Abstract
Melanoma, which evolves from melanocytes, is the most malignant skin cancer and is highly fatal, although it only accounts for 4% of all skin cancers. Numerous studies have demonstrated that melanoma has a large tumor mutational burden, which means that melanoma has great potential to achieve immune evasion. Tumor-associated macrophages (TAMs) are an important component of both the immune system and tumor microenvironment. Several studies have demonstrated their double-edged sword effects on melanoma. The present review focuses on the role of TAMs in melanoma development, including regulation of proliferation, invasion, metastasis, angiogenesis and chemical resistance of melanoma. Furthermore, the existing mechanisms of action of the TAM-targeting treatments for melanoma are reviewed. More broadly, the weak points of existing research and the direction of future research are finally identified and described.
Keywords: macrophages, melanoma, double-edged sword, targeted therapy
1. Introduction
Melanoma evolves from melanocytes, which are mainly melanin-producing cells, is the most malignant skin cancer and is highly fatal, although it only accounts for 4% of all skin cancers (1). Globally, it affected 324,600 individuals in 2020, resulting in 57,000 deaths (2). A report from 2017 estimated that melanoma would result in 20,000 new cases annually in mainland China (3). According to annual reports on the status of cancer in the United States published in 2020, the incidence of melanoma is increasing continuously regardless of gender (4,5).
Although current therapies, including immune checkpoint treatment, targeted therapies, radiotherapy and chemotherapy, have resulted in a sustained reduction in the death rates of melanoma (6.1% annually) (4), treatments for melanoma still have room for advancement due to drug resistance (6). Numerous studies have demonstrated that melanoma has a large tumor mutational burden, meaning that melanoma has great potential to achieve immune evasion (7-9). Thus, the understanding of the detailed mechanism underlying immunosuppression in melanoma has become increasingly important. It is now widely accepted that the tumor microenvironment (TME), the complex ecosystem in which tumor cells reside and interact with various types of cells (10), has an important impact on tumor progression and drug resistance (11).
Macrophages are an important component of both the immune system and TME (12), and their infiltration into the tumor is associated with poor prognoses in most solid tumors (13-18). A number of studies have reported their double-edged sword effects on melanoma (19-21). As innate immune cells, macrophages can kill tumor cells via different extracellular mechanisms through phagocytosis, antigen presentation and T cell regulation, resulting in early tumor cell elimination (22,23). Along with tumor progression, the M2-type polarization of macrophages is induced by various signaling factors from the tumor and other stromal cells to promote tumor progression and threaten the life of the patient (22,23). The present review discusses the tumor-suppressive roles, tumor-promoting roles and potential clinical applications of macrophages in melanoma.
2. Overview of macrophages in the TME
Inflammation is an outstanding hallmark of cancer and is important for promoting tumor progression (10). For a number of cancer types, inflammation is an enabling characteristic that precedes malignant transformation with a subsequent shift to immunosuppressive TMEs (24).
Macrophages have different origins. The monocyte-macrophage lineage derives from precursor cells in the bone marrow and is driven by granulocyte-macrophage colony-stimulating factor (GM-CSF) (25). Tissue-resident macrophages with the ability to self-maintain originate from the yolk sac or fetal liver precursors during fetal development and show specialized phenotypes depending on the specific organ (26,27). TAMs are considered to be derived from both circulating monocytes and tissue-resident populations (28,29).
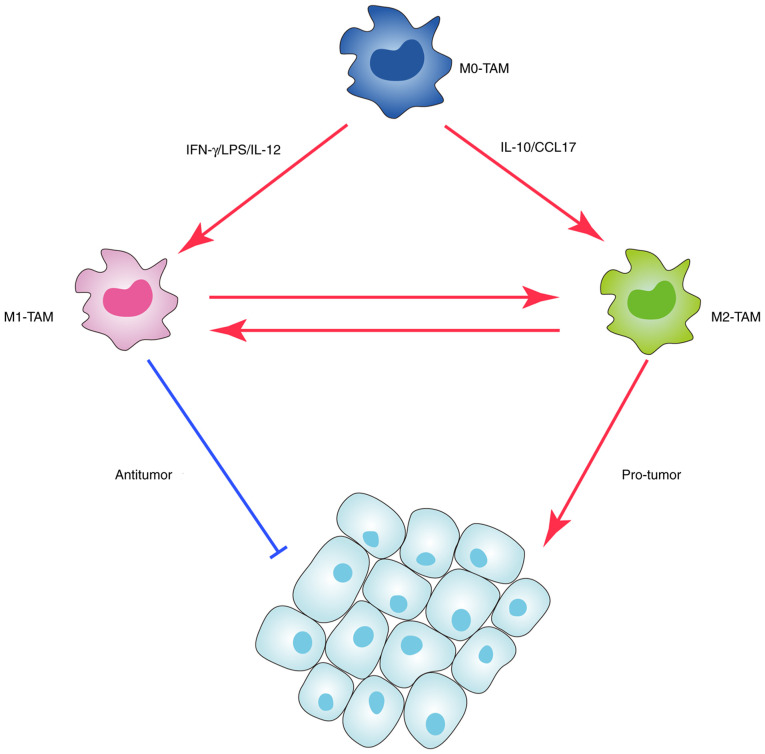
In regard to the phenotypic trait, macrophages can be classified into two categories: Classically activated (M1) and alternatively activated (M2) macrophages (Fig. 1). M1 macrophages can activate the adaptive immune system and are characterized by high expression levels of IL-12 and major histocompatibility complex class II, and low expression levels of IL-10 and arginase (30). M2 macrophages highly express the following: Arginase 1, a member of the arginase family; CD206, an important pattern recognition receptor and endocytic receptor in the innate immune system; IL-10, a well-recognized inflammatory and immunosuppressive factor; C-C motif chemokine ligand (CCL) 17; and CCL22, which can attract immune cells to specific locations (31). In the TME, M1 macrophages have antitumor abilities due to pro-inflammatory responses and the ability to produce pro-inflammatory factors such as IL-6, IL-12, C-X-C motif chemokine ligand 10 and tumor necrosis factor (TNF), whereas M2 macrophages have pro-tumor abilities (32,33). Among these, M2 macrophages are similar to TAMs in their phenotypic trait. Studies have demonstrated that the presence of TAMs is associated with poor survival in various tumor types (13,14,34). However, a number of studies have reported that various subtypes of macrophages exist, some of which spread along the spectrum of macrophage phenotypes and have distinct functions (35,36). This reflects the complexity of the TME. During the process of tumor development and aggravation, macrophages, as compartments of intratumor heterogeneity, also evolve under selective pressure, such as low pH, hypoxia, oxidative stress and nutritional deprivation (37).
