Abstract
Objective
To assess the effect of pretreatment body mass index (BMI) and the extent of change in BMI (ΔBMI) during the treatment course on the treatment outcomes in patients with nasopharyngeal carcinoma (NPC) receiving volumetric modulated arc therapy (VMAT).
Methods
Data pertaining to 498 consecutive NPC patients with stage I–IVA disease who received VMAT between January 2010 and November 2011 at a single center were retrospectively analyzed. Univariate Kaplan-Meier and multivariate Cox regression analyses were used to evaluate the prognostic significance of pretreatment BMI and ΔBMI. Receiver operating characteristic (ROC) curve analysis was used to determine the optimal cut-off point of ΔBMI.
Results
The 5-year loco-regional failure-free (L-FFR), distant failure-free survival (D-FFR), disease-free survival (DFS), and overall survival (OS) rates were 90.6%, 83.7%, 71.5% and 79.3%, respectively. The 5-year L-FFR, D-FFR, DFS, OS rates for NPC patients with ΔBMI ≤1 kg/m2 vs ΔBMI >1 kg/m2 were 92.3% vs 89.3% (P = .137), 90.9% vs 78.5% (P < .001), 80.4% vs 65.1% (P < .001), and 88.0% vs 73.0% (P < .001), respectively. ΔBMI >1 kg/m2 was an independent predictor of D-FFR (P = .002), DFS (P = .002), and OS (P = .001).
Conclusions
ΔBMI during treatment course may have a significant impact on the prognosis of NPC patients receiving VMAT.
Keywords: body mass index, prognosis, nasopharyngeal carcinoma, volumetric modulated arc therapy
Introduction
Nasopharyngeal carcinoma (NPC) is a particularly common high-grade malignant head and neck tumor in Southeast and East Asia. 1 Radiotherapy is the main treatment modality for NPC. Volumetric modulated arc therapy (VMAT), a novel form of intensity modulated radiation therapy (IMRT), has been widely accepted in lieu of static IMRT for NPC over the past years owing to the advantages of shortened treatment time and dosimetric benefits.2-4
Body mass index (BMI), which is calculated as weight in kilograms divided by the square of the height in meters (kg/m2), is a simple index of nutritional status in adults. BMI has been shown to be associated with the prognosis of cancer; however, the relationship has not been fully elucidated. In some studies, higher BMI was found to be associated with a favorable prognosis for various tumors, such as head and neck cancer, 5 esophageal cancer, 6 and colon cancer. 7 However, in some other studies, higher BMI was associated with a worse prognosis in patients with prostate cancer 8 and breast cancer. 9 The prognostic relevance of BMI in the context of NPC is not well characterized. To the best of our knowledge, no studies have addressed the prognostic relevance of the extent of decrease in BMI in NPC patients treated with VMAT. In a study by Huang et al, 10 pretreatment BMI was found to be an independent prognostic factor for patients with locoregionally advanced NPC treated with chemoradiotherapy. Lin et al 11 found no significant relationship of BMI and percent weight loss on survival of NPC patients receiving IMRT. However the studies were based on conventional radiation therapy and IMRT, respectively. Currently, VMAT is being widely used in the treatment of NPC; therefore, further studies are required to assess the effect of BMI on outcomes of VMAT. The aim of this study was to assess the prognostic significance of BMI, including pretreatment BMI and the extent of decrease in BMI during treatment of NPC patients with VMAT.
Methods and Materials
Patients
Between January 2010 and November 2011, a total of 508 patients with newly-diagnosed NPC were treated primarily with VMAT at our institution. The inclusion criteria were: (1) stage I-IVA disease according to the 8th edition of the AJCC/UICC TNM staging system; (2) confirmed by pathology; (3) treated by VMAT. The exclusion criteria were as follows: (1) diagnosed with a previous malignancy or other concomitant malignant disease; (2) pregnancy or lactation; (3) metastatic disease at diagnosis. Based on the criteria, 498 patients were included in this retrospective study. The characteristics of patients are listed in Table 1. The study protocol was approved by the ethics committee of our institution. The participants were telephonically contacted to obtain verbal informed consent for retrospective analysis of the pertinent clinical information. Additionally, we de-identified all patient details. The reporting of this study conforms to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. 12
Table 1.
