Abstract
The selective serotonin reuptake inhibitors (SSRIs) citalopram and escitalopram are associated with QT prolongation, which increases the risk of serious arrhythmia. Consequently, regulatory agencies issued safety warnings in 2011. This study aimed to investigate the risk of serious arrhythmia following initiation of citalopram or escitalopram compared to other SSRIs and the risk in the periods before and after the warnings were issued. We conducted a series of nationwide cohort studies emulating a target trial using Danish healthcare register data from January 1, 2002, to December 31, 2016. We included patients (aged ≥65 years) who filled an SSRI prescription with a 1‐year washout period before the index date. The outcome was an event of serious arrhythmia. Individuals were followed for a maximum of 6 months using an intention‐to‐treat approach. Log‐binomial regression analyses were performed, estimating risk ratios (RRs) and 95% confidence intervals (CIs) adjusting for age and sex, comorbidities, and comedications with propensity scores. Dose–response effects were not investigated because dosage instructions were not available. We included 167,366 (146,014 individuals), 40,113 (37,069 individuals), and 50,281 (44,754 individuals) person‐trials of citalopram, escitalopram, and other SSRIs, respectively. In total, there were 228 events of serious arrhythmia. No difference in risk was observed in the entire study period for either citalopram (0.87 [0.62–1.22]) or escitalopram (0.85 [0.53–1.40]). We identified lower point estimates after the safety warning, RR 0.54 (95% CI 0.31–0.93) for citalopram and 0.58 (0.20–1.63) for escitalopram. Initiation of citalopram and escitalopram was not associated with an increased risk of serious arrhythmia. However, lower point estimates were observed after the safety warning.
Study Highlights
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
The selective serotonin reuptake inhibitors (SSRIs) citalopram and escitalopram have been associated with an increased risk of QT prolongation, causing regulatory agencies to issue drug safety warnings. Conflicting evidence has arisen from observational studies regarding the association between citalopram and escitalopram use and QT prolongation‐related arrhythmia and cardiac arrest.
WHAT QUESTION DID THE STUDY ADDRESS?
We aimed to estimate the relative risk of serious arrhythmia among citalopram and escitalopram initiators, compared to other SSRIs and to compare the risk before and after the warnings.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
Using emulation of a target trial, we found no increased risk of serious arrhythmia after initiation of citalopram or escitalopram. Lower risk estimates were identified after the safety warnings.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
The pro‐arrhythmic effects related to the QT‐prolonging potential of citalopram and escitalopram appear to be manageable in clinical practice. Future studies could shed light on whether the distribution of risk factors for arrhythmias change over time among SSRI users.
INTRODUCTION
Citalopram and escitalopram belong to the drug class selective serotonin reuptake inhibitors (SSRIs). They are most often used to treat depression, but they are also prescribed for a range of anxiety disorders. Both drugs are well‐tolerated, efficacious, and generally have a favorable safety profile compared to the tricyclic antidepressants that they have now largely replaced. 1 , 2 Citalopram, escitalopram, and sertraline are the most frequently initiated SSRIs in Denmark. 3 However, the use of citalopram and escitalopram has been linked to prolongation of the QT interval. 4 , 5 , 6 , 7 A prolonged QT interval is caused by irregularities in the heart’s electrical conduction, either congenital or acquired. Prolonged QT increases the risk of serious arrhythmia, including Torsade des Pointes, ventricular tachycardia, and ventricular fibrillation. Acquired QT prolongation may develop due to drugs that block hERG potassium channels. This results in a slower potassium efflux and a longer repolarization period, which increases the risk of developing a serious arrhythmia. 8 , 9 Therefore, there are suspicions that citalopram and escitalopram might be implicated in triggering serious arrhythmias. Based on an unpublished randomized trial showing dose‐dependent QT prolongation and individual case safety reports, the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) updated the Prescription Information and Summary of Product Characteristics for citalopram in 2011 and issued a drug safety communication to healthcare professionals. The warning regarded dose reduction recommendations, contraindications, precautions, and interactions. 10 , 11 A similar warning was also issued by the EMA regarding escitalopram, the S‐enantiomer of citalopram. 12 In contrast, the FDA did not issue a warning for escitalopram, as the unpublished trial did not show a dose‐dependent prolonged QT interval that was clinically significant for this drug. 10 In addition, the FDA dose recommendation for escitalopram in older adults was already lower than the EMA recommendation. 12 , 13 The association with arrhythmias has not been confirmed by clinical trials due to the rare nature of this outcome. Conflicting evidence has arisen from observational studies in which the majority have not identified increased risks. 14 , 15 , 16 , 17 , 18 , 19 However, three studies did. 20 , 21 , 22 The first study was published in 2011 by Weeke et al. It was a nationwide Danish case‐time‐control study that examined the risk of cardiac arrest associated with episodes of use of each SSRI compared to episodes of no use. 20 In a Canadian cohort study from 2016, Qirjazi et al. identified a small increased risk of ventricular arrhythmia among users of citalopram compared to users of paroxetine and sertraline. 21 Recently, a Danish case–control study found higher rates of out‐of‐hospital cardiac arrest among high‐dose citalopram users (>20 mg) and high‐dose escitalopram users (>10 mg) compared to users of sertraline. 22 To our knowledge, no study has identified an increased risk of serious arrhythmia following initiation of escitalopram compared to other SSRIs. Moreover, no study has compared the risk in the periods before and after the safety warning. In addition, in a signal detection study investigating individual SSRIs and their risk of serious medical events, we identified a potential safety signal of cardiac arrest among users of citalopram compared to users of other SSRIs. 23 Therefore, in the current study, we investigate the potential safety signal for citalopram in more detail and also assess the risk of arrhythmia and cardiac arrest in initiators of escitalopram. Finally, we compare the risk of serious arrhythmia before and after the safety warnings were issued.
