Abstract
Emerging data evaluated the possible link between the Coronavirus 19 (COVID-19) vaccine and acute flares of rheumatic autoimmune diseases. However, the association between the COVID-19 vaccine and the development of de-novo rheumatic autoimmune diseases remained unclear. We report the first case series of three male patients who developed new-onset systemic lupus erythematosus following receiving Pfizer BNT162b2 mRNA vaccination. The clinical characteristics share some similarities with drug-induced lupus. More patients with SLE following COVID-19 may be diagnosed in the future. Additional studies will provide more significant insights into the possible immunogenic influence of the COVID-19 vaccine.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00296-022-05203-3.
Keywords: Systemic lupus erythematosus, COVID-19, Vaccine
Introduction
The Coronavirus 2019 (COVID-19) pandemic has substantial influence globally. COVID-19 vaccine is highly effective in preventing COVID-19 complications, including hospitalization and death [1]. However, patients with autoimmune rheumatic diseases (ARD), including systemic lupus erythematosus (SLE), are of great concern regarding COVID-19-related adverse effects. SLE patients have a higher risk of COVID-19-related complications, including mortality than age, sex-adjusted controls, and other ARD conditions [2, 3]. In addition, disease-modifying anti-rheumatic drugs (DMARDs) interfere with the immunogenicity of the COVID-19 vaccines, which subsequently impairs their effectiveness among ARD and SLE patients [4, 5].
The risk of ARD flares following the COVID-19 vaccines has raised some concerns. The COVID-19 vaccine is safe among SLE patients, with a low rate of severe adverse effects [6]. The VACOLUP study reported that a flare following COVID-19 vaccination occurred in only 3% of SLE patients [7]. However, in further research, up to a third of SLE patients were reported to flare after receiving the COVID-19 vaccine, mostly with mild symptoms not requiring hospitalizations [8].
New-onset SLE among previously healthy patients vaccinated against COVID-19 has recently emerged sporadically. We report the new onset of SLE following COVID-19 vaccination among three patients and a relevant literature review on the topic.
Case series
The clinical, laboratory, and therapeutic characteristics of our patients are shown in Table 1. We obtained written informed consent from all patients before conducting this analysis. ANA test was conducted via indirect immunofluorescence (IIF) assays using human epithelial type 2 cells (HEp-2 cells). Our institute’s laboratory cutoff for positive ANA is ≥ 1:160, corresponding to the 95th percentile of local age-matched and gender-matched healthy individuals. Additional extractable nuclear antigen antibodies (ENAS) were tested using a multiplex flow immune assay. This assay test for the significant ENAS of autoimmune disease. Only in the case of a positive anti-double-strand-DNA result, a confirmation test using indirect immunofluorescence Crithidia is performed. In the current manuscript, we reported only the positive results of the ENAS tests.
Table 1.
