Abstract
Disclaimer
This article is based on recommendations from the 12th WALT Congress, Nice, October 3-6, 2018, and a follow-up review of the existing data and the clinical observations of an international multidisciplinary panel of clinicians and researchers with expertise in the area of supportive care in cancer and/or PBM clinical application and dosimetry. This article is informational in nature. As with all clinical materials, this paper should be used with a clear understanding that continued research and practice could result in new insights and recommendations. The review reflects the collective opinion and, as such, does not necessarily represent the opinion of any individual author. In no event shall the authors be liable for any decision made or action taken in reliance on the proposed protocols.
Objective
This position paper reviews the potential prophylactic and therapeutic effects of photobiomodulation (PBM) on side effects of cancer therapy, including chemotherapy (CT), radiation therapy (RT), and hematopoietic stem cell transplantation (HSCT).
Background
There is a considerable body of evidence supporting the efficacy of PBM for preventing oral mucositis (OM) in patients undergoing RT for head and neck cancer (HNC), CT, or HSCT. This could enhance patients’ quality of life, adherence to the prescribed cancer therapy, and treatment outcomes while reducing the cost of cancer care.
Methods
A literature review on PBM effectiveness and dosimetry considerations for managing certain complications of cancer therapy were conducted. A systematic review was conducted when numerous randomized controlled trials were available. Results were presented and discussed at an international consensus meeting at the World Association of photobiomoduLation Therapy (WALT) meeting in 2018 that included world expert oncologists, radiation oncologists, oral oncologists, and oral medicine professionals, physicists, engineers, and oncology researchers. The potential mechanism of action of PBM and evidence of PBM efficacy through reported outcomes for individual indications were assessed.
Results
There is a large body of evidence demonstrating the efficacy of PBM for preventing OM in certain cancer patient populations, as recently outlined by the Multinational Association for Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO). Building on these, the WALT group outlines evidence and prescribed PBM treatment parameters for prophylactic and therapeutic use in supportive care for radiodermatitis, dysphagia, xerostomia, dysgeusia, trismus, mucosal and bone necrosis, lymphedema, hand-foot syndrome, alopecia, oral and dermatologic chronic graft-versus-host disease, voice/speech alterations, peripheral neuropathy, and late fibrosis amongst cancer survivors.
Conclusions
There is robust evidence for using PBM to prevent and treat a broad range of complications in cancer care. Specific clinical practice guidelines or evidence-based expert consensus recommendations are provided. These recommendations are aimed at improving the clinical utilization of PBM therapy in supportive cancer care and promoting research in this field. It is anticipated these guidelines will be revised periodically.
Keywords: photobiomodulation (PBM), cancer supportive care, guidelines, recommendations, mucositis, dermatitis, cancer-treatment side effects
1 Introduction
Despite the ongoing improvements in cancer therapy, it is still associated with severe life-impairing side effects. Both treatment- and patient-related risk factors determine the severity of the complications. Moreover, they negatively impact the patients’ quality of life (QoL) and daily activities. Therefore, effective supportive care strategies are necessary (1). The biological effects of photobiomodulation (PBM) therapy were discovered by Endre Mester in 1965 (2). Currently, PBM is defined as: “the use of non-ionizing optical radiation in the visible and near-infrared spectral range is absorbed by endogenous chromophores to elicit photophysical and photochemical events at various biological scales without eliciting thermal damage, leading to physiological changes and therapeutic benefits” (3). There is a considerable body of evidence supporting the efficacy of PBM for the prevention of oral mucositis (OM) in patients undergoing radiotherapy (RT) for head and neck cancer (HNC), chemotherapy (CT), or hematopoietic stem cell transplantation (HSCT) (4). Recent advances in understanding the mechanisms of action of PBM and dosimetry parameters of PBM have resulted in examining other oncology-related conditions that may lead to effective management of a broader range of complications associated with cancer treatment. This could improve overall QoL, adherence to cancer treatment regimens, and their outcomes while reducing cost of care (5).
2 Methods
This position paper was developed based on reviewing existing literature on PBM in supportive cancer care. For oral mucositis, a systematic review was feasible. For all other indications, expert consensus opinions are based on a narrative review of the literature and the personal experiences of contributing experts. A level of evidence was assigned to these indications based on the Somerfield criteria based on the type and design of the study (6). The level of evidence for each intervention is then translated into a guideline recommendation where Level I or II evidence is achieved by at least one well-designed randomized controlled trial (RCT). A suggestion was possible for lower-level evidence, but only when consistent evidence from multiple studies and panel consensus on the interpretation of this evidence. Thus, to enable maximal, practical clinical use and promote future research for PBM in supportive cancer care, WALT makes recommendations in two categories as clinical practice guidelines and consensus expert opinion. We strongly recommend clinical judgment of the provider and individual patient scenario be carefully éaccounted for in interpreting and applying these WALT PBM recommendations.
3 Results
The reader is referred to comprehensive reviews in these WALT series for a detailed discussion of PBM parameters. A brief review of critical PBM parameters is presented below.
3.1 PBM parameters
PBM parameters using low-level lasers or light-emitting diodes (LEDs) in cancer supportive care (summarized in Table 1 ) are usually within the red and near-infrared (NIR) wavelength range between 600 nanometers (nm) and around 1,000 nm, with a power density from 5 (mW)/cm2 to 150 mW/cm2 (7). The duration of application varies according to the site, but it may well be within 30 - 60 seconds per point. While shorter efficacious treatment times have been used (2-10sec per point, multiple spots that are clinically laborious), this could be attributed to cumulative PBM dose effects. The therapeutic dosage is depicted as the energy density measured in Joules as J/cm2 and varies between 0.1 to 12 J/cm2 as per current literature. Low-level laser systems used include helium-neon (HeNe), neodymium-doped yttrium aluminum garnet (Nd : YAG), gallium aluminum arsenide (GaAlAs) diode lasers, indium gallium aluminum phosphorus (InGaAlP), and non-thermal, non-ablative carbon dioxide (CO2) lasers (8). In recent years, LEDs with wavelengths in the red or NIR regions have become increasingly common due to their safety, low cost, and suitability for home use (8, 9).
Table 1.
WALT 2022 recommendations for PBM in the management of OM – updated MASCC/ISSO guidelines from Zadik et al., 2019 (4).
| Cancer Treatment Modality | Treatment Intent | PBM Treatment Parameters | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Device Parameters | Delivery parameters | |||||||||||
| Wavelength(nm) | Irradiance (Power Density) (mW/cm2) | Time per spot(sec) | Fluence (Energy Density) (J/cm2) | Photon Fluence (p.J/cm2) | Einstein(E) | Spot size(cm2) | Number of spots | Distance from the tissue | Frequency | Duration | ||
| HSCT | Prevention | 632.8 | 31.25 | 40 | 1.0 | 2 | 0.4 | 0.8 | 18 | < 1 cm | Daily | From day after cessation of conditioning for 5 days |
| 650 | 1000 * | 2 | 2.0 | 3.8 | 0.8 | 0.04 | 54-70 | In contact | Daily | From 1st day of conditioning till day + 2 post-HSCT (for 7-13 days) | ||
| RT | Prevention | 632.8 | 24 | 125 | 3.0 | 6 | 1.3 | 1 | 12 | < 1 cm | 5 days/wk | Entire RT course |
| RT-CT | Prevention | 660 | 417 * | 10 | 4.2 | 8.0 | 1.7 | 0.24 | 72 | In contact | 5 days/wk | Entire RT course |
| 660 | 625 * | 10 | 6.2 | 11.8 | 2.6 | 0.04 | 69 | In contact | 3 days/wk (alternate days) | Entire RT course | ||
*Potential thermal effect; The clinician is advised to pay attention to the combination of specific parameters.
CT, chemotherapy; HSCT, hematopoietic stem cell transplantation; IO, intra-oral; NR, not reported; PBM, photobiomodulation; RT, radiotherapy; wk, week;
1 Einstein = 4.5 p.J/cm2 which is the photonic fluence at 810 nm that is equivalent to the conventional fluence of 3 J/cm2 (62).
Photon Energy at 632 nm = 2 eV and 650 and 660 nm = 1.9 eV.
The biological effects of PBM on the exposed tissues depend upon a number of variables, including the location of the cells in the field of exposure, cell type, molecular and redox state of the cell, the tissue microenvironment, PBM parameters such as wavelength, power density, type of delivery as in pulsing or continuous, beam or spot size, and duration of exposure (10, 11). It is well known that PBM therapy exhibits a biphasic dose-response that warrants optimal tissue-specific irradiation dose parameters. In other words, doses lower than the optimal value may have a diminished effect, while doses higher than optimal can have no beneficial or even adverse therapeutic outcomes (12, 13). The effect of such a phenomenon has been consistently evident in published data on the effectiveness and particular disparity of PBM therapy in cancer complications.
Titrating adequate doses and defining the essential PBM parameters as per evidence gathered in a systematic way for each indication is a prerequisite for the successful use of this treatment modality. Without standardization in beam measurement, dose calculation, and the correct reporting of these parameters, studies will not be reproducible, and outcomes will not be consistent. A common misconception is that energy (in J) or energy density (J/cm2) is all that is necessary to replicate a successful treatment, irrespective of the original power, power density, and duration parameters (14, 15). In addition, it is not uncommon to find discrepancies between the specifications provided by a device manufacturer and the actual performance of the device (16). Therefore, routine device maintenance, including power measurements, should be carried out regularly in the context of research trials and clinical practice.
3.2 Safety considerations
During the course of more than two decades of PBM use in the management of OM in HNC patients, limited significant adverse effects have been reported. Only one study reported a burning sensation following PBM therapy in 50% of pediatric patients (9 out 18) (17). Given its diverse biological impact, consideration of PBM on tumor response to therapy and/or tumor behaviors remains a critical question that has yet to be definitively answered. Given tumor genomic heterogeneity, it seems likely that the effect of PBM on tumor behavior, like drugs or biologicals, is not uniform and might provide an explanation that addresses the contradictions of observations reported in the literature. Even tumors of similar histological characterization (i.e., squamous cell cancers of the mouth) vary, as is illustrated by 35% expressing dysregulations in the PI3K pathway, a common PBM target (18). It is important to note the limitations of in vitro studies in oncology versus the systems approach and clinical outcomes that are required. Clinical trials focus more on the effects of PBM on epithelial and connective tissue interactions, micro-environment, immune recognition, and on immune function. While cell culture studies may provide some insight into potential mechanisms, in vivo and human trial data are mandatory, and no firm conclusions can be drawn using tissue culture studies alone.
3.2.1 In vitro and in vivo safety data
It is unlikely that PBM has carcinogenic effects in normal cells. The non-ionizing wavelengths of the red and NIR spectrum used in PBM are far longer than the safety limit of 320 nm for DNA damage (19–21). No signs of malignant transformation in non-malignant epithelial cells and fibroblasts were observed following exposure to PBM with a wavelength of 660 nm, 350 mW for 15 minutes during three consecutive days (22). In addition, no malignant transformation of normal breast epithelial cells was detected in an in vitro study comparing the effects of different doses and wavelengths of PBM during multiple exposures (23).
Conflicting data refute or support the potential for PBM to impact tumor activity and responsiveness. As noted above, given the lack of uniformity, which characterizes tumor biology, it seems probable that tumors might vary widely in how they react to the range of biomodulatory activities that results from PBM exposure. The literature is rich with papers in this space. Many of the pathways associated with negative tumor behaviors are induced by PBM, including cell proliferation and anti-apoptosis. In fact, the effects of PBM on cell proliferation and differentiation have been investigated in cell culture systems in vitro using malignant cell lines, and have generated contradictory data across a range of different tumor cell lines and PBM parameters (24–29). For example, a study with laryngeal carcinoma cells demonstrated proliferation after 809 nm GaAIAs laser irradiation at energy densities between 1.96 and 7.84 J/cm2 (19). Another study also found increased cell proliferation of HEp2 carcinoma cells after PBM exposure at different wavelengths (685 nm and 830 nm) and doses (30). In a study comparing PBM administered to normal osteoblasts and osteosarcoma cells with a range of different wavelengths and doses, only 10 J/cm2 from an 830 nm laser was able to enhance osteoblast proliferation, whereas energy densities of 1 J/cm2, 5 J/cm2, and 10 J/cm2 from a 780 nm laser decreased proliferation. Osteosarcoma cells were unaffected by 830 nm laser irradiation, whereas 670 nm laser had a mild proliferative effect (31).
An in vitro study compared the effects of different doses of PBM at various wavelengths on human breast carcinoma and melanoma cell lines (23). Although certain doses of PBM increased breast carcinoma cell proliferation, multiple exposures had either no effect or showed negative dose-response relationships. PBM (660nm) administered in low doses (1 J/cm2) increased in vitro proliferation and potentially increased invasive potential of tongue squamous cell carcinoma cells (32). Similarly, another in vitro study suggested that PBM (660 nm or 780 nm, 40 mW, 2.05, 3.07 or 6.15 J/cm2) might stimulate oral dysplastic and oral cancer cells by modulating the Akt/mTOR/CyclinD1 signaling pathway to produce a more aggressive behavior (33). PBM exposure to three HNC cell lines was noted to result in the proliferation of cells in each tumor line, but not in a normal tissue control (34). A systematic review demonstrated that the effect of PBM on tumor cells depends highly on the PBM parameters used (35). While the limits of basing broad-reaching conclusions on in vitro assays have been noted, collectively, it would be irresponsible to ignore the possibility that PBM could, in some cases, negatively impact tumor behavior. Investigating and understanding how PBM may modify tumor behaviors, both positively and negatively, is a research priority.
Direct investigation of the radiation effects of PBM as it affects tumor response is limited, but as with other forms of cytotoxic cancer therapy, it is likely that PBM may have the ability to affect tumor response to radiation in ways, which are informed, not only by the dose, fractions, and timing of PBM or radiotherapy (RT) but by the tumor. While the data is sparse and limited to in vitro systems, there is evidence to suggest that, in some cases, PBM may act as a radiosensitizer (36). High fluences (120 J/cm2) have been noted to up-regulate the activity of survivin, a member of the inhibitor of apoptosis family (IAP), mediating self-protection during tumor cell apoptosis (37). An in vitro study observed a pro-apoptotic effect of PBM in oral squamous cell carcinoma (OSCC) cells in the absence of radiation and no anti-apoptotic effects occurred that might promote tumor cell resistance to cancer therapy (22). Increased apoptosis of human osteosarcoma cells was also induced by the administration of NIR (810 nm, continuous-wave, 20 mW/cm², 1.5 J/cm²) prior to NPe6-mediated photodynamic therapy as a result of increased cellular ATP and a higher uptake of the photosensitizer (38). On the potential enhancement of ionizing RT and CT, a study demonstrated that PBM applied shortly before RT increased the loco-regional blood flow that contributed to better local oxygenation (39). A study with an orthotopic mouse model of head and neck squamous cell carcinoma (HNSCC) demonstrated that PBM does not protect the tumor from the cytotoxic effects of RT (40).
In contrast, a decreased mitotic rate was found in gingival SCC after PBM at 805 nm and energy density of 4 J/cm2 and 20 J/cm2 (25), whereas no effect on cell proliferation or protein expression of osteosarcoma cells was found when PBM was administered with a wavelength of 830 nm (41). PBM (808 nm; 5.85 and 7.8 J/cm2) had an inhibitory effect on the proliferation of a human hepatoma cells line (42), and a study with that glioblastoma/astrocytoma cells demonstrated a slightly decreased mitotic rate after PBM at 805 nm and 5–20 J/cm2 (43). Similarly, 808 nm laser irradiation with an energy density of more than 5 J/cm2 inhibited cell proliferation of glioblastoma cells in vitro (44). Moreover, a study observed growth inhibition of cancer cell lines at relatively high cumulative PBM doses (45). This prompted another study to hypothesize that PBM may have a therapeutic potential in lung cancer (46). PBM administered at a dose of 150 J/cm2 appeared safe, with only minor effects on B16F10 melanoma cell proliferation in vitro, and had no significant effect on tumor growth in vivo. Only a high-power density (2.5 W/cm2) combined with a very high dose of 1050 J/cm2 could induce melanoma tumor growth in vivo (47). Current reports from in vitro studies suggest that PBM may favor tumor progression for OSCC through the activation of the Akt/mTOR pathway (33), cellular proliferation (32) (34), and cellular migration (48), while there are other reports on reduction in tumor growth (48, 49). These controversial results, in addition, to being in-vitro evaluations, could be due to the differences in poorly documented parameters, and experimental model systems (e.g; cell confluency, media conditions, etc) (50). It is important to note that the results suggesting PBM tumor enhancement was not replicable in other studies.
PBM (660 nm, 30mW, 424 mW/cm2, 56.4 J/cm2, 133 sec, 4 J) applied to chemically-induced OSCC in hamster cheek pouch tissue increased the growth and severity of OSCC (51). However, different results were demonstrated in a mouse model, which was used to study the effects of PBM on multiple UV-induced skin tumors. The experimental mice received full-body PBM for 37 days (670 nm, twice a day, 5 J/cm2), whereas control animals received sham PBM. There was no enhanced tumor growth. Moreover, there was even a small but significant reduction in tumor area in the PBM group, which could be explained by a local photodynamic effect or PBM-induced antitumor immune activity (52). Similar results were reported in a rat study showing that small tumors exposed to PBM receded and completely disappeared (53). It was hypothesized that upregulation of ATP signaling by PBM promoted apoptosis and differentiation of tumor cells, thereby slowing tumor proliferation (54, 55). In an animal model of leukemia, all the animals developed chemotherapy-induced alopecia (CIA). In order to stimulate hair regrowth, rats in the experimental group received PBM, whereas control animals received sham treatment. There was no significant difference in leukemia development between the two groups, as 22% of the PBM-treated animals and 20% of the control animals remained leukemia-free. As such, PBM therapy did not negatively influence the efficacy of CT (56). A study with xenograft melanoma and OSCC mouse models demonstrated that PBM reduced tumor growth and invasiveness. This study suggests that PBM can indirectly attack tumor tissue by stimulating anti-tumor immunity and normalizing tumor vessels after PBM (49). Several clinical studies on the impact of PBM treatments on tumor burden are evidenced by their use for managing oral mucositis (57–59). These reports clearly demonstrate the reduced tumor incidences, both recurrences at the primary site and secondaries, in these patients attributed to several factors such as the ability to complete prescribed oncotherapy regimens, improved host health and resilience, and thus improved anti-tumor responses. While direct PBM contributions to these anti-tumor responses are plausible, it remains to be thoroughly investigated (60, 61).
The results from these studies suggest that different tumor cells may have distinct responses to specific PBM parameters and doses. In part, these differences may also be explained by variations in the cellular microenvironment since these have been shown to affect cellular signal transduction pathways to PBM exposure. The microenvironment of tumor cells varies among in vitro studies and differs significantly from that found in animal models. Moreover, it is clear that additional studies using in vivo systems and different tumor lines are needed to better understand the differences in tumor response to PBM and how pre-treatment molecular and genomic characterization of malignancies can be used to determine the appropriate fit for PBM like other aspects of precision medicine. Based on this current evidence, the WALT expert panel concludes that PBM is safe for use in cancer-bearing patients, but does not recommend direct treatments over the tumor site. The ‘one-size-fits-all’ protocols or ‘point-and-shoot’ approach should no longer be acceptable for PBM treatments for its broad range of clinical applications. Rationalized dosimetry, including individual wavelength photon energy (photonic fluence), appropriate delivery technique, and clinical judgment of targeted biological responses are essential for optimal clinical therapeutic outcomes (62, 63).
3.2.2 Human clinical safety data
A clinical study reported no differences in cancer recurrence rates for patients receiving PBM for lymphedema following breast cancer treatment compared to controls (64). A recent RCT in which PBM was administered for prevention of OM during CRT in HNC patients (diagnosed with SCC of the nasopharynx, oropharynx, and hypopharynx) reported that at a median follow-up of 18 months (range 10-48 months), patients treated with PBM had better locoregional disease control and improved progression-free or overall survival (65). A recent retrospective analysis on 152 advanced OSCC patients examined the outcome of cancer therapy and the incidence of tumor recurrence after PBM (660 nm, 40 mW, 10 J/cm2) for the prevention of OM. Results showed that prophylactic PBM did not influence treatment outcome of primary cancer, recurrence, the development of new primary tumors, or survival in advanced OSCC patients (66), bearing in mind the individual tumor response in the era of precision oncology.
PBM in the red or NIR spectrum may be safe and effective in managing several complications of cancer therapy and hence should be considered for cancer patients (67). Nevertheless, as robust evidence for the lack of malignant cell protection or enhancement of tumor growth has not been published, and vigilance remains warranted. Given the lack of definitive data with respect to long-term survival and in recognition of the complexities, which govern tumor responsiveness, it is incumbent on the clinician to fully inform patients of the potential benefits and risks associated with PBM. Given its tremendous potential in the oncology population, aggressive pre-clinical and clinical investigations are critical to fully understand those parameters, which define tumor effects and patients’ response or non-responsiveness to PBM benefits.
