Abstract
Erwinia chrysanthemi produces a battery of hydrolases and lyases which are very effective in the maceration of plant cell walls. Although two endoglucanases (CelZ and CelY; formerly EGZ and EGY) are produced, CelZ represents approximately 95% of the total carboxymethyl cellulase activity. In this study, we have examined the effectiveness of CelY and CelZ alone and of combinations of both enzymes using carboxymethyl cellulose (CMC) and amorphous cellulose (acid-swollen cellulose) as substrates. Synergy was observed with both substrates. Maximal synergy (1.8-fold) was observed for combinations containing primarily CelZ; the ratio of enzyme activities produced was similar to those produced by cultures of E. chrysanthemi. CelY and CelZ were quite different in substrate preference. CelY was unable to hydrolyze soluble cellooligosaccharides (cellotetraose and cellopentaose) but hydrolyzed CMC to fragments averaging 10.7 glucosyl units. In contrast, CelZ readily hydrolyzed cellotetraose, cellopentaose, and amorphous cellulose to produce cellobiose and cellotriose as dominant products. CelZ hydrolyzed CMC to fragments averaging 3.6 glucosyl units. In combination, CelZ and CelY hydrolyzed CMC to products averaging 2.3 glucosyl units. Synergy did not require the simultaneous presence of both enzymes. Enzymatic modification of the substrate by CelY increased the rate and extent of hydrolysis by CelZ. Full synergy was retained by the sequential hydrolysis of CMC, provided CelY was used as the first enzyme. A general mechanism is proposed to explain the synergy between these two enzymes based primarily on differences in substrate preference.
The hydrolysis of cellulose into soluble sugars by microbial systems offers the potential to provide a renewable feedstock for the production of fuels and chemicals (10, 17, 22). However, the crystalline structure and insoluble nature of cellulose represents a formidable challenge for enzymatic hydrolysis. Interactions between different cellulase enzymes and substrates are quite complex (2, 5, 20, 24, 36). The solubilization of crystalline cellulose by the fungus Trichoderma longibranchiatum has been extensively studied as a model primarily due to its commercial utility. Cellulases produced by T. longibranchiatum can be divided into three classes: endoglucanases (carboxymethyl cellulases [CMCases]), which hydrolyze amorphous regions of cellulose; exoglucanases (cellobiohydrolases), which progressively cleave cellobiose units from the ends of crystalline or amorphous cellulose; and β-glucosidases (cellobiases), which hydrolyze soluble cellooligosaccharides into glucose (5, 20). Multiple enzymes of each type are produced by T. longibranchiatum. Combinations of these fungal enzymes function in a synergistic fashion (23, 24, 32, 35, 36). Bacteria also produce multiple enzymes for cellulose hydrolysis (5, 21, 25). Synergy has been demonstrated for combinations of bacterial exoglucanases and endoglucanases (3, 12, 23, 28) and for combinations of bacterial endoglucanases and fungal exoglucanases (2, 18, 33). In nature, it is likely that enzymes from many different organisms function together during cellulose hydrolysis.
Our laboratory is developing recombinant strains of ethanologenic Escherichia coli and Klebsiella oxytoca that produce bacterial cellulases and reduce the amount of fungal cellulase required for biofuel production (17). In previous studies, we have expressed the celZ gene, which encodes the major endoglucanase (CelZ; formerly EGZ) from Erwinia chrysanthemi, at high levels in E. coli (39) and K. oxytoca (38). Expression and secretion were facilitated in both recombinant hosts by adding the out genes, which encode a type II protein transport system from E. chrysanthemi (14, 38, 39).
E. chrysanthemi produces a battery of hydrolase and lyase enzymes which are very effective in the maceration of plant tissues (9, 29, 31). This organism produces two different endoglucanases, CelY (formerly EGY) and CelZ (7, 8, 13). With carboxymethyl cellulose (CMC) as a substrate, 95% of the total endoglucanase activity was attributed to CelZ while only 5% of the activity was attributed to CelY. Although the latter percentage indicates a minor activity, the retention of both enzymes during evolution suggests that the combination of CelY and CelZ is beneficial for the efficient hydrolysis of cellulose. Genes encoding both activities have been previously cloned and sequenced (7, 13). Based on their deduced amino acid sequences, CelY and CelZ have been assigned to different families of glycohydrolases, family 8 and family 5 (25), respectively. In recombinant E. coli, the celY gene from E. chrysanthemi was poorly expressed due to promoter structure (13).
In this study, we have constructed plasmids that express higher levels of CelY in recombinant E. coli. Surprisingly, 90% of the CelY activity was secreted as an extracellular product by a native E. coli secretion system. Using recombinant CelY and CelZ, the combined actions of both enzymes were investigated using CMC and acid-swollen cellulose as substrates. Synergy was observed with both substrates.
MATERIALS AND METHODS
Bacteria, plasmids, and culture conditions.
Bacterial strains and plasmids used in this study are listed in Table 1. E. coli DH5α and TOPO10F′ were used as hosts for plasmid constructions. The celZ gene was previously cloned in our laboratory from E. chrysanthemi P86021 (4). The celY gene was cloned by Guiseppi et al. (13) from E. chrysanthemi 3937. The out genes were cloned by He et al. (14) from E. chrysanthemi EC16.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| Strains | ||
| Escherichia coli | ||
| DH5α | lacZΔM15 recA | Bethesda Research Laboratory |
| B | Prototrophic | ATCC 11303 |
| HB101 | recA lacY recA | ATCC 37159 |
| TOP10F′ | This strain expresses the lac repressor (lacIq gene) from an F episome | Invitrogen |
| Plasmids | ||
| pCR2.1-TOPO | TOPO TA cloning vector, Apr Kmr | Invitrogen |
| pRK2013 | Kmr mobilizing helper plasmid (mob+) | ATCCa |
| pCPP2006 | Spr, ca. 40-kbp plasmid carrying the complete out genes from E. chrysanthemi EC16 | 14 |
| pLOI1620 | Apr, celZ gene and its native promoter from E. chrysanthemi P86021 | 4 |
| pMH18 | Apr, celY gene and its native promoter from E. chrysanthemi 3937 | 13 |
| pLOI2311 | celY gene (without native promoter), cloned into pCR2.1-TOPO vector and oriented for expression from the lac promoter | This study |
ATCC, American Type Culture Collection.