Patients’ Characteristics.
| Characteristic | Number of patient (%) |
|---|---|
| Age (years) | |
| ≤50 | 322 (64.7%) |
| >50 | 176 (35.3%) |
| Gender | |
| Male | 383 (76.9) |
| Female | 115 (23.1%) |
| T-stage | |
| T1 | 39 (7.8%) |
| T2 | 149 (29.9%) |
| T3 | 214 (43.0%) |
| T4 | 96 (19.3%) |
| N-stage | |
| N0 | 127 (25.5%) |
| N1 | 242 (48.6%) |
| N2 | 106 (21.3%) |
| N3 | 23 (4.6%) |
| TNM stage | |
| I | 11 (2.2%) |
| II | 128 (25.7%) |
| III | 248 (49.8%) |
| IV | 111 (22.3%) |
| Pretreatment BMI value | |
| <18.5 Kg/m2 | 33 (6.6%) |
| 18.5-22.9 Kg/m2 | 232 (46.6%) |
| 23.0-27.4 Kg/m2 | 207 (41.6%) |
| ≥27.5 Kg/m2 | 26 (5.2%) |
| ΔBMI value | |
| ≤1 Kg/m2 | 209 (42.0%) |
| >1 Kg/m2 | 289 (58.0%) |
Pre-RT BMI, pretreatment BMI; ΔBMI, Difference value of BMI before treatment and end of radiotherapy.
BMI Measurement
BMI was calculated as the patient’s weight (in kilograms) divided by the patient’s height squared (in meters). Pretreatment BMI was measured 1 week before the start of treatment. Posttreatment BMI was defined as the BMI at the last day of radiotherapy. Change in BMI value during treatment (ΔBMI) was calculated using the formula: pretreatment BMI minus posttreatment BMI. Patients were divided into four groups based on the pretreatment BMI: underweight (BMI<18.5 kg/m2), normal weight (BMI 18.5-22.9 kg/m2), overweight (BMI 23.0-27.4 kg/m2), and obese (BMI ≥27.4 kg/m2) according to the World Health Organization classifications for Asian populations.
Radiotherapy
All patients in this study were treated with VMAT. The target volumes were outlined manually according to our treatment protocol described elsewhere. 13 The primary gross tumor volume (GTV-P) and the cervical metastatic lymph nodes (GTV-N) included all gross disease as determined by imaging (CT and MRI fusion), clinical, and endoscopic findings. The clinical target volumes (CTVs) were designed to encompass microscopic disease including the high-risk region (CTV-1) and the subclinical prophylactic low-risk region (CTV-2). Levels II, III, IV, and V can be incorporated into CTV of the neck nodal regions (CTV-N). The planning target volume (PTV) was created based on each volume with an additional 3-mm margin, allowing for setup variability. Organs at risk (OARs) included the brain stem, spinal cord, optic nerve, optic chiasm, temporal lobe, crystal, and parotid gland.
A total dose of 69.7-70 Gy administered in 31-35 fractions (2-2.25 Gy/fraction) was set to the PTV of GTV-P and GTV-N. While a total dose of 59.5-62 Gy at 1.7-2 Gy/fraction was set to the PTV of CTV-1 and 52.7-56 Gy at 1.6-1.8 Gy/fraction was set to the PTV of CTV-2 and CTV-N.
Chemotherapy
Patients with stage III-IV disease received 2-4 cycles of cisplatin-based neoadjuvant chemotherapy and 1-2 cycles of concurrent chemotherapy. Patients with stage T1-2N1 disease underwent 1-2 cycles of concurrent chemotherapy. Patients at stage T1-2N0 only received radiation. Neoadjuvant chemotherapy consisted of gemcitabine (1000 mg/m2 on days 1 and 8) plus cisplatin (80 mg/m2 on days 1-3) or paclitaxel (135 mg/m2 on day 1) plus cisplatin (80 mg/m2 on days 1-3). Concurrent chemotherapy based on cisplatin (80 mg/m2 on days 1-3) was administered during the course of radiotherapy. Chemotherapy was repeated every 21 days.
Nutritional Support
This was a retrospective study, and there was no standardized protocol for nutritional support. All patients were encouraged to take regular meals during treatment. None of the patients received prophylactic or reactive feeding tubes during the treatment. Parenteral nutrition was not a routine nutritional support in this population either.
Follow-Up
Follow-up was performed every 3 months for the first 2 years and every 6 months for the first 5 years. Thereafter, follow-up was performed once a year. Follow-up included complete physical, hematologic, and biochemical examinations, chest radiograph, abdominal ultrasonography, and nasopharyngoscopy examination. MRI/CT of the nasopharynx, CT chest, and abdominal ultrasound were performed every 6-12 months routinely. 18F-Fluorodeoxyglucose positron emission tomography and CT (PET-CT) was performed at the discretion of the treating physician as per need. All local or regional relapse was confirmed by pathology.
