Abstract
Diabetic nephropathy (DN) is one of the main causes of chronic renal failure, which is also the final cause of mortality in ~30% of diabetic patients. 1, 2, 3, 4, 6-penta-O-galloyl-β-D-glucose (PGG) from Galla rhois has anti-inflammation, anti-oxidation and angiogenesis effects. The present study aimed to explore the protective effects on diabetic nephropathy rats by alleviating inflammation and oxidative stress and the underlying mechanism. High-fat diet/STZ induced rats and high glucose (HG) induced podocytes (MPC5) were used to simulate the DN in vivo and in vitro. The blood glucose level was measured using a blood glucose meter and renal function was determined by an automatic biochemical analyzer. The pathological changes and renal fibrosis were observed through hematoxylin and eosin, periodic acid-Schiff and Masson staining. The expression of nephrin in tissues, fibrosis-related proteins in tissues, MAPK/NF-κB and ERK/nuclear factor erythroid-derived 2-related factor 2 (Nrf2)/hemeoxygenase-1 (HO-1) signaling pathway related proteins in tissues and apoptosis related proteins in tissues and podocytes was detected by western blotting. The inflammatory response and oxidative stress in tissues and podocytes were determined by respective commercial kits and apoptosis in tissues and podocytes was detected by TUNEL assay. The viability of podocytes treated with PGG with or without HG was analyzed by CCK-8 assay. As a result, the blood glucose level, urinary albumin/creatinine ratio, blood urea nitrogen and serum creatinine in blood were all increased and nephrin expression was decreased. The pathological changes and renal fibrosis were aggravated and the inflammation, oxidative stress and apoptosis in renal tissues were enhanced. The above effects were reversed by PGG treatment dose-dependently. MAPK/NF-κB and ERK/Nrf2/HO-1 signaling pathways were activated in DN rats and were suppressed by PGG treatment. The reduced viability and increased apoptosis, inflammation and oxidative stress in MPC5 cells were shown in HG induction, which was reversed by PGG treatment. However, P79350 (p38 agonist) and LM22B-10 (ERK1/2 agonist) weakened the effect of PGG. In conclusion, PGG protects against DN kidney injury by alleviating inflammation and oxidative stress by suppressing the MAPK/NF-κB and ERK/Nrf2/HO-1 signaling pathways.
Keywords: 1,2,3,4,6-penta-O-galloyl-β-D-glucose; inflammation; oxidative stress; diabetic nephropathy
Introduction
Diabetes mellitus (DM) is a general term for a series of abnormal metabolic disorders mainly manifested by chronic hyperglycemia, which is usually caused by insulin secretion imbalance or insulin function imbalance or both (1). DM can lead to a variety of complications with macrovascular and microvascular as the core pathological features (2). Diabetic nephropathy (DN) is the most common chronic diabetic microvascular complication (3). The standard of living of the Chinese is increasing as is the incidence of DN (4-6). Since 2011, DN has become the main cause of hospitalization and development of end-stage renal disease in kidney patients in China (7). DN has a long course of disease, high disability mortality and poor prognosis, which brings dual effects of quality of life and economic burden to patients (8,9).
The course of inflammatory response serves an important role in the occurrence and development of DN (10). According to relevant studies, inflammation and DN complement each other and the occurrence and development of DN is mainly mediated by the process of inflammatory response, with a very close relationship between the two (11-13). A previous study has shown that oxidative stress and inflammation are involved in the occurrence and development of DN and oxidative stress and inflammation are important and even central links in the pathogenesis of DN (14).
Galla rhois is a traditional herbal medicine that has been reported to alleviate inflammation in acute kidney injury (15). 1,2,3,4,6-penta-O-galloyl-β-D-glucose (PGG), a polyphenolic substance, is the main component of Galla rhois and an effective anti-inflammatory drug (15). PGG inhibits MCP-5 and MMP-9 progenitor cells to exert anti-inflammatory effects on activated microglias to delay the progression of Alzheimer's disease (AD) (16) and PGG inhibits inflammation by inhibiting MyD88/NF-κB and MyD88/MAPK signaling pathways in colitis (17). In addition, a previous study has reported that after 21 days of PGG treatment, the biochemical changes induced by high fat diet (HFD) are alleviated, the oral glucose tolerance is improved and the HFD-induced diabetes is improved in C57BL/6 mice (18), PGG can improve renal tubule injury in ischemia-reperfusion injury-induced acute kidney injury rats, including renal tubule dysfunction and microvascular inflammation (15) and PGG reduces renal crystallization and oxidative stress in a hyperoxaluric rat model (19). However, the therapeutic effects of PGG in subjects with DN have not been reported.
DN inflammatory damage includes high expression of inflammatory factors and activation of inflammatory signaling pathways. Among them, p38MAPK inflammatory signaling pathway serves an important role in renal tissue lesions induced by inflammation (20). The activation of the NF-κB inflammatory pathway can increase the expression of various pro-inflammatory factors and mediate inflammatory activities and also cause the activation of TGF-β/Smad pathway to promote renal fibrosis in DN (21). MAPK/NF-κB signaling pathway is suppressed in the treatment of hirudin (22) and celastrol (23) for DN rats. Studies have indicated that PGG inhibits LPS/IFNγ-activated BV-2 microglia proinflammatory response (24) and PGG exhibits skin-protective activity in skin damage induced by UVB radiation (25) all through the MAPK/NF-κB signaling pathway.
Nuclear factor erythroid-derived 2-related factor 2 (Nrf2) is a key factor in endogenous antioxidant mechanism, which can further induce the expression of downstream signal molecules such as hemeoxygenase-1 (HO-1) by activating Nrf2 expression, thereby serving an antioxidant stress role (26). Phosphorylation of ERK can promote the activation of Nrf2 in cells, which can dissociate Nrf2 and cytoskeleton related proteins into human nucleus and initiate the expression of downstream target gene HO-1 of Nrf2(27). The ERK/Nrf2/HO-1 pathway is involved in the protection of phosphocreatine and curcumin for diabetes-induced kidney injury and improve inflammatory response and oxidative stress (28,29). PGG has been reported to protect PC12 cells from MPP(+)-mediated cell death (30) and exert its hepatoprotective effects (31) by stimulating Nrf2 nuclear translocation and upregulating HO-1 expression.
The present study aimed to examine the potential role of PGG as a molecule protecting diabetes-kidney injury in diabetic nephropathy rats and MPC5 cells induced by high glucose (HG) through the MAPK/NF-κB and ERK/Nrf2/HO-1 signaling pathways.
Materials and methods
Compounds
PGG (>98% purity) was purchased from Medchem Express. PGG was dissolved in dimethyl sulfoxide (DMSO) and diluted in culture medium to a final concentration ≤0.1% (v/v) to avoid toxicity. Based on moisture, total ash and gallic acid content, high-quality dry Galla rhois powder was purchased from Sichuan market.
The Galla rhois powder (20 g) was weighed and placed in a triangular flask of 500 and 20 ml of 60% ethanol solution was added. After soaking for 24 h, the mixture was extracted by ethanol reflux method for twice consecutively, each time for 1.5 h. The filtrate was combined after vacuum extraction and then enriched with reduced pressure. The obtained extract was freeze-dried at -50˚C and the dried powder was dissolved in DMSO solution to prepare 100 mg/ml Galla rhois extract (GRE) for later use.
