Abstract
Background
Omicron variant (B.1.1.529) is a dominant variant worldwide. However, the risk factors for Omicron variant clearance are yet unknown. The present study aimed to investigate the risk factors for early viral clearance of Omicron variant in patients with a history of inactivated vaccine injection.
Methods
Demographic, clinical, and epidemiological data from 187 patients were collected retrospectively during the Omicron variant wave.
Results
73/187 and 114/187 patients were administered two and three doses of vaccine, respectively. The median duration of SARS-CoV-2 RNA positivity was 9 days, and the difference between patients with two and three vaccine injections was insignificant (P = 0.722). Fever was the most common symptom (125/187), and most patients (98.4%) had a fever for < 7 days. The RNA was undetectable in 65/187 patients on day 7. Univariable logistic analysis showed that baseline glucose, uric acid, lymphocytes count, platelet count, and CD4+ T lymphocyte count were associated with SARS-CoV-2 RNA-positivity on day 7. Multivariable analysis showed that glucose ≥ 6.1 mmol/L and CD4+T lymphocytes count were independent risk factors for RNA positivity on day 7. 163/187 patients had an undetectable RNA test on day 14, and uric acid was the only independent risk factor for RNA positivity. Moreover, baseline glucose was negatively correlated with uric acid and CD4+ and CD8+ T cell count, while uric acid was positively correlated with CD4+ and CD8+ T cell count.
Conclusions
Omicron variant clearance was delayed in breakthrough cases with elevated fasting blood glucose, irrespective of the doses of inactivated vaccine.
Keywords: SARS-CoV-2, COVID-19, Omicron, Glucose, Uric acid
Background
In the past 2 years, approximately 527 million patients have been infected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and 6.3 million were deceased globally. Omicron variant (B.1.1.529), first reported in November 2021, has become the dominant variant worldwide [1, 2]. Although its virulence is weak, SARS-CoV-2 is more transmissible and infectious than other variants of concern [1, 3].
The spike receptor-binding domain (RBD) in the Omicron variant has enhanced binding properties with the human angiotensin-converting enzyme 2 (ACE2) receptor, which facilitates cell permeability [4]. Since RBD is the primary target of neutralizing antibodies upon vaccination or infection, whether mutations in RBD can affect the neutralizing activity of monoclonal antibodies (mAbs), leading to immune escape, is a significant concern [5, 6]. Moreover, mutations in the amino-terminal domain (NTD) also reduce the protectivity of the vaccine. Data from a multicenter retrospective study from Singapore showed that viral load was similar between vaccinated and unvaccinated patients at diagnosis. The mRNA vaccine caused a rapid decline in the Delta variant’s viral load of the Delta variant [7]. Another study showed that the viral load was lower in patients infected within 6 months of vaccination compared to the unvaccinated individuals, while no significant difference was detected in the viral load between patients vaccinated > 6 months before infection and unvaccinated individuals [8]. However, data about convalescent plasma or vaccines against the Omicron variant are disappointing [9–12]. Sera from convalescent patients infected by the Delta variant or vaccinated individuals showed low or no neutralizing activity against the Omicron variant [9, 11]. Although antibodies generated by a booster vaccine show a lower response against the Omicron variant than Delta, accumulating evidence warranted the administration of a booster vaccine dose [11, 13].
Based on previous studies, blood glucose predicted the mortality of coronavirus disease 2019 (COVID-19), even in patients without a history of diabetes [14–16]. High fasting plasma glucose level (≥ 6.1 mmol/L) at admission is an independent predictor for prolonged SARS-CoV-2 shedding [17]. Individuals with hyperglycemia had lower spike IgG titer than those with normoglycemia after two doses of the BNT162b2 vaccine, indicating that hyperglycemia is associated with a poor humoral immune response [18]. In young adults with type 1 diabetes, the elevated glucose level is positively associated with persistent symptoms [19]. The in vitro experiment confirmed that increased glucose concentration decreases the viability of A549 cells and induces ACE2 overexpression [20]. In addition, viral loads are high, and respiratory tissue damage is severe in the diabetic mice model [21].
To date, the risk factors for Omicron variant clearance are yet unknown. Herein, we investigated the risk factors for 7- and 14-day viral clearance post-infection of Omicron variant in patients with a history of inactivated vaccine injection.