Patient’s clinical, immunological and therapeutic characteristics in the current series
| Patient number | Age/sex | Time after COVID-19 vaccination | Type of vaccination | Clinical features | Immunological features | EULAR/ACR SLE classification criteria | Treatment | SLEDAI 2K (first visit) | SLEDAI 2K (last visit) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 24/m | Seven days after the first dose | Pfizer BNT162b2 mRNA | Psoriasiform papulosquamous rash, a non-scarring alopecia, arthritis | Positive ANA 1/160, Ribosomal P, chromatin, and low C3 | 13 | HCQ 200 mg BID, topical steroids, and etoricoxib | 10 | 4 |
| 2 | 23/m | One month after the second dose | Pfizer BNT162b2 mRNA | Pancytopenia, fever, malar rash, oral ulcers, non-resolving headache, lymphadenopathy | Positive ANA 1/160, Ro/SSA, beta 2 glycoprotein IgG, direct Coombs | 14 | HCQ 200 mg BID, Prednisone 1 mg/kg, azathioprine 2 mg/kg, Granulocyte colony-stimulating factor and revolade | 15 | 1 |
| 3 | 56/m | One month after second dose | Pfizer BNT162b2 mRNA | Arthritis, lymphadenopathy | Positive ANA 1/160, double-stranded DNA, smith, low c3 | 15 | HCQ 200 mg BID and etoricoxib | 8 | 4 |
COVID-19 coronavirus 19, ANA antinuclear antibodies, EULAR/ACR European League Against Rheumatism and the American College of Rheumatology, BID twice daily, DNA deoxyribonucleic acid, mRNA messenger ribonucleic acid, SLEDA 2K systemic lupus erythematosus disease activity index 2000, HCQ hydroxychloroquine
The first patient is a 24-year-old male with no pre-morbidities or family history of autoimmune conditions. A rash appeared on the head, neck, and arms 3 days after he was vaccinated. In addition, he also reported a morning’s stiffness of an hour of both wrists. On examination, there were psoriasiform–papulosquamous plaques over the face, neck, and arms, non-scarring hair loss over the head, and stress pain at the wrists without effusion. Initial laboratory results displayed a normal range of complete blood count and blood chemistry panels, a positive antinuclear antibody (ANA) (1:160) with a speckled pattern, and positive anti chromatin (nucleosomal) and ribosome P antibodies (1.6 IU/mL, > 8.0 IU/mL, respectively). The C3 and C4 levels were 70 mg/dL (normal range 90–180 mg/dL) and 21 mg/dL (normal range 10–40 mg/dL), respectively. The patient started hydroxychloroquine 200 mg BID, mometasone furoate cream 0.1% QD, and etoricoxib 90 mg QD as needed. In addition, the patient was instructed to avoid direct exposure to the sun and to use broad-spectrum sunscreens. The rash and arthritis resolved during the following months, and the alopecia substantially improved.
The second patient is a 23-year-old male with no pre-morbidities or family history of autoimmune conditions. He was admitted to the hematology ward due to pancytopenia and fever, which started a month following the BNT162b2 mRNA COVID-19 vaccine administration. In addition, he reported a new non-resolving headache, oral ulcers, and malar rash over the face. The complete blood count at admission showed leukopenia 1800/µL (normal range 4800–10,000 µL), neutropenia 210 µL (normal range 1900–8000 µL), hemoglobin 9.1 g/dL (normal range 14.0–18.0 g/dL), and thrombocytopenia 12 µL (normal range 130–400 µL). The patient had positive ANA (1:160) with a fine-speckled pattern, positive anti-Ro/SSA (1.2 IU/mL), and a positive anti-beta 2 glycoprotein IgG (18.7 U/mL). In addition, there were positive direct Coombs tests but normal levels of haptoglobin. Serological tests were negative for active Epstein–Barr virus, cytomegalovirus, Human immunodeficiency virus, Parvo-B19 virus, hepatitis B, and C. The patient underwent positron emission tomography–computed tomography (PET/CT) during hospitalization. The test showed an enlargement of right sub-clavicular and axillary lymph nodes at the same side the COVID-19 vaccine was administrated. Axillary lymph node biopsy revealed follicular hyperplasia without signs of malignancy, infection, or Kikuchi–Fujimoto disease. Bone marrow biopsy showed general hyperplasia without sign of malignancy or infection. The patient started 1 mg/kg prednisone, hydroxychloroquine 200 mg BID, Filgrastim [a granulocyte colony-stimulating factor (G-CSF)], and Eltrombopag 50 mg QD. In addition, we added azathioprine 2 mg/kg a month later, parallel to tapping the prednisone dosage to complete cessation in 6 months.