E. coli cultures were grown at 37°C in Luria-Bertani (LB) broth (1) containing, per liter, 10 g of Difco (Detroit, Mich.) tryptone, 5 g of Difco yeast extract, and 5 g of sodium chloride or on solid LB medium containing agar (1.5%). Clones were screened for endoglucanase production using the Congo red method (34). Indicator plates were prepared by supplementing LB agar with low-viscosity CMC (0.3%). Ampicillin (50 μg/ml), kanamycin (50 μg/ml), and spectinomycin (100 μg/ml) were added as appropriate for selection.
Genetic methods.
Standard methods were used for plasmid construction and analyses (1). The coding region for celY was amplified by PCR using pMH18 as the template with the following primer pair: N terminus 5′CTGTTCCGTTACCAACAC3′ and C terminus 5′GTGAATGGGATCACGAGT3′. The E. chrysanthemi out genes (pCPP2006) were transferred by conjugation using pRK2013 for mobilization (39). DNA was sequenced by the dideoxy method using a LI-COR model 4000-L DNA sequencer and fluorescent primers.
Enzyme assay.
Endoglucanase activity was determined in vitro using CMC as a substrate. Appropriate dilutions of cell-free culture broth (extracellular activity) or broth containing cells that had been disrupted by ultrasound (total activity) were assayed at 35°C in 50 mM citrate buffer (pH 5.2) containing low-viscosity CMC (20 g per liter). Reactions were terminated by heating in a boiling water bath for 10 min. Reducing sugars were measured using 3,5-dinitrosalicylic acid reagent with glucose as a standard (34). Enzyme activity (CMCase) is expressed as micromoles of reducing sugar released per minute (in international units). Results are averages of two or more determinations.
Synergism.
Stationary-phase cultures of DH5α(pLOI1620 plus pCPP2006) and DH5α(pLOI2311) were sonicated and centrifuged as previously described (39) as a source of CelZ and CelY, respectively. These were diluted as necessary to provide equal CMCase activities. Mixtures of CelZ and CelY were tested for synergy at 35°C in 50 mM citrate buffer (pH 5.2) containing CMC (20 g per liter) or acid-swollen cellulose (20 g per liter). For tests with Avicel (20 g per liter), enzyme preparations were mixed without prior dilution. Hydrolyzed samples of acid-swollen cellulose and Avicel were centrifuged (10,000 × g, 5 min) to remove insoluble material prior to the determination of concentrations of reducing sugars.
The effects of sequential additions of CelZ and CelY were also investigated. Substrates were hydrolyzed with a single enzyme for 4 h and then inactivated by boiling for 20 min. After the hydrolysate cooled, the second enzyme was added and incubated for an additional 4 h. Control experiments were conducted with both enzymes together (4 h) and with each enzyme alone (4 h). Samples were analyzed for reducing sugar. In some cases, products were also analyzed by thin-layer chromatography.
The degree of synergism for enzyme mixtures was calculated as the observed activity divided by the sum of predicted contributions from CelY alone and CelZ alone (28).
Hydrolysis products from soluble cellooligosaccharides and cellulose.
Hydrolysis products from cellobiose, cellotriose, cellotetraose, cellopentaose, acid-swollen cellulose (34), and Avicel were analyzed by thin-layer chromatography. For tests with soluble cellooligosaccharides, 15 μl of a 1% substrate was mixed with 45 μl of crude enzyme (0.07 IU) and incubated at 35°C for 2 h, and the reaction was terminated by heating in a boiling water bath. Avicel (2%, 48-h incubation) and acid-swollen cellulose (2%, 6-h incubation) were digested with different concentrations of endoglucanse, namely, 8 IU of CMCase/ml and 0.8 IU of CMCase/ml, respectively. Again, reactions were terminated by heating in a boiling water bath.
Hydrolysis products were separated for approximately 4 h using Whatman 250-μm-thick Silica gel 150A plates with the solvent system described by Kim (19). By volume, this solvent contained 6 parts chloroform, 7 parts acetic acid, and 1 part water. Sugars were visualized by spraying with 6.5 mM N-(1-naphthyl)ethylenediamine dihydrochloride and heating at 100°C for approximately 10 min (6).
Materials and chemicals.
Tryptone and yeast extract were products of Difco. Antibiotics, low-viscosity CMC, cellobiose, cellotriose, and cellotetraose were obtained from the Sigma Chemical Co. (St. Louis, Mo.). Cellopentaose was obtained from V-Lab (Covington, La.). Avicel was purchased from Fluka Chemika (Buchs, Switzerland).
RESULTS
Production of CelY and CelZ by recombinant E. coli.
As reported previously (7, 13), low levels of CelY activity were produced by native E. chrysanthemi 3937 and by recombinant E. coli harboring plasmid pMH18. Poor expression from the high-copy-number plasmid in E. coli was attributed to promoter function and a putative requirement for a celY activator protein (13). A new clone was constructed to produce higher levels of CelY for our investigations of synergy. The CelY coding region (without promoter) was amplified by PCR and cloned behind the lac promoter in pCR2.1-TOPO. The resulting plasmid, pLOI2311, was strongly positive on CMCase indicator plates. Replacement of the native promoter with the lac promoter increased celY expression by approximately 10-fold, from 165 to 1,800 IU/liter (Table 2). Approximately 90% of CelY activity was found in the extracellular milieu. Expression of celZ was included for comparison (Table 2). High levels of CelZ were produced by E. coli harboring plasmid pLOI1620. Extracellular CelZ and total CelZ activities were further increased by addition of the E. chrysanthemi out genes (pCPP2006) as reported previously (39). Unlike CelZ activity, however, CelY activity was not affected by the presence of out genes. Maximal CelY and CelZ activities were obtained from 24-h cultures. The supernatants from disrupted cultures of DH5α containing pLOI2311 or pLOI1620 and pCPP2006 (out genes) were used as a source of CelY or CelZ, respectively, for further investigations.
