Abstract
Introduction
Shellfish allergy is an important cause of food allergies worldwide. Both in vivo and in vitro diagnostics failure nowadays is caused by the poor quality of the extracts associated with the scarce availability of allergenic molecules in the market. It is known that not all patients with shellfish allergies experience adverse reactions to mollusks. It is still unclear how to detect and diagnose these patients correctly.
Aim
To investigate the features of shrimp-allergic patients either reactive or tolerant to mollusks, with the currently available diagnostic methods.
Methods
Nineteen centers, scattered throughout Italy, participated in the real-life study, enrolling patients allergic to shrimp with or without associated reactions to mollusks. Patients underwent skin tests using commercial extracts or fresh raw and cooked shrimp and mollusks, and IgE reactivity to currently available allergenic extracts and molecules was measured in vitro.
Results
Two hundred and forty-seven individuals with a self reported adverse reactions to shrimp participated in the study; of these 47.8% reported an adverse reaction to mollusks ingestion (cephalopod and/or bivalve). Neither of the tests used, in vivo nor in vitro, was able to detect all selected patients. Accordingly, a great heterogeneity of results was observed: in vivo and in vitro tests agreed in 52% and 62% of cases. Skin tests were able to identify the mollusk reactors (p < 0.001), also using fresh cooked or raw food (p < 0.001). The reactivity profile of mollusk reactors was dominated by Pen m 1, over Pen m 2 and Pen m 4 compared to tolerant subjects, but 33% of patients were not detected by any of the available molecules. Overall, a higher frequency of IgE rectivity to shrimp was recorded in northern Italy, while mollusk reactivity was more frequent in the center-south.
Conclusion
The current diagnostic methods are inadequate to predict the cross-reactivity between crustaceans and mollusks. The detection of mollusks hypersensitivity should still rely on skin tests with fresh material. The exclusion of mollusks from shrimp allergic patients’ diets should occur when clinical history, available diagnostic instruments, and/or tolerance tests support such a decision.
Keywords: Multiplex analysis, IgE diagnosis, Tropomyosin, Crustaceans, Mollusks, Food allergy, Urticaria/angioedema, Anaphylaxis
Key messages
-
1
Current diagnostic methods are inadequate to predict cross-reactivity between crustaceans and mollusks;
-
2.
The detection of mollusks hypersensitivity must still rely on skin tests with fresh material (and oral challenges where possible);
-
3.
Clinically, there is no need to exclude a priori mollusks from shrimp allergic patients' diets;
Introduction
Background/rationale
Shellfish is included among the “Big Eight” food groups responsible for most cases of food allergy.1 It is estimated that depending on the different geographic areas, about 3% of the general population is allergic to shellfish.1,2 This generic term includes many different invertebrate species which are divided into 2 large groups: crustaceans (shrimps, crabs, lobsters, etc) and mollusks (Bivalvia, such as clam, mussels, scallops or oysters, and Cephalopoda, like squids, cuttlefish or octopuses, etc).3,4 These taxonomic classifications are essential to predict the structural, immunological, and allergological similarities that underlie a possible cross-reactivity.5 The availability and consumption of shellfish vary greatly in different parts of the world, representing the second cause of primary food allergy in Italy after lipid transfer proteins.6 Even eating habits, including the different methods of food processing, may exert a strong impact on the incidence and severity of allergy to crustaceans and mollusks, since in some cases physical treatments can increase or reduce IgE reactivity,7 depending on the molecule involved in patient sensitization.8 So far, it has been argued that due to the cross-reactivity among allergens in invertebrates, primarily tropomyosin, the patient allergic to crustaceans should also exclude cephalopods and Bivalvia from the diet. However, recent studies seem to suggest that this is not the case; in fact, a significant proportion of crustacean allergic patients report that they usually tolerate other invertebrates.9
Both in vivo and in vitro diagnostics failure nowadays is caused in part by the poor quality of the extracts, and extract-based diagnostics can also be limited by cross-reactivity, failing to detect IgE crosslinking.10,11 It has been widely demonstrated that diagnostics with commercially available shellfish extracts do not always lead to the correct identification of sensitized patients.12 Also, from the point of view of a molecular approach, another main diagnostic problem is that all the components commercially available for in vitro tests come from crustaceans and not from mollusks. Consequently, IgE diagnostic approach for mollusks adverse reaction is often indirectly based on a presumptive but not fully demonstrated cross-reactivity, unless tests with fresh, raw or cooked cephalopod or Bivalvia are carried out, with all the drawbacks associated with such an approach.
Objectives
The main aims of this study were: (i) to investigate the sensitization pattern characteristics of patients allergic to shrimp with or without cross-reactivity to cephalopods and/or bivalve mollusks; (ii) to define the molecular profile in both cross-reacting and non-cross-reacting individuals; (iii) to highlight any differences in the severity of the allergic reactions; and (iv) to investigate the geographical differences in shellfish sensitization profiles in Italy.
Patients and methods
Study design
We conducted a multicentre cross-sectional observational clinical survey on shellfish allergy, aimed at identifying how many shrimp-allergic patients are also mollusk- and/or shrimp-reactive and which are the best tools to identify them.
