Abstract
Chikungunya is an arboviral disease caused by a virus with wide geographical distribution in endemic areas. This case report documents a patient with antisynthetase syndrome post‐chikungunya infection. Autoimmune diseases result from breakdown of immune tolerance. Among all triggers, viruses represent the greatest environmental potential to precipitate inflammatory myopathy.
Keywords: case report, chikungunya virus, myopathy, myositis
Short abstract
Viral infections can trigger inflammatory myopathies. Although rare, antisynthetase syndrome can develop post‐Chikungunya infection presenting with a wide range of symptoms including interstitial lung disease and have to be early recognized.
1. INTRODUCTION
Chikungunya virus (CHIKV) is an alphavirus endemic in Africa, Asia, and South America and can be found in tropical areas with seasonal characteristics, with outbreaks reported in several countries worldwide. 1 , 2 Chikungunya presents a broad symptom spectrum, similar to other arboviruses, including fever, rash, myalgia, arthralgia, and headache. 3 When duration of symptoms persists beyond three months, it reaches the chronic phase, with persistence of arthritis and joint tenosynovitis. 1
CHIKV infection can be a trigger for onset of autoimmune diseases in genetically predisposed individuals. 2 In this scenario, inflammatory myopathy is an extremely rare presentation of CHIKV scarcely described in the literature. This article aims to report a rare case of an antisynthetase syndrome (ASS) post‐chikungunya infection, characterized by muscle weakness, interstitial lung disease, and positive anti‐Jo‐1.
2. CASE PRESENTATION
We report a 38‐year‐old female patient, previously healthy, presented with high fever, adynamia, diffuse myalgia, and polyarthralgia of hands, wrists, elbows, and knees. She sought medical attention and was diagnosed with CHIKV infection, confirmed by a positive IgM enzyme‐linked immunosorbent assay (ELISA) serological study in two samples. Joint pain and myalgia persisted, and after two months, she presented progressive complaints of fatigue, difficulty combing her hair, walking, and getting out of bed. She had frequent falls, dysphagia for solids, dysphonia, and dyspnea on mild exertion and was bedridden. She was then admitted to the hospital for investigation.
On physical examination, she presented decreased proximal limb muscle strength, grade 2 in lower limbs and grade 3 in upper limbs, according to Medical Research Council (MRC) scale. Pulmonary auscultation presented fine bilateral basilar crackles. Manual muscle test (MMT) of 64 out of 150 points. Locomotor examination showed arthritis in knees and ankles bilaterally.
Complementary examinations revealed elevated creatine phosphokinase (CPK: 25150 U/L [VR: 0 A 170 U/L]) and increased transaminases (TGO: 377 U/L and TGP: 264 U/L [VR: 0 A 31 U/L]). Anti‐Jo‐1 antibody was reagent (141 U/ml [VR: reagent >10 U/ml]). Antinuclear antibody (ANA) with a mixed pattern of 1/80 fine dotted nuclear type and 1/160 dotted cytoplasmic type. Electroneuromyography of four limbs confirmed a pattern of myopathy, affecting mostly proximal muscles. Magnetic resonance imaging (MRI) of thighs showed diffuse edema of muscles, without areas of liposubstitution, suggesting an acute inflammatory process (Figures 1 and 2). Chest computed tomography (CT) scan showed ground‐glass opacities in bases up to the middle third of lungs, compatible with nonspecific interstitial pneumopathy (NSIP).
FIGURE 1.
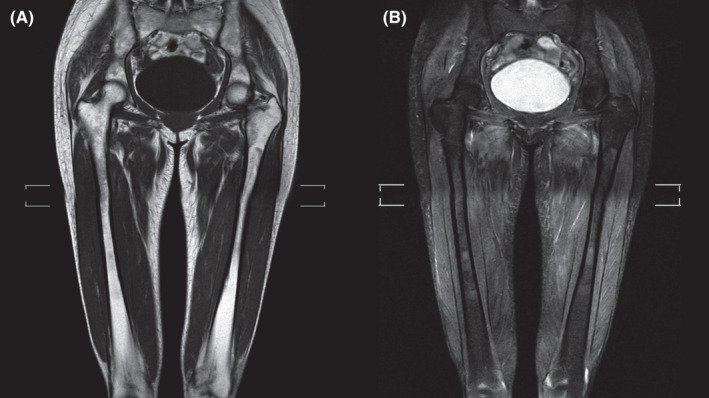
Magnetic resonance imaging (MRI) of hip and thighs showing diffuse muscular edema affecting the pelvic girdles and thighs, with the absence of muscle liposubstitution. (A) Coronal T1. (B) Coronal T2 with fat saturation (SPAIR technique)
FIGURE 2.