TABLE 2.
Effects of E. chrysanthemi out genes on the expression and secretion of celY and celZ in E. coli DH5α
| Enzyme expressed | Promoter | Growth (h) | Without out genes
|
With out genes (+ pCCP2006)
|
||||
|---|---|---|---|---|---|---|---|---|
| Extracellular CMCasea (IU per liter) | Total CMCase (IU per liter) | Apparent secretion (%) | Extracellular CMCase (IU per liter) | Total CMCase (IU per liter) | Apparent secretion (%) | |||
| CelY | Native promoter (pMH18) | 24 | 136 | 165 | 82 | 136 | 180 | 76 |
| lac promoter (pLOI2311) | 8 | 208 | 266 | 78 | NDb | ND | ND | |
| 16 | 1,420 | 1,590 | 90 | ND | ND | ND | ||
| 24 | 1,650 | 1,800 | 90 | 1,360 | 1,510 | 90 | ||
| CelZ | Native plus lac promoter (pLOI1620) | 8 | 130 | 1,320 | 10 | 6,710 | 7,460 | 90 |
| 16 | 1,200 | 9,030 | 13 | 13,400 | 19,700 | 68 | ||
| 24 | 1,800 | 12,500 | 14 | 23,600 | 36,800 | 64 | ||
CMCase activity secreted or released in the culture supernatant.
ND, not determined.
Synergistic action of CelY and CelZ with CMC as a substrate.
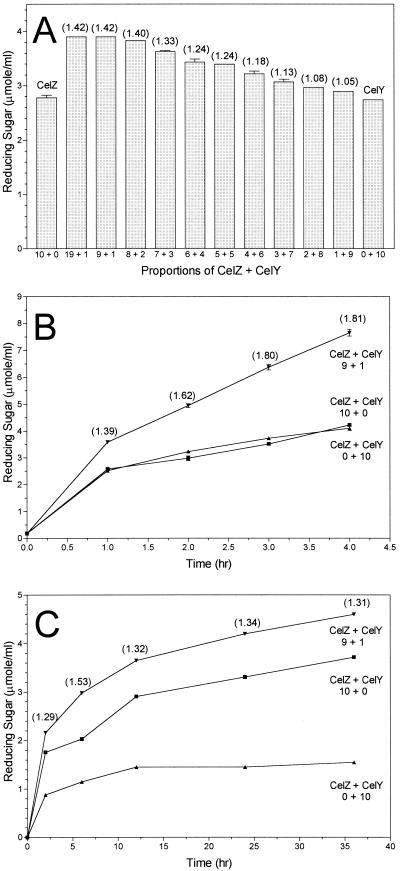
Initial experiments examining the combined actions of CelY and CelZ were conducted with CMC (20 g per liter) for a single incubation time (Fig. 1A). Disrupted cell preparations containing CelY and CelZ were each diluted to equal activities (CMCase) and combined in different proportions to maintain a constant sum of individual activities. CelY and CelZ were tested individually as controls. Activities of CelY and CelZ in all mixtures were significantly higher than that of either enzyme assayed alone, indicating a synergistic interaction. The synergistic effect increased with the proportion of CelZ. Maximal synergy (1.42) was observed with ratios of CelZ to CelY activities of 9 to 1 and 19 to 1.
FIG. 1.
Synergistic action of CelY and CelZ. Both enzymes were diluted to equal CMCase activities (1.5 IU/ml). Calculated synergies are shown in parentheses. (A) Effect of enzyme ratios on synergy. Different amounts of CelY and CelZ were combined to maintain a constant predicted activity (0.15 IU/ml) based on the contributions of individual enzymes. Assays were incubated with CMC for 1 h at 35°C, and reactions were terminated by boiling. Numbers on the x axes indicate the proportions of CelZ and CelY. Synergy is shown above each bar. (B) Hydrolysis of CMC by CelZ and CelY, alone and in combination (9 parts CelZ to 1 part CelY). All assay mixtures contained equal total activities (0.15 IU/ml) based on the sum of individual CelY and CelZ activities. Synergy is shown above each point for the combination of both enzymes. (C) Hydrolysis of acid-swollen cellulose by CelZ and CelY, alone and in combination. A 9-to-1 ratio of CelZ to CelY was used for the combined enzyme reaction. All assay mixtures contained 1.5 IU/ml based on the sum of individual CelY and CelZ activities. Synergy is shown above each point for the combination of both enzymes.
Further experiments examined the effect of incubation time using CMC as the substrate and an activity ratio of 9 to 1 for CelZ and CelY, respectively (Fig. 1B). CelZ and CelY alone were included as controls. The synergistic effect of combining CelZ and CelY was clearly evident as increases in the rate and extent of hydrolysis. Calculated synergy increased with incubation time. At the end of the incubation (4 h), the concentration of reducing sugars was 1.8-fold higher in the mixed enzyme preparation than that predicted by the arithmetic sum of individual CelZ and CelY activities.
Effect of substrate (CMC) concentration on synergy.
A previous study has determined that synergy in other systems is affected by substrate concentration (37). This was also true for synergy between CelZ and CelY (Table 3). Increasing the CMC concentration from 2.5 to 20 g per liter increased the observed synergy from 1.12 to 1.89. Based on the specific activities of CelZ and CelY and a maximal synergism of 1.89, the enzyme turnover rate for the combination was 8-fold that of purified CelY alone and 1.5-fold that of purified CelZ alone.
TABLE 3.