Liquid chromatography-mass spectrometry (LC-MS) detection of Galla rhois
Sample (200 mg) was transferred to a 2-ml EP tube. Subsequently, 0.6 ml 2-chlorophenylalanine in methanol (4 ppm; -20˚C) was added and the sample was vortexed for 30 sec. Glass beads (100 mg) were added to the mixture which was then put into a tissue grinder and ground for 60 sec at 55 Hz. After ultrasound for 15 min at room temperature, the mixture was centrifuged at 16,000 x g at 4˚C for 10 min. Then, 300 µl supernatant was taken and filtered through 0.22 µm membrane, which was collected into a detection bottle for LC-MS detection.
Chromatographic separation was used with an ACQUITY UPLC HSS T3 (150x2.1 mm; 1.8 µm; Waters Corporation) column maintained at 40˚C. The temperature of the autosampler was 8˚C. Gradient elution of analytes was carried out with 0.1% formic acid in water (C) and 0.1% formic acid in acetonitrile (D) or 5 mM ammonium formate in water (A) and acetonitrile (B) at a flow rate of 0.25 ml/min. Injection of 2 µl of each sample was performed after equilibration. An increasing linear gradient of solvent B (v/v) was used as follows: ~0-1 min, 2% B/D; ~1-9 min, 2~50% B/D; ~9-12 min, 50~98% B/D; ~12-13.5 min, 98% B/D; ~13.5-14 min, ~98-2% B/D; ~14-20 min, 2% Dpositive model (~14-17 min; 2% B-negative model).
The electrospray ionization mass spectrometry (ESI-MS) experiments were used with the spray voltage of 3.5 and 2.5 kV in positive and negative modes, respectively. Sheath gas and auxiliary gas were set at 30 and 10 arbitrary units, respectively. The capillary temperature was 325˚C. respectively. The Orbitrap analyzer scanned over a mass range of m/z 81-1 000 for full scan at a mass resolution of 70,000. Data dependent acquisition MS/MS experiments were performed with high-energy collisional dissociation scan. The normalized collision energy was 30 eV. Dynamic exclusion was implemented to remove some unnecessary information in MS/MS spectra. The information of PGG in LC-MS detection is shown as Table I.
Table I.
The data of isolated 1, 2, 3, 4, 6-penta-O-galloyl-β-D-glucose in liquid chromatography-mass spectrometry detection.
| Name | Mass-to-charge ratio (m/z) | Retention time | ppm | Formula | Ionization mode | P-value |
|---|---|---|---|---|---|---|
| Pentagalloylglucose | 939.1230577 | 522.631 | 12.94042809 | C41H32O26 | [M-H]- | 6.01972x10-7 |
Animal model and grouping
Male SD rats (age, 6 weeks; n=30; weight 200-250 g) were purchased from Shanghai Jiesijie Experimental Animal Co., Ltd and housed in standard environmental conditions at 22±2˚C and 55-60% humidity, with free access to food and water and a 12 h light-dark cycle. Rats in Control group (n=6) were fed a normal healthy diet and other rats were fed a high-fat diet (HFD; main ingredients, 60.7% basic feed, 10% lard, 15% sucrose, 10% egg yolk powder, 4% cholesterol and 0.3% cholate) for 4 weeks. After that, HFD-fed rats were intraperitoneally injected with 1% STZ solution (35 mg/kg) to induce the diabetic model and rats in the Control group were injected with the same amount of sodium citrate buffer. After 72 h, blood was collected from the tail vein and blood glucose level was measured using a blood glucose meter (Thermo Fisher Scientific, Inc.) after a 12-h fast. Fasting blood glucose level was ≥16.7 mmol/l, indicating that the diabetic rat model was successful (32,33). Subsequently, diabetic rats were randomly divided into four groups including DN group, DN + PGG-L (low; 5 mg/kg) group, DN + PGG-H (high; 20 mg/kg) group and DN + GRE (25 mg/kg) group (n=6 for each). Diabetic rats were continuously fed with HFD for 8 weeks in DN group. Treatment group rats were respectively administered with 5 mg/kg PGG, 20 mg/kg PGG and 25 mg/kg GRE by gavage every day, which were accompanied by HFD for 8 weeks. At the end of 8 weeks of treatment, blood was collected from the tail vein and blood glucose level was measured using a blood glucose meter (Thermo Fisher Scientific, Inc.) after a 12-h fast. At the end of 0 and 8 weeks of treatment, the rats were placed in a separate metabolic cage for 24 h to collect urine and the urinary albumin/creatinine ratio (ACR) was determined by an automatic biochemical analyzer. All rats were euthanized by intraperitoneal injection of pentobarbital sodium (165 mg/kg; i.p.) and the renal tissues were isolated. All experiments were approved and supervised by the Animal Care and Use Committee and the Animal Ethics Committee of Beichen District Hospital of Traditional Chinese Medicine (approval no. 202001; Tianjin, China).
Monitoring of renal function
The urinary ACR was determined by an automatic biochemical analyzer on the 0th day and 56th day. The levels of blood urea nitrogen (BUN) and serum creatinine (Scr) in blood was also measured by an automatic biochemical analyzer on the 56th day.
Hematoxylin and eosin (H&E), periodic acid-Schiff (PAS) and Masson staining
The renal tissues were fixed in paraformaldehyde for 24 h at 4˚C, washed in an ethanol series, embedded in paraffin and sectioned at 4 µm. The paraffin sections were heated in a constant temperature oven at 40˚C, dewaxed in xylene, rehydrated using a descending ethanol gradient from 100 to 70% and washed in PBS. For H&E staining, the sections were stained using hematoxylin for 5-10 min at room temperature and stained with eosin for 1 min at room temperature. For PAS staining, slides were incubated with periodic acid for 5 min and Schiff's reagent was added for incubated for 15 min at room temperature, followed by counterstaining with hematoxylin for 1 min. For Masson staining, sections were incubated in Masson-ponceau for 5-10 min at 37˚C and stained with aniline blue for 5 min at 37˚C. The pathological changes and renal fibrosis were observed by light microscopy (Olympus Corporation).
Cell culture and processing
Conditionally immortalized mouse podocytes (MPC5), purchased from Shanghai Enzyme-linked Biotechnology Co., Ltd. The cells were cultured in RPMI 1640 medium (HyClone; Cytiva) supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), 10 U/ml of interferon-γ (IFN-γ; Invitrogen; Thermo Fisher Scientific, Inc.) and 1% penicillin-streptomycin (MilliporeSigma) at 37˚C and 5% CO2. Subsequently, the cells in Control group, mannitol (MA) and high glucose (HG) group were in turn induced with normal glucose (5 mM glucose), MA (30 mM) and HG (30 mM glucose) for 48 h. HG-induced MPC5 cells were also treated with PGG with and without p79350 (p38 agonist) and LM22B-10 (ERK1/2 agonist).
CCK-8 assay
MPC5 cells were seeded into 96-well plates at density of 5x103 cells per well. One experiment was that MPC5 cells were treated with only PGG at different concentrations (20, 40 and 80 µM) for 24 h. Another experiment included six groups: Control group; MPC5 cells were induced with normal glucose (5 mM glucose); MA group; MPC5 cells were induced with mannitol (30 mM); HG group; MPC5 cells were induced with high glucose (30 mM glucose); HG + PGG (20, 40 and 80 µM) groups; MPC5 cells pre-treated with different concentrations (20, 40 and 80 µM) respectively for 2 h were induced by HG for 48 h. After indicated treatment, cells in each well were incubated with 10 µl CCK-8 solution (Beyotime Institute of Biotechnology). Optical density (OD) of each well was detected at 450 nm by a microplate reader (Thermo Fisher Scientific, Inc.).