Figure 1.
Classification of TAMs and their dual roles in tumors. TAM, tumor-associated macrophage; LPS, lipopolysaccharide; CCL17, C-C motif chemokine ligand 17.
Macrophages participate in tumor progression by interacting with both tumor and other stromal cells. Tumor cells and other malignant structures reverse the function of macrophages. It will become an adjunct to the tumor. Macrophages can promote tumor proliferation, angiogenesis, immune evasion, invasion and metastasis (38). Thus, an increasing number of studies have been performed to improve the treatment of patients with tumors by restoring tumor-killing abilities, reshaping the plasticity of TAMs from M2 into M1, or depleting M2 macrophages (39,40).
3. Double-edged sword effect of TAMs in melanoma
As previously mentioned, TAMs can be classified into two categories, M1 and M2, which have the opposite effect on tumor development. In this section, the tumor-promoting and tumor-suppressing effects of M2 and M1, respectively, are reviewed with a focus on their role in regulating the proliferation, invasion, metastasis, angiogenesis and chemical resistance of melanoma (Table I).
Table I.
Double-edged sword effect of TAMs in melanoma.
| First author/s, year | TAM Classification | Mechanisms | Effects | (Refs.) |
|---|---|---|---|---|
| Johansson et al, 2020 | M1 | Increased expression of Cx43 to induce M1 polarization | Inhibiting the invasion and migration | (44) |
| Kou et al, 2017 | M2 | TRIM59 loss in M2 macrophages | Promoting the invasion and migration | (45) |
| Tian et al, 2019 | M1 | IL-9-induced cytotoxicity of M1 macrophages | Decreasing metastatic ability | (46) |
| Shoshan et al, 2016 | M2 | NFAT1 binds to IL-2 and regulates its expression, thereby promoting T cell activation | Increasing metastatic ability | (50) |
| Liu et al, 2018 | M1 | Activation of NKT cells promotes the polarization of M1-TAMs | Inhibiting the growth of melanoma | (51) |
| Paul et al, 2019 | M2 | Exosomal miR-125b-5p combines with LIPA in macrophages to induce M2 polarization | Inhibiting the growth of melanoma | (52) |
| Yamada et al, 2016 | M1 | Unknown | Triggering the immune response and normalizing irregular tumor vascular network | (55) |
| Jarosz-Biej et al, 2018 | M2 | Melanoma exosomes enhance HIF-2α activity in M2-like TAMs | Promoting vasculature for better reconstruction | (56) |
| Ribas et al, 2016 | M2 | High IL-34 expression | Inducing melanoma resistance to PD-1 inhibitors | (66) |
| Han et al, 2018 | M2 | Exosomal PD-L1 induces M2 macrophage polarization | Results in anti-PD-1/PD-L1 therapy resistance | (69) |
| Liu et al, 2021 | M1 | Blocking the binding of Lgr4 and its ligands R-spondin 1-4 on TAMs to induce the polarization of M1 macrophages | Improving the efficacy of PD-1 immunotherapy | (58) |
| Heldin et al, 2012 | M1 | Blockade of TGF-βR to induce M1-TAMs | Increasing the efficacy of doxorubicin chemotherapy | (71) |
Cx43, connexin 43; HIF-2α, hypoxia-inducible factor 2α; Lgr4, leucine rich repeat containing G protein-coupled receptor 4; LIPA, lysosomal acid lipase; miR, microRNA; NFAT1, nuclear factor of activated T cell transcription factor 1; NKT cells, natural killer T cells; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; TAM, tumor-associated macrophage; TGF-βR, TGF-β receptor; TRIM59; tripartite motif-containing 59.
Regulating tumor proliferation, invasion and metastasis
M1 polarization of macrophages inhibits the proliferation of melanoma (41). By contrast, an increased number of M2 macrophages promotes melanoma growth (42). Furthermore, a study found that macrophages deficient in integrin β3 induced the polarization of M2 macrophages to promote melanoma growth (43). The results of the survival analysis of patients with melanoma treated with isolated hepatic perfusion also indicated that M1 macrophages, rather than M2 macrophages, were associated with longer overall survival, which is due to the inhibition of melanoma growth by M1 macrophages (44). In terms of invasion and migration of melanoma, Kou et al (45) reported that increased expression of Connexin 43, a vital gap junction protein in the TME, induced M1 polarization, thereby inhibiting the invasion and migration of melanoma cells in vitro. However, another study (46) demonstrated that M2 macrophages lacking tripartite motif 59 (TRIM59), which belongs to the TRIM family of proteins (47), promoted melanoma migration and invasion in a Transwell assay. Further research has demonstrated that M2 macrophages lacking TRIM59 promote the expression of MMP-9 and mucosal vascular addressin cell adhesion molecule 1, which are related to the invasion and migration of melanoma cells (46). In vivo models have been widely used to investigate the metastatic ability of melanoma. Park et al (48) established a xenogeneic model by planting melanoma cells overexpressing IL-9 in mice and found that the level of lung metastasis of melanoma was lower than that of the wild-type melanoma cells. M1 macrophages in the lungs and spleen were increased. Through in vitro experiments, this study also demonstrated that the IL-9-induced cytotoxicity in M1 macrophages was enhanced. However, to the best of our knowledge, the inhibitory effect of M1 macrophages on melanoma has not been directly confirmed in vivo, which is a limitation of current research. Eliminating M1 macrophages in mice or using immunodeficient mice may be helpful to further verify these results.
Furthermore, crosstalk between TAMs and other immune cells is also an important mechanism that affects tumor proliferation, invasion and metastasis (49). Nuclear factor of activated T cells (NFAT1) is a transcription factor that can bind to IL-2 and regulate its expression, thereby promoting T cell activation (50). Notably, NFAT1 has been demonstrated to increase the infiltration of M2-TAMs, thereby serving a critical role in enhancing TAM-mediated promotion of growth and metastasis in malignant melanoma (51). However, the activation of natural killer T cells promotes the polarization of M1-TAMs, inhibiting the growth of melanoma (52). Notably, the information exchange between melanoma cells and macrophages also serves an important role in the progression of melanoma (53). Gerloff et al (54) reported that melanoma delivered microRNA (miR)-125b-5p into macrophages through exosomes in vitro. Subsequently, miR-125b-5p is combined with lysosomal acid lipase A in macrophages, which in turn contributes to M2 macrophage polarization (54). Since exosomes can carry the genetic molecules of the source cell, they may also serve an important role in cancer suppression. However, this possibility requires further exploration.