Methods
Patients
A total of 239 patients with COVID-19 were admitted to the Third People’s Hospital of Changzhou between March 13, 2022 and May 10, 2022. All the patients were diagnosed according to the Chinese guidelines for the diagnosis and treatment of COVID-19 [22]. COVID-19 was diagnosed based on the epidemiological history and the positive RNA test in the throat swabs. Biochemical examination and a computed tomography (CT) scan were given routinely at admission. According to the guidelines for the prevention and treatment of diabetes, the disease was diagnosed based on the history of the patient, glycated hemoglobin (HbA1c), and repeated fasting glucose tests [23]. Demographic, clinical, and epidemiological data were retrospectively collected from electronic medical records and a laboratory information management system.
Reverse transcriptase-polymerase chain reaction (RT-PCR) assay
COVID-19 was confirmed based on the RT-PCR assay performed by the Laboratory of the Third People’s Hospital of Changzhou using a commercial kit (Jiangsu Bioperfectus Technologies Co., Taizhou, China). According to the manufacturer’s instructions, cycle threshold (Ct) values of ORF1ab and N genes < 35 indicated a positive RNA test. Duplicate tests were performed at 24 h if the result was negative (Ct ≥ 35).
Statistical analysis
All analyses were performed using SPSS 25.0 software (Armonk, NY, USA). Data were expressed as median (interquartile range, IQR) or frequencies and compared using the Kruskal–Wallis test or chi-square test. Correlation analysis was performed using Spearman’s correlation test. Logistic regression analysis was conducted to identify the independent risk factors for viral clearance. A two-sided P-value < 0.05 was considered statistically significant.
Results
Baseline characteristics of patients with COVID-19
Among the 239 patients with COVID-19, 52 were excluded because of the undefined history of the vaccine. Data from 187 patients were analyzed, including 73 patients who had two doses of vaccine injection and 114 patients who received three doses. The duration from the first, second, and third vaccine injections to infection was defined as T1, T2, and T3, respectively. Only 5 patients had a T2 < 6 months, and none of the patients had a T3 < 1 month.
For comorbidity, 3 patients had chronic hepatitis B, 8 had diabetes, 17 had hypertension, 1 had pulmonary tuberculosis, and 1 had chronic kidney disease. None of the patients was administered glucocorticoid or immunosuppressor at admission or during hospitalization.
A total of 43 patients had elevated fasting blood glucose (≥ 6.1 mmol/L) at admission, among whom 7 patients had a diabetes history, and 1 patient was diagnosed with diabetes during hospitalization. Among the 35 patients who were not diagnosed with diabetes, the fasting blood glucose in 17 patients decreased to a normal level in a week without any hypoglycemic treatment, and 18 patients with slightly elevated glucose (≥ 6.1 mmol/L and < 7.5 mmol/L) did not undergo a repeat test. In addition, only 9 patients had a glucose ≥ 7.9 mmol/L at admission, and 4 patients had a normal glucose level in a week and were excluded from diabetes.
Fever was the most common symptom (125/187, 66.8%), and most patients (123/125, 98.4%) had a fever duration of < 7 days. Other symptoms included cough (30/187, 16.0%), pharyngalgia (22/187, 11.8%), muscular soreness (9/187, 4.8%), headache (9/187, 3.2%), and diarrhea (6/187, 3.2%). According to CT scans, 13 individuals were diagnosed with unilateral pneumonia and 6 with bilateral pneumonia, while none developed severe pneumonia or ARDS. The median duration of SARS-CoV-2 RNA-positivity was 9 days, and the difference between patients with two and three doses of vaccine injection was insignificant (Z = 0.356, P = 0.722).
Characteristics of patients with and without SARS-CoV-2 clearance on day 7
As shown in Table 1, 65 (34.8%) patients had an undetectable RNA test on day 7. No significant difference was detected in age, diabetes, T1, T2, and duration of fever between patients with and without SARS-CoV-2 clearance. Patients with viral clearance on day 7 had a lower glucose level than those with a positive RNA test (Z = 3.943, P < 0.01). In addition, neutrophils were higher, while the lymphocyte and platelet counts were lower in patients with a positive RNA test than those with a negative RNA test (Z = 2.160, 2.014, and 2.114, respectively; P < 0.05). Moreover, CD4+ T lymphocytes were higher in patients with viral clearance on day 7 (Z = 2.647, P < 0.01).
Table 1.