Statistical Analysis
Statistical analysis was performed using SPSS version 13.0 software (SPSS Inc. Chicago, IL) and R 2.15.3 software. Survival outcomes, including 5-year local failure-free rate (L-FFR), 5-year distant failure-free rate (D-FFR), 5-year disease-free survival (DFS), and 5-year overall survival (OS) were calculated from the date of diagnosis to the most recent follow-up or to the date of recurrence, metastasis, or death. Kaplan-Meier method was used for survival analysis and between-group differences were assessed using the log-rank test. Cox proportional hazards regression analysis was performed to identify the prognostic factors. P values < .05 were considered indicative of statistical significance.
Receiver operating characteristic (ROC) curve analysis was performed to determine the optimal cut-off point of ΔBMI to predict mortality. The optimal cut-off point was the minimal distance to the ideal point (sensitivity = specificity = 100%) on the ROC curve. It provides a criterion for choosing the ‘‘optimal’’ threshold value based on the Youden index. 14 The study population was then stratified according to the optimal cut-off point.
Results
Baseline Characteristics and Follow-Up
The baseline characteristics of the 498 patients are summarized in Table 1. The mean pretreatment BMI in our cohort was 22.80 kg/m2 (range 15.01-35.06). Patients were divided into four groups based on the pretreatment BMI according to the WHO BMI subgroups for Asian populations. A total of 33 patients (6.6%) were underweight, 232 (46.6%) had a normal BMI, and 207 (41.6%) were overweight; only 26 (5.2%) were obese. The mean ΔBMI was 1.29 kg/m2 (range −1.65-5.75). The median duration of follow-up in our cohort was 68 months (range 4-110).
Optimal ΔBMI Cut-Off Point
The area under the ROC curve was .598 (95% CI 0.540-.656; P = .002). The cut-off point of ΔBMI was 1.04 kg/m2 (sensitivity: 75.7%; specificity: 46.8%). Therefore, we selected the cut-off point as 1 kg/m2 to classify patients intoΔBMI≤1 kg/m2 group and ΔBMI>1 kg/m2 group for survival analysis.
Treatment Outcomes
The 5-year L-FFR, D-FFR, DFS, and OS rates in the overall cohort were 90.6%, 83.7%, 71.5%, and 79.3% respectively. Forty-seven patients had local or regional failure, 81 patients developed distant metastasis, and 10 patients had distant metastasis with local or regional failure.
The prognostic significance of age, sex, N-stage, T-stage, primary tumor volume (PTV), pretreatment BMI, and ΔBMI was analyzed. The results of univariate analysis are shown in Table 2. On multivariate analysis, age [relative risk (RR) = 2.457, P < .001), N-stage (RR = 1.807, P = .004), PTV (RR = 2.259, P < .001), and ΔBMI (RR = 2.197, P = .001) were adverse prognostic factors for OS (Table 3). None of these factors were independent prognostic factors for L-FFR. N-stage (RR = 2.623, P < .001), PTV (RR = 1.900, P = .007), and ΔBMI (RR = 2.306, P = .002) showed an independent association with D-FFR. Age (RR = 1.942, P < .001), N-stage (RR = 2.623, P < .001), PTV (RR = 1.900, P = .001), and ΔBMI (RR = 1.800, P = .002) were adverse prognostic factors for DFS. Pretreatment BMI was not found to be an independent prognostic factor for L-FFR, D-FFR, DFS, or OS in our cohort.
Table 2.
Univariate analysis of predictive factors for the patients with NPC.
| Variable | 5-year LFR (%) | P-value | 5-year D-FFS (%) | P-value | 5-year DFS (%) | P-value | 5-year OS (%) | P-value | |
|---|---|---|---|---|---|---|---|---|---|
| Gender | Male | 90.3 | .673 | 82.8 | .240 | 70.0 | .201 | 78.1 | .179 |
| Female | 91.3 | 87.0 | 76.5 | 83.5 | |||||
| Age | ≤50 y | 92.3 | .033 | 83.6 | .863 | 76.2 | <.001 | 84.5 | <.001 |
| >50 y | 87.4 | 84.0 | 62.9 | 69.7 | |||||
| N-stage | N0 | 93.7 | .325 | 92.1 | <.001 | 81.1 | <.001 | 85.0 | .003 |
| N1 | 90.1 | 86.8 | 74.0 | 81.8 | |||||
| N2 | 88.7 | 69.8 | 56.6 | 69.8 | |||||
| N3 | 87.0 | 69.6 | 60.9 | 65.2 | |||||
| T-stage | T1 | 94.9 | .016 | 92.3 | <.001 | 87.2 | <.001 | 94.9 | <.001 |
| T2 | 91.9 | 87.2 | 77.9 | 85.2 | |||||
| T3 | 92.1 | 85.5 | 73.,8 | 80.8 | |||||
| T4 | 83.3 | 70.8 | 50.0 | 60.4 | |||||
| PTV | ≤15 mL | 95.1 | .188 | 91.3 | <.001 | 81.6 | <.001 | 87.4 | <.001 |
| 15-25 mL | 89.2 | 86.5 | 76.6 | 82.9 | |||||
| 25-50 mL | 90.4 | 85.5 | 72.9 | 81.9 | |||||
| >50 mL | 88.1 | 72.0 | 55.9 | 65.3 | |||||
| Pre-BMI | ≤23 Kg/m2 | 90.9 | .864 | 82.3 | .312 | 70.6 | .520 | 79.2 | .884 |
| >23 Kg/m2 | 90.1 | 85.4 | 72.5 | 79.4 | |||||
| ΔBMI | ≤1 Kg/m2 | 92.3 | .137 | 90.9 | <.001 | 80.4 | <.001 | 88.0 | <.001 |
| >1 Kg/m2 | 89.3 | 78.5 | 65.1 | 73.0 | |||||
Pre-BMI, pretreatment BMI; ΔBMI, Difference value of BMI before treatment and end of radiotherapy; 5-y L-FFR, 5-year locol-regional failure-free rate; 5-y D-FFS, 5-year distant failure-free survival; 5-y DFS, 5-year disease free survival; 5-y OS, 5-year overall survival.