Regulating angiogenesis in melanoma
The present review further explores the role of TAMs in angiogenesis in melanoma. Increased M2 polarization of TAMs has been found to stimulate tumor angiogenesis, leading to tumor progression (55). By contrast, M1-like TAMs trigger immune responses and normalize irregular tumor vascular networks, which sensitize cancer cells to chemotherapy and radiotherapy and further suppress tumor growth (56). This specific mechanism can be attributed to the induction of GM-CSF expression in endothelial cells by melanoma exosomes, thereby enhancing the activity of hypoxia-inducible factor-2α (HIF-2α) in M2-like TAMs. HIF-2α further attenuates VEGF activity by inducing the production of soluble VEGFR-1, promoting improved tissue and vasculature patency, which favors tumor growth (57). However, the results of the studies performed so far are controversial. Jarosz-Biej et al (56) analyzed the tissues of 43 patients with melanoma and found that a higher blood vessel density was positively associated with an increased number of M1-like TAMs.
Regulating the resistance to melanoma treatment
Recent research has also indicated that macrophages serve a role in melanoma resistance (58,59). Due to the different phenotypes of macrophages, these can promote resistance in melanoma on one hand and also improve the efficacy of drugs in the treatment of melanoma on the other hand (60). Durable responses in melanoma treatment have been achieved with immunotherapies that target immune checkpoint molecules, such as cytotoxic T-lymphocyte antigen 4 (CTLA4) (61-63) and programmed cell death protein 1 (PD-1) (64,65). However, 25% of patients with melanoma who have shown an objective response to PD-1 blockers also develop resistance (66). This finding has prompted scientists to explore the mechanism of melanoma resistance to PD-1 inhibitors (67,68). Melanoma resection specimens, which have been collected from patients with refractory metastatic melanoma who were treated with nivolumab, a PD-1 inhibitor for immunotherapy, exhibit high expression levels of IL-34. Importantly, high expression levels of IL-34 have been found to be positively associated with increased frequencies of M2-polarization TAMs (69). This finding suggests that M2-TAMs may be related to melanoma resistance to PD-1 inhibitors. In vitro experiments performed by Liu et al (58) further demonstrated that melanoma cell-derived exosomes carrying relatively large amounts of programmed death-ligand 1 (PD-L1) could induce M2 macrophages polarization, eventually resulting in anti-PD-1/PD-L1 therapy resistance. Furthermore, another study has demonstrated that blocking the binding of G protein-coupled receptor 4 on TAM to its ligand R-spondin 1-4 can reduce the polarization of M2 macrophages on the one hand, and promote the polarization of M1 macrophages on the other hand, further improving the efficacy of PD-1 immunotherapy in melanoma treatment (70). Notably, interactions among immune cells may also be involved in melanoma resistance. In particular, myeloid-derived suppressor cells interact with autoimmune macrophages and inhibit the cell surface expression of CD40 and the production of IL-27(19). Furthermore, low CD40/IL-27 signaling in tumors is associated with high TAM infiltration and immune checkpoint blockade (ICB) therapy resistance in both murine and human melanoma (19). In addition to ICB, macrophages have also been found to serve a role in the resistance of melanoma to chemotherapeutics. It has been reported that the combination of transforming growth factor-β (TGF-β) and TGF-β receptor (TGF-βR) contributes to the drug resistance and invasiveness of tumor cells and weakens the antitumor immune response (71). A study has demonstrated that the blockade of TGF-βR can trigger reprogramming into an antitumor M1-TAM phenotype, thereby increasing the efficacy of doxorubicin chemotherapy (72). Macrophages have also been demonstrated to secrete TNFα, inducing melanoma resistance to MAPK pathway inhibitors (59). The aforementioned studies indicate that macrophages may be involved in melanoma resistance to multiple drugs. Further research is required to explore the mechanism by which macrophages cause drug resistance.
4. TAM-targeting therapies in melanoma
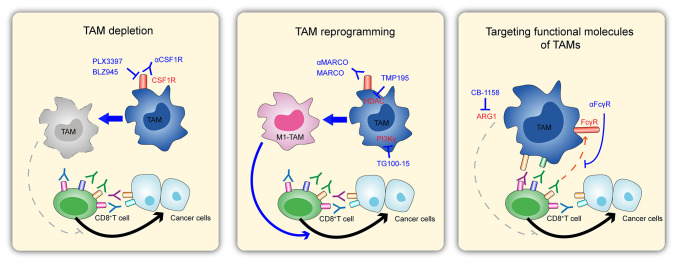
Targeting TAMs can improve antitumor immune responses (73). Given these profound effects exerted by macrophages on the progression of melanoma and several other tumors, targeting macrophages is considered a promising potential therapeutic strategy. Conventional therapies, including surgery, chemotherapy, radiotherapy and targeted therapy, in addition to reducing or reprogramming TAMs, are the two primary approaches to melanoma treatment (74). The current TAM-related approaches for melanoma treatment are described subsequently (Fig. 2).
Figure 2.
Potential strategies for treating melanoma by targeting macrophages. (Left) Depleting TAMs by regulating CSF1R receptors, thereby interfering with tumor killing by CD8+ T cells. (Middle) Enhanced tumor killing by CD8+ T cells by reprogramming TAMs to promote M1-type polarization of macrophages. (Right) Regulation of tumor killing by CD8+ T cells by targeting functional molecules of TAMs. This figure has been adapted from Fig. 1C of the article ‘Targeting Tumor-Associated Macrophages as a Potential Strategy to Enhance the Response to Immune Checkpoint Inhibitors’ (100). Front. Cell Dev. Biol., 04 April 2018 | https://doi.org/10.3389/fcell.2018.00 © 2018 Cassetta and Kitamura. ARG1, arginase 1; CSF1R, colony-stimulating factor 1 receptor; FcγR, Fc-γ receptor; HDAC, histone deacetylase; MARCO, macrophage receptor with collagenous structure; PLX3397, pexidartinib; TAM, tumor-associated macrophage.
Reducing the number of TAMs in melanoma: Deleting or inhibiting recruitment
Direct deletion of TAMs is an attractive option based on the idea that removing a tumor would improve the prognosis of a patient with melanoma. For instance, colony-stimulating factor 1 receptor (CSF1R) can control the differentiation, proliferation and survival of macrophages (75), and is present in the vast majority of macrophages. Targeting CSF1R seems to be an effective method for depleting TAMs in tumors, therefore, it has been studied in different tumors (74). In some tumor types, clinical trials have indicated that targeting CSF1R, or combining it with other therapies, can result in improved treatment outcomes (74). In addition, there is currently a clinical trial targeting the CSF1R axis in melanoma; this is, howwver, unable to provide definitive conclusions at this time (76).