3.3 Clinical indications
Virtually all conditions modulated by PBM (e.g., inflammation, ulceration, edema, pain, fibrosis, neurological and muscular injury) are thought to be involved in the pathogenesis of RT, HSCT, CT, or CRT-induced complications in patients treated for cancer.
3.3.1 Acute oral mucositis
Oral mucositis (OM) is defined as an injury to the mucous lining of the oral cavity due to chemical irritations, CT, or RT. The incidence of OM is 59-100% in patients with oral or oropharyngeal cancer undergoing RT to the head and neck, and approximately 80% of the patients receiving myeloablative HSCT and high dose-conditioning regimen. OM is common in patients treated with CT for hematological cancer and occurs less frequently in patients receiving CT for solid cancers, affecting 15-80%. In recent years, mucosal injury has become prevalent in patients treated with certain types of targeted therapy and immunotherapy. Considering that the pathogenesis and course of OM depend on the clinical circumstances, the management of OM is discussed separately for each cancer type and treatment that causes it (68).
The underlying pathophysiology of OM is related to multiple factors. It consists of simultaneous, interrelated events that develop in a progressive mode in multiple tissue regions, including the epithelium and the connective tissue. As such, a five-phase model of OM was developed based on extensive research (69, 70). The key players in the development of OM are the excessive reactive oxygen species (ROS) production (71) and the activation of nuclear factor kappa B (NFκB). Studies also demonstrate that microvascular damage, the production of pro-inflammatory cytokines, host-microbiome interactions, and extracellular matrix (ECM) alterations are implicated in the pathogenesis of OM (72). Recent advances in understanding the pathogenesis of OM highlight emerging mediators of toxicity and potential insights from technological advances in mucositis research (68). This review noted that the precise etiopathology of OM induced by targeted therapy or immunotherapy requires further investigations.
Clinically OM is characterized by erythematous mucosal changes, which can develop into oral ulcerations (4, 5, 69, 73). It can significantly impair the patients’ QoL and functional status and interfere with the cancer treatment regimen. Furthermore, OM may increase the risk for bacteremia and septicemia in immunosuppressed patients and is associated with increased mortality at day 100 post-HSCT (74). Interestingly, acute OM may be associated with an increased risk of chronic mucositis in HNC patients (75, 76). Up to now, effective management strategies for OM are still scarce (77), and pain control is often insufficient (73). However, several interventions reached the level of evidence that allowed the Mucositis Study Group (MSG) of the Multinational Association for Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO) to recommend Clinical Practice Guidelines or suggest their use in specific patient populations (HNC, hematological cancer, solid cancers), cancer therapy modality (RT for HNC, HSCT, CT, combination of RT-CT), and for a specific purpose (prevention/treatment) (78). The reader is advised to read all the MASCC/ISOO guidelines, the methods used to develop them, and the general concepts of application.
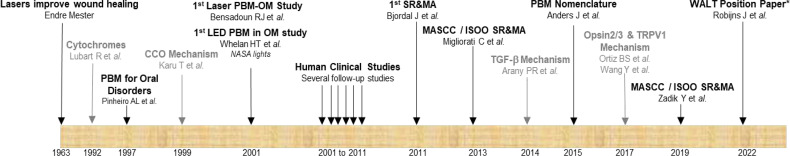
PBM is one such intervention, and this section will only describe the core of the MASCC/ISOO guidelines regarding PBM (79). Since the discovery of the wound healing effects of PBM in 1963 by Dr. Endre Mester, much research has been done regarding PBM in general. In supportive cancer care, the main focus has been OM prevention and management. Figure 1 shows a brief timeline of the advances in the field of PBM as relevant to OM. The first clinical study on OM and PBM was conducted in 2001, which was followed by many follow-up clinical trials that have enabled a significant body of literature. This led to several systematic reviews and meta-analyses with MASCC/ISOO guidelines first published in 2013 that was recently updated in 2019. The MSG of MASCC/ISOO identified strong evidence in favor of PBM to prevent OM in three categories reflecting discrete oncotherapy scenarios ( Table 1 ). This strong evidence includes multiple RCTs that consistently showed significant positive results regarding OM prevention (4). For each category, recommendations were made in favor of effective protocols supported by evidence obtained in an RCT with no major study-design flaw and that was reproducible. Importantly, since there may be more than one effective protocol for each of these categories, they recommended that the specific physical PBM setting of the selected protocol be followed thoroughly for optimal results. In other words, PBM therapy outcomes will be unpredictable if the PBM settings and delivery approaches are randomly combined from various protocols. In the WALT guidelines below, we summarize our best understanding of these parameters to suggest a starting point for clinical treatments, irrespective of a specific device, and wavelength. It is well appreciated that research about new protocols, adjustments to the individual photometric features, pediatric-population specific protocols, and technical innovations of PBM devices may lead to further refinement and update of the current recommendations. There remains some uncertainty on the potential for malignant transformation when PBM is applied directly to the tumor site, which should be avoided (4). Informed consent from the patients is essential, including information about the expected benefit and potential risks of PBM should be explicitly communicated.
Figure 1.
Timeline of advances in the application of PBM therapy for oral mucositis. The grey font represents basic science advances. *current WALT position paper. Abbreviations used SR&MA – Systematic review and meta-analysis; OM –Oral Mucositis; PBM - Photobiomodulation; MASCC – Multinational Association for Supportive Care in Cancer; ISOO – International Society for Oral oncology; WALT – World Association for Photobiomodulation Therapy; CCO – Cytochrome C Oxidase; TGF-β – Transforming Growth Factor beta 1; TRPV1 - Transient Receptor Potential-V1 .
Further, there have been several studies directly investigating the efficacy of PBM for OM in pediatric patients with cancer of different etiologies undergoing CT, mixed RT/CRT, or mixed HSCT/CT (17, 80–96). The study settings ranged from case series, cohort studies, and non-randomized trials to RCTs. The results are promising as demonstrated in the meta-analysis by Patel et al. in 2021 (97). They showed that PBM could significantly reduce the incidence of severe OM in pediatric patients based on 16 studies. However, due to significant differences in PBM protocols in these trials (as IN the MASCC/ISOO analysis), it is hard to make definite recommendations for pediatric patients at the moment (97–100). Nonetheless, these studies emphasize the non-invasive, patient-friendly, safe and well-tolerated therapy PBM represents. Hence, we include a PBM protocol for the prevention and treatment of OM.
3.3.1.1 WALT recommendation 2022: clinical practice guidelines
For Prevention of oral mucositis with an intra-oral device, WALT recommends a visible wavelength (630-680 nm) LED/Laser device with a power density (treatment surface irradiance) of 10-50 mW/cm2 for a total dose of 1.2 Einstein (photon fluence at 650 nm = 5.7 p.J/cm2) of per treatment field performed within 30 to 120 min prior to oncotherapy. Other wavelengths (400-1100 nm) may be used with suitable adjustment to dosing, but treatments must be monitored to ensure a non-thermal (< 45 °C) process.
For Treatments of oral mucositis with an intra-oral device, similar device parameters for a total dose of 2.5 Einstein (photon fluence at 650 nm = 11.4 p.J/cm2) should be used and repeated 3 - 4 times a week for at least 15-20 sessions or until healing after the end of oncotherapy.
For Prevention of oral mucositis with a transcutaneous device, WALT recommends a near-infrared wavelength (800-1100 nm) LED/Laser device with a power density (treatment surface irradiance) of 30-150 mW/cm2 for a total dose 1 Einstein (photon fluence at 810nm = 4.5 p.J/cm2) per treatment field performed within 30 to 120 min prior to oncotherapy.
For treatments of oral mucositis with a transcutaneous device, similar device parameters for a total dose of 6 1.3 Einstein (photon fluence at 810nm = 9 p.J/cm2) should be used and repeated 3 - 4 times a week for atleast 15-20 sessions or until healing after the end of oncotherapy. Other wavelengths (400-1100 nm) may be used with suitable adjustment to dosing, but treatments must be monitored to ensure they are non-thermal (< 45°C).
Consideration for Pediatric protocol: As there is inadequate data, we suggest the same protocols as in adults for both trans-cutaneous and intra-oral approaches (wavelengths, fluence per fraction, irradiance, number of treatments per week, total number of PBM treatments) be used for preventive and curative intent. We did note that the trans-cutaneous devices appear to be more clinically practical, have higher patient acceptance, and hence are easier to implement in children. Better-designed, multicenter clinical research studies using these recommendations as a starting point are essential to further optimize and validate PBM treatments for this specific application.
3.3.2 Xerostomia and hyposalivation
One of the common oral complications related to therapy for HNC is hyposalivation, and its accompanying symptom is termed xerostomia (i.e., subjective sensation of dry mouth). Saliva plays a key role in oral mucosal integrity, oral caloric intake, taste perception, and speech (101). A substantial decrease in salivary function reduces QoL and increases the burden of long-term dental care (102, 103). Dry mouth affects about 74% of patients immediately after RT and increases to 85% two years after conventional RT to the head and neck area (104, 105). While IMRT may preserve some of the major salivary glands, 68% of patients develop xerostomia two years post-RT (106). Hyposalivation and xerostomia occur following CT, radioactive iodine treatment, HSCT, targeted therapy, and immunotherapy. About 40% of patients experience dry mouth during allo-HSCT, and increasing to 79% of patients at about 7 years post allo-HSCT. It is suspected that much of the late dry mouth is due to chronic graft-versus-host disease (GVHD) (105). Moreover, it may occur secondary to medications used to support the cancer patient (e.g., opioid analgesics, centrally acting pain medications) and with dehydration. Saliva is an important factor in the maintenance of mucosal integrity, promotion of oral wound healing, taste perception, formation of food bolus, initiation of food ingestion, and swallowing and speech (101). Further, it has a function in maintaining the integrity of the oral structure, in lubrication and hydration, and in modifying the bacterial metabolism and adherence to the tooth surface. Hyposalivation can negatively impact the patients’ QoL and lead to an increased risk of dental caries and tooth loss (102, 103).
A systemic review followed the methods described above, and similar to the MASCC/ISOO approach, added the reproducibility variable as an exclusion criterion. The details of this systematic review can be found in Heiskanen, 2019 (79). Briefly, six controlled clinical trials on PBM and xerostomia/hyposalivation were identified, two reported on the same patient population and were considered as a single study for the purpose of our analysis ( Table 1 ). Four studies examined patients with HNC treated with RT or RT combined with CT, and one study was in patients undergoing HSCT. Considering that salivary gland damage caused by RT may be different from the damage caused by high-dose CT, each patient population is considered separately (107–112).
Regarding RT or RT-CT induced salivary gland damage, when categorizing the studies according to the aim of the PBM, there was one RCT for prevention (106, 107) and one for treatment of salivary hypofunction (109). In addition, for the prevention of salivary hypofunction in HNC patients, there was a comparative study (110), and for the treatment of salivary hypofunction in HNC patients, there was an additional before-and-after study (108). The protocols used in these studies varied regarding the laser type, the approach (extra- or intra-oral), number of sites applied, power, irradiance, time per point, fluence, and timing relative to the RT. All the studies had relatively low power, and none included placebo or were double-blind. The results for the prevention or treatment of RT/RT-CT associated salivary hypofunction were mixed with some studies demonstrating benefit in objective outcome measures (106–108) and subjective outcome measures (110), whereas other trials reported no effect for these measures (109) or for critical outcome measures (110). For patients undergoing HSCT, a single RCT assessed the effect of PBM on xerostomia (110). The primary endpoint of the study was the prevention of OM, but also data was collected for xerostomia. The study showed a significant improvement in the xerostomia score in the PBM group compared to the control group when the scores were accumulated until day 21 post-HSCT. This study did not assess objective outcome measures for salivary gland dysfunction (111).
In summary, the evidence about PBM for cancer therapy-associated salivary gland dysfunction is limited. Some evidence indicates that there is a promising potential for this therapy for the prevention or treatment of salivary gland dysfunction. More research is essential ( Table 2 ).
Table 2.
WALT 2022 recommendations for PBM in managing salivary gland dysfunction in cancer patients - updated from Heiskanen et al., 2020 (78).
| Ref. | Patient population | Treatment Intent | Study design | PBM Treatment Parameters | Efficacy | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Route of delivery | Wavelength (nm) | Laser type | Approach | Power(mW) | Irradiance(mW/cm2) | Time(sec) | Fluence (J/cm2) | Photon Fluence (p.J/cm2) | Einstein(E) | |||||
| Gonnelli 2016 (108) | HNC | P | RCT | Combo | 660 +780 + clinical care | InGalAlP | IO (660 nm) |
40 | Calculated:1000 | 10 | 10 | 19 | 4.2 | Yes, objective outcome measures |
| EO (780 nm) |
15 | Calculated:375 | 10 | 3.8 | 5.7 | 1.3 | ||||||||
| Palma 2017 (110) | HNC | T | Before-and-after | Combo | 808 | InGalAlP | IO | 30 | 750 | 10 | 7.5 | 11.2 | 2.5 | Yes, objective and subjective outcome measures |
| EO | ||||||||||||||
| Saleh 2014 (111) | HNC | T | RCT | Combo | 830 | GaAlAr | IO | 100 | 3570 | 20 | 71 | 106.5 | 23.7 | Not effective in both objective and subjective outcome measures |
| EO | ||||||||||||||
| Simoes 2010 (112) | HNC | P | Comparative | Intraoral | 660 | AlGaInP | IO | 40 | Calculated:1111 | 6 | 6 | 11.4 | 2.5 | Yes, subjective outcome measures |
| Cowen 1997 (71) | Hematologic cancer | P | RCT | Intraoral | 632.8 | HeNe | IO | 60 | Calculated:150 | 10 | 1.5 | 3 | 0.7 | Yes, subjective outcome measures |
intra-oral; EO, extra-oral; HNC head and neck cancer; HSCT, hematopoietic stem cell transplantation; P, prevention; Ref, reference; RCT, randomized controlled trial; T, therapy
1 Einstein = 4.5 p.J/cm2 which is the photonic fluence at 810 nm that is equivalent to the conventional fluence of 3 J/cm2 (62).
Photon Energy at 632 nm = 2 eV and 660 nm = 1.9 eV, 780 = eV, 808 = 1.5 eV, and 830 nm = 1.5eV.
3.3.2.1 WALT recommendation 2022: expert consensus opinion
There is inadequate data to provide clinical treatment guidelines. Hence, we provide the following technical recommendations to further clinical treatments and research studies for PBM therapy in managing oncotherapy-associated xerostomia and hyposalivation. WALT recommends treatments with a transcutaneous PBM device using a visible or near-infrared wavelength (400-1100 nm) LED/Laser device with a power density (treatment surface irradiance) of 10-150 mW/cm2 for a total dose 2 Einstein (photon fluence at 810 nm = 9 p.J/cm2) per treatment field performed. Treatments should be repeated 2 to 3 times a week for at least 3 to 4 weeks, or clinical benefit is evident. It is noted that this protocol may have to be repeated after 3 to 6 months for sustained benefits. Better-designed, multicenter clinical research studies using these recommendations as a starting point are essential to further optimize and validate PBM treatments for this specific application.
3.3.3 Acute dysphagia
Patients undergoing RT or CRT for HNC have an increased risk on developing dysphagia depending upon treatment volumes, which is characterized by pain and difficulty in swallowing. The prevalence of dysphagia in general oncologic patients is 15.4%. This disorder may involve changes in neuromusculoskeletal structure and function, anatomical alterations, changes in salivary flow and consistency, adverse effects during oncologic surgeries in the head and neck area, and/or odynophagia. These negatively impact the patients’ QoL and can lead to nutritional deficiencies, which can result in increased health care costs (111, 112). The pathophysiology is complex, and multiple mechanisms may overlap, which may change along the course of the disease and treatment. There is a strong relationship between OM, xerostomia, and dysphagia. Moreover, there seems to be a significant association between these oral problems and the patient’s Karnofsky level (113).
In a double-blind RCT with patients with hematologic malignancies submitted to high dose CRT followed by an autologous peripheral SCT or bone marrow transplant (BMT), patients were preventively treated with intraoral PBM for OM. A significant improvement of the ability to swallow was noted (12.8 ± 3.1 L+ vs. 19.8 ± 4.6 L-, p= 0,01), as well as improved saliva production (5.2 ± 1.3 L+ versus 16.2 ± 2.4, p= 0,005) in patients treated with PBM versus the control group (110). Two prospective RCTs with HNC patients treated with intraoral PBM have been published in which mucositis-related dysphagia was a secondary endpoint. A double-blind, phase III study did not demonstrate a reduction in the incidence of grade 3 pharyngeal dysphagia using low-dose PBM (0.1J/point) five times per week (70). However, the other RCT showed a significant reduction of dysphagia by using a combination of intraoral PBM five times per week before each RT session and total parenteral nutrition (TPN) (114). A prospective, randomized, double-blind, phase III trial with HNC patients evaluated the use of intraoral PBM for the prevention and treatment of OM. The study showed that gastrostomy need was significantly lower in PBM patients than in placebo patients (p= 0.01). The QoL of the PBM group was significantly better (64). Few PBM studies have reported dysphagia as an endpoint. As such, currently, it is difficult to provide suggestions on the use of PBM for the management of dysphagia. Therefore, it is recommended that future clinical trials need to include dysphagia as a primary endpoint.
3.3.3.1 WALT recommendation 2022: expert consensus opinion
There is inadequate data to provide clinical treatment guidelines. Hence, we provide the following technical recommendations to further clinical treatments and research studies for PBM therapy in managing oncotherapy-associated acute dysphagia. WALT recommends a near-infrared wavelength 810 nm LED/Laser device with a power density (treatment surface irradiance) of 30-150 mW/cm2 for a total dose 1 Einstein (photon fluence at 810 n = 4.5 p.J/cm2) per treatment field. Treatments may be repeated 2 -3 times a week, for at least 3 to 4 weeks or till improvements are evident. Better-designed, multicenter clinical research studies using these recommendations as a starting point are essential to further optimize and validate PBM treatments for this specific application.
3.3.4 Acute radiation dermatitis
Up to 95% of the patients undergoing RT develop acute radiodermatitis (ARD), which is an inflammatory skin reaction inflicted by cellular injury due to ionizing radiation. Skin reactions typically become visible two to three weeks after the first RT session and can be graded based on the criteria of the Radiation Therapy Oncology Group (RTOG), from erythema, and dry desquamation (grade 1), patchy moist desquamation (grade 2), confluent moist desquamation (grade 3), or in rare cases necrosis with hemorrhage and ulceration can occur (grade 4) (115, 116). The severity of ARD is dependent on several patient and treatment-related characteristics (117, 118).
The pathophysiology of ARD is complex, and the severity depends on the survival of the actively proliferating basal cells in the epidermis. In the first phase, an erythematous skin reaction develops caused by vascular damage leading to an increase in vascular permeability and vasodilation. This is followed by an inflammatory reaction characterized by transendothelial migration of circulatory immune cells to the irradiated skin driven by the production of cytokines and chemokines. The skin compensates by up-regulating the proliferation of the basal epidermal stem cells, leading to dry desquamation when the turnover of the new cells is faster than the shedding of the old ones. When the basal stem cells become depleted, moist desquamation arises (119). ARD is a distressing and painful side effect of RT, which can lead to problems in the patients’ daily life, negatively affecting their QoL. In cases of severe ARD, interruption of RT can be necessary, which can negatively affect the treatment outcome and overall patient survival (120). Guidelines for prevention and management of ARD put forth by MASCC recommend the implementation of daily hygiene practices and the application of potent topical steroids (120–123).
The use of PBM for the prevention and management of ARD was first reported in a case-control study. Three breast cancer patients with RT-induced skin ulcers were treated with PBM, which demonstrated improved wound healing (124, 125). A prospective intervention trial with a retrospective control group showed that PBM reduced the incidence of grade 2≥ ARD in breast cancer patients (126). In contrast, an RCT was not able to replicate these results (127). In a prospective, quasi-experimental intervention trial (DERMIS) with breast cancer patients undergoing RT, PBM was applied twice per week in a therapeutic manner. Results demonstrated that PBM prevented the aggravation of ARD and improved the patients’ QoL towards the end of RT (128). In a prospective intervention trial with breast cancer patients, they applied PBM from the start of RT twice per week and showed that PBM was associated with a reduced incidence of severe ARD (129). The TRANSDERMIS trial was a patient-blinded RCT investigating the use of PBM in the prevention of ARD in breast cancer patients. PBM was applied twice weekly during the full course of RT. The trial showed that PBM was able to prevent the development of grade 2≥ ARD both by subjective and objective outcome measures and, the QoL measures were significantly better in the PBM treated group (130, 131). A recent RCT demonstrated that PBM improved ARD in HNC patients (132). The DERMISHEAD study (ClinicalTrials.gov Identifier: NCT02738268) is an actively enrolling placebo-controlled RCT, investigating the efficacy of PBM in preventing ARD in HNC patients (133).