Setting
Nineteen allergy centers scattered throughout Italy participated in the study. Doctors consecutively enrolled as many patients as possible with an unequivocal allergy to shrimp between May 15 and November 15, 2021.
Participants
The main eligibility criterion was a self-reported history of food allergy to shrimp, after ingestion, and the selection methods of the participants were the presence of a positive skin prick test (SPT) with commercial shrimp extract and/or prick to prick (PTP) skin test with fresh raw or cooked shrimp, or the presence of IgE to shrimp. The source of the participants was the allergy clinics participating in the study, where patients were interviewed closely about shellfish allergy and/or tolerance. The diagnosis of tolerance was based on a patient's history of consumption without any problem.
Variables
Shellfish-induced allergic reactions reported by patients included both mild (oral allergy syndrome or isolated gastrointestinal reactions) and moderate-severe reactions (urticaria/angioedema, anaphylaxis).
Given the observational nature of the study, no randomization procedure was implemented during enrollment.
Data sources/measurement
The clinical histories were confirmed by a positive PTP with fresh food (shrimp, mussel or clam for bivalves, and octopus or squid for cephalopods either raw or cooked) and/or SPT with commercial extracts of shrimp and mollusks currently available in Italy (Alk-Abello’ s.p.a., Allergy Therapeutics Italia, Anallergo, FirmaSrl, Roxall Italia, Lofarma, and Stallergenes Italia Srl) and/or by the detection of IgE specific for shellfish extracts by ImmunoCAP (shrimp, bivalve, evaluated by mussel and/or clam, and cephalopods, tested with octopus and/or squid), and Pen a 1 (tropomyosin) (Thermo Fisher, Uppsala, Sweden).
In a subset randomly selected (98 individuals, 48 from North Italy and 50 from Center-South Italy) a more in-depth evaluation was carried out by the Allergy Explorer-ALEX2® (Macroarray Diagnostics, Vienna) multiplex platform evaluating a broader profile of shellfish molecules,13 including Pen m 1 (tropomyosin), Pen m 2 (arginine kinase), Pen m 3 (myosin light chain), and Pen m 4 (sarcoplasmic calcium-binding protein) all from Black-Tiger shrimp (Penaeus monodon), Cra c 6 (the troponin-c from brown shrimp, Crangon crangon), Der p 10 (tropomyosin), and Der p 11 (paramyosin) both from Dermatophagoides pteronyssinus, as well as of shellfish extract including crab, lobster, northern shrimp, white shrimp, squid, mussel, oyster, clam, and scallop. IgE levels >0.3kUA/L were considered positive as per the manufacturer's instructions.
Bias
The diagnosis of shellfish allergy was not confirmed by blinded or open oral food challenges due to both the severity of several reactions and the fact that many centers were not sufficiently equipped in terms of facilities or personnel to manage possible severe allergic reactions.
Study size
The study size was reached by enrolling the maximum number of patients in the 6 months set for the study by the participating centers.
Quantitative variables
Study participants, all allergic to shrimp, were grouped as reactors or tolerant to mollusks. Further grouping was obtained for the reactors, based on the outcome reported from the clinical history, as a mild or moderate-severe adverse reaction. Further grouping was achieved by the participants' reactivity to SPT with commercial products or PTP with fresh foods and/or at specific IgE levels, either via extracts or molecular components.
Statistical methods
The sampled data were recorded and analyzed by SPSS (version 27.0.1.0 SPSS, Inc, Chicago, III).
In univariate analysis, the non-parametric Mann-Whitney U test (2 groups) was first used to compare continuous IgE values in subjects with or without a given clinical involvement; then, each variable of interest was dichotomized (as negative or positive) to study the proportion of subjects with symptoms in the two groups thus obtained.
Pearson's χ2 test or Fisher's exact test (used for two-by-two contingency tables with less than 50 cases) were used to assessing if paired observations on two variables expressed in a contingency table, were independent of each other.
We performed multiple logistic regression for the clinical variables with dichotomous scores (present, absent) to see whether the association between clinical symptoms and different shellfish allergens reactivity was present after simultaneously adjusting for the other variables of interest.
The degree of relationship between the quantitative variables studied was analyzed using the Spearman Correlation (rho) test, and the most commonly used bivariate correlation technique. A value of p < 0.05 was considered statistically significant.
To provide a visual representation of the distribution of the different molecules in panallergen families, we have produced Venn diagrams using the VennMaster 0.38 package14
Ethical issues
The study was approved by the Ethical Committee of the coordinating centre (IDI-IRCCS CE | 667/2021). Data collection takes place anonymously, using only data obtained through routine specialist surveys. Recruited patients gave informed consent to the use of their clinical data in an anonymous form.
Results
Participants
Two hundred forty-seven individuals (M 48.2%, F 51.8%; mean age 39 ± 17, range 2–79 years) enrolled in the 19 centers participating in the study, represented the study group.
Descriptive data
Forty-four per cent (n = 108) of the patients came from central-southern Italy, while the remaining 56% (n = 139) of cases had been recruited in northern Italy. The repository Figure 1 and the associated Table detail the number of patients recruited at each center, including the demographics and clinical features (eg, sex, age, house dust mite reactivity, and percentage of subjects from northern/central-southern Italy of each group).