Reducing the number of TAMs in the TME by inhibiting their recruitment is another approach to melanoma treatment (77). For example, the CCL2-C-C motif chemokine receptor 2 axis often recruits monocytes, causing TAM expansion, and inhibition of CCL2 can delay tumor progression in a number of experimental tumor models, including melanoma. However, the studies on this approach are insufficient, and more evidence is required.
Activating macrophages in melanoma
It has been confirmed that among the tumor cells, TAMs can have antitumor effects and suppress tumor growth by activating immune responses, although other TAMs promote tumors (78). This suggests that TAMs are flexible and reprogramming them to treat tumors would be a reasonable therapeutic approach. Several studies have focused on this topic (79,80). Evidence has demonstrated that melanoma cells can block macrophage activation by suppressing toll-like receptor (TLR) signaling (81). A clinical study has been performed to test the efficiency and safety of TLR7 ligands (852A) in the treatment of melanoma (82). Combining an agonist of TLR (3M-052 for TLR7/8), which polarizes macrophages towards a pro-inflammatory phenotype, with a checkpoint blockade is more efficient than a checkpoint blockade alone in the treatment of B16-F10 melanomas (82). Targeting the macrophage receptor with collagenous structure (MARCO) with anti-MARCO antibodies could also improve the efficiency of immunotherapy (anti-CTLA4) in a B16 melanoma mouse model (83).
Among various stimulating factors, GM-CSF is widely known to induce macrophages to become tumoricidal not only in melanoma but also in various other tumors, and has been approved for the treatment of unresectable stage IIIB-IVM1a melanoma under certain circumstances (in those who received treatment with GM-CSF as part of combination therapy or in an adjuvant setting) (84). For example, GM-CSF combined with ipilimumab resulted in longer overall survival and lower toxicity, but no difference in progression-free survival was observed (85). By using an indirect treatment comparison in melanoma, a systematic review has revealed that GM-CSF shows improved therapeutic effects compared with glycoprotein peptide vaccines and is at least as good as dacarbazine (86). However, the tumoricidal role of GM-CSF may also not be related to macrophages, because it is also involved in the development and maturation of dendritic cells (DCs) and in the activation and proliferation of T cells (87). The different dependencies of GM-CSF on macrophages, DCs and T cells still remain unclear. IFN-γ, monocyte chemoattractant protein-1, IL-1β and galectin-9 have also been reported as macrophage activators that inhibit tumor growth (88). However, there is still a lack of clinical trials to validate treatment options.
Other approaches: Adoptive macrophage therapy
Adoptive cellular therapy and chimeric antigen receptor (CAR) T cells have achieved marked success in the treatment of lymphoma and leukemia, among others (89,90). Therefore, the adoptive transfer of engineered active macrophages may also be a feasible approach for melanoma treatment. These macrophages may become cytotoxic to tumor cells after artificial administration of special drugs, cytokines and even gene editing (91,92). In 1974, Fidler (93) demonstrated that intravenous injection of specifically activated macrophages by supernatants from lymphocytes can decrease lung metastases of melanoma. Another study also demonstrated the efficiency of the adoptive transfer of activated macrophages (using GM-CSF or muramyl dipeptide) (94,95). However, this is far from any clinical application of adoptive macrophage therapy, as the mechanism of action of adoptive macrophage therapy is not fully understood. Notably, an increasing number of applications of CAR-macrophages in tumors have been reported. Zhang et al (96) developed induced pluripotent stem cells, which have been derived from engineered CAR-macrophages that can be used to kill cancer cells. Additionally, Chen et al (97) have reported that CAR-macrophages could be used as a novel immunotherapy candidate against solid tumors. Furthermore, Klichinsky et al (98) have demonstrated that CAR-macrophages could induce a pro-inflammatory TME and boost antitumor T cell activity in two solid tumor xenograft mouse models. However, the application of CAR-macrophages in melanoma has not yet been reported and could be a potential future research direction.
5. Conclusion and future perspectives
TAMs can be classified as M1 or M2 macrophages. M1 macrophages can activate the adaptive immune system, whereas M2 macrophages have pro-tumor abilities. The present review aims to explore the current knowledge on the role of TAMs in melanoma development through the regulation of proliferation, invasion, metastasis, angiogenesis and chemical resistance of melanoma. Macrophage function and polarization are regulated by multiple TME-based factors. The TAM-activating molecules listed in Table I are expected to be potential candidates for targeted intervention in melanoma progression. Interestingly, the crosstalk between TAMs and other immune cells is also an important mechanism that affects tumor proliferation, invasion and metastasis. Furthermore, the participation of exosomes in the polarization process of TAMs is expected to become a future research topic. Notably, macrophages can adopt different activation states, and the repolarization of TAMs into antitumor M1 macrophages is a promising therapeutic option.
The present review describes three macrophage-based melanoma treatment strategies: Depletion of TAMs in melanoma, activation of macrophages in melanoma and adoptive macrophage therapy. However, the mechanism of action of macrophages in melanoma is not yet fully understood. Notably, an increasing number of applications of CAR-macrophages have been reported in several tumors, including leukemia (96), ovarian cancer (98) and breast cancer (99), but not in melanoma. Therefore, this could be a potential future research direction. Further exploration of the role and mechanism of TAMs in the occurrence and development of melanoma may provide a basis for improved treatment of melanoma.
Acknowledgements
Not applicable.