METHODS
Data sources
We obtained data from the Danish national healthcare registries and used each person’s unique civil registration number (CPR) to link information from the included registries. The National Prescription Registry was used to identify SSRI exposure and comedications based on anatomical therapeutic chemical (ATC) classification codes. 24 , 25 We used the Danish National Patient Registry to identify exclusion criteria and events of serious arrhythmia and comorbidities. 26 Diagnosis codes were coded as International Statistical Classification of Diseases and Related Health Problems (ICD) 10th revision. 27 Procedure codes were coded with Nordic Classification of Surgical Procedures (NCSP). 28 The Danish Register of Causes of Death and the Danish Civil Registration System were used to identify deaths and migrations, respectively. 29 , 30
Study cohort
A nationwide registry‐based cohort study was conducted, emulating a hypothetical randomized trial (Table S1). We included patients aged 65 years and above, who redeemed a prescription of an SSRI after a washout period of at least 1 year between January 1, 2002, and December 31, 2016. The analysis was restricted to older adults (≥65 years) due to a low incidence of the outcome in younger adults. We excluded patients with <365 days of observation time before the index date, more than one SSRI on the index date (start of first SSRI prescription), and a previous diagnosis of serious arrhythmia 5 years before the index date. Moreover, patients with HIV, end‐stage renal diseases, liver diseases, severe respiratory diseases, other end‐stage diseases, organ transplants, congenital abnormalities, and substance misuse any time before the index date were excluded (Figure 1). A graphical depiction of the study design is illustrated in Figure S1. To increase the number of exposures and outcomes, we allowed individuals to enter the study more than once when fulfilling the eligibility, exposure washout, and exclusion criteria. 31 , 32 Hence, individuals could be included in multiple person‐trials, as illustrated in Figure 2. Here, three examples of individuals are illustrated: individual one, who enters two person‐trials, as the eligibility, exposure washout, and exclusion criteria are fulfilled two times. Individual two also enters twice, but at the second entry, the individual experiences the event of interest. Within the follow‐up period of the first entry, the individual fills a prescription which is not included because the 1‐year exposure washout is not fulfilled. After the second inclusion, the individual fills another prescription, which is not included because an exclusion criterion is not fulfilled (5‐year outcome washout). Finally, individual three enters three times. In this scenario, the individual fills three prescriptions that fulfill the eligibility, exposure washout, and exclusion criteria. In addition, three prescriptions are redeemed, which are not included because the exposure washout is not fulfilled. Definitions of exposures, outcomes, exclusion criteria, comorbidities, and comedications are provided in Table S2.
FIGURE 1.
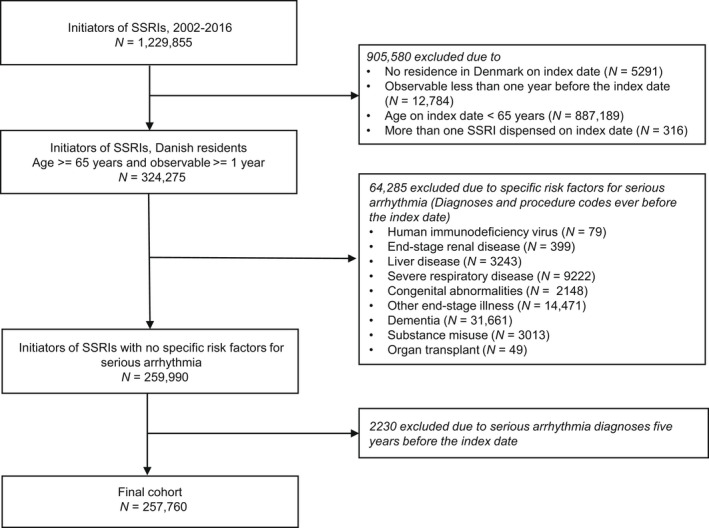
Flow chart from initiators of selective serotonin reuptake inhibitors (SSRIs) to the final cohort.
FIGURE 2.
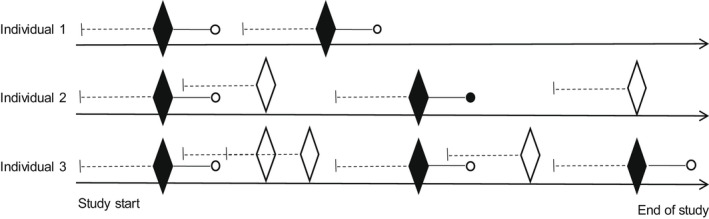
Repeated inclusions.  Prescription included as a person‐trial (eligibility, exposure washout, and exclusion criteria fulfilled);
Prescription included as a person‐trial (eligibility, exposure washout, and exclusion criteria fulfilled);  Prescription not included (criteria not fulfilled); ● Outcome within 6 months after the index date; ○ Censoring (death, emigration, end of follow‐up, or end of study);
Prescription not included (criteria not fulfilled); ● Outcome within 6 months after the index date; ○ Censoring (death, emigration, end of follow‐up, or end of study);  1‐year washout for the exposure.
1‐year washout for the exposure.