The third patient is a 56-year-old male without past medical conditions or a family history of ARD. He was admitted to the internal ward due to joint pain and swollen left axillary lymph nodes. The symptoms occurred a month following receiving the BNT162b2 mRNA COVID-19 vaccine on the left hand. On physical examination, there was bilateral knee and left wrist arthritis. In addition, there was a left-sided palpable, soft, and tender axillary lymph node. Complete blood count and chemistry were normal. The patient had positive ANA (1:160) with a homogenous pattern, maximal levels of anti-double-stranded DNA (> 200 IU/mL confirmed by Crithidia luciliae), and maximal levels of positive anti-smith > 8.0 IU/mL. C3 level was 65 mg/dL and C4 level was 11 mg/dL. Additional urinalysis and immunological tests were normal. A total body computed tomography showed enlargement of the left axillary lymph node. A biopsy of this region showed follicular hyperplasia without signs of malignancy, infection, or Kikuchi–Fujimoto disease. We started hydroxychloroquine 200 mg BID and etoricoxib 90 mg QD as needed. A resolution of arthritis was noted but not of axillary lymphadenopathy.
Literature review
We conducted a literature search in Pubmed/Medline, Google Scholar, and Cochrane databases of English peer-reviewed new cases of SLE following any COVID-19 vaccine. Our search included the keywords “systemic lupus erythematosus,” “SLE” with “COVID-19 “, “vaccine,” “vaccination,” or “SARS CoV-2” between December 2020 and March 2022 (search strategy flowchart is presented in Supplementary Fig. 1). We expanded the search by reviewing the references for each case. We excluded cases of patients diagnosed with a highly suggestive background of SLE or antiphospholipid syndrome before receiving the COVID-19 vaccination or those who did not fulfill the 2019 EULAR/ACR SLE classification criteria [9]. We also excluded cases of subacute cutaneous lupus erythematosus without systemic manifestations. Although we did not limit the time between receiving a vaccination and the onset of symptoms, all cases occurred within a month following the COVID-19 vaccination. Our search identified six cases summarized in Table 2.
Table 2.
Summary of previous case reports of new-onset of systemic lupus erythematosus following COVID-19 vaccine
| References | Age/sex | Time after COVID-19 vaccination | Type of vaccination | Clinical features | Immunological features | EULAR/ACR SLE classification criteria | Treatment |
|---|---|---|---|---|---|---|---|
| Kim et al. [10] | 60/f | Not specified | Not specified | Class III lupus nephritis, pancytopenia, fever, pneumonitis | Positive ANA 1/1280, double-stranded DNA, smith, low c3 and c4 | 20 | Pulse methylprednisolone, I.V Cyclophosphamide, oral prednisolone (1 mg/kg) and hydroxychloroquine (100 mg BID) |
| Zavala-Miranda et al. [11] | 23/f | Two weeks after the first dose | Astra-Zeneca (ChAdOx1-S) | Class V lupus nephritis, lymphopenia | Positive ANA 1/1280, double-stranded DNA, low c3 and c4 | 20 | Mycophenolate mofetil, high-dose glucocorticoids, hydroxychloroquine, and diuretics (the exact dosages not specified) |
| Baez-Negron et al. [12] | 27/f | Two weeks after the second dose | Moderna mRNA-1273 | Tiredness, weight loss, arthritis, and later proteinuria | Positive ANA 1/160, Ro/SSA, La/SSB, double-stranded DNA, low C4 | 15 | Hydroxychloroquine (300 mg Q.D.), prednisone 20 mg, and subsequently mycophenolate mofetil 1 g BID |
| Mousa et al. [13] | 22/f | One week after the first dose | Pfizer BNT162b2 mRNA | Pancytopenia, cutaneous vasculitis, pancreatitis | Positive ANA 1/2000, double-stranded DNA, low c3 and c4 | 19 | Pulse methylprednisolone, oral prednisolone (40 mg), hydroxychloroquine (200 mg Q.D.), and azathioprine 50 mg |
| Nune et al. [15] | 24/m | Two weeks after the second dose | Pfizer BNT162b2 mRNA | Fever, arthritis, oral ulcers, leukopenia, lymphopenia, lymphadenopathy | Positive ANA 1/2560, double-stranded DNA, low c3 and c4 | 20 | Prednisone (1 mg/kg) and Methotrexate 15 mg/week |