Effect of substrate concentration on synergy
| Amt of CMC substrate (g/liter) | Concn of reducing sugar released (μmol/ml)a
|
Avg synergyb ± SD | ||
|---|---|---|---|---|
| CelZ | CelY | CelZ + CelY | ||
| 20 | 3.98 ± 0.04 | 3.83 ± 0.04 | 7.51 ± 0.07 | 1.89 ± 0.02 |
| 10 | 4.53 ± 0.01 | 2.91 ± 0.07 | 5.38 ± 0.04 | 1.25 ± 0.01 |
| 5.0 | 2.87 ± 0.01 | 1.18 ± 0.04 | 2.92 ± 0.04 | 1.08 ± 0.02 |
| 2.5 | 1.42 ± 0.01 | 0.50 ± 0.04 | 1.49 ± 0.01 | 1.12 ± 0.01 |
Averages ± standard deviations. CelZ and CelY were diluted to equal CMCase activities. Reaction mixtures (0.15 IU/ml) with both CelZ and CelY contained 9 parts CelZ and 1 part CelY. As controls, CelZ (0.15 IU/ml) and CelY (0.15 IU/ml) were each tested individually.
Synergy was calculated as the observed activity divided by the sum of predicted contributions from CelY alone (10%) plus CelZ alone (90%).
CelY was more sensitive to substrate concentration than CelZ. Increasing the CMC concentration resulted in an eightfold increase in reducing sugar products with CelY but only a threefold increase with CelZ. Based on a double reciprocal plot of the data in Table 3, apparent Km values of 104, 12, and 38 g per liter were estimated for CelY, CelZ, and the combination of both enzymes (9 parts CelZ plus 1 part CelY), respectively. The higher apparent Km for CelY is consistent with a requirement for longer substrate molecules.
The extent of CMC hydrolysis was also examined by determining the approximate sizes of hydrolysis products. CMC (1.25 g per liter) was incubated (4 h, 0.75 IU of CMCase/ml) with CelY, CelZ, and a combination of both enzymes (9 parts CelZ plus 1 part CelY). Chain length was estimated based on results of the reducing sugar assay before (250 glucosyl units) and after incubation. The average chain length was substantially reduced by all three enzyme preparations. CelZ was more effective in reducing chain length than CelY, with CelZ reducing chain length to 3.6 glucosyl residues, versus 10.7 with CelY. The combination of both enzymes resulted in a synergistic action. Simultaneous hydrolysis with both enzymes reduced the average size of the hydrolysis products to 2.3 glucosyl residues, which is 36% smaller than with CelZ alone and 79% smaller than with CelY alone. These results confirm that CelZ readily hydrolyzes both large CMC polymers and smaller saccharides. The action of CelY appears more limited in that it hydrolyzes primarily large polymers with greater than 10 glucosyl units.
Sequential and simultaneous hydrolysis of CMC with CelZ and CelY.
The mechanism of synergistic action between CelZ and CelY was further investigated by comparing the effects of sequential hydrolysis with individual endoglucanases to those of simultaneous hydrolysis by a mixture of both enzymes (Table 4). Again, synergy was observed for the simultaneous actions of both enzymes. No synergy was observed for the sequential hydrolysis of CMC when CelZ was used as the first enzyme and CelY was used as the second enzyme (after heat inactivation of CelZ). In contrast, full synergy was retained when CMC was first treated with CelY and then with CelZ (after heat inactivation of CelY). These results indicate that synergy can be achieved by the independent activities of CelY and CelZ. Enzymatic modification of the substrate by CelY increased the rate and extent of subsequent hydrolysis by CelZ. These results provide further evidence that CelY and CelZ function quite differently. CelY appears primarily to reduce the chain lengths of large polymers, while CelZ appears to act more randomly, hydrolyzing both large and small substrate molecules.
TABLE 4.
Sequential and simultaneous hydrolysis of CMC by CelZ and CelY
| Enzyme (relative proportion)a | Measured reducing sugar released (μmol/ml)b | Predicted activity from the arithmetic sum of CelY and CelZ (μmol/ml)c | Synergybd |
|---|---|---|---|
| CelZ (10 parts) + CelY (0 parts) | 4.65 ± 0.08 | 4.65 | 1.00 ± 0.02 |
| CelZ (0 parts) + CelY (10 parts) | 4.14 ± 0.04 | 4.14 | 1.00 ± 0.01 |
| CelZ (9 parts) + CelY (1 part) (simultaneously) | 8.28 ± 0.08 | 4.60 | 1.80 ± 0.02 |
| CelZ (9 parts) and then CelY (1 part) (sequentially) | 4.86 ± 0.23 | 4.60 | 1.06 ± 0.05 |
| CelY (1 part) and then CelZ (9 parts) (sequentially) | 8.75 ± 0.14 | 4.60 | 1.90 ± 0.03 |
CelZ and CelY were diluted to equal CMCase activities. Both simultaneous and sequential hydrolysis reactions (0.15 IU/ml) were investigated using 9 parts CelZ and 1 part CelY. In the sequential hydrolysis experiments, the first enzyme was incubated with the substrate for 4 h and inactivated by boiling for 20 min. After cooling, the second enzyme was added and incubated for an additional 4 h. All reactions were terminated by boiling.
Averages ± standard deviations (three experiments).
Calculated sum of individual CelY and CelZ activities.
Synergy was calculated as the observed activity divided by the sum of predicted contributions from CelY alone (10%) plus CelZ alone (90%).
Synergistic action on acid-swollen and crystalline cellulose.
Potential synergy was investigated using acid-swollen cellulose as the substrate and a 9 to 1 ratio of CelZ to CelY based on CMCase activities (Fig. 1C). Since the activities of CelZ and CelY with acid-swollen cellulose are lower than those with CMC (7), enzyme loading (1.5 IU) and incubation times were increased. When assayed individually with acid-swollen cellulose, CelY was approximately one-third as active as CelZ. However, the combination of these two enzymes was significantly more active than was predicted by the arithmetic sum of individual activities at all time points. The degree of synergy was essentially constant (1.36 ± 0.17) during the 36-h period of incubation.