Exposures
We had two sets of exposures. These were initiators of citalopram (ATC code: N06AB04 and escitalopram N06AB10). The comparator group consisted of initiators of other SSRIs, being sertraline (N06AB06), paroxetine (N06AB05), fluvoxamine (N06AB08), and fluoxetine (N06AB03). Patients were followed from the prescription fill (index date) until the occurrence of serious arrhythmia or censoring (emigration, death, end of follow‐up period, or end of study period), whichever came first.
Outcome
The study outcome was an inpatient admission with a main diagnosis of serious arrhythmia with a washout period 5 years before the index date. We defined serious arrhythmia by the following ICD‐10 codes: I47.2 (ventricular tachycardia), I49.0 (ventricular fibrillation and flutter), I49.3 (ventricular premature depolarization), and I46 (cardiac arrest). 33
Covariates
The selection of covariates to adjust for potential confounding was based on recent reviews on arrhythmia and a previous study investigating the same outcome. 17 , 33 , 34 , 35 , 36 The covariates consisted of age, sex, comorbidities, and comedications. The comorbidities were defined as in and outpatient diagnoses of acute coronary syndrome, arrhythmia, arterial disease, cardiac surgery, valve disorders, venous thromboembolism, cerebrovascular disease, heart failure/cardiomyopathy, other ischemic heart disease, cancer, obesity, renal diseases, respiratory diseases, and psychiatric and neurologic diseases occurring 5 years before the index date. Moreover, thyroid disturbances, alcohol misuse, smoking, arrhythmia, and obesity included diagnoses and drug proxies based on prescriptions filled 6 months before the index date. Comedications were a fill of a prescription of antihypertensives, corticosteroids, anti‐dementia drugs, antidepressants, antipsychotics, digoxin, antiepileptics, hormone replacement treatment, antidiabetics, lipid‐lowering drugs, nitrates, nonsteroidal anti‐inflammatory drugs (NSAIDs), opioids, anticoagulants, anxiolytics, and antiplatelet drugs that occurred 6 months before the index date. More information is provided in Table S2.
Statistical analysis
Incidence rates (IRs) with 95% confidence intervals (CIs) were calculated using the gamma inverse cumulative distribution function. We used log‐binomial regression models to estimate risk ratios (RRs) and 95% CIs. Because individuals were allowed to enter the study more than once, we applied the generalized estimating equation method with an independent correlation structure to account for the correlation within individuals. 37 , 38 We estimated RRs adjusted for age and sex, and propensity score quintiles. Logistic regression models were used to estimate the propensity scores by index year with the exposure as the dependent variable and the risk factors as independent variables. 39 A supplementary analysis was conducted with propensity score matching. It was performed using one‐to‐one propensity score greedy nearest neighbor matching. The matching was done exactly on the index year. No replacement was applied but we applied a maximum caliper distance of 0.2 of the logit of the propensity score. Standardized mean differences were calculated for post‐matching assessment, where values below 25% were considered well balanced. 40
The risk before and after the safety warnings was further investigated. For citalopram, the period before the warning was defined from the start of the study on January 1, 2002, to October 27, 2011, the date of the EMA Pharmacovigilance Working Party plenary meeting discussing citalopram. 11 The period after the warning was from October 28, 2011, to the end of study on December 31, 2016. For escitalopram, the period before was defined from January 1, 2002, until November 24, 2011. 12 The period after was defined from November 25, 2011, until December 31, 2016.
Sensitivity analyses
To allow the safety warning to have an effect, we estimated the risk in the after periods in two sensitivity analyses. These had a 1‐month and 6‐month gap from the EMA meeting to the beginning of the after period. Hence, in the 1‐month gap analysis, the after period started on November 27, 2011, and December 25, 2011. In the 6‐month gap analysis, the after period started on April 27, 2012, and May 25, 2012, for citalopram and escitalopram, respectively. Moreover, a sensitivity analysis including only ventricular tachycardia and ventricular fibrillation and flutter in the definition of the outcome was conducted to compare results to a previous study. 21
Ethics statement
The study was approved by the Danish Data Protection Agency through the University of Copenhagen (0421‐0022/18‐7000). Ethical approval is not required for register‐based research in Denmark. 41
RESULTS
Cohort characteristics
In total, there were 167,366 (146,014 individuals), 40,113 (37,069 individuals), and 50,281 (44,754 individuals) person‐trials of citalopram, escitalopram, and other SSRIs included in the study. In total, 17.0% of individuals were eligible for the study more than once. The age distribution was similar among the groups. However, initiators of the comparator SSRIs were slightly older than initiators of citalopram and escitalopram. There were more initiators in the age groups 65–74 years and 75–84 years than 85 years and above across all exposure and comparator groups, and the majority were women (63.5–65.4%). More information about cohort characteristics is provided in Table 1. In the matched analysis, 49,823 (47,454 and 44,338 individuals) and 27,231 (25,737 and 25,019 individuals) matched pairs of citalopram and escitalopram initiators and their comparators were included. The age and sex distributions in these cohorts were similar to the unmatched cohorts. The standardized mean differences ranged from 0.28% to 20.58%. Before and after the warnings were issued, there were 126,546 and 40,820 person‐trials of citalopram and 34,351 and 5762 person‐trials of escitalopram, respectively. The annual number of SSRI initiators dropped during the study period, most pronounced for citalopram and escitalopram in the period from 2011 and onward (Figure S3).
TABLE 1.