Western blotting
Renal tissues or MPC5 cells were lysed in RIPA lysis buffer (P0013B, Beyotime Institute of Biotechnology), which was extracted by a total protein extraction kit (cat. no. AMJ-KT0007; AmyJet Scientific, Inc.) to obtain the total protein. The concentration of total protein was determined by a BCA kit (cat. no. KTD3001, AmyJet Scientific, Inc.). After that, 50 µg total protein was separated on a 10% SDS-PAGE, transferred to a PVDF membrane and blocked with 5% non-fat milk at room temperature for 1 h. Then, the membrane was incubated with the primary antibodies to nephrin (cat. no. ab216341; 1:1,000; Abcam), TGF-β (cat. no. ab215715; 1:1,000; Abcam), Collagen (Col) I (cat. no. ab270993; 1:1,000; Abcam), Col IV (cat. no. ab6586; 1:1,000; Abcam), Bcl-2 (cat. no. ab182858; 1:2,000; Abcam), Bax (cat. no. ab32503; 1:1,000; Abcam) and cleaved caspase 3 (cat. no. ab214430; 1:5,000; Abcam), p-p38 (cat. no. 4511; 1:1,000; Cell signaling pathway), p-ERK1/2 (cat. no. 9101; 1:1,000; Cell signaling pathway), p-p65 (cat. no. ab76302; 1:1,000; Abcam), caspase 3 (cat. no. ab184787; 1:2,000; Abcam), p38 (cat. no. 8690; 1:1,000; Cell signaling pathway), ERK1/2 (cat. no. 4695; 1:1,000; Cell signaling pathway), p65 (cat. no. ab32536; 1:1,000; Abcam), Nrf2 (cat. no. ab92946; 1:1,000; Abcam), HO-1 (cat. no. ab52947; 1:2,000; Abcam) and β-actin (cat. no. ab8227; 1:1,000; Abcam) overnight at 4˚C. The secondary antibody Goat Anti-Rabbit IgG H&L (cat. no. ab6721; 1:2,000; Abcam) was incubated for 1 h at room temperature. The gray values of each protein bands were analyzed by ImageJ v3.0 (National Institutes of Health) and β-actin was used as a loading control.
TUNEL assay
Renal tissue sections and MPC5 cells on coverslips were treated with 20 g/ml proteinase K and stained as recommended using the In Situ Cell Death Detection kit (Nanjing KeyGen Biotech Co., Ltd.) according to the manufacturer's instructions (34). Cell nuclei were stained with DAPI (1 mg/ml) at room temperature for 10 min in the dark. Images of three fields of view were randomly captured under a fluorescence microscope.
Detection of inflammatory factors
The concentration of inflammatory factors in renal tissues or cell culture medium was quantified via ELISA (22). For tissue homogenates, renal tissues (1 g) were added with pre-chilled homogenate buffer solution, which was homogenized and centrifuged (2,000 x g; 15 min; 4˚C) to collect supernatant of renal tissues. For cell culture medium, the cell medium of MPC5 cells was centrifuged at 2.000 x g for 5 min at 4˚C. The following ELISA kits were used for tissues: TNF-α (cat. no. K1052-100; AmyJet Scientific, Inc.), IL-1β (cat. no. E-EL-R0012c; Elabscience Biotechnology, Inc.), IL-6 (cat. no. ab119548; Abcam) and myeloperoxidase (MPO; cat. no. ab105136; Abcam); or for cell culture medium: TNF-α (cat. no. PT512; Beyotime Institute of Biotechnology), IL-1β (cat. no. PI301; Beyotime Institute of Biotechnology) and IL-6 (vPI326; Beyotime Institute of Biotechnology) according to the manufacturer's instructions.
Determination of oxidative stress indicators
The activities of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), reactive oxygen species (ROS) and the levels of malondialdehyde (MDA) in the renal homogenates and cell supernatant were measured using commercial kits of SOD (cat. no. A001-3-2), GSH-Px (cat. no. A005-1-2), ROS (cat. no. E004-1-1) and MDA (cat. no. A003-1-2) (All from Nanjing Jiancheng Bioengineering Institute) according to the manufacturer's instruction.
Statistical analysis
All the data herein are shown as mean ± SD and analyzed using GraphPad Prism 8 (GraphPad Software, Inc.). All data were analyzed by Sapiro-Wilk (S-W) to evaluate whether they fitted the normal distribution. When data fitted the normal distribution, the significant difference among multiple groups was determined via one-way of variance (ANOVA) test with post hoc Tukey's test. As the histopathology scores were categorical data, they were statistically analyzed using the Kruskal-Wallis test. P<0.05 was considered to indicate a statistically significant difference.
Results
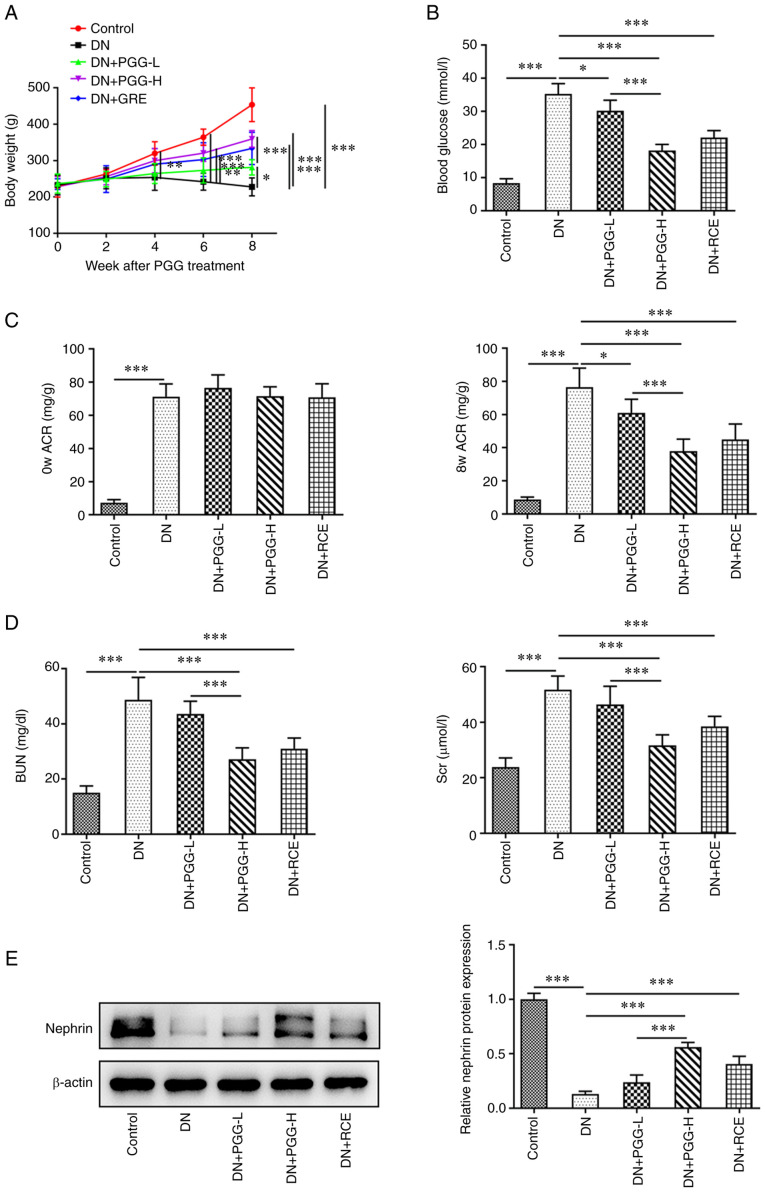
PGG improves renal function in diabetic rats
The body weight of DN rats was decreased and PGG treatment improved the body weight of DN rats. The improvement of 20 mg/kg PGG for the body weight of DN rats was greater compared with that of 5 mg/kg PGG. The body weight of DN rats could also be improved by GRE (Fig. 1A). The blood glucose was increased in DN rats and gradually decreased after PGG from 5 to 20 mg/kg. GRE could also downregulated the blood glucose in DN rats (Fig. 1B). The ACR was increased in DN rats at day 0 and was decreased after PGG treatment and GRE treatment for 8 weeks (Fig. 1C). The levels of BUN and Scr in DN rats rose, but were reduced by the treatment of PGG and GRE (Fig. 1D). The expression of nephrin was lower in DN rats but was upregulated following PGG treatment and GRE treatment (Fig. 1E).