Table 3.
Multivariate Analysis of Predictive Factors for the Patients With NPC.
| Variable | 5-year L-FFR | 5-year D-FFS | 5-year DFS | 5-year OS | ||||
|---|---|---|---|---|---|---|---|---|
| RR,95% CI P | RR,95% CI P | RR,95% CI P | RR,95% CI P | |||||
| Age | ||||||||
| ≤50 vs >50 | 1.607 | .155 | 1.183 | .476 | 1.942 | <.001 | 2.457 | <.001 |
| .836-3.091 | .745-1.879 | 1.388-2.716 | 1.654-3.650 | |||||
| T-stage | ||||||||
| T1-2 vs T3-4 | 1.511 | .261 | 1.377 | .139 | 1.377 | .139 | 1.648 | .058 |
| .736-3.105 | .902-2.103 | .902-2.103 | .984-2.763 | |||||
| N-stage | ||||||||
| N0-1 vs N2-3 | 1.475 | .222 | 2.623 | <.001 | 1.880 | <.001 | 1.807 | .004 |
| .791-2.749 | 1.683-4.087 | 1.330-2.658 | 1.203-2.715 | |||||
| PTV | ||||||||
| ≤50 mL vs >50 mL | 1.607 | .155 | 1.900 | .007 | 1.990 | <.001 | 2.259 | <.001 |
| .836-3.091 | 1.190-3.034 | 1.389-2.852 | 1.398-3.465 | |||||
| Pre-BMI | ||||||||
| ≤23 Kg/m2 vs >23 Kg/m2 | .977 | .938 | .746 | .746 | .830 | .291 | .881 | .538 |
| .536-1.778 | .472-1.180 | .588-1.173 | .588-1.319 | |||||
| ΔBMI | ||||||||
| ≤1 Kg/m2 vs >1 Kg/m2 | 1.438 | .258 | 2.306 | .002 | 1.800 | .002 | 2.197 | .001 |
| .767-2.699 | 1.350-3.939 | 1.233-2.628 | 1.379-3.500 | |||||
RR, risk ratio; CI, confidence interval; Pre-BMI, pretreatment BMI; ΔBMI, Difference value of BMI before treatment and end of radiotherapy; 5-y L-FFR, 5-year locol-regional failure-free rate; 5-y D-FFS, 5-year distant failure-free survival; 5-y DFS, 5-year disease free survival; 5-y OS, 5-year overall survival.