3.3.4.1 WALT recommendation 2022: expert consensus opinion
There is inadequate data to provide clinical treatment guidelines. Hence, we provide the following technical recommendations to further clinical treatments and research studies for PBM therapy in managing oncotherapy-associated radiation dermatitis. WALT recommends treatments with a transcutaneous PBM device using a visible or near-infrared wavelength (400-1100 nm) LED/Laser device with a power density (treatment surface irradiance) of 10-150 mW/cm2 for a total dose of 1 Einstein (photon fluence at 810 nm = 4.5 p.J/cm2) treatment field performed within 30 to 120 min prior to oncotherapy. Treatments should be repeated 3 - 4 times a week for at least 5 to 6 weeks, or clinical benefit is evident. When ARD is associated with inflammation of subcutaneous tissue, WALT recommends using a near-infrared (730 – 800 nm) transcutaneous LED/laser device with a dose 2 Einstein (photon fluence at 810 nm = 9 p.J/cm2) per treatment field 3-4 times a week, for at least 5 to 6 weeks. Better-designed, multicenter clinical research studies using these recommendations as a starting point are essential to further optimize and validate PBM treatments for this specific application.
3.3.5 Lymphedema
As a consequence of cancer treatment, Lymphedema is apparent in breast cancer and HNC patients HNC) (134, 135). About 20% of breast cancer patients can develop lymphedema in the upper extremity after cancer treatment (134). In the case of HNC, it is often an undervalued late effect, but it has been diminished with the introduction of Intensity-Modulated Radiation Therapy (IMRT). In HNC patients, lymphedema may develop externally, on the face and neck, and/or internally involving the larynx and pharynx. External lymphedema may negatively impact the patients’ body image, while internal lymphedema may disturb the normal breathing process, lead to dysphagia and trismus, and impact speech. The incidence of lymphedema in HNC patients is relatively high. For example, a single-center study on 81 HNC patients reported an incidence of 75%, with 10% external, 39% internal, and 51% experiencing both types of lymphedema. Pharyngeal carcinoma patients had the highest risk. Chronic lymphedema that develops later (2–6 months after) may resolve spontaneously in some patients, but not in all (135). The pathobiology of lymphedema consists of an initiation where disruption of lymphatic structures occurs by surgery or RT, leading to the accumulation of lymph fluid in the interstitial tissues. Inflammatory cells will infiltrate the affected tissue. This inflammatory reaction will be enhanced by the recruitment of additional immune cells from the circulation by cytokines and chemokines that remain in the tissue due to lymphatic dysfunction. This will ultimately lead to additional soft tissue damage and fibrosis, worsening the lymphatic function even more (134, 136).
The current treatment of lymphedema is based on symptom management and prevention of disease progression. As such, the main therapeutic option for lymphedema is complete decongestive therapy (CDT) (137–139). There was only one case-control study that showed a beneficial effect of PBM in the management of lymphedema in HNC patients (140). However, no prospective trial has been published to date, and further research is recommended. Also, in breast cancer patients, PBM has been investigated as a potential treatment for post-mastectomy lymphedema. The possible underlying mechanism of PBM for this indication is the stimulation of lymphangiogenesis, enhancement of lymphatic motility, and reduction of lymphatic fibrosis. A meta-analysis showed moderate evidence for the effectiveness of PBM in the reduction of arm swelling and pain in women with breast cancer-related lymphedema (98). Additionally, results demonstrated that the combination of PBM with CDT was more effective in reducing the arm volume than with CDT alone (141–144). An RCT with 40 breast cancer patients demonstrated a pain decrease of 50% and significantly higher grip strength after PBM (145). Another RCT evaluated the effectiveness of PBM as a complementary treatment to CDT for managing lymphedema in breast cancer patients 12 months post-intervention. Results demonstrated that the combination of PBM and CDT significantly decreased the number of lymphedema symptoms and relieved their impaired limb mobility symptoms. In addition, PBM reduced their emotional distress from lymphedema symptoms, such as sadness and self-perception (146).
3.3.5.1 WALT recommendation 2022: expert consensus opinion
There is inadequate data to provide clinical treatment guidelines. Hence, we provide the following technical recommendations to further clinical treatments and research studies for PBM therapy in managing oncotherapy-associated lymphedema. WALT recommends treatments with a transcutaneous PBM device using a visible or near-infrared wavelength (400-1100 nm) LED/Laser device with a power density (treatment surface irradiance) of 10-150 mW/cm2 for a total dose of 2 Einstein (photon fluence at 810 nm = 9 p.J/cm2) per treatment field. Treatments should be repeated 2 to 3 times a week for at least 3 to 4 weeks, or clinical benefit is evident. Better-designed, multicenter clinical research studies using these recommendations as a starting point are essential to further optimize and validate PBM treatments for this specific application.
3.3.6 Dysgeusia
Dysgeusia is a taste disorder characterized by a persistent gustatory sensation in the absence of taste stimulants or distorted gustatory perception. The complete loss of gustatory perception (ageusia) is rare, as most of the patients experience a reduction in the intensity of gustatory perception (hypogeusia). This disorder may be temporary or, in some cases, permanent (147, 148). Taste dysfunction in cancer patients can lead to decreased food intake and subsequent malnutrition and weight loss, resulting in a decreased QoL and increased healthcare costs (149, 150). In one report, the prevalence of dysgeusia in patients submitted to induction CT exclusively was 56.3%, to RT exclusively 66.5%, and to CRT 76%. About 15% of the patients continued to experience this side effect up to one-year post-treatment (151).
The pathogenesis of dysgeusia is complex and not well understood because there are multiple mechanical and chemical factors and clinical conditions (systemic factors and diseases; hyposalivation; oral, dental, and oropharyngeal pathologies) that can impact the gustatory perception (152). The mechanism by which CT and RT cause taste alterations is believed to be due to neurological damage, a decrease in the number of receptor cells, and/or an alteration of cell structure (151). Evaluating the severity of dysgeusia is complicated because of the heterogeneity of the methods, which can be qualitative, sip and spit tests, and/or patient surveys (150). No standard management approaches for dysgeusia have been established (151).
Other than a single case report with a positive outcome (153), there are no prospective clinical trials evaluating the use of PBM in the management of dysgeusia. Therefore, the potential utility of PBM in the management of dysgeusia in cancer patients currently remains limited.
3.3.6.1 WALT Recommendation 2022: Expert Consensus Opinion
There is inadequate data to provide clinical treatment guidelines. Hence, we provide the following technical recommendations to further clinical treatments and research studies for PBM therapy in managing oncotherapy-associated dysgeusia. WALT recommends treatments with an intraoral visible (red 630-680 nm) or transcutaneous near-infrared (800-1100 nm) wavelength LED/laser device with a power density (treatment surface irradiance) of 10-150 mW/cm2 for a total dose of 2 Einstein (photon fluence at 810 nm = 9 p.J/cm2) per treatment field performed. Treatments should be repeated 3 to 4 times a week for 3 to 4 weeks, or clinical benefit is evident.Better-designed, multicenter clinical research studies using these recommendations as a starting point are essential to further optimize and validate PBM treatments for this specific application.
3.3.7 Trismus
Trismus is a restriction in jaw movement, which may be due to tumor, local infection, tissue fibrosis, pain upon mouth opening or a tonic contraction of the muscles of mastication (131). It has been defined variously as a mouth opening of less than 40 or less than 20 mm, whereas less restrictive classifications also have been used (154). Trismus is caused by tumor invasion or RT of the masticatory muscles or the temporomandibular joint (154, 155). The weighted prevalence of trismus is estimated to be 25% following conventional RT, 5% following IMRT, and 31% for CRT (156). The risk of trismus increases when the cumulative radiation dose is higher than 60 Gy (157). However, the most important risk factor is the inclusion of the lateral pterygoid muscles in the high-dose RT field (158). Trismus typically develops 3-6 months post-RT and frequently becomes a lifelong problem (155, 159).
As demonstrated by several studies, fibrosis seems to be an important event in the development of RT-induced trismus. Other factors enhancing the development of trismus are post-surgical scar tissue, nerve damage, or a combination of these factors (155). Mandibular hypomobility eventually leads to muscle shortening and possibly temporomandibular joint dysfunction (156). The number of negative consequences of trismus and orofacial pain for the patients’ general health is high, including a reduction in nutritional intake, difficulty speaking, compromised oral health, and a poor QoL (160). Trismus can be prevented by avoiding RT to the masticatory structures. However, early interventions can also be used to minimize trismus (161–163).
There is a single case report study that investigated the use of PBM in the management of trismus two months post-RT. At the final PBM session, the patient’s mouth opening increased from 20 to 30 mm, and the pain decreased from a visual analog score (VAS) from 9 to 1. These positive results persisted up to one year after the final PBM session (164). The main rationale for a possible clinical benefit of PBM in the management of trismus is the potential of PBM to reduce fibrosis and promote muscle regeneration. Due to the limited amount of clinical data, no recommendations are possible on the use of PBM for the management of trismus.
3.3.7.1 WALT Recommendation 2022: Expert Consensus Opinion
There is inadequate data to provide clinical treatment guidelines. Hence, we provide the following technical recommendations to further clinical treatments and research studies for PBM therapy in managing oncotherapy-associated trismus. WALT recommends treatments with a transcutaneous near-infrared (800-1100 nm) wavelength LED/laser device with a power density (treatment surface irradiance) of 10-150 mW/cm2 for a total dose of 2 Einstein (photon fluence at 810 nm = 9 p.J/cm2) per treatment field performed. Treatments should be repeated 3 to 4 times a week for 4 to 6 weeks, or clinical benefit is evident.Better-designed, multicenter clinical research studies using these recommendations as a starting point are essential to further optimize and validate PBM treatments for this specific application.
3.3.8 Bone Necrosis
Bone necrosis can occur due to RT of the HNC region, which is specified as osteoradionecrosis (ORN), or due to specific medication, also termed medication-related osteonecrosis of the jaw (MRONJ) (165, 166) (167, 168). RT can cause vascular occlusion leading to the loss of osteocytes, which can result in bone necrosis (165, 166). Mandibular ORN prevalence is estimated between 5%-15%, but due to the improvements in the RT technique and the introduction of IMRT, less than 5% of patients develop ORN. Moreover, suitable pre-RT dental care can also help to prevent ORN (155, 169). To date, the underlying mechanism of ORN is still not completely clear. Most obvious, RT induces a fibro-atrophic process, which will consist of free radical formation, endothelial dysfunction, inflammation, microvascular thrombosis, fibrosis, and remodeling. This will eventually result in bone and tissue necrosis (170). Several factors can increase the risk of ORN, including inflammatory dental disease, soft tissue trauma, and dental surgical procedures to the bone in sites of high dose RT. An important risk factor of ORN is removing diseased teeth after RT. However, bone necrosis can also develop due to periodontal disease, trauma, or in a spontaneous manner (171, 172). Removing compromised teeth before RT and proper dental care during and following RT is essential to prevent ORN (165, 166).
Medication-related osteonecrosis of the jaw (MRONJ) can occur in cancer patients undergoing treatment with angiogenesis inhibitors (e.g., bevacizumab, sunitinib) for advanced HNC and in patients with bone metastases treated with bisphosphonates (167, 168). Bevacizumab is an antibody that blocks vascular endothelial growth factor (173), which can lead to tissue ischemia (174–176). In addition, it may inhibit proper wound healing and stimulate oral mucosal breakdown leading to exposure of the necrotic jawbone (177). Sunitinib is a TKI that can also cause MRONJ by blocking several pathways related to angiogenesis (178, 179). Bisphosphonates inhibit bone turnover by inducing osteoclastic apoptosis and inhibiting osteoblast-mediated osteoclastic activity (168). The incidence of MRONJ ranges from 0.8% to 12%. The American Association of Oral and Maxillofacial Surgeons (AAOMS) recommends good oral hygiene, the use of pharmacological therapy (e.g., antibiotics, pain medication), and, in case of persisting exposed bone, surgical removal (180).
In an in vivo study, PBM applied on rat bone before and during RT had a positive effect (181), and comparable results were described by another animal study (182). However, another in vivo study found that PBM was not able to repair the RT-induced bone damage (183). To our knowledge, no clinical studies on the effects of PBM for ORN have been conducted. Although, several studies demonstrated a beneficial effect of PBM in the management of MRONJ induced by bisphosphonates (184–190). A review concluded that the use of PBM might have a significant advantage above classical therapy as the overall complete response rate was 55% for the patients that underwent PBM, while this was only 30% for the patients receiving the classical care (191). A study in a rodent wound healing model found evidence that both laser and LED were capable of stimulating angiogenesis in vivo (192). Up to now, no clinical trials on the management of MRONJ induced by angiogenesis inhibitors have been found in the literature. Rapid repair of chronic soft tissue necrosis in previously radiation-treated HNC patients has been reported in 2-4 weeks of PBM in a small series of cases (193). This finding was confirmed by similar findings in another case report of another institution (194). This case report describes an additional case of a persisting radiation-associated mucosal necrotic lesion, treated at another institution with rapid and early improvement and complete resolution in 6 weeks, comparable to the three cases previously reported (195). These cases provide clinical evidence of rapid repair of radiation-induced chronic mucosal ulceration due to PBM.
3.3.8.1 WALT Recommendation 2022: Expert Consensus Opinion
There is inadequate data to provide clinical treatment guidelines. Hence, we provide the following technical recommendations to further clinical treatments and research studies for PBM therapy in managing oncotherapy-associated bone necrosis. WALT recommends treatments with an intraoral visible (red 630-680 nm) or transcutaneous near-infrared (800-1100 nm) wavelength LED/laser device with a power density (treatment surface irradiance) of 10-150 mW/cm2 for a total dose of 2 Einstein (photon fluence at 810 nm = 9 p.J/cm2) per treatment field performed. Treatments should be repeated 3 to 4 times a week for 4 to 6 weeks, or clinical benefit is evident. Better-designed, multicenter clinical research studies using these recommendations as a starting point are essential to further optimize and validate PBM treatments for this specific application.
3.3.9 Voice and Speech Alterations
The main communication tools of a person are his/her voice and speech, and they play an important role in a person’s identity and personality. The quality of the voice is determined by the movement and characteristics of the vocal cords, while the quality of speech is dependent on the resonance characteristics of the vocal tract. Any alteration to the muscle or tissue properties of the articulator structures can affect the coordinated volitional movements leading to speaking problems. Impairments of the voice and speech can significantly diminish the patient’s QoL. However, these complications do not receive much supportive care during cancer therapy. In addition, they are likely under-reported in efforts to preserve organ function after cancer therapy (196, 197).
The pathophysiology of voice and speech problems resembles that of dysphagia, which may include neuromuscular weakness due to tumor invasion. Mucositis of the soft palate and laryngeal soft tissues, fibrosis or vocal fold atrophy, edema and atrophy of laryngeal and pharyngeal tissues, and altered saliva or xerostomia can lead to CRT-induced voice and/or speech alterations (198, 199). Long-term functional impairment of the voice and/or speech may be prevented by new RT delivery techniques, including IMRT, which are designed to spare anatomical structures that are involved with voice and/or speech. Further, early speech rehabilitation may also help (200).
Currently, there are no exact figures of the prevalence of speech and voice dysfunction in advanced HNC patients treated with (C)RT. Therefore, prospective studies are needed that will include baseline measurements and standardized multidimensional assessment of functional aspects of voice and speech (196). To our knowledge, there are no studies on the effect of PBM on the quality of speech and voicing in HNC patients. Still, PBM may preserve the function of the anatomical structures involved directly and could have indirect benefits by decreasing hyposalivation.
3.3.9.1 WALT Recommendation 2022: Expert Consensus Opinion
There is inadequate data to provide clinical treatment guidelines. Hence, we provide the following technical recommendations to further clinical treatments and research studies for PBM therapy in managing oncotherapy-associated voice and speech alterations. WALT recommends treatments with a transcutaneous near-infrared (800-1100 nm) wavelength LED/laser device with a power density (treatment surface irradiance) of 10-150 mW/cm2 for a total dose of 2 Einstein (photon fluence at 810 nm = 9 p.J/cm2) treatment field performed. Treatments should be repeated 3 to 4 times a week for 2 to 4 weeks, or clinical benefit is evident. Better-designed, multicenter clinical research studies using these recommendations as a starting point are essential to further optimize and validate PBM treatments for this specific application.
3.3.10 Palmar-plantar erythrodysesthesia
Palmar-Plantar Eythrodysesthesia (PPE), also known as hand-foot syndrome, is a side effect of many classic CT agents and newer molecular targeted therapies (201, 202). PPE is characterized by redness, swelling, and pain on the palms of the hands and/or the soles of the feet, that when severe, can progress to frank blistering. It sometimes occurs elsewhere on the skin, such as the knees or elbows, but this is less common (201, 202). The most conventional cytotoxic drugs that can cause this syndrome include doxorubicin, cytarabine, docetaxel, capecitabine, or 5-fluorouracil. Also, multitargeted TKIs such as sorafenib, sunitinib, regorafenib, and others that target angiogenesis is associated with PPE. PPE is usually the worst during the first 6 weeks of treatment with targeted therapy, although with conventional CT, the condition may not present until 2-3 months after initiation of therapy (203–205).
The underlying mechanism is still not clear. There are several factors that could explain the development of PPE in hand palms and foot soles, such as the rapid cell division, gravitational forces, specific vascular anatomy, temperature gradients, as well as increased drug concentration in the eccrine sweat glands (203–205). Management of PPE consists of discontinuation of the drug and symptomatic treatment to provide relief, diminish edema, and prevent superinfection. Symptom management includes wound care, pain medication, and/or the application of topical alcohol-free (anti-inflammatory) emollients (206).
The evidence regarding the use of PBM for the management of PPE is limited. A single clinical trial with patients that presented PPE due to active CT or targeted therapy was conducted. Patients served as their own control, as one body site was treated with PBM while the other one was treated with sham laser. Preliminary results of 31 patients demonstrated no significant effect of PBM on the severity of PPE when both body sites were compared at the end of the therapy. However, patients’ pain decreased, and patient satisfaction was higher due to PBM (207).
3.3.10.1 WALT Recommendation 2022: Expert Consensus Opinion
There is inadequate data to provide clinical treatment guidelines. Hence, we provide the following technical recommendations to further clinical treatments and research studies for PBM therapy in managing oncotherapy-associated Palmar-Plantar Eythrodysesthesia. WALT recommends treatments with an intraoral visible (red 630-680 nm) or transcutaneous near-infrared (800-1100 nm) wavelength LED/Laser device with a power density (treatment surface irradiance) of 10-150 mW/cm2 for a total dose of 2 Einstein (photon fluence at 810 nm = 9 p.J/cm2) per treatment field performed. For early lesions, a LED/Laser device with a red wavelength (630-660 nm) in direct contact with the skin can also be used. Treatments should be repeated 2 to 3 times a week for 2 to 4 weeks, or clinical benefit is evident. Better-designed, multicenter clinical research studies using these recommendations as a starting point are essential to further optimize and validate PBM treatments for this specific application.
3.3.11 Graft versus host disease
One of the complications that occur following allogenic HSCT is graft-versus-host disease (GVHD). This side effect is accompanied by skin, digestive, and oral problems. The mechanism underlying GVHD is based on an immune reaction caused by the immune cells from the non-identical donor (the graft) that recognize the transplant recipient (the host) as foreign. When it occurs in the first 100 days after transplantation, it is defined as the acute form, while after this time period, it is called chronic GVHD, which can persist for months to years. Oral problems occur mostly during the chronic GVHD and are characterized by white and red changes on oral mucosa with or without ulceration and may be complicated by dry mouth. Moreover, oral lichen planus GVHD can occur as a single manifestation of GVHD, which often resembles classic oral lichen planus, an inflammatory condition that affects oral mucous membranes. Oral lichen planus is characterized by white, lacy patches; red, swollen tissues; or open ulcers. Clinical symptoms range from a painful, burning sensation, a diminished taste, a loss of appetite, and hyposalivation in some extreme cases (208, 209).
Acute GVHD may appear in 20%–40% of patients receiving an allogeneic HSCT from a sibling with an identical human leukocyte antigen (HLA) and in over 50% of patients receiving an allogeneic HSCT from an unrelated donor. The incidence of chronic GVHD ranges from 30% to 70% (210–212). Management of oral GVHD is based on good oral hygiene and/or systemic/local treatment with steroids or other immunomodulatory medicines. However, this seems to be insufficient in some cases (208, 209). PBM has been evaluated in the treatment of oral lichen planus with positive outcomes (213–220). Based upon this, PBM has been utilized for local treatment of oral GVHD in patients with continuing symptoms and signs despite systemic and topical therapies with corticosteroids and other immunosuppressants. Two reports of patients who were treated with PBM showed mucosal lichenoid change; particularly, ulceration and erythema were improved in 3-4 weeks of treatment and oral pain, and dry mouth improved in most patients (221, 222). These findings suggest that PBM may represent an additional approach for the management of oral GVHD and suggest that controlled studies should be conducted to confirm the efficacy of PBM therapy in oral GVHD and to determine optimal PBM therapy protocols.