| Patil et al. [14] | 22/f | Ten days after the first dose | Astra-Zeneca (ChAdOx1-S) | Fever, arthritis, rash, lymphadenopathy, anemia, pedal edema | Positive ANA 1/320, double-stranded DNA, histone | 20 | Hydroxychloroquine (400 mg Q.D.), prednisone 50 mg, and mycophenolate mofetil 2 g BID |
COVID-19 coronavirus 19, ANA antinuclear antibodies, BID twice daily, Q.D. once daily, mRNA messenger ribonucleic acid, DNA deoxyribonucleic acid, EULAR/ACR European League Against Rheumatism and the American College of Rheumatology
Discussion
We identified six case reports in the literature of new-onset SLE following the COVID-19 vaccine. Most cases of SLE were observed among women younger than 30 years (five out of six). The clinical picture described in these cases has shown considerable variation, and three reports described the presentation of lupus nephritis. Kim et al. described a 60-year-old female who developed class III lupus nephritis and pancytopenia (the exact time interval and vaccine type are not specified). The patients required treatment with high-dose glucocorticoids and intravenous cyclophosphamide [10]. Zavala-Miranda et al. described a 23-year-old female who developed class V lupus nephritis 2 weeks following the first dose of the Astra-Zeneca vaccine. The patient was treated with high-dose glucocorticoids and mycophenolate [11]. The third report by Baez-Negro’n et al. described a 27-year-old female who developed tiredness and symmetrical polyarthritis 2 weeks following the second dose of the Moderna mRNA-1273 vaccine [12]. Over several months she developed mild proteinuria (urine protein creatinine ratio 640 mg) and was treated with prednisone and mycophenolate. Although kidney biopsy was not performed, the patients had elevated anti-dsDNA antibodies, low C4 levels, and proteinuria, supporting the possible diagnosis of lupus nephritis in this case. A fourth patient was a 22-year-old female who presented 1 week following the Pfizer BNT162b2 mRNA vaccine. The patient had pancytopenia, cutaneous vasculitis, and pancreatitis [13]. She was treated with high-dose glucocorticoids, hydroxychloroquine, and azathioprine. The last two patients did not have significant organ involvement besides fever, arthritis, and cytopenias [14, 15].
The current research is the first reported case series of three patients who developed SLE following the COVID-19 vaccine. Although our series is composed of a relatively small number of patients, it has several advantages (Fig. 1). First, all patients in this series were evaluated in the same rheumatological clinic; they were diagnosed based on the EULAR/ACR SLE classification criteria and treated according to the 2019 EULAR recommendations for the management update of systemic lupus erythematosus [16, 17]. Second, all the patients in this series were men, as opposed to a significant predominance of SLE among women [18]. Third, SLE activity during the initial stage was high. One patient had severe disease activity (SLEDAI 2K of 15 points), and two had moderate activity (SLEDAI 2K of 10 and 8 points). Fortunately, no major organ was involved, and a few months after treatment initiation, all patients fulfilled the EULAR definition of low disease activity [17]. The lack of female predominance and the non-major organ involvement reported in drug-induced lupus may suggest that the mechanism of post-COVID-19 vaccine SLE has similar features [19]. Of note, anti-histone antibodies did not test in any of the patients in this series. Yet, these antibodies are not pathognomonic for drug-induced lupus and are also observed in 80% of primary SLE patients [20, 21]. We also acknowledge that cutaneous biopsy could support the diagnosis of SLE in two of our patients, yet this procedure was not conducted.
Fig. 1.