Figure 1.
PGG improves renal function in diabetic rats. (A) The body weight of DN rats with or without PGG treatment. (B) The blood glucose in DN rats with or without PGG treatment was detected by a blood glucose meter. The (C) ACR and levels of (D) BUN and Scr in DN rats with or without PGG treatment was measured by an automatic biochemical analyzer. (E) The expression of nephrin in DN rats with or without PGG treatment was analyzed by western blotting. *P<0.05, **P<0.01 and ***P<0.001. PGG, 1, 2, 3, 4, 6-penta-O-galloyl-β-D-glucose; DN, diabetic nephropathy ACR, albumin/creatinine ratio; BUN, blood urea nitrogen; Scr, serum creatinine; GRE, Galla rhois extract; -L, low; 5 mg/kg; -H, high; 20 mg/kg.
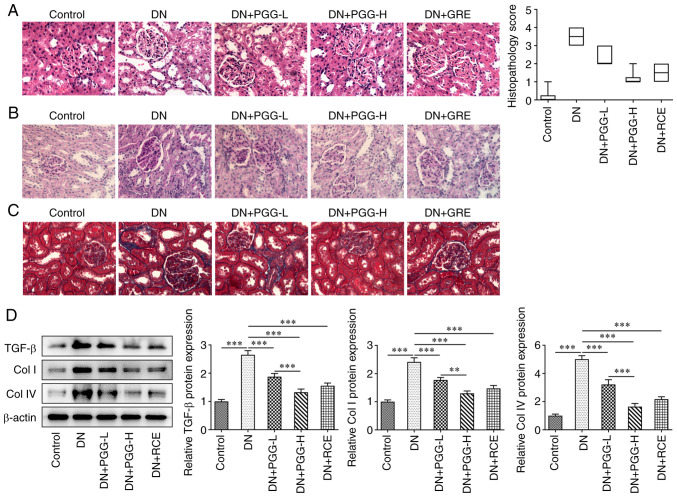
PGG improves renal pathological changes in diabetic rats
Renal structure damage was observed on inner medulla, outer medulla and cortex following H&E staining (Fig. 2A). Glomerular fibrosis clearly occurred in DN rats as shown by PAS staining (Fig. 2B). Following Masson staining blue-stained collagen fibers were observed in the glomeruli and tubules of the DN rats, which was higher compared with the Control group, indicating that collagen fibers were precipitated (Fig. 2C). However, administration of PGG or GRE reversed this. In DN rats, TGF-β, Col I and Col IV protein expression were increased significantly. After treatment with PGG or GRE, the expression of TGF-β, Col I and Col IV were clearly reduced (Fig. 2D).
Figure 2.
PGG improves renal pathological changes in diabetic rats. The pathological changes, fibrosis and collagen deposition in renal tissues were in turn detected by (A) hematoxylin and eosin, (B) periodic acid-Schiff and (C) Masson staining. Magnification, x400. (D) The expression of fibrosis related proteins in DN rats with or without PGG treatment was detected by western blotting. **P<0.01 and ***P<0.001. PGG, 1, 2, 3, 4, 6-penta-O-galloyl-β-D-glucose; DN, diabetic nephropathy; GRE, Galla rhois extract; -L, low; 5 mg/kg; -H, high; 20 mg/kg.
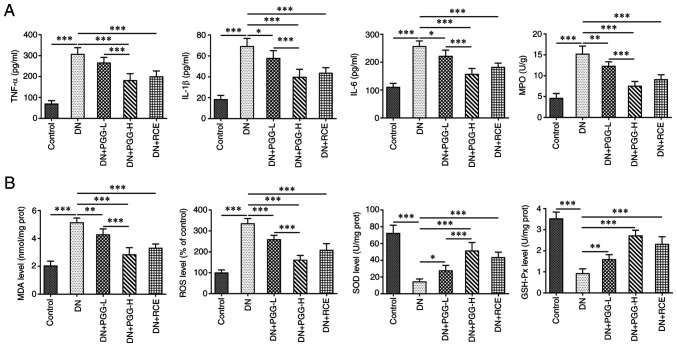
PGG improves renal inflammatory response and oxidative stress levels in diabetic rats
Compared with the Control group, the levels of TNF-α, IL-1β, IL-6 and MPO in the DN group were significantly elevated (Fig. 3A) and the levels of MDA and ROS were significantly increased in the DN group and levels of SOD and GSH-Px were significantly decreased (Fig. 3B). Notably, these inflammation and oxidative stress events were improved under PGG treatment in a concentration-dependent manner or GRE treatment.
Figure 3.
PGG improves renal inflammatory response and oxidative stress levels in diabetic rats. The levels of (A) inflammatory factors and (B) oxidative stress related indicators in DN rats with or without PGG treatment were respectively determined by their commercial kits. *P<0.05, **P<0.01 and ***P<0.001. PGG, 1, 2, 3, 4, 6-penta-O-galloyl-β-D-glucose; DN, diabetic nephropathy; GRE, Galla rhois extract; -L, low; 5 mg/kg; -H, high; 20 mg/kg; MPO, myeloperoxidase; MDA, malondialdehyde; ROS, reactive oxygen species; SOD, superoxide dismutase; GSH-Px, glutathione peroxidase.
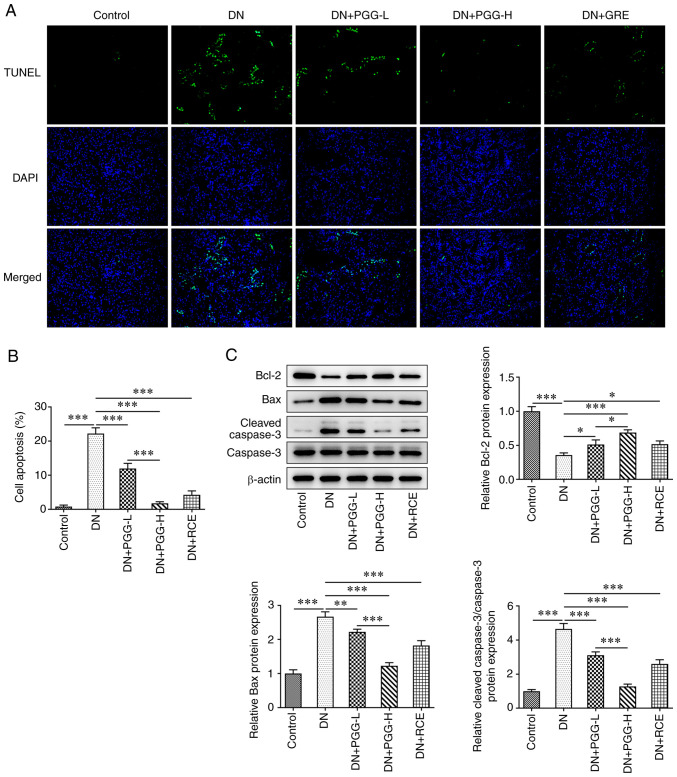
PGG inhibits the apoptosis of podocytes in diabetic rats
The apoptosis of podocytes detected by TUNEL assay indicated that the percent of apoptotic cells was significantly exhibited in the DN group, as shown in Fig. 4A and B. In the DN group, Bax and cleaved caspase 3 protein expression were increased significantly while Bcl-2 expression was significantly reduced (Fig. 4C). Following treatment with PGG in different concentrations or GRE, the apoptosis of podocytes was suppressed and Bax and cleaved caspase 3 protein expression were decreased while Bcl-2 expression was evidently increased.