3.3.11.1 WALT Recommendation 2022: Expert Consensus Opinion
There is inadequate data to provide clinical treatment guidelines. Hence, we provide the following technical recommendations to further clinical treatments and research studies for PBM therapy in managing oncotherapy-associated Graft-versus-Host Disease. WALT recommends treatments with a transcutaneous near-infrared (800-1100 nm) wavelength LED/laser device with a power density (treatment surface irradiance) of 10-150 mW/cm2 for a total dose of 2 Einstein (photon fluence at 810 nm = 9 p.J/cm2) per treatment field performed. For early lesions, a LED/Laser device with a red wavelength (630-660 nm) in direct contact with the skin has been noted to be beneficial as well. Treatments should be repeated 3 to 4 times a week for 4 to 6 weeks, or clinical benefit is evident.Better-designedd, multicenter clinical research studies using these recommendations as a starting point are essential to further optimize and validate PBM treatments for this specific application.
3.3.12 Peripheral neuropathy
Chemotherapy-induced peripheral neuropathy (CIPN) is a common side effect of CT, with an incidence of 68% in the first month after CT (223). The main neurotoxic CT agents are taxanes, platinum drugs, vinca alkaloids, thalidomide, and bortezomib. Symptoms related to CIPN are typically symmetric and bilateral and include paresthesia, numbness, burning pain, loss of temperature sensation, and loss of tendon reflexes typically appearing in distal extremities, indicating increased vulnerability of neurons with the longest axons (224, 225). Sensory neurons are particularly affected, while motor, autonomic, or CNS involvement is rare. This selective vulnerability likely relates to the permeability of the blood-nerve barrier at the level of the dorsal root ganglion (226). CIPN impairs patients’ daily activities because of comorbidities such as psychological distress, fall risk, and poor sleep quality resulting in a significant decrease in QoL (227). Furthermore, CIPN represents a heavy economic burden (228). The pathogenesis of CIPN can be explained by the fact that neurotoxic CT agents cause mitochondrial DNA damage, stabilize or destabilize microtubule formation, or have an anti-angiogenic effect. However, the exact pathophysiology of CIPN is still not clear (226).
Pharmacological symptom management (e.g., anti-depressants or pain medication) provides a limited benefit (229, 230). In severe cases, CT dose delays and/or reductions are necessary, which can affect treatment outcomes (224). There are no widely accepted evidence-based measures to prevent or minimize CIPN (231). Several animal studies have been performed to investigate the effectiveness of PBM in the management of CIPN (232–234). They demonstrated that PBM is able to reduce neuropathic pain, promote functional recovery of peripheral nerves, and stimulate axonal growth and regeneration. Moreover, an in vivo study showed a reduction of cold and mechanical allodynia after PBM (233). Furthermore, research with beneficial results has been reported for PBM in patients with diabetic neuropathy, demonstrating that the nerve conduction velocity significantly improved after PBM (230, 235–237).
Three clinical trials have been conducted investigating the effect of PBM on the management of CIPN. A single group prospective trial with breast cancer patients observed a decreased Brief Pain Index (BPI) after PBM (238). According to an RCT, PBM induced a significant reduction in the modified Total Neuropathy Score (mTNS) in patients with cancer from different etiologies (239). Another prospective, single-arm study with gastrointestinal cancer patients observed a significant reduction in neurotoxicity symptoms after PBM (240, 241).
3.3.12.1 WALT Recommendation 2022: Expert Consensus Opinion
There is inadequate data to provide clinical treatment guidelines. Hence, we provide the following technical recommendations to further clinical treatments and research studies for PBM therapy in managing oncotherapy-associated peripheral neuropathy. WALT recommends treatments with a transcutaneous near-infrared (800-1100 nm) wavelength LED/laser device with a power density (treatment surface irradiance) of 10-150 mW/cm2 for a total dose of 2 Einstein (photon fluence at 810 nm = 9 p.J/cm2) per treatment field performed. Treatments should be repeated 3 to 4 times a week for 4 to 6 weeks, or clinical benefit is evident. Better-designed, multicenter clinical research studies using these recommendations as a starting point are essential to further optimize and validate PBM treatments for this specific application.
3.3.13 Radiation-induced fibrosis
The soft tissue and lymphatic complications of HNC radiation are ubiquitous, as more than 50% of the patients develop severe fibrosis (242). Radiation-induced fibrosis (RIF) can adversely impact patients’ QoL. A particularly important manifestation of RIF includes impaired swallowing and aspiration due to mucosal and stromal fibrosis of the neck and pharyngeal musculature, laryngeal lymphedema, and stiffness of the tissue architecture that result in substantial physical symptom burden and functional loss (135). The extent of RIF depends on multiple factors, including the tumor site, the affected adjacent organs, and the dose prescribed (243).
The underlying mechanism of RIF in normal tissues has been extensively studied. Ionizing radiation generates ROS and nitrogen species (NO) that lead to localized inflammation, which in the acute setting causes irritation of the skin and the epithelial lining of adjacent organs (70). The acute inflammatory process evolves into a chronic inflammatory state and tissue remodeling several months after RT, which can progress over many years, causing lymphedema, fibrosis, pain, atrophy, and organ dysfunction (243, 244). Transforming growth factor-beta (TGF-β) serves as one of the primary mediators in this response along with a host of other cytokines and growth factors (243). The current treatment of RIF involves taking pentoxifylline/vitamin E primarily daily for several years with modest benefit. Despite elucidation of molecular mechanisms of RIF secondary to RT in animal models, to date, no effective treatment measures are available (245).
The use of PBM in the management of fibrosis has been investigated in studies in vitro (246–249). The available evidence demonstrates that PBM can regulate the main targets of the fibrosis pathway ranging from the reduction of the fibroblast proliferation and migration speed, inhibition of the production of TGF-β and the related pathway, to the downregulation of the production and deposition of collagen (247). There are currently no published clinical studies using PBM to address RIF (250). Currently, investigators at New York University are evaluating the feasibility, safety, and tolerability of providing PBM for the treatment of RIF in HNC patients treated with RT. Moreover, they are investigating the corresponding metabolic changes in the microenvironment.
3.3.13.1 WALT Recommendation 2022: Expert Consensus Opinion
There is inadequate data to provide clinical treatment guidelines. Hence, we provide the following technical recommendations to further clinical treatments and research studies for PBM therapy in managing radiation-induced fibrosis.
for prevention with a transcutaneous device, WALT recommends a near-infrared wavelength (800-1100 nm) LED/Laser device with a power density (treatment surface irradiance) of 10-150 mW/cm2 for a total dose of 2 Einstein (photon fluence at 810 nm = 9 p.J/cm2) per treatment field. Treatments should be performed 30 to 120 min before each treatment for the duration of the radiation treatment,
for treatments with a transcutaneous device, WALT recommends a near-infrared wavelength (800-1100 nm) LED/Laser device with a power density (treatment surface irradiance) of 10-150 mW/cm2 for a total dose of 2 Einstein (photon fluence at 810 nm = 9 p.J/cm2) per treatment field. Treatments should be repeated 3 to 4 times a week for 6 to 8 weeks, or clinical benefit is evident.
Better-designed, multicenter clinical research studies using these recommendations as a starting point are essential to further optimize and validate PBM treatments for this specific application.
3.3.14 Chemotherapy-induced alopecia
Chemotherapy-induced alopecia (CIA) is a common side effect of CT, affecting approximately 65% of patients undergoing CT, although this figure is highly dependent on the CT agent used (251). CIA is a direct effect of the cytotoxic CT on the rapidly dividing cells, including the hair matrix cells (251). This side effect is considered as one of the most distressing and traumatizing experiences for cancer patients, particularly in women (252, 253). During and even after CT, CIA will hinder patients’ QoL and their ability to cope effectively with their disease. Since hair is an important indicator of femininity, attractiveness, and personality, loss of hair could lead to body dissatisfaction and poor post-treatment adjustment (254). In addition, CIA can induce physical pain such as headaches and pain on the scalp (255). There are no approved pharmacologic therapies for CIA (251). The effect of topical or oral application of minoxidil has been investigated with limited success (256, 257). The only available preventive measure is based on scalp cooling (258). Nevertheless, this treatment has a highly variable success rate and brings along certain complications (e.g., feeling cold, dry skin, nausea, mild headaches) (258).
Concerning the use of PBM and hair loss, positive results were demonstrated in clinical trials applying PBM for the treatment of androgenetic alopecia and alopecia areata (259–261). An in vivo study investigated the effectiveness of PBM in CIA in rats. By 15 days post-CT, the PBM-treated rats showed hair regrowth while none of the sham-treated rats did. PBM probably stimulated the proliferation of hair-matrix keratinocyte stem cells or activated dermal papilla cells (56). No clinical trials on the use of PBM for CIA are available.
3.3.14.1 WALT Recommendation 2022: Expert Consensus Opinion
There is inadequate data to provide clinical treatment guidelines. Hence, we provide the following technical recommendations to further clinical treatments and research studies for PBM therapy in managing chemotherapy-induced alopecia. WALT recommends treatments with a transcutaneous visible to near-infrared (400-1100 nm) wavelength LED/laser device with a power density (treatment surface irradiance) of 10-150 mW/cm2 for a total dose of 2 Einstein (photon fluence at 810 nm = 9 p.J/cm2) per treatment field performed. The red (630-680 nm) wavelength has shown the most efficacy in several studies. Treatments should be repeated 3 to 4 times a week for 6 to 8 weeks, or clinical benefit is evident. Better-designed, multicenter clinical research studies using these recommendations as a starting point are essential to further optimize and validate PBM treatments for this specific application.
4 Conclusions and future perspectives
Cancer therapy is still associated with a wide range of acute and late complications that impair the patients’ QoL. Based on the evidence collected in this review, PBM has the potential to become a new preventive and/or therapeutic option for a broad range of acute and chronic side effects associated with cancer therapy. The effectiveness of PBM for the prevention and management of OM has already been demonstrated. As such, PBM has been taken up in the general treatment guidelines developed by the MASCC/ISOO, the European Society for Medical Oncology (ESMO), and the National Institute for Health and Care Excellence (NICE) (4, 262, 263).
The aim of this position paper is to provide scientific evidence for the use of PBM in various cancer therapy-related side effects, and a rigorous effort is made to indicate specific PBM parameters ( Table 3 ). Future investigations should be performed to better define optimal PBM parameters (irradiation and treatment) for each of the complications. Concerning the safety of PBM in oncologic patients, in vitro, in vivo, and even recent clinical data have been generated. Even though the clinical trial results did not demonstrate adverse events associated with PBM, it is still necessary to treat cancer patients with caution when applying PBM. More in vivo studies with animal tumor models need to be developed to elucidate the effect of PBM on the tumor and its microenvironment. Large clinical trials with a wide variety of cancer patients in which different PBM parameters are tested, and that include a long follow-up period of at least five years, are required to conclude that PBM does not negatively affect cancer progression and overall survival.
Table 3.
WALT 2022 recommendations for PBM treatments in prevention and/or management of cancer therapy-related complications.
| Complication | PBM Treatment Parameters | Level of Evidence*I to V | Level of Evidence Recommendation or Expert Opinion | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Device parameters | Delivery parameters | |||||||||||||
| Route of delivery | Beam Mode(Continuous and/or Pulsed) | Wavelength(nm) | Power(mW) | Irradiance (mW/cm²) | Time(sec) | Specified at 810 nm | Treatment area | Distance from tissue(Contact/non-contact) | Frequency(No. sessions/week andTotal sessions) | |||||
| Fluence(J/cm²)(Prophylactic or Curative intent) | Photon Fluence (p.J/cm2) | Einstein(E) | ||||||||||||
| Acute Radiodermatitis | External | CW &/P | 630-904 | 20-150 | 20-150 | TBD | 3 | 4.5 | 1 | TBD | TBD | Daily10->30 | II | Expert opinion |
| 6 | 9 | 2 | ||||||||||||
| Lymphedema | External | CW &/P | 750-904 | 20-150 | 20-150 (Red)20-80 (IR) | TBD | 2 | 3 | 0.7 | TBD | TBD | 3 times a week for 4 - 6 weeks | III | Expert Opinion |
| 6 | 9 | 2 | ||||||||||||
| Radiation Fibrosis | External & Internal | CW &/P | 750-850 | 20-150 | 20-150 (Red)20-80 (IR) | TBD | 2 | 3 | 0.7 | TBD | TBD | 3 times a week for 4 - 6 weeks | NA | Expert Opinion |
| 6 | 9 | 2 | ||||||||||||
| Palmar-Plantar Erythrodysesthesia | External | CW &/P | 630-680+750-850 | 20-150 | 20-150 (Red)20-80 (IR) | TBD | 2 | 3 | 0.7 | TBD | TBD | 3 times a week for 4 - 6 weeks | V | Expert Opinion |
| 6 | 9 | 2 | ||||||||||||
| Graft versus Host disease | External & Internal | CW &/P | 630-680+750-850 | 20-150 | 20-150 (Red)20-80 (IR) | TBD | 2 | 3 | 0.7 | TBD | TBD | 3 times a week for 4 - 6 weeks | IV | Expert Opinion |
| 6 | 9 | 2 | ||||||||||||
| Dysphagia | External & Internal | CW &/P | 630-680+750-850 | 20-150 | 20-150 (Red)20-80 (IR) | TBD | 2 | 3 | 0.7 | TBD | TBD | 3 times a week for 4 - 6 weeks | V | Expert Opinion |
| 6 | 9 | 2 | ||||||||||||
| Dysgeusia | External & Internal | CW &/P | 630-680+750-850 | 20-150 | 20-150 (Red)20-80 (IR) | TBD | 2 | 3 | 0.7 | TBD | TBD | 3 times a week for 4 - 6 weeks | V | Expert Opinion |
| 6 | 9 | 2 | ||||||||||||
| Trismus | External & Internal | CW &/P | 630-680+750-850 | 20-150 | 20-150 (Red)20-80 (IR) | TBD | 2 | 3 | 0.7 | TBD | TBD | 3 times a week for 4 - 6 weeks | V | Expert opinion |
| 6 | 9 | 2 | ||||||||||||
| Osteonecrosis and Mucosal necrosis | External & Internal | CW &/P | 630-680+750-850 | 20-150 | 20-150 (Red)20-80 (IR) | TBD | 2 | 3 | 0.7 | TBD | TBD | 3 times a week for 4 - 6 weeks | IV | Expert Opinion |
| 6 | 9 | 2 | ||||||||||||
| Voice and/or Speech alterations | External | CW &/P | 630-680+750-850 | 20-150 | 20-150 (Red)20-80 (IR) | TBD | 2 | 3 | 0.7 | TBD | TBD | 3 times a week for 4 - 6 weeks | NA | Expert Opinion |
| 6 | 9 | 2 | ||||||||||||
| Chemotherapy-Induced Peripheral Neuropathy | External | CW &/P | 780-970 | 80-120 | 20-150 (Red)20-80 (IR) | TBD | 7.5 | 11.2 | 2.5 | TBD | TBD | 3 times a week for 4 - 6 weeks | IV | Expert Opinion |
| 48 | 72 | 16 | ||||||||||||
| Chemotherapy-Induced Alopecia | External | CW &/P | 630-680+750-850 | 20-150 | 20-150 (Red)20-80 (IR) | TBD | 2 | 3 | 0.7 | TBD | TBD | 3 times a week for 4 - 6 weeks | NA | Expert Opinion |
| 6 | 9 | 2 | ||||||||||||
| Periodontal lesions after Chemotherapy and Radiotherapy | Internal | CW &/P | 630-660+810-830 | 20-150 | 20-150 (Red)20-80 (IR) | TBD | 2 | 3 | 0.7 | TBD | TBD | Daily10 to 30 | NA | Expert Opinion |
| 6 | 9 | 2 | ||||||||||||
NA, not applicable TBD: to be decided by the operator
These proposed protocols are based on expert opinion and do not exclude other protocols. *Level I: Evidence obtained from meta-analysis of multiple, well-designed, controlled studies; randomized trials with low false-positive and false-negative errors (high power); Level II: Evidence obtained from at least 1 well-designed experimental study; randomized trials with high false-positive and/or false-negative errors (low power); Level III: Evidence obtained from well-designed, quasi-experimental studies such as nonrandomized, controlled single-group, pre-test, and post-test comparison, cohort, time, or matched case-control series; Level IV: Evidence obtained from well-designed, non-experimental studies, such as comparative and correlational descriptive and case studies; Level V: Evidence obtained from case reports and clinical examples (48).
1 Einstein = 4.5 p.J/cm2 which is the photonic fluence at 810 nm that is equivalent to the conventional fluence of 3 J/cm2 (62).
Photon Energy at 632 nm = 2 eV, 660 nm = 1.9 eV, 680 nm = 1.8 eV, 750 nm = 1.6 eV, 780 = 1.5 eV, 808 = 1.5 eV, and 850 nm = 1.5eV, 904 nm = 1.4 eV.
An emerging approach in general medicine, which also seems to be important when applying PBM in cancer patients, is precision medicine. It is based on taking into account the patient’s personal lifestyle, environment, and variability in gene expression. Each cell type and especially tumor cells may have different responses to certain PBM parameters and doses, which are caused by variations in the cellular microenvironment. As such, more personalized PBM protocols for each indication will be necessary for the future. PBM in the daily clinical oncology practice setting may address a number of significant and common adverse effects (264). This may eventually reduce the incidence, duration, and severity of these devastating effects. Thereby, the patient will experience less pain and discomfort during and after their cancer therapy, which enables them to perform their daily activities during active cancer treatment and throughout survival. This will eventually result in improved patients’ QoL and optimization of their specific cancer treatment. Further, the treatment compliance of the patient will increase, resulting in an improved success rate of the cancer therapy. Thus, patient care will advance, which will ultimately result in increased patient survival.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Funding
MRH was supported by US NIH Grants R01AI050875 and R21AI121700.
Acknowledgments
The authors want to thank Laure Alston, James Carroll, Vedang Kothari, and Damien Vila for their contributions during WALT 2018 Congress.