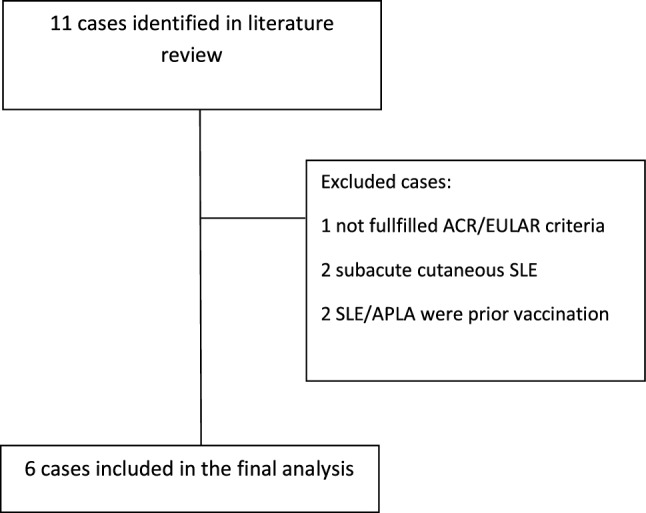
Literature strategy flowchart
It has been postulated that some vaccines can trigger the new onset of SLE. Two mechanisms have been suggested to explain the association between the foreign antigen (of the vaccine) and autoimmunity: molecular mimicry and activation of antigen-presenting cells toll-like receptors (TLRs) [22]. In addition, there are anecdotal reports in the literature of de-novo lupus that emerge after certain vaccines (e.g., hepatitis B, tetanus, and human papillomavirus) [23–25]. For instance, Gatto et al. reported six cases of SLE following anti-human papillomavirus vaccine uptake [25]. Yet, unlike our cohort, all of these patients had a family susceptibility to autoimmunity. Moreover, vaccines (including those mentioned above) are safe for most patients, and their protective effect mounts these anecdotal reports [26].
Other than SLE, the diagnosis of new-onset autoimmune conditions following COVID-19 vaccination has been reported previously (rheumatoid arthritis, immune thrombotic thrombocytopenia, autoimmune liver diseases, Guillain–Barré syndrome, IgA nephropathy) [27]. Two mechanisms have been suggested to explain the association between vaccination and the development of autoimmune conditions. The former is molecular antigen mimicry induced by a vaccine (e.g., against hepatitis B, human papillomavirus, and influenza) that may generate a new-onset or flare of autoimmune response [23, 24]. The latter is related to vaccine adjuvant activation, which activates endosomal Toll-like receptors (TLRs) TLR-7 and TLR-8, triggers the NLR pyrin domain containing 3 (NLRP3) inflammasome, and produces type I interferon [25, 26]. Both mechanisms have been proposed to explain a possible relationship between certain vaccines and SLE diagnosis [28, 29]. In addition, molecular mimicry induced by the bindings of hapten to a drug or its metabolite was also suggested as a possible mechanism in the pathogenesis of drug-induced lupus [30].
Conclusion
We report the first case series of three male patients who developed new-onset SLE following the COVID-19 vaccine. Although causality between vaccination and SLE diagnosis is difficult to determine, the very low incidence of SLE among men supports the conjecture that the COVID-19 vaccine can trigger autoimmunity by molecular mimicry or vaccine adjuvant. Furthermore, male predominance and rapid clinical improvement after treatment initiation support the possibility that the COVID-19 vaccine may induce autoimmune reactions similar to other regimens that cause drug-induced lupus. Thus, it is likely that clinician’s awareness of this phenomenon may improve the management of COVID-19 and its implications.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
IS and MAS are responsible for study conception and design. IS and LZ extracted the data. IS and LZ drafted the manuscript. TP, YR, and MAS gave critical revisions.
Declarations
Conflict of interest
All authors declare no conflicts of interest
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Iftach Sagy, Email: iftachsagy@gmail.com.
Lior Zeller, Email: zellerl@bgu.ac.il.
Yael Raviv, Email: YaelR2@clalit.org.il.
Tzvika Porges, Email: TzviPo@clalit.org.il.