Prognostic Value of the ΔBMI Cut-Off Point
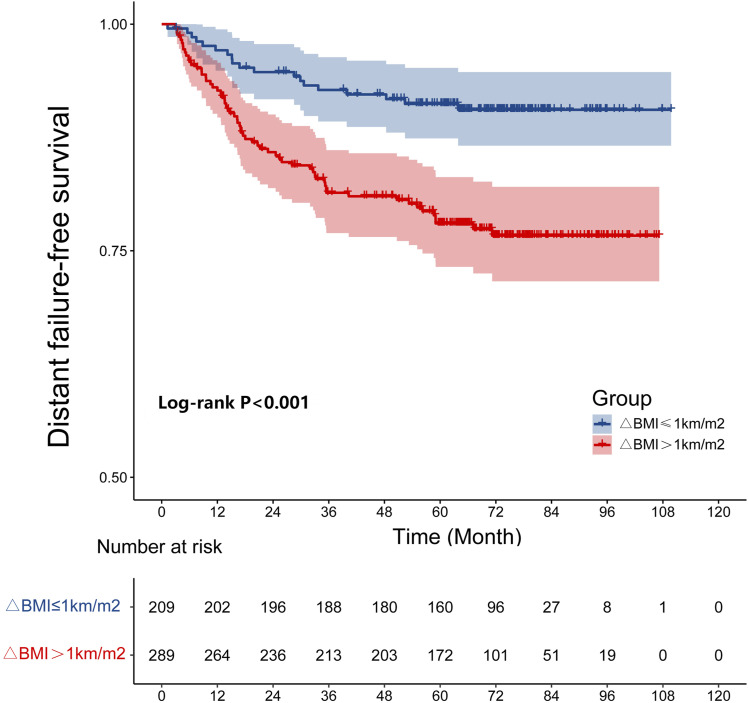
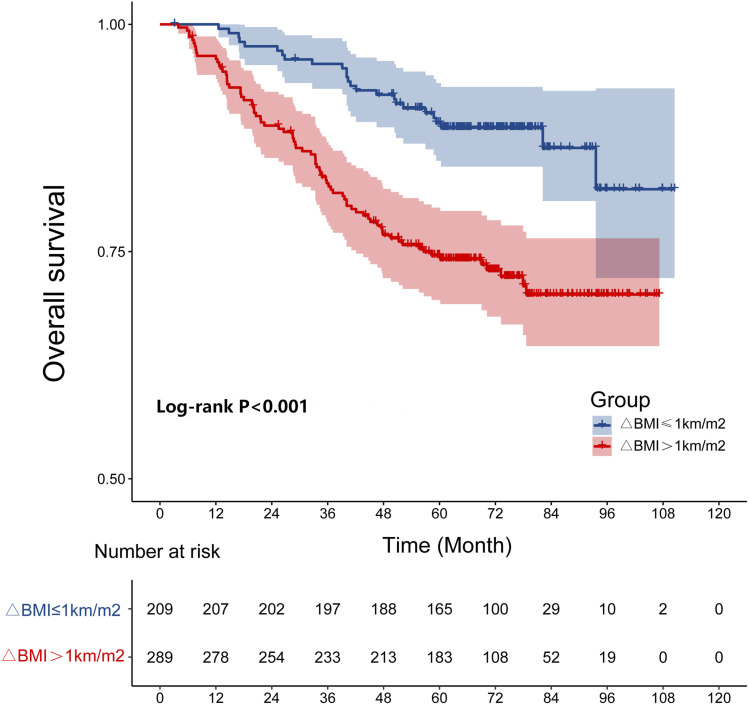
In the study, the 5-year L-FFR, D-FFR, DFS, OS rates for NPC patients with a ΔBMI ≤1 kg/m2 vs ΔBMI >1 kg/m2 were 92.3% vs 89.3% (P = .137), 90.9% vs 78.5% (P < .001, Figure 1), 80.4% vs 65.1% (P < .001), and 88.0% vs 73.0% (P < .001, Figure 2), respectively. Overall, patients with a ΔBMI >1 kg/m2 had significantly poorer D-FFR, DFS, and OS than patients with ΔBMI ≤1 kg/m2. In multivariate analysis, ΔBMI using the cut-off point was an independent prognostic factor for D-FFR (P = .002), DFS (P = .002), and OS (P = .001).
Figure 1.
The cumulative distant failure-free survival curves by ΔBMI (≤1 and >1 Kg/m2).
Figure 2.
The cumulative overall survival curves by ΔBMI (≤1 and >1 Kg/m2).
Discussion
In the study, we investigated the prognostic relevance of BMI in NPC patients treated with VMAT. In our cohort, pretreatment BMI had no significant effect on treatment outcomes. However, ΔBMI was associated with D-FFR, DFS, and OS. To the best of our knowledge, this is the first study to investigate the prognostic value of change in BMI during the treatment course in NPC patients undergoing VMAT.
Numerous studies have confirmed that IMRT is an outstanding radiotherapeutic technique for NPC. Nonetheless, the prolonged treatment time of IMRT may limit the radiotherapy accuracy because of increased intra-fractional patient movements. 15 Additionally, the increased dose to OARs, compared with VMAT, may increase the incidence of radiation-induced secondary cancers.16-18 Therefore VMAT has gradually replaced IMRT due to shortened treatment time and dosimetric benefits. Several studies have found that VMAT can deliver dosimetric benefits to some extent compared with static IMRT in treatment plan.3,4 Although many studies have indicated the relationship of BMI and percent weight loss with survival of NPC patients, there is a paucity of published data pertaining to VMAT. Thus we conducted this study to assess the correlation between BMI and prognosis in NPC treated with VMAT.