Conflict of interest
MRH declares the following potential conflicts of interest. Scientific Advisory Boards: Transdermal Cap Inc, Cleveland, OH; BeWell Global Inc, Wan Chai, Hong Kong; Hologenix Inc. Santa Monica, CA; LumiThera Inc, Poulsbo, WA; Vielight, Toronto, Canada; Bright Photomedicine, Sao Paulo, Brazil; Quantum Dynamics LLC, Cambridge, MA; Global Photon Inc, Bee Cave, TX; Medical Coherence, Boston MA; NeuroThera, Newark DE; JOOVV Inc, Minneapolis-St. Paul MN; AIRx Medical, Pleasanton CA; FIR Industries, Inc. Ramsey, NJ; UVLRx Therapeutics, Oldsmar, FL; Ultralux UV Inc, Lansing MI; Illumiheal & Petthera, Shoreline, WA; MB Lasertherapy, Houston, TX; ARRC LED, San Clemente, CA; Varuna Biomedical Corp. Incline Village, NV; Niraxx Light Therapeutics, Inc, Boston, MA. Consulting; Lexington Int, Boca Raton, FL; USHIO Corp, Japan; Merck KGaA, Darmstadt, Germany; Philips Electronics Nederland B.V. Eindhoven, Netherlands; Johnson & Johnson Inc, Philadelphia, PA; Sanofi-Aventis Deutschland GmbH, Frankfurt am Main, Germany. Stockholdings: Global Photon Inc, Bee Cave, TX; Mitonix, Newark, DE, JR, JL and JM are part of the Limburg Clinical Research Center (LCRC) and were supported by the foundation Limburg Sterk Merk, the province of Limburg, the Flemish government, Hasselt University, Ziekenhuis Oost-Limburg, Jessa Hospital, Kom op Tegen Kanker, Limburgs Kankerfonds, Limburgse Kankersamenwerking, and ASA Srl. SE discloses conflicts of interest related to treatment for cGVHD consulting Falk Pharma GmbH. PRA has been supported by travel or serves as a consultant for Mureva, Vielight, Thor Photomedicine, Kerber Applied Research, Lumithera, Jooov, and NST consulting.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Contributor Information
Collaborators: “Cancer Supportive Care” WALT Working Group
References
- 1. Klastersky J, Fontaine C, Force BSCT. Supportive care in cancer patients: a constantly evolving field. Curr Opin Oncol (2019) 31(4):257–8. doi: 10.1097/CCO.0000000000000542 [DOI] [PubMed] [Google Scholar]
- 2. Mester A, Mester A. The history of photobiomodulation: endre mester (1903-1984). Photomedicine Laser Surg (2017) 35(8):393–4. doi: 10.1089/pho.2017.4332 [DOI] [PubMed] [Google Scholar]
- 3. Anders JJ, Arany PR, Baxter GD, Lanzafame RJ. Light-emitting diode therapy and low-level light therapy are photobiomodulation therapy. Photobiomodul Photomed Laser Surg (2019) 37(2):63–5. doi: 10.1089/photob.2018.4600 [DOI] [PubMed] [Google Scholar]
- 4. Zadik Y, Arany PR, Fregnani ER, Bossi P, Antunes HS, Bensadoun RJ, et al. Systematic review of photobiomodulation for the management of oral mucositis in cancer patients and clinical practice guidelines. Supportive Care Cancer (2019) 27(10):3969–83. doi: 10.1007/s00520-019-04890-2 [DOI] [PubMed] [Google Scholar]
- 5. Zecha JA, Raber-Durlacher JE, Nair RG, Epstein JB, Elad S, Hamblin MR, et al. Low-level laser therapy/photobiomodulation in the management of side effects of chemoradiation therapy in head and neck cancer: part 2: proposed applications and treatment protocols. Supportive Care Cancer (2016) 24(6):2793–805. doi: 10.1007/s00520-016-3153-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Somerfield M, Padberg J, Pfister D, Bennett CL, Recht A, Smith TJ, et al. ASCO clinical practice guidelines: process, progress, pitfalls, and prospects. Class Pap Curr Comments (2000) 4(4):881–6. [Google Scholar]
- 7. Zecha JA, Raber-Durlacher JE, Nair RG, Epstein JB, Sonis ST, Elad S, et al. Low level laser therapy/photobiomodulation in the management of side effects of chemoradiation therapy in head and neck cancer: part 1: mechanisms of action, dosimetric, and safety considerations. Supportive Care Cancer (2016) 24(6):2781–92. doi: 10.1007/s00520-016-3152-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hamblin M, Ferraresi C, Huang YY, de Freitas LF, Carroll J. Low-level light therapy: Photobiomodulation. Bellingham, Washington USA: SPIE press; (2018). 390 p. [Google Scholar]
- 9. Gavish L, Houreld NN. Therapeutic efficacy of home-use photobiomodulation devices: a systematic literature review. Photobiomodul Photomed Laser Surg (2019) 37(1):4–16. doi: 10.1089/photob.2018.4512 [DOI] [PubMed] [Google Scholar]
- 10. Huang YY, Sharma SK, Carroll J, Hamblin MR. Biphasic dose response in low level light therapy - an update. Dose-response (2011) 9(4):602–18. doi: 10.2203/dose-response.11-009.Hamblin [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bensadoun RJ, Nair RG. Low-level laser therapy in the prevention and treatment of cancer therapy-induced mucositis: 2012 state of the art based on literature review and meta-analysis. Curr Opin Oncol (2012) 24(4):363–70. doi: 10.1097/CCO.0b013e328352eaa3 [DOI] [PubMed] [Google Scholar]
- 12. Huang YY, Chen AC, Carroll JD, Hamblin MR. Biphasic dose response in low level light therapy. Dose-response: Publ Int Hormesis Society (2009) 7(4):358–83. doi: 10.2203/dose-response.09-027.Hamblin [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chung H, Dai T, Sharma SK, Huang YY, Carroll JD, Hamblin MR. The nuts and bolts of low-level laser (light) therapy. Ann Biomed engineering (2012) 40(2):516–33. doi: 10.1007/s10439-011-0454-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bjordal JM. Low level laser therapy (LLLT) and world association for laser therapy (walt) dosage recommendations. Photomed Laser Surg (2012) 30(2):61–2. doi: 10.1089/pho.2012.9893 [DOI] [PubMed] [Google Scholar]
- 15. Jenkins PA, Carroll JD. How to report low-level laser therapy (LLLT)/photomedicine dose and beam parameters in clinical and laboratory studies. Photomedicine Laser Surg (2011) 29(12):785–7. doi: 10.1089/pho.2011.9895 [DOI] [PubMed] [Google Scholar]
- 16. Guirro RR, Weis LC. Radiant power determination of low-level laser therapy equipment and characterization of its clinical use procedures. Photomed Laser Surg (2009) 27(4):633–9. doi: 10.1089/pho.2008.2361 [DOI] [PubMed] [Google Scholar]
- 17. Chermetz M, Gobbo M, Ronfani L, Ottaviani G, Zanazzo GA, Verzegnassi F, et al. Class IV laser therapy as treatment for chemotherapy-induced oral mucositis in onco-haematological paediatric patients: a prospective study. Int J Paediatr Dent (2014) 24(6):441–9. doi: 10.1111/ipd.12090 [DOI] [PubMed] [Google Scholar]
- 18. Hedberg ML, Peyser ND, Bauman JE, Gooding WE, Li H, Bhola NE, et al. Use of nonsteroidal anti-inflammatory drugs predicts improved patient survival for PIK3CA-altered head and neck cancer. J Exp Med (2019) 216(2):419–27. doi: 10.1084/jem.20181936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mester E, Ludany G, Sellyei M, Szende B. [On the biologic effect of laser rays]. Bull Soc Int Chir (1968) 27(1):68–73. [PubMed] [Google Scholar]
- 20. Mester E, Sellyei M, Tota J. The effect of laser beam on Ehrlich ascites tumor cells in vitro . Orv Hetil (1968) 109(46):2551–2. [PubMed] [Google Scholar]
- 21. Mester E, Juhasz J, Varga P, Karika G. Lasers in clinical practice. Acta Chir Acad Sci Hung (1968) 9(3):349–57. [PubMed] [Google Scholar]
- 22. Schartinger VH, Galvan O, Riechelmann H, Dudas J. Differential responses of fibroblasts, non-neoplastic epithelial cells, and oral carcinoma cells to low-level laser therapy. Support Care Cancer (2012) 20(3):523–9. doi: 10.1007/s00520-011-1113-0 [DOI] [PubMed] [Google Scholar]
- 23. Powell K, Low P, McDonnell PA, Laakso EL, Ralph SJ. The effect of laser irradiation on proliferation of human breast carcinoma, melanoma, and immortalized mammary epithelial cells. Photomed Laser Surg (2010) 28(1):115–23. doi: 10.1089/pho.2008.2445 [DOI] [PubMed] [Google Scholar]
- 24. Marchesini R, Dasdia T, Melloni E, Rocca E. Effect of low-energy laser irradiation on colony formation capability in different human tumor cells in vitro. Lasers Surg Med (1989) 9(1):59–62. doi: 10.1002/lsm.1900090112 [DOI] [PubMed] [Google Scholar]
- 25. Schaffer M, Sroka R, Fuchs C, Schrader-Reichardt U, Schaffer PM, Busch M, et al. Biomodulative effects induced by 805 nm laser light irradiation of normal and tumor cells. J Photochem Photobiol B (1997) 40(3):253–7. doi: 10.1016/S1011-1344(97)00065-1 [DOI] [PubMed] [Google Scholar]
- 26. Ocana-Quero JM, Gomez-Villamandos R, Moreno-Millan M, Santisteban-Valenzuela JM. Helium-neon (he-ne) laser irradiation increases the incidence of unreduced bovine oocytes during the first meiotic division in vitro. Lasers Med Sci (1998) 13(4):260–4. doi: 10.1007/s101030050005 [DOI] [PubMed] [Google Scholar]
- 27. Pinheiro AL, do Nascliento SC, de Vieira AL, Rolim AB, da Silva PS, Brugnera A., Jr. Does LLLT stimulate laryngeal carcinoma cells? an in vitro study. Braz Dent J (2002) 13(2):109–12. doi: 10.1590/S0103-64402002000200006 [DOI] [PubMed] [Google Scholar]
- 28. Kreisler M, Christoffers AB, Willershausen B, d’Hoedt B. Low-level 809 nm GaAlAs laser irradiation increases the proliferation rate of human laryngeal carcinoma cells in vitro. Lasers Med Sci (2003) 18(2):100–3. doi: 10.1007/s10103-003-0265-7 [DOI] [PubMed] [Google Scholar]
- 29. de Castro JL, Pinheiro AL, Werneck CE, Soares CP. The effect of laser therapy on the proliferation of oral KB carcinoma cells: an in vitro study. Photomedicine Laser Surg (2005) 23(6):586–9. doi: 10.1089/pho.2005.23.586 [DOI] [PubMed] [Google Scholar]
- 30. Werneck CE, Pinheiro AL, Pacheco MT, Soares CP, de Castro JL. Laser light is capable of inducing proliferation of carcinoma cells in culture: a spectroscopic in vitro study. Photomedicine Laser Surg (2005) 23(3):300–3. doi: 10.1089/pho.2005.23.300 [DOI] [PubMed] [Google Scholar]
- 31. Renno AC, McDonnell PA, Parizotto NA, Laakso EL. The effects of laser irradiation on osteoblast and osteosarcoma cell proliferation and differentiation in vitro. Photomedicine Laser Surg (2007) 25(4):275–80. doi: 10.1089/pho.2007.2055 [DOI] [PubMed] [Google Scholar]
- 32. Gomes Henriques AC, Ginani F, Oliveira RM, Keesen TS, Galvao Barboza CA, Oliveira Rocha HA, et al. Low-level laser therapy promotes proliferation and invasion of oral squamous cell carcinoma cells. Lasers Med Sci (2014) 29(4):1385–95. doi: 10.1007/s10103-014-1535-2 [DOI] [PubMed] [Google Scholar]
- 33. Sperandio FF, Giudice FS, Correa L, Pinto DS, Jr., Hamblin MR, de Sousa SC. Low-level laser therapy can produce increased aggressiveness of dysplastic and oral cancer cell lines by modulation of Akt/mTOR signaling pathway. J Biophotonics (2013) 6(10):839–47. doi: 10.1002/jbio.201300015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bamps M, Dok R, Nuyts S. Low-level laser therapy stimulates proliferation in head and neck squamous cell carcinoma cells. Front Oncol (2018) 8:343. doi: 10.3389/fonc.2018.00343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. da Silva JL, Silva-de-Oliveira AFS, Andraus RAC, Maia LP. Effects of low level laser therapy in cancer cells-a systematic review of the literature. Lasers Med Sci (2020) 35(3):523–9. doi: 10.1007/s10103-019-02824-2 [DOI] [PubMed] [Google Scholar]
- 36. Djavid GE, Bigdeli B, Goliaei B, Nikoofar A, Hamblin MR. Photobiomodulation leads to enhanced radiosensitivity through induction of apoptosis and autophagy in human cervical cancer cells. J Biophotonics (2017) 10(12):1732–42. doi: 10.1002/jbio.201700004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chu J, Wu S, Xing D. Survivin mediates self-protection through ROS/cdc25c/CDK1 signaling pathway during tumor cell apoptosis induced by high fluence low-power laser irradiation. Cancer Letters (2010) 297(2):207–19. doi: 10.1016/j.canlet.2010.05.013 [DOI] [PubMed] [Google Scholar]
- 38. Tsai SR, Yin R, Huang YY, Sheu BC, Lee SC, Hamblin MR. Low-level light therapy potentiates NPe6-mediated photodynamic therapy in a human osteosarcoma cell line via increased ATP. Photodiagnosis Photodyn Ther (2015) 12(1):123–30. doi: 10.1016/j.pdpdt.2014.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schaffer M, Bonel H, Sroka R, Schaffer PM, Busch M, Reiser M, et al. Effects of 780 nm diode laser irradiation on blood microcirculation: preliminary findings on time-dependent T1-weighted contrast-enhanced magnetic resonance imaging (MRI). J Photochem Photobiol B (2000) 54(1):55–60. doi: 10.1016/S1011-1344(99)00155-4 [DOI] [PubMed] [Google Scholar]
- 40. Barasch A, Li H, Rajasekhar VK, Raber-Durlacher J, Epstein JB, Carroll J, et al. Photobiomodulation effects on head and neck squamous cell carcinoma (HNSCC) in an orthotopic animal model. Support Care Cancer (2020) 28(6):2721–7. doi: 10.1007/s00520-019-05060-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Coombe AR, Ho CT, Darendeliler MA, Hunter N, Philips JR, Chapple CC, et al. The effects of low level laser irradiation on osteoblastic cells. Clin Orthod Res (2001) 4(1):3–14. doi: 10.1034/j.1600-0544.2001.040102.x [DOI] [PubMed] [Google Scholar]
- 42. Liu YH, Cheng CC, Ho CC, Pei RJ, Lee KY, Yeh KT, et al. Effects of diode 808 nm GaAlAs low-power laser irradiation on inhibition of the proliferation of human hepatoma cells in vitro and their possible mechanism. Res Commun Mol Pathol Pharmacol (2004) 115-116:185–201. [PubMed] [Google Scholar]
- 43. Sroka R, Schaffer M, Fuchs C, Pongratz T, Schrader-Reichard U, Busch M, et al. Effects on the mitosis of normal and tumor cells induced by light treatment of different wavelengths. Lasers Surg Med (1999) 25(3):263–71. doi: [DOI] [PubMed] [Google Scholar]
- 44. Murayama H, Sadakane K, Yamanoha B, Kogure S. Low-power 808-nm laser irradiation inhibits cell proliferation of a human-derived glioblastoma cell line in vitro. Lasers Med Sci (2012) 27(1):87–93. doi: 10.1007/s10103-011-0924-z [DOI] [PubMed] [Google Scholar]
- 45. Al-Watban FA, Andres BL. Laser biomodulation of normal and neoplastic cells. Lasers Med Sci (2012) 27(5):1039–43. doi: 10.1007/s10103-011-1040-9 [DOI] [PubMed] [Google Scholar]
- 46. Crous AM, Abrahamse H. Lung cancer stem cells and low-intensity laser irradiation: a potential future therapy? Stem Cell Res Ther (2013) 4(5):129. doi: 10.1186/scrt340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Frigo L, Luppi JS, Favero GM, Maria DA, Penna SC, Bjordal JM, et al. The effect of low-level laser irradiation (In-Ga-Al-AsP - 660 nm) on melanoma in vitro and in vivo. BMC Cancer (2009) 9:404. doi: 10.1186/1471-2407-9-404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schalch TD, Fernandes MH, Destro Rodrigues MFS, Guimaraes DM, Nunes FD, Rodrigues JC, et al. Photobiomodulation is associated with a decrease in cell viability and migration in oral squamous cell carcinoma. Lasers Med Sci (2019) 34(3):629–36. doi: 10.1007/s10103-018-2640-4 [DOI] [PubMed] [Google Scholar]
- 49. Ottaviani G, Martinelli V, Rupel K, Caronni N, Naseem A, Zandona L, et al. Laser therapy inhibits tumor growth in mice by promoting immune surveillance and vessel normalization. EBioMedicine (2016) 11:165–72. doi: 10.1016/j.ebiom.2016.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Silveira FM, Paglioni MP, Marques MM, Santos-Silva AR, Migliorati CA, Arany P, et al. Examining tumor modulating effects of photobiomodulation therapy on head and neck squamous cell carcinomas. Photochem Photobiol Sci (2019) 18(7):1621–37. doi: 10.1039/C9PP00120D [DOI] [PubMed] [Google Scholar]
- 51. de CMJS, Pinheiro AN, de Oliveira SC, Aciole GT, Sousa JA, Canguss MC, et al. Influence of laser phototherapy (lambda660 nm) on the outcome of oral chemical carcinogenesis on the hamster cheek pouch model: histological study. Photomedicine Laser Surg (2011) 29(11):741–5. doi: 10.1089/pho.2010.2896 [DOI] [PubMed] [Google Scholar]
- 52. Myakishev-Rempel M, Stadler I, Brondon P, Axe DR, Friedman M, Nardia FB, et al. A preliminary study of the safety of red light phototherapy of tissues harboring cancer. Photomed Laser Surg (2012) 30(9):551–8. doi: 10.1089/pho.2011.3186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mikhailov VA, Skobelkin OK, Denisov IN, Frank GA, Voltchenko NN. Investigations on the influence of low level diode laser irradiation on the growth of experimental tumours. LASER Ther (1993) 5(1):33–8. doi: 10.5978/islsm.93-OR-03 [DOI] [Google Scholar]
- 54. Shabbir M, Ryten M, Thompson C, Mikhailidis D, Burnstock G. Purinergic receptor-mediated effects of ATP in high-grade bladder cancer. BJU Int (2008) 101(1):106–12. doi: 10.1111/j.1464-410X.2007.07286.x [DOI] [PubMed] [Google Scholar]
- 55. Karu T. Mitochondrial mechanisms of photobiomodulation in context of new data about multiple roles of ATP. Photomed Laser Surg (2010) 28(2):159–60. doi: 10.1089/pho.2010.2789 [DOI] [PubMed] [Google Scholar]
- 56. Wikramanayake TC, Villasante AC, Mauro LM, Nouri K, Schachner LA, Perez CI, et al. Low-level laser treatment accelerated hair regrowth in a rat model of chemotherapy-induced alopecia (CIA). Lasers Med Sci (2013) 28(3):701–6. doi: 10.1007/s10103-012-1139-7 [DOI] [PubMed] [Google Scholar]
- 57. Antunes HS, Herchenhorn D, Small IA, Araujo CMM, Viegas CMP, de Assis Ramos G, et al. Long-term survival of a randomized phase III trial of head and neck cancer patients receiving concurrent chemoradiation therapy with or without low-level laser therapy (LLLT) to prevent oral mucositis. Oral Oncol (2017) 71:11–5. doi: 10.1016/j.oraloncology.2017.05.018 [DOI] [PubMed] [Google Scholar]
- 58. Bensadoun RJ, Epstein JB, Nair RG, Barasch A, Raber-Durlacher JE, Migliorati C, et al. Safety and efficacy of photobiomodulation therapy in oncology: A systematic review. Cancer Med (2020) 9(22):8279–300. doi: 10.1002/cam4.3582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bezinelli LM, Correa L, Vogel C, Kutner JM, Ribeiro AF, Hamerschlak N, et al. Long-term safety of photobiomodulation therapy for oral mucositis in hematopoietic cell transplantation patients: a 15-year retrospective study. Support Care Cancer (2021) 29(11):6891–902. doi: 10.1007/s00520-021-06268-9 [DOI] [PubMed] [Google Scholar]
- 60. Arany PR. Healing tumors with light: science fiction or the future of medicine? Photomed Laser Surg (2018) 36(5):227–9. doi: 10.1089/pho.2018.4457 [DOI] [PubMed] [Google Scholar]
- 61. Hamblin MR, Nelson ST, Strahan JR. Photobiomodulation and cancer: what is the truth? Photomed Laser Surg (2018) 36(5):241–5. doi: 10.1089/pho.2017.4401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Arany PR. Photobiomodulation therapy–easy to do, but difficult to get right. Laser Focus World (2019). https://www.laserfocusworld.com/lasers-sources/article/14037967/photobiomodulation-therapyeasy-to-do-but-difficult-to-get-right. [Google Scholar]