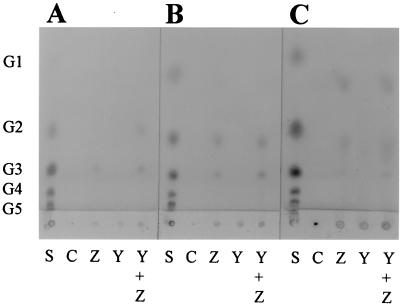
The hydrolysis products from acid-swollen cellulose (6 h) were analyzed by thin-layer chromatography (Fig. 2A and B). No soluble saccharides were observed after incubation with CelY alone. Cellobiose and cellotriose were the primary products from hydrolysis with CelZ alone and a combination of CelY and CelZ. With the combination of both enzymes, higher product levels were evident as darker and larger spots, confirming a synergistic action.
FIG. 2.
Thin-layer chromatography analysis of the hydrolysis products from acid-swollen cellulose and Avicel. Abbreviations for y axis: G1, glucose; G2, cellobiose; G3, cellotriose; G4, cellotetraose; and G5, cellopentaose. Lanes: S, mixed-cellooligosaccharide standard; C, control lacking enzyme; Z, CelZ; Y, CelY; and Y + Z, CelY plus CelZ. (A) Acid-swollen cellulose (6-h incubation, 1-μl loading); (B) acid-swollen cellulose (6-h incubation, 2-μl loading); (C) Avicel (48-h incubation, 10-μl loading).
The synergistic action of CelZ and CelY was also investigated with Avicel (Fig. 2C), a highly crystalline cellulose. Small amounts of cellobiose and cellotriose were observed as hydrolysis products with CelZ alone and with the mixture of CelY and CelZ. Due to low activity with Avicel, large loadings (10 μl) were required on thin-layer plates to visualize products. Note that this additional salt increased the relative migrations of oligosaccharide products in comparison to those of the standards. No cellooligosaccharide spots were observed with CelY alone. Again, synergism was evident with the combination of CelY and CelZ. We observed larger and more intense spots corresponding to cellobiose, cellotriose, and cellotetraose with the enzymes combined than with CelZ alone. The low activity with Avicel as a substrate and the relatively low levels of products are consistent with the hydrolysis of the amorphous rather than the crystalline regions of Avicel. These results indicate that the synergistic action of CelZ and CelY is not limited to a model substrate such as CMC. Synergistic hydrolysis was also observed for acid-swollen cellulose and the amorphous regions of Avicel.
Hydrolysis of cellooligosaccharides.
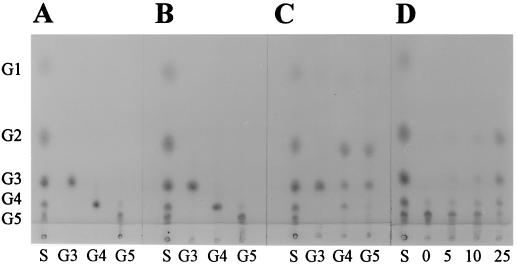
The substrate specificities of CelZ and CelY were further investigated using soluble cellooligosaccharides (cellobiose, cellotriose, cellotetraose, and cellopentaose). Hydrolysis products were analyzed by thin-layer chromatography (Fig. 3). Cellobiose was not hydrolyzed by CelY, CelZ, or a combination of both enzymes (data not shown). None of the cellooligosaccharides was hydrolyzed by CelY alone (Fig. 3B). In contrast, CelZ hydrolyzed cellotetraose and cellopentaose but not cellotriose (Fig. 3C). CelZ hydrolysis products from cellotetraose were primarily cellobiose, with lesser amounts of cellotriose and glucose. With cellopentaose as the substrate, CelZ produced approximately equal amounts of cellobiose and cellotriose, indicating a preferential attack on the second or third glycosidic bond. This conclusion was further confirmed by examining samples at various times during the incubation of cellopentaose with CelZ (Fig. 3D). Cellobiose and cellotriose progressively accumulated during incubation, with a corresponding reduction in cellopentaose. Thus, unlike with CelY, which requires large substrates, CelZ hydrolyzes soluble cellooligosaccharides containing 4 or more glucosyl units.
FIG. 3.
Hydrolysis of cellooligosaccharides by CelZ and CelY. Each test contained approximately 0.07 IU of CMCase per ml (2-h incubation, 35°C). Abbreviations: S, mixed-cellooligosaccharide standard; G1, glucose; G2, cellobiose; G3, cellotriose; G4, cellotetraose; and G5, cellopentaose. (A) Before hydrolysis; (B) after incubation with CelY; (C) after incubation with CelZ; (D) CelZ hydrolysis of cellopentaose after different periods of incubation (0, 5, 10, and 25 min).
DISCUSSION
E. chrysanthemi CelZ and CelY are typical endoglucanases in that both have high activities with CMC as a substrate and little activity with crystalline cellulose (7). However, the structures of these enzymes are quite different, with minimal sequence identity (13). Each has been assigned to a different glycohydrolase family (25), and only CelZ contains a cellulose-binding domain (25). In E. chrysanthemi, 95% of the total endoglucanase activity (CMCase) is attributed to CelZ and 5% is attributed to CelY (7, 13). To be effective during the maceration of plant cell walls, these enzymes must be secreted into the extracellular milieu. CelZ is secreted using a type II secretion system which requires the sec and out genes (14, 39). In recombinant E. coli containing the out genes, approximately half of the total CelZ activity was recovered in the culture supernatant. In contrast, 90% of CelY was secreted as an extracellular product in recombinant E. coli and this secretion was not affected by the presence of out genes. This gene also contains an N-terminal leader sequence (13) and is presumed to utilize a type IV secretion system (16), similar to that proposed for the CelL endoglucanase in Pseudomonas solanacearum (15). The use of two different routes for the extracellular secretion of E. chrysanthemi endoglucanases may facilitate higher levels of endoglucanase production.