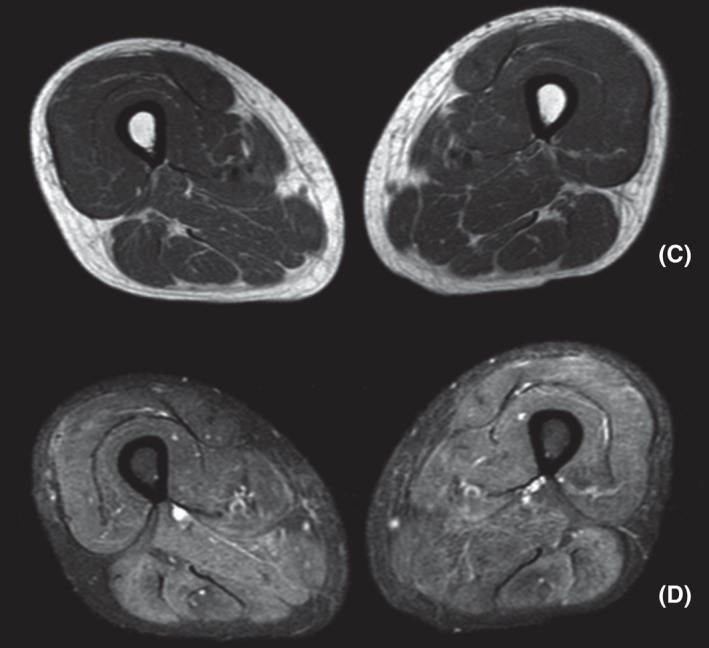
(C) Axial T1: mild muscle volume reduction, without evidence of liposubstitution. (D) Axial T2 with fat saturation (SPAIR technique): diffuse edema of musculature of the anterior, posterior, and medial compartments
The diagnosis of ASS, an immune‐mediated inflammatory myopathy, post‐chikungunya infection, was then established. Two months after the acute‐phase symptoms of chikungunya infection solved with persistence of only pain and myalgia, the patient presented with a compatible clinical of ASS, with proximal muscle weakness in all four limbs, MRI of thighs showing a muscular inflammatory process, electroneuromyography with a myopathic pattern, interstitial pneumopathy, and a positive Anti‐Jo1 antibody, in addition to joint involvement.
Other etiological causes were investigated and ruled out, such as viral infections by hepatitis virus, cytomegalovirus, HIV, syphilis, and COVID‐19, metabolic disorders, use of previous medications, and screening for neoplasms, confirming chikungunya as the trigger for myopathy.
Pulse therapy was started with methylprednisolone for 3 days and human immunoglobulin for 6 monthly cycles. The patient had clinical recovery and a gradual decrease in muscle enzymes, reaching 257 U/L (VR: 0–170 U/L) after 6 months. She was also given monthly intravenous cyclophosphamide for a period of 6 months due to pulmonary involvement. The patient maintained motor and respiratory physical therapy, evolving with grade 5 muscle strength in the four limbs, with no complaints of fatigue, dysphagia, or dysphonia.