- 63. Young NC, Maxiamano V, Arany PR. Thermodynamic basis for multi-wavelength dosing for photobiomodulation therapy to direct odontoblast differentiation. J Biophotonics (2022) 15(6):e202100398. doi: 10.1002/jbio.202100398 [DOI] [PubMed] [Google Scholar]
- 64. Piller N, Dawson R, Rice J, De Vries D. Is there a link between LE treatment and breast cancer reoccurence? J Lymphoedema (2011) 15(6):202100398. [Google Scholar]
- 65. Antunes HS, Herchenhorn D, Small IA, Araujo CM, Viegas CM, Cabral E, et al. Phase III trial of low-level laser therapy to prevent oral mucositis in head and neck cancer patients treated with concurrent chemoradiation. Radiother Oncol (2013) 109(2):297–302. doi: 10.1016/j.radonc.2013.08.010 [DOI] [PubMed] [Google Scholar]
- 66. Brandao TB, Morais-Faria K, Ribeiro ACP, Rivera C, Salvajoli JV, Lopes MA, et al. Locally advanced oral squamous cell carcinoma patients treated with photobiomodulation for prevention of oral mucositis: retrospective outcomes and safety analyses. Support Care Cancer (2018) 26(7):2417–23. doi: 10.1007/s00520-018-4046-z [DOI] [PubMed] [Google Scholar]
- 67. de Pauli Paglioni M, Araujo ALD, Arboleda LPA, Palmier NR, Fonseca JM, Gomes-Silva W, et al. Tumor safety and side effects of photobiomodulation therapy used for prevention and management of cancer treatment toxicities. A systematic review. Oral Oncol (2019) 93:21–8. doi: 10.1016/j.oraloncology.2019.04.004 [DOI] [PubMed] [Google Scholar]
- 68. Bowen J, Al-Dasooqi N, Bossi P, Wardill H, Van Sebille Y, Al-Azri A, et al. The pathogenesis of mucositis: updated perspectives and emerging targets. Support Care Cancer (2019) 27(10):4023–33. doi: 10.1007/s00520-019-04893-z [DOI] [PubMed] [Google Scholar]
- 69. Epstein JB, Thariat J, Bensadoun RJ, Barasch A, Murphy BA, Kolnick L, et al. Oral complications of cancer and cancer therapy: from cancer treatment to survivorship. CA Cancer J Clin (2012) 62(6):400–22. doi: 10.3322/caac.21157 [DOI] [PubMed] [Google Scholar]
- 70. Sonis ST. A biological approach to mucositis. J Support Oncol (2004) 2(1):21–32. [PubMed] [Google Scholar]
- 71. Gouvea de Lima A, Villar RC, de Castro G, Jr., Antequera R, Gil E, Rosalmeida MC, et al. Oral mucositis prevention by low-level laser therapy in head-and-neck cancer patients undergoing concurrent chemoradiotherapy: a phase III randomized study. Int J Radiat Oncol Biol Phys (2012) 82(1):270–5. doi: 10.1016/j.ijrobp.2010.10.012 [DOI] [PubMed] [Google Scholar]
- 72. Vasconcelos RM, Sanfilippo N, Paster BJ, Kerr AR, Li Y, Ramalho L, et al. Host-microbiome cross-talk in oral mucositis. J Dent Res (2016) 95(7):725–33. doi: 10.1177/0022034516641890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Elting LS, Keefe DM, Sonis ST, Garden AS, Spijkervet FK, Barasch A, et al. Patient-reported measurements of oral mucositis in head and neck cancer patients treated with radiotherapy with or without chemotherapy: demonstration of increased frequency, severity, resistance to palliation, and impact on quality of life. Cancer (2008) 113(10):2704–13. doi: 10.1002/cncr.23898 [DOI] [PubMed] [Google Scholar]
- 74. Sonis ST, Oster G, Fuchs H, Bellm L, Bradford WZ, Edelsberg J, et al. Oral mucositis and the clinical and economic outcomes of hematopoietic stem-cell transplantation. J Clin Oncol (2001) 19(8):2201–5. doi: 10.1200/JCO.2001.19.8.2201 [DOI] [PubMed] [Google Scholar]
- 75. Lalla RV, Treister N, Sollecito T, Schmidt B, Patton LL, Mohammadi K, et al. Oral complications at 6 months after radiation therapy for head and neck cancer. Oral Dis (2017) 23(8):1134–43. doi: 10.1111/odi.12710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Elad S, Zadik Y. Chronic oral mucositis after radiotherapy to the head and neck: a new insight. Support Care Cancer (2016) 24(11):4825–30. doi: 10.1007/s00520-016-3337-5 [DOI] [PubMed] [Google Scholar]
- 77. Lalla RV, Bowen J, Barasch A, Elting L, Epstein J, Keefe DM, et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer (2014) 120(10):1453–61. doi: 10.1002/cncr.28592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Elad S. The MASCC/ISOO mucositis guidelines 2019: the second set of articles and future directions. Support Care Cancer (2020) 28(5):2445–7. doi: 10.1007/s00520-019-05153-w [DOI] [PubMed] [Google Scholar]
- 79. Heiskanen V, Zadik Y, Elad S. Photobiomodulation therapy for cancer treatment-related salivary gland dysfunction: a systematic review. Photobiomodul Photomed Laser Surg (2020) 38(6):340–7. doi: 10.1089/photob.2019.4767 [DOI] [PubMed] [Google Scholar]
- 80. Nunes LFM, de Arruda JAA, Souza AF, Silva RCC, Lanza CRM, Kakehasi FM, et al. Prophylactic photobiomodulation therapy using 660 nm diode laser for oral mucositis in paediatric patients under chemotherapy: 5-year experience from a Brazilian referral service. Lasers Med Sci (2020) 35(8):1857–66. doi: 10.1007/s10103-020-03060-9 [DOI] [PubMed] [Google Scholar]
- 81. Abramoff MM, Lopes NN, Lopes LA, Dib LL, Guilherme A, Caran EM, et al. Low-level laser therapy in the prevention and treatment of chemotherapy-induced oral mucositis in young patients. Photomed Laser Surg (2008) 26(4):393–400. doi: 10.1089/pho.2007.2144 [DOI] [PubMed] [Google Scholar]
- 82. Medeiros-Filho JB, Maia Filho EM, Ferreira MC. Laser and photochemotherapy for the treatment of oral mucositis in young patients: Randomized clinical trial. Photodiagnosis Photodyn Ther (2017) 18:39–45. doi: 10.1016/j.pdpdt.2017.01.004 [DOI] [PubMed] [Google Scholar]
- 83. Noirrit-Esclassan E, Valera MC, Vignes E, Munzer C, Bonal S, Daries M, et al. Photobiomodulation with a combination of two wavelengths in the treatment of oral mucositis in children: The PEDIALASE feasibility study. Arch Pediatr (2019) 26(5):268–74. doi: 10.1016/j.arcped.2019.05.012 [DOI] [PubMed] [Google Scholar]
- 84. Cruz LB, Ribeiro AS, Rech A, Rosa LG, Castro CG, Jr., Brunetto AL. Influence of low-energy laser in the prevention of oral mucositis in children with cancer receiving chemotherapy. Pediatr Blood Cancer (2007) 48(4):435–40. doi: 10.1002/pbc.20943 [DOI] [PubMed] [Google Scholar]
- 85. Whelan HT, Connelly JF, Hodgson BD, Barbeau L, Post AC, Bullard G, et al. NASA Light-emitting diodes for the prevention of oral mucositis in pediatric bone marrow transplant patients. J Clin Laser Med Surg (2002) 20(6):319–24. doi: 10.1089/104454702320901107 [DOI] [PubMed] [Google Scholar]
- 86. de Castro JF, Abreu EG, Correia AV, da Mota Vasconcelos Brasil C, da Cruz Perez DE, de Paula Ramos Pedrosa F. Low-level laser in prevention and treatment of oral mucositis in pediatric patients with acute lymphoblastic leukemia. Photomed Laser Surg (2013) 31(12):613–8. doi: 10.1089/pho.2012.3327 [DOI] [PubMed] [Google Scholar]
- 87. Treister NS, London WB, Guo D, Malsch M, Verrill K, Brewer J, et al. A feasibility study evaluating extraoral photobiomodulation therapy for prevention of mucositis in pediatric hematopoietic cell transplantation. Photomed Laser Surg (2016) 34(4):178–84. doi: 10.1089/pho.2015.4021 [DOI] [PubMed] [Google Scholar]
- 88. Amadori F, Bardellini E, Conti G, Pedrini N, Schumacher RF, Majorana A. Low-level laser therapy for treatment of chemotherapy-induced oral mucositis in childhood: a randomized double-blind controlled study. Lasers Med Sci (2016) 31(6):1231–6. doi: 10.1007/s10103-016-1975-y [DOI] [PubMed] [Google Scholar]
- 89. Kuhn A, Porto FA, Miraglia P, Brunetto AL. Low-level infrared laser therapy in chemotherapy-induced oral mucositis: a randomized placebo-controlled trial in children. J Pediatr Hematol Oncol (2009) 31(1):33–7. doi: 10.1097/MPH.0b013e318192cb8e [DOI] [PubMed] [Google Scholar]
- 90. Vitale MC, Modaffari C, Decembrino N, Zhou FX, Zecca M, Defabianis P. Preliminary study in a new protocol for the treatment of oral mucositis in pediatric patients undergoing hematopoietic stem cell transplantation (HSCT) and chemotherapy (CT). Lasers Med Sci (2017) 32(6):1423–8. doi: 10.1007/s10103-017-2266-y [DOI] [PubMed] [Google Scholar]
- 91. Soto M, Lalla RV, Gouveia RV, Zecchin VG, Seber A, Lopes NN. Pilot study on the efficacy of combined intraoral and extraoral low-level laser therapy for prevention of oral mucositis in pediatric patients undergoing hematopoietic stem cell transplantation. Photomed Laser Surg (2015) 33(11):540–6. doi: 10.1089/pho.2015.3954 [DOI] [PubMed] [Google Scholar]
- 92. Eduardo Fde P, Bezinelli LM, de Carvalho DL, Lopes RM, Fernandes JF, Brumatti M, et al. Oral mucositis in pediatric patients undergoing hematopoietic stem cell transplantation: clinical outcomes in a context of specialized oral care using low-level laser therapy. Pediatr Transplant (2015) 19(3):316–25. doi: 10.1111/petr.12440 [DOI] [PubMed] [Google Scholar]
- 93. Eduardo FP, Bezinelli L, Luiz AC, Correa L, Vogel C, Eduardo CP. Severity of oral mucositis in patients undergoing hematopoietic cell transplantation and an oral laser phototherapy protocol: a survey of 30 patients. Photomed Laser Surg (2009) 27(1):137–44. doi: 10.1089/pho.2007.2225 [DOI] [PubMed] [Google Scholar]
- 94. Weissheimer C, Curra M, Gregianin LJ, Daudt LE, Wagner VP, Martins MAT, et al. New photobiomodulation protocol prevents oral mucositis in hematopoietic stem cell transplantation recipients-a retrospective study. Lasers Med Sci (2017) 32(9):2013–21. doi: 10.1007/s10103-017-2314-7 [DOI] [PubMed] [Google Scholar]
- 95. Tomazevic T, Potocnik U, Cizerl D, Jazbec J. Optimization of photobiomodulation protocol for chemotherapy-induced mucositis in pediatric patients. Photobiomodul Photomed Laser Surg (2020) 38(8):466–71. doi: 10.1089/photob.2019.4794 [DOI] [PubMed] [Google Scholar]
- 96. Gobbo M, Verzegnassi F, Ronfani L, Zanon D, Melchionda F, Bagattoni S, et al. Multicenter randomized, double-blind controlled trial to evaluate the efficacy of laser therapy for the treatment of severe oral mucositis induced by chemotherapy in children: laMPO RCT. Pediatr Blood Cancer (2018) 65(8):e27098. doi: 10.1002/pbc.27098 [DOI] [PubMed] [Google Scholar]
- 97. Patel P, Robinson PD, Baggott C, Gibson P, Ljungman G, Massey N, et al. Clinical practice guideline for the prevention of oral and oropharyngeal mucositis in pediatric cancer and hematopoietic stem cell transplant patients: 2021 update. Eur J Cancer (2021) 154:92–101. doi: 10.1016/j.ejca.2021.05.013 [DOI] [PubMed] [Google Scholar]
- 98. He M, Zhang B, Shen N, Wu N, Sun J. A systematic review and meta-analysis of the effect of low-level laser therapy (LLLT) on chemotherapy-induced oral mucositis in pediatric and young patients. Eur J Pediatr (2018) 177(1):7–17. doi: 10.1007/s00431-017-3043-4 [DOI] [PubMed] [Google Scholar]
- 99. Miranda-Silva W, Gomes-Silva W, Zadik Y, Yarom N, Al-Azri AR, Hong CHL, et al. MASCC/ISOO clinical practice guidelines for the management of mucositis: sub-analysis of current interventions for the management of oral mucositis in pediatric cancer patients. Support Care Cancer (2021) 29(7):3539–62. doi: 10.1007/s00520-020-05803-4 [DOI] [PubMed] [Google Scholar]
- 100. Miranda-Silva W, da Fonseca FP, Gomes AA, Mafra ABB, Rocha V, Fregnani ER. Oral mucositis in paediatric cancer patients undergoing allogeneic hematopoietic stem cell transplantation preventively treated with professional dental care and photobiomodulation: Incidence and risk factors. Int J Paediatr Dent (2022) 32(2):251–63. doi: 10.1111/ipd.12850 [DOI] [PubMed] [Google Scholar]
- 101. Epstein JB, Robertson M, Emerton S, Phillips N, Stevenson-Moore P. Quality of life and oral function in patients treated with radiation therapy for head and neck cancer. Head Neck (2001) 23(5):389–98. doi: 10.1002/hed.1049 [DOI] [PubMed] [Google Scholar]
- 102. Shiboski CH, Hodgson TA, Ship JA, Schiodt M. Management of salivary hypofunction during and after radiotherapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod (2007) 103 Suppl:S66 e1–19. doi: 10.1016/j.tripleo.2006.11.013 [DOI] [PubMed] [Google Scholar]
- 103. Peterson D, Jensen SB. Oral complications of nonsurgical cancer therapies: diagnosis and treatment. In: Glick M, editor. Burket’s oral medicine. People’s Medical Publishing House, USA. (2014). p. 201–18. [Google Scholar]
- 104. Jensen SB, Pedersen AM, Vissink A, Andersen E, Brown CG, Davies AN, et al. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: prevalence, severity and impact on quality of life. Supportive Care Cancer (2010) 18(8):1039–60. doi: 10.1007/s00520-010-0827-8 [DOI] [PubMed] [Google Scholar]
- 105. Vissink A, van Luijk P, Langendijk JA, Coppes RP. Current ideas to reduce or salvage radiation damage to salivary glands. Oral Dis (2015) 21(1):e1–10. doi: 10.1111/odi.12222 [DOI] [PubMed] [Google Scholar]
- 106. Gonnelli FA, Palma LF, Giordani AJ, Deboni AL, Dias RS, Segreto RA, et al. Low-level laser for mitigation of low salivary flow rate in head and neck cancer patients undergoing radiochemotherapy: a prospective longitudinal study. Photomedicine Laser Surg (2016) 34(8):326–30. doi: 10.1089/pho.2016.4104 [DOI] [PubMed] [Google Scholar]
- 107. Gonnelli FA, Palma LF, Giordani AJ, Deboni AL, Dias RS, Segreto RA, et al. Low-level laser therapy for the prevention of low salivary flow rate after radiotherapy and chemotherapy in patients with head and neck cancer. Radiol Bras (2016) 49(2):86–91. doi: 10.1590/0100-3984.2014.0144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Palma LF, Gonnelli FAS, Marcucci M, Dias RS, Giordani AJ, Segreto RA, et al. Impact of low-level laser therapy on hyposalivation, salivary pH, and quality of life in head and neck cancer patients post-radiotherapy. Lasers Med Sci (2017) 32(4):827–32. doi: 10.1007/s10103-017-2180-3 [DOI] [PubMed] [Google Scholar]
- 109. Saleh J, Figueiredo MA, Cherubini K, Braga-Filho A, Salum FG. Effect of low-level laser therapy on radiotherapy-induced hyposalivation and xerostomia: a pilot study. Photomedicine Laser Surg (2014) 32(10):546–52. doi: 10.1089/pho.2014.3741 [DOI] [PubMed] [Google Scholar]
- 110. Simoes A, de Campos L, de Souza DN, de Matos JA, Freitas PM, Nicolau J. Laser phototherapy as topical prophylaxis against radiation-induced xerostomia. Photomedicine Laser Surg (2010) 28(3):357–63. doi: 10.1089/pho.2009.2486 [DOI] [PubMed] [Google Scholar]
- 111. Cowen D, Tardieu C, Schubert M, Peterson D, Resbeut M, Faucher C, et al. Low energy helium-neon laser in the prevention of oral mucositis in patients undergoing bone marrow transplant: results of a double blind randomized trial. Int J Radiat Oncol Biol Phys (1997) 38(4):697–703. doi: 10.1016/S0360-3016(97)00076-X [DOI] [PubMed] [Google Scholar]
- 112. Dysphagia Section OCSGMAoSCiCISoOO. Raber-Durlacher JE, Brennan MT, Verdonck-de Leeuw IM, Gibson RJ, Eilers JG, et al. Swallowing dysfunction in cancer patients. Supportive Care Cancer (2012) 20(3):433–43. doi: 10.1007/s00520-011-1342-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Russi EG, Corvo R, Merlotti A, Alterio D, Franco P, Pergolizzi S, et al. Swallowing dysfunction in head and neck cancer patients treated by radiotherapy: review and recommendations of the supportive task group of the Italian association of radiation oncology. Cancer Treat Rev (2012) 38(8):1033–49. doi: 10.1016/j.ctrv.2012.04.002 [DOI] [PubMed] [Google Scholar]
- 114. Mercadante S, Aielli F, Adile C, Ferrera P, Valle A, Fusco F, et al. Prevalence of oral mucositis, dry mouth, and dysphagia in advanced cancer patients. Supportive Care Cancer (2015) 23(11):3249–55. doi: 10.1007/s00520-015-2720-y [DOI] [PubMed] [Google Scholar]
- 115. Gautam AP, Fernandes DJ, Vidyasagar MS, Maiya AG, Vadhiraja BM. Low level laser therapy for concurrent chemoradiotherapy induced oral mucositis in head and neck cancer patients - a triple blinded randomized controlled trial. Radiotherapy oncology: J Eur Soc Ther Radiol Oncol (2012) 104(3):349–54. doi: 10.1016/j.radonc.2012.06.011 [DOI] [PubMed] [Google Scholar]
- 116. Cox JD, Stetz J, Pajak TF. Toxicity criteria of the radiation therapy oncology group (RTOG) and the European organization for research and treatment of cancer (EORTC). Int J Radiat oncology biology physics (1995) 31(5):1341–6. doi: 10.1016/0360-3016(95)00060-C [DOI] [PubMed] [Google Scholar]
- 117. Chen AP, Setser A, Anadkat MJ, Cotliar J, Olsen EA, Garden BC, et al. Grading dermatologic adverse events of cancer treatments: the common terminology criteria for adverse events version 4.0. J Am Acad Dermatol (2012) 67(5):1025–39. doi: 10.1016/j.jaad.2012.02.010 [DOI] [PubMed] [Google Scholar]
- 118. Twardella D, Popanda O, Helmbold I, Ebbeler R, Benner A, von Fournier D, et al. Personal characteristics, therapy modalities and individual DNA repair capacity as predictive factors of acute skin toxicity in an unselected cohort of breast cancer patients receiving radiotherapy. Radiotherapy Oncol (2003) 69(2):145–53. doi: 10.1016/S0167-8140(03)00166-X [DOI] [PubMed] [Google Scholar]
- 119. De Langhe S, Mulliez T, Veldeman L, Remouchamps V, van Greveling A, Gilsoul M, et al. Factors modifying the risk for developing acute skin toxicity after whole-breast intensity modulated radiotherapy. BMC cancer (2014) 14:711. doi: 10.1186/1471-2407-14-711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Robijns J, Laubach H-J. Acute and chronic radiodermatitis: clinical signs, pathophysiology, risk factors and management options. J Egyptian Women’s Dermatologic Soc (2018) 15(1):2–9. doi: 10.1097/01.EWX.0000529960.52517.4c [DOI] [Google Scholar]
- 121. Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol (2006) 54(1):28–46. doi: 10.1016/j.jaad.2005.08.054 [DOI] [PubMed] [Google Scholar]
- 122. Mendelsohn FA, Divino CM, Reis ED, Kerstein MD. Wound care after radiation therapy. Adv skin Wound Care (2002) 15(5):216–24. doi: 10.1097/00129334-200209000-00007 [DOI] [PubMed] [Google Scholar]
- 123. Haubner F, Ohmann E, Pohl F, Strutz J, Gassner HG. Wound healing after radiation therapy: review of the literature. Radiat Oncol (2012) 7:162. doi: 10.1186/1748-717X-7-162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Wong RK, Bensadoun RJ, Boers-Doets CB, Bryce J, Chan A, Epstein JB, et al. Clinical practice guidelines for the prevention and treatment of acute and late radiation reactions from the MASCC skin toxicity study group. Support Care Cancer (2013) 21(10):2933–48. doi: 10.1007/s00520-013-1896-2 [DOI] [PubMed] [Google Scholar]
- 125. Schindl M, Kerschan K, Schindl A, Schon H, Heinzl H, Schindl L. Induction of complete wound healing in recalcitrant ulcers by low-intensity laser irradiation depends on ulcer cause and size. Photodermatology photoimmunology photomedicine (1999) 15(1):18–21. doi: 10.1111/j.1600-0781.1999.tb00047.x [DOI] [PubMed] [Google Scholar]