Park et al 6 found that higher pretreatment BMI may be associated with better prognosis in Korean patients with head and neck cancer. In the studies by Hung et al 10 and Shen et al, 19 pretreatment BMI was an independent prognostic factor for patients with NPC treated with chemoradiotherapy, which was contrary to our results. In our study, multivariate analysis showed no significant influence of pretreatment BMI on the risk of L-FFR, D-FFR, DFS, or OS. One possible explanation was that the previous study was based on conventional two-dimensional radiotherapy. Therefore the conclusions may not be directly extrapolatable to patients receiving VMAT. VMAT can provide improved treatment outcomes and lower incidence of toxicity. 20 Less side effects of radiotherapy minimize the effect on nutrition status during the treatment course, thus decreasing the importance of pretreatment BMI.
Moreover, we analyzed the prognostic relevance of ΔBMI in patients with NPC receiving VMAT. We further performed ROC curve analysis to determine the optimal ΔBMI cut-off point for the purpose of risk stratification. Cut-off point associated with optimal sensitivity and specificity should be used in clinical practice. 21 Hence, we defined the critical cut-off point according to maximized Youden index and practicability of clinical application. A cut-off value of 1 kg/m2 was chosen for predicting the treatment outcomes of NPC undergoing VMAT. Zeng et al 22 also found that critical weight loss predicts poor prognosis in NPC patients. Li et al 23 found that weight loss had an adverse effect on the prognosis of NPC patients. However, the radiotherapy techniques used in these studies were different. In the present study, we just focused on NPC patients treated with VAMT. Thus the study is predominantly different from the previous studies.
Based on the current study, ΔBMI had a significant prognostic value in NPC patients receiving VMAT. The potential reasons are as follows. Severe decrease in BMI during the treatment course is indicative of malnutrition, which increases the toxicity and reduces the patient’s ability to tolerate treatment, including surgery, radiotherapy, and chemotherapy. Patients with malnutrition experience more treatment delays or interruptions and poor overall survival. In addition, the immune system is highly dependent on adequate nutrition. So malnutrition also impairs the immune status leading to poorer therapeutic effect.24,25 Moreover, Langius et al 26 reported that patients with severe weight loss had significantly lower numbers of T cells and iNKT cells. Unfortunately, it was demonstrated that a severe deficiency of peripheral blood iNKT cells in patients with head and neck cancer was significantly related to poor clinical outcome. 27 However, it is crucial to note that the result was related to the extent of decrease in BMI. Our study showed that 5-year D-FFR, DFS, OS rates were significantly higher in patients with ΔBMI ≤1 kg/m2.
Advances in the radiotherapy techniques have facilitated higher rates of local control. Distant metastasis has gradually become the major reason for treatment failure in NPC patients. 28 Therefore, identification of effective markers of distant metastasis is a key imperative for NPC patients in addition to the N-stage. Our research demonstrated that ΔBMI (RR = 2.306, P = .002) is a potential convenient prognostic factor for D-FFS.
Several factors associated with unfavorable prognosis of NPC have been identified, including larger primary tumor volume, 29 advanced N stage, lower hemoglobin (Hb) level, 30 conspicuous decrease in Hb level during treatment course, 31 and overexpression of vascular endothelial growth factor (VEGF) 32 or epidermal growth factor receptor (EGFR). 33 However, the prognostic relevance of BMI as a convenient marker has largely been ignored. Our research revealed that ΔBMI might be a simple and reliable prognostic factor in NPC patients receiving VMAT. Therefore we believe that active and optimal treatment should be considered for patients who show a conspicuous decrease in BMI in order to prevent continued decline of BMI. Xu et al 34 reported that percutaneous endoscopic gastrostomy can confer survival benefit in NPC patients treated with concurrent chemoradiotherapy. Meng et al 35 demonstrated that early nutritional intervention can improve outcomes of NPC patients. Nevertheless, the appropriate treatment approach and time-point for intervention in NPC patients who show a conspicuous decrease in BMI during VMAT is not clear.
Despite the positive findings, some limitations of our study should be acknowledged. The single-center scope of the study and the retrospective study design are the principal limitations of the study. Thus, due caution should be exercised while interpreting the findings. A prospective multi-center study is warranted to obtain more robust evidence.
Conclusion
ΔBMI is a potential predictor of survival outcomes in NPC patients receiving VMAT. Nutritional intervention including the placement of feeding tubes is highly recommended for NPC patients as clinically indicated. Concerted efforts should be made to investigate the appropriate treatment strategy for NPC patients who exhibit a conspicuous decrease in BMI while undergoing VMAT.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Startup Fund for scientific research, Fujian Medical University [grant number: 2018QH1230].