Outcome data
All patients selected for the study reported a clinical history suggesting an adverse reaction to shrimp confirmed by positive in vivo and/or in vitro testing, but only 47.8% of them (n = 118) reported adverse reactions after the ingestion of cephalopods or bivalves; of these, 38.5% (n = 95) experienced urticaria/angioedema, and 9.3% (n = 23) anaphylaxis. Eight per cent of the patients who could not tolerate the mollusks also reported rhinitis symptoms, and 4.9% dyspnoea and wheezing. No age and sex differences were detected concerning symptom severity.
Main results
Neither of the tests used, in vivo nor in vitro, was able to detect all selected patients as positive. Indeed, 60.9% of the patients scored positive on SPT with shrimp extract; 78.3% scored positive on PTP with fresh food, 65,8% with cooked food, and 72.4% showed specific IgE to shrimp by ImmunoCAP. Thirty-nine (16.6%) individuals were negative for skin tests with both commercial extracts and raw or cooked shrimp but scored positive on specific IgE assays.
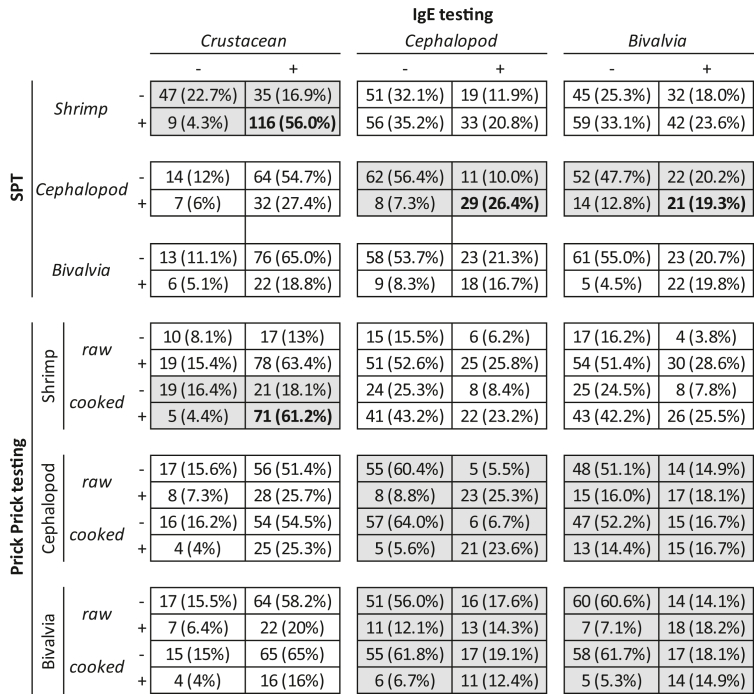
As shown in Table 1, a concordance between commercial SPT and IgE assay for shrimp was found only in 56% of patients; concordance rose to 62% between PTP with raw or cooked fresh shrimp and the in vitro test. Comparing the in vivo and in vitro evaluations with cephalopods (squid or octopus) or bivalves (mussels or clams), an even higher heterogeneity of results was observed (Table 1).
Table 1.
Italian Multicenter real-life Study on mollusk allergy in shrimp allergic patients, May 15 to November 15, 2021. Concordance among commercial SPT, PTP and ImmunoCAP IgE assay for Crustaceans and Mollusks are shown (shaded when p<0.05)
No correlation was found comparing IgE levels to shellfish extracts, shrimp, cephalopods, and bivalve, except for a moderate relationship between cephalopods and mussels (rho 0.634, p < 0.001).
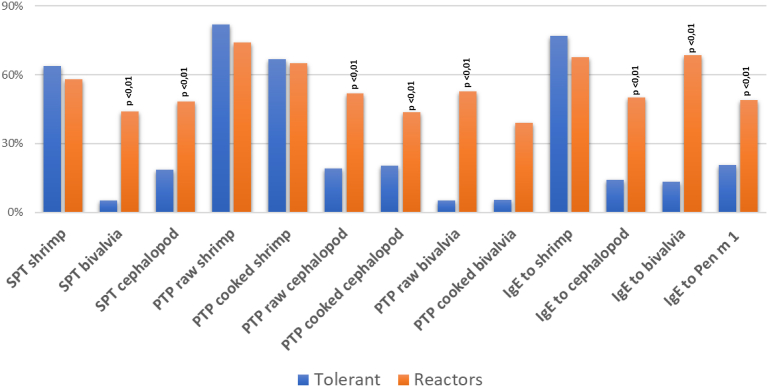
To evaluate the prevalence of IgE reactivity to mollusks in shrimp reactive subjects, our patients were extensively studied in vitro with both extracts and allergenic molecules, using singleplex and multiplex methods. Fig. 1 shows the reactivity to the different diagnostic approaches, in vivo and in vitro, in mollusk tolerant patients. The mollusk reactor participants showed a significant (<0.01) greater frequency of reactivity to molluscs when evaluated with both skin tests (both SPT and PTP), and IgE (both multiplex and singleplex systems). Interestingly, also the in vitro reactivity to tropomyosin was more frequently observed in subjects reactors to molluscs.