Baseline characteristics of SSRI initiators
| Citalopram | Escitalopram | Other SSRIs | |
|---|---|---|---|
| N (%) | N = 167,366 | N = 40,113 | N = 50,281 |
| Unique individuals a | 146,014 | 37,069 | 44,754 |
| Trials per unique individual, N | |||
| 1 | 117,171 | 27,228 | 31,730 |
| 2 | 36,078 | 9231 | 12,463 |
| 3 | 10,396 | 2697 | 4118 |
| 4 | 2836 | 716 | 1372 |
| 5 | 682 | 173 | 410 |
| 6 | 146 | 38 | 146 |
| 7–8 | 57 | 30 | 42 |
| Sex, female | 106,222 (63.47) | 25,535 (63.66) | 32,859 (65.35) |
| Age, years, 65–74 | 65,030 (38.85) | 16,960 (42.28) | 25,796 (51.30) |
| Age, years, 75–84 | 66,587 (39.79) | 15,321 (38.19) | 17,726 (35.25) |
| Age, years, 85 and above | 35,749 (21.36) | 7832 (19.52) | 6759 (13.44) |
| Comorbidities | |||
| Acute coronary syndrome | 3348 (2.00) | 867 (2.16) | 863 (1.72) |
| Arterial disease | 6135 (3.67) | 1478 (3.68) | 1595 (3.17) |
| Cancer | 21,053 (12.58) | 5458 (13.61) | 5459 (10.86) |
| Cardiac surgery/invasive cardiac procedure | 6514 (3.89) | 1603 (4.00) | 1786 (3.55) |
| Cerebrovascular disease | 15,356 (9.18) | 3405 (8.49) | 2886 (5.74) |
| Heart failure/cardiomyopathy | 11,346 (6.78) | 2707 (6.75) | 2354 (4.68) |
| Serious neurologic disease | 961 (0.57) | 213 (0.53) | 200 (0.40) |
| Other ischemic heart disease | 21,117 (12.62) | 5108 (12.73) | 5354 (10.65) |
| Renal disease | 4037 (2.41) | 964 (2.40) | 819 (1.63) |
| Respiratory disease | 20,781 (12.42) | 4890 (12.19) | 5675 (11.29) |
| Valve disorders | 5590 (3.34) | 1386 (3.46) | 1499 (2.98) |
| Venous thromboembolism | 3878 (2.32) | 986 (2.46) | 1013 (2.01) |
| Comedications | |||
| ACE inhibitors and angiotensin receptor blockers | 56,074 (33.50) | 13,513 (33.69) | 16,919 (33.65) |
| Anticoagulants | 12,178 (7.28) | 2792 (6.96) | 2896 (5.76) |
| Anxiolytics | 78,435 (46.86) | 20,778 (51.80) | 24,578 (48.88) |
| Beta‐blocker | 40,002 (23.90) | 9248 (23.05) | 11,341 (22.56) |
| Calcium channel blockers | 37,220 (22.24) | 8459 (21.09) | 10,821 (21.52) |
| Corticosteroids, inhalants | 9768 (5.84) | 2453 (6.12) | 3216 (6.40) |
| Antidepressants | 19,535 (11.67) | 7039 (17.55) | 8675 (17.25) |
| Digoxin | 12,211 (7.30) | 2823 (7.04) | 2326 (4.63) |
| Antiepileptic drugs | 8641 (5.16) | 2398 (5.98) | 2889 (5.75) |
| Hormone replacement therapy | 16,750 (10.01) | 4486 (11.18) | 6137 (12.21) |
| Insulin | 5104 (3.05) | 1184 (2.95) | 1398 (2.78) |
| Lipid‐lowering drugs | 43,985 (26.28) | 10,419 (25.97) | 14,001 (27.85) |
| Loop diuretics | 32,785 (19.59) | 7448 (18.57) | 7180 (14.28) |
| Nitrates | 13,039 (7.79) | 2938 (7.32) | 3053 (6.07) |
| NSAIDs | 39,161 (23.40) | 9564 (23.84) | 10,895 (21.67) |
| Opioids | 44,614 (26.66) | 10,977 (27.37) | 11,887 (23.64) |
| Oral corticosteroids | 16,871 (10.08) | 4148 (10.34) | 4383 (8.72) |
| Oral antidiabetics | 12,858 (7.68) | 2880 (7.18) | 3947 (7.85) |
| Other diuretics | 46,096 (27.54) | 10,457 (26.07) | 11,403 (22.68) |
| Anti‐Parkinson’s drug | 4109 (2.46) | 1100 (2.74) | 1055 (2.10) |
| Platelet inhibitors | 66,061 (39.47) | 14,873 (37.08) | 15,700 (31.22) |
| Xantines | 1337 (0.80) | 281 (0.70) | 292 (0.58) |
| Alcohol related diseases and drugs for alcohol misuse | 3798 (2.27) | 1028 (2.56) | 1336 (2.66) |
| Arrhythmia and antiarrhythmic drugs | 22,874 (13.67) | 5371 (13.39) | 5438 (10.82) |
| Obesity and drugs to treat obesity | 3267 (1.95) | 777 (1.94) | 1314 (2.61) |
| Psychiatric disorders and antipsychotics | 22,598 (13.50) | 8082 (20.15) | 7868 (15.65) |
| Smoking‐related disorders and drugs to treat smoking dependence | 27,022 (16.15) | 6321 (15.76) | 7929 (15.77) |
| Thyroid disturbances | 14,639 (8.75) | 3645 (9.09) | 4575 (9.10) |
Abbreviations: ACE, angiotensin‐converting enzyme; NSAIDs, non‐steroid anti‐inflammatory drugs; SSRIs, selective serotonin reuptake inhibitors.