Amir Bieber, Email: amir.bieber@gmail.com.
Mahmoud Abu-Shakra, Email: Mahmouds2@clalit.org.il.
References
- 1.Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. New Engl J Med. 2021;384(15):1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strangfeld A, Schäfer M, Gianfrancesco MA, Lawson-Tovey S, Liew JW, Ljung L, et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2021;80(7):930–942. doi: 10.1136/annrheumdis-2020-219498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gianfrancesco M, Hyrich KL, Al-Adely S, Carmona L, Danila MI, Gossec L, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2020;79(7):859–866. doi: 10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geisen UM, Berner DK, Tran F, Sümbül M, Vullriede L, Ciripoi M, et al. Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann Rheum Dis. 2021;80(10):1306–1311. doi: 10.1136/annrheumdis-2021-220272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Izmirly PM, Kim MY, Samanovic M, Fernandez-Ruiz R, Ohana S, Deonaraine KK, et al. Evaluation of immune response and disease status in systemic lupus erythematosus patients following SARS–CoV-2 vaccination. Arthritis Rheumatol. 2022;74(2):284–294. doi: 10.1002/art.41937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartels LE, Ammitzbøll C, Andersen JB, Vils SR, Mistegaard CE, Johannsen AD, et al. Local and systemic reactogenicity of COVID-19 vaccine BNT162b2 in patients with systemic lupus erythematosus and rheumatoid arthritis. Rheumatol Int. 2021;41(11):1925–1931. doi: 10.1007/s00296-021-04972-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Felten R. Tolerance of COVID-19 vaccination in patients with systemic lupus erythematosus: the international VACOLUP study. Lancet Rheumatol. 2021;3(9):e613–e615. doi: 10.1016/S2665-9913(21)00221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zavala-Flores E, Salcedo-Matienzo J, Quiroz-Alva A, Berrocal-Kasay A. Side effects and flares risk after SARS-CoV-2 vaccination in patients with systemic lupus erythematosus. Clin Rheumatol. 2022 doi: 10.1007/s10067-021-05980-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dörner T, Furie R. Novel paradigms in systemic lupus erythematosus. Lancet. 2019;393(10188):2344–2358. doi: 10.1016/S0140-6736(19)30546-X. [DOI] [PubMed] [Google Scholar]
- 10.Kim HJ, Jung M, Lim BJ, Han SH. New-onset class III lupus nephritis with multi-organ involvement after COVID-19 vaccination. Kidney Int. 2022;101(4):826–828. doi: 10.1016/j.kint.2022.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zavala-Miranda MF, González-Ibarra SG, Pérez-Arias AA, Uribe-Uribe NO, Mejia-Vilet JM. New-onset systemic lupus erythematosus beginning as class V lupus nephritis after COVID-19 vaccination. Kidney Int. 2021;100(6):1340–1341. doi: 10.1016/j.kint.2021.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Báez-Negrón L, Vilá LM. New-onset systemic lupus erythematosus after mRNA SARS-CoV-2 vaccination. Case Rep Rheumatol. 2022;202:1–4. doi: 10.1155/2022/6436839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Am N, Saleh AM, Khalid A, Alshaya AK, Alanazi SMM. Systemic lupus erythematosus with acute pancreatitis and vasculitic rash following COVID-19 vaccine: a case report and literature review. Clin Rheumatol. 2022 doi: 10.1007/s10067-022-06097-zs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patil S, Patil A. Systemic lupus erythematosus after COVID-19 vaccination: a case report. J Cosmet Dermatol. 2021;20(10):3103–3104. doi: 10.1111/jocd.14386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nune A, Iyengar KP, Ish P, Varupula B, Musat CA, Sapkota HR. The emergence of new-onset SLE following SARS-CoV-2 vaccination. QJM An Int J Med. 2021;114(10):739–740. doi: 10.1093/qjmed/hcab229. [DOI] [PubMed] [Google Scholar]