Fig. 1.
The figure shows the prevalence of reactivity to the tests performed in the Italian Multicenter real-life Study on mollusk allergy in shrimp allergic patients, May 15 to November 15, 2021. (skin prick test with commercial extracts, prick - prick with fresh foods, both cooked or raw, and in vitro dosage of specific IgE to extract with ImmunoCAP singleplex method) respectively, in mollusk tolerant subjects, or with a history of an adverse reaction to cephalopods or bivalves. Significant differences are indicated in the figures (p < 0.01)
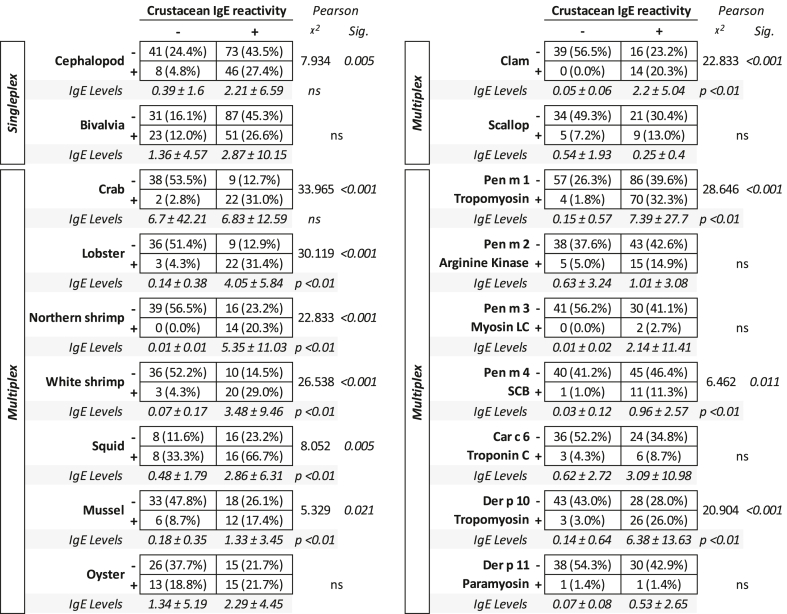
As shown in Table 2, IgE sensitization to shrimp was significantly associated with hypersensitivity to cephalopods (p < 0.005), but not to bivalves on singleplex testing. Notably, specific IgE levels did not differ in the two groups.
Table 2.
Mollusks extract and molecules IgE sensitization frequency and levels as per multiplex (ALEX2 test) and singleplex (ImmunoCAP) detection in individuals with or without IgE reactivity to shrimp (even if positive after skin tests, SPT and/or PTP, with shrimp). ns = not significant
On the other hand, when shrimp immunoreactive patients were evaluated by the multiplex system, they were also more frequently positive for crab, lobster, northern and white shrimp, squid, mussel, and clam, but not for oyster and scallop (Table 2). Crustacean reactivity was accompanied by significantly higher levels of specific IgE in all extracts tested, except for crab, oyster, and scallop, where specific IgE levels did not differ.
From the molecular point of view, IgE reactivity to crustaceans was associated with a significantly higher frequency and higher levels of specific IgE to tropomyosins (both Pen m 1 and Der p 10) and sarcoplasmic calcium-binding protein (Pen m 4), in comparison with the patient that scored negative for specific IgE to crustaceans (Table 2).
Other analyses
Sensitization profiles and clinical correlations
One hundred-eighteen (47.8%) patients reported also moderate (80.5%) to severe (19.5%) reactions after ingestion of mollusks. There was no difference in the frequency of reactive episodes related to sex or age.
Interestingly, a higher risk of developing a severe reaction after mollusks intake was associated with skin test reactivity to commercial extracts of cephalopod (OR: 4.81; CI 1.8–9.3; p < 0.001) or bivalves (OR: 14.970; CI 4.2–53.3; p < 0.001). Similarly, mollusk reactors showed a high frequency of reactivity to both raw (OR: 4.492; CI 1.9–10.4; p < 0.001) and cooked squid (OR: 3.040; CI 1.3–7.3; p < 0.001), and to both raw (OR: 20.907; CI 5.8–75.2; p < 0.001) and cooked clam (OR: 17.249; CI 3.1–41.1; p < 0.001).