Except for the number of unique individuals, all counts and percentages refer to the number of person‐trials.
Citalopram and escitalopram and risk of serious arrhythmia
There were 225 events of serious arrhythmia observed in the study population. Of these, 147 occurred in citalopram initiators, 32 in escitalopram initiators, and 46 in the comparator group. The IRs among initiators of citalopram, escitalopram, and comparators were 19.2 (16.3–22.6), 17.5 (12.0–24.7), and 19.9 (14.6–26.6) per 10,000 person‐years, respectively. The RRs of serious arrhythmia among initiators of citalopram or escitalopram compared to other SSRIs are provided in Table 2. In general, we observed no significantly increased risks during the study period for either citalopram (RR 0.87 [0.62–1.22]) or escitalopram (RR 0.86 [0.53–1.40]). In the sensitivity analysis, including only ventricular tachycardia and ventricular fibrillation and flutter in the definition of the outcome, the point estimates were higher than the main analysis. Although, due to fewer events, CIs were also wider (Table S3).
TABLE 2.
Risk ratios of serious arrhythmia with citalopram and escitalopram compared to other SSRIs
| RR (95% CIs) | Crude | Age and sex adjusted | Propensity score adjusted |
|---|---|---|---|
| Whole study period | |||
| Citalopram | 0.96 (0.69–1.34) | 0.90 (0.64–1.26) | 0.87 (0.62–1.22) |
| Escitalopram | 0.87 (0.56–1.37) | 0.81 (0.51–1.28) | 0.86 (0.53–1.40) |
| Period before | |||
| Citalopram | 1.34 (0.84–2.15) | 1.26 (0.77–2.03) | 1.18 (0.72–1.96) |
| Escitalopram | 1.19 (0.67–2.11) | 1.06 (0.59–1.90) | 0.94 (0.51–1.72) |
| Period after | |||
| Citalopram | 0.59 (0.35–1.00) | 0.55 (0.32–0.94) | 0.54 (0.31–0.93) |
| Escitalopram | 0.57 (0.20–1.62) | 0.55 (0.19–1.59) | 0.58 (0.20–1.63) |
Note: Estimates provided both as crude, age and sex adjusted, and propensity score adjusted in the whole study period (January 1, 2002, to December 31, 2016), before and after the European Medicines Agency’s warning was issued.
Abbreviations: CIs, confidence intervals; RR, risk ratio; SSRIs, selective serotonin reuptake inhibitors.
We observed a significantly reduced risk of serious arrhythmia among initiators of citalopram after the warning (RR 0.54 [0.31–0.93]). The crude, age and sex adjusted, and propensity score matched analyses showed similar estimates (Table S3). The point estimates for escitalopram were similar but with wider and insignificant CIs (Table 2). In the sensitivity analyses introducing gaps of 1 month and 6 months in the after period, both citalopram and escitalopram initiation showed similar patterns as the analysis of the after period in the main analysis (Table S3).
DISCUSSION
In this large nationwide cohort study of older adults that emulated a target trial, no overall increased risk of serious arrhythmia was observed among initiators of citalopram or escitalopram compared to other SSRIs. Higher point estimates of RRs were observed in the period before the safety warnings were issued. Given the upper confidence limits, we cannot rule out an increased RR below 22% and 40% among citalopram and escitalopram initiators, respectively.
Previous studies have also investigated the risk of QT prolongation‐related arrhythmias (out‐of‐hospital cardiac arrest, sudden cardiac death, and ventricular arrhythmias) following SSRI initiation. A study by Leonard et al. using Medicaid claims data from 1999 to 2003, comparing citalopram to paroxetine, found no significant increased risk of sudden cardiac death or ventricular arrhythmia. 16 Another study conducted with Medicaid data from 1998 to 2011 by Ray et al., found no evidence of a significantly increased risk of sudden unexpected death, sudden cardiac death, or mortality with high doses of citalopram or escitalopram versus equivalent doses of fluoxetine, paroxetine, and sertraline. 17 Moreover, a cohort study conducted in Taiwan by Lin et al. on data from 2000 to 2011 found that citalopram and escitalopram exposure was not associated with a higher risk of arrhythmia compared to other SSRIs. 18 Studies with different designs (no use instead of active comparators) have also investigated the risk of serious arrhythmia. A UK study by Coupland et al. examined the risk of arrhythmia in adults (20–64 years) from 2000 to 2011 and found no significant increased risk of arrhythmia for patients treated with citalopram, even at high doses (>40 mg) compared to episodes of no use. 14 A cohort study from the United States, published in 2013 by Zivin et al., was neither able to identify increased risk in higher doses of citalopram nor that the risk differed from equivalent doses of sertraline. In contrast, they found lower risks among patients treated with medium doses (21–40 mg) compared to high doses (>40 mg). However, the authors state that this finding may be due to residual confounding because patients with multiple comorbidities were less likely to receive high doses. 15 Nevertheless, three previous studies have found an increased risk for citalopram but not for escitalopram. Weeke et al. 20 conducted a study on Danish registry data, which examined the risk of out‐of‐hospital cardiac arrest among citalopram and escitalopram users between 2001 and 2007. Qirjazi et al. 21 conducted a cohort study from 2002 to 2012 examining the risk of arrhythmia among older adults (≥65) newly prescribed citalopram and escitalopram, compared to sertraline and paroxetine. A recent nested case–control study from Denmark using data from the Danish Cardiac Arrest Registry found higher rates of out‐of‐hospital cardiac arrest among users of high‐dose citalopram (>20 mg) and escitalopram (>10 mg) compared to users of sertraline. 22
Our study differs from the existing studies by emulating a target trial in which individuals can be eligible for inclusion multiple times. Moreover, two of the previous studies did not include older adults in their cohorts, 14 , 16 whereas three included both younger and older adults. 15 , 17 , 18 Hence, our results should be cautiously compared to studies including young adults. We limited our analysis to adults aged 65 years and above due to a very low number of events in younger age groups. Weeke et al. examined the risk in older adults comparing episodes of use to no use. However, they defined their outcome as patients who had an out‐of‐hospital cardiac arrest and used the Danish Cardiac Arrest Registry as their data source, as opposed to our study, which used in‐hospital diagnosis codes. The study by Qirjazi et al. also consisted entirely of older adults. However, the definition of the outcome differed. Our study used two more diagnostic codes to define the outcome: premature ventricular depolarization and cardiac arrest. The sensitivity analysis excluding these diagnosis codes showed higher point estimates. However, the propensity score adjusted analysis was not possible among initiators of escitalopram because of too few events.