3. DISCUSSION
Chikungunya is an arboviral disease caused by an RNA virus with wide geographical distribution in endemic areas and other tropical areas with seasonal characteristics that are favorable to development of disease vectors (Aedes aegypti and Aedes albopictus). 4 The chance of large outbreaks of CHIKV worldwide is high, especially in developing countries due to abundance of vectors. 5 In 2007, an infected tourist from India introduced chikungunya to northern Italy, resulting in the identification of 292 suspected cases. 6
Chikungunya is self‐limited and has a broad clinical spectrum, being characterized by a characteristic triad consisting of fever, arthralgia, and rash. 5 The clinical course of the disease can be divided into acute and chronic phases. In acute phase, there may be fever, symmetrical polyarthralgia, headache, nausea, vomiting, fatigue, and myalgia. Arthralgia affects large and small joints and preferentially involves more distal regions such as ankles, wrists, and phalanges. 4 Joint pain may persist, characterizing onset of chronic phase, which lasts for 12 weeks to years. 4 The prevalence of chronic phase is highly variable and may affect more than half of the patients. 1 Complications of cardiovascular, renal, respiratory, hepatic, gastrointestinal, and adrenal systems are associated with the infection and are referred to as atypical features. 3
In addition, CHIKV, like other viruses, can act as a trigger for autoimmune diseases, among them, autoimmune inflammatory myopathies, a group of diseases characterized by the presence of inflammation of skeletal muscle and subdivided into dermatomyositis (DM), polymyositis (PM), necrotizing autoimmune myopathy (MAN), inclusion body myositis (IBM), and antisynthetase syndrome, according to clinical, histological, immunopathological, and autoantibody characteristics. 7
Autoimmune diseases are the result of hyperstimulation of immunity, caused by breakdown of immune tolerance, which has a multifactorial etiology. Among all potential triggers for development of autoimmune diseases, viruses represent the greatest environmental potential to trigger inflammatory myopathy (IM) in genetically susceptible individuals. Inflammatory myopathy has been observed during or after viral infection, but attempts to amplify viruses from muscles, including coxsackievirus, influenza, paramyxovirus, mumps, cytomegalovirus, and Epstein‐Barr virus, have failed. The best‐
studied viral relationship so far has been with retrovirus. 7 However, there are fewer case reports in the literature highlighting association of CHIKV with the development of autoimmune diseases, especially inflammatory myopathies. 5 , 8
Dev et al (2019) reported a case of inflammatory myositis following chikungunya infection in 2019 in India. 8 Martins et al (2016) reported a case of a patient presenting with myositis and encephalitis post‐CHIKV infection in Brazil. 5 However, this is the first case of antisynthetase syndrome post‐chikungunya infection characterized in literature.
There are possible hypothesized mechanisms for development of post‐infection‐induced autoimmune disease. Autoreactive T cells can be activated by cross‐linking with a viral antigen that bears similarity to autoantigen and become pathogenic. Stimulation of Toll‐like receptors and other pattern‐recognition receptors on antigen‐presenting cells leads to production of pro‐inflammatory mediators, which can trigger tissue damage. Tissue destruction by T cells and inflammatory mediators further increase release of autoantigens, potentiating the immune response. 2
Another possible mechanism is the evasion of host's innate and adaptive immune responses by the virus, as seen in mice showing a chronic inflammatory process in ankles after inoculation with CHIKV RNA and persistence of the virus in the local tissue. Furthermore, several alphaviruses, including CHIKV, have been shown to antagonize STAT activation by type I and type II interferon. 9
Antisynthetase syndrome is a rare disease with unknown incidence and a prevalence twice higher in females. It presents a broad clinical picture, with proximal muscle weakness, arthritis, arthralgia, Raynaud's phenomenon, fever, “mechanic's hands,” and interstitial lung disease. It is related to the presence of anti‐synthetase autoantibodies, especially anti‐Jo‐1, and to histological presentation of perimysial and perifascicular muscle fiber necrosis. Pulmonary involvement is related to high morbidity and mortality rates, especially interstitial lung disease (ILD), which can present with dyspnea, cough, and chest pain. 7
In this case, the patient presented with proximal muscle weakness in all four limbs, MRI of thighs showing a muscular inflammatory process, electroneuromyography with a myopathic pattern, interstitial pneumopathy, and a reagent Anti‐Jo1 antibody, in addition to joint involvement. The case report presented some limitations, such as the fact that the patient did not perform a muscle biopsy, due to lack of availability of the procedure at the service. The patient also did not present mechanic's hands, a classic but not specific sign. 10
Moreover, although acute symptoms presentation and the temporal correlation corroborates the possible association between the Chikungunya infection and antisyntethase syndrome, this correlation is not entirely defined. The patient's CHIKV‐like manifestation may correspond to an ASS presentation from the onset of symptoms instead of CHIKV acute‐phase symptoms. In this scenario, the CHIKV‐IgM seropositivity would be due to a previous infection, since the seropositivity can last up to 12–36 months 11 with or without symptomatic presentation of the infection. Adding to that, State Health Department's consulted data show an incidence of reported cases of CHIKV in the patient's neighborhood in all the last years. Thus, the epidemiological link exists both in the patient's symptomatic period and in the years before. Therefore, it is not possible to exclude the possibility of a late‐diagnosed CHIKV with further symptomatic ASS uncorrelated, even though it is not the most plausible scenario.