CelZ and CelY act synergistically during the hydrolysis of amorphous cellulose and CMC. This result was unanticipated, since a prior study with these enzymes failed to observe synergy (7). Since no quantitative results or details were provided, this discrepancy is attributed to differences in methodology. In our experiments, the extent of synergy was dependent upon the ratio of the two enzymes, substrate concentration, and the period of incubation. Maximal synergy was observed for enzyme mixtures containing 90 to 95% CelZ (CMCase activity basis). Based on the specific activities for purified CelZ (200 μmol/mg of protein; molecular weight, 45,000) and CelY (33 μmol/mg of protein; molecular weight, 35,000) with CMC as a substrate (7), the 9:1 and 19:1 mixtures of CelZ to CelY correspond to molar enzyme ratios of 1.2 to 1 and 2.4 to 1, respectively.
Synergy has been extensively documented for many combinations of endoglucanase with exoglucanase (24, 35, 36) and for combinations of exoglucanases (3, 12, 23, 28). However, synergy between two endoglucanases is unusual. Two previous reports of increased activity with mixtures of endoglucanases have been attributed to possible contamination with an exoglucanase (20, 27). Contamination was very unlikely in our study due to the use of recombinant enzyme preparations. A third recent report has demonstrated synergy between endoglucanases from two different species of Gloeophyllum, G. trabeum and G. sepiarium (23). With softwood-dissolving pulp as a substrate, the combined activities of both enzymes was 108% of that predicted by the sum of individual activities.
The synergistic action of E. chrysanthemi CelY and CelZ was much more pronounced than that observed with the Gloeophyllum endoglucanases (23). In both cases, synergy appears to result from differences in substrate preferences and modes of action. CelY failed to hydrolyze soluble cellooligosaccharides and produced no soluble products during the hydrolysis of amorphous cellulose. With this enzyme, hydrolysis products from CMC averaged 10 glucosyl units. CelY exhibited a ninefold-higher apparent Km for CMC than CelZ. In contrast, CelZ hydrolyzed both long-chain substrates and soluble cellooligosaccharides (4 or more glucosyl residues). Cellobiose and cellotriose were produced as primary products from the hydrolysis of amorphous cellulose by CelZ.
Equal levels of synergy were observed when both enzymes were present simultaneously and after the sequential addition of CelY and CelZ (after heat inactivation of CelY). No synergy was observed when CelZ was used as the first enzyme. Thus, only CelY can independently modify the substrate to increase digestibility. This action by CelY is consistent with a preference for long substrate molecules in converting CMC or amorphous cellulose into a modified substrate containing fragments of intermediate lengths rather than a random assortment of sizes. The smaller products from CMC are soluble and are observed as an increase in the concentration of reducing sugar. The low apparent activity of CelY with acid-swollen cellulose is consistent with the removal of longer products (6 or more glucosyl units) as insoluble material during centrifugation. The increase in the concentration of soluble reducing sugar observed as synergy with amorphous cellulose is proposed to result from an increase in soluble products from the CelZ-mediated hydrolysis of intermediate-length cellooligosaccharides (CelY products), which produces diffusable substrates that are further hydrolyzed by CelZ. Analogous activities also increase the efficiency of CMC depolymerization. The enhanced activity of CelZ on CelY-modified substrates is thus proposed to result from increases in the rate of production and effective concentration of small, rapidly diffusing substrate molecules.
Both CelY and CelZ have been retained during the evolution of E. chrysanthemi and are presumed to have unique features which contribute to the success of this organism among the biota. Our results establish that these two enzymes have different but complementary requirements for substrate length which result in synergistic hydrolysis of acid-swollen cellulose (amorphous) and CMC. Maximal synergy was observed with enzyme mixtures containing small amounts of CelY activity relative to that of CelZ (1 to 19, similar to the ratio produced by E. chrysanthemi in nature [7, 13]). The synergistic action of CelY and CelZ resulting from complementary differences in substrate preference may have provided an important evolutionary advantage for the retention of both endoglucanase enzymes. Previous investigators (26, 30) have proposed analogous differences in substrate preferences as a rationale for the retention of multiple pectate lyase (pel) genes by E. chrysanthemi.
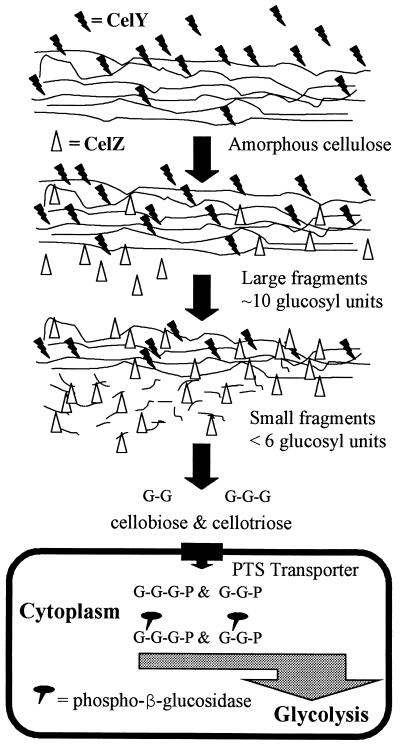
Figure 4 shows a cartoon model for the digestion of amorphous cellulose by E. chrysanthemi. This organism appears to use a combination of three glucosidase enzymes (CelY, CelZ, and phospho-β-glucosidase). Nicks are inserted into amorphous cellulose at relatively long intervals by CelY to reduce the average chain length and thus minimize the number of CelZ-catalyzed events required to create soluble fragments of 2 to 6 glucosyl units. Resulting soluble fragments are further hydrolyzed by CelZ to dimers and trimers. Dimers (and presumably trimers also) are then transported into the cell by the phosphoenolpyruvate-dependent phosphotransferase system for cellobiose (11) and hydrolyzed by the cytoplasmic phospho-β-glucosidase. The resulting products, glucose and glucose-6-phosphate, enter glycolysis for further metabolism. Compared to the number of organisms able to use glucose, relatively few organisms are capable of cellobiose uptake and direct intracellular metabolism. By avoiding complete extracellular hydrolysis to glucose through the actions of CelY, CelZ, and an active transport system for cellobiose and cellotriose, E. chrysanthemi has evolved to minimize the availability of cellulose-derived products to competing organisms in the environment.
FIG. 4.