Characteristics of patients with and without SARS-CoV-2 on day 7
| Variables | Positive (n = 122) | Negative (n = 65) | Z or χ2 | P value |
|---|---|---|---|---|
| Age, years | 47.5(34–52) | 43(33–50.5) | 1.495 | 0.14 |
| Male, n(%) | 90(73.8) | 53(81.5) | 1.422 | 0.23 |
| Diabetes, n(%) | 6(4.9) | 2(3.1) | 0.351 | 0.72 |
| T1,days | 305.5(263.8–348.5) | 315.0(265.5–348.0) | 0.065 | 0.95 |
| T2,days | 275.5(233.5–310.3) | 274.0(234.5–315.0) | 0.031 | 0.98 |
| Duration of fever, days | 2.0(0–3.0) | 1.0(0–2.0) | 1.277 | 0.20 |
| Laboratory findings | ||||
| ALT, U/L | 20.2(15.0–33.2) | 21.7(14.8–41.5) | 0.555 | 0.58 |
| AST, U/L | 19.5(16.0–25.0) | 20.0(16.5–25.5) | 0.526 | 0.60 |
| Glucose, mmol/L | 5.4(4.8–6.3) | 4.8(4.5–5.5) | 3.943 | < 0.01 |
| Creatinine, µmol/L | 69.3(58.1–76.9) | 69.5(58.9–79.6) | 0.546 | 0.59 |
| Uric acid, µmol/L | 304.6(259.5–363.9) | 329.8(273.3–395.3) | 1.806 | 0.07 |
| CRP, mg/L | 4.0(2.0–11.1) | 4.7(1.9–9.3) | 0.338 | 0.74 |
| Neutrophils, E + 09/L | 4.2(3.2–5.3) | 3.6(2.4–5.0) | 2.160 | 0.03 |
| Lymphocytes, E + 09/L | 1.1(0.7–1.4) | 1.2(0.9–1.6) | 2.014 | 0.04 |
| Platelets, E + 09/L | 202.0 (174.8–234.3) | 224.0(188.5–257.5) | 2.114 | 0.04 |
| CD4+T | 424.0(275.8–647.8) | 550.0(412.0–705.5) | 2.647 | < 0.01 |
| CD8+T | 310.5(203.8–483.0) | 359.0(260.5–494.0) | 1.550 | 0.12 |
| B cells count | 123.0(79.0–233.0) | 158.0(99.0–258.0) | 1.963 | 0.05 |
| CT findings | ||||
| Unilateral pneumonia, n(%) | 10(8.20) | 3(4.62) | 0.841 | 0.55 |
| Bilateral pneumonia, n(%) | 4(3.28) | 2(3.08) | 0.006 | 0.99 |
Data were expressed as median (IQR) for continuous variables and n (%) for categorical values, and were compared using Kruskal–Wallis test or Chi-square test. T1, duration from the first infection of vaccine to diagnosis; T2, duration from the second infection of vaccine to diagnosis; ALT, alanine aminotransferase; AST, aspartate transaminase; CRP, C-reaction protein; CT, computer tomography
Risk factors for SARS-CoV-2 RNA positivity on day 7
As shown in Table 2, the univariable logistic analysis showed that baseline glucose, uric acid, lymphocyte count, platelet count, and CD4+T lymphocyte count are associated with SARS-CoV-2 RNA positivity on day 7. Then, multivariable analysis showed that baseline glucose and CD4+T lymphocytes count were independent risk factors for SARS-CoV-2 RNA positivity on day 7 (odds ratio (OR) = 0.631 and 1.001, 95% confidence interval (CI): 0.456–0.874 and 1.000–1.002, P < 0.01 and P = 0.02, respectively).
Table 2.
Risk factors for SARS-CoV2 clearance on day 7
| Baseline variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Odds ratio | 95%CI | P | Odds ratio | 95%CI | P | |
| Age (years) | 0.979 | 0.955–1.005 | 0.11 | |||
| Sex | 0.637 | 0.302–1.342 | 0.24 | |||
| T1 | 0.999 | 0.994–1.005 | 0.87 | |||
| T2 | 1.000 | 0.995–1.006 | 0.91 | |||
| ALT | 1.004 | 0.991–1.017 | 0.53 | |||
| Glucose | 0.607 | 0.433–0.851 | < 0.01 | 0.631 | 0.456–0.874 | < 0.01 |
| Creatinine | 1.006 | 0.987–1.025 | 0.55 | |||
| Uric acid | 1.003 | 1.000–1.007 | 0.07 | |||
| Lymphocytes | 1.668 | 1.026–2.712 | 0.04 | |||
| Platelets | 1.006 | 1.000–1.011 | 0.04 | |||
| CD4 + T cells | 1.001 | 1.000–1.002 | < 0.01 | 1.001 | 1.000–1.002 | 0.02 |
| CD8 + T cells | 1.001 | 0.999–1.002 | 0.28 | |||
| B cells | 1.002 | 1.000–1.004 | 0.11 | |||
CI, 95% confidence interval; T1, duration from the first infection of vaccine to diagnosis; T2, duration from the second infection of vaccine to diagnosis; ALT, alanine aminotransferase
More patients had a glucose level ≥ 6.1 mmol/L at admission in the RNA-positive group (35/122, 28.7%) compared to those in the RNA-negative group (8/65, 12.3%) (χ2 = 6.426, P = 0.01). Then, the multivariable analysis used glucose ≥ 6.1 mmol/L as a categorical value, and the data showed that glucose ≥ 6.1 mmol/L and CD4+ T lymphocytes count were independent risk factors for SARS-CoV-2 RNA-positivity on day 7 (OR = 0.351 and 1.001, 95% CI: 0.150–0.825 and 1.000–1.002, P = 0.02 and P = 0.01, respectively).