Figure 4.
PGG inhibits the apoptosis of podocytes in diabetic rats. (A and B) The apoptosis of podocytes in DN rats with or without PGG treatment was detected by TUNEL assay. Magnification, x200. (C) The expression of apoptosis-related proteins in DN rats with or without PGG treatment was detected by western blotting. *P<0.05, **P<0.01 and ***P<0.001. PGG, 1, 2, 3, 4, 6-penta-O-galloyl-β-D-glucose; DN, diabetic nephropathy.
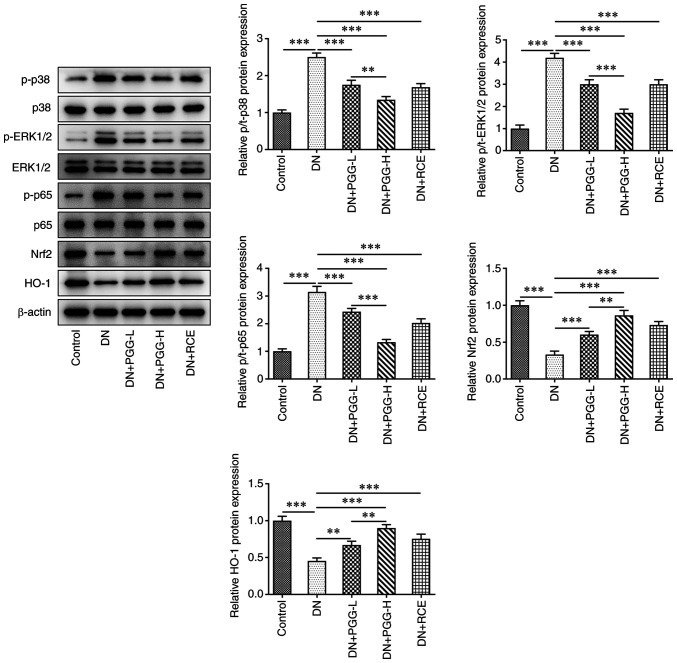
PGG regulates the MAPK/NF-κB and ERK/Nrf2/HO-1 signaling pathways
The expression of p-p38, p-ERK1/2 and p-p65 was promoted while the expression of Nrf2 and HO-1 was suppressed. Conversely, PGG treatment or GRE treatment improved this. The results showed that PGG suppressed the MAPK/NF-κB and ERK/Nrf2/HO-1 signaling pathways (Fig. 5).
Figure 5.
PGG regulates the MAPK/NF-κB and ERK/Nrf2/HO-1 signaling pathways. The expression of related proteins in MAPK/NF-κB and ERK/Nrf2/HO-1 signaling pathways in DN rats with or without PGG treatment was detected by western blotting. **P<0.01 and ***P<0.001. PGG, 1, 2, 3, 4, 6-penta-O-galloyl-β-D-glucose; Nrf2, nuclear factor erythroid-derived 2-related factor 2; HO-1, hemeoxygenase-1; DN, diabetic nephropathy; p-, phosphorylated; t-, total.
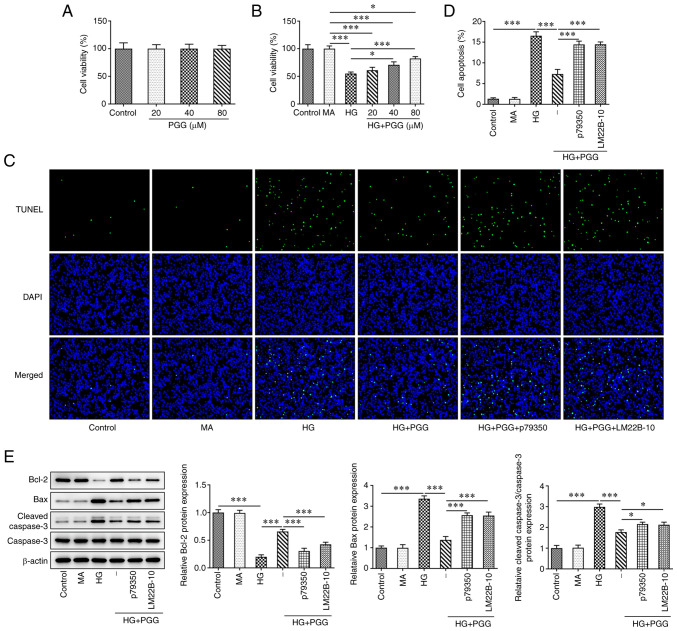
P79350 and LM22B-10 reverses the protective effect of PGG on viability and apoptosis of HG-induced MPC5 cells
After MPC5 cells were treated with PGG at different concentrations (20, 40 and 80 µM), the viability was not obviously changed (Fig. 6A). HG induction decreased the viability of MPC5 cells, which was increased by the PGG treatment or GRE treatment (Fig. 6B). The apoptosis of MPC5 cells was increased in HG group, which was decreased by PGG treatment (Fig. 6C and D). The Bcl-2 expression was reduced and the expression of Bax and cleaved caspase 3 was increased in HG-induced MPC5 cells, which were reverse by PGG treatment (Fig. 6E). However, the protective effect of PGG on podocytes lesion induced by HG could be reversed by P79350 (p38 agonist) and LM22B-10 (ERK1/2 agonist).
Figure 6.
P79350 and LM22B-10 reverses the protective effect of PGG on viability and apoptosis of HG-induced MPC5 cells. (A) The MPC5 cell viability treated by PGG was determined by CCK-8 assay. The (B) viability and (C and D) apoptosis of HG-induced MPC5 cells with or without PGG treatment were determined by CCK-8 and TUNEL assays. Magnification, x200. (E) The expression of apoptosis-related proteins in HG-induced MPC5 cells with or without PGG treatment was detected by western blotting. *P<0.05 and ***P<0.001. PGG, 1, 2, 3, 4, 6-penta-O-galloyl-β-D-glucose; HG, high glucose; MPC5, conditionally immortalized mouse podocytes.