Ethics Approval: The study protocol was approved by the ethics committee of our institution (YKT2020-O11-12). The participants were telephonically contacted to obtain verbal informed consent for retrospective analysis of the pertinent clinical information. Additionally, we de-identified all patient details.
ORCID iD
Chuanben Chen https://orcid.org/0000-0002-5994-8897
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [DOI] [PubMed] [Google Scholar]
- 2.Gomez-Millan Barrachina J, Jerez Sainz I, Perez Rozos A, et al. Potential advantages of volumetric arc therapy in head and neck cancer. Head Neck. 2015;37:909-914. [DOI] [PubMed] [Google Scholar]
- 3.Anamalayil SJ, Teo BK, Lin A, Lustig RA, Ahn PH. Effects of full-neck volumetric-modulated arc therapy vs split-field intensity-modulated head and neck radiation therapy on low neck targets and structures. Br J Radiol. 2016;89:1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dai X, Zhao Y, Liang Z, et al. Volumetric-modulated arc therapy for oropharyngeal carcinoma: adosimetric and delivery efficiency comparison with staticfield IMRT. Phys Med. 2015;31:54-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pai PC, Chuang CC, Tseng CK, et al. Impact of pretreatment body mass index on patients with head-and-neck cancer treated with radiation. Int J Radiat Oncol Biol Phys. 2012;83(1):e93-e100. [DOI] [PubMed] [Google Scholar]
- 6.Park SM, Lim MK, Shin SA, Yun YH. Impact of prediagnosis smoking, alcohol, obesity, and insulin resistance on survival in male cancer patients: National Health Insurance Corporation Study. J Clin Oncol. 2006;24(31):5017-5024. [DOI] [PubMed] [Google Scholar]
- 7.Simkens LH, Koopman M, Mol L, et al. Influence of body mass index on outcome in advanced colorectal cancer patients receiving chemotherapy with or without targeted therapy. Eur J Cancer. 2011;47(17):2560-2567. [DOI] [PubMed] [Google Scholar]
- 8.Efstathiou JA, Chen MH, Renshaw AA, Loffredo MJ, D’Amico AV. Influence of body mass index on prostate-specific antigen failure after androgen suppression and radiation therapy for localized prostate cancer. Cancer. 2007;109(8):1493-1498. [DOI] [PubMed] [Google Scholar]
- 9.Dawood S, Broglio K, Gonzalez-Angulo AM, et al. Prognostic value of body mass index in locally advanced breast cancer. Clin Cancer Res. 2008;14(6):1718-1725. [DOI] [PubMed] [Google Scholar]
- 10.Huang PY, Wang CT, Cao KJ, et al. Pretreatment body mass index as an independent prognostic factor in patients with locoregionally advanced nasopharyngeal carcinoma treated with chemoradiotherapy: findings from a randomised trial. Eur J Cancer. 2013;49(8):1923-1931. [DOI] [PubMed] [Google Scholar]
- 11.Lin YH, Chang KP, Lin YS, Chang TS. Evaluation of effect of body mass index and weight loss on survival of patients with nasopharyngeal carcinoma treated with intensity-modulated radiation therapy. Radiat Oncol. 2015;10:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573-577. [DOI] [PubMed] [Google Scholar]
- 13.Chen C, Lin X, Pan J, Fei Z, Chen L, Bai P. Is it necessary to repeat CT imaging and replanning during the course of intensity-modulated radiation therapy for locoregionally advanced nasopharyngeal carcinoma? Jpn J Radiol. 2013;31(9):593-599. [DOI] [PubMed] [Google Scholar]
- 14.Greiner M, Pfeiffer D, Smith RD. Principles and practical application of the receiver-operating characteristic analysis for diagnostic tests. Prev Vet Med. 2000;45:23-41. [DOI] [PubMed] [Google Scholar]
- 15.Hoogeman MS, Nuyttens JJ, Levendag PC, Heijmen BJ. Time dependence of intrafraction patient motion assessed by repeat stereoscopic imaging. Int J Radiat Oncol Biol Phys. 2008;70(2):609-618. [DOI] [PubMed] [Google Scholar]
- 16.Hall EJ. Intensity-modulated radiation therapy, protons, and the risk of second cancers. Int J Radiat Oncol Biol Phys. 2006;65(1):1-7. [DOI] [PubMed] [Google Scholar]
- 17.Hall EJ, Wuu CS. Radiation-induced second cancers: the impact of 3D-CRT and IMRT. Int J Radiat Oncol Biol Phys. 2003;56(1):83-88. [DOI] [PubMed] [Google Scholar]