Characteristics of patients with and without SARS-CoV-2 clearance on day 14
As shown in Table 3, most patients had an undetectable RNA test on day 14 (163/187, 87.2%). Patients with viral clearance at day 14 had higher uric acid levels and lymphocyte counts than those with a positive RNA test (Z = 2.997 and 2.084, P < 0.01 and = 0.04, respectively). The CD4+ and CD8+ T cell count was high in patients with viral clearance (Z = 2.382 and 2.094, both P < 0.05).
Table 3.
Characteristics of patients with and without SARS-CoV-2 on day 14
| Variables | Positive (n = 24) | Negative (n = 163) | Z or χ2 | P value |
|---|---|---|---|---|
| Age, years | 46.5(40.0–56.5) | 46.0(34.0–51.0) | 1.132 | 0.26 |
| Male, n (%) | 17(70.8) | 126(77.3) | 0.486 | 0.49 |
| Diabetes, n (%) | 2(8.3) | 6(3.7) | 1.106 | 0.27 |
| T1, days | 290.0(258.5–321.3) | 315.0(266.0–352.0) | 1.737 | 0.08 |
| T2, days | 270.0(221.5–291.8) | 275.0(234.0–317.0) | 0.998 | 0.32 |
| Duration of fever, days | 2.0(0–4.0) | 1.0(0–3.0) | 0.537 | 0.59 |
| Laboratory findings | ||||
| ALT, U/L | 19.6(13.0–26.2) | 21.1(15.3–37.1) | 1.636 | 0.10 |
| AST, U/L | 19.0(16.0–22.0) | 20.0(16.0–25.0) | 0.670 | 0.50 |
| Glucose, mmol/L | 5.4(4.8–6.7) | 5.1(4.7–5.9) | 1.526 | 0.13 |
| Creatinine, µmol/L | 75.8(55.9–82.7) | 69.0(58.5–76.9) | 1.539 | 0.12 |
| Uric acid, µmol/L | 269.3(224.2–316.8) | 322.4(270.3–380.2) | 2.997 | < 0.01 |
| CRP, mg/L | 6.8(3.4–11.1) | 3.9(1.8–9.6) | 1.695 | 0.09 |
| Neutrophils, E + 09/L | 3.6(2.9–4.6) | 4.1(3.0–5.3) | 1.244 | 0.21 |
| Lymphocytes, E + 09/L | 0.8(0.6–1.3) | 1.1(0.8–1.5) | 2.084 | 0.04 |
| Platelets, E + 09/L | 193.5(161.3–222.5) | 209.0(179.0–252.0) | 1.810 | 0.07 |
| CD4+T | 387.5(238.0–503.0) | 482.0(332.0–693.0) | 2.382 | 0.02 |
| CD8+T | 291.0(162.5–384.0) | 346.0(220.0–495.0) | 2.094 | 0.04 |
| B cells count | 11.4(7.8–16.2) | 11.8(7.9–16.2) | 0.215 | 0.83 |
| CT findings | ||||
| Unilateral pneumonia, n(%) | 6(25.0) | 7(4.3) | 13.865 | < 0.01 |
| Bilateral pneumonia, n(%) | 1(4.2) | 5(3.1) | 0.081 | 0.57 |
Data were expressed as median (IQR) for continuous variables and n (%) for categorical values, and were compared using Kruskal–Wallis test or Chi-square test. T1, duration from the first infection of vaccine to diagnosis; T2, duration from the second infection of vaccine to diagnosis; ALT, alanine aminotransferase; AST, aspartate transaminase; CRP, C-reaction protein; CT, computer tomography
Risk factors for SARS-CoV-2 RNA positivity on day 14
As shown in Table 4, the univariate logistic analysis showed that baseline uric acid, lymphocyte count, platelet count, and CD4+ and CD8+ T lymphocyte count were associated with SARS-CoV-2 RNA-positivity on day 14. Then, multivariate analysis showed that only baseline uric acid is the independent risk factor for SARS-CoV-2 RNA positivity on day 14 (OR = 1.008, 95% CI: 1.002–1.014, P = 0.01).