To our knowledge, this study is the only one that has examined the risk before and after the safety warnings were issued. We observed a drop in the overall number of initiators of SSRIs, particularly citalopram and escitalopram, which may be explained by the safety warnings in 2011 leading to initiation of other SSRIs, such as sertraline rather than citalopram and escitalopram. Our findings showed higher point estimates of serious arrhythmia before the warning and lower point estimates in the period after the warning for both drugs. This finding may be due to prescribers being more aware of patients at cardiovascular risk after the warning. In our previous signal detection study with annual sequential cohorts, we detected a potential safety signal of cardiac arrest among new users of citalopram compared to other SSRIs. 23 The current study examined the potential association in more detail with confounder adjustment tailored to serious arrhythmia and an emulated target trial design. The signal was not present at the end of our study, which is consistent with the current analysis showing a declining RR after the warning.
Our study has several strengths. Most importantly, we allowed patients to be included in multiple person‐trials, which increased the power of our study. Moreover, we used nationwide registry data that covered all new SSRI users from January 1, 2002, to December 31, 2016, thus avoiding selection bias. The outcome serious arrhythmia is known to have high validity. Sundbøll et al. conducted a population‐based validation study, which examined the positive predictive values (PPVs) for cardiovascular diagnoses in the Danish National Patient Registry. They found a PPV of 79% (67–88%) for ventricular tachycardia and ventricular fibrillation and flutter given as a primary diagnosis. For cardiac arrest, they found a PPV of 99% (92–100%). 42 Unfortunately, the study did not examine premature depolarization. To reduce confounding by indication, we utilized a new user active comparator design by comparing the risk among initiators of citalopram and escitalopram to the risk among initiators of other SSRIs. We excluded patients with risk factors for serious arrhythmia. In addition, we adjusted for a large set of potential confounders covering comedications and comorbidities through propensity score methods. Potential confounders were selected based on recent literature reviews and a previous study investigating the same outcome. 33 , 34 , 35 , 36 , 43 The robustness of our findings was checked in the analyses investigating the risk in the period after the warning. We did this by allowing a 1 month and 6 month gap from when the warnings were issued to the beginning of the after period.
The study also has some limitations. We used redeemed prescriptions rather than actual SSRI intake to define our exposure and comedications. We believe this exposure misclassification is similar among initiators of our exposure and comparator drugs. Moreover, we used in‐hospital diagnoses from admissions to define the outcome and comorbidities. We believe, however, that those who survive cardiac arrest outside a hospital will eventually be admitted to a hospital and that any potential outcome misclassification is nondifferential, as serious arrhythmias leading to hospital admission presumably occurs equally frequent in users of citalopram than users of other SSRIs. Even though we could adjust for several potential confounders, some residual confounding cannot be excluded. We used proxies for lifestyle factors that cannot be measured in our data directly. These covered smoking, body mass index, and alcohol use and were included as ICD‐10 and ATC codes for smoking‐related disorders, obesity, and alcohol misuse. Last, we could not study dose–response effects because dosage instructions are not recorded in the data. A recent Swedish study indicate that low doses are generally being prescribed in older adults (≥65 years) treated with citalopram and escitalopram. The mean (SD) prescribed daily doses in these patients were 17.6 mg (±6.0) for citalopram and 9.9 mg (±3.9) for escitalopram. 44 This means that the risk associated with higher doses of SSRIs may be diluted substantially. The change that we saw in the risk before and after the warnings may indicate that lower doses were used after the warnings or that clinicians became aware of risk factors, which we were not able to adjust for in our study.
CONCLUSION
In this large, population‐based cohort study, we observed no increased risk of serious arrhythmia among initiators of citalopram or escitalopram compared to initiators of other SSRIs. However, point estimates of serious arrhythmia risk were lower in the period after the regulatory agencies issued a safety warning.
AUTHOR CONTRIBUTIONS
All authors wrote the manuscript. All authors designed the research. M.Aa. and M.An. performed the research. M.Aa. and M.An. analyzed the data.
Funding information
The Pharmacovigilance Research Center is supported by a grant from the Novo Nordisk Foundation (NNF15SA0018404) to the University of Copenhagen. This includes the professorship of M.An. and part of the PhD study of M.Aa.