Funding Statement
Funding: This study was funded by the Traditional Chinese Medicine Specialist Inheritance Studio (grant no. GZS2020022) and the Zhejiang Medical and Health Research Project (grant no. 2020KY447).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
QZ conceived, wrote and reviewed the manuscript. TF wrote the manuscript. SW, SC and HY participated in performing the literature review and drawing Figs. 1 and 2. MT and YC were involved in reviewing the manuscript, agreed to be accountable for all aspects of the work and provided final approval of the version to be submitted. YC acquired the funding. All authors have read and approved the final manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Tsao H, Atkins MB, Sober AJ. Management of cutaneous melanoma. N Engl J Med. 2004;351:998–1012. doi: 10.1056/NEJMra041245. [DOI] [PubMed] [Google Scholar]
- 2.Wang H, Yang L, Wang D, Zhang Q, Zhang L. Pro-tumor activities of macrophages in the progression of melanoma. Hum Vaccin Immunother. 2017;13:1556–1562. doi: 10.1080/21645515.2017.1312043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo J, Qin S, Liang J, Lin T, Si L, Chen X, Chi Z, Cui C, Du N, Fan Y, et al. Chinese guidelines on the diagnosis and treatment of melanoma (2015 edition) Ann Transl Med. 2015;3(322) doi: 10.3978/j.issn.2305-5839.2015.12.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henley SJ, Ward EM, Scott S, Ma J, Anderson RN, Firth AU, Thomas CC, Islami F, Weir HK, Lewis DR, et al. Annual report to the nation on the status of cancer, part I: National cancer statistics. Cancer. 2020;126:2225–2249. doi: 10.1002/cncr.32802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Islami F, Ward EM, Sung H, Cronin KA, Tangka FKL, Sherman RL, Zhao J, Anderson RN, Henley SJ, Yabroff KR, et al. Annual report to the nation on the status of cancer, part 1: National cancer statistics. J Natl Cancer Inst. 2021;113:1648–1669. doi: 10.1093/jnci/djab131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mandalà M, Voit C. Targeting BRAF in melanoma: Biological and clinical challenges. Crit Rev Oncol Hematol. 2013;87:239–255. doi: 10.1016/j.critrevonc.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Fu Y, Liu S, Zeng S, Shen H. From bench to bed: The tumor immune microenvironment and current immunotherapeutic strategies for hepatocellular carcinoma. J Exp Clin Cancer Res. 2019;38(396) doi: 10.1186/s13046-019-1396-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Effern M, Glodde N, Braun M, Liebing J, Boll HN, Yong M, Bawden E, Hinze D, van den Boorn-Konijnenberg D, Daoud M, et al. Adoptive T cell therapy targeting different gene products reveals diverse and context-dependent immune evasion in melanoma. Immunity. 2020;53:564–580.e9. doi: 10.1016/j.immuni.2020.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Marzagalli M, Ebelt ND, Manuel ER. Unraveling the crosstalk between melanoma and immune cells in the tumor microenvironment. Semin Cancer Biol. 2019;59:236–250. doi: 10.1016/j.semcancer.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Morad G, Helmink BA, Sharma P, Wargo JA. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell. 2021;184:5309–5337. doi: 10.1016/j.cell.2021.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kashfi K, Kannikal J, Nath N. Macrophage reprogramming and cancer therapeutics: Role of iNOS-derived NO. Cells. 2021;10(3194) doi: 10.3390/cells10113194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH, Wang XZ, Zhao YW, Wei YQ. Prognostic significance of tumor-associated macrophages in solid tumor: A meta-analysis of the literature. PLoS One. 2012;7(e50946) doi: 10.1371/journal.pone.0050946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, Nair VS, Xu Y, Khuong A, Hoang CD, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015;21:938–945. doi: 10.1038/nm.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo B, Cen H, Tan X, Ke Q. Meta-analysis of the prognostic and clinical value of tumor-associated macrophages in adult classical Hodgkin lymphoma. BMC Med. 2016;14(159) doi: 10.1186/s12916-016-0711-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mei J, Xiao Z, Guo C, Pu Q, Ma L, Liu C, Lin F, Liao H, You Z, Liu L. Prognostic impact of tumor-associated macrophage infiltration in non-small cell lung cancer: A systemic review and meta-analysis. Oncotarget. 2016;7:34217–34228. doi: 10.18632/oncotarget.9079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin S, Huang J, Li Z, Zhang J, Luo J, Lu C, Xu H, Xu H. The prognostic and clinicopathological significance of tumor-associated macrophages in patients with gastric cancer: A meta-analysis. PLoS One. 2017;12(e0170042) doi: 10.1371/journal.pone.0170042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao X, Qu J, Sun Y, Wang J, Liu X, Wang F, Zhang H, Wang W, Ma X, Gao X, Zhang S. Prognostic significance of tumor-associated macrophages in breast cancer: A meta-analysis of the literature. Oncotarget. 2017;8:30576–30586. doi: 10.18632/oncotarget.15736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valencia JC, Erwin-Cohen RA, Clavijo PE, Allen C, Sanford ME, Day CP, Hess MM, Johnson M, Yin J, Fenimore JM, et al. Myeloid-derived suppressive cell expansion promotes melanoma growth and autoimmunity by inhibiting CD40/IL27 regulation in macrophages. Cancer Res. 2021;81:5977–5990. doi: 10.1158/0008-5472.CAN-21-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harjes U. Educating macrophages in melanoma. Nat Rev Cancer. 2021;21(4) doi: 10.1038/s41568-020-00317-x. [DOI] [PubMed] [Google Scholar]