Table 4.
Risk factors for SARS-CoV2 clearance on day 14
| Baseline variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Odds ratio | 95%CI | P | Odds ratio | 95%CI | P | |
| Age (years) | 0.973 | 0.938–1.009 | 0.14 | |||
| Sex | 0.713 | 0.275–1.850 | 0.49 | |||
| T1 | 1.007 | 0.998–1.016 | 0.12 | |||
| T2 | 1.004 | 0.997–1.012 | 0.27 | |||
| ALT | 1.015 | 0.990–1.042 | 0.24 | |||
| Glucose | 0.899 | 0.734–1.101 | 0.30 | |||
| Creatinine | 0.980 | 0.956–1.005 | 0.11 | |||
| Uric acid | 1.008 | 1.002–1.014 | 0.01 | 1.008 | 1.002–1.014 | 0.01 |
| Lymphocytes | 2.677 | 0.986–7.266 | 0.05 | |||
| Platelet | 1.009 | 1.000–1.018 | 0.05 | |||
| CD4+T cells | 1.002 | 1.000–1.004 | 0.04 | |||
| CD8+T cells | 1.003 | 1.000–1.006 | 0.03 | |||
| B cells | 1.003 | 0.999–1.007 | 0.20 | |||
CI, 95% confidence interval; T1, duration from the first infection of vaccine to diagnosis; T2, duration from the second infection of vaccine to diagnosis; ALT, alanine aminotransferase
Adjusted multivariable analysis was conducted when CD4+ and CD8+ T lymphocyte count was deleted, and data showed that uric acid is the only independent risk factor for SARS-CoV-2 RNA positivity on day 14.
Correlation between glucose, uric acid, CD4+T, and CD8+T cell count
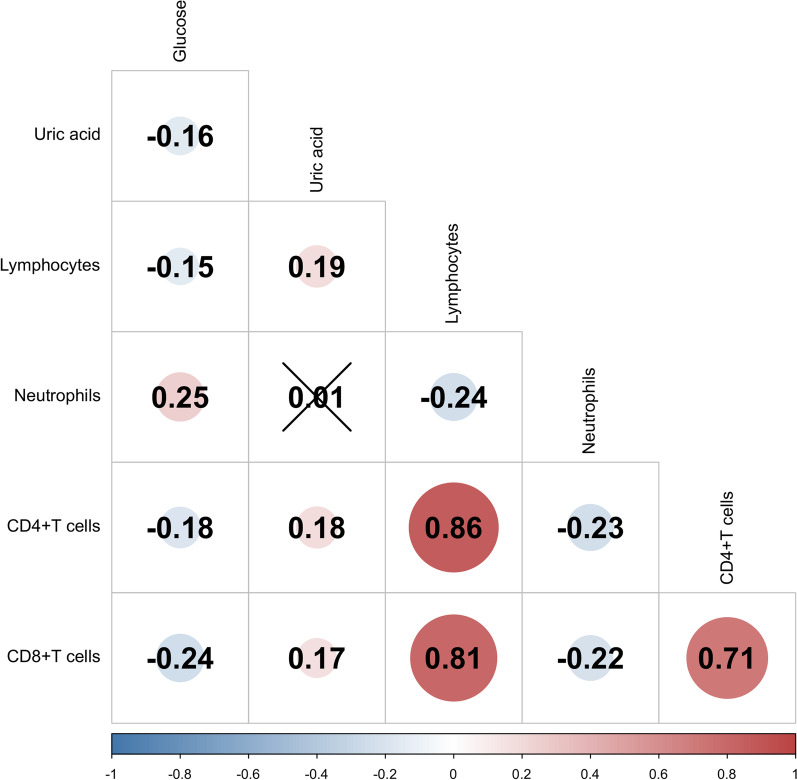
As shown in Fig. 1, baseline glucose was negative with uric acid, lymphocyte count, CD4+ T cell count, and CD8+ T cell count (all P < 0.05), while uric acid was positively correlated with CD4+ T cell and CD8+ T cell count (both P < 0.05). In addition, there was a positive correlation between CD4+ T and CD8+ T cell count (r = 0.710, P < 0.01).
Fig. 1.