CONFLICT OF INTEREST
At the time of the study, M.L.D.B. was an employee of the Copenhagen Centre for Regulatory Science (CORS). CORS is a cross‐faculty university‐anchored institution involving various public (Danish Medicines Agency, Copenhagen University) and private (Novo Nordisk A/S, Lundbeck A/S, Ferring pharmaceuticals A/S, LEO pharma A/S) stakeholders as well as patient organizations (Rare Diseases Denmark). The center is purely devoted to the scientific aspects of the regulatory field and has a patient‐oriented focus, and the research is not a company‐specific product or directly company related. Currently, M.L.D.B. is employed by Utrecht University to conduct research under the umbrella of the Utrecht Centre for Pharmaceutical Policy and Regulation. This center receives no direct funding or donations from private parties, including the pharma industry. Research funding from public–private partnerships (e.g., IMI, The Escher Project; http://escher.lygature.org/), is accepted under the condition that no company‐specific study is conducted. The center has received unrestricted research funding from public sources (e.g., World Health Organization, Netherlands Organization for Health Research and Development, the Dutch National Health Care Institute, EC Horizon 2020, the Dutch Medicines Evaluation Board, and the Dutch Ministry of Health). M.An. has participated in research projects funded by AstraZeneca, H. Lundbeck & Mertz, Novartis, and Pfizer, and has received fees for leading courses and teaching from Atrium, the Danish Association of the Pharmaceutical Industry. All other authors declared no competing interests for this work.
Supporting information
Appendix S1
ACKNOWLEDGMENT
The authors gratefully acknowledge Kathrine Pape for her contribution to discussions on the study design.
Aakjær M, Werther SK, De Bruin ML, Andersen M. Serious arrhythmia in initiators of citalopram, escitalopram, and other selective serotonin reuptake inhibitors: A population‐based cohort study in older adults. Clin Transl Sci. 2022;15:2105‐2115. doi: 10.1111/cts.13319
REFERENCES
- 1. Chu A, Wadhwa R. Selective Serotonin Reuptake Inhibitors. In: StatPearls [internet]. StatPearls Publishing; 2021. Accessed December 7, 2021. http://www.ncbi.nlm.nih.gov/books/NBK554406/. [PubMed] [Google Scholar]
- 2. RADS . Behandlingsvejledning for almen praksis – Unipolar depression . 2015.
- 3. Forns J, Pottegård A, Reinders T, et al. Antidepressant use in Denmark, Germany, Spain, and Sweden between 2009 and 2014: incidence and comorbidities of antidepressant initiators. J Affect Disord. 2019;249:242‐252. [DOI] [PubMed] [Google Scholar]
- 4. Castro VM, Clements CC, Murphy SN, et al. QT interval and antidepressant use: a cross sectional study of electronic health records. BMJ. 2013;346:f288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jayasinghe R, Kovoor P. Drugs and the QTc interval . Accessed December 7, 2021. https://www.nps.org.au/australian‐prescriber/articles/drugs‐and‐the‐qtc‐interval.
- 6. Zhang Y, Baranchuk A, Hancox JC. Towards limiting QT interval prolongation and arrhythmia risk in citalopram use. Cardiol J. 2014;21(5):454‐457. [DOI] [PubMed] [Google Scholar]
- 7. Ojero‐Senard A, Benevent J, Bondon‐Guitton E, et al. A comparative study of QT prolongation with serotonin reuptake inhibitors. Psychopharmacology (Berl). 2017;234(20):3075‐3081. [DOI] [PubMed] [Google Scholar]
- 8. Stroobandt RX, Barold SS, Sinnaeve AF. ECG from Basics to Essentials Step by Step. John Wiley & Sons; 2016. [Google Scholar]
- 9. Fanoe S, Kristensen D, Fink‐Jensen A, et al. Risk of arrhythmia induced by psychotropic medications: a proposal for clinical management. Eur Heart J. 2014;35(20):1306‐1315. [DOI] [PubMed] [Google Scholar]
- 10. Center for Drug Evaluation and Research . FDA Drug Safety Communication: Revised Recommendations for Celexa (citalopram hydrobromide) Related to a Potential Risk of Abnormal Heart Rhythms with High Doses. FDA; [Internet]. 2019. Accessed January 27, 2022. https://www.fda.gov/drugs/drug‐safety‐and‐availability/fda‐drug‐safety‐communication‐revised‐recommendations‐celexa‐citalopram‐hydrobromide‐related. [Google Scholar]
- 11. European Medicines Agency . Pharmacovigilance Working Party (PhVWP). October 2011 Plenary Meeting. HMA Management Group; 2011. [Google Scholar]
- 12. European Medicines Agency . Pharmacovigilance Working Party (PhVWP). November 2011 Plenary Meeting. HMA Management Group; 2011. [Google Scholar]
- 13. Food and Drug Administration . Lexapro (escitalopram oxalate). The U.S. Food and Drug Administration; 2002. [Google Scholar]
- 14. Coupland C, Hill T, Morriss R, Moore M, Arthur A, Hippisley‐Cox J. Antidepressant use and risk of cardiovascular outcomes in people aged 20 to 64: cohort study using primary care database. BMJ. 2016;352:i1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zivin K, Pfeiffer PN, Bohnert ASB, et al. Evaluation of the FDA warning against prescribing citalopram at doses exceeding 40 mg. Am J Psychiatry. 2013;170(6):642‐650. [DOI] [PubMed] [Google Scholar]