- 16.Aringer M, Costenbader K, Daikh D, Brinks R, Mosca M, Ramsey-Goldman R, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. 2019;71(9):1400–1412. doi: 10.1002/art.40930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fanouriakis A, Kostopoulou M, Alunno A, Aringer M, Bajema I, Boletis JN, et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis. 2019;78(6):736–745. doi: 10.1136/annrheumdis-2019-215089. [DOI] [PubMed] [Google Scholar]
- 18.Pons-Estel GJ, Ugarte-Gil MF, Alarcón GS. Epidemiology of systemic lupus erythematosus. Expert Rev Clin Immunol. 2017;13(8):799–814. doi: 10.1080/1744666X.2017.1327352. [DOI] [PubMed] [Google Scholar]
- 19.Vasoo S. Drug-induced lupus: an update. Lupus. 2006;15(11):757–761. doi: 10.1177/0961203306070000. [DOI] [PubMed] [Google Scholar]
- 20.Vedove CD, Giglio MD, Schena D, Girolomoni G. Drug-induced lupus erythematosus. Arch Dermatol Res. 2009;301:99–105. doi: 10.1007/s00403-008-0895-5. [DOI] [PubMed] [Google Scholar]
- 21.Gómez-Puerta JA, Burlingame RW, Cervera R. Anti-chromatin (anti-nucleosome) antibodies: diagnostic and clinical value. Autoimmun Rev. 2008;7(8):606–611. doi: 10.1016/j.autrev.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Millet A, Decaux O, Perlat A, Grosbois B, Jego P. Systemic lupus erythematosus and vaccination. Eur J Intern Med. 2009;20(3):236–241. doi: 10.1016/j.ejim.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 23.Ayvazian LF, Badger TL. Disseminated lupus erythematosus occurring among student nurses. N Engl J Med. 1948;239(16):565–570. doi: 10.1056/NEJM194810142391601. [DOI] [PubMed] [Google Scholar]
- 24.Tudela P, Martí S, Bonal J. Systemic lupus erythematosus and vaccination against hepatitis B. Nephron. 1992;62(2):236–236. doi: 10.1159/000187043. [DOI] [PubMed] [Google Scholar]
- 25.Gatto M, Agmon-Levin N, Soriano A, Manna R, Maoz-Segal R, Kivity S, et al. Human papillomavirus vaccine and systemic lupus erythematosus. Clin Rheumatol. 2013;32(9):1301–1307. doi: 10.1007/s10067-013-2266-7. [DOI] [PubMed] [Google Scholar]
- 26.Soriano A, Nesher G, Shoenfeld Y. Predicting post-vaccination autoimmunity: who might be at risk? Pharmacol Res. 2015;92:18–22. doi: 10.1016/j.phrs.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y, Xu Z, Wang P, Li XM, Shuai ZW, Ye DQ, et al. New-onset autoimmune phenomena post-COVID-19 vaccination. Immunology. 2022;165(4):386–401. doi: 10.1111/imm.13443. [DOI] [PubMed] [Google Scholar]
- 28.Agmon-Levin N, Zafrir Y, Paz Z, Shilton T, Zandman-Goddard G, Shoenfeld Y. Ten cases of systemic lupus erythematosus related to hepatitis B vaccine. Lupus. 2009;18(13):1192–1197. doi: 10.1177/0961203309345732. [DOI] [PubMed] [Google Scholar]
- 29.Soldevilla H, Briones S, Navarra S. Systemic lupus erythematosus following HPV immunization or infection? Lupus. 2012;21(2):158–161. doi: 10.1177/0961203311429556. [DOI] [PubMed] [Google Scholar]
- 30.Griem P, Wulferink M, Sachs B, Gleichmann E. Allergic and autoimmune reactions to xenobiotics: how do they arise? Immunol Today. 1998;19(3):133–141. doi: 10.1016/S0167-5699(98)80012-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