Correlation between serum glucose, uric acid, CD4+T and CD8+T cells count in Omicron variant infected patients
Discussion
In the present study, we investigated the risk factors for early clearance of Omicron variant in patients with a history of inactivated vaccine injection. On day 7 after admission, 34.8% of patients had an undetectable RNA test, and baseline glucose ≥ 6.1 mmol/L and CD4+ T lymphocyte count were independent risk factors for SARS-CoV-2 RNA positivity. On day 14 after admission, SARS-CoV-2 RNA became undetectable in 87.2% of patients, and uric acid was the only independent risk factor for RNA-positivity.
The knowledge of prolonged SARS-CoV-2 shedding during Omicron waves is limited, especially for patients receiving inactivated vaccine injections. For most mAbs, the neutralizing activity against the Omicron variant is limited [10, 11, 24]. Wang et al. reported that the seroconversion rate of neutralizing antibodies was just 3.3% for individuals who received two doses of inactivated vaccine, while the rate was 95% for those who received three doses of vaccine [25]. Nonetheless, the neutralizing antibody titer induced by three vaccine doses was 16.5-fold low for the Omicron variant [25]. In the present study, the duration of SARS-CoV-2 shedding was similar between patients who received two and three doses of the vaccine. Thus, it can be speculated that mutations in RBD affect the neutralizing activity of antibodies induced by the inactivated vaccine. Since most patients were vaccinated > 6 months before infection, the long interval from vaccination to infection may account for the non-optimal protection activity. These results suggest that effective vaccine strategies are warranted.
Several studies indicated that glucose at admission is an independent risk factor for the poor prognosis of COVID-19 during the Delta variant wave [15, 16, 20]. Moreover, high glucose at admission is an independent predictor for prolonged SARS-CoV-2 shedding, irrespective of a history of diabetes [17]. Similar results were obtained in the present study during the Omicron variant wave. Glucose-like metabolite deficiency may facilitate viral entry and replication, as described previously [21]. Another mechanism is that the production of neutralizing antibodies is reduced in diabetes [18, 26]. In addition, our results showed that CD4+ T cell count is low in patients without early viral clearance and is negatively related to glucose. Thus, glucose and cellular immunity regulation may be an appropriate strategy for managing COVID-19.
The present data showed that uric acid is the only independent predictor for SARS-CoV-2-positivity on day 14, negatively correlated with glucose, and positively correlated with CD4+ and CD8+ T cells. Interestingly, the patients with lower serum uric acid levels experienced severe symptoms than those with higher uric acid levels [27, 28]. SARS-CoV-2 induces low serum uric acid [28] level and causes hyperglycemia in a cat model [29]. The interactions between uric acid, glucose, impaired cellular or humoral immunity, and viral replication are yet to be elucidated. Although diabetes is detected in only a few cases in the present study, elevated fasting blood glucose was a common phenomenon at admission; however, the underlying mechanisms are yet unknown. Moreover, the effect of hypoglycemic agents, such as sodium-glucose cotransporter 2 (SGLT2) inhibitors, on COVID-19 remains an interesting issue worth exploration [30].
The main limitation of the present study is that neutralizing antibodies at admission are not estimated. Regarding the Omicron variant spread, data from the present study highlighted the potential predictive value of metabolic factors, such as glucose and uric acid, in clinical practice. Typically, the benefits of the vaccine outweigh the potential risks [31, 32]; hence, vaccination during the pandemic of the Omicron variant should be administered without hesitation. Since outbreaks of COVID-19 occurred in different cities, a multicenter study with large sample size is warranted. Another limitation is that only 3 patients received HbA1c tests; otherwise, sufficient data could have been collected.
Conclusion
Omicron variant clearance was delayed in breakthrough cases with elevated fasting blood glucose, regardless of the inactivated vaccine doses.
Acknowledgements
Not applicable.
Abbreviations
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- RBD
Receptor-binding domain
- ACE2
Angiotensin-converting enzyme 2
- mAbs
Monoclonal antibodies
- NTD
Amino-terminal domain
- COVID-19
Coronavirus disease 2019
- RT-PCR
Reverse transcriptase-polymerase chain reaction
- Ct
Cycle threshold
- IQR
Interquartile range
- CT
Computed tomography
- OR
Odds ratio
- CI
Confidence interval
Author contributions
Conceptualization: YX and J-GZ. Data curation: X-JZ, G-CS, H-FL, WZ, S-QZ, Z-YH, and L-GL. Formal analysis: YX, X-JZ, G-CS, and H-FL. Funding acquisition: YX, X-JZ, S-QZ. Writing: YX, X-JZ, and G-JZ. X-JZ and G-CS contributed equally to this work. All authors read and approved the final manuscript.