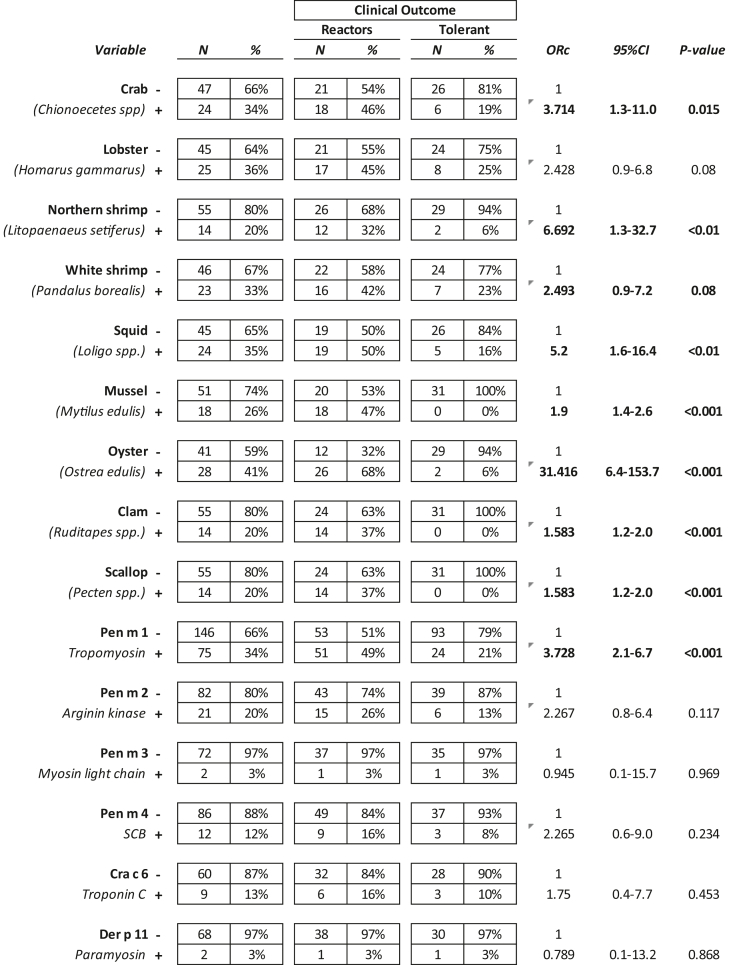
The multiple logistic regression analysis, when simultaneously adjusted for the presence of specific IgE in all the extracts studied, age, and sex, showed a significant relationship between a history of adverse reaction to mollusks and IgE reactivity to both cephalopods (ORadj 4.0, 95% CI 1.5–10.7, p < 0,01) or bivalve (ORadj 7.6, 95% CI 3.1–18.6, p < 0.0001) extracts. Moreover, as shown in Table 3, allergy to mollusks was associated with IgE reactivity to northern shrimp (Litopaenaeussetiferus), squid (Loligo spp.), mussel (Mytilus edulis), oyster (Ostrea edulis), clam (Ruditapes spp.), and scallop (Pecten spp.) extracts. Pen m 1 (Penaeus monodon tropomyosin) hypersensitivity was significantly associated with an increased risk of severe reaction to mollusks.
Table 3.
Extract- and molecule-IgE sensitization frequency in shrimp-allergic individuals reactors or tolerant mollusks. ns = not significant
Three patients scoring SPT positive to shrimp showed in vitro reactivity to bivalves (both oyster and mussel) in 2 cases and squid in 1 case, but no sign of IgE reactivity against any of the extracts and molecules of crustaceans in vitro, with only a low reactivity in one case to paramyosin (0.24 kU/L) and tropomyosin (0.39 kU/L) from house dust mite (Der p 11 and Der p 10 respectively).
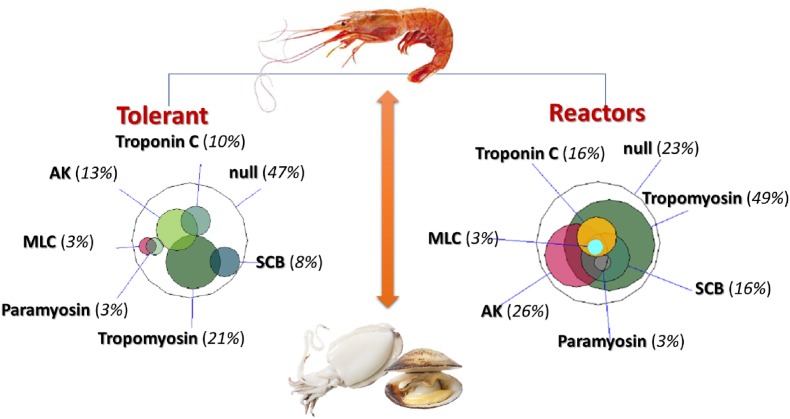
As summarized in Fig. 2, the molecular sensitization profile was dominated by Pen m 1, but this occurred more frequently in those who did not tolerate mollusks. Moreover, mollusks reactors showed also a two times higher frequency of sensitization to arginine kinase and sarcoplasmic calcium-binding protein (Fig. 2). Interestingly, the number of shrimp allergic patients who did not react to any of the molecules currently available for in vitro diagnostics was very high, particularly among those who tolerated cephalopods and bivalves (47%).
Fig. 2.
Molecular sensitization profile in 98 patients allergic to crustaceans that were tolerant 40 patients) or reactors (58 patients) to cephalopods and/or bivalves. The area-proportional Venn diagrams show the logical relationship between the single molecules currently available for shrimp allergy diagnosis in the fraction of tolerant patients and the fraction who react to mollusks. The squid picture is given as an example of cephalopods and the mussel as an example of bivalves. Null refers to the percentage of patients not reactive to any molecule tested; AK: Arginine kinase; SCB: Sarcoplasmic calcium-binding protein; MLC: Myosin light chain
The proportion of patients scoring positive or negative on the different in vivo and in vitro tests performed in the study in the light of their reactivity or tolerance to mollusks is summarized in Fig. 1, showing a greater frequency of Bivalvia and cephalopod reactivity both with in vivo (both SPT and PTP), and in vitro (both multiplex and singleplex systems) in mollusks reactors.