- 21.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–6173. doi: 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- 22.Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14:399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeNardo DG, Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol. 2019;19:369–382. doi: 10.1038/s41577-019-0127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Undi RB, Filiberti A, Ali N, Huycke MM. Cellular carcinogenesis: Role of polarized macrophages in cancer initiation. Cancers (Basel) 2022;14(2811) doi: 10.3390/cancers14112811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hong IS. Stimulatory versus suppressive effects of GM-CSF on tumor progression in multiple cancer types. Exp Mol Med. 2016;48(e242) doi: 10.1038/emm.2016.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mass E, Ballesteros I, Farlik M, Halbritter F, Günther P, Crozet L, Jacome-Galarza CE, Händler K, Klughammer J, Kobayashi Y, et al. Specification of tissue-resident macrophages during organogenesis. Science. 2016;353(aaf4238) doi: 10.1126/science.aaf4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ginhoux F, Guilliams M. Tissue-resident macrophage ontogeny and homeostasis. Immunity. 2016;44:439–449. doi: 10.1016/j.immuni.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 28.Bowman RL, Klemm F, Akkari L, Pyonteck SM, Sevenich L, Quail DF, Dhara S, Simpson K, Gardner EE, Iacobuzio-Donahue CA, et al. Macrophage ontogeny underlies differences in tumor-specific education in brain malignancies. Cell Rep. 2016;17:2445–2459. doi: 10.1016/j.celrep.2016.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Movahedi K, Laoui D, Gysemans C, Baeten M, Stangé G, Van den Bossche J, Mack M, Pipeleers D, In't Veld P, De Baetselier P, Van Ginderachter JA. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70:5728–5739. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- 30.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 31.Yunna C, Mengru H, Lei W, Weidong C. Macrophage M1/M2 polarization. Eur J Pharmacol. 2020;877(173090) doi: 10.1016/j.ejphar.2020.173090. [DOI] [PubMed] [Google Scholar]
- 32.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ivashkiv LB. Epigenetic regulation of macrophage polarization and function. Trends Immunol. 2013;34:216–223. doi: 10.1016/j.it.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Komohara Y, Jinushi M, Takeya M. Clinical significance of macrophage heterogeneity in human malignant tumors. Cancer Sci. 2014;105:1–8. doi: 10.1111/cas.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: Enabling diversity with identity. Nat Rev Immunol. 2011;11:750–761. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- 37.Vitale I, Shema E, Loi S, Galluzzi L. Intratumoral heterogeneity in cancer progression and response to immunotherapy. Nat Med. 2021;27:212–224. doi: 10.1038/s41591-021-01233-9. [DOI] [PubMed] [Google Scholar]
- 38.Pathria P, Louis TL, Varner JA. Targeting tumor-associated macrophages in cancer. Trends Immunol. 2019;40:310–327. doi: 10.1016/j.it.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 39.Xiao H, Guo Y, Li B, Li X, Wang Y, Han S, Cheng D, Shuai X. M2-like tumor-associated macrophage-targeted codelivery of STAT6 inhibitor and IKKβ siRNA induces M2-to-M1 repolarization for cancer immunotherapy with low immune side effects. ACS Cent Sci. 2020;6:1208–1222. doi: 10.1021/acscentsci.9b01235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jayasingam SD, Citartan M, Thang TH, Mat Zin AA, Ang KC, Ch'ng ES. Evaluating the polarization of tumor-associated macrophages Into M1 and M2 phenotypes in human cancer tissue: Technicalities and challenges in routine clinical practice. Front Oncol. 2019;9(1512) doi: 10.3389/fonc.2019.01512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo H, Zhou L, Guo J, Huang X, Gu J. Endostatin inhibits the proliferation and migration of B16 cells by inducing macrophage polarity to M1-type. Mol Med Rep. 2021;24(841) doi: 10.3892/mmr.2021.12481. [DOI] [PubMed] [Google Scholar]
- 42.Muniz-Bongers LR, McClain CB, Saxena M, Bongers G, Merad M, Bhardwaj N. MMP2 and TLRs modulate immune responses in the tumor microenvironment. JCI Insight. 2021;6(e144913) doi: 10.1172/jci.insight.144913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Su X, Esser AK, Amend SR, Xiang J, Xu Y, Ross MH, Fox GC, Kobayashi T, Steri V, Roomp K, et al. Antagonizing integrin β3 increases immunosuppression in cancer. Cancer Res. 2016;76:3484–3495. doi: 10.1158/0008-5472.CAN-15-2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johansson J, Siarov J, Kiffin R, Mölne J, Mattsson J, Naredi P, Olofsson Bagge R, Martner A, Lindnér P. Presence of tumor-infiltrating CD8+ T cells and macrophages correlates to longer overall survival in patients undergoing isolated hepatic perfusion for uveal melanoma liver metastasis. Oncoimmunology. 2020;9(1854519) doi: 10.1080/2162402X.2020.1854519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kou Y, Ji L, Wang H, Wang W, Zheng H, Zou J, Liu L, Qi X, Liu Z, Du B, Lu L. Connexin 43 upregulation by dioscin inhibits melanoma progression via suppressing malignancy and inducing M1 polarization. Int J Cancer. 2017;141:1690–1703. doi: 10.1002/ijc.30872. [DOI] [PubMed] [Google Scholar]
- 46.Tian Y, Guo Y, Zhu P, Zhang D, Liu S, Tang M, Wang Y, Jin Z, Li D, Yan D, et al. TRIM59 loss in M2 macrophages promotes melanoma migration and invasion by upregulating MMP-9 and Madcam1. Aging (Albany NY) 2019;11:8623–8641. doi: 10.18632/aging.102351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y, Yang WB. Down-regulation of tripartite motif protein 59 inhibits proliferation, migration and invasion in breast cancer cells. Biomed Pharmacother. 2017;89:462–467. doi: 10.1016/j.biopha.2017.02.039. [DOI] [PubMed] [Google Scholar]
- 48.Park SM, Do-Thi VA, Lee JO, Lee H, Kim YS. Interleukin-9 inhibits lung metastasis of melanoma through stimulating anti-tumor M1 macrophages. Mol Cells. 2020;43:479–490. doi: 10.14348/molcells.2020.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shu Y, Cheng P. Targeting tumor-associated macrophages for cancer immunotherapy. Biochim Biophys Acta Rev Cancer. 2020;1874(188434) doi: 10.1016/j.bbcan.2020.188434. [DOI] [PubMed] [Google Scholar]
- 50.Shoshan E, Braeuer RR, Kamiya T, Mobley AK, Huang L, Vasquez ME, Velazquez-Torres G, Chakravarti N, Ivan C, Prieto V, et al. NFAT1 directly regulates IL8 and MMP3 to promote melanoma tumor growth and metastasis. Cancer Res. 2016;76:3145–3155. doi: 10.1158/0008-5472.CAN-15-2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu H, Yang L, Qi M, Zhang J. NFAT1 enhances the effects of tumor-associated macrophages on promoting malignant melanoma growth and metastasis. Biosci Rep. 2018;38(BSR20181604) doi: 10.1042/BSR20181604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paul S, Chhatar S, Mishra A, Lal G. Natural killer T cell activation increases iNOS+CD206- M1 macrophage and controls the growth of solid tumor. J Immunother Cancer. 2019;7(208) doi: 10.1186/s40425-019-0697-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Di Martile M, Farini V, Consonni FM, Trisciuoglio D, Desideri M, Valentini E, D'Aguanno S, Tupone MG, Buglioni S, Ercolani C, et al. Melanoma-specific bcl-2 promotes a protumoral M2-like phenotype by tumor-associated macrophages. J Immunother Cancer. 