactions. Thus far, only one of these operons, the mrp operon of B. subtilis, has been shown to catalyze different transport reactions that relate to different gene products within the operon. The major finding of the current study is that MrpF can function in cholate and Na+ efflux independently of any other mrp gene product, whereas MrpA-dependent Na+/H+ antiport activity and Na+ resistance are highly dependent upon other mrp gene products, probably requiring all six of them. MrpA is a strong candidate for a major, if not sole, structural gene for Mrp-encoded Na+/H+ antiport, since the antiport activity of mrpA mutant VK1A, which has elevated levels of all the remaining mrp genes, has low Na+ resistance and efflux activities. In addition, mrpA overexpression in mutant VK6 (polar) elevates the Na+ resistance of that strain more than overexpression of any of the other mrp genes.
We tentatively hypothesize that the Na+ efflux catalyzed by MrpF is coupled to solute efflux (e.g., endogenous cholate-like substrate and/or exogenous cholate-like compounds) rather than being a true Na+/H+ antiport mode of this independent transporter. However, the assays conducted to date of MrpF-dependent cholate and Na+ efflux have not shown that there actually is coupling between the two substrates. In the whole cells in which the assays have thus far been conducted, there are complications of high contaminating Na+ levels, multiple antiporters apart from mrp, and the potential presence of an endogenous substrate that could substitute for preloaded cholate. Attempts to assess coupling of cholate efflux by MrpF in vesicles from the current B. subtilis strains were not undertaken because of their substantial Na+/H+ antiport activities as well as difficulties that we have had in making good everted vesicle preparations from these strains. Future studies will attempt to clarify the possible Na+-cholate coupling in everted vesicles from appropriate Escherichia coli strains if, as is now anticipated, expression of MrpF alone yields an active transporter. Moreover, attempts will be made to identify a transport activity for additional mrp gene products, especially MrpB and MrpC.
The basis for the MrpB-dependent increase in the Na+ resistance of strain VK6 at pH 7.0 is of interest. The Block+ program for motif analysis (4) does not provide useful clues vis à vis a specific MrpB transport activity, but indicates that MrpC has some similarities to Na+-coupled organic acid transporters. If there were one or more additional contributors to overall Mrp-dependent Na+ efflux, that would explain why mrpA and mrpF mutants have slightly but reproducibly higher Na+ resistance than the mrp null strain VKN1 at pH 7.0. No attempt can be made to interpret the differences in MIC at pH 8.3, since the levels of Na+ that are toxic to the mrp mutants at that pH are already in the range of contaminating Na+, and thus assessments of differences are unlikely to be accurate. The striking growth deficit of mrpA and mrpD mutants in the absence of added Na+ at pH 8.3 might in fact reflect a much greater Na+ sensitivity, effected by contaminating levels, in these strains. The evident importance of mrpD at high pH is intriguing, especially since its overexpression does not increase the Na+- resistance of polar mrpA strain VK6, and thus it is unlikely to be an antiport protein itself.
The studies of MrpA-dependent Na+ efflux in the right-side-out vesicles of VK1A/mrpA support the earlier indication from whole-cell assays (9) that the Na+/H+ antiporter can function as a secondary, proton motive force-dependent antiporter. However, although not shown, we were unable to demonstrate efflux at pH 8.0 and 8.3 in these vesicles even though Mrp-dependent Na+ efflux in malate-utilizing cells is clearly an important function at this pH. The possibility of a primary coupling mode for Mrp-dependent Na+/H+ antiport, using redox energy, is underscored by the importance and efficacy of the Mrp system in cells at elevated pH; by the complex dependence of the antiport on multiple mrp gene products; by the importance of MrpD at elevated pH; and by the strong sequence similarity between several mrp gene products—especially MrpA, MrpB, MrpE, and MrpD—to hydrophobic subunits of energy-coupled NADH dehydrogenase and to regions of other redox proteins (as analyzed via BLAST [1] and Block+ [4]). To explore possible primary energization, we are undertaking studies of the Bacillus mrp operons expressed in various E. coli strains. If a redox-dependent activity and complex formation are supported, it will be important to identify the electron donors and acceptors which would facilitate any subsequent efforts to study a purified Mrp complex.
Another final set of observations of interest in the current study emerge from the Northern analyses. Evidently, deletion of any of the mrp genes results in a significant increase in mrp expression, with the effect being somewhat smaller in mrpC and mrpF mutants. A simple interpretation would be that a rise in cytoplasmic Na+ leads to the overexpression. Even if correct, there are many features left to elucidate, including the basis for the difference between the effect in mrpC and mrpF mutant strains as well as the elements and mechanism of the putative regulatory effect. Another finding that will merit further exploration is that expression of maeN, which encodes an Na+-malate symporter, is partially coordinated with expression of mrp except that its basal expression is much greater and lower rather than greater levels of maeN RNA are observed in the mrpA mutant VK1A. The greatly reduced levels of maeN RNA in the VK1A strain may reflect disruption of some feature in the regulatory interaction between maeN and mrp that is lost during construction of the mrpA deletion. The regulatory coupling of maeN and mrp suggests the importance under at least some growth conditions of coordinating a full Na+ cycle. Such a cycle would encompass Na+/malate symport and Na+ reextrusion in exchange for H+ and also coupled to (MrpF-dependent) efflux of external chaotropes (e.g., cholate) and perhaps additional metabolic by-products. Under alkaline pH conditions, such an Na+-coupled cycle could be particularly important for achieving both substrate uptake and cytoplasmic pH regulation, and its efficacy would be enhanced if a primary, redox-coupled energization option existed for one or more of the efflux activities.