Model illustrating the utilization of amorphous cellulose by E. chrysanthemi. Three glucosidases are used for the catabolism of amorphous cellulose. Two of these, CelY and CelZ, are extracellular endoglucanases which function together in a synergistic fashion. CelY requires large substrate molecules and hydrolyzes these into shorter, insoluble fragments. CelY does not hydrolyze soluble cellooligosaccharides (2 to 5 glucosyl residues). CelZ readily hydrolyzes soluble cellooligosaccharides (cellopentaose and cellotetraose) and amorphous fragments of intermediate lengths to produce cellobiose and cellotriose. Cellobiose (G-G) and cellotriose (G-G-G) are phosphorylated (P) during cellular uptake by a phosphoenolpyruvate-dependent phosphotransferase system (PTS). Hydrolysis is completed intracellularly by a third enzyme, phospho-β-glucosidase. Resulting monomeric products (glucose and glucose-6-phosphate) are metabolized by glycolysis.
ACKNOWLEDGMENTS
We thank F. Barras for sharing plasmid pMH18, which contains the celY gene from E. chrysanthemi 3937, and A. Collmer for sharing plasmid pCPP2006, which contains the out genes from E. chrysanthemi EC16.
This research was supported in part by grants from the U.S. Department of Agriculture, National Research Initiative (98-35504-6177); the U.S. Department of Energy, Office of Basic Energy Science (FG02-96ER20222); and the Florida Agricultural Experiment Station, University of Florida.
Footnotes
Florida Agricultural Experiment Journal Series no. R-07249.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Deidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1987. [Google Scholar]
- 2.Baker J O, Adney W S, Thomas S R, Nieves R A, Chou Y C, Vinzant T B, Tucker M P, Laymon R A, Himmel M E. Synergism between purified bacterial and fungal cellulases. In: Saddler J N, Himmel M E, editors. Enzymatic degradation of insoluble carbohydrates. American Chemical Society Symposium Series 618. Washington, D.C.: American Chemical Society; 1995. pp. 113–141. [Google Scholar]
- 3.Barr B K, Hsieh Y L, Ganem B, Wilson D B. Identification of two functionally different classes of exocellulases. Biochemistry. 1996;35:586–592. doi: 10.1021/bi9520388. [DOI] [PubMed] [Google Scholar]
- 4.Beall D S, Ingram L O. Genetic engineering of soft-rot bacteria for ethanol production from lignocellullose. J Ind Microbiol. 1993;11:151–155. [Google Scholar]
- 5.Beguin P, Aubert J P. The biological degradation of cellulose. FEMS Microbiol Rev. 1994;13:25–58. doi: 10.1111/j.1574-6976.1994.tb00033.x. [DOI] [PubMed] [Google Scholar]
- 6.Bounias M. N-(1-naphthyl)ethylenediamine dihydrochloride as a new reagent for nanomole quantification of sugars on thin-layer plates by a mathematical calibration process. Anal Biochem. 1980;106:291–295. doi: 10.1016/0003-2697(80)90523-0. [DOI] [PubMed] [Google Scholar]
- 7.Boyer M H, Cami B, Chambost J P, Magnan M, Cattaneo J. Characterization of a new endoglucanase from Erwinia chrysanthemi. Eur J Biochem. 1987;162:311–316. doi: 10.1111/j.1432-1033.1987.tb10602.x. [DOI] [PubMed] [Google Scholar]
- 8.Boyer M H, Chambost J P, Magnan M, Cattaneo J. Carboxymethyl-cellulase from Erwinia chrysanthemi. II. purification and partial characterization of an endo-β-1,4-glucanase. J Biotechnol. 1984;1:241–252. [Google Scholar]
- 9.Collmer A, Keen N T. The role of pectic enzymes in plant pathogenesis. Annu Rev Phytopathol. 1996;24:383–409. [Google Scholar]
- 10.Dale B E. Biobased industrial products: bioprocess engineering when cost really counts. Biotechnol Prog. 1999;15:775–776. doi: 10.1021/bp990286f. [DOI] [PubMed] [Google Scholar]
- 11.El Hassouni M, Henrissat B, Chippaux M, Barras F. Nucleotide sequences of the arb genes, which control β-glucoside utilization in Erwinia chrysanthemi: comparison with the Escherichia coli bgl operon and evidence for a new β-glycohydrolase family including enzymes from eubacteria, archeabacteria, and humans. J Bacteriol. 1992;174:765–777. doi: 10.1128/jb.174.3.765-777.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilkes N R, Kwan E, Kilburn D G, Miller R C, Warren R A J. Attack of carboxymethylcellulose at opposite ends by two cellobiohydrolases from Cellulomonas fimi. J Biotechnol. 1997;57:83–90. [Google Scholar]
- 13.Guiseppi A, Aymeric J L, Cami B, Barras F, Creuzet N. Sequence analysis of the cellulase-encoding celY gene of Erwinia chrysanthemi: a possible case of interspecies gene transfer. Gene. 1991;106:109–114. doi: 10.1016/0378-1119(91)90573-t. [DOI] [PubMed] [Google Scholar]
- 14.He S Y, Lindeberg M, Chatterjee A K, Collmer A. Cloned Erwinia chrysanthemi out genes enable Escherichia coli to selectively secrete a diverse family of heterologous proteins to its milieu. Proc Natl Acad Sci USA. 1991;88:1079–1083. doi: 10.1073/pnas.88.3.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang J Z, Schell M A. Role of the two-component leader sequence and mature amino acid sequences in extracellular export of endoglucanase EGL from Pseudomonas solanacearum. J Bacteriol. 1992;174:1314–1323. doi: 10.1128/jb.174.4.1314-1323.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hueck C J. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol Mol Biol Rev. 1998;62:379–433. doi: 10.1128/mmbr.62.2.379-433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ingram L O, Aldrich H C, Borges A C C, Causey T B, Martinez A, Morales F, Saleh A Z, Underwood S A, Yomano L P, York S W, Zaldivar J, Zhou S. Enteric bacterial catalysts for fuel ethanol production. Biotechnol Prog. 1999;15:855–866. doi: 10.1021/bp9901062. [DOI] [PubMed] [Google Scholar]