- 16. Leonard CE, Bilker WB, Newcomb C, Kimmel SE, Hennessy S. Antidepressants and the risk of sudden cardiac death and ventricular arrhythmia. Pharmacoepidemiol Drug Saf. 2011;20(9):903‐913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ray WA, Chung CP, Murray KT, Hall K, Stein CM. High‐dose citalopram and escitalopram and the risk of out‐of‐hospital death. J Clin Psychiatry. 2017;78(2):190‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lin Y‐T, Lu T‐S, Hansen RA, Wang C‐C. Selective serotonin reuptake inhibitor use and risk of arrhythmia: a nationwide, Population‐Based Cohort Study, Clin Ther. 2019;41(6):1128‐1138.e8. [DOI] [PubMed] [Google Scholar]
- 19. Wu C‐S, Tsai Y‐T, Hsiung CA, Tsai H‐J. Comparative risk of ventricular arrhythmia and sudden cardiac death across antidepressants in patients with depressive disorders. J Clin Psychopharmacol. 2017;37(1):32‐39. [DOI] [PubMed] [Google Scholar]
- 20. Weeke P, Jensen A, Folke F, et al. Antidepressant use and risk of out‐of‐hospital cardiac arrest: a nationwide case‐time‐control study. Clin Pharmacol Ther. 2012;92(1):72‐79. [DOI] [PubMed] [Google Scholar]
- 21. Qirjazi E, McArthur E, Nash DM, et al. Risk of ventricular arrhythmia with citalopram and escitalopram: a population‐based study. PLoS One. 2016;11(8):e0160768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eroglu TE, Barcella CA, Gerds TA, et al. Risk of out‐of‐hospital cardiac arrest in antidepressant drug users. Br J Clin Pharmacol. 2022;1‐10. https://doi.org/10.1111/bcp.15224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aakjær M, De Bruin ML, Kulahci M, Andersen M. Surveillance of antidepressant safety (SADS): active signal detection of serious medical events following SSRI and SNRI initiation using big healthcare data. Drug Saf. 2021;44:1215‐1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kildemoes HW, Sørensen HT, Hallas J. The Danish National Prescription Registry. Scand J Public Health. 2011;39(7 Suppl):38‐41. [DOI] [PubMed] [Google Scholar]
- 25. WHOCC – ATC/DDD Index [Internet] . [cited 2020 Sep 22]. https://www.whocc.no/atc_ddd_index/
- 26. Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39(7 Suppl):30‐33. [DOI] [PubMed] [Google Scholar]
- 27. ICD‐10 Version:2016 [Internet] . [cited 2022 Jan 5]. https://icd.who.int/browse10/2016/en
- 28. Nordic Medico‐Statistical Committee . NOMESCO Classification of Surgical Procedures. Nordic Medico‐Statistical Committee; 2010. [Google Scholar]
- 29. Helweg‐Larsen K. The Danish register of causes of death. Scand J Public Health. 2011;39(7 Suppl):26‐29. [DOI] [PubMed] [Google Scholar]
- 30. Pedersen CB. The Danish civil registration system. Scand J Public Health. 2011;39(7 Suppl):22‐25. [DOI] [PubMed] [Google Scholar]
- 31. Hernán MA, Robins JM. Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol. 2016;183(8):758‐764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Danaei G, Rodríguez LAG, Cantero OF, Logan R, Hernán MA. Observational data for comparative effectiveness research: an emulation of randomised trials of statins and primary prevention of coronary heart disease. Stat Methods Med Res. 2013;22(1):70‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Inghammar M, Svanström H, Melbye M, Pasternak B, Hviid A. Oral fluoroquinolone use and serious arrhythmia: bi‐national cohort study. BMJ. 2016;352:i843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Roden DM. Drug‐induced prolongation of the QT interval. N Engl J Med. 2004;350(10):1013‐1022. [DOI] [PubMed] [Google Scholar]
- 35. Tisdale JE, Chung MK, Campbell KB, et al. Drug‐induced arrhythmias: a scientific statement from the American Heart Association. Circulation. 2020;142(15):e214‐e233. [DOI] [PubMed] [Google Scholar]
- 36. Vandael E, Vandenberk B, Vandenberghe J, Willems R, Foulon V. Risk factors for QTc‐prolongation: systematic review of the evidence. Int J Clin Pharmacol. 2017;39(1):16‐25. [DOI] [PubMed] [Google Scholar]
- 37. SAS Institute Inc. , Cary, NC, USA. SAS/STAT(R) 13.1 User's Guide – The GENMOD Procedure. 2013;221.
- 38. Hanley JA, Negassa A, deB Edwardes MD, Forrester JE. Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol. 2003;157(4):364‐375. [DOI] [PubMed] [Google Scholar]
- 39. Rosenbaum P, Rubin D. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41‐55. [Google Scholar]
- 40. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res. 2011;46(3):399‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thygesen LC, Daasnes C, Thaulow I, Brønnum‐Hansen H. Introduction to Danish (nationwide) registers on health and social issues: structure, access, legislation, and archiving. Scand J Public Health. 2011;39(7 Suppl):12‐16. [DOI] [PubMed] [Google Scholar]
- 42. Sundbøll J, Adelborg K, Munch T, et al. Positive predictive value of cardiovascular diagnoses in the Danish National Patient Registry: a validation study. BMJ Open 2016;6(11):e012832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. John RM, Tedrow UB, Koplan BA, et al. Ventricular arrhythmias and sudden cardiac death. Lancet Lond Engl. 2012;380(9852):1520‐1529. [DOI] [PubMed] [Google Scholar]
- 44. Lisinski A, Hieronymus F, Eriksson E, Wallerstedt SM. Low SSRI dosing in clinical practice‐a register‐based longitudinal study. Acta Psychiatr Scand. 2021;143(5):434‐443. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