ACKNOWLEDGMENTS
This work was supported in part by research grants DE-FG02-86ER13559 from the Department of Energy and GM28454 from the National Institute of General Medical Sciences to T.A.K. and from the Inoue Enryo Memorial Foundation for Promoting Science to M.I.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E F, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Dimari J F, Bechhofer D. Initiation of mRNA decay in Bacillus subtilis. Mol Microbiol. 1991;7:705–717. doi: 10.1111/j.1365-2958.1993.tb01161.x. [DOI] [PubMed] [Google Scholar]
- 3.Hamamoto T, Hashimoto M, Hino M, Kitada M, Seto Y, Kudo T, Horikoshi K. Characterization of a gene responsible for the Na+/H+ antiporter system of alkalophilic Bacillus species strain C-125. Mol Microbiol. 1994;14:939–946. doi: 10.1111/j.1365-2958.1994.tb01329.x. [DOI] [PubMed] [Google Scholar]
- 4.Henikoff S, Henikoff J G. Protein family classification based on searching a database of blocks. Genomics. 1994;19:97–107. doi: 10.1006/geno.1994.1018. [DOI] [PubMed] [Google Scholar]
- 5.Henner D J. Inducible expression of regulatory genes in Bacillus subtilis. Methods Enzymol. 1990;185:223–228. doi: 10.1016/0076-6879(90)85022-g. [DOI] [PubMed] [Google Scholar]
- 6.Hiramatsu T, Kodama K, Kuroda T, Mizushima T, Tsuchiya T. A putative multisubunit Na+/H+ antiporter from Staphylococcus aureus. J Bacteriol. 1998;180:6442–6448. doi: 10.1128/jb.180.24.6642-6648.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horton R M. In vitro recombination and mutagenesis of DNA. Methods Mol Biol. 1996;67:141–149. doi: 10.1385/0-89603-483-6:141. [DOI] [PubMed] [Google Scholar]
- 8.Ireton D D, Rudner Z, Siranosian K J, Grossman A D. Integration of multiple developmental signals in Bacillus subtilis through Spo0A transcription factor. Genes Dev. 1993;7:283–294. doi: 10.1101/gad.7.2.283. [DOI] [PubMed] [Google Scholar]
- 9.Ito M, Guffanti A A, Oudega B, Krulwich T A. mrp, a multigene, multifunctional locus in Bacillus subtilis with roles in resistance to cholate and to Na+ and in pH homeostasis. J Bacteriol. 1999;181:2394–2402. doi: 10.1128/jb.181.8.2394-2402.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jack D L, Storms M L, Tchieu J H, Paulsen I T, Saier M H., Jr A broad-specificity multidrug efflux pump requiring a pair of homologous SMR-type proteins. J Bacteriol. 2000;182:2311–2313. doi: 10.1128/jb.182.8.2311-2313.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaback H R. Bacterial membranes. Methods Enzymol. 1971;22:99–120. [Google Scholar]
- 12.Kosono S, Morotomi S, Kitada M, Kudo T. Analyses of a Bacillus subtilis homologue of the Na+/H+ antiporter gene which is important for pH homeostasis of alkaliphilic Bacillus sp. C-125. Biochim Biophys Acta. 1999;1409:171–175. doi: 10.1016/s0005-2728(98)00157-1. [DOI] [PubMed] [Google Scholar]
- 13.Kosono S, Ohashi Y, Kawamura F, Kitada M, Kudo T. Function of a principal Na+/H+ antiporter, ShaA, is required for initiation of sporulation in Bacillus subtilis. J Bacteriol. 2000;182:898–904. doi: 10.1128/jb.182.4.898-904.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krulwich T A, Guffanti A A, Ito M. pH tolerance in Bacillus: alkaliphiles vs non-alkaliphiles. In: Chadwick D J, Cardew G, editors. Bacterial responses to pH. Chichester, England: Wiley; 1999. pp. 167–182. [DOI] [PubMed] [Google Scholar]
- 15.Krulwich T A, Ito M, Gilmour R, Hicks D B, Guffanti A A. Energetics of alkaliphilic Bacillus species: physiology and molecules. Adv Microb Physiol. 1998;40:410–438. doi: 10.1016/s0065-2911(08)60136-8. [DOI] [PubMed] [Google Scholar]
- 16.Kudo T, Hino M, Kitada M, Horikoshi K. DNA sequences required for the alkalophily of Bacillus sp. strain C-125 are located close together on its chromosomal DNA. J Bacteriol. 1990;172:7282–7283. doi: 10.1128/jb.172.12.7282-7283.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:205–275. [PubMed] [Google Scholar]
- 18.Masaoka Y, Ueno Y, Morita Y, Kuroda T, Mizushima T, Tsuchiya T. A two-component multidrug efflux pump, EbrAB, in Bacillus subtilis. J Bacteriol. 2000;182:2307–2310. doi: 10.1128/jb.182.8.2307-2310.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy E, Huwyler L, Bastos M D D. Transposon Tn554: complete nucleotide sequence and isolation of transposition defective and antibiotic-sensitive mutants. EMBO J. 1985;4:3357–3365. doi: 10.1002/j.1460-2075.1985.tb04089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Padan E, Krulwich T A. Sodium stress. In: Storz G, Hengge-Aronis R, editors. Bacterial stress responses. Washington, D.C.: ASM Press; 2000. pp. 117–130. [Google Scholar]
- 21.Putnoky P, Kerezt A, Nakamura T, Endre G, Grosskopf E, Kiss P, Kondorosi A. The pha cluster of Rhizobium meliloti involved in pH adaptation and symbiosis encodes a novel type of K+ efflux system. Mol Microbiol. 1998;28:1091–1101. doi: 10.1046/j.1365-2958.1998.00868.x. [DOI] [PubMed] [Google Scholar]