- 18.Irwin D C, Spezio M, Walker L P, Wilson D B. Activity studies of eight purified cellulases: specificity, synergism, and binding domain effects. Biotechnol Bioeng. 1993;42:1002–1013. doi: 10.1002/bit.260420811. [DOI] [PubMed] [Google Scholar]
- 19.Kim C H. Characterization and substrate specificity of an endo-β-1,4-d-glucanase I (Avicelase I) from an extracellular multienzyme complex of Bacillus circulans. Appl Environ Microbiol. 1995;61:959–965. doi: 10.1128/aem.61.3.959-965.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klyosov A A. Trends in biochemistry and enzymology of cellulose degradation. Biochemistry. 1990;27:10477–10585. doi: 10.1021/bi00499a001. [DOI] [PubMed] [Google Scholar]
- 21.Linden J C, Shiang M. Bacterial cellulases: regulation of synthesis. In: Leatbam G F, Himmel M E, editors. Enzymes in biomass conversion. American Chemical Society Symposium Series 460. Washington, D.C.: American Chemical Society; 1991. pp. 331–349. [Google Scholar]
- 22.Lynd L E, Wyman C E, Gerngross T U. Biocommodity engineering. Biotechnol Prog. 1999;15:777–793. doi: 10.1021/bp990109e. [DOI] [PubMed] [Google Scholar]
- 23.Mansfield S D, Saddler J N, Gubitz G M. Characterization of endoglucanases from the brown rot fungi Gloeophyllum sepiarium and Gloeophyllum trabeum. Enzyme Microb Technol. 1998;23:133–140. [Google Scholar]
- 24.Nidetzky B, Steiner W, Claeyssens M. Synergistic interaction of cellulases from Trichoderma reesei during cellulose degradation. In: Saddler J N, Himmel M E, editors. Enzymatic degradation of insoluble carbohydrates. American Chemical Society Symposium Series 618. Washington, D.C.: American Chemical Society; 1995. pp. 90–112. [Google Scholar]
- 25.Ohmiya K, Sakka K, Karita S, Kimura T. Structure of cellulases and their applications. Biotechnol Genet Eng Rev. 1997;14:365–414. doi: 10.1080/02648725.1997.10647949. [DOI] [PubMed] [Google Scholar]
- 26.Preston J F, Rice J D, Ingram L O, Keen N T. Differential depolymerization mechanisms of pectate lyases secreted by Erwinia chrysanthemi EC16. J Bacteriol. 1992;174:2039–2042. doi: 10.1128/jb.174.6.2039-2042.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rao M, Deshpande V, Mishrat C. Purification, characterization, and synergistic action of endoglucanases from Fusarium lini. Biotechnol Bioeng. 1986;28:1100–1105. doi: 10.1002/bit.260280722. [DOI] [PubMed] [Google Scholar]
- 28.Riedel K, Ritter J, Bronnenmeier K. Synergistic interaction of the Clostridium stercorarium cellulases Avicelase I (CelZ) and Avicelase II (CelY) in the degradation of microcrystalline cellulose. FEMS Microbiol Lett. 1997;147:239–243. [Google Scholar]
- 29.Robert-Baudouy T. Molecular biology of Erwinia: from soft-rot to antileukaemics. Trends Biotechnol. 1991;9:325–329. [Google Scholar]
- 30.Roy C, Kester H, Visser J, Schevckik V, Hugouvieux-Cotte-Pattat N, Robert-Baudouy J, Benen J. Modes of action of five different endopectate lyases from Erwinia chrysanthemi 3937. J Bacteriol. 1999;181:3705–3709. doi: 10.1128/jb.181.12.3705-3709.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Starr M P, Chatterjee A K. The genus Erwinia: enterobacteria pathogenic to plants and animals. Annu Rev Microbiol. 1972;26:389–426. doi: 10.1146/annurev.mi.26.100172.002133. [DOI] [PubMed] [Google Scholar]
- 32.Tomme P, Heriban V, Claeyssens M. Adsorption of two cellobiohydrolases from Trichoderma reesei to avicel: evidence for “exo-exo” synergism and possible “loose complex” formation. Biotechnol Lett. 1990;12:525–530. [Google Scholar]
- 33.Tomme P, Warren R A J, Gilkes N R. Cellulose hydrolysis by bacteria and fungi. Adv Microbiol Physiol. 1995;37:1–81. doi: 10.1016/s0065-2911(08)60143-5. [DOI] [PubMed] [Google Scholar]
- 34.Wood T M, Bhat K M. Methods for measuring cellulase activities. Methods Enzymol. 1988;160:87–112. [Google Scholar]
- 35.Wood T M, McCrae S I, Bhat K M. The mechanism of fungal cellulase action: synergism between enzyme components of Penicillium pinophilum cellulase in solubilizing hydrogen bond-ordered cellulose. Biochem J. 1989;260:37–43. doi: 10.1042/bj2600037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woodward J. Synergism in cellulase systems. Bioresour Technol. 1991;36:67–75. [Google Scholar]
- 37.Woodward J, Hayes M K, Lee N E. Hydrolysis of cellulose by saturating and non-saturating concentrations of cellulose: implications for synergism. Biotechnology. 1988;6:301–304. [Google Scholar]
- 38.Zhou S, Ingram L O. Engineering endoglucanase-secreting strains of ethanologenic Klebsiella oxytoca P2. J Ind Microbiol Biotechnol. 1999;22:600–607. doi: 10.1038/sj.jim.2900666. [DOI] [PubMed] [Google Scholar]
- 39.Zhou S, Yomano L P, Saleh A Z, Davis F C, Aldrich H C, Ingram L O. Enhancement of expression and apparent secretion of Erwinia chrysanthemi endoglucanase (encoded by celZ) in Escherichia coli B. Appl Environ Microbiol. 1999;65:2439–2445. doi: 10.1128/aem.65.6.2439-2445.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]