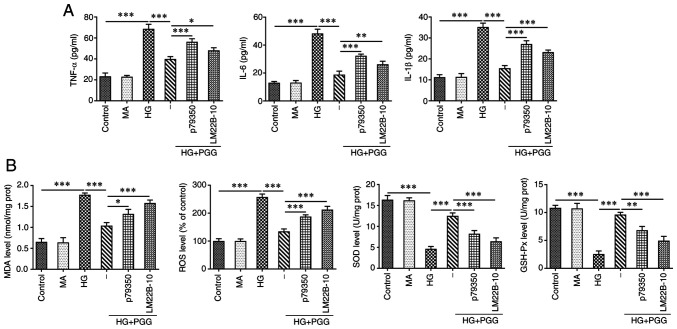
P79350 and LM22B-10 reverses the protective effect of PGG on inflammation and oxidative stress of HG-induced MPC5 cells
As shown in Fig. 7A, the levels of inflammatory factors TNF-α, IL-6 and IL-1β were increased in HG-induced MPC5 cells, which was reduced by PGG. The result reflecting the oxidative stress indicated that the levels of MDA and ROS were increased while levels of SOD and GSH-Px were decreased in HG-induced MPC5 cells and PGG could reverse the above phenomenon in HG-induced MPC5 cells (Fig. 7B). P79350 (p38 agonist) and LM22B-10 (ERK1/2 agonist) could weaken the protective effect of PGG on inflammation and oxidative stress.
Figure 7.
P79350 and LM22B-10 reverses the protective effect of PGG on inflammation and oxidative stress of HG-induced MPC5 cells. The levels of inflammatory factors (A) and oxidative stress related indicators (B) in HG-induced MPC5 cells with or without PGG treatment were respectively determined by their commercial kits. *P<0.05, **P<0.01 and ***P<0.001. PGG, 1, 2, 3, 4, 6-penta-O-galloyl-β-D-glucose; HG, high glucose; MPC5, conditionally immortalized mouse podocytes; MA, mannitol.
Discussion
Oxidative stress refers to the imbalance of the homeostasis of the body's oxidation and antioxidant systems, resulting in the excessive production of ROS, which exceeds the normal scavenging capacity of the body, thus causing oxidative damage to tissues, especially mitochondrial damage (35). Son et al (36) found that evident lipid peroxidation and ROS increase occur when renal cells were exposed to hyperglycemia and the oxidative stress state of cells was significantly reduced after the intervention with antioxidant. The expression of a number of inflammatory factors such as cell adhesion factors chemokines and proinflammatory factors in renal tissue of DN patients is increased (37). It has been shown that elevated ROS levels in the kidneys of patients with DN mediate infiltration of macrophages and recruitment of inflammatory cells and promote the production of inflammatory factors (IL-1β, IL-6, TNF-α, MCP-1, TGF-β and NF-κB), which serve a key role in initiating diabetic kidney injury (38). The present study also found that the inflammation and oxidative stress were occurred in diabetic nephropathy rats.
Clinical studies show that anti-inflammatory and antioxidant drugs significantly reduce urinary albumin excretion in DN patients and reduce levels of TNF-α and MDA in vivo (39). Lipoic acid (LA) not only reduces oxidative stress, but also serves an important role in inflammation. Wang et al (40) showed that LA can reduce MDA level and increase SOD activity in serum and renal cortex of diabetic rats. Clinical treatment also shows that LA treatment can significantly reduce oxidative stress-related indexes in diabetic patients, alleviate DN and improve kidney injury (41). The above studies indicate that the alleviation of inflammation and oxidative stress can improve kidney injury in DN. The present study also showed that inflammation and oxidative stress was suppressed by PGG to protect the DN. GRE is a single herb which contains PGG. The results also indicated that GRE could alleviate the inflammation and oxidative stress in DN rats.
In the state of hyperglycemia, inflammation of renal tissue is initiated through a variety of signaling pathways, among which the p38MAPK signaling pathway is a classic inflammatory signaling pathway. Once this signaling pathway is activated, the course of DN will be accelerated. Activation of p38MAPK signaling pathway can indirectly or directly lead to the production of inflammatory factors (including IL-1β and TNF-α) through phosphorylation of different transcription factors, including NF-κB and participate in disease inflammation (42). It was found that by inhibiting the phosphorylation of p38MAPK, the release of pro-inflammatory factors could be inhibited and the progression of DN could be delayed (43).
The ERK/Nrf2/HO-1 signaling pathway serves a protective role in DN kidney injury. Shopit et al (28) demonstrate that phosphocreatine protects against kidney injury by decreasing the ERK expression and activating the Nrf2/HO-1 pathway. The expression of p-ERK is significantly higher in DN mouse model and HK-2 cells treated with HG, while the expression of p-ERK in RTN1A silenced HK-2 cells treated with HG is significantly lower than that in the control group (44). ROS accumulation in Nrf2 knockout DN mice results in renal damage (45). Chen et al (46) also found that collagen formation and renal interstitial fibrosis in DN rats can be improved by naringin and the mechanism is related to the activation of Nrf2. HO-1 is a downstream protein of Nrf2, which can effectively reduce inflammatory response and oxidative stress damage and serve a protective role in cells (47). The phosphocreatine effect against kidney injury may be ascribed to its antioxidant properties by decreasing the ERK expression and activating the Nrf2/HO-1 pathway (28). The above studies demonstrate that induction and activation of Nrf2/HO-1 pathway can reduce renal damage caused by oxidative stress. The present study also found that expression of p-p38, p-p65 and p-ERK1/2 was increased and expression of Nrf2 and HO-1 was decreased in DN rats, which was reversed by treatment of PGG or GRE. As P79350 is a p38 agonist and LM22B-10 is an ERK1/2 agonist, the application of P79350 and LM22B-10 will result in the activation of MAPK/NF-κB and ERK/Nrf2/HO-1 signaling pathways. In the present study, PGG suppressed the MAPK/NF-κB and ERK/Nrf2/HO-1 signaling pathways. The p38 agonist (P79350) and ERK1/2 agonist (LM22B-10) were used to reversely validate that PGG protected the DN via the MAPK/NF-κB and ERK/Nrf2/HO-1 signaling pathways. In fact, PGG protected the DN via the MAPK/NF-κB and ERK/Nrf2/HO-1 signaling pathways.
In conclusion, PGG alleviated inflammation and oxidative stress in DN rats through suppressing the MAPK/NF-κB and ERK/Nrf2/HO-1 signaling pathways. First, the renal function of DN rats was significantly declined and renal fibrosis of DN rats was significantly aggravated. However, PGG significantly ameliorated renal function and decreased renal fibrosis in DN rats. Second, the inflammation, oxidative stress and podocyte apoptosis were clearly increased in DN rats and reversed by PGG. Third, the MAPK/NF-κB and ERK/Nrf2/HO-1 signaling pathways were activated in DN rats and inhibited by PGG. However, P79350 (p38 agonist) and LM22B-10 (ERK1/2 agonist) weakened the effect of PGG by promoting apoptosis, inflammation and oxidative stress in HG-induced MPC5 cells. There also existed limitation in this study; it was uncertain how much PGG was in the RCE and the concentration will be studied in the future using HPLC to perform a quantitative analysis.
Acknowledgements
Not applicable.