2020;8(e000489) doi: 10.1136/jitc-2019-000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gerloff D, Lützkendorf J, Moritz RKC, Wersig T, Mäder K, Müller LP, Sunderkötter C. Melanoma-derived exosomal miR-125b-5p educates tumor associated macrophages (TAMs) by targeting lysosomal acid lipase A (LIPA) Cancers (Basel) 2020;12(464) doi: 10.3390/cancers12020464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamada K, Uchiyama A, Uehara A, Perera B, Ogino S, Yokoyama Y, Takeuchi Y, Udey MC, Ishikawa O, Motegi S. MFG-E8 drives melanoma growth by stimulating mesenchymal stromal cell-induced angiogenesis and M2 polarization of tumor-associated macrophages. Cancer Res. 2016;76:4283–4292. doi: 10.1158/0008-5472.CAN-15-2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jarosz-Biej M, Kamińska N, Matuszczak S, Cichoń T, Pamuła-Piłat J, Czapla J, Smolarczyk R, Skwarzyńska D, Kulik K, Szala S. M1-like macrophages change tumor blood vessels and microenvironment in murine melanoma. PLoS One. 2018;13(e0191012) doi: 10.1371/journal.pone.0191012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hood JL. Melanoma exosome induction of endothelial cell GM-CSF in pre-metastatic lymph nodes may result in different M1 and M2 macrophage mediated angiogenic processes. Med Hypotheses. 2016;94:118–122. doi: 10.1016/j.mehy.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu N, Zhang J, Yin M, Liu H, Zhang X, Li J, Yan B, Guo Y, Zhou J, Tao J, et al. Inhibition of xCT suppresses the efficacy of anti-PD-1/L1 melanoma treatment through exosomal PD-L1-induced macrophage M2 polarization. Mol Ther. 2021;29:2321–2334. doi: 10.1016/j.ymthe.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith MP, Sanchez-Laorden B, O'Brien K, Brunton H, Ferguson J, Young H, Dhomen N, Flaherty KT, Frederick DT, Cooper ZA, et al. The immune microenvironment confers resistance to MAPK pathway inhibitors through macrophage-derived TNFα. Cancer Discov. 2014;4:1214–1229. doi: 10.1158/2159-8290.CD-13-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ruffell B, Coussens LM. Macrophages and therapeutic resistance in cancer. Cancer Cell. 2015;27:462–472. doi: 10.1016/j.ccell.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, Patt D, Chen TT, Berman DM, Wolchok JD. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015;33:1889–1894. doi: 10.1200/JCO.2014.56.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Prieto PA, Yang JC, Sherry RM, Hughes MS, Kammula US, White DE, Levy CL, Rosenberg SA, Phan GQ. CTLA-4 blockade with ipilimumab: Long-term follow-up of 177 patients with metastatic melanoma. Clin Cancer Res. 2012;18:2039–2047. doi: 10.1158/1078-0432.CCR-11-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eroglu Z, Kim DW, Wang X, Camacho LH, Chmielowski B, Seja E, Villanueva A, Ruchalski K, Glaspy JA, Kim KB, et al. Long term survival with cytotoxic T lymphocyte-associated antigen 4 blockade using tremelimumab. Eur J Cancer. 2015;51:2689–2697. doi: 10.1016/j.ejca.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 66.Ribas A, Hamid O, Daud A, Hodi FS, Wolchok JD, Kefford R, Joshua AM, Patnaik A, Hwu WJ, Weber JS, et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA. 2016;315:1600–1609. doi: 10.1001/jama.2016.4059. [DOI] [PubMed] [Google Scholar]
- 67.Ribas A, Medina T, Kirkwood JM, Zakharia Y, Gonzalez R, Davar D, Chmielowski B, Campbell KM, Bao R, Kelley H, et al. Overcoming PD-1 blockade resistance with CpG-A toll-like receptor 9 agonist vidutolimod in patients with metastatic melanoma. Cancer Discov. 2021;11:2998–3007. doi: 10.1158/2159-8290.CD-21-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakamura T, Sato T, Endo R, Sasaki S, Takahashi N, Sato Y, Hyodo M, Hayakawa Y, Harashima H. STING agonist loaded lipid nanoparticles overcome anti-PD-1 resistance in melanoma lung metastasis via NK cell activation. J Immunother Cancer. 2021;9(e002852) doi: 10.1136/jitc-2021-002852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Han N, Baghdadi M, Ishikawa K, Endo H, Kobayashi T, Wada H, Imafuku K, Hata H, Seino KI. Enhanced IL-34 expression in nivolumab-resistant metastatic melanoma. Inflamm Regen. 2018;38(3) doi: 10.1186/s41232-018-0060-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tan B, Shi X, Zhang J, Qin J, Zhang N, Ren H, Qian M, Siwko S, Carmon K, Liu Q, et al. Inhibition of rspo-Lgr4 facilitates checkpoint blockade therapy by switching macrophage polarization. Cancer Res. 2018;78:4929–4942. doi: 10.1158/0008-5472.CAN-18-0152. [DOI] [PubMed] [Google Scholar]
- 71.Heldin CH, Vanlandewijck M, Moustakas A. Regulation of EMT by TGFβ in cancer. FEBS Lett. 2012;586:1959–1970. doi: 10.1016/j.febslet.2012.02.037. [DOI] [PubMed] [Google Scholar]
- 72.Mardomi A, Ghollasi M, Korani M, Panahi M, Parsa-Kondelaji M, Sabzichi M, Salimi A. Blockade of TGF-βR improves the efficacy of doxorubicin by modulating the tumor cell motility and affecting the immune cells in a melanoma model. Naunyn Schmiedebergs Arch Pharmacol. 2021;394:2309–2322. doi: 10.1007/s00210-021-02134-x. [DOI] [PubMed] [Google Scholar]
- 73.Anfray C, Ummarino A, Andón FT, Allavena P. Current strategies to target tumor-associated-macrophages to improve anti-tumor immune responses. Cells. 2019;9(46) doi: 10.3390/cells9010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cassetta L, Pollard JW. Targeting macrophages: Therapeutic approaches in cancer. Nat Rev Drug Discov. 2018;17:887–904. doi: 10.1038/nrd.2018.169. [DOI] [PubMed] [Google Scholar]
- 75.Stanley ER, Chitu V. CSF-1 receptor signaling in myeloid cells. Cold Spring Harb Perspect Biol. 2014;6(a021857) doi: 10.1101/cshperspect.a021857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weiss SA, Djureinovic D, Jessel S, Krykbaeva I, Zhang L, Jilaveanu L, Ralabate A, Johnson B, Levit NS, Anderson G, et al. A phase I study of APX005M and cabiralizumab with or without nivolumab in patients with melanoma, kidney cancer, or non-small cell lung cancer resistant to anti-PD-1/PD-L1. Clin Cancer Res. 2021;27:4757–4767. doi: 10.1158/1078-0432.CCR-21-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fang WB, Yao M, Brummer G, Acevedo D, Alhakamy N, Berkland C, Cheng N. Targeted gene silencing of CCL2 inhibits triple negative breast cancer progression by blocking cancer stem cell renewal and M2 macrophage recruitment. Oncotarget. 2016;7:49349–49367. doi: 10.18632/oncotarget.9885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kwan A, Winder N, Atkinson E, Al-Janabi H, Allen RJ, Hughes R, Moamin M, Louie R, Evans D, Hutchinson M, et al. Macrophages mediate the antitumor effects of the oncolytic virus HSV1716 in mammary tumors. Mol Cancer Ther. 2021;20:589–601. doi: 10.1158/1535-7163.MCT-20-0748. [DOI] [PubMed] [Google Scholar]