- 18.Patil VM, Kapoor R, Chakraborty S, Ghoshal S, Oinam AS, Sharma SC. Dosimetric risk estimates of radiation-induced malignancies after intensity modulated radiotherapy. J Cancer Res Ther. 2010;6(4):442-447. [DOI] [PubMed] [Google Scholar]
- 19.Shen GP, Xu FH, He F, et al. Pretreatment lifestyle behaviors as survival predictors for patients with nasopharyngeal carcinoma. PLoS One. 2012;7:e36515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo R, Tang LL, Mao YP, et al. Clinical outcomes of volume-modulated arc therapy in 205 patients with nasopharyngeal carcinoma: an analysis of survival and treatment toxicities. PLoS One. 2015;10:e0129679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39:561-577. [PubMed] [Google Scholar]
- 22.Zeng Q, Shen LJ, Guo X, Guo XM, Qian CN, Wu PH. Critical weight loss predicts poor prognosis in nasopharyngeal carcinoma. BMC Cancer. 2016;16:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li G, Jiang XY, Qiu B, Shen LJ, Chen C, Xia YF. Vicious circle of acute radiation toxicities and weight loss predicts poor prognosis for nasopharyngeal carcinoma patients receiving intensity modulated radiotherapy. J Cancer. 2017;8(5):832-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Cutsem E, Arends J. The causes and consequences of cancer associated malnutrition. Eur J Oncol Nurs. 2005;9(suppl 2):S51-S63. [DOI] [PubMed] [Google Scholar]
- 25.Capuano G, Gentile PC, Bianciardi F, Tosti M, Palladino A, Di Palma M. Prevalence and influence of malnutrition on quality of life and performance status in patients with locally advanced head and neck cancer before treatment. Support Care Cancer. 2010;18(4):433-437. [DOI] [PubMed] [Google Scholar]
- 26.Langius JA, Bakker S, Rietveld DH, et al. Critical weight loss is a major prognostic indicator for disease-specific survival in patients with head and neck cancer receiving radiotherapy. Br J Cancer. 2013;109:1093-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molling JW, Langius JA, Langendijk JA, et al. Low levels of circulating invariant natural killer T cells predict poor clinical outcome in patients with head and neck squamous cell carcinoma. J Clin Oncol. 2007;25:862-868. [DOI] [PubMed] [Google Scholar]
- 28.Lee AW, Lin JC, Ng WT. Current management of nasopharyngeal cancer. Semin Radiat Oncol. 2012;22(3):233-244. [DOI] [PubMed] [Google Scholar]
- 29.Chen C, Fei Z, Pan J, Bai P, Chen L. Significance of primary tumor volume and T-stage on prognosis in nasopharyngeal carcinoma treated with intensity-modulated radiation therapy. Jpn J Clin Oncol. 2011;41:537-542. [DOI] [PubMed] [Google Scholar]
- 30.Chua DT, Sham JS, Choy DT. Prognostic impact of hemoglobin levels on treatment outcome in patients with nasopharyngealcarcinoma treated with sequential chemoradiotherapy or radiotherapy alone. Cancer. 2004;101(2):307-316. [DOI] [PubMed] [Google Scholar]
- 31.Gao J, Tao YL, Li G, Yi W, Xia YF. Involvement of difference in decrease of hemoglobin level in poor prognosis of Stage I and II nasopharyngeal carcinoma: implication in outcome of radiotherapy. Int J Radiat Oncol Biol Phys. 2012;82(4):1471-1478. [DOI] [PubMed] [Google Scholar]
- 32.Li YH, Hu CF, Shao Q, et al. Elevated expressions of survivin and VEGF protein are strong independent predictors of survival in advanced nasopharyngeal carcinoma. J Transl Med. 2008;6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chua DT, Nicholls JM, Sham JS, Au GK. Prognostic value of epidermal growth factor receptor expression in patients with advanced stage nasopharyngeal carcinoma treated with induction chemotherapy and radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59(1):11-20. [DOI] [PubMed] [Google Scholar]
- 34.Xu Y, Chen M, Guo Q, et al. Percutaneous endoscopic gastrostomy can improve survival outcomes in patients with N3 nasopharyngeal carcinoma undergoing concurrent chemoradiotherapy. Oral Oncol. 2021;121:105435. [DOI] [PubMed] [Google Scholar]
- 35.Meng L, Wei J, Ji R, et al. Effect of early nutrition intervention on advanced nasopharyngeal carcinoma patients receiving chemoradiotherapy. J Cancer. 2019;10(16):3650-3656. [DOI] [PMC free article] [PubMed] [Google Scholar]