- 126. Schindl A, Schindl M, Schindl L, Jurecka W, Honigsmann H, Breier F. Increased dermal angiogenesis after low-intensity laser therapy for a chronic radiation ulcer determined by a video measuring system. J Am Acad Dermatol (1999) 40(3):481–4. doi: 10.1016/S0190-9622(99)70503-7 [DOI] [PubMed] [Google Scholar]
- 127. DeLand MM, Weiss RA, McDaniel DH, Geronemus RG. Treatment of radiation-induced dermatitis with light-emitting diode (LED) photomodulation. Lasers Surg Med (2007) 39(2):164–8. doi: 10.1002/lsm.20455 [DOI] [PubMed] [Google Scholar]
- 128. Fife D, Rayhan DJ, Behnam S, Ortiz A, Elkeeb L, Aquino L, et al. A randomized, controlled, double-blind study of light emitting diode photomodulation for the prevention of radiation dermatitis in patients with breast cancer. Dermatologic Surg (2010) 36(12):1921–7. doi: 10.1111/j.1524-4725.2010.01801.x [DOI] [PubMed] [Google Scholar]
- 129. Censabella S, Claes S, Robijns J, Bulens P, Mebis J. Photobiomodulation for the management of radiation dermatitis: the DERMIS trial, a pilot study of MLS((R)) laser therapy in breast cancer patients. Support Care Cancer (2016) 24(9):3925–33. doi: 10.1007/s00520-016-3232-0 [DOI] [PubMed] [Google Scholar]
- 130. Strouthos I, Chatzikonstantinou G, Tselis N, Bon D, Karagiannis E, Zoga E, et al. Photobiomodulation therapy for the management of radiation-induced dermatitis: A single-institution experience of adjuvant radiotherapy in breast cancer patients after breast conserving surgery. Strahlentherapie und Onkologie: Organ der Deutschen Rontgengesellschaft [et al] (2017) 193(6):491–8. doi: 10.1007/s00066-017-1117-x [DOI] [PubMed] [Google Scholar]
- 131. Robijns J, Censabella S, Claes S, Pannekoeke L, Busse L, Colson D, et al. Biophysical skin measurements to evaluate the effectiveness of photobiomodulation therapy in the prevention of acute radiation dermatitis in breast cancer patients. Supportive Care Cancer (2019) 27(4):1245. doi: 10.1007/s00520-018-4487-4 [DOI] [PubMed] [Google Scholar]
- 132. Robijns J, Censabella S, Claes S, Pannekoeke L, Busse L, Colson D, et al. Prevention of acute radiodermatitis by photobiomodulation: A randomized, placebo-controlled trial in breast cancer patients (TRANSDERMIS trial). Lasers Surg Med (2018) 50(7):763–71. doi: 10.1002/lsm.22804 [DOI] [PubMed] [Google Scholar]
- 133. Zhang X, Li H, Li Q, Li Y, Li C, Zhu M, et al. Application of red light phototherapy in the treatment of radioactive dermatitis in patients with head and neck cancer. World J Surg Oncol (2018) 16(1):222. doi: 10.1186/s12957-018-1522-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Robijns J, Lodewijckx J, Bensadoun RJ, Mebis J. A narrative review on the use of photobiomodulation therapy for the prevention and management of acute radiodermatitis: proposed mechanisms, current clinical outcomes, and preliminary guidance for clinical studies. Photobiomodul Photomed Laser Surg (2020) 38(6):332–9. doi: 10.1089/photob.2019.4761 [DOI] [PubMed] [Google Scholar]
- 135. DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol (2013) 14(6):500–15. doi: 10.1016/S1470-2045(13)70076-7 [DOI] [PubMed] [Google Scholar]
- 136. Deng J, Ridner SH, Murphy BA. Lymphedema in patients with head and neck cancer. Oncol Nurs Forum (2011) 38(1):E1–10. doi: 10.1188/11.ONF.E1-E10 [DOI] [PubMed] [Google Scholar]
- 137. Deng J, Ridner SH, Dietrich MS, Wells N, Wallston KA, Sinard RJ, et al. Prevalence of secondary lymphedema in patients with head and neck cancer. J Pain symptom management (2012) 43(2):244–52. doi: 10.1016/j.jpainsymman.2011.03.019 [DOI] [PubMed] [Google Scholar]
- 138. Hwang JM, Hwang JH, Kim TW, Lee SY, Chang HJ, Chu IH. Long-term effects of complex decongestive therapy in breast cancer patients with arm lymphedema after axillary dissection. Ann Rehabil Med (2013) 37(5):690–7. doi: 10.5535/arm.2013.37.5.690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Smith BG, Lewin JS. Lymphedema management in head and neck cancer. Curr Opin Otolaryngol Head Neck Surg (2010) 18(3):153–8. doi: 10.1097/MOO.0b013e3283393799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Lee N, Wigg J, Carroll J. The use of low level light therapy in the treatment of head and neck oedema. J Lymphoedema (2013) 8(1):35–42. [Google Scholar]
- 141. Robijns J, Censabella S, Bulens P, Maes A, Noé L, Brosens M, et al. The role of photobiomodulation therapy in the care of cancer patients: review of the literature. BELG J Med Oncol (2017) 11(8):364–74. [Google Scholar]
- 142. Nair RG, Bensadoun RJ. Mitigation of cancer therapy side-effects with light. Morgan & Claypool Publishers, San Rafael (California, USA) (2016). [Google Scholar]
- 143. Bensadoun RJ. Photobiomodulation or low-level laser therapy in the management of cancer therapy-induced mucositis, dermatitis and lymphedema. Curr Opin Oncol (2018) 30(4):226–32. doi: 10.1097/CCO.0000000000000452 [DOI] [PubMed] [Google Scholar]
- 144. Smoot B, Chiavola-Larson L, Lee J, Manibusan H, Allen DD. Effect of low-level laser therapy on pain and swelling in women with breast cancer-related lymphedema: a systematic review and meta-analysis. J Cancer survivorship: Res Pract (2014) 9(2):287–304. doi: 10.1007/s11764-014-0411-1 [DOI] [PubMed] [Google Scholar]
- 145. Storz MA, Gronwald B, Gottschling S, Schope J, Mavrova R, Baum S. Photobiomodulation therapy in breast cancer-related lymphedema: a randomized placebo-controlled trial. Photodermatology photoimmunology photomedicine (2017) 33(1):32–40. doi: 10.1111/phpp.12284 [DOI] [PubMed] [Google Scholar]
- 146. Kilmartin L, Denham T, Fu MR, Yu G, Kuo TT, Axelrod D, et al. Complementary low-level laser therapy for breast cancer-related lymphedema: a pilot, double-blind, randomized, placebo-controlled study. Lasers Med Sci (2020) 35(1):95–105. doi: 10.1007/s10103-019-02798-1 [DOI] [PubMed] [Google Scholar]
- 147. Brand JG. Within reach of an end to unnecessary bitterness? Lancet (2000) 356(9239):1371–2. doi: 10.1016/S0140-6736(00)02836-1 [DOI] [PubMed] [Google Scholar]
- 148. Heckmann SM, Hujoel P, Habiger S, Friess W, Wichmann M, Heckmann JG, et al. Zinc gluconate in the treatment of dysgeusia–a randomized clinical trial. J Dent Res (2005) 84(1):35–8. doi: 10.1177/154405910508400105 [DOI] [PubMed] [Google Scholar]
- 149. Boltong A, Aranda S, Keast R, Wynne R, Francis PA, Chirgwin J, et al. A prospective cohort study of the effects of adjuvant breast cancer chemotherapy on taste function, food liking, appetite and associated nutritional outcomes. PloS One (2014) 9(7):e103512. doi: 10.1371/journal.pone.0103512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Ponticelli E, Clari M, Frigerio S, De Clemente A, Bergese I, Scavino E, et al. Dysgeusia and health-related quality of life of cancer patients receiving chemotherapy: A cross-sectional study. Eur J Cancer Care (2017) 26(2):e12633. doi: 10.1111/ecc.12633 [DOI] [PubMed] [Google Scholar]
- 151. Hovan AJ, Williams PM, Stevenson-Moore P, Wahlin YB, Ohrn KE, Elting LS, et al. A systematic review of dysgeusia induced by cancer therapies. Supportive Care Cancer (2010) 18(8):1081–7. doi: 10.1007/s00520-010-0902-1 [DOI] [PubMed] [Google Scholar]
- 152. Epstein JB, Smutzer G, Doty RL. Understanding the impact of taste changes in oncology care. Supportive Care Cancer (2016) 24(4):1917–31. doi: 10.1007/s00520-016-3083-8 [DOI] [PubMed] [Google Scholar]
- 153. Mobadder ME, Farhat F, Mobadder WE, Nammour S. Photobiomodulation therapy in the treatment of oral mucositis, dysgeusia and oral dryness as side-effects of head and neck radiotherapy in a cancer patient: A case report. Dent J (Basel) (2018) 6(4):1–6. doi: 10.3390/dj6040064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Dijkstra PU, Huisman PM, Roodenburg JL. Criteria for trismus in head and neck oncology. Int J Oral Maxillofac Surg (2006) 35(4):337–42. doi: 10.1016/j.ijom.2005.08.001 [DOI] [PubMed] [Google Scholar]
- 155. Vissink A, Jansma J, Spijkervet FK, Burlage FR, Coppes RP. Oral sequelae of head and neck radiotherapy. Crit Rev Oral Biol Med (2003) 14(3):199–212. doi: 10.1177/154411130301400305 [DOI] [PubMed] [Google Scholar]
- 156. Bensadoun RJ, Riesenbeck D, Lockhart PB, Elting LS, Spijkervet FK, Brennan MT, et al. A systematic review of trismus induced by cancer therapies in head and neck cancer patients. Supportive Care Cancer (2010) 18(8):1033–8. doi: 10.1007/s00520-010-0847-4 [DOI] [PubMed] [Google Scholar]
- 157. Teguh DN, Levendag PC, Voet P, van der Est H, Noever I, de Kruijf W, et al. Trismus in patients with oropharyngeal cancer: relationship with dose in structures of mastication apparatus. Head Neck (2008) 30(5):622–30. doi: 10.1002/hed.20760 [DOI] [PubMed] [Google Scholar]
- 158. Goldstein M, Maxymiw WG, Cummings BJ, Wood RE. The effects of antitumor irradiation on mandibular opening and mobility: a prospective study of 58 patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod (1999) 88(3):365–73. doi: 10.1016/S1079-2104(99)70044-2 [DOI] [PubMed] [Google Scholar]
- 159. Wang CJ, Huang EY, Hsu HC, Chen HC, Fang FM, Hsiung CY. The degree and time-course assessment of radiation-induced trismus occurring after radiotherapy for nasopharyngeal cancer. Laryngoscope (2005) 115(8):1458–60. doi: 10.1097/01.mlg.0000171019.80351.46 [DOI] [PubMed] [Google Scholar]
- 160. Dijkstra PU, Kalk WW, Roodenburg JL. Trismus in head and neck oncology: a systematic review. Oral Oncol (2004) 40(9):879–89. doi: 10.1016/j.oraloncology.2004.04.003 [DOI] [PubMed] [Google Scholar]
- 161. van der Molen L, van Rossum MA, Burkhead LM, Smeele LE, Hilgers FJ. Functional outcomes and rehabilitation strategies in patients treated with chemoradiotherapy for advanced head and neck cancer: a systematic review. Eur Arch Otorhinolaryngol (2009) 266(6):889–900. doi: 10.1007/s00405-008-0817-3 [DOI] [PubMed] [Google Scholar]
- 162. van der Molen L, Heemsbergen WD, de Jong R, van Rossum MA, Smeele LE, Rasch CR, et al. Dysphagia and trismus after concomitant chemo-Intensity-Modulated radiation therapy (chemo-IMRT) in advanced head and neck cancer; dose-effect relationships for swallowing and mastication structures. Radiotherapy Oncol (2013) 106(3):364–9. doi: 10.1016/j.radonc.2013.03.005 [DOI] [PubMed] [Google Scholar]
- 163. Carnaby-Mann G, Crary MA, Schmalfuss I, Amdur R. “Pharyngocise”: randomized controlled trial of preventative exercises to maintain muscle structure and swallowing function during head-and-neck chemoradiotherapy. Int J Radiat oncology biology physics (2012) 83(1):210–9. doi: 10.1016/j.ijrobp.2011.06.1954 [DOI] [PubMed] [Google Scholar]
- 164. Bernal Rodriguez CG, CdP E, Aranha ACC, de Freitas PM. Photobiomodulation with low-level laser in the treatment of trismus after radiotherapy: a case report. Photobiomodulation Photomedicine Laser Surg (2019) 37(4):240–3. doi: 10.1089/photob.2018.4524 [DOI] [PubMed] [Google Scholar]
- 165. Chrcanovic BR, Reher P, Sousa AA, Harris M. Osteoradionecrosis of the jaws–a current overview–part 1: Physiopathology and risk and predisposing factors. Oral Maxillofac Surg (2010) 14(1):3–16. doi: 10.1007/s10006-009-0198-9 [DOI] [PubMed] [Google Scholar]
- 166. Chrcanovic BR, Reher P, Sousa AA, Harris M. Osteoradionecrosis of the jaws–a current overview–part 2: dental management and therapeutic options for treatment. Oral Maxillofac Surg (2010) 14(2):81–95. doi: 10.1007/s10006-010-0205-1 [DOI] [PubMed] [Google Scholar]
- 167. Kurzweg T, Mockelmann N, Laban S, Knecht R. Current treatment options for recurrent/metastatic head and neck cancer: a post-ASCO 2011 update and review of last year’s literature. Eur Arch Otorhinolaryngol (2012) 269(10):2157–67. doi: 10.1007/s00405-012-1998-3 [DOI] [PubMed] [Google Scholar]
- 168. Paulo S, Abrantes AM, Laranjo M, Carvalho L, Serra A, Botelho MF, et al. Bisphosphonate-related osteonecrosis of the jaw: specificities. Oncol Rev (2014) 8(2):254. doi: 10.4081/oncol.2014.254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Epstein JB, Wong FL, Stevenson-Moore P. Osteoradionecrosis: clinical experience and a proposal for classification. J Oral Maxillofac Surg (1987) 45(2):104–10. doi: 10.1016/0278-2391(87)90399-5 [DOI] [PubMed] [Google Scholar]
- 170. Delanian S, Lefaix JL. The radiation-induced fibroatrophic process: therapeutic perspective via the antioxidant pathway. Radiotherapy Oncol (2004) 73(2):119–31. doi: 10.1016/j.radonc.2004.08.021 [DOI] [PubMed] [Google Scholar]
- 171. Bagan JV, Jimenez Y, Hernandez S, Murillo J, Diaz JM, Poveda R, et al. Osteonecrosis of the jaws by intravenous bisphosphonates and osteoradionecrosis: a comparative study. Med Oral Patol Oral Cir Bucal (2009) 14(12):e616–9. doi: 10.4317/medoral.14.e616 [DOI] [PubMed] [Google Scholar]
- 172. Madrid C, Abarca M, Bouferrache K. Osteoradionecrosis: an update. Oral Oncol (2010) 46(6):471–4. doi: 10.1016/j.oraloncology.2010.03.017 [DOI] [PubMed] [Google Scholar]
- 173. West C, Azria D, Chang-Claude J, Davidson S, Lambin P, Rosenstein B, et al. The REQUITE project: validating predictive models and biomarkers of radiotherapy toxicity to reduce side-effects and improve quality of life in cancer survivors. Clin Oncol (2014) 26(12):739–42. doi: 10.1016/j.clon.2014.09.008 [DOI] [PubMed] [Google Scholar]
- 174. Binello PB, Bandelloni R, Labanca M, Buffoli B, Rezzani R, Rodella LF. Osteonecrosis and the jaws and bevacizumab therapy: A case report. Int J Immunopathology Pharmacol (2012) 25(3):789–91. doi: 10.1177/039463201202500328 [DOI] [PubMed] [Google Scholar]
- 175. Sivolella S, Lumachi F, Stellini E, Favero L. Denosumab and anti-angiogenetic drug-related osteonecrosis of the jaw: an uncommon but potentially severe disease. Anticancer Res (2013) 33(5):1793–7. [PubMed] [Google Scholar]
- 176. Estilo CL, Fornier M, Farooki A, Carlson D, Bohle G, 3rd, Huryn JM. Osteonecrosis of the jaw related to bevacizumab. J Clin Oncol (2008) 26(24):4037–8. doi: 10.1200/JCO.2007.15.5424 [DOI] [PubMed] [Google Scholar]
- 177. Santos-Silva AR, Belizario Rosa GA, Castro Junior G, Dias RB, Prado Ribeiro AC, Brandao TB. Osteonecrosis of the mandible associated with bevacizumab therapy. Oral surgery Oral medicine Oral Pathol Oral radiology (2013) 115(6):e32–6. doi: 10.1016/j.oooo.2013.02.001 [DOI] [PubMed] [Google Scholar]
- 178. Koch FP, Walter C, Hansen T, Jager E, Wagner W. Osteonecrosis of the jaw related to sunitinib. Oral Maxillofac Surg (2011) 15(1):63–6. doi: 10.1007/s10006-010-0224-y [DOI] [PubMed] [Google Scholar]
- 179. Nicolatou-Galitis O, Migkou M, Psyrri A, Bamias A, Pectasides D, Economopoulos T, et al. Gingival bleeding and jaw bone necrosis in patients with metastatic renal cell carcinoma receiving sunitinib: report of 2 cases with clinical implications. Oral surgery Oral medicine Oral Pathol Oral radiology (2012) 113(2):234–8. doi: 10.1016/j.tripleo.2011.08.024 [DOI] [PubMed] [Google Scholar]
- 180. Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, Mehrotra B, et al. American Association of oral and maxillofacial surgeons position paper on medication-related osteonecrosis of the jaw–2014 update. J Oral Maxillofac Surg (2014) 72(10):1938–56. doi: 10.1016/j.joms.2014.04.031 [DOI] [PubMed] [Google Scholar]
- 181. Da Cunha SS, Sarmento V, Ramalho LM, De Almeida D, Veeck EB, Da Costa NP, et al. Effect of laser therapy on bone tissue submitted to radiotherapy: experimental study in rats. Photomedicine Laser Surg (2007) 25(3):197–204. doi: 10.1089/pho.2007.2002 [DOI] [PubMed] [Google Scholar]
- 182. El-Maghraby EM, El-Rouby DH, Saafan AM. Assessment of the effect of low-energy diode laser irradiation on gamma irradiated rats’ mandibles. Arch Oral Biol (2013) 58(7):796–805. doi: 10.1016/j.archoralbio.2012.10.003 [DOI] [PubMed] [Google Scholar]
- 183. Batista JD, Zanetta-Barbosa D, Cardoso SV, Dechichi P, Rocha FS, Pagnoncelli RM. Effect of low-level laser therapy on repair of the bone compromised by radiotherapy. Lasers Med Sci (2014) 29(6):1913–8. doi: 10.1007/s10103-014-1602-8 [DOI] [PubMed] [Google Scholar]
- 184. Scoletta M, Arduino PG, Dalmasso P, Broccoletti R, Mozzati M. Treatment outcomes in patients with bisphosphonate-related osteonecrosis of the jaws: a prospective study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod (2010) 110(1):46–53. doi: 10.1016/j.tripleo.2010.02.020 [DOI] [PubMed] [Google Scholar]
- 185. Romeo U, Galanakis A, Marias C, Vecchio AD, Tenore G, Palaia G, et al. Observation of pain control in patients with bisphosphonate-induced osteonecrosis using low level laser therapy: preliminary results. Photomedicine Laser Surg (2011) 29(7):447–52. doi: 10.1089/pho.2010.2835 [DOI] [PubMed] [Google Scholar]
- 186. Vescovi P, Merigo E, Meleti M, Manfredi M, Guidotti R, Nammour S. Bisphosphonates-related osteonecrosis of the jaws: a concise review of the literature and a report of a single-centre experience with 151 patients. J Oral Pathol Med (2012) 41(3):214–21. doi: 10.1111/j.1600-0714.2011.01091.x [DOI] [PubMed] [Google Scholar]
- 187. Angiero F, Sannino C, Borloni R, Crippa R, Benedicenti S, Romanos GE. Osteonecrosis of the jaws caused by bisphosphonates: evaluation of a new therapeutic approach using the er : YAG laser. Lasers Med Sci (2009) 24(6):849–56. doi: 10.1007/s10103-009-0654-7 [DOI] [PubMed] [Google Scholar]
- 188. Atalay B, Yalcin S, Emes Y, Aktas I, Aybar B, Issever H, et al. Bisphosphonate-related osteonecrosis: laser-assisted surgical treatment or conventional surgery? Lasers Med Sci (2011) 26(6):815–23. doi: 10.1007/s10103-011-0974-2 [DOI] [PubMed] [Google Scholar]
- 189. Martins MA, Martins MD, Lascala CA, Curi MM, Migliorati CA, Tenis CA, et al. Association of laser phototherapy with PRP improves healing of bisphosphonate-related osteonecrosis of the jaws in cancer patients: a preliminary study. Oral Oncol (2012) 48(1):79–84. doi: 10.1016/j.oraloncology.2011.08.010 [DOI] [PubMed] [Google Scholar]
- 190. Altay MA, Tasar F, Tosun E, Kan B. Low-level laser therapy supported surgical treatment of bisphosphonate related osteonecrosis of jaws: a retrospective analysis of 11 cases. Photomedicine Laser Surg (2014) 32(8):468–75. doi: 10.1089/pho.2014.3742 [DOI] [PubMed] [Google Scholar]
- 191. Latifyan S, Genot MT, Klastersky J. Bisphosphonate-related osteonecrosis of the jaw: a review of the potential efficacy of low-level laser therapy. Supportive Care Cancer (2016) 24(9):3687–93. doi: 10.1007/s00520-016-3139-9 [DOI] [PubMed] [Google Scholar]
- 192. Colombo F, Neto Ade A, Sousa AP, Marchionni AM, Pinheiro AL, Reis SR. Effect of low-level laser therapy (lambda660 nm) on angiogenesis in wound healing: a immunohistochemical study in a rodent model. Braz Dent J (2013) 24(4):308–12. doi: 10.1590/0103-6440201301867 [DOI] [PubMed] [Google Scholar]