Funding
This study was supported by the 333 High-level Talents Project of Jiangsu Province (LGY2020032), the Science and Technology Project of Changzhou (CJ20200057), Qingmiao Talents Cultivation Project of Changzhou Health Commission (CZQM2020089), Project of High-quality Education Resources of Nanjing Medical University (2021H049).
Availability of data and materials
Derived data are available on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Third People’s Hospital of Changzhou, and was performed in accordance with the 2013 Declaration of Helsinki.
Consent for publication
Written consent was exempted for this retrospective study.
Competing interests
The authors declare no conflict of interest.
Footnotes
Xiu-Jun Zhang and Guo-Can Si contributed equally to this work
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yuan Xue, Email: xueyuan80908@163.com.
Guojun Zheng, Email: immu_zheng@126.com.
References
- 1.Bhattacharyya RP, Hanage WP. Challenges in inferring intrinsic severity of the SARS-CoV-2 omicron variant. N Engl J Med. 2022;386(7):e14. doi: 10.1056/NEJMp2119682. [DOI] [PubMed] [Google Scholar]
- 2.Mohapatra RK, Tiwari R, Sarangi AK, et al. Omicron (B11529) variant of SARS-CoV-2: concerns, challenges, and recent updates. J Med Virol. 2022;94(6):2336–2342. doi: 10.1002/jmv.27633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo Y, Han J, Zhang Y, et al. SARS-CoV-2 omicron variant: epidemiological features, biological characteristics, and clinical significance. Front Immunol. 2022 doi: 10.3389/fimmu.2022.877101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Islam F, Dhawan M, Nafady MH, et al. Understanding the omicron variant (B.1.1.529) of SARS-CoV-2: mutational impacts, concerns, and the possible solutions. Ann Med Surg (Lond) 2022 doi: 10.1016/j.amsu.2022.103737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rossler A, Knabl L, von Laer D, Kimpel J. Neutralization Profile after recovery from SARS-CoV-2 omicron infection. N Engl J Med. 2022;386(18):1764–1766. doi: 10.1056/NEJMc2201607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu J, Collier AY, Rowe M, et al. Neutralization of the SARS-CoV-2 omicron BA.1 and BA.2 variants. N Engl J Med. 2022;386(16):1579–1580. doi: 10.1056/NEJMc2201849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chia PY, Ong SWX, Chiew CJ, et al. Virological and serological kinetics of SARS-CoV-2 Delta variant vaccine breakthrough infections: a multicentre cohort study. Clin Microbiol Infect. 2022;28(4):612 e611–612 e617. doi: 10.1016/j.cmi.2021.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bramante CT, Proper JL, Boulware DR, et al. Vaccination against SARS-CoV-2 is associated with a lower viral load and likelihood of systemic symptoms. Open Forum Infect Dis. 2022;9(5):ofac066. doi: 10.1093/ofid/ofac066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dejnirattisai W, Shaw RH, Supasa P, et al. Reduced neutralisation of SARS-CoV-2 omicron B.1.1.529 variant by post-immunisation serum. Lancet. 2022;399(10321):234–236. doi: 10.1016/S0140-6736(21)02844-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffmann M, Kruger N, Schulz S, et al. The Omicron variant is highly resistant against antibody-mediated neutralization: implications for control of the COVID-19 pandemic. Cell. 2022;185(3):447–456 e411. doi: 10.1016/j.cell.2021.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Planas D, Saunders N, Maes P, et al. Considerable escape of SARS-CoV-2 omicron to antibody neutralization. Nature. 2022;602(7898):671–675. doi: 10.1038/s41586-021-04389-z. [DOI] [PubMed] [Google Scholar]
- 12.Rossler A, Riepler L, Bante D, von Laer D, Kimpel J. SARS-CoV-2 omicron variant neutralization in serum from vaccinated and convalescent persons. N Engl J Med. 2022;386(7):698–700. doi: 10.1056/NEJMc2119236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schulz SR, Hoffmann M, Roth E, et al. Augmented neutralization of SARS-CoV-2 omicron variant by boost vaccination and monoclonal antibodies. Eur J Immunol. 2022;52(6):970–977. doi: 10.1002/eji.202249841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang MC, Hwang JM, Jeon JH, et al. Fasting plasma glucose level independently predicts the mortality of patients with coronavirus disease 2019 infection: a multicenter retrospective cohort study. Endocrinol Metab (Seoul). 2020;35(3):595–601. doi: 10.3803/EnM.2020.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Long H, Li J, Li R, et al. Plasma glucose levels and diabetes are independent predictors for mortality in patients with COVID-19. Epidemiol Infect. 2022;150:e106. doi: 10.1017/S095026882200022X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang P, Wang N, Wang J, et al. Admission fasting plasma glucose is an independent risk factor for 28-day mortality in patients with COVID-19. J Med Virol. 2021;93(4):2168–2176. doi: 10.1002/jmv.26608. [DOI] [PubMed] [Google Scholar]