- 79.Dai X, Lu L, Deng S, Meng J, Wan C, Huang J, Sun Y, Hu Y, Wu B, Wu G, et al. USP7 targeting modulates anti-tumor immune response by reprogramming tumor-associated macrophages in lung cancer. Theranostics. 2020;10:9332–9347. doi: 10.7150/thno.47137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wan C, Sun Y, Tian Y, Lu L, Dai X, Meng J, Huang J, He Q, Wu B, Zhang Z, et al. Irradiated tumor cell-derived microparticles mediate tumor eradication via cell killing and immune reprogramming. Sci Adv. 2020;6(eaay9789) doi: 10.1126/sciadv.aay9789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thomas G, Micci L, Yang W, Katakowski J, Oderup C, Sundar P, Wang X, Geles KG, Potluri S, Salek-Ardakani S. Intra-tumoral activation of endosomal TLR pathways reveals a distinct role for TLR3 agonist dependent type-1 interferons in shaping the tumor immune microenvironment. Front Oncol. 2021;11(711673) doi: 10.3389/fonc.2021.711673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Singh M, Khong H, Dai Z, Huang XF, Wargo JA, Cooper ZA, Vasilakos JP, Hwu P, Overwijk WW. Effective innate and adaptive antimelanoma immunity through localized TLR7/8 activation. J Immunol. 2014;193:4722–4731. doi: 10.4049/jimmunol.1401160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Georgoudaki AM, Prokopec KE, Boura VF, Hellqvist E, Sohn S, Östling J, Dahan R, Harris RA, Rantalainen M, Klevebring D, et al. Reprogramming tumor-associated macrophages by antibody targeting inhibits cancer progression and metastasis. Cell Rep. 2016;15:2000–2011. doi: 10.1016/j.celrep.2016.04.084. [DOI] [PubMed] [Google Scholar]
- 84.Hoeller C, Michielin O, Ascierto PA, Szabo Z, Blank CU. Systematic review of the use of granulocyte-macrophage colony-stimulating factor in patients with advanced melanoma. Cancer Immunol Immunother. 2016;65:1015–1034. doi: 10.1007/s00262-016-1860-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hodi FS, Lee S, McDermott DF, Rao UN, Butterfield LH, Tarhini AA, Leming P, Puzanov I, Shin D, Kirkwood JM. Ipilimumab plus sargramostim vs ipilimumab alone for treatment of metastatic melanoma: A randomized clinical trial. JAMA. 2014;312:1744–1753. doi: 10.1001/jama.2014.13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Quinn C, Ma Q, Kudlac A, Palmer S, Barber B, Zhao Z. Relative efficacy of granulocyte-macrophage colony-stimulating factor, dacarbazine, and glycoprotein 100 in metastatic melanoma: An indirect treatment comparison. Adv Ther. 2017;34:495–512. doi: 10.1007/s12325-016-0464-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shi Y, Liu CH, Roberts AI, Das J, Xu G, Ren G, Zhang Y, Zhang L, Yuan ZR, Tan HS, et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: What we do and don't know. Cell Res. 2006;16:126–133. doi: 10.1038/sj.cr.7310017. [DOI] [PubMed] [Google Scholar]
- 88.Wang H, Zhang L, Yang L, Liu C, Zhang Q, Zhang L. Targeting macrophage anti-tumor activity to suppress melanoma progression. Oncotarget. 2017;8:18486–18496. doi: 10.18632/oncotarget.14474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Singh AK, McGuirk JP. CAR T cells: Continuation in a revolution of immunotherapy. Lancet Oncol. 2020;21:e168–e178. doi: 10.1016/S1470-2045(19)30823-X. [DOI] [PubMed] [Google Scholar]
- 90.Khalil DN, Smith EL, Brentjens RJ, Wolchok JD. The future of cancer treatment: Immunomodulation, CARs and combination immunotherapy. Nat Rev Clin Oncol. 2016;13:273–290. doi: 10.1038/nrclinonc.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sloas C, Gill S, Klichinsky M. Engineered CAR-macrophages as adoptive immunotherapies for solid tumors. Front Immunol. 2021;12(783305) doi: 10.3389/fimmu.2021.783305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu J, Gao W, Tang Q, Yu Y, You W, Wu Z, Fan Y, Zhang L, Wu C, Han G, et al. M2 macrophage-derived exosomes facilitate HCC metastasis by transferring αM β2 integrin to tumor cells. Hepatology. 2021;73:1365–1380. doi: 10.1002/hep.31432. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 93.Fidler IJ. Inhibition of pulmonary metastasis by intravenous injection of specifically activated macrophages. Cancer Res. 1974;34:1074–1078. [PubMed] [Google Scholar]
- 94.Chakraborty NG, Okino T, Stabach P, Padula SJ, Yamase H, Morse E, Sha'afi RI, Twardzik DR, Shultz LJ, Mukherji B. Adoptive transfer of activated human autologous macrophages results in regression of transplanted human melanoma cells in SCID mice. In Vivo. 1991;5:609–614. [PubMed] [Google Scholar]
- 95.Sone S, Fidler IJ. In vitro activation of tumoricidal properties in rat alveolar macrophages by synthetic muramyl dipeptide encapsulated in liposomes. Cell Immunol. 1981;57:42–50. doi: 10.1016/0008-8749(81)90118-0. [DOI] [PubMed] [Google Scholar]
- 96.Zhang L, Tian L, Dai X, Yu H, Wang J, Lei A, Zhu M, Xu J, Zhao W, Zhu Y, et al. Pluripotent stem cell-derived CAR-macrophage cells with antigen-dependent anti-cancer cell functions. J Hematol Oncol. 2020;13(153) doi: 10.1186/s13045-020-00983-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen Y, Yu Z, Tan X, Jiang H, Xu Z, Fang Y, Han D, Hong W, Wei W, Tu J. CAR-macrophage: A new immunotherapy candidate against solid tumors. Biomed Pharmacother. 2021;139(111605) doi: 10.1016/j.biopha.2021.111605. [DOI] [PubMed] [Google Scholar]
- 98.Klichinsky M, Ruella M, Shestova O, Lu XM, Best A, Zeeman M, Schmierer M, Gabrusiewicz K, Anderson NR, Petty NE, et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat Biotechnol. 2020;38:947–953. doi: 10.1038/s41587-020-0462-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang W, Liu L, Su H, Liu Q, Shen J, Dai H, Zheng W, Lu Y, Zhang W, Bei Y, Shen P. Chimeric antigen receptor macrophage therapy for breast tumours mediated by targeting the tumour extracellular matrix. Br J Cancer. 2019;121:837–845. doi: 10.1038/s41416-019-0578-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cassetta L, Kitamura T. Targeting tumor-associated macrophages as a potential strategy to enhance the response to immune checkpoint inhibitors. Front Cell Dev Biol. 2018;6(38) doi: 10.3389/fcell.2018.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.