- 193. Epstein JB, Song PY, Ho AS, Larian B, Asher A, Bensadoun RJ. Photobiomodulation therapy: management of mucosal necrosis of the oropharynx in previously treated head and neck cancer patients. Supportive Care Cancer (2017) 25(4):1031–4. doi: 10.1007/s00520-016-3525-3 [DOI] [PubMed] [Google Scholar]
- 194. de Bataille C, Sibaud V, Prioul A, Laprie A, Vigarios E. Management of radiation-induced mucosal necrosis with photobiomodulation therapy. Supportive Care Cancer (2018) 26(8):2491–2. doi: 10.1007/s00520-017-3899-x [DOI] [PubMed] [Google Scholar]
- 195. Epstein JB, Song P, Ho A, Larian B, Asher A, Bensadoun RJ. Management of radiation-induced mucosal necrosis with photobiomodulation therapy. Supportive Care Cancer (2018) 26(8):2493. doi: 10.1007/s00520-018-4228-8 [DOI] [PubMed] [Google Scholar]
- 196. Jacobi I, van der Molen L, Huiskens H, van Rossum MA, Hilgers FJM. Voice and speech outcomes of chemoradiation for advanced head and neck cancer: a systematic review. Eur Arch oto-rhino-laryngology (2010) 267(10):1495–505. doi: 10.1007/s00405-010-1316-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. van der Molen L, van Rossum MA, Jacobi I, van Son RJ, Smeele LE, Rasch CR, et al. Pre- and posttreatment voice and speech outcomes in patients with advanced head and neck cancer treated with chemoradiotherapy: expert listeners’ and patient’s perception. J voice: Off J Voice Foundation (2012) 26(5):664.e25–33. doi: 10.1016/j.jvoice.2011.08.016 [DOI] [PubMed] [Google Scholar]
- 198. Meleca RJ, Dworkin JP, Kewson DT, Stachler RJ, Hill SL. Functional outcomes following nonsurgical treatment for advanced-stage laryngeal carcinoma. Laryngoscope (2003) 113(4):720–8. doi: 10.1097/00005537-200304000-00025 [DOI] [PubMed] [Google Scholar]
- 199. Johns MM, Kolachala V, Berg E, Muller S, Creighton FX, Branski RC. Radiation fibrosis of the vocal fold: from man to mouse. Laryngoscope (2012) 122 Suppl 5:S107–25. doi: 10.1002/lary.23735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. de Bruijn MJ, ten Bosch L, Kuik DJ, Quene H, Langendijk JA, Leemans CR, et al. Objective acoustic-phonetic speech analysis in patients treated for oral or oropharyngeal cancer. Folia Phoniatr Logop (2009) 61(3):180–7. doi: 10.1159/000219953 [DOI] [PubMed] [Google Scholar]
- 201. Hunter KU, Schipper M, Feng FY, Lyden T, Haxer M, Murdoch-Kinch CA, et al. Toxicities affecting quality of life after chemo-IMRT of oropharyngeal cancer: prospective study of patient-reported, observer-rated, and objective outcomes. Int J Radiat oncology biology physics (2013) 85(4):935–40. doi: 10.1016/j.ijrobp.2012.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Miller KK, Gorcey L, McLellan BN. Chemotherapy-induced hand-foot syndrome and nail changes: a review of clinical presentation, etiology, pathogenesis, and management. J Am Acad Dermatol (2014) 71(4):787–94. doi: 10.1016/j.jaad.2014.03.019 [DOI] [PubMed] [Google Scholar]
- 203. Wilkes GM, Doyle D. Palmar-plantar erythrodysesthesia. Clin J Oncol Nurs (2005) 9(1):103–6. doi: 10.1188/05.CJON.103-106 [DOI] [PubMed] [Google Scholar]
- 204. Webster-Gandy JD, How C, Harrold K. Palmar-plantar erythrodysesthesia (PPE): a literature review with commentary on experience in a cancer centre. Eur J Oncol Nurs (2007) 11(3):238–46. doi: 10.1016/j.ejon.2006.10.004 [DOI] [PubMed] [Google Scholar]
- 205. Lipworth AD, Robert C, Zhu AX. Hand-foot syndrome (hand-foot skin reaction, palmar-plantar erythrodysesthesia): focus on sorafenib and sunitinib. Oncology (2009) 77(5):257–71. doi: 10.1159/000258880 [DOI] [PubMed] [Google Scholar]
- 206. Chidharla A, Kasi A. Cancer, chemotherapy acral erythema (Palmar-plantar erythrodysesthesia, palmoplantar erythrodysesthesia, hand-foot syndrome). StatPearls Publishing, Treasure Island; (Florida, USA) (2020). [Google Scholar]
- 207. Abstracts of the MASCC/ISOO annual meeting 2018. Supportive Care Cancer (2018) 26(suppl 2):39–364. doi: 10.1007/s00520-018-4193-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet (2009) 373(9674):1550–61. doi: 10.1016/S0140-6736(09)60237-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Epstein JB, Raber-Durlacher JE, Huysmans MC, Schoordijk MCE, Cheng JE, Bensadoun RJ, et al. Photobiomodulation therapy alleviates tissue fibroses associated with chronic graft-versus-host disease: two case reports and putative anti-fibrotic roles of tgf-beta. Photomed Laser Surg (2018) 36(2):92–9. doi: 10.1089/pho.2017.4297 [DOI] [PubMed] [Google Scholar]
- 210. Aladag E, Kelkitli E, Goker H. Acute graft-versus-host disease: a brief review. Turk J Haematol (2020) 37(1):1–4. doi: 10.4274/tjh.galenos.2019.2019.0157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Justiz Vaillant AA, Mohammadi O. Graft versus host disease. StatPearls Publishing, Treasure Island (Florida, USA) (2020). [PubMed] [Google Scholar]
- 212. Gonzalez RM, Pidala J. Evolving therapeutic options for chronic graft-versus-Host disease. Pharmacotherapy (2020) 40(8):756–72. doi: 10.1002/phar.2427 [DOI] [PubMed] [Google Scholar]
- 213. Elshenawy HM, Eldin AM, Abdelmonem MA. Clinical assessment of the efficiency of low level laser therapy in the treatment of oral lichen planus. Open Access Maced J Med Sci (2015) 3(4):717–21. doi: 10.3889/oamjms.2015.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Derikvand N, Ghasemi SS, Moharami M, Shafiei E, Chiniforush N. Management of oral lichen planus by 980 nm diode laser. J Lasers Med Sci (2017) 8(3):150–4. doi: 10.15171/jlms.2017.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Al-Maweri SA, Kalakonda B, Al-Soneidar WA, Al-Shamiri HM, Alakhali MS, Alaizari N. Efficacy of low-level laser therapy in management of symptomatic oral lichen planus: a systematic review. Lasers Med Sci (2017) 32(6):1429–37. doi: 10.1007/s10103-017-2233-7 [DOI] [PubMed] [Google Scholar]
- 216. Mutafchieva MZ, Draganova-Filipova MN, Zagorchev PI, Tomov GT. Effects of low level laser therapy on erosive-atrophic oral lichen planus. Folia Med (Plovdiv) (2018) 60(3):417–24. doi: 10.2478/folmed-2018-0008 [DOI] [PubMed] [Google Scholar]
- 217. Hoseinpour Jajarm H, Asadi R, Bardideh E, Shafaee H, Khazaei Y, Emadzadeh M. The effects of photodynamic and low-level laser therapy for treatment of oral lichen planus-a systematic review and meta-analysis. Photodiagnosis Photodyn Ther (2018) 23:254–60. doi: 10.1016/j.pdpdt.2018.07.001 [DOI] [PubMed] [Google Scholar]
- 218. Bhattacharya PT, Patil K, Guledgud MV. Effectiveness of 904nm gallium-arsenide diode laser in treatment of oral lichen planus: Report of 2 cases. Actas Dermosifiliogr (2019) 110(4):325–7. doi: 10.1016/j.adengl.2019.02.009 [DOI] [PubMed] [Google Scholar]
- 219. Mirza S, Rehman N, Alrahlah A, Alamri WR, Vohra F. Efficacy of photodynamic therapy or low level laser therapy against steroid therapy in the treatment of erosive-atrophic oral lichen planus. Photodiagnosis Photodyn Ther (2018) 21:404–8. doi: 10.1016/j.pdpdt.2018.02.001 [DOI] [PubMed] [Google Scholar]
- 220. Akram Z, Abduljabbar T, Vohra F, Javed F. Efficacy of low-level laser therapy compared to steroid therapy in the treatment of oral lichen planus: A systematic review. J Oral Pathol Med (2018) 47(1):11–7. doi: 10.1111/jop.12619 [DOI] [PubMed] [Google Scholar]
- 221. Epstein JB, Raber-Durlacher JE, Lill M, Linhares YP, Chang J, Barasch A, et al. Photobiomodulation therapy in the management of chronic oral graft-versus-host disease. Supportive Care Cancer (2017) 25(2):357–64. doi: 10.1007/s00520-016-3401-1 [DOI] [PubMed] [Google Scholar]
- 222. Elad S, Or R, Shapira MY, Haviv A, Galili D, Garfunkel AA, et al. CO2 laser in oral graft-versus-host disease: a pilot study. Bone Marrow Transplant (2003) 32(10):1031–4. doi: 10.1038/sj.bmt.1704272 [DOI] [PubMed] [Google Scholar]
- 223. Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, MacLeod MR, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain (2014) 155(12):2461–70. doi: 10.1016/j.pain.2014.09.020 [DOI] [PubMed] [Google Scholar]
- 224. Rivera E, Cianfrocca M. Overview of neuropathy associated with taxanes for the treatment of metastatic breast cancer. Cancer Chemother Pharmacol (2015) 75(4):659–70. doi: 10.1007/s00280-014-2607-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. LaPointe NE, Morfini G, Brady ST, Feinstein SC, Wilson L, Jordan MA. Effects of eribulin, vincristine, paclitaxel and ixabepilone on fast axonal transport and kinesin-1 driven microtubule gliding: implications for chemotherapy-induced peripheral neuropathy. Neurotoxicology (2013) 37:231–9. doi: 10.1016/j.neuro.2013.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Staff NP, Grisold A, Grisold W, Windebank AJ. Chemotherapy-induced peripheral neuropathy: A current review. Ann neurology (2017) 81(6):772–81. doi: 10.1002/ana.24951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Hong JS, Tian J, Wu LH. The influence of chemotherapy-induced neurotoxicity on psychological distress and sleep disturbance in cancer patients. Curr Oncol (2014) 21(4):174–80. doi: 10.3747/co.21.1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Pike CT, Birnbaum HG, Muehlenbein CE, Pohl GM, Natale RB. Healthcare costs and workloss burden of patients with chemotherapy-associated peripheral neuropathy in breast, ovarian, head and neck, and nonsmall cell lung cancer. Chemotherapy Res Practice (2012) 2012:913848. doi: 10.1155/2012/913848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Izycki D, Niezgoda AA, Kazmierczak M, Piorunek T, Izycka N, Karaszewska B, et al. Chemotherapy-induced peripheral neuropathy - diagnosis, evolution and treatment. Ginekologia polska (2016) 87(7):516–21. doi: 10.5603/GP.2016.0036 [DOI] [PubMed] [Google Scholar]
- 230. Khamseh ME, Kazemikho N, Aghili R, Forough B, Lajevardi M, Hashem Dabaghian F, et al. Diabetic distal symmetric polyneuropathy: effect of low-intensity laser therapy. Lasers Med science (2011) 26(6):831–5. doi: 10.1007/s10103-011-0977-z [DOI] [PubMed] [Google Scholar]
- 231. De Iuliis F, Taglieri L, Salerno G, Lanza R, Scarpa S. Taxane induced neuropathy in patients affected by breast cancer: Literature review. Crit Rev Oncol Hematol (2015) 96(1):34–45. doi: 10.1016/j.critrevonc.2015.04.011 [DOI] [PubMed] [Google Scholar]
- 232. Hsieh YL, Chou LW, Chang PL, Yang CC, Kao MJ, Hong CZ. Low-level laser therapy alleviates neuropathic pain and promotes function recovery in rats with chronic constriction injury: possible involvements in hypoxia-inducible factor 1alpha (HIF-1alpha). J Comp Neurol (2012) 520(13):2903–16. doi: 10.1002/cne.23072 [DOI] [PubMed] [Google Scholar]
- 233. Hsieh YL, Fan YC, Yang CC. Low-level laser therapy alleviates mechanical and cold allodynia induced by oxaliplatin administration in rats. Supportive Care Cancer (2016) 24(1):233–42. doi: 10.1007/s00520-015-2773-y [DOI] [PubMed] [Google Scholar]
- 234. de Andrade AL, Bossini PS, do Canto De Souza AL, Sanchez AD, Parizotto NA. Effect of photobiomodulation therapy (808 nm) in the control of neuropathic pain in mice. Lasers Med Sci (2017) 32(4):865–72. doi: 10.1007/s10103-017-2186-x [DOI] [PubMed] [Google Scholar]
- 235. Bashiri H. Evaluation of low level laser therapy in reducing diabetic polyneuropathy related pain and sensorimotor disorders. Acta Med Iranica (2013) 51(8):543–7. [PubMed] [Google Scholar]
- 236. Yamany AA, Sayed HM. Effect of low level laser therapy on neurovascular function of diabetic peripheral neuropathy. J Advanced Res (2012) 3(1):21–8. doi: 10.1016/j.jare.2011.02.009 [DOI] [Google Scholar]
- 237. Kumar S, Maiya AG, Hande HM, Vidyasagar S, Rao K, Rajagopal KV. Efficacy of low level laser therapy on painful diabetic peripheral neuropathy. Laser Ther (2015) 24(3):195–200. doi: 10.5978/islsm.15-OR-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Yamada K, Kaise H, Ogata A. Low-level laser therapy for symptoms induced by breast cancer treatments. ASCO San Antonio Breast Cancer Symposium (SABCS) (2010). weblink: www.sabcs.org. [Google Scholar]
- 239. Argenta PA, Ballman KV, Geller MA, Carson LF, Ghebre R, Mullany SA, et al. The effect of photobiomodulation on chemotherapy-induced peripheral neuropathy: A randomized, sham-controlled clinical trial. Gynecologic Oncol (2017) 144(1):159–66. doi: 10.1016/j.ygyno.2016.11.013 [DOI] [PubMed] [Google Scholar]
- 240. Hsieh YL, Chou LW, Hong SF, Chang FC, Tseng SW, Huang CC, et al. Laser acupuncture attenuates oxaliplatin-induced peripheral neuropathy in patients with gastrointestinal cancer: a pilot prospective cohort study. Acupuncture medicine: J Br Med Acupuncture Soc (2016) 34(5):398–405. doi: 10.1136/acupmed-2016-011112 [DOI] [PubMed] [Google Scholar]
- 241. Lodewijckx J, Robijns J, Bensadoun RJ, Mebis J. Photobiomodulation therapy for the management of chemotherapy-induced peripheral neuropathy: an overview. Photobiomodul Photomed Laser Surg (2020) 38(6):348–54. doi: 10.1089/photob.2019.4771 [DOI] [PubMed] [Google Scholar]
- 242. Ridner SH, Dietrich MS, Niermann K, Cmelak A, Mannion K, Murphy B. A prospective study of the lymphedema and fibrosis continuum in patients with head and neck cancer. Lymphatic Res Biol (2016) 14(4):198–205. doi: 10.1089/lrb.2016.0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Straub JM, New J, Hamilton CD, Lominska C, Shnayder Y, Thomas SM. Radiation-induced fibrosis: mechanisms and implications for therapy. J Cancer Res Clin Oncol (2015) 141(11):1985–94. doi: 10.1007/s00432-015-1974-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Dorr W, Hendry JH. Consequential late effects in normal tissues. Radiotherapy oncology: J Eur Soc Ther Radiol Oncol (2001) 61(3):223–31. doi: 10.1016/S0167-8140(01)00429-7 [DOI] [PubMed] [Google Scholar]
- 245. Villa A, Sonis S. Toxicities associated with head and neck cancer treatment and oncology-related clinical trials. Curr Problems Cancer (2016) 40(5-6):244–57. doi: 10.1016/j.currproblcancer.2016.06.001 [DOI] [PubMed] [Google Scholar]
- 246. Mamalis A, Koo E, Tepper C, Jagdeo J. MicroRNA expression analysis of human skin fibroblasts treated with high-fluence light-emitting diode-red light. J Biophotonics (2018) 11:e201800207. doi: 10.1002/jbio.201800207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Mamalis A, Siegel D, Jagdeo J. Visible red light emitting diode photobiomodulation for skin fibrosis: key molecular pathways. Curr Dermatol Rep (2016) 5:121–8. doi: 10.1007/s13671-016-0141-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Mamalis A, Jagdeo J. The combination of resveratrol and high-fluence light emitting diode-red light produces synergistic photobotanical inhibition of fibroblast proliferation and collagen synthesis: a novel treatment for skin fibrosis. Dermatologic Surg (2017) 43(1):81–6. doi: 10.1097/DSS.0000000000000921 [DOI] [PubMed] [Google Scholar]
- 249. Mamalis A, Koo E, Garcha M, Murphy WJ, Isseroff RR, Jagdeo J. High fluence light emitting diode-generated red light modulates characteristics associated with skin fibrosis. J Biophotonics (2016) 9(11-12):1167–79. doi: 10.1002/jbio.201600059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Tam M, Arany PR, Robijns J, Vasconcelos R, Corby P, Hu K. Photobiomodulation therapy to mitigate radiation fibrosis syndrome. Photobiomodul Photomed Laser Surg (2020) 38(6):355–63. doi: 10.1089/photob.2019.4766 [DOI] [PubMed] [Google Scholar]
- 251. Trueb RM. Chemotherapy-induced hair loss. Skin Ther letter (2010) 15(7):5–7. [PubMed] [Google Scholar]
- 252. Rosman S. Cancer and stigma: experience of patients with chemotherapy-induced alopecia. Patient Educ counseling (2004) 52(3):333–9. doi: 10.1016/S0738-3991(03)00040-5 [DOI] [PubMed] [Google Scholar]
- 253. Browall M, Gaston-Johansson F, Danielson E. Postmenopausal women with breast cancer: their experiences of the chemotherapy treatment period. Cancer nursing (2006) 29(1):34–42. doi: 10.1097/00002820-200601000-00006 [DOI] [PubMed] [Google Scholar]
- 254. Hesketh PJ, Batchelor D, Golant M, Lyman GH, Rhodes N, Yardley D. Chemotherapy-induced alopecia: psychosocial impact and therapeutic approaches. Supportive Care Cancer (2004) 12(8):543–9. doi: 10.1007/s00520-003-0562-5 [DOI] [PubMed] [Google Scholar]
- 255. Choi EK, Kim IR, Chang O, Kang D, Nam SJ, Lee JE, et al. Impact of chemotherapy-induced alopecia distress on body image, psychosocial well-being, and depression in breast cancer patients. Psycho-oncology (2014) 23(10):1103–10. doi: 10.1002/pon.3531 [DOI] [PubMed] [Google Scholar]
- 256. Duvic M, Lemak NA, Valero V, Hymes SR, Farmer KL, Hortobagyi GN, et al. A randomized trial of minoxidil in chemotherapy-induced alopecia. J Am Acad Dermatol (1996) 35(1):74–8. doi: 10.1016/S0190-9622(96)90500-9 [DOI] [PubMed] [Google Scholar]
- 257. Yang X, Thai KE. Treatment of permanent chemotherapy-induced alopecia with low dose oral minoxidil. Australas J Dermatol (2016) 57(4):e130–e2. doi: 10.1111/ajd.12350 [DOI] [PubMed] [Google Scholar]
- 258. Ross M, Fischer-Cartlidge E. Scalp cooling: a literature review of efficacy, safety, and tolerability for chemotherapy-induced alopecia. Clin J Oncol Nurs (2017) 21(2):226–33. doi: 10.1188/17.CJON.226-233 [DOI] [PubMed] [Google Scholar]
- 259. Dodd EM, Winter MA, Hordinsky MK, Sadick NS, Farah RS. Photobiomodulation therapy for androgenetic alopecia: A clinician’s guide to home-use devices cleared by the federal drug administration. J cosmetic laser Ther (2017) 9:1–9. doi: 10.1080/14764172.2017.1383613 [DOI] [PubMed] [Google Scholar]
- 260. Hamblin MR. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS biophysics (2017) 4(3):337–61. doi: 10.3934/biophy.2017.3.337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Darwin E, Heyes A, Hirt PA, Wikramanayake TC, Jimenez JJ. Low-level laser therapy for the treatment of androgenic alopecia: a review. Lasers Med Sci (2018) 33(2):425–34. doi: 10.1007/s10103-017-2385-5 [DOI] [PubMed] [Google Scholar]
- 262. Peterson DE, Bensadoun RJ, Roila F, Group EGW. Management of oral and gastrointestinal mucositis: ESMO clinical practice guidelines. Ann Oncol (2011) 22 Suppl 6:vi78–84. doi: 10.1093/annonc/mdr391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Interventional procedure overview of low-level laser therapy for preventing or treating oral mucositis caused by radiotherapy or chemotherapy (2018). Available at: https://www.nice.org.uk/guidance/ipg615.
- 264. Pedersen AM, Bardow A, Jensen SB, Nauntofte B. Saliva and gastrointestinal functions of taste, mastication, swallowing and digestion. Oral Dis (2002) 8(3):117–29. doi: 10.1034/j.1601-0825.2002.02851.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.