Funding Statement
Funding: The present study was supported by the Tianjin Beichen District science and technology plan project (grant no. SHGY2020054) and the Scientific research project of integrated traditional Chinese and Western medicine of Tianjin Municipal Health Commission and Tianjin Administration of traditional Chinese Medicine (grant no. 2021123).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
DW, YL and LD were responsible for designing the study, collecting the data, performing the statistical analysis and drafting the manuscript. YW, CZ, WW, YuZ and YiZ collected the data, performed the statistical analysis and conducted the literature search. TY supervised the project, helped to design the study, analyzed the data and wrote the manuscript. DW and YL confirm the authenticity of all the raw data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All experiments were approved and supervised by the Animal Care and Use Committee and the Animal Ethics Committee of Beichen District Hospital of Traditional Chinese Medicine (approval no. 202001; Tianjin, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Petersmann A, Müller-Wieland D, Müller UA, Landgraf R, Nauck M, Freckmann G, Heinemann L, Schleicher E. Definition, classification and diagnosis of diabetes mellitus. Exp Clin Endocrinol Diabetes. 2019;127:S1–S7. doi: 10.1055/a-1018-9078. [DOI] [PubMed] [Google Scholar]
- 2.Cole JB, Florez JC. Genetics of diabetes mellitus and diabetes complications. Nat Rev Nephrol. 2020;16:377–390. doi: 10.1038/s41581-020-0278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sagoo MK, Gnudi L. Diabetic nephropathy: An overview. Methods Mol Biol. 2020;2067:3–7. doi: 10.1007/978-1-4939-9841-8_1. [DOI] [PubMed] [Google Scholar]
- 4.Nanditha A, Ma RC, Ramachandran A, Snehalatha C, Chan JC, Chia KS, Shaw JE, Zimmet PZ. Diabetes in Asia and the pacific: Implications for the global epidemic. Diabetes Care. 2016;39:472–485. doi: 10.2337/dc15-1536. [DOI] [PubMed] [Google Scholar]
- 5.Wang L, Gao P, Zhang M, Huang Z, Zhang D, Deng Q, Li Y, Zhao Z, Qin X, Jin D, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA. 2017;317:2515–2523. doi: 10.1001/jama.2017.7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma RCW. Epidemiology of diabetes and diabetic complications in China. Diabetologia. 2018;61:1249–1260. doi: 10.1007/s00125-018-4557-7. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L, Long J, Jiang W, Shi Y, He X, Zhou Z, Li Y, Yeung RO, Wang J, Matsushita K, et al. Trends in chronic kidney disease in china. N Engl J Med. 2016;375:905–906. doi: 10.1056/NEJMc1602469. [DOI] [PubMed] [Google Scholar]
- 8.Umanath K, Lewis JB. Update on diabetic nephropathy: Core curriculum 2018. Am J Kidney Dis. 2018;71:884–895. doi: 10.1053/j.ajkd.2017.10.026. [DOI] [PubMed] [Google Scholar]
- 9.Arokiasamy P, Salvi S, Mani S. Global burden of diabetes mellitus. In: Handbook of Global Health. Haring R, Kickbusch I, Ganten D and Moeti M (eds). Springer International Publishing, Cham, pp1-44, 2021. [Google Scholar]
- 10.Yan D, Tu Y, Jiang F, Wang J, Zhang R, Sun X, Wang T, Wang S, Bao Y, Hu C, Jia W. Uric Acid is independently associated with diabetic kidney disease: A cross-sectional study in a Chinese population. PLoS One. 2015;10(e0129797) doi: 10.1371/journal.pone.0129797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang YH, Lei CC, Lin KC, Chang DM, Hsieh CH, Lee YJ. Serum uric acid level as an indicator for CKD regression and progression in patients with type 2 diabetes mellitus-a 4.6-year cohort study. Diabetes Metab Res Rev. 2016;32:557–564. doi: 10.1002/dmrr.2768. [DOI] [PubMed] [Google Scholar]
- 12.Wada J, Makino H. Inflammation and the pathogenesis of diabetic nephropathy. Clin Sci (Lond) 2013;124:139–152. doi: 10.1042/CS20120198. [DOI] [PubMed] [Google Scholar]
- 13.Moreno JA, Gomez-Guerrero C, Mas S, Sanz AB, Lorenzo O, Ruiz-Ortega M, Opazo L, Mezzano S, Egido J. Targeting inflammation in diabetic nephropathy: A tale of hope. Expert Opin Investig Drugs. 2018;27:917–930. doi: 10.1080/13543784.2018.1538352. [DOI] [PubMed] [Google Scholar]
- 14.Zhao LL, Makinde EA, Shah MA, Olatunji OJ, Panichayupakaranant P. Rhinacanthins-rich extract and rhinacanthin C ameliorate oxidative stress and inflammation in streptozotocin-nicotinamide-induced diabetic nephropathy. J Food Biochem. 2019;43(e12812) doi: 10.1111/jfbc.12812. [DOI] [PubMed] [Google Scholar]
- 15.Park JH, Kho MC, Oh HC, Kim YC, Yoon JJ, Lee YJ, Kang DG, Lee HS. 1,[Formula: See text]2,[Formula: See text]3,[Formula: See text]4,[Formula: See text]6-Penta-O-Galloyl-β-D-glucose from galla rhois ameliorates renal tubular injury and microvascular inflammation in acute kidney injury rats. Am J Chin Med. 2018;46:785–800. doi: 10.1142/S0192415X18500416. [DOI] [PubMed] [Google Scholar]
- 16.Mendonca P, Taka E, Bauer D, Cobourne-Duval M, Soliman KF. The attenuating effects of 1,2,3,4,6 penta-O-galloyl-β-d-glucose on inflammatory cytokines release from activated BV-2 microglial cells. J Neuroimmunol. 2017;305:9–15. doi: 10.1016/j.jneuroim.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jang SE, Hyam SR, Jeong JJ, Han MJ, Kim DH. Penta-O-galloyl-β-D-glucose ameliorates inflammation by inhibiting MyD88/NF-κB and MyD88/MAPK signalling pathways. Br J Pharmacol. 2013;170:1078–1091. doi: 10.1111/bph.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohan CG, Viswanatha GL, Savinay G, Rajendra CE, Halemani PD. 1,2,3,4,6 Penta-O-galloyl-β-d-glucose, a bioactivity guided isolated compound from Mangifera indica inhibits 11β-HSD-1 and ameliorates high fat diet-induced diabetes in C57BL/6 mice. Phytomedicine. 2013;20:417–426. doi: 10.1016/j.phymed.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 19.Lee HJ, Jeong SJ, Lee HJ, Lee EO, Bae H, Lieske JC, Kim SH. 1,2,3,4,6-Penta-O-galloyl-beta-D-glucose reduces renal crystallization and oxidative stress in a hyperoxaluric rat model. Kidney Int. 2011;79:538–545. doi: 10.1038/ki.2010.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Navarro-González JF, Mora-Fernández C, de Fuentes MM, García-Pérez J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev Nephrol. 2011;7:327–340. doi: 10.1038/nrneph.2011.51. [DOI] [PubMed] [Google Scholar]
- 21.Xu L, Shen P, Bi Y, Chen J, Xiao Z, Zhang X, Wang Z. Danshen injection ameliorates STZ-induced diabetic nephropathy in association with suppression of oxidative stress, pro-inflammatory factors and fibrosis. Int Immunopharmacol. 2016;38:385–394. doi: 10.1016/j.intimp.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 22.Han J, Pang X, Zhang Y, Peng Z, Shi X, Xing Y. Hirudin protects against kidney damage in streptozotocin-induced diabetic nephropathy rats by inhibiting inflammation via P38 MAPK/NF-κB pathway. Drug Des Devel Ther. 2020;14:3223–3234. doi: 10.2147/DDDT.S257613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang M, Chen Y, Yang MJ, Fan XR, Xie H, Zhang L, Nie YS, Yan M. Celastrol attenuates renal injury in diabetic rats via MAPK/NF-κB pathway. Phytother Res. 2019;33:1191–1198. doi: 10.1002/ptr.6314. [DOI] [PubMed] [Google Scholar]