- 17.Zhao Y, Xing H. Influence of fasting plasma glucose level on admission of COVID-19 patients: a retrospective study. J Diabetes Res. 2022 doi: 10.1155/2022/7424748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Islam Z, Yamamoto S, Mizoue T, et al. Association of impaired fasting glucose and diabetes with SARS-CoV-2 spike antibody titers after the BNT162b2 vaccine among health care workers in a tertiary hospital in Japan. Vaccines (Basel) 2022 doi: 10.3390/vaccines10050776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nimri R, Marianna R, Yackobovitch-Gavan M, et al. Symptoms and glycemic control in young people with type 1 diabetes following SARS-CoV-2 infection: an observational study. J Clin Endocrinol Metab. 2022 doi: 10.1210/clinem/dgac288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vargas-Rodriguez JR, Valdes Aguayo JJ, Garza-Veloz I, et al. Sustained hyperglycemia and its relationship with the outcome of hospitalized patients with severe COVID-19 potential role of ACE2 upregulation. J Pers Med. 2022 doi: 10.3390/jpm12050805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tong L, Xiao X, Li M, et al. A glucose-like metabolite deficient in diabetes inhibits cellular entry of SARS-CoV-2. Nat Metab. 2022 doi: 10.1038/s42255-022-00567-z. [DOI] [PubMed] [Google Scholar]
- 22.China NHCotPsRo Diagnosis and treatment plan for COVID-19(trial version 9) Int J Epidemiol Infect Dis. 2022 doi: 10.3760/cma.j.cn331340-20220325-00065. [DOI] [Google Scholar]
- 23.Chinese Diabetes S National Office for Primary Diabetes C. National handbook for the prevention and control of diabetes in primary care. Zhonghua Nei Ke Za Zhi. 2019;58(10):713–735. doi: 10.3760/cma.j.issn.0578-1426.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Iketani S, Liu L, Guo Y, et al. Antibody evasion properties of SARS-CoV-2 omicron sublineages. Nature. 2022;604(7906):553–556. doi: 10.1038/s41586-022-04594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang K, Jia Z, Bao L, et al. Memory B cell repertoire from triple vaccinees against diverse SARS-CoV-2 variants. Nature. 2022;603(7903):919–925. doi: 10.1038/s41586-022-04466-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D’Addio F, Sabiu G, Usuelli V, et al. Immunogenicity and safety of SARS-CoV-2 mRNA vaccines in a cohort of patients with type 1 diabetes. Diabetes. 2022 doi: 10.2337/db22-0053. [DOI] [PubMed] [Google Scholar]
- 27.Chen B, Lu C, Gu HQ, et al. Serum uric acid concentrations and risk of adverse outcomes in patients with COVID-19. Front Endocrinol (Lausanne) 2021 doi: 10.3389/fendo.2021.633767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu F, Guo Y, Lin J, et al. Association of serum uric acid levels with COVID-19 severity. BMC Endocr Disord. 2021;21(1):97. doi: 10.1186/s12902-021-00745-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y, Gao J, Huang K, et al. SARS-CoV-2 infection causes hyperglycemia in cats. J Infect Dis. 2022 doi: 10.1093/infdis/jiac143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu B, Li S, Kang B, Zhou J. The current role of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes mellitus management. Cardiovasc Diabetol. 2022;21(1):83. doi: 10.1186/s12933-022-01512-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bjork J, Bonander C, Moghaddassi M, et al. COVID-19 vaccine effectiveness against severe disease from SARS-CoV-2 Omicron BA.1 and BA.2 subvariants—surveillance results from southern Sweden, December 2021 to March 2022. Euro Surveill. 2022 doi: 10.2807/1560-7917.ES.2022.27.18.2200322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng SM, Mok CKP, Chan KC, et al. SARS-CoV-2 Omicron variant BA.2 neutralisation in sera of people with Comirnaty or CoronaVac vaccination, infection or breakthrough infection, Hong Kong, 2020 to 2022. Euro Surveill. 2022 doi: 10.2807/1560-7917.ES.2022.27.18.2200178. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Derived data are available on reasonable request.