Geographical differences in shellfish sensitization profiles
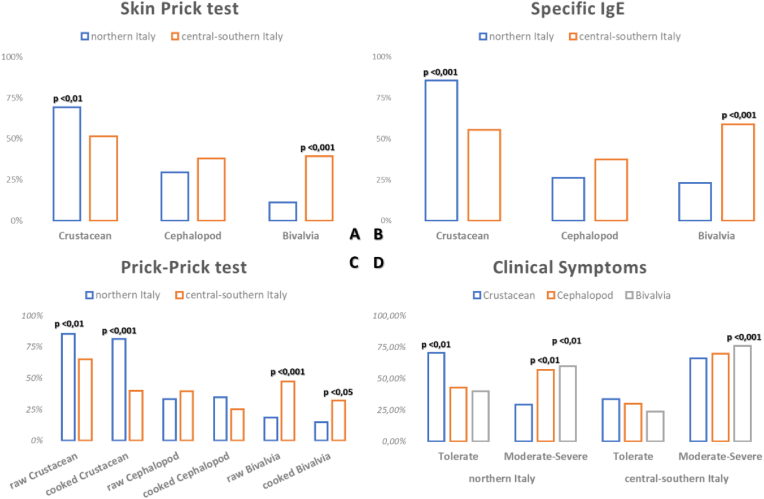
We also analyzed the data as a function of the geographical distribution of patients, distinguishing between northern and centre-southern areas of Italy. We found a higher frequency of crustaceans reactivity in northern Italy, irrespective of the diagnostic tests used (skin prick test, PTP test with fresh or cooked food, and specific IgE measurement for allergen extracts), whilst in centre-southern Italy a more frequent sensitization to Bivalvia was recorded (Fig. 3A–C). This observation was in keeping with a higher frequency of reactions to bivalves in the south, whilst the sensitization levels to cephalopods were comparable among the south and north (Fig. 3 D).
Fig. 3.
The figure shows the comparison of the results of the tests performed in the population of the Italian Multicenter real-life Study on mollusk allergy in shrimp allergic patients. in the north compared with central-southern Italy. The results of SPTs with commercial extracts are shown in A, the differences in the IgE assay with the singleplex method (ImmunoCAP) in B, the in vivo tests with fresh, cooked or raw food in C, and the different tolerance profiles in D. to the respective foods indicated in the figure, between north and central-southern Italy. The significant differences are shown directly within the figure
Discussion
Key results
In our multicenter study, involving 19 centers scattered throughout Italy, we evaluated the sensitization profile of allergic subjects to shrimp reactive or not reactive also to mollusks.
We confirmed once more that the diagnostics with both extracts and the currently available molecules are inadequate to detect properly patients hypersensitive to crustaceans and mollusks.15 We observed a great heterogeneity of results from one patient to another using the routine diagnostic approaches, including SPT with commercial extracts, PTP with fresh material, and specific IgE measurements. We previously showed the unreliability of commercial extracts for SPT available on the market due to differences in allergenic protein concentrations, thus potentially leading to confounding results.12
The currently available molecular diagnostics for shellfish shows 2 major pitfalls. First, not all allergen molecules are present on the diagnostic platforms and, secondly, all the molecules available derive from crustaceans and none from mollusks. We found that the molecular profile of patients allergic to shrimp and reactive or tolerant to mollusks shows differences in the frequency of sensitization to tropomyosin, AK and SCB, indicating that a larger and more specific number of molecules might lead to a more defined and clearer pattern of sensitization to mollusk allergy (Fig. 2). The molecular diagnosis of mollusk allergy is therefore often indirect, based on the presumption of cross-reactivity with crustaceans, unless proven with the oral food challenge. Of the 58 shellfish molecules currently registered as allergens by the WHO/IUIS Allergen Nomenclature Sub-Committee, only 8 belong to mollusks (see Table in the repository). If one considers, for instance, that the tropomyosins from mollusks share no more than 60% amino acid sequence identity with the other allergenic tropomyosins isolated so far from crustaceans, insects, mites, and fish,2 one can easily figure out that this might lead to a failure in the detection of allergic patients.16 A 2018 study showed that in the pacific oyster extract, along with many specific allergens of invertebrates present in fish, and mites, it is possible to isolate allergens from other different biological sources such as pollen and fungi, thus prompting interesting scenarios about possible unexpected sources of sensitization.17
Another interesting point concerns the differences in sensitization profiles observed in the different geographic areas of the country. We detected that in the north sensitization to crustaceans was much more frequent than in the center/south, where in turn sensitization to cephalopods and bivalves was more prevalent. These differences might be the result of different culinary habits between the north (where the way of life is more similar to that in Central and Northern Europe) and the south, where habits are similar to those in other Mediterranean countries such as Greece or Spain.9,18. However, at present, there are no studies evaluating the different culinary habits and their impact on allergy sensitization in Italy. When dietary and nutritional patterns were studied in an elderly rural population in Italy, higher consumption of animal fats, sugar and alcoholic beverages was observed in northern Italy, while in southern Italy a higher intake of fruit, vegetables, fish and olive oil, with significant differences between women and men.19 In addition, geographical differences could also underlie different sensitization mechanisms according to different environmental exposure.15 Furthermore, possible occupational exposure should be considered.20,21 These points need to be addressed in future studies.