- 24.Mendonca P, Taka E, Bauer D, Reams RR, Soliman KFA. The attenuating effects of 1,2,3,4,6 penta-O-galloyl-β-d-glucose on pro-inflammatory responses of LPS/IFNγ-activated BV-2 microglial cells through NFκB and MAPK signaling pathways. J Neuroimmunol. 2018;324:43–53. doi: 10.1016/j.jneuroim.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim BH, Choi MS, Lee HG, Lee SH, Noh KH, Kwon S, Jeong AJ, Lee H, Yi EH, Park JY, et al. Photoprotective potential of penta-O-Galloyl-β-DGlucose by targeting NF-κB and MAPK signaling in UVB radiation-induced human dermal fibroblasts and mouse skin. Mol Cells. 2015;38:982–990. doi: 10.14348/molcells.2015.0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee DS, Kim KS, Ko W, Li B, Jeong GS, Jang JH, Oh H, Kim YC. The cytoprotective effect of sulfuretin against tert-butyl hydroperoxide-induced hepatotoxicity through Nrf2/ARE and JNK/ERK MAPK-mediated heme oxygenase-1 expression. Int J Mol Sci. 2014;15:8863–8877. doi: 10.3390/ijms15058863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shopit A, Niu M, Wang H, Tang Z, Li X, Tesfaldet T, Ai J, Ahmad N, Al-Azab M, Tang Z. Protection of diabetes-induced kidney injury by phosphocreatine via the regulation of ERK/Nrf2/HO-1 signaling pathway. Life Sci. 2020;242(117248) doi: 10.1016/j.lfs.2019.117248. [DOI] [PubMed] [Google Scholar]
- 29.Bucolo C, Drago F, Maisto R, Romano GL, D'Agata V, Maugeri G, Giunta S. Curcumin prevents high glucose damage in retinal pigment epithelial cells through ERK1/2-mediated activation of the Nrf2/HO-1 pathway. J Cell Physiol. 2019;234:17295–17304. doi: 10.1002/jcp.28347. [DOI] [PubMed] [Google Scholar]
- 30.Chen H, Li H, Cao F, Zhen L, Bai J, Yuan S, Mei Y. 1,2,3,4,6-penta-O-galloyl-β-D-glucose protects PC12 cells from MPP(+)-mediated cell death by inducing heme oxygenase-1 in an ERK- and Akt-dependent manner. J Huazhong Univ Sci Technolog Med Sci. 2012;32:737–745. doi: 10.1007/s11596-012-1027-1. [DOI] [PubMed] [Google Scholar]
- 31.Pae HO, Oh GS, Jeong SO, Jeong GS, Lee BS, Choi BM, Lee HB, Chung HT. 1,2,3,4,6-penta-O-galloyl-beta-D-glucose up-regulates heme oxygenase-1 expression by stimulating Nrf2 nuclear translocation in an extracellular signal-regulated kinase-dependent manner in HepG2 cells. World J Gastroenterol. 2006;12:214–221. doi: 10.3748/wjg.v12.i2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lertpatipanpong P, Lee J, Kim I, Eling T, Oh SY, Seong JK, Baek SJ. The anti-diabetic effects of NAG-1/GDF15 on HFD/STZ-induced mice. Sci Rep. 2021;11(15027) doi: 10.1038/s41598-021-94581-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han X, Tao YL, Deng YP, Yu JW, Cai J, Ren GF, Sun YN, Jiang GJ. Metformin ameliorates insulitis in STZ-induced diabetic mice. PeerJ. 2017;5(e3155) doi: 10.7717/peerj.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan Y, Fan H, Zhu B, Zhou Y, Liu Q, Li P. Astragaloside IV protects against diabetic nephropathy via activating eNOS in streptozotocin diabetes-induced rats. BMC Complement Altern Med. 2019;19(355) doi: 10.1186/s12906-019-2728-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, Cavan D, Shaw JE, Makaroff LE. IDF diabetes atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 36.Son SM, Whalin MK, Harrison DG, Taylor WR, Griendling KK. Oxidative stress and diabetic vascular complications. Curr Diab Rep. 2004;4:247–252. doi: 10.1007/s11892-004-0075-8. [DOI] [PubMed] [Google Scholar]
- 37.Peng W, Huang S, Shen L, Tang Y, Li H, Shi Y. Long noncoding RNA NONHSAG053901 promotes diabetic nephropathy via stimulating Egr-1/TGF-β-mediated renal inflammation. J Cell Physiol. 2019;234:18492–18503. doi: 10.1002/jcp.28485. [DOI] [PubMed] [Google Scholar]
- 38.Matoba K, Takeda Y, Nagai Y, Kawanami D, Utsunomiya K, Nishimura R. Unraveling the role of inflammation in the Pathogenesis of Diabetic kidney disease. Int J Mol Sci. 2019;20(3393) doi: 10.3390/ijms20143393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eitah HE, Maklad YA, Abdelkader NF, El Din AA, Badawi MA, Kenawy SA. Modulating impacts of quercetin/sitagliptin combination on streptozotocin-induced diabetes mellitus in rats. Toxicol Appl Pharmacol. 2019;365:30–40. doi: 10.1016/j.taap.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 40.Wang L, Wu CG, Fang CQ, Gao J, Liu YZ, Chen Y, Chen YN, Xu ZG. The protective effect of α-Lipoic acid on mitochondria in the kidney of diabetic rats. Int J Clin Exp Med. 2013;6:90–97. [PMC free article] [PubMed] [Google Scholar]
- 41.Brami C, Bao T, Deng G. Natural products and complementary therapies for chemotherapy-induced peripheral neuropathy: A systematic review. Crit Rev Oncol Hematol. 2016;98:325–334. doi: 10.1016/j.critrevonc.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Niederlechner S, Baird C, Wischmeyer PE. P38MAP kinase, but not phosphoinositol-3 kinase, signal downstream of glutamine-mediated fibronectin-integrin signaling after intestinal injury. Nutr J. 2013;12(88) doi: 10.1186/1475-2891-12-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fang Y, Tian X, Bai S, Fan J, Hou W, Tong H, Li D. Autologous transplantation of adipose-derived mesenchymal stem cells ameliorates streptozotocin-induced diabetic nephropathy in rats by inhibiting oxidative stress, pro-inflammatory cytokines and the p38 MAPK signaling pathway. Int J Mol Med. 2012;30:85–92. doi: 10.3892/ijmm.2012.977. [DOI] [PubMed] [Google Scholar]
- 44.Zhao LM, Yang SF, Chen PF, Cheng WD, Yun-Hui MA. RTN1A induces renal tubular epithelial cells to secrete VEGF and IL-8 and promotes diabetic nephropathy renal fibrosis via ERK signaling pathway. Chinese Journal of Pathophysiology. 2018;34:2233–2239. (In Chinese) [Google Scholar]
- 45.Zheng H, Whitman SA, Wu W, Wondrak GT, Wong PK, Fang D, Zhang DD. Therapeutic potential of Nrf2 activators in streptozotocin-induced diabetic nephropathy. Diabetes. 2011;60:3055–3066. doi: 10.2337/db11-0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen F, Zhang N, Ma X, Huang T, Shao Y, Wu C, Wang Q. Naringin alleviates diabetic kidney disease through inhibiting oxidative stress and inflammatory reaction. PLoS One. 2015;10(e0143868) doi: 10.1371/journal.pone.0143868. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Zhou Y, Wang X, Ying W, Wu D, Zhong P. Cryptotanshinone attenuates inflammatory response of microglial cells via the Nrf2/HO-1 pathway. Front Neurosci. 2019;13(852) doi: 10.3389/fnins.2019.00852. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.