4. CONCLUSION
More studies are needed to highlight and detail the pathophysiology of CHIKV in development of autoimmune diseases in genetically susceptible individuals, as well as its diverse clinical presentations. It is known that viral infections can be triggers for inflammatory myopathies and therefore attention should be given to patients who persist with muscle weakness and myalgia after a chikungunya infection.
AUTHOR CONTRIBUTION
Raiza Cansian Tuão and Ketty Lysie Libardi Lira Machado: conceptualized the study, wrote, revised, and edited the manuscript, and analyzed and interpreted the muscle magnetic resonance images. Mariana de Oliveira Macabú, Paula dos Santos Athayde, Isac Ribeiro Moulaz, and Barbara Ferraço Dalmaso: wrote, revised, and edited the manuscript. Valéria Valim Cristo conceptualized and revised the manuscript. All authors were involved in editing and approving the manuscript.
CONFLICTS OF INTEREST
No conflict of interest.
ETHICAL APPROVAL
This case report was carried out following the recommendations of the ethics committee of the Cassiano Antonio Moraes University Hospital ‐ HUCAM, Brazil.
CONSENT
Written informed consent was obtained from the patient for the publication of this case report.
ACKNOWLEDGMENT
No acknowledgments to be made.
Tuão RC, Macabú MdO, Athayde PdS, et al. Antisynthetase Syndrome after chikungunya infection: A case report. Clin Case Rep. 2022;10:e05877. doi: 10.1002/ccr3.5877
Institution where the article was developed/written: Federal University of Espírito Santo.
Funding information
None
DATA AVAILABILITY STATEMENT
All data included in this report are accurate to the best of our knowledge. We will make available data (images and reports) upon request.
REFERENCES
- 1. Ministério da Saúde, Brazil: Chikungunya clinical management. 2017;1:1‐67. https://bvsms.saude.gov.br/bvs/publicacoes/chikungunya_manejo_clinico.pdf. Accessed October 10, 2021. [Google Scholar]
- 2. Getts MT, Miller SD. 99th Dahlem conference on infection, inflammation and chronic inflammatory disorders: triggering of autoimmune diseases by infections. Clin Exp Immunol. 2010;160:15‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vairo F, Haider N, Kock R, Ntoumi F, Ippolito G, Zumla A. Chikungunya: epidemiology, pathogenesis, clinical features, management, and prevention. Infect Dis Clin North Am. 2019;33:1003‐1025. [DOI] [PubMed] [Google Scholar]
- 4. Castro APCR, Lima RAN, Santos J. Chikungunya: vision of the pain clinician. Revista Dor (online). 2016;17:299‐302. [Google Scholar]
- 5. Martins HA, Bernardino SN, Santos CC, Ribas VR. Chikungunya and myositis: a case report in Brazil. J Clin Diagn Res. 2016;10:OD05‐OD06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Choudhary N, Makhija P, Puri V, Khwaja GA, Duggal A. An unusual case of myelitis with myositis. J Clin Diagn Res. 2016;10:OD19‐OD20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dalakas MC. Inflammatory myopathies: update on diagnosis, pathogenesis and therapies, and COVID‐19‐related implications. Acta Myol. 2020;39:289‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dev N, Kumar R, Gogna A, Sharma S. Chikungunya‐induced inflammatory myositis: a case report in India. Trop Doct. 2019;49:241‐243. [DOI] [PubMed] [Google Scholar]
- 9. Morrison TE, Oko L, Montgomery SA, et al. A mouse model of chikungunya virus‐induced musculoskeletal inflammatory disease: evidence of arthritis, tenosynovitis, myositis, and persistence. Am J Pathol. 2011;178:32‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carboni RCS, Pinto GLB, Shinjo SK. High YKL‐40 serum levels and its expression in the muscle tissues of patients with antisynthetase syndrome. Adv. Rheumal. 2021;61:44. [DOI] [PubMed] [Google Scholar]
- 11. Costa DMDN, Coêlho MRCD, Gouveia PADC, et al. Long‐term persistence of serum‐specific anti‐chikungunya igm antibody ‐ a case series of Brazilian patients. Rev Soc Bras Med Trop. 2021;12(54):e0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data included in this report are accurate to the best of our knowledge. We will make available data (images and reports) upon request.