The finding of 3 single patients (1.2%) who were in vitro mono-reactors to bivalves (both oyster and mussel) but not to shrimp (the reactivity to the shrimp was recorded only by in vivo tests), allows us to speculate that primary sensitization to mollusks may be, albeit rarely, possible, sometimes associated with sensitization also to house dust mites.22 A study designed to verify whether reactivity is always secondary to sensitization to mite allergens should be carried out, focusing on a pediatric-only population.
Limitations
Objective limitations of the study are the absence of oral food challenge in patient selection, which was based only on history, and the use of heterogeneous commercial preparations, both for in vivo and in vitro diagnostics, where the producers do not declare always the exact species of crustacean used in the preparation.
Due to the real-life, multi-center nature of the study, which included 19 different centers, not the same products and procedures were used by all participating professionals. Thus, in some centers the reaction to bivalves has been verified with clams, cooked or raw, and in others with mussels.
Interpretation
In conclusion, our multicenter real-life study on a large sample of shrimp allergic patients study shows the following:
-
(i)
We confirm that current diagnostic methods are inadequate to predict cross-reactivity between shrimp and mollusks due to the lack of specific mollusk allergens and because these are only partially cross-reactive to crustacean homologue proteins;
-
(ii)
The detection of mollusk hypersensitivity must still rely on PTP with fresh material (and oral challenges where possible);
-
(iii)
Clinically, the exclusion of mollusks from shrimp-allergic patients' diets, should occur after appropriate investigation, when available diagnostic instruments support such a decision, even if at present only oral food challenge may be a predictor of tolerance;
Abbreviations
SPT, skin prick test; PTP, prick-prick test; AK, Arginine kinase; SCB, Sarcoplasmic calcium-binding protein; MLC, Myosin light chain; WHO/IUIS, World Health Organization/International Union of Immunological Societies; OR, odds ratio.
Funding information
This study was funded, in part, by the Italian Ministry of Health, Current Research Program 2021–2023.
Author contribution statement
ES, EC, and GM carried out the experiments and data collection. ES; AA; EE; IB; FC; EC; FB; GD; AD; LF; FLR; LML; DM; GMa; GMe; MM; EN; RO; EAP; SP; AR; FR; AR; MG; LC, VP; DV and RA recruited the patients. ES performed the statistical analysis. ES and RA conceived the study and assisted in data interpretation. All authors reviewed, edited and approved the final manuscript.
All Authors consent for publication, and confirm that this manuscript is original, has not been published before, is not currently being considered for publication elsewhere, and has not been posted to a preprint server.
Availability of data and materials
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Ethical approval statement
The research was conducted ethically following the World Medical Association Declaration of Helsinki. The study was approved by the Ethical Committee of the coordinating centre (IDI-IRCCS CE | 667/2021). Data collection takes place anonymously, using only data obtained through routine specialist surveys. Recruited patients gave informed consent to the use of their clinical data in an anonymous form.
Declaration of competing interest
ES has received consultant arrangements and speakers’ bureau participation from Stallergenes, Thermo Fisher Scientific, and non-financial support from Microarray Diagnostics, Vienna, all outside the submitted work. LC has received honoraria from Malesci, Menarini, Mylan and Thermo Fisher Scientific. DV and RA received honoraria from Thermo Fisher Scientific.
Acknowledgements
We are grateful to all the doctors, nurses, and other medical professionals and staff who take care of COVID-19 patients evaluated in the study.
Footnotes
Full list of author information is available at the end of the article
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2022.100685.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Davis C.M., Gupta R.S., Aktas O.N., Diaz V., Kamath S.D., Lopata A.L. Clinical management of seafood allergy. J Allergy Clin Immunol Pract. 2020;8:37–44. doi: 10.1016/j.jaip.2019.10.019. [DOI] [PubMed] [Google Scholar]
- 2.Ruethers T., Taki A.C., Johnston E.B., et al. Seafood allergy: a comprehensive review of fish and shellfish allergens. Mol Immunol. 2018;100:28–57. doi: 10.1016/j.molimm.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Tong W.S., Yuen A.W.T., CYY Wai, Leung N.Y.H. 2018. Diagnosis of Fish and Shellfish Allergies; pp. 247–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopata A.L., Hehir R.E.O., Lehrer S.B. 2010. Shellfish Allergy Clinical & Experimental Allergy; pp. 850–858. [DOI] [PubMed] [Google Scholar]
- 5.Faber M.A., Pascal M., Kharbouchi O El, Sabato V., Hagendorens M.M. Decuyper II et al. Shellfish allergens : tropomyosin and beyond. Allergy. 2017;72:842–848. doi: 10.1111/all.13115. [DOI] [PubMed] [Google Scholar]
- 6.Giuffrida M.G., Villalta D., Mistrello G., Amato S., Asero R. Shrimp allergy beyond tropomyosin in Italy: clinical relevance of arginine kinase, sarcoplasmic calcium binding protein and hemocyanin. Eur Ann Allergy Clin Immunol. 2014;46:172–177. [PubMed] [Google Scholar]
- 7.Nowak-Wegrzyn A., Fiocchi A. Rare, medium, or well done? The effect of heating and food matrix on food protein allergenicity. Curr Opin Allergy Clin Immunol. 2009;9:234–237. doi: 10.1097/ACI.0b013e32832b88e7. [DOI] [PubMed] [Google Scholar]
- 8.Kamath S.D., Rahman A.M.A., Voskamp A., Komoda T., Rolland J.M., Lopata A.L. 2014. Effect of Heat Processing on Antibody Reactivity to Allergen Variants and Fragments of Black Tiger Prawn : A Comprehensive Allergenomic Approach; pp. 1144–1155. [DOI] [PubMed] [Google Scholar]
- 9.Azofra J., Echechipía S., Irazábal B., et al. Heterogeneity in allergy to mollusks: a clinical-immunological study in a population from the north of Spain. J Investig Allergol Clin Immunol. 2017;27:252–260. doi: 10.18176/jiaci.0137. [DOI] [PubMed] [Google Scholar]
- 10.Yang A.C., Arruda L.K., Santos B.R., Barbosa M.C.R., Chapman M.D. 2018. Measurement of IgE Antibodies to Shrimp Tropomyosin Is Superior to Skin Prick Testing with Commercial Extract and Measurement of IgE to Shrimp for Predicting Clinically Relevant Allergic Reactions after Shrimp Ingestion; pp. 872–878. [DOI] [PubMed] [Google Scholar]
- 11.Ruethers T., Koeberl M., Lopata A.L., et al. 2019. Variability of Allergens in Commercial Fish Extracts for Skin Prick Testing; pp. 1–12. [DOI] [PubMed] [Google Scholar]
- 12.Asero R., Scala E., Villalta D., et al. Vol. 27. 2017. Shrimp allergy: analysis of commercially available extracts for in vivo diagnosis; pp. 175–182. [DOI] [PubMed] [Google Scholar]
- 13.Scala E., Caprini E., Abeni D., et al. A qualitative and quantitative comparison of IgE antibody profiles with two multiplex platforms for component-resolved diagnostics in allergic patients. Clin Exp Allergy. 2021;51:1603–1612. doi: 10.1111/cea.14016. [DOI] [PubMed] [Google Scholar]
- 14.Kestler H.A., Müller A., Kraus J.M., et al. VennMaster: area-proportional Euler diagrams for functional GO analysis of microarrays. BMC Bioinf. 2008;9:67. doi: 10.1186/1471-2105-9-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scala E., Villalta D., Meneguzzi G., Brusca I., Cecchi L. Comparison of the performance of skin prick and isac tests in the diagnosis of allergy. Eur Ann Allergy Clin Immunol. 2020;52:258–267. doi: 10.23822/EurAnnACI.1764-1489.135. [DOI] [PubMed] [Google Scholar]
- 16.Celi G., Brusca I., Scala E., et al. House dust mite allergy and shrimp allergy : a complex interaction. Eur Ann Allergy Clin Immunol. 2020;52:205–209. doi: 10.23822/EurAnnACI.1764-1489.108. [DOI] [PubMed] [Google Scholar]
- 17.Nugraha R., Kamath S.D., Johnston E., et al. Rapid and comprehensive discovery of unreported shellfish allergens using large-scale transcriptomic and proteomic resources. J Allergy Clin Immunol. 2018;141:1501–1504.e8. doi: 10.1016/j.jaci.2017.11.028. [DOI] [PubMed] [Google Scholar]
- 18.Veronese N., Notarnicola M., Cisternino A.M., et al. Trends in adherence to the Mediterranean diet in South Italy: a cross sectional study. Nutr Metabol Cardiovasc Dis. 2020;30:410–417. doi: 10.1016/j.numecd.2019.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Corrêa Leite M.L., Nicolosi A., Cristina S., Hauser W.A., Pugliese P., Nappi G. Dietary and nutritional patterns in an elderly rural population in Northern and Southern Italy: (I). A cluster analysis of food consumption. Eur J Clin Nutr. 2003;57:1514–1521. doi: 10.1038/sj.ejcn.1601719. [DOI] [PubMed] [Google Scholar]
- 20.Wiszniewska M., Tymoszuk D., Pas-Wyroślak A., et al. Occupational allergy to squid (Loligo vulgaris). Occupational allergy to squid (Loligo vulgaris) Occup Med. 2013;63:298–300. doi: 10.1093/occmed/kqt025. [DOI] [PubMed] [Google Scholar]
- 21.Jeebhay M.F., Robins T.G., Lehrer S.B., Lopata A.L. Occupational seafood allergy: a review. Occup Environ Med. 2001;58:553–562. doi: 10.1136/oem.58.9.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asero R., Pravettoni V., Scala E., Villalta D. House dust mite-shrimp allergen interrelationships. Curr Allergy Asthma Rep. 2020;20:3–7. doi: 10.1007/s11882-